Abstract
Objectives:
Varying statin exposures in bacteremic patients have different impacts on mortality. Among patients with adherent statin use, we sought to evaluate the impact of statin continuation on inpatient mortality in bacteremic patients.
Methods:
A retrospective cohort study was conducted using Optum ClinformaticsTM with matched Premier Hospital data (October 2009–March 2013). Patients with a primary diagnosis of bacteremia and 6 months of continuous enrollment prior to the admission, receiving antibiotics at least 2 days of antibiotics during the first 3 days of admission, were selected for inclusion. Furthermore, patients demonstrating adherent statin use based on 90 days of continuous therapy prior to admission were included. We then compared those continuing statin therapy for at least the first 5 days after admission and those not continuing during the admission.
Results:
Simvastatin (53.2%) and atorvastatin (33.8%) were the most commonly used statins among the 633 patients who met our inclusion and exclusion criteria. Propensity score adjusted Cox proportional hazards regression models demonstrated significantly lower inpatient mortality among those continuing statin therapy compared with those not continuing (n = 232 vs 401, adjusted hazard ratio 0.25, 95% confidence interval 0.08–0.79).
Conclusion:
Among patients adherent to their statin therapy prior to a bacteremia hospitalization, continued statin use after admission increased survival by 75% compared with those not continuing.
Keywords: Bacteremia, mortality, statins, exposure patterns, protective effects
Introduction
Statins have been associated with improved survival among patients with infections,1–7 in meta-analyses,8,9 randomized trials, and observational studies.4,5,7,10 The proposed pleiotropic effects with statins, including a reduced inflammatory response,11,12 suggest that the critical period of exposure would be from time of onset of infection through the initial period of antibiotic therapy.5,10,13 Importantly, that period of onset is likely to occur outside of the healthcare setting, and therefore there will be a delay between onset and presentation to receive care. In previous research, definitions of statin exposure vary widely between studies, tend to be overly broad, and rarely take into account statin therapy adherence.3–5 This study sought to evaluate the impact of statin continuation during hospital admission compared with non-continuation, in a cohort of adherent statin users.
Methods
A retrospective cohort study design was used to assess inpatient mortality among adherent statin users. This study was conducted using de-identified Optum ClinformaticsTM (OptumInsight, Eden Prairie, MN) with matched Premier Hospital data (1 October 2009–31 March 2013), which is an administrative claims database from a large commercial health plan (Optum Clinformatics) matched with hospital data (Premier). Included in the analysis were adult patients (>18 years) with a primary diagnosis of bacteremia (International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes 003.1, 020.2, 022.3, 036.2, 038.0, 038.1, 038.10–038.12, 038.19, 038.2, 038.3, 038.40–038.44, 038.49, 038.8, 038.9, 054.5, 449, 771.81, 995.91, 995.92, and 790.7).14 We only included patients with hospital admissions between 1 April 2010 and 31 March 2013, to allow for a continuous enrollment period of 6 months prior to admission. Antibiotic therapy for each patient during the hospital stay was assessed. Patients who received at least 2 consecutive days of at least one antibiotic therapy for bacteremia15–18 within the first 3 days of the admission were included. For patients with multiple admissions for bacteremia, only the first admission was included. Medication use was identified from both outpatient prescriptions and medications given during the hospital stay. Charlson and Elixhauser comorbidity scores were defined using diagnosis codes.14
Using pharmacy claims, we identified prevalent statins users demonstrating adherence to their statin therapy, which was defined as patients who, irrespective of their statin initiation time, had at least 90 days of continuous statin exposure (i.e. atorvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin) in the 90 days prior to hospitalization. In terms of adherence measures, the proportion of days covered was therefore 100% for all included patients, at least 90 days of supply dispensed in the 90 days prior to admission. The proportion of days covered (PDC) of ⩾80% is commonly considered as good adherence for statins.19,20 Statin exposure was further categorized as statin continuation, with at least 5 days of statin therapy after admission, and non-continuation after admission. Inpatient mortality was defined as death occurring during the hospital stay.
