Abstract
Background and Purpose:
The aim of this study was to investigate the survival outcomes and safety of hypofractioned stereotactic radiotherapy as a salvage treatment for recurrent high-grade glioma.
Patients and Methods:
Between March 2012 and March 2017, 32 consecutive patients (12 women, 20 men) treated in a single center were retrospectively included in this study. Grade III gliomas were diagnosed in 14 patients and grade IV in 18 patients. Thirty-four lesions were treated with hypofractionated stereotactic radiotherapy on a linear accelerator. Hypofractionated stereotactic radiotherapy delivered a median dose of 30 Gy (27-30) in 6 fractions (3-6) of 5 Gy (5-9). The treatment plans were normalized to 100% at the isocenter and prescribed to the 80% isodose line. Clinical outcomes and prognostic factors were analyzed.
Results:
Median follow-up was 20.9 months. Median overall survival following hypofractionated stereotactic radiotherapy was 15.6 months (median overall survival for patients with glioblastoma and grade III glioma was 8.2 and 19.5 months, respectively; P = .0496) and progression-free survival was 3.7 months (median progression-free survival for patients with glioblastoma and grade III glioma was 3.6 and 4.5 months, respectively; P = .2424). In multivariate analysis, tumor grade III (P = .0027), an Eastern Cooperative Oncology Group status <2 at the time of reirradiation (P = .0023), and a mean dose >35 Gy (P = .0055) significantly improved overall survival. A maximum reirradiation dose above 38 Gy (P = .0179) was significantly associated with longer progression-free survival.
Conclusion:
Hypofractionated stereotactic radiotherapy is well tolerated and offers an effective salvage option for the treatment of recurrent high-grade gliomas with encouraging overall survival. Our results suggest that the dose distribution had an impact on survival.
Keywords: recurrence, high-grade glioma, hypofractionated, stereotactic radiotherapy, salvage
Introduction
High-grade gliomas (HGGs) are the most frequent brain tumors in adults, with an annual incidence of 6 cases per one hundred thousand worldwide.1
The main current recommendation for treatment is full microsurgical resection for all patients. If this is not feasible, a stereotactic biopsy should be carried out. After surgery, patients should receive radiotherapy and chemotherapy based on histology and molecular analysis.2-6
Unfortunately, these malignant brain tumors are radioresistant and chemoresistant and have a very poor prognosis. Also, the risk of relapse and progression is inevitable.2,7
The management of tumor recurrence is not standardized and may be difficult. It requires an individual evaluation based on age, performance status, histology, extent of the initial resection, type of response to initial therapy, time since diagnosis, and recurrence size.8,9
The treatment of HGG recurrences and progressions must be evaluated in multidisciplinary tumor board and a surgical treatment must be systematically discussed. The other possible treatments include radiotherapy, chemotherapy (temozolomide [TMZ]; fotemustine; lomustine, procarbazine, and vincristine [PCV], etc), targeted therapies (bevacizumab), or novel agents which are proposed in the framework of clinical trials.10
For the treatment of HGG recurrence by radiotherapy, many approaches have been studied, including brachytherapy, single-fraction radiosurgery, hypofractionated stereotactic radiotherapy (HFSRT), or conventional fractionated radiotherapy10-18; however, none of these treatments demonstrated significant improvement in a phase III study.
Nevertheless, stereotactic radiotherapy is an interesting approach because it is minimally invasive, ambulatory, short-lasting, and well tolerated.17 Several studies19-46 have reported the feasibility of HFSRT as it shows potential efficacy and acceptable toxicity for the treatment of recurrent HGGs.
The aim of this study was to evaluate the efficacy of and safety to HFSRT as a salvage treatment for patients suffering from HGG relapse in our cancer center and to compare these results with the literature.
Material and Methods
Between March 2012 and March 2017, 32 consecutive patients with recurrent HGG received HFSRT at the Department of Radiation Oncology of Georges-François Leclerc Cancer Center in Dijon, Burgundy, France.
Eligibility Criteria
The study was approved by our institutional review board. The study included patients with HGG diagnosed on the initial pathological analysis and patients who presented a transformation from a low-grade lesion into a high-grade lesion during follow-up (contrast-enhanced magnetic resonance imaging [MRI]). All patients underwent neurosurgery followed by fractionated brain irradiation with a standard dose (54 or 60 Gy) with or without chemotherapy. Tumor progression or recurrence was assessed by MRI scans during follow-up or when the neurological condition of patients deteriorated. The decision to treat the relapse with HFSRT was confirmed in a multidisciplinary neuro-oncology tumor board.
