Abstract
Stereotactic body radiation therapy and stereotactic radiosurgery have become important treatment options for the treatment of spinal malignancies. A better understanding of dose tolerances with more conformal technology have allowed administration of higher and more ablative doses. In this review, the framework for approaching a patient with spinal metastases and primary tumors will be discussed as well as details on the delivery of this treatment.
Keywords: SBRT, spinal malignancies, tumor ablation, image-guided
Introduction
Stereotactic body radiotherapy (SBRT) involves the precise delivery of high dose per fraction radiotherapy (RT) to extracranial tumors. Its predecessor, stereotactic radiosurgery (SRS) to the brain, was first described by Leksell in 1951 and utilized a collimator helmet rigidly fixed to the skull for precise target localization accuracy.1,2 Although the spine shares some advantages with the brain as a treatment target, SBRT did not emerge until around 40 years later due to limitations in immobilization and localization outside the cranium as well as treatment planning technology.3
An individual vertebral body, such as the skull, is a bony structure that experiences minimal interfraction change and little intrafraction movement, in contrast to less rigid sites such as the lung or gastrointestinal (GI) tract.4 Although early endeavors attempted the use of rigid, surgically implanted frames similar to brain SRS, physicians soon found that the spine lends itself well to X-ray, and more recently computed tomography (CT)-based, image guidance due to its irregular shape and contrast from surrounding soft tissues.5–7
The evolution of treatment planning technology has also been closely tied with the development of spine SBRT. Without the benefit of a helmet to focus numerous beams at a single, precise isocenter simultaneously, as in frame-based radiosurgery, safe delivery of doses to irregular structures such as the the spine requires utilization of multiple beam angles or arcs. Intensity-modulated RT, developed in the 1980s to 1990s, and more recently volumetric-modulated arc therapy (VMAT) in particular have allowed for more conformal dose planning than was previously possible with conventional linear accelerator-based treatment, allowing safe delivery of ablative doses while sparing the spinal cord and other neighboring structures.8 As a result, high-dose treatment can be delivered to the spine in short courses of 1 to 5 fractions, that is, SBRT, and is now used in a variety of clinical settings including for metastatic disease and primary tumors of the spine.
Biology
The efficacy of SRS and SBRT lies in ability to deliver highly ablative doses to the spine with the help of image-guided technology. Recent studies of survivors with metastatic disease living longer than 5 years found local tumor control over 80% following high-dose, single-fraction RT.9 At the present time, there are no randomized trials confirming the superiority of single-fraction radiosurgery to SBRT. However, it is posited that exposing tumors to a dose per fraction of at least 8 Gy may active radiobiological pathways leading to tumor cell death through mechanisms apart from mitotic catastrophe and apoptosis.10
The dominant form of cell death when irradiated with conventional methods is apoptosis. Proposed mechanisms of increased cell death with SBRT include radiation-induced tumor antigen-specific immune response, endothelial/vascular injury, or increased cell kill secondary to higher delivered dose.11–19 Preclinical studies support the hypothesis that radiation-induced immunogenic tumor cell death contributes to an in situ vaccine.20,21 This idea has been further expanded with evidence showing radiation induces an immunogenic tumor cell death and alters the tumor microenvironment to enhance recruitment of antitumor T cells.22–24 A proposed mechanism of secondary immunogenic effects is shown in Figure 1. Within the application of SBRT, a preclinical study by Dewan et al found that fractionated local RT synergized with cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) blockade to induce antitumor T cells immunity and inhibit a second palpable tumor outside the radiation field. The difference between single fraction and SBRT was in its ability to synergize with CTLA-4 blockade.12
Figure 1.
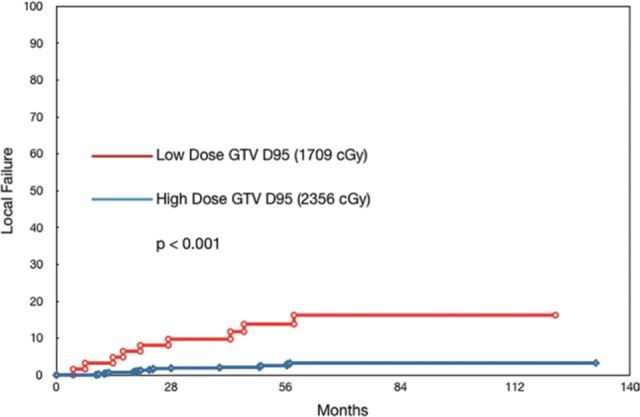
Local failure for groups receiving lower or higher single-fraction doses to the GTV. GTV indicates gross tumor volume. Reproduced with permission from Yamada et al. 25
Ablative doses have shown to cause significant vascular damage, resulting in reduced blood perfusion and subsequent damage to the intratumor microenvironment leading to induced tumor cell death. A preclinical study of rodents and human tumor xenografts found that doses above 10 Gy per fraction resulted in severe vascular damage compared to 5 to 10 Gy per fraction.18 Following ablative doses, another preclinical study found ceramide-mediated signaling of tumor necrosis factor-α and FasL-mediated apoptosis of cells,14 supporting the likely tumoricidal effect of ablative doses.
Indications
Metastatic Disease
The most common application of spine SBRT is in the most common type of tumor seen in the spine, metastatic disease, which represents over 90% of all spinal tumors.26 The utilization of spine SBRT for metastatic disease has increased significantly since the early 2000s.27
As noted earlier, there are radiobiological advantages to higher dose per fraction and shorter overall treatment times in that tumors are more likely to be ablated. This may result in more sustained pain control and less likelihood of the need for retreatment, which is especially important as new therapies continue to increase the life expectancy of patients with certain metastatic cancers.28–32 Unfortunately, Radiation Therapy Oncology Group (RTOG) 9714, comparing protracted (30 Gy per 10 fractions) versus single fraction (8 Gy), the 3-month pain response was only 66%, which begs the question whether that can be improved upon and if so whether SBRT could be the treatment to do so.28 Currently, RTOG 0631 is an ongoing phase III trial that will compare pain relief and quality of life between SRS and conventionally fractionated RT.
Practically speaking, a shorter treatment course is also much more convenient for patients, especially those with limited mobility or those who may be traveling a long distance for therapy. However, patients do need to be able to tolerate a longer treatment time per fraction than with conventional treatment, which can often be completed in less than 10 minutes. Gerstzen et al, for example, cited an average treatment time of 90 minutes using CyberKnife.33 As always, treatment decision-making needs to be multifactorial and individualized to the situation when considering SBRT.
Clinical decision-making framework: neurologic, oncologic, mechanical and systemic
While the clinical use of SBRT continues to expand, it is important to point out that not every spinal metastasis is amenable to spine SBRT, and for some it is simply not necessary when conventional fractionation would be sufficiently effective.27,34 The neurologic, oncologic, mechanical and systemic framework was introduced in 2013 to help guide such therapeutic decisions for spinal metastases, including in the presence of epidural spinal cord compression. Neurologic assessment includes evaluation of the degree of spinal cord compression as well as the presence of functional radiculopathy. Oncologic considerations include radiosensitivity of the tumor. Breast, prostate, ovarian, and neuroendocrine tumors are generally considered radiosensitive, while renal, thyroid, hepatocellular, colon, non-small cell lung carcinoma, sarcoma, and melanoma tend to be more radioresistant.
Mechanically, a patient may have pathologic fractures for which surgical intervention may be warranted. The Spinal Instability Neoplastic Score (SINS) is a validated tool to help assess the degree of mechanical instability.35 Systemic disease is also assessed, taking into account projected survival and likelihood of tolerating the treatment. The recommendations for each combination of factors are listed in Table 1.
Table 1.
NOMS Framework by Laufer et al.34
| Neurologic | Oncologic | Mechanical | Systemic | Decision |
|---|---|---|---|---|
| Low-grade ESCC + no myelopathy | Radiosensitive | Stable | cEBRT | |
| Radiosensitive | Unstable | Stabilization → cEBRT | ||
| Radioresistant | Stable | SRS | ||
| Radioresistant | Unstable | Stabilization → SRS | ||
| High-grade ESCC ± myelopathy | Radiosensitive | Stable | cEBRT | |
| Radiosensitive | Unstable | Stabilization → cEBRT | ||
| Radioresistant | Stable | Tolerate surgery | Decomp/stab → SRS | |
| Radioresistant | Stable | Unable to tolerate | cEBRT | |
| Radioresistant | Unstable | Tolerate surgery | Decomp/stab → SRS | |
| Radioresistant | Unstable | Unable to tolerate | Stabilization → cEBRT |
Abbreviations: ESCC, epidural spinal cord compression; NOMS, neurologic, oncologic, mechanical, and systemic; SRS, stereotactic radiosurgery.