To identify baseline differences between the statin continuation and non-continuation groups, we reviewed demographic and clinical data including current and prior comorbidities.14 For categorical variables, if the assumptions for the chi-square test were not met, the Fisher’s exact test was utilized. For continuous variables, the non-parametric Wilcoxon Rank Sum test was used.
We developed a propensity score for statin continuation versus non-continuation. The propensity score was therefore the predicted probability of statin therapy continuation, as calculated from the baseline covariates included in an unconditional logistic regression model which was built with manual backward elimination.21–23 Patients from the statin continuation and non-continuation groups were stratified by propensity score quintile to achieve homogeneity between exposure groups within quintiles of the predicted probability of statin continuation.24 To evaluate the association of continued statin use and in-hospital mortality, we used a Cox proportional hazards model. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and statistical significance was considered a p-value of ⩽0.05. This study was reviewed and approved as exempt by the University of Rhode Island’s Institutional Review Board.
Results
We identified 633 patients who met our inclusion and exclusion criteria (Figure 1). This included 232 patients with statin continuation and 401 with non-continuation. In comparing the statin continuation and non-continuation groups (Table 1), age (median 62 vs 61 years) and gender (43% vs 44% females) were similar. The median Charlson comorbidity index during admission (2.0 vs 2.0, p = 0.57; Table 2) and during the 6 months prior to admission (3.0 vs 3.0, p = 0. 97) was the same in both the groups. Admission from the emergency room occurred for 97% of patients with statin continuation and 95% with non-continuation (p = 0.29). Marital status, race, region, and admitting physician specialty were also similar between the statin continuation versus non-continuation groups. Simvastatin (53.2%) and atorvastatin (33.8%) were the most commonly used statins. Inpatient mortality was significantly lower (2.59% vs 10.97%, p = 0.0002) and length of stay was higher (median 6.0, interquartile range (IQR) 5.0–9.0 vs 5.0 days, IQR 3.0–9.0, p < 0.0001) in those continuing statins compared with those not continuing. The final propensity score model c-statistic was 0.88, suggesting a strong model for predicting the probability of statin continuation.23 Among bacteremic patients with adherent statin use prior to admission, the propensity score adjusted Cox proportional hazards regression model evaluating time to inpatient mortality demonstrated significantly lower inpatient mortality among those continuing statin therapy (hazard ratio (HR) 0.25, 95% CI 0.08–0.79) compared with those not continuing after admission.
Figure 1.
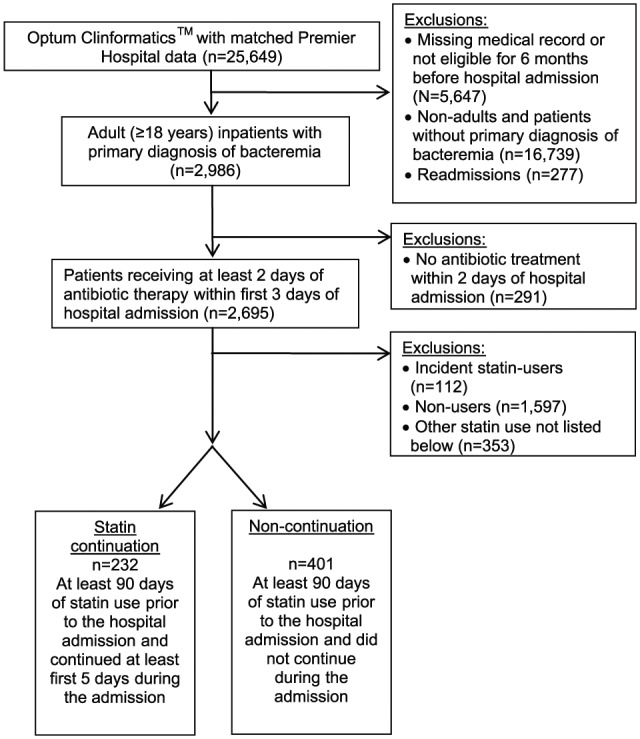
Study cohort identification.
Table 1.