Treatment Planning
Computed tomography (CT) simulations with slice thickness of 1.25 mm were performed, using a LightSpeed RT16 Vision (GE Health Care, Milwaukee, Wisconsin). During the planning CT, patients were fitted with a thermoplastic mask system dedicated to stereotactic treatment to ensure immobilization and reproducibility. Patients were treated with a stereotactic approach, using intensity-modulated radiation therapy (5-7 static fields) or volumetric-modulated arc therapy (1-4 arcs) technology with a Varian linear accelerator (Varian Medical Systems, Palo Alto, California): Trilogy with SonArray patient positioning system and Bite-Block system. Since 2015, a NovalisTx with BrainLAB and Exatrac systems (BrainLAB, Munich, Germany) has been used.
The dose prescribed for reirradiation was based on the localization of prior radiation therapy, the site of the lesion, and its proximity to organs at risk or the recurrence volume.
A total dose of 30 Gy in 6 fractions with 2 or 3 fractions per week was delivered, corresponding to a biologically effective dose (BED) of 80 Gy (α/β = 3) and 45 Gy (α/β = 10). For a reirradiation in the initial planning target volume (PTV), a cumulative BED with the first course (60 Gy in 30 fractions) corresponded to 180 Gy (α/β = 3) and 117 Gy (α/β = 10). One patient, who had 2 lesions, was treated with 27 Gy in 3 fractions with 3 fractions per week on each lesion (BED = 108 Gy; α/β = 3-51.3 Gy; α/β = 10 and a cumulative BED of 208 Gy [α/β = 3] or 123.3 Gy [α/β = 10]). The treatment plans were normalized to 100% at the isocenter and prescribed to the 80% isodose line. Treatment was planned using the fusion of CT and MRI images. The clinical target volume (CTV) corresponded to the gross target volume obtained using contrast-enhanced T1-weighted MRI, edema (T2 FLAIR) was not included in the CTV. This volume was expanded by margins of 2 or 3 mm to generate the PTV, except for one of the first patients treated with a 5 mm PTV. The medullary canal, brainstem, whole brain, normal brain (whole brain minus PTV minus cerebellum), anterior and posterior chambers of eyeballs, chiasma, optical nerves, and cochlea, defined as organ at risks, were delineated.
The Eclipse Treatment Planning System (version 11) was used with Analytical Anisotropic Algorithm model to plan dosimetry. A Patient-Specific Quality Assurance has been performed before start of treatment.
Concomitant Drugs
Most patients (31; 96.9%) were treated without chemotherapy. One (3.1%) patient received concomitant bevacizumab (10 mg/kg, every 2 weeks).
Follow-Up
Clinical and radiological data for follow-up were collected at the first medical consultation after HFSRT (for adjuvant chemotherapy or systematic follow-up), and after each medical consultation with a radiological (MRI) evaluation and at each change of therapeutic line. This radiological evaluation has been performed every 3 months after reirradiation.
The primary endpoint of this study was survival. Overall survival (OS) was calculated from the end of the HFSRT. Progression-free survival (PFS) was calculated from the end of HSFRT until tumor progression or death (by any cause). Tumor progression was defined according to response assessment in neuro-oncology criteria. The secondary endpoint of this study was toxicity, which was classified according to the common terminology criteria for adverse events v 4.03.
Statistical Analysis
Categorical variables are presented as percentages and were compared using the χ2 or Fisher test. Continuous variables are described as means (with standard deviations) and medians (with ranges) and were compared using the Student or Wilcoxon test in case of non-normal distribution. The median survival time was estimated using the reverse Kaplan-Meier method. Survival probabilities were estimated using the Kaplan-Meier method and the log-rank test was used to compare survival curves. Hazard ratios and their 95% confidence interval for univariate and multivariate analysis of OS were estimated using a Cox proportional hazards regression model. Correlations between covariables were tested for eligible variables. To prevent collinearity, when 2 variables were significantly correlated, one variable was retained according to its clinical relevance or to the value of the likelihood ratio. Statistical analyses were performed using SAS 9.3 software. All tests were 2 sided, and P values were considered significant when less than .05.