If a patient presents with a high-grade cord compression from a radioresistant tumor such as renal cell carcinoma, for example, but the spine is mechanically stable, the recommendation would be for decompression followed by SRS. If a patient has no epidural involvement or cord compression from a radiosensitive tumor such as lymphoma, seminoma, or myeloma, and is mechanically stable, then conventionally fractionated External Beam Radiation Therapy (EBRT) should be adequately effective.34 This framework allows for personalization of therapy, steering toward decision-making that minimizes both treatment- and tumor-specific risks.
Single fraction
Single-fraction treatment is often used for patients with early-stage disease who either have radioresistant histology and/or favorable prognosis, that is, those likely to live long enough to experience the benefits of an ablative treatment over a conventionally fractionated palliative one. Good candidates would typically not have high-grade epidural cord compression or received prior RT because spinal cord integrity may be further endangered either from short-term tumor swelling or even from the radiation itself. In those cases, hypofractionated or conventional treatment is likely more appropriate. Additional factors to consider in patients undergoing reirradiation are discussed further in the “Reirradiation” section.
RTOG 9714 evaluated single-fraction treatments of 8 Gy to the spine; however, single-fraction doses have escalated significantly since then.28 Memorial Sloan Kettering Cancer Center (MSKCC) currently utilizes 24 Gy as the standard single-fraction spine SBRT dose.25 The most recent outcomes reported from that institution by Yamada et al consisted of 811 spine metastases in 657 patients, treated to a median of 24 Gy (range 16-26 Gy). With a median follow-up of 26.9 months, local failure was <1% and 3.1% at 12 and 48 months, respectively. With subgroup analysis of lesions receiving lower (median 17.09 Gy to the gross tumor volume [GTV]) and higher doses (median 23.56 Gy to the GTV), local failure rates were 14% and 2.1% at 48 months, respectively, suggesting a benefit of higher doses that was independent of histology.25 The resulting local failure curves are shown in Figure 1. The MSKCC also reported on a cohort of thirty-one 5-year survivors of spine SBRT, 80% of whom were treated for metastases. With a median follow-up of 6.1 years, 91.6% of lesions remained stable. Of the 3 patients who failed locally, 1 had been treated for a recurrent primary chordoma, not a metastasis.9 These results suggest that the treatment is not only effective locally but that this response is durable in long-term survivors.
Another large institutional experience from University of Pittsburgh Medical Center (UPMC) was reported in 2007 and consisted of 500 spinal metastases treated with single-fraction radiation doses ranging from 12.5 to 25 Gy (mean 20, median 19). Eighty-six percent experienced long-term pain control, and long-term tumor control was achieved in 90% of lesions treated upfront and 88% of lesions that had been previously irradiated.33
A phase I/II study from University of Texas MD Anderson Cancer Center (MDACC) consisted of 61 patients treated with single-fraction doses of 16 of 18 Gy to the clinical target volume (CTV)/GTV for nonrenal cell histologies (30 lesions) and 16 of 24 Gy to the CTV/GTV for renal cell histology (33 lesions). At a mean follow-up of 20 months, they found 88% and 64% 18-month local control (LC) and overall survival (OS), respectively, with only 2 patients experiencing grade 2 or higher side effects.36
Other reports have spanned wide single-fraction dose ranges from 10 to 24 Gy with similarly encouraging LC, symptomatic improvement, and safety outcomes, a summary of which is shown in Table 2. An RTOG phase II/III study was initiated comparing 16 or 18 Gy with 8 Gy, of which the phase II feasibility results are available but the phase III results are still pending.37 Given the prior comparison of the low- and high-dose cohorts by Yamada et al report showing inferior LC in the 17 Gy range being evaluated in RTOG 0631, it is possible that the full benefit of single-fraction SBRT may not be apparent from its eventual results.25
Table 2.
Selected Studies Utilizing Single-Fraction SRS for Spine Metastases.a
| Author | Tumors, Pts | Histology (% Radioresistant) | Dose, Gy: GTV/CTV If Applicable | Follow-Up, Months | Response | Survival |
|---|---|---|---|---|---|---|
| Yamada et al 25 | 811 (657) | Mixed (46) | 24 (median, range 16-26) | 26.9 (mean) | 96.5% LC | NR |
| Bate et al 38 | 24 (single-fraction SRS alone arm) | Mixed | 22 (median, range 16-23) | 9.8 months SRS alone | 95.8% LC (1 year) | NR |
| Sellin et al 39 | 40 (37) | RCC (100) | 24 (median; 13/37 pts treated with hypofractionated RT) | 49.0 months (median, range 38.2-75.8 months) | 57% LC | 16.3 months median (range 7.4-25.3 months) |
| Folkert et al 40 | 68 (single-fraction arm) | High-grade sarcoma (100) | 24 (median, range 18-24) single-fraction arms) | 12.3 (median including all arms) | 90.8% (12 months) | 70.7% at 12 months |
| Chang et al 41 | 131 (93) | Mixed (≥24.4) | 19.9 (median) | 23.7 (median) | 89.2% radiological and pain control (1 year) | 19 months, median 32.4 months mean |
| Garg et al 36 | 63 (61) | Mixed (≥78) | 18/16 (non-RCC) 24/18 (RCC) |
20 (mean) | 88% LC (18 months) | 64% at 18 months, median 30 months |
| Staehler et al 42 | 105 (55) (spine arm) | RCC (100) | 20 (median) | 16.3 (median, range 1-31) | 94.1% (12 months) | 17.4 months median |
| Amdur et al 43 | 25 (21) | Mixed (≥39) | 15 | 8 (median) | 95% LC | 25% (1 year) |
| Ryu et al 44 | 61 (49) | Mixed (≥8.7) | 10-16 | NR | 46% complete pain relief, 18.9% partial, 16.2% stable; 84% pain control (1 year) |
NR |
| Gerszten et al 33 | 500 (393) | Mixed (≥40) | 20 (mean, range 12.5-25) | 21 (median) | 88% LC | NR |
Abbreviations: CTV, clinical target volume; GTV, gross tumor volume; LC, local control; NR, not reported; pts, patients; RCC, renal cell carcinoma; SRS, stereotactic radiosurgery.
a Radioresistant indicates renal, thyroid, hepatocellular, colon, non-small cell lung carcinoma, sarcoma, and melanoma.
Hypofractionation
Delivery of radiation in shorter than “conventional” fractionation regimens is generally termed hypofractionation. In the case of SBRT, this would typically entail more than 1 but still 5 or fewer treatments. Patients considered for hypofraction are those for whom ablative treatment is still desired and worthwhile but for who single-fraction treatment may not be appropriate, including those with moderate epidural cord compression or who have undergone prior RT.
A phase I/II study from MDACC enrolled 63 patients with 74 spine metastases, of who the first 32 received 30 Gy in 5 fractions and the later patients received 27 Gy in 3 fractions. They found a progression-free incidence of 84% at 1 year and did not find a difference in outcomes between the 2 fractionation regimens. They reported that the change in fractionation was made due to “lengthy treatment duration” for the longer regimen.45 The MDACC later undertook another phase I/II study of 149 patients receiving spine SBRT utilizing mostly 3-fraction regimens totaling 27 to 30 Gy. They noted significant improvement in pain and lower utilization of opioid pain medication during the 6 months following treatment. They also noted 80.5% and 72.5% progression-free survival (PFS) at 1 and 2 years, respectively.46
A 2014 multi-institutional retrospective study of 387 spinal metastases treated with SBRT to median dose of 24 Gy in a median of 3 fractions. They also compared the subgroups receiving 1 to 5 fractions (n = 352) with those receiving 6 to 20 fractions (n = 35) and found no statistically significant differences in LC or OS; however, given the small number of patients in the 6 to 20 fraction group, the study may not have been sufficiently powered to detect a difference. Two-year LC was 83.9% overall.47 A summary table of selected hypofractionation data can be seen in Table 3.
Table 3.