Demographic and hospitalization-related characteristics among adherent statin users prior to admission.
| Characteristics | Statin continuation (N = 232) |
Non-continuation (N = 401) |
p value | ||
|---|---|---|---|---|---|
| N /median |
% /IQR |
N /median |
% /IQR |
||
| Age (years) | 62 | 55–76 | 61 | 56–68 | 0.1928 |
| Gender | 0.6749 | ||||
| Female | 99 | 42.67 | 178 | 44.39 | |
| Male | 133 | 57.33 | 223 | 55.61 | |
| Race | 0.8387 | ||||
| Black | 17 | 7.3 | 34 | 8.5 | |
| Other | 33 | 14.2 | 53 | 13.2 | |
| White | 182 | 78.5 | 314 | 78.3 | |
| Census region | 0.1168 | ||||
| East North Central | 45 | 19.4 | 57 | 14.21 | |
| East South Central | 5 | 2.16 | 5 | 1.25 | |
| Middle Atlantic | 10 | 4.31 | 21 | 5.24 | |
| Mountain | 13 | 5.60 | 22 | 5.49 | |
| New England | 7 | 3.02 | 5 | 1.25 | |
| Pacific | 27 | 11.64 | 32 | 7.98 | |
| South Atlantic | 64 | 27.59 | 153 | 38.15 | |
| West North Central | 27 | 11.64 | 45 | 11.22 | |
| West South Central | 34 | 14.66 | 61 | 15.21 | |
| Admission Type | 0.2932 | ||||
| Emergency | 225 | 96.98 | 382 | 95.26 | |
| Non-emergency | 7 | 3.02 | 19 | 4.74 | |
| Admitting physician facility | 0.7205 | ||||
| Intensive care/surgery | 8 | 3.45 | 18 | 4.49 | |
| Medicine | 93 | 40.09 | 167 | 41.65 | |
| Other | 131 | 56.47 | 216 | 53.87 | |
| Diagnosis-related group (DRG) description | 0.1988 | ||||
| Non-ventilation | 224 | 96.55 | 378 | 94.26 | |
| Ventilation | 8 | 3.45 | 23 | 5.74 | |
| Hospital admission year | 0.2553 | ||||
| 2010 | 41 | 17.67 | 85 | 21.20 | |
| 2011 | 95 | 40.95 | 143 | 35.66 | |
| 2012 | 96 | 41.38 | 173 | 43.15 | |
Data are median and interquartile range (IQR) or number and percent of patients.
Table 2.
Clinical characteristics and health service utilization among adherent statin users prior to admission.
| Characteristics | Statin continuation (N = 232) |
Non-continuation (N = 401) |
p value | ||
|---|---|---|---|---|---|
| N /median |
% /IQR |
N /median |
% /IQR |
||
| Comorbidities (during admission) | |||||
| Charlson score (median and IQR) | 2 | 1–4 | 2 | 0–3 | 0.5708 |
| Elixhauser score (median and IQR) | 4 | 3–6 | 4 | 3–6 | 0.3973 |
| Chronic renal disease | 61 | 26.29 | 78 | 19.45 | 0.0451 |
| Coagulation and hemorrhagic disorders | 27 | 11.64 | 84 | 20.95 | 0.0030 |
| Coagulopathy | 25 | 10.78 | 75 | 18.70 | 0.0084 |
| Congestive heart failure nonhypertensive | 64 | 27.59 | 60 | 14.96 | 0.0001 |
| Coronary atherosclerosis and other heart diseases | 87 | 37.50 | 115 | 28.68 | 0.0218 |
| Diabetes mellitus with complication | 61 | 26.29 | 69 | 17.21 | 0.0064 |
| Dyslipidemia including hyperlipidemia | 161 | 69.40 | 208 | 51.87 | <0.0001 |
| Infective arthritis | 15 | 6.47 | 11 | 2.74 | 0.0230 |
| Liver disease | 12 | 5.17 | 47 | 11.72 | 0.0063 |
| Malignant neoplasm | 6 | 2.59 | 54 | 13.47 | <0.0001 |
| Metastatic cancer | 3 | 1.29 | 30 | 7.48 | 0.0003 |
| Mild liver disease | 11 | 4.74 | 40 | 9.98 | 0.0197 |
| Obesity | 69 | 29.74 | 83 | 20.70 | 0.0103 |
| Poisoning by medication and drugs | 20 | 8.62 | 69 | 17.21 | 0.0027 |
| Solid tumor without metastasis | 5 | 2.16 | 49 | 12.22 | <0.0001 |
| Weight loss | 22 | 9.48 | 68 | 16.96 | 0.0095 |
| Medication use (during admission) | |||||
| Anti-hypertensive medication | 207 | 89.22 | 274 | 68.33 | <0.0001 |
| Comorbidities (6 months prior to admission) | |||||