Results
Patients
The characteristics of 32 patients are resumed in Table 1. The median age at HGG diagnosis was 57.5 (29-76) years. There were 20 (62.5%) men and 12 (37.5%) women. At the moment of recurrence, all patients presented an HGG: 18 (56.25%) glioblastoma (GBM) and 14 (43.75%) grade III gliomas. According to the 2007 World Health Organization classification in force at the time of diagnosis, there were distributed as follows: 9 (28.13%) oligodendrogliomas, 3 (9.38%) astrocytomas, and 2 (6.25%) oligoastrocytomas. Seven patients (21.88%) presented a transformation from low grade to high grade, whose 2 patients with a histological confirmation.
Table 1.
Patients and Initial Tumor Characteristics.
| Patients | N = 32 |
| Women | 12 (37.5%) |
| Men | 20 (62.5%) |
| Median age at HGG diagnosis | 57.5 (29.0-76.0) |
| Pathology | |
| Oligodendroglioma | 9 (28.1%) |
| Oligoastrocytoma | 2 (6.3%) |
| Astrocytoma | 3 (9.4%) |
| Glioblastoma | 18 (56.3%) |
| Methylation MGMT | |
| Yes | 7 (21.9%) |
| No | 6 (18.8%) |
| Unknown | 19 (59.4%) |
| Mutation IDH1 | |
| Yes | 1 (3.1%) |
| No | 5 (15.6%) |
| Unknown | 26 (81.3%) |
| 1p19q codeletion | |
| No | 4 (12.5%) |
| Unknown | 28 (87.5%) |
| Treatment characteristics | |
| Extent of surgery | |
| Gross total resection | 6 (19.4%) |
| Subtotal resection | 17 (54.8%) |
| Stereotactic biopsy | 8 (25.8%) |
| Unknown | 1 (3.1%) |
| Salvage surgery prior to initial irradiation | |
| Subtotal resection | 2 (6.3%) |
| Unknown | 1 (3.1%) |
| No | 29 (90.6%) |
| Chemotherapy prior to initial irradiation | 8 (25%) |
| Radiochemotherapy | |
| Radiotherapy alone | 6 (18.75%) |
| Radio chemotherapy | 26 (81.25%) |
| Dose | |
| 60 Gy/30 fr | 30 (93.75%) |
| 54 Gy/27 fr | 2 (6.25%) |
| Concomitant chemotherapy | |
| TMZ | 22 (84.6%) |
| TMZ + bevacizumab | 4 (15.4%) |
Abbreviations: fr, fractions; HGG, high-grade glioma; MGMT, O6-methylguanin-DNA-methyltransferase; TMZ, temozolomide.
O6-methylguanin-DNA-methyltransferase status was known for 13 patients (40.7%), of whom 7 (21.9%) showed hypermethylation. An IDH1 mutation was identified for 1 patient (3.1%) and was negative for 5 (15.6%) others. 1p19q codeletion status was known for 4 patients (12.5%) and was negative.
Primary Treatment
All patients had undergone at least one neurosurgical intervention. At the initial diagnosis, gross total resection was performed in 6 patients (18.75%), subtotal resection was performed in 17 patients (53.13%), a stereotactic biopsy was done in 8 patients (25%), and the surgical status was unknown for 1 patient (3.1%). Three patients (9.38%) underwent a second surgery prior to the radiation therapy.
All of the patients received a full course of radiation therapy with a median dose of 60 Gy (54-60) in conventional fractionation; 2 patients (6.25%) received 54 Gy in 27 fractions and the other (93.75%) patients 60 Gy in 30 fractions. Six patients (18.75%) had radiotherapy alone and 26 (81.25%) received concomitant chemotherapy according to the Stupp protocol.2 Four patients also had concomitant bevacizumab as part of a protocol.
Disease Evolution
The median time between HGG diagnosis and the first recurrence or progression was 1.3 (0-8.4) years and time between the initial radiation therapy and the first recurrence was 1.2 (0.08-11.3) years. The median number of recurrences prior to the HFSRT was 2 (1-5), and 18 patients (56.25%) received 1 to 3 systemic salvage therapies with various agents such as PCV, TMZ, bevacizumab, lomustine, and fotemustine.
Seven patients (21.88%) had salvage neurosurgery (4 with macroscopic resection and 3 with subtotal surgery), 1 patient (3.1%) had 2 surgeries: the first macroscopic and the second subtotal.
Recurrence at the Time of HSFRT
At the time of the HSFRT, the median age was 61.5 (33-77) years. Ten (31.3%), 14 (43.8%), and 8 (25%) patients had an Eastern Cooperative Oncology Group (ECOG) status of 0, 1, or 2, respectively. The recursive partitioning analysis status was III for 7 (21.9%), IV for 12 (37.5%), V for 11 (34.4%), and VI for 2 (6.3%) patients.