Selected Studies Utilizing Hypofractonated SRS for Spine Metastases.a
| Author | Tumors, Pts | Histology (% Radioresistant) | Dose, Gy/Number Fractions | Follow-Up, months | Response | Survival |
|---|---|---|---|---|---|---|
| Park et al 48 | 59 (39) | Mixed (≥8.5) | 27/3 (median) | 7.4 (median) | 93.2% LC probability (1 year) | 47.4% (1 year) |
| Anand et al 49 | 76 (52) | Mixed (≥13.5) | 24-7/3 (for 71 lesions; 5 received 14-18/1) | 8.48 (median) | 94% LC (1 year) | 68% (1 year) |
| Bishop et al 50 | 332 (285) | Mixed (49) | 43 (median)/1-3 | 19 (median, 33 for living patients) | 88% LC (1 year) | 64% (1 year) |
| Bate et al 38 | 24 (hypofractonated SRS alone arm) | Mixed | 20-30/2-5 | 9.8 (median SRS alone) | 95.8% LC (1 year) | NR |
| Guckenberger et al 47 | 387 (301) | Mixed (≥35) | 24/3 (median, range 8-60/1-20) | 11.8 (median) | 89.9% LC (1 year) | 64.9% (1 year, median 19.5 months) |
| Thibault et al 51 | 71 (37) | RCC (100) | 24/2 (median) | 12.3 (median) | 83% LC (1 year) | 64% (1 year) |
| Sohn et al 52 | 13 (13) | RCC (100) | 38 (mean)/4 (median) | NR | 85.7% LC (1 year) | 15 months (median) |
| Folkert et al 40 | 52 (hypofractionation arm) | Sarcoma (100) | 28.5 (median)/3-6 | 12.3 (median including all arms) | 84.1% (12 months) | 46.2% (12 months) |
| Wang et al 46 | 166 (149) | Mixed (≥69) | 27-30/3 | 15.9 | 80.5% PFS (1 year) | 68.5% (1 year, median 23 months) |
| Gill et al 53 | 20 | Mixed (60) | 30/5 | 34 (median) | 80% LC (1 year) | 80% (1 year) |
| Ahmed et al 54 | 85 (66) | Mixed (≥52) | 24/3 (median) | 8.2 (mean) | 91.2% (1 year) without prior RT | 59% (1 year) without prior RT |
| Nguyen et al 55 | 55 (48) | RCC (100) | 27/3 (median) | 13.1 (median) | 82.1% PFS (1 year) 52% pain free (1 year) | |
| Sahgal et al 56 | 23 (14) without prior RT | Mixed (65) | 24/3 (median) | 9 (median) | 78% LC | NR |
| Nelson et al 57 | 33 (32) | Mixed (≥31) | 18/3 (median) | 6 (median) | 4 treatment failures | NR |
| Gibbs et al 58 | 102 (74) | Mixed | 16-25/1-5 | 9 (mean) | 84% improvement in symptomatic patients | 49% |
| Chang et al 45 | 74 (63) | Mixed (≥57) | 30/5 or 27/3 | 21.3 (median) | 84% PFS (1 year) | 69.8% (1 year, median survival 24.3 months) |
Abbreviations: LC, local control; NR, not reported; PFS, progression-free survival; pts, patients; RCC, renal cell carcinoma; SRS, stereotactic radiosurgery.
a Radioresistant indicates renal, thyroid, hepatocellular, colon, non-small cell lung carcinoma, sarcoma, and melanoma or as defined by study authors.
A recent National Cancer Database (NCDB) analysis highlighted the trend toward increased utilization of SBRT between 2004 and 2013. Although single fractionation was the most common regimen, used in 38% of patients, the use of hypofractionated regimens combined outpaced it, including 26% of patients receiving 3 fractions and 25% receiving 5 fractions.27 In addition to RTOG 0631 which is comparing single dosing regimens, the National Cancer Institute of Canada (NCIC) is also comparing hypofractionation regimens, specifically 20 Gy in 5 fractions and 24 Gy in 2 fractions along with 8 Gy in 1 fraction. As more data continue to become available, they may shed more light on the specific benefits of different fractionation regimens. In the meantime, hypofractionation appears to be both safe and efficacious in patients who may not be as well suited to single fractionation including those with moderate cord compression, or those who have received prior radiation treatment. A summary of selected studies utilizing hypofractionated regimens is seen in Table 3.
Reirradiation
Patients who have undergone prior radiation treatment are of particular interest with regard to spine SBRT because they may not be candidates for significant doses of additional radiation using conventional fields due to toxicity risks, most notably to the spinal cord. Thus, the benefits of a more conformal dose distribution and sharper gradient are of particular importance. In the aforementioned study of 500 patients treated at UPMC, 69% were undergoing reirradiation, and in that subgroup, LC remained high at 88% with long-term pain control of 86%.33
Damast et al evaluated 94 patients who had experienced in-field recurrences after 30 Gy delivered in 10 fractions. These patients were then treated with either a more traditional 20 Gy in 5 fractions or a more aggressive 30 Gy in 5 fractions. Local failure was significantly reduced with the higher doses, 45% versus 26% at 1 year (P = .04), and no patients developed myelopathy.59
Another study of reirradiation in 215 patients at 7 institutions incorporated a heterogeneous mix of prior treatment and retreatment regimes. The median prior dosing was 30 Gy in 10 fractions with a median retreatment dosing of 18 Gy in 1 fraction, given at a median of 13.1 months after prior RT. At 6 and 12 months, LC remained high at 93% and 83%, respectively.60
A prospective study of reirradiation was performed at MDACC including 59 patients and utilized dosing of 30 Gy in 5 fractions or 27 Gy in 3 fractions. At a mean follow-up of 17.6 months, 1-year LC and survival were both 76% and freedom from neurologic deterioration was 92% at 1 year.61
Considering that patients undergoing reirradiation are a generally less favorable group than those undergoing de novo treatment, a median LC rate of 76% (range 66%-90%) in retreated patients reported by 1 review paper as well as improvement in pain scores from 65% to 81% are certainly encouraging.62 Table 4 contains further detail regarding selected reirradiation studies.
Table 4.
Selected Studies Utilizing SBRT Reirradiation for Spine Metastases.a
| Author | Tumors, Pts | Histology (% Radioresistant) | Dose, Gy/ Number Fractions (If Applicable) | Follow-Up, months | Response | Survival |
|---|---|---|---|---|---|---|
| Hashmi et al 60 | 247 (215) | Mixed (≥39) | 16.6/1 (median single fx) or 24/3 (median multiple fx) | 8.1 (median) | 83% LC (1 year) | 48% (1 year) |
| Kawashiro et al 63 | 23 | Mixed (≥34) | 24.5/5 (median) | 10 (median) | 88% 1 year | 45% (1 year, median 12 months) |
| Thibault et al 64 | 56 (40) | Mixed (≥48) | 30/4 (median) | 6.8 (median) | 81% 1 year | 48% (1 year, median 10 months) |
| Chang et al 41 | 59 (54) | Mixed (≥24) | 20.6 (median) | 17.3 (median) | 80.8% radiological and pain control (1 year) | 20.7 mean, 11.0 median |
| Ahmed et al 54 | 22 | Mixed (≥52) | 24/3 (median) | 8.2 (mean) | 83.3% (1 year) | 28% (1 year) |
| Damast et al 59 | 97 (95) | Mixed (78) | 20-30/5 | 12.1 (median) | 66% LC (1 year) | 13.6 months median |
| Garg et al 61 | 63 (59) | Mixed (≥54) | 30/5 or 27/3 | 17.6 (mean) | 76% LC (1 year) | 76% OS (1 year) |
| Mahadevan et al 65 | 81 (60) | Mixed (≥57) | 24/3 or 25-30/5-6 | 12 (median) | 93% stable or improved disease, 65% pain relief | 11 months (median) |
| Choi et al 66 | 51 (42) | Mixed (≥31) | 20/2 (median) | 7 (median) | 73% LC (1 year) | 68% (1 year) |
| Sahgal et al 56 | 37 (25) | Mixed (8) | 24/3 (median) | 7 (median) | 96% PFS (1 year) | NR |
| Gerszten et al 67 | 68 (50) (96% prior RT) | Breast (0) | 15-22.5/1 (mean 19) | 16 (median) | 100% LC, 96% long-term pain improvement | NR |
| Hamilton et al 5 | 5 (5) | Mixed | 10/1 (median) | 6 (median) | 100% LC | 60% |
Abbreviations: fx, fraction; LC, local control; NR, not reported; OS, overall survival; PFS, progression free survival; pts, patients; SBRT, stereotactic body radiation therapy.
a Radioresistant indicates renal, thyroid, hepatocellular, colon, non-small cell lung carcinoma, sarcoma, and melanoma or as defined by study authors.