| Charlson score (median and IQR) | 3 | 1–5 | 3 | 2–6 | 0. 9720 |
| Elixhauser score (median and IQR) | 6 | 5–9 | 5 | 3–9 | 0.7992 |
| History of any cancer | 35 | 15.09 | 102 | 25.44 | 0.0023 |
| History of chronic kidney disease | 47 | 20.26 | 57 | 14.21 | 0.048 |
| History of condition with dizziness or vertigo | 32 | 13.79 | 25 | 6.23 | 0.0014 |
| History of dyslipidemia including hyperlipidemia | 175 | 75.43 | 263 | 65.59 | 0.0097 |
| History of history of other Immunocompromise | 14 | 6.03 | 46 | 11.47 | 0.0244 |
| History of maintenance chemotherapy radiotherapy | 8 | 3.45 | 43 | 10.72 | 0.0012 |
| History of malignant neoplasm | 24 | 10.34 | 76 | 18.95 | 0.0042 |
| History of metastatic cancer | 7 | 3.02 | 37 | 9.23 | 0.0031 |
| History of other and ill-defined cerebrovascular diseases | 15 | 6.47 | 12 | 2.99 | 0.0372 |
| History of other eye disorder | 27 | 11.64 | 28 | 6.98 | 0.0451 |
| History of solid tumor without metastasis | 24 | 10.34 | 74 | 18.45 | 0.0066 |
| Medication use history (6 months prior to admission) | |||||
| History of diabetic medication | 74 | 31.9 | 112 | 27.93 | 0.0081 |
| History of anti-hypertensive medication | 199 | 85.78 | 317 | 79.05 | 0.0357 |
Data are median and interquartile range (IQR) or number and percent of patients.
Discussion
In this retrospective cohort study among privately insured patients with bacteremia, we observed higher survival among the statin continuation group compared with the non-continuation group. These results support statin continuation through the period of inflammation, as the inflammatory response has been found to be lower among patients taking statins around the time the infection develops.25,26 The specific mechanism by which mortality is reduced among statin users with bacteremic infections still remains undefined, however, a proposed mechanism has been the moderation of the overall inflammatory response.27 Other previously observed anti-inflammatory effects with statins have included lowering of C-reactive protein (CRP), chemokine release (MCP-1, RANTES), cytokines (IL-1β, TNF α, IL-6, IL-8), and adhesion molecules (P-selectin, VLA 4, CD11a, CD11b, CD18).28,29 Statins may also have a direct antimicrobial effect,30 and possible antibacterial activity of statins against a variety of pathogens may be attributed to their ability to suppress cell growth, and to promote apoptosis.31–33
Contrary to our findings, a recent randomized controlled trial (RCT) did not observe benefits of statin continuation on inflammatory parameters and sepsis among adherent statin users.34 However, the aforementioned study has several methodological issues as pointed out in a correspondence by Bostock and Vizcaychipi,35 including a vague primary endpoint, lack of information regarding previous statin therapy duration, and use of the Mann–Whitney test to evaluate the matched groups. The limitations of a number of previous studies evaluating protective effects of statins were (a) control for few confounders,2,7,10,27,36 (b) lack of information about pre-hospitalization medication use,10,36,37 (c) combined incident and adherent statin use,2,27,37 and (d) combined pre-hospital and post-hospital use.37,38 These limitations may explain the conflicting findings between studies in regard to the impact of statin use on mortality among patients with infections.