The median time between the HGG diagnosis and HFSRT was 2 (0.6-13.4) years while the time between the primary radiotherapy and reirradiation was 1.9 (0.5-13.2) years.
Two patients (6.25%) presented bifocal recurrence at the time of the HSFRT. The characteristics of the 34 lesions treated with HFSRT are resumed in Table 2.
Table 2.
Patients, Recurrent Tumor, and HFSRT Characteristics.
| Patients | N = 32 |
| Median age at stereotactic radiotherapy [min-max] | 61.5 [33.0-77.0] |
| ECOG status: 0/1/2 | 10 (31.3%)/14 (43.8%)/8 (25.0%) |
| RPA status: III/IV/V/VI | 7 (21.9%)/12 (37.5%)/11 (34.4%)/2 (6.3%) |
| Number of patients with salvage surgery prior to HSFRT | 7 (21.9%) |
| Number of patients with chemotherapy prior to HSFRT | 18 (56.25%) |
| Recurrent tumor at time of HSFRT | |
| Median time from initial irradiation (years) | 1.9 [0.08-13.2] |
| Number of lesions | 34 |
| Median tumor volume (cm3) | 6.1 [0.1-42.2] |
| HSFRT characteristics | N = 34 |
| Dose: 27 Gy / 30 Gy | 2 (5.9%)/32 (94.1%) |
| Number of fractions: 3 / 6 | 2 (5.9%)/32 (94.1%) |
| Dose per fraction: 9 Gy / 5 Gy | 2 (5.9%)/32 (94.1%) |
| Isodose: 80% | 34 (100%) |
| PTV margins: 2 mm/3 mm/5 mm | 18 (52.9%)/15 (44.1%)/1 (2.9%) |
| Concomitant drug: bevacizumab | 1 (2.9%) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HFSRT, hypofractionated stereotactic radiotherapy; PTV, planning target volume; RPA, recursive partitioning analysis.
The majority of recurrences (23; 67.7%) were localized within the initial PTV, 2 (5.9%) were localized outside and 1 (2.9%) was on the periphery (defined as 1 cm on either side of the initial PTV boundaries). For 8 (23.5%), the relationship with the initial PTV was unknown (initial dosimetric data were lost when computer versions were updated).
HFSRT Characteristics
The median tumor volume was of 6.1 (0.1-42.2) cm3, the PTV was 15 (0.6-67.5) cm3, and the prescription volume (isodose line 80%) was 19.1 (1.4-66.6) cm3. The median maximum dose (Dmax), median minimum dose (Dmin), and median mean dose (Dmean) were 38.7 (32.7-42.0), 29.1 (14.0-32.4), and 35.1 (31.5-37.5) Gy, respectively.
Most patients (24; 75%) were subsequently treated with various agents such as TMZ, bevacizumab, fotemustine, lomustine, PCV, erlotinib, afatinib, or C-MET inhibitor after the HFSRT and/or at the new recurrence.
At the time of analysis, no patients had undergone another surgery following the HFSRT.
Survival
The median follow-up was 20.9 (2.8-47.4) months. At the time of the analysis, 20 patients (62.5%) had died.
OS following HFSRT
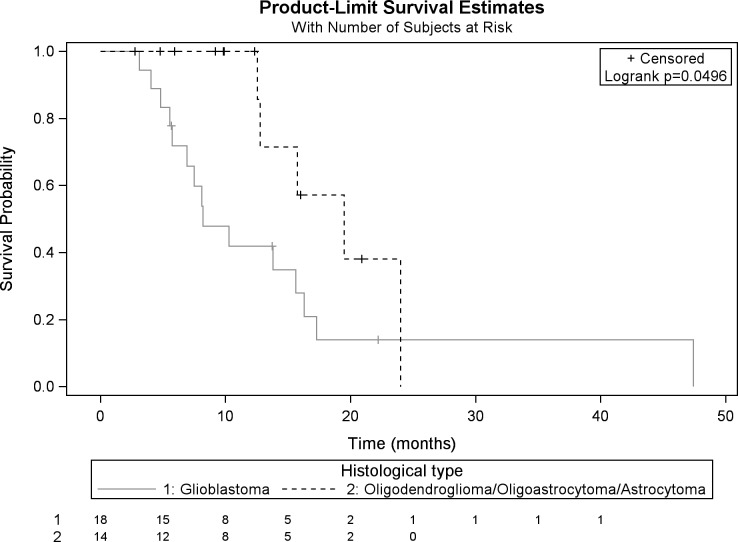
Median OS calculated from the reirradiation was 15.6 (8.2-17.3) months. The survival rate at 6 and 12 months was 83.4% and 64.6%, respectively. Median OS for patients with GBM was 8.2 (5.7-17.3) months and that for patients with grade III glioma was 19.5 (12.6-24) months (Figure 1).