Postoperative irradiation
Treatment doses are typically similar in the postoperative setting to those in the definitive setting. If a cord compression has been relieved and a greater distance has been created between the vertebral body and the cord itself, hypofractionation or even single-fraction treatment may become feasible when it had not been previously.
The largest study of postoperative SBRT to the spine is the retrospective report by Laufer et al of 186 patients treated with either 24 Gy in a single fraction, 27 to 30 Gy in 3 fractions, termed “high-dose hypofractionation,” or 18 to 26 Gy in 5 to 6 fractions, termed “low-dose hypofractionation.” Overall rate of local progression was 16.4% at 1 year for SRS, 4.1% in the high-dose hypofractionation group, and 22.6% in the low-dose hypofractionation group (P = .04).34
A review by Redmond et al estimated the crude LC following postoperative SBRT to be 88.6% (range 70%-100%) based on a combined 426 patients.68 Tao et al also evaluated the postoperative patients treated on the phase I/II trials at MDACC, not included in the prior review, and found 85% LC and 74% OS at 1 year.69
Massicotte et al described their experience treating 10 patients using a minimal access spine surgery followed by SBRT, which resulted in 70% LC and 80% OS at a median of 13 months.70 As with other spine SBRT uses, its combination with surgery continues to evolve, and thus far the data appear quite promising. A summary of postoperative studies is shown in Table 5.
Table 5.
Selected Studies Utilizing Postoperative SBRT for Spine Metastases.a
| Author | Tumors, Pts | Histology (% Radioresistant) | Dose, Gy: GTV/CTV If Applicable | Follow-Up, months | Response | Survival |
|---|---|---|---|---|---|---|
| Tao et al 69 | 69 (66) | Mixed (≥85) | 16-24/1 or 27/3 or 30/5 | 30 (median) | 85% LC (1 year) | 74% (1 year, median 29 months) |
| Bate et al 38 | 21 | Mixed | Single fraction: 22 (median, range 16-22), hypofractionated: 20-30/2-5 | 13.7 | 90.5% (1 year) | NR |
| Laufer et al 71 | (186) | Mixed (77) | 18-36/5-6 or 24-30/3 or 24/1 | 7.6 (median) | 83.6% LC (1 year) >95% LC with SRS (1 year) | 26.3% PFS |
| Al-Omair et al 72 | (80) | Mixed (≥40) | 18-26/1 or 18-40/3-5 | 8.3 (median) | 84% LC (1 year) | 64% (1 year) |
| Massicotte et al 70 | 10 | Mixed (40) | 18-35/1-5 | 13 (median) | 70% LC | 80% OS |
| Rock et al 73 | 18 | Mixed (including primary) | 11.4/1 (mean) | 7 (median) | 92% neurological stability or improvement | NR |
| Gerszten et al 74 | 26 (26) | Mixed (≥15) | 16-20/1 | 16 (median) | 92% pain improvement | NR |
Abbreviations: CTV, clinical target volume; GTV, gross tumor volume; LC, local control; NR, not reported; OS, overall survival; PFS, progression-free survival; pts, patients; SBRT, stereotactic body radiation therapy; SRS, stereotactic radiosurgery.
a Radioresistant indicates renal, thyroid, hepatocellular, colon, non-small cell lung carcinoma, sarcoma, and melanoma or as defined by study authors.
Oligometastatic disease
Another consideration that has arisen in recent years is the oligometastatic state. Although the presence of spinal metastases still classifies patients as stage IV, there is evidence to suggest that certain patients with limited metastatic disease burden, for example, 3 or fewer sites, may experience long-term survival with aggressive therapy, including SBRT.75,76
Investigators from MDACC looked at the 38 oligometastatic patients treated on their phase I/II trials, 53% of who received 27 Gy in 3 fractions, 87% of who had spinal disease as the only metastatic site, and 42% of who had radioresistant histology. They found 2- and 5-year OS to be 84% and 60%, with PFS 82% and 78%, respectively. Five percent of patients experienced G3-4 toxicity, which is important to note that with longer life expectancy comes higher risk of detriment from treatment-related complications.77
A study from Australia reported on 60 patients with 72 oligometastases treated with a median of 24 Gy in 2 fractions. At a median follow-up of 21 months, widespread failure-free survival at 1 and 2 years were 67% and 59%, respectively, with OS rates at 1 and 2 years of 90% and 76%, and freedom from local progression of 92% and 86%, respectively.78
Another study from Rochester prospectively treated patients who had oligometastatic disease to 1 organ treated with SBRT, mostly using 10 Gy × 5 fractions. They included 9 patients with bone-confined disease of who 8 were alive with no new metastases at a median 41-month follow-up for the entire group.79 They later reported 6-year OS, Freedom From Distant Metastases (FFDM), and LC rates were 9%, 13%, and 65%, respectively, for the whole group. Within the subgroup of patients with breast cancer who had a median 4.5-year follow-up, there was a significant improvement in overall survival (OS) and Distant Metastases Free Survival (DMFS) when bony oligometastases were treated with SBRT, compared with other sites. None of 17 such lesions treated recurred during the reported follow-up.80
The landscape of metastatic disease continues to grow in complexity, especially based on disease burden and histology and further highlights the need for individualized treatment decision making. Patients with oligometastatic disease represent, in a way, the ideal candidate for SBRT because they likely have the most to gain from tumor ablation as opposed to simple palliation. Further data are likely forthcoming on this population, as certain patients continue to experience longer survival in conjunction with new systemic therapies and more aggressive treatment to metastatic sites, including SBRT.
Primary Malignant Spinal Tumors
Although vastly less common than metastases, many primary tumors of the spine are also well suited for short-course ablative therapy. These include extradural bone tumors such as chordoma, chondrosarcoma, osteosarcoma, and Ewing sarcoma as well as intradural extramedullary neurogenic tumors such as meningiomas, schwannoma, neurofibromas, and malignant peripheral nerve sheath tumors (MPNSTs). Intramedullary tumors can also occur, including ependymomas, astrocytomas but will not be discussed here.
Primary bone tumors are most commonly found in the base of skull and sacrum as well as mobile spine, and standard-of-care therapy consists of maximal safe resection followed by adjuvant radiation. Patients have historically been plagued by recurrences.81
Chordomas are the most widely studied of the primary spine tumors; however, due to their rarity, the available data are relatively heterogeneous. These tumors are difficult to control even with en bloc resection. The Cleveland Clinic reported on 12 spine chordomas in 8 patients treated to a median dose of 16 Gy (range 11-16 Gy), 7 of which were also resected. At a median follow-up of 9.7 months, admittedly a relatively short interval, they found 75% LC.82 Radiosurgery utilizing 24 Gy in a single fraction can result in up to 95% LC at 2 years median follow-up.83
Lockney et al also looked a cohort of 12 patients with chordoma treated with cytoreductive separation surgery followed by SBRT (24 Gy in 1 fraction or 24-36 Gy in 3 fractions) with a minimum of 6 months follow-up imaging. Of the 5 patients treated upfront, median post-SBRT follow-up time of 65.9 months with an LC rate of 80%. Of the 7 patients undergoing salvage therapy, the LC rate was 57.1%, and the median follow-up duration was 10.7 months with median time to progression of 2.9 months. Of the patients, 18% had RT-related major complications, including dysphagia, mucositis, and vocal cord paralysis.84
Another study included 20 patients with either chordoma or chondrosarcoma, 20% of which were in sacrum and 15% in spine (remainder skull base). They were treated to a median dose of 37.5 Gy in 5 fractions (range 25-40 Gy). At a median follow-up of 28 months, crude OS and local relapse free survival (LRFS) were 90%. Two patients experienced G4-5 toxicity, including one from radiation vasculopathy.85
These high rates of LC for chordomas and chondrosarcomas, particularly in the Lockney et al cohort treated upfront with intralesional resection followed by SBRT, are highly encouraging considering that a more traditional en bloc surgery with wide margins is often associated with significant morbidity.84 Along with proton and other particle beam therapies that are also being investigated, high dose per fraction SBRT may now represent another promising alternative to en bloc resection.86
The data on neurogenic tumors of the spine treated with SBRT are even less robust, again due to their rarity. One report included 58 patients treated with 66 spinal SRS procedures (39 of which used SRS as the primary modality) for 110 tumors. In all, 47 patients had schwannomas, 7 had neurofibromas, and 4 had MPNSTs. Outcomes varied widely with histology, with benign tumors experiencing 95.4% LC over an average of 44 months. They also found that NF1-related tumors had much shorted median survival of 1.13 years compared to sporadic MPNSTs, whose median survival was 5.8 years.87
Although the abovementioned findings are interesting to note, the patient numbers are limited, and the study designs are retrospective in nature. As a result, spinal SBRT remains a reasonable consideration in combination with surgery or as the primary treatment modality in patients for who surgery is either contraindicated or undesired.