In terms of contrasting results between studies assessing the effects of statin continuation, a prospective cohort study conducted in Spain evaluated the survival benefits with statin use in S. aureus bacteremic (SAB) patients with at least 30 days prior statin use and continuation until SAB diagnosis, and did not observe significant protective effect of statins on 30-day mortality (odds ratio (OR) = 0.35; 95% CI: 0.10–1.23; p = 0.10).4 Conversely, a recent multicenter RCT conducted among 250 critically ill patients with severe sepsis (123 statins, 127 placebo) reported a significantly lower 28-day mortality rate in the adherent statin continuation group compared with the placebo group (5% vs 28%; p = 0.01).39 A retrospective cohort study conducted at a Veterans Affairs Medical Center in Washington among bacteremic patients, identified a therapeutic benefit with statin continuation (n = 35 vs 353, adjusted OR 0.13, 95% CI 0.02–0.99) compared with non-statin users.7
The results of this study have potential limitations. In our primary analysis, we evaluated adherent statin users, potentially leading to healthy user bias. We used time-varying analytic methods to mitigate the impact of survival bias. However, in the comparison group, one death was observed within the first 5 days of admission, and had this patient been excluded, the HR may have been closer to 1. Furthermore, the sample size of our study was small and we could not study the protective effects of each statin separately due to the small numbers. The effect of statins on inpatient mortality in patients with sepsis may be different for individual statins.40 We also could not assess the dose-dependent effects, changes in statin therapy (drug or dose) prior to admission, at admission, or during the admission, or the effects of adherence due to low sample sizes. In our review of statin doses, dispensing quantity in incident users mostly reflected moderate to high doses. As we used an administrative claims database for our analysis, we assumed outpatient statin exposure to be equivalent to filling a prescription. Furthermore, there is a possibility of statins having a different impact on clinical outcomes based on the causative pathogen, since the mechanism of action is not exactly known and it may vary for different pathogens. Microbiology data was not available for a potential causative pathogen, but we identified organisms using ICD-9 codes, where available. Bacteremic treatment varies by organism type and we were only able to use general inclusion criteria of having received an antibiotic which may be used for bacteremia.15–18 Since we only evaluated a general bacteremic population, our results may not be generalized to pathogen-specific bacteremias. Despite using propensity scores to control for confounding, we could not control for unmeasured confounding. Specifically, bacteremia severity scores and bacteremia source were not available from the data source, although we did control for ventilation status and sepsis severity using diagnosis-related groups (DRG).
In conclusion, our retrospective cohort study quantified the effect of adherent statin continuation on clinical outcomes such as inpatient mortality and hospital length of stay among bacteremic patients in a real-world clinical population. We observed significant reduction in inpatient morality among adherent statin continuation for at least the first few days after hospitalization compared with non-continuation during admission. Our results possibly hint at the necessity of statin exposure through the period of inflammation development as the inflammatory response has been found to be decreasing among patients consuming statins at the same time as developing infection.25,26 Further unaddressed questions related to this research question include appropriate statin exposure time and duration needed for the maximum clinical benefits, and differences in the magnitude of each statin’s protective effects.
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs. Substantial contributions to the conception and design of the work, or the acquisition, or analysis and interpretation of data are as follows. Design: A.M.P., K.L.L., and A.R.C.; Data: A.M.P., K.L.L., T.T.T., and A.R.C. Drafting the manuscript or revising it critically for important intellectual content: A.M.P., K.L.L., T.T.T., and A.R.C. Final approval of the version to be published: A.M.P., K.L.L., T.T.T., and A.R.C. Accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: A.M.P. and A.R.C. Presented, in part, at the 32nd International Conference on Pharmacoepidemiology and Therapeutic Risk Management, 28 August 2016.
Footnotes
Authors’ note: Tristan T Timbrook is now affiliated with Department of Pharmacy, University of Utah Health, Salt Lake City, Utah, USA.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ajinkya Pawar has no conflicts to disclose. Aisling Caffrey has received research funding from Pfizer, Merck (Cubist), and The Medicines Company. Tristan Timbrook has received honoraria for speaking and/or consulting from BioFire Diagnostics, GenMark Diagnostics, and Roche Diagnostics. Kerry LaPlante has received research funding or acted as a scientific advisor for Allergan, Bard, Merck (Cubist), Pfizer, and The Medicines Company.