Figure 1.
Kaplan-Meier OS after HFSRT for patients with GBM and grade III glioma. OS indicates overall survival; HFSRT, hypofractionated stereotactic radiotherapy; GBM, glioblastoma.
In univariate analysis, the initial irradiation technique, the initial T2 FLAIR volume, concomitant bevacizumab with the primary irradiation, reirradiation tumor volumes, and reirradiation mean dose were significant prognostic factors (P < .05) of OS (Table 3). In multivariate analysis, tumor grade III (P = .0027), a mean dose >35 Gy (P = .0055), and an ECOG status <2 at the time of reirradiation (P = .0023) significantly improved OS (Table 3).
Table 3.
Univariate and Multivariate Analysis: Prognostic Factors for OS.
| HR | 95% CI | P Value | |
|---|---|---|---|
| Univariate analysis for OS following HSFRT | |||
| Initial irradiation technique: IMRT vs 3D | 0.227 | 0.056-0.926 | .0388 |
| Initial T2 FLAIR volume: >100 vs ≤100 cm3 | 4.147 | 1.085-15.856 | .0376 |
| Initial irradiation with concomitant bevacizumab: yes vs no | 4.853 | 1.505-15.649 | .0082 |
| HSFRT GTV volume: >6 vs ≤6 cm3 | 5.185 | 1.691-15.900 | .0040 |
| HSFRT PTV volume: >15 vs ≤15 cm3 | 3.281 | 1.169-9.208 | .0240 |
| Prescription volume (isodose line 80%): >19 vs ≤19 cm3 | 3.281 | 1.169-9.208 | .0240 |
| Multivariate analysis for OS following HSFRT | |||
| Tumor grade: grade IV vs grade III | 6.234 | 1.887-20.591 | .0027 |
| Stereotactic mean dose: >35 Gy vs ≤35 Gy | 0.219 | 0.075-0.639 | .0055 |
| ECOG status: 2 vs 0-1 | 8.115 | 2.108-31.240 | .0023 |
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; GTV, gross tumor volume; HR, hazard ratio; HFSRT, hypofractionated stereotactic radiotherapy; IMRT, intensity-modulated radiation therapy; PTV, planning target volume; OS, overall survival.
Progression-free survival
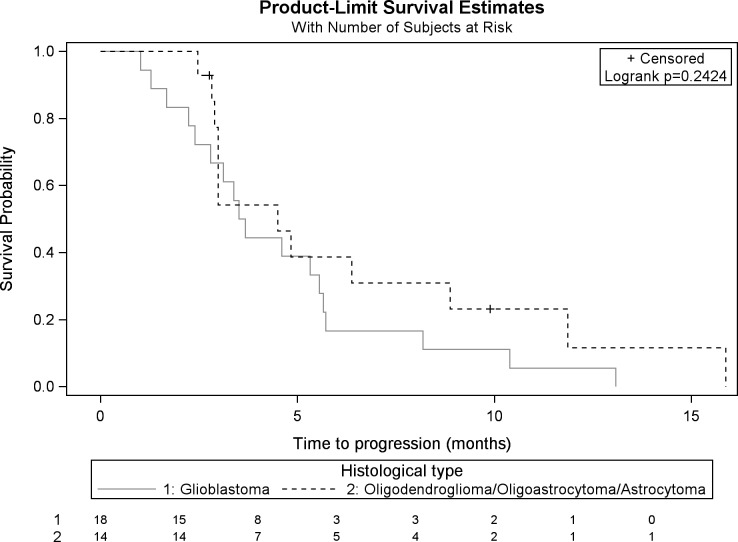
The PFS after HFSRT was 3.7 (3-5.7) months overall: 3.6 (2.4-5.6) months for patients with GBM and 4.5 (2.9-8.9) months for patients with grade III glioma (Figure 2).
Figure 2.