Toxicity
Stereotactic body radiation therapy for spinal metastases is highly ablative; however, there are several potential complications that can occur, which are often dependent on the location of the lesion treated. Acute toxicities can include nausea, fatigue, dermatitis, esophagitis, and myelitis. Late toxicities are more significant and can include esophageal stenosis, fistula, ulcer formation, vertebral compression fracture (VCF) as well as spinal cord injury.
Of these, VCF and spinal cord injury have been well characterized and extensively reported in the literature. Sahgal et al reported pooled outcomes from 410 spine segments that were treated with spine SBRT. The 1- and 2-year VCF incidence rates were 12.35% and 13.49%, respectively, with median time to fracture of 2.46 months.88 Significant predictors for compression fracture were dose per fraction >19 Gy, lytic tumors, baseline spinal misalignment, and baseline presence of a compression fracture. In another study, the 5-year cumulative incidence rate of symptomatic compression fractures requiring interventions was <10% among patients who received SRS to 24 Gy.89 The experience from MSKCC of single-fraction spinal SRS with 24 Gy revealed a radiographic VCF rate of 36% of which 14% became symptomatic and required intervention.9 Treatment of VCFs after SBRT for spinal metastases include percutaneous cement augmentation with vertebroplasty and kyphoplasty as they provide pain relief and mechanical support; however, preventative strategies are still under investigation.90
Spinal cord injury, or more specifically radiation-induced myelopathy (RM), is the most potentially debilitating complication, and the risk is accepted to be less than 1%. A dosimetric analysis of 19 patients was conducted by Sahgal et al who underwent reirradiation after conventional treatment. Of those who had RM, the median re-RT point dose maximum in the RM group was 123.4 Gy versus 25 Gy in the non-RM group, which was significantly different. The Biologic Effective Dose (BED) from the initial course of radiation with conventional RT was not significantly different between the 2 groups. The recommended dose constraints based on this analysis were to limit the cumulative BED to <140 Gy for the thecal sac point dose maximum and limit the maximum SBRT BED to 50 Gy for the thecal sac point dose maximum. Furthermore, a 5-month period between radiation treatments was considered safe.91
Recommendations from a retrospective study by Saghal et al modeling 9 patients who had RM following SBRT to the spine to 66 patients who did not have RM were to limit the thecal sac maximum point volume–dose to 12.4 Gy in 1 fraction, 20.3 Gy in 3 fractions, and 25.3 Gy in 5 fractions in order to reduce the risk of RM to less than 5%.92 A comprehensive study examining dose–volume data for de novo SBRT spine cases found the risk of spinal cord injury to be ≤1% with 13 Gy in single fraction and 20 Gy in 3 fractions. In the reirradiation setting, the estimated risk level was 0.4% for 10 Gy and 0.6% for 14 Gy in 5 fractions.93
With regard to esophageal toxicity, Cox et al reported on 204 patients treated to a median dose of 24 Gy in 1 fraction, of who 31 (15%) patients experienced acute and 24 (12%) patients experienced late esophageal toxicity. Overall, 14 patients (6.8%) had grade 3 or higher esophageal toxicity according to CTCAE 4.0.94 In the secondary analysis of the MDACC phase I/II studies, esophageal toxicity rates were also low. Ten (15%) patients and 8 (12%) patients had GI toxicities including esophagitis as well as dysphagia, nausea, vomiting, anorrhexia, and diarrhea. There were no cases of grade 3 or higher GI toxicity.69
Technical Considerations
Technological advances in the field have allowed providers to deliver SBRT safely and accurately. Delivery begins with comprehensive imaging, which may include preoperative and postoperative T1-weighted magnetic resonance imaging (MRI) and cord delineation on T2-weighted MRI or CT myelogram of at least 1 vertebral body above and below the area of interest. Specifically, the use of T1-weighted imaging (T1WI) as well as STIR sequences has been recommended by the International Spine Radiosurgery Consortium (ISRC) and the SPIne assessment in Neuro-Oncology group.95–97 The T1WI is used to delineate infiltrative disease in the marrow, while T2WI depicts extraosseous extension of tumor into the epidural space. Coregistration is done with high-resolution CT scan from the simulation to optimize target delineation. Slice thickness of the treatment planning CT scan should not be more than 2 mm (preferably 1 mm). Immobilization during simulation may consist of molded alpha cradles with lateral support paddles as well as customized masks for lesions at T5 and above.98 An image of one such immobilization device, the CDR frame, can be seen in Figure 2.
Figure 2.
CDR cradle. Permission obtained from CDR Systems.
Contouring
There are several consensus guidelines published to help guide contouring and target delineation to standardize the nomenclature and delivery of spinal stereotactic RT. Namely, the ISRC guidelines from 201295 and the postoperative guidelines from 201799 gathered an expert panel to define the GTV when present, CTV, and planning target volume. At least 50% of radiation oncologists and an increasing number of neurosurgeons are treating spinal metastases with SRS/SBRT, the need for following a standardized approach is even more important. Selected details are shown in Figures 3 to 5, and further elements of contouring can be found in the references mentioned earlier.
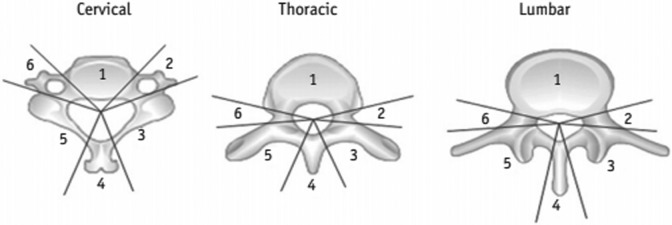
Figure 3.
Delineation of anatomic portions of the spine. 1 = vertebral body, 2/6 = pedicle, 3/5 = lamina and transverse process, 4 = spinous process.95
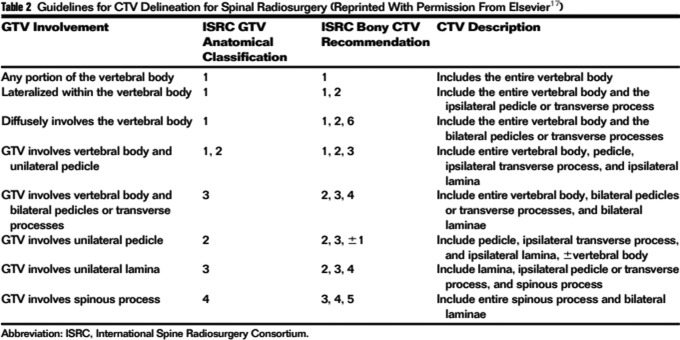
Figure 4.
General CTV contouring recommendations.95 CTV indicates clinical target volume.
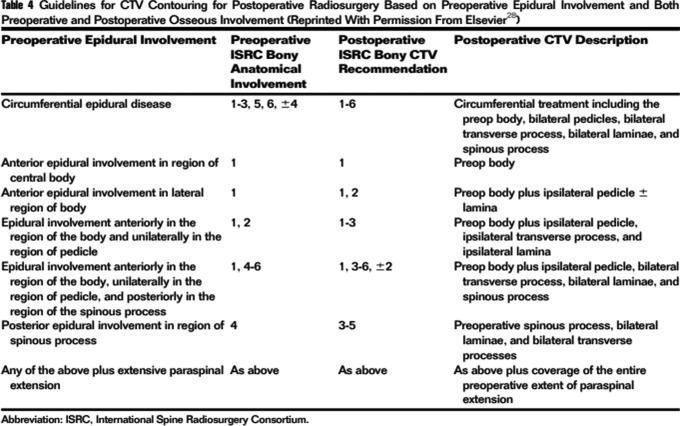
Figure 5.