Ethical approval: This study, which used existing de-identified data, was determined to be exempt from 45 CFR Part 46 by the University of Rhode Island’s Institutional Review Board per exemption 45 CFR 46.1010(b)(4).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent is not required in research determined to be exempt from 45 CFR Part 46.
ORCID iD: Aisling R Caffrey  https://orcid.org/0000-0002-4180-027X
https://orcid.org/0000-0002-4180-027X
References
- 1. Mortensen EM, Restrepo MI, Copeland LA, et al. Impact of previous statin and angiotensin II receptor blocker use on mortality in patients hospitalized with sepsis. Pharmacotherapy 2007; 27: 1619–1626. [DOI] [PubMed] [Google Scholar]
- 2. Dobesh PP, Klepser DG, McGuire TR, et al. Reduction in mortality associated with statin therapy in patients with severe sepsis. Pharmacotherapy 2009; 29: 621–630. [DOI] [PubMed] [Google Scholar]
- 3. Tseng MY, Hutchinson PJ, Czosnyka M, et al. Effects of acute pravastatin treatment on intensity of rescue therapy, length of inpatient stay, and 6-month outcome in patients after aneurysmal subarachnoid hemorrhage. Stroke 2007; 38: 1545–1550. [DOI] [PubMed] [Google Scholar]
- 4. Lopez-Cortes LE, Galvez-Acebal J, Del Toro MD, et al. Effect of statin therapy in the outcome of bloodstream infections due to Staphylococcus aureus: a prospective cohort study. PLoS ONE 2013; 8: e82958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kruger P, Fitzsimmons K, Cook D, et al. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med 2006; 32: 75–79. [DOI] [PubMed] [Google Scholar]
- 6. Hsu J, Andes DR, Knasinski V, et al. Statins are associated with improved outcomes of bloodstream infection in solid-organ transplant recipients. Eur J Clin Microbiol Infect Dis 2009; 28: 1343–1351. [DOI] [PubMed] [Google Scholar]
- 7. Liappis AP, Kan VL, Rochester CG, et al. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis 2001; 33: 1352–1357. [DOI] [PubMed] [Google Scholar]
- 8. Ma Y, Wen X, Peng J, et al. Systematic review and meta-analysis on the association between outpatient statins use and infectious disease-related mortality. PLoS ONE 2012; 7: e51548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janda S, Young A, Fitzgerald JM, et al. The effect of statins on mortality from severe infections and sepsis: a systematic review and meta-analysis. J Crit Care 2010; 25: 656.e7–652.e7. [DOI] [PubMed] [Google Scholar]
- 10. Thomsen RW, Hundborg HH, Johnsen SP, et al. Statin use and mortality within 180 days after bacteremia: a population-based cohort study. Crit Care Med 2006; 34: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 11. Fehr T, Kahlert C, Fierz W, et al. Statin-induced immunomodulatory effects on human T cells in vivo. Atherosclerosis 2004; 175: 83–90. [DOI] [PubMed] [Google Scholar]
- 12. Kwak B, Mulhaupt F, Myit S, et al. Statins as a newly recognized type of immunomodulator. Nat Med 2000; 6: 1399–1402. [DOI] [PubMed] [Google Scholar]
- 13. Caffrey AR, Timbrook TT, Noh E, et al. Evidence to support continuation of statin therapy in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2017; 61: e02228–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clinical Classifications Software (CCS) Healthcare Cost Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality, 2009. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/AppendixASingleDX.txt (accessed 27 November 2015). [Google Scholar]
- 15. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010; 50: 625–663. [DOI] [PubMed] [Google Scholar]
- 16. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11: 79–109. [DOI] [PubMed] [Google Scholar]
- 17. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59: e10–e52. [DOI] [PubMed] [Google Scholar]
- 18. Timsit JF, Laupland KB. Update on bloodstream infections in ICUs. Curr Opin Crit Care 2012; 18: 479–486. [DOI] [PubMed] [Google Scholar]