Kaplan-Meier PFS after HFSRT for patients with GBM and grade III glioma. PFS indicates progression-free survival; HFSRT, hypofractionated stereotactic radiotherapy; GBM, glioblastoma.
The median time to the first MRI evaluation after HSFRT was 3 (1-10) months.
The first progression after HFSRT based on MRI evaluation was located inside the PTV for 25 patients (44%). It was outside the PTV in 36%, and both inside and outside in 20%.
The maximum reirradiation dose above 38 Gy was a significant prognostic factor of PFS (P = .0179) in multivariate analysis (Table 4).
Table 4.
Univariate and Multivariate Analysis: Prognostic Factors for PFS.
| HR | 95% CI | P Value | |
|---|---|---|---|
| Univariate analysis for PFS | |||
| Maximum dose: >38 Gy vs ≤38 Gy | 0.74 | 0.146-0.958 | .0405 |
| Multivariate analysis for PFS | |||
| Stereotactic maximum dose: >38 Gy vs ≤38 Gy | 0.317 | 0.122-0.820 | .0179 |
Abbreviations: CI, confidence interval; HR, hazard ratio; PFS, progression-free survival.
Toxicity
Treatment was completed in all patients in the specified time. All patients were included in the analysis. One patient was lost to follow-up at the end of the HFSRT. Treatment was well tolerated, no acute toxicity >grade 2 was observed, and the neurological deteriorations correlated with neoplastic progression during the follow-up. Nevertheless, one patient presented homonymous hemianopsia during the HFSRT, but this resolved during the follow-up. Ten patients (31.25%) had suspected radionecrosis. If in doubt between radionecrosis or progression, a new MRI at 2 months or a multimodal MRI has been proposed. In 6 patients, this suspicion corresponded to tumor progression. For the other patients, radionecrosis was suggested on multimodal MRI. These patients had asymptomatic radionecrosis at the time of diagnosis.
Discussion
The standard of care for patients with recurrent GBM or grade III glioma has not yet been clearly defined, and many approaches are available for salvage strategies, including surgery, reirradiation, or systemic agents.10-18
In the current study, we evaluated the feasibility of HFSRT as a salvage treatment for HGG. Our patients were long survivors, as the median time between HGG diagnosis and the first relapse and the time between the initial radiotherapy and HFSRT were 1.3 and 1.9 years, respectively. This can be explained by the large proportion of patients with grade III glioma (43.75%). In addition, 7 patients presented a transformation from low-grade glioma to HGG with a slow disease evolution. Furthermore, most patients had experienced several relapses between the initial radiation therapy and the HFSRT (median number: 2), and management was often multimodal with different treatments (new surgery, chemotherapy).
Fogh et al 35 reported that patients with early relapse from initial irradiation (<6 months) had a more unfavorable prognosis, suggesting they should not qualify for salvage therapy.
The HFSRT appeared to be a feasible and a short minimally invasive approach for the treatment of HGG recurrence in eloquent and/or previously irradiated areas. Indeed, this is particularly important because relapse of HGG principally occurs within the 2 cm around the initial tumor site.17,47
In our study, treatment was well tolerated and did not block the possibility of further treatments, as most patients were treated with various agents after HFSRT. These results suggest that this technique is safe.
In the literature, many authors have studied hypofractionated or moderately fractionated stereotactic radiotherapy delivered with a linear accelerator for the management of HGG recurrence and also concluded that HFSRT reirradiation for HGG recurrence is feasible with minimal adverse effects19-44,46 (details of these studies are summarized in Table 5. The studies with Gamma Knife or CyberKnife as well single-fraction radiosurgery studies were not included).
Table 5.
Review of the Literature Including HFSRT With LINAC for Reirradiation of Recurrent HGG With or Without Chemotherapy.
| Authors and Year | Number of Patients | Median Age | GBM/Grade III | Median KPS | Total Dose; Dose per Fraction (Gy) | Number of Fractions; Number of fr per Week | PTV Margins (mm) | IDS | Median Tumor Volume (cm3) | Median Time Between Initial Irradiation and HFSRT (Months) | Surgery Before HFSRT | Associated Chemotherapy | OS (Months) | PFS (Months) | Prognostic Factors | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laing et al 199319 | 22 | 34 (14-56) | 12/7 | 70 | 20-50; 5 | 5; daily | 2 | 80-90 | 25 (1-93) | 20 | 6 | – | 9.8 | – | – | Neurological deterioration: 5 |
| Glass et al 199720 | 20 | 44 (6-73) | 13/7 | 90 | 42; 