Postoperative CTV contouring recommendations.99 CTV indicates clinical target volume.
Another important consideration is the physiologic movement of the spinal cord and cauda equina; thus, accurate assessment of its position is important in planning SBRT to the spine. A study utilizing dynamic axial and sagittal MRI found minor but still present oscillatory motion of critical neural tissues; thus, a planning organ-at-risk margin may be necessary to ensure an increased level of safety.100 Additionally, residual target motion, defined as target movement between image-guided corrections, has shown to result in uncertainties for the dose delivered to the spinal cord; thus, more frequent imaging may be warranted to decrease discrepancies between planned and treated targets.101
Treatment Planning and Dose Constraints
Commonly used fractionation schemes include 18 to 24 Gy in 1 fraction, 24 Gy in 2 fractions, 24 to 30 Gy in 3 fractions, 30 Gy in 4 fractions, and 30 to 40 Gy in 5 fractions.10 As our understanding of organs-at-risk tolerances have evolved in this field, the current accepted constraint is to a maximum dose of 1200 to 1400 cGy for the spinal cord and 1600 to 1800 cGy for the cauda equina when prescribing single-fraction RT. The general rule is to meet constraints for a threshold <5% risk of serious adverse effects.
Follow-Up
Both clinician- and patient-reported outcomes are incorporated into the posttreatment follow-up and is well described in the most recent SPIne Response Assessment in Neuro-Oncology group report (Laufer, 2018 #266). The most commonly used assessments are the SINS for mechanical spinal stability, the Bilsky epidural spinal cord compression score for cord compression, the Karnofsky Performance Status for survival prediction, and the American Spinal Injury Association Impairment Scale for neurologic deficit status. There still exists a wide range of assessment tools used by clinicians; thus, studies to validate these instruments are ongoing to determine standard tools that most accurately describe outcomes.
Future Directions
As the trend toward personalized medicine continues to be emphasized, the possibilities of combination therapy with separation surgery, SRS/SBRT, and systemic therapy are growing. Furthermore, toxicity with combined treatment has been well tolerated. A phase I trial of interleukin-2 and SBRT showed an overall response rate of 62.5% in 7 patients with minimal toxicity for metastatic spinal melanoma.102 Another report of patients treated for metastatic renal cell carcinoma with SRS plus sunitinib or sorafenib showed LC was 90.4% with only one grade 1 abdominal pain.42 While the typical practice is to hold targeted therapy 3 to 5 days before and after SRS, the concurrent use and optimal timing for the synergistic effect has yet to be determined.103
Reports of the abscopal effect have also been demonstrated in murine models with hypofractionated radiation and immunotherapy,12,104,105 in addition to evidence showing elevated activated CD4 T cells and reduced levels of suppressor cells after ipilimumab and RT were administered.19 Still, our understanding of combined treatment in this setting is limited, and prospective clinical studies are needed to better elucidate outcomes and toxicities associated with the concurrent use of chemotherapy and targeted agents.
Finally, SBRT technologies continue to advance with improved on-board imaging with the C-arm gantry, integrated MRI-linear accelerators, and multiisocentric treatments of extracranial sites with the latest Gamma Knife models.106
Conclusions
In conclusion, SRS and SBRT for the treatment of spinal metastases is an effective and safe method, offering patients a durable response with good quality of life. The advanced image-guided technology and conformal planning in the primary, postoperative, and reirradiation settings have allowed accurate delivery of ablative doses to metastatic disease. Further prospective clinical studies are needed to better elucidate the role of concurrent chemotherapy and immunotherapy to harness the immune system for more optimal outcomes.
Abbreviations
- CT
computed tomography
- CTLA-4
cytotoxic T-lymphocyte–associated antigen 4
- CTV
clinical target volume
- GI
gastrointestinal
- GTV
gross tumor volume
- LC
local control
- MDACC
University of Texas MD Anderson Cancer Center
- MPNST
malignant peripheral nerve sheath tumor
- MRI
magnetic resonance imaging
- MSKCC
Memorial Sloan Kettering Cancer Center
- OS
overall survival
- PFS
progression-free survival
- RM
radiation myelopathy
- RT
radiotherapy
- SBRT
stereotactic body radiation therapy
- SINS
Spinal Instability Neoplastic Score
- SRS
stereotactic radiosurgery
- UPMC
University of Pittsburgh Medical Center
- VCF
vertebral compression fracture
- VMAT
volumetric-modulated arc therapy
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Virginia W. Osborn, MD  http://orcid.org/0000-0001-8700-4861
http://orcid.org/0000-0001-8700-4861
References
- 1. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102(4):316–319. [PubMed] [Google Scholar]
- 2. Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psychiatry. 1983;46(9):797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blomgren H, Lax I, Naslund I, Svanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34(6):861–870. [DOI] [PubMed] [Google Scholar]
- 4. Li W, Purdie TG, Taremi M, et al. Effect of immobilization and performance status on intrafraction motion for stereotactic lung radiotherapy: analysis of 133 patients. Int J Radiat Oncol Biol Phys. 2011;81(5):1568–1575. [DOI] [PubMed] [Google Scholar]
- 5. Hamilton AJ, Lulu BA, Fosmire H, Stea B, Cassady JR. Preliminary clinical experience with linear accelerator-based spinal stereotactic radiosurgery. Neurosurgery. 1995;36(2):311–319. [DOI] [PubMed] [Google Scholar]
- 6. Ryu S, Fang Yin F, Rock J, et al. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97(8):2013–2018. [DOI] [PubMed] [Google Scholar]
- 7. Uematsu M, Shioda A, Tahara K, et al. Focal, high dose, and fractionated modified stereotactic radiation therapy for lung carcinoma patients: a preliminary experience. Cancer. 1998;82(6):1062–1070. [DOI] [PubMed] [Google Scholar]
- 8. Brahme A, Roos JE, Lax I. Solution of an integral equation encountered in rotation therapy. Phys Med Biol. 1982;27(10):1221–1229. [DOI] [PubMed] [Google Scholar]
- 9. Moussazadeh N, Lis E, Katsoulakis E, et al. Five-year outcomes of high-dose single-fraction spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2015;93(2):361–367. [DOI] [PubMed] [Google Scholar]
- 10. Tseng CL, Eppinga W, Charest-Morin R, et al. Spine stereotactic body radiotherapy: indications, outcomes, and points of caution. Global Spine J. 2017;7(2):179–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88(2):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8(2):89–91. [DOI] [PubMed] [Google Scholar]
- 14. Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155–1159. [DOI] [PubMed] [Google Scholar]
- 15. Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18(4):240–243. [DOI] [PubMed] [Google Scholar]
- 16. Kocher M, Treuer H, Voges J, Hoevels M, Sturm V, Muller RP. Computer simulation of cytotoxic and vascular effects of radiosurgery in solid and necrotic brain metastases. Radiother Oncol. 2000;54(2):149–156. [DOI] [PubMed] [Google Scholar]
- 17. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–7523. [DOI] [PubMed] [Google Scholar]
- 18. Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res. 2012;177(3):311–327. [DOI] [PubMed] [Google Scholar]
- 19. Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Formenti SC, Demaria S. Local control by radiotherapy: is that all there is? Breast Cancer Res. 2008;10(6):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Obeid M, Panaretakis T, Joza N, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14(10):1848–1850. [DOI] [PubMed] [Google Scholar]
- 22. Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. [DOI] [PubMed] [Google Scholar]
- 23. Demaria S, Formenti SC. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int J Radiat Biol. 2007;83(11-12):819–825. [DOI] [PubMed] [Google Scholar]