- 19. Kronish IM, Ross JS, Zhao H, et al. Impact of hospitalization for acute myocardial infarction on adherence to statins among older adults. Circ Cardiovasc Qual Outcomes 2016; 9: 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shroufi A, Powles JW. Adherence and chemoprevention in major cardiovascular disease: a simulation study of the benefits of additional use of statins. J Epidemiol Community Health 2010; 64: 109–113. [DOI] [PubMed] [Google Scholar]
- 21. D’Agostino RB., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17: 2265–2281. [DOI] [PubMed] [Google Scholar]
- 22. Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997; 127: 757–763. [DOI] [PubMed] [Google Scholar]
- 23. Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York: Wiley, 2000. [Google Scholar]
- 24. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Falagas ME, Makris GC, Matthaiou DK, et al. Statins for infection and sepsis: a systematic review of the clinical evidence. J Antimicrob Chemother 2008; 61: 774–785. [DOI] [PubMed] [Google Scholar]
- 26. Almog Y, Novack V, Eisinger M, et al. The effect of statin therapy on infection-related mortality in patients with atherosclerotic diseases. Crit Care Med 2007; 35: 372–378. [DOI] [PubMed] [Google Scholar]
- 27. O’Neal HR, Jr, Koyama T, Koehler EA, et al. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med 2011; 39: 1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Loecker I, Preiser JC. Statins in the critically ill. Ann Intensive Care 2012; 2: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arnaud C, Burger F, Steffens S, et al. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol 2005; 25: 1231–1236. [DOI] [PubMed] [Google Scholar]
- 30. Terblanche M, Almog Y, Rosenson RS, et al. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Dis 2007; 7: 358–368. [DOI] [PubMed] [Google Scholar]
- 31. Yamazaki H, Suzuki M, Aoki T, et al. Influence of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors on ubiquinone levels in rat skeletal muscle and heart: relationship to cytotoxicity and inhibitory activity for cholesterol synthesis in human skeletal muscle cells. J Atheroscler Thromb 2006; 13: 295–307. [DOI] [PubMed] [Google Scholar]
- 32. Muck AO, Seeger H, Wallwiener D. Class-specific pro-apoptotic effect of statins on human vascular endothelial cells. Z Kardiol 2004; 93: 398–402. [DOI] [PubMed] [Google Scholar]
- 33. Tapia-Perez JH, Kirches E, Mawrin C, et al. Cytotoxic effect of different statins and thiazolidinediones on malignant glioma cells. Cancer Chemother Pharmacol 2011; 67: 1193–1201. [DOI] [PubMed] [Google Scholar]
- 34. Kruger PS, Harward ML, Jones MA, et al. Continuation of statin therapy in patients with presumed infection: a randomized controlled trial. Am J Respir Crit Care Med 2011; 183: 774–781. [DOI] [PubMed] [Google Scholar]
- 35. Bostock GD, Vizcaychipi MP. Continuation of statin therapy in patients with presumed infection. Am J Respir Crit Care Med 2012; 185: 456. [DOI] [PubMed] [Google Scholar]
- 36. Leung S, Pokharel R, Gong MN. Statins and outcomes in patients with bloodstream infection: a propensity-matched analysis. Crit Care Med 2012; 40: 1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmidt H, Hennen R, Keller A, et al. Association of statin therapy and increased survival in patients with multiple organ dysfunction syndrome. Intensive Care Med 2006; 32: 1248–1251. [DOI] [PubMed] [Google Scholar]
- 38. Yang KC, Chien JY, Tseng WK, et al. Statins do not improve short-term survival in an oriental population with sepsis. Am J Emerg Med 2007; 25: 494–501. [DOI] [PubMed] [Google Scholar]
- 39. Kruger P, Bailey M, Bellomo R, et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med 2013; 187: 743–750. [DOI] [PubMed] [Google Scholar]
- 40. Ouellette DR, Moscoso EE, Corrales JP, et al. Sepsis outcomes in patients receiving statins prior to hospitalization for sepsis: comparison of in-hospital mortality rates between patients who received atorvastatin and those who received simvastatin. Ann Intensive Care 2015; 5: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]