- 24. Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181(5):3099–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamada Y, Katsoulakis E, Laufer I, et al. The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg Focus. 2017;42(1):E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simmons ED, Zheng Y. Vertebral tumors: surgical versus nonsurgical treatment. Clin Orthop Relat Res. 2006;443:233–247. [DOI] [PubMed] [Google Scholar]
- 27. McClelland S, Kim E III, Passias PG, Murphy JD, Attia A, Jaboin JJ. Spinal stereotactic body radiotherapy in the United States: a decade-long nationwide analysis of patient demographics, practice patterns, and trends over time. J Clin Neurosci. 2017;46:109–112. [DOI] [PubMed] [Google Scholar]
- 28. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798–804. [DOI] [PubMed] [Google Scholar]
- 29. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. [DOI] [PubMed] [Google Scholar]
- 30. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. [DOI] [PubMed] [Google Scholar]
- 31. Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976). 2007;32(2):193–199. [DOI] [PubMed] [Google Scholar]
- 34. Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18(6):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fourney DR, Frangou EM, Ryken TC, et al. Spinal Instability Neoplastic Score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011;29(22):3072–3077. [DOI] [PubMed] [Google Scholar]
- 36. Garg AK, Shiu AS, Yang J, et al. Phase 1/2 trial of single-session stereotactic body radiotherapy for previously unirradiated spinal metastases. Cancer. 2012;118(20):5069–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ryu S, Pugh SL, Gerszten PC, et al. RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1-3) spine metastases: phase 2 results. Pract Radiat Oncol. 2014;4(2):76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bate BG, Khan NR, Kimball BY, Gabrick K, Weaver J. Stereotactic radiosurgery for spinal metastases with or without separation surgery. J Neurosurg Spine. 2015;22(4):409–415. [DOI] [PubMed] [Google Scholar]
- 39. Sellin JN, Reichardt W, Bishop AJ, et al. Factors affecting survival in 37 consecutive patients undergoing de novo stereotactic radiosurgery for contiguous sites of vertebral body metastasis from renal cell carcinoma. J Neurosurg Spine. 2015;22(1):52–59. [DOI] [PubMed] [Google Scholar]
- 40. Folkert MR, Bilsky MH, Tom AK, et al. Outcomes and toxicity for hypofractionated and single-fraction image-guided stereotactic radiosurgery for sarcomas metastasizing to the spine. Int J Radiat Oncol Biol Phys. 2014;88(5):1085–1091. [DOI] [PubMed] [Google Scholar]
- 41. Chang UK, Cho WI, Kim MS, Cho CK, Lee DH, Rhee CH. Local tumor control after retreatment of spinal metastasis using stereotactic body radiotherapy; comparison with initial treatment group. Acta Oncol. 2012;51(5):589–595. [DOI] [PubMed] [Google Scholar]
- 42. Staehler M, Haseke N, Nuhn P, et al. Simultaneous anti-angiogenic therapy and single-fraction radiosurgery in clinically relevant metastases from renal cell carcinoma. BJU Int. 2011;108(5):673–678. [DOI] [PubMed] [Google Scholar]
- 43. Amdur RJ, Bennett J, Olivier K, et al. A prospective, phase II study demonstrating the potential value and limitation of radiosurgery for spine metastases. Am J Clin Oncol. 2009;32(5):515–520. [DOI] [PubMed] [Google Scholar]
- 44. Ryu S, Jin R, Jin JY, et al. Pain control by image-guided radiosurgery for solitary spinal metastasis. J Pain Symptom Manage. 2008;35(3):292–298. [DOI] [PubMed] [Google Scholar]
- 45. Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7(2):151–160. [DOI] [PubMed] [Google Scholar]
- 46. Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1-2 trial. Lancet Oncol. 2012;13(4):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guckenberger M, Mantel F, Gerszten PC, et al. Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: a multi-institutional analysis. Radiat Oncol. 2014;9:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Park HJ, Kim HJ, Won JH, Lee SC, Chang AR. Stereotactic body radiotherapy (SBRT) for spinal metastases: who will benefit the most from SBRT? Technol Cancer Res Treat. 2015;14(2):159–167. [DOI] [PubMed] [Google Scholar]
- 49. Anand AK, Venkadamanickam G, Punnakal AU, et al. Hypofractionated stereotactic body radiotherapy in spinal metastasis – with or without epidural extension. Clin Oncol (R Coll Radiol). 2015;27(6):345–352. [DOI] [PubMed] [Google Scholar]
- 50. Bishop AJ, Tao R, Rebueno NC, et al. Outcomes for spine stereotactic body radiation therapy and an analysis of predictors of local recurrence. Int J Radiat Oncol Biol Phys. 2015;92(5):1016–1026. [DOI] [PubMed] [Google Scholar]
- 51. Thibault I, Al-Omair A, Masucci GL, et al. Spine stereotactic body radiotherapy for renal cell cancer spinal metastases: analysis of outcomes and risk of vertebral compression fracture. J Neurosurg Spine. 2014;21(5):711–718. [DOI] [PubMed] [Google Scholar]
- 52. Sohn S, Chung CK, Sohn MJ, et al. Stereotactic radiosurgery compared with external radiation therapy as a primary treatment in spine metastasis from renal cell carcinoma: a multicenter, matched-pair study. J Neurooncol. 2014;119(1):121–128. [DOI] [PubMed] [Google Scholar]
- 53. Gill B, Oermann E, Ju A, et al. Fiducial-free CyberKnife stereotactic body radiation therapy (SBRT) for single vertebral body metastases: acceptable local control and normal tissue tolerance with 5 fraction approach. Front Oncol. 2012;2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ahmed KA, Stauder MC, Miller RC, et al. Stereotactic body radiation therapy in spinal metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):e803–809. [DOI] [PubMed] [Google Scholar]
- 55. Nguyen QN, Shiu AS, Rhines LD, et al. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(4):1185–1192. [DOI] [PubMed] [Google Scholar]
- 56. Sahgal A, Ames C, Chou D, et al. Stereotactic body radiotherapy is effective salvage therapy for patients with prior radiation of spinal metastases. Int J Radiat Oncol Biol Phys. 2009;74(3):723–731. [DOI] [PubMed] [Google Scholar]
- 57. Nelson JW, Yoo DS, Sampson JH, et al. Stereotactic body radiotherapy for lesions of the spine and paraspinal regions. Int J Radiat Oncol Biol Phys. 2009;73(5):1369–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gibbs IC, Kamnerdsupaphon P, Ryu MR, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82(2):185–190. [DOI] [PubMed] [Google Scholar]
- 59. Damast S, Wright J, Bilsky M, et al. Impact of dose on local failure rates after image-guided reirradiation of recurrent paraspinal metastases. Int J Radiat Oncol Biol Phys. 2011;81(3):819–826. [DOI] [PubMed] [Google Scholar]
- 60. Hashmi A, Guckenberger M, Kersh R, et al. Re-irradiation stereotactic body radiotherapy for spinal metastases: a multi-institutional outcome analysis. J Neurosurg Spine. 2016;25(5):646–653. [DOI] [PubMed] [Google Scholar]
- 61. Garg AK, Wang XS, Shiu AS, et al. Prospective evaluation of spinal reirradiation by using stereotactic body radiation therapy: The University of Texas MD Anderson Cancer Center experience. Cancer. 2011;117(15):3509–3516. [DOI] [PubMed] [Google Scholar]
- 62. Myrehaug S, Sahgal A, Hayashi M, et al. Reirradiation spine stereotactic body radiation therapy for spinal metastases: systematic review. J Neurosurg Spine. 2017;27(4):428–435. [DOI] [PubMed] [Google Scholar]
- 63. Kawashiro S, Harada H, Katagiri H, et al. Reirradiation of spinal metastases with intensity-modulated radiation therapy: an analysis of 23 patients. J Radiat Res. 2016;57(2):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thibault I, Campbell M, Tseng CL, et al. Salvage stereotactic body radiotherapy (SBRT) following in-field failure of initial SBRT for spinal metastases. Int J Radiat Oncol Biol Phys. 2015;93(2):353–360. [DOI] [PubMed] [Google Scholar]
- 65. Mahadevan A, Floyd S, Wong E, Jeyapalan S, Groff M, Kasper E. Stereotactic body radiotherapy reirradiation for recurrent epidural spinal metastases. Int J Radiat Oncol Biol Phys. 2011;81(5):1500–1505. [DOI] [PubMed] [Google Scholar]
- 66. Choi CY, Adler JR, Gibbs IC, et al. Stereotactic radiosurgery for treatment of spinal metastases recurring in close proximity to previously irradiated spinal cord. Int J Radiat Oncol Biol Phys. 2010;78(2):499–506. [DOI] [PubMed] [Google Scholar]
- 67. Gerszten PC, Burton SA, Welch WC, et al. Single-fraction radiosurgery for the treatment of spinal breast metastases. Cancer. 2005;104(10):2244–2254. [DOI] [PubMed] [Google Scholar]
- 68. Redmond KJ, Lo SS, Fisher C, Sahgal A. Postoperative stereotactic body radiation therapy (SBRT) for spine metastases: a critical review to guide practice. Int J Radiat Oncol Biol Phys. 2016;95(5):1414–1428. [DOI] [PubMed] [Google Scholar]
- 69. Tao R, Bishop AJ, Brownlee Z, et al. Stereotactic body radiation therapy for spinal metastases in the postoperative setting: a secondary analysis of mature phase 1-2 trials. Int J Radiat Oncol Biol Phys. 2016;95(5):1405–1413. [DOI] [PubMed] [Google Scholar]
- 70. Massicotte E, Foote M, Reddy R, Sahgal A. Minimal access spine surgery (MASS) for decompression and stabilization performed as an out-patient procedure for metastatic spinal tumours followed by spine stereotactic body radiotherapy (SBRT): first report of technique and preliminary outcomes. Technol Cancer Res Treat. 2012;11(1):15–25. [DOI] [PubMed] [Google Scholar]
- 71. Laufer I, Iorgulescu JB, Chapman T, et al. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine. 2013;18(3):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Al-Omair A, Masucci L, Masson-Cote L, et al. Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro Oncol. 2013;15(10):1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rock JP, Ryu S, Shukairy MS, et al. Postoperative radiosurgery for malignant spinal tumors. Neurosurgery. 2006;58(5):891–898; discussion 891-898. [DOI] [PubMed] [Google Scholar]
- 74. Gerszten PC, Germanwala A, Burton SA, et al. Combination kyphoplasty and spinal radiosurgery: a new treatment paradigm for pathological fractures. Neurosurg Focus. 2005;18(3):e8. [DOI] [PubMed] [Google Scholar]
- 75. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8–10. [DOI] [PubMed] [Google Scholar]
- 76. Milano MT, Katz AW, Muhs AG, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer. 2008;112(3):650–658. [DOI] [PubMed] [Google Scholar]
- 77. Ho JC, Tang C, Deegan BJ, et al. The use of spine stereotactic radiosurgery for oligometastatic disease. J Neurosurg Spine. 2016;25(2):239–247. [DOI] [PubMed] [Google Scholar]
- 78. Chang JH, Gandhidasan S, Finnigan R, et al. Stereotactic ablative body radiotherapy for the treatment of spinal oligometastases. Clin Oncol (R Coll Radiol). 2017;29(7):e119–e125. [DOI] [PubMed] [Google Scholar]
- 79. Milano MT, Katz AW, Okunieff P. Patterns of recurrence after curative-intent radiation for oligometastases confined to one organ. Am J Clin Oncol. 2010;33(2):157–163. [DOI] [PubMed] [Google Scholar]
- 80. Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83(3):878–886. [DOI] [PubMed] [Google Scholar]
- 81. Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13(2):e69–76. [DOI] [PubMed] [Google Scholar]
- 82. Jung EW, Jung DL, Balagamwala EH, et al. Single-fraction spine stereotactic body radiation therapy for the treatment of chordoma. Technol Cancer Res Treat. 2017;16(3):302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yamada Y, Laufer I, Cox BW, et al. Preliminary results of high-dose single-fraction radiotherapy for the management of chordomas of the spine and sacrum. Neurosurgery. 2013;73(4):673–680; discussion 680. [DOI] [PubMed] [Google Scholar]
- 84. Lockney DT, Shub T, Hopkins B, et al. Spinal stereotactic body radiotherapy following intralesional curettage with separation surgery for initial or salvage chordoma treatment. Neurosurg Focus. 2017;42(1):E4. [DOI] [PubMed] [Google Scholar]
- 85. Vasudevan HN, Raleigh DR, Johnson J, et al. Management of chordoma and chondrosarcoma with fractionated stereotactic radiotherapy. Front Surg. 2017;4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Park L, Delaney TF, Liebsch NJ, et al. Sacral chordomas: impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys. 2006;65(5):1514–1521. [DOI] [PubMed] [Google Scholar]
- 87. Shin DW, Sohn MJ, Kim HS, et al. Clinical analysis of spinal stereotactic radiosurgery in the treatment of neurogenic tumors. J Neurosurg Spine. 2015;23(4):429–437. [DOI] [PubMed] [Google Scholar]
- 88. Sahgal A, Atenafu EG, Chao S, et al. Vertebral compression fracture after spine stereotactic body radiotherapy: a multi-institutional analysis with a focus on radiation dose and the Spinal Instability Neoplastic Score. J Clin Oncol. 2013;31(27):3426–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Virk MS, Han JE, Reiner AS, et al. Frequency of symptomatic vertebral body compression fractures requiring intervention following single-fraction stereotactic radiosurgery for spinal metastases. Neurosurg Focus. 2017;42(1):E8. [DOI] [PubMed] [Google Scholar]
- 90. Sahgal A, Whyne CM, Ma L, Larson DA, Fehlings MG. Vertebral compression fracture after stereotactic body radiotherapy for spinal metastases. Lancet Oncol. 2013;14(8):e310–e320. [DOI] [PubMed] [Google Scholar]
- 91. Sahgal A, Ma L, Weinberg V, et al. Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(1):107–116. [DOI] [PubMed] [Google Scholar]
- 92. Sahgal A, Weinberg V, Ma L, et al. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys. 2013;85(2):341–347. [DOI] [PubMed] [Google Scholar]
- 93. Grimm J, Sahgal A, Soltys SG, et al. Estimated risk level of unified stereotactic body radiation therapy dose tolerance limits for spinal cord. Semin Radiat Oncol. 2016;26(2):165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cox BW, Jackson A, Hunt M, Bilsky M, Yamada Y. Esophageal toxicity from high-dose, single-fraction paraspinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83(5):e661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cox BW, Spratt DE, Lovelock M, et al. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83(5):e597–605. [DOI] [PubMed] [Google Scholar]
- 96. Thibault I, Chang EL, Sheehan J, et al. Response assessment after stereotactic body radiotherapy for spinal metastasis: a report from the SPIne response assessment in Neuro-Oncology (SPINO) group. Lancet Oncol. 2015;16(16):e595–603. [DOI] [PubMed] [Google Scholar]
- 97. Sahgal A, Bilsky M, Chang EL, et al. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J Neurosurg Spine. 2011;14(2):151–166. [DOI] [PubMed] [Google Scholar]
- 98. Lovelock DM, Hua C, Wang P, et al. Accurate setup of paraspinal patients using a noninvasive patient immobilization cradle and portal imaging. Med Phys. 2005;32(8):2606–2614. [DOI] [PubMed] [Google Scholar]
- 99. Redmond KJ, Robertson S, Lo SS, et al. Consensus contouring guidelines for postoperative stereotactic body radiation therapy for metastatic solid tumor malignancies to the spine. Int J Radiat Oncol Biol Phys. 2017;97(1):64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tseng CL, Sussman MS, Atenafu EG, et al. Magnetic resonance imaging assessment of spinal cord and cauda equina motion in supine patients with spinal metastases planned for spine stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2015;91(5):995–1002. [DOI] [PubMed] [Google Scholar]
- 101. Chuang C, Sahgal A, Lee L, et al. Effects of residual target motion for image-tracked spine radiosurgery. Med Phys. 2007;34(11):4484–4490. [DOI] [PubMed] [Google Scholar]
- 102. Seung SK, Curti BD, Crittenden M, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2 – tumor and immunological responses. Sci Transl Med. 2012;4(137):137ra174. [DOI] [PubMed] [Google Scholar]
- 103. Mazeron R, Anderson B, Supiot S, Paris F, Deutsch E. Current state of knowledge regarding the use of antiangiogenic agents with radiation therapy. Cancer Treat Rev. 2011;37(6):476–486. [DOI] [PubMed] [Google Scholar]
- 104. Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63(3):655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2 Pt 1):728–734. [PubMed] [Google Scholar]
- 106. Ma L, Wang L, Tseng CL, Sahgal A. Emerging technologies in stereotactic body radiotherapy. Chin Clin Oncol. 2017;6(suppl 2):S12. [DOI] [PubMed] [Google Scholar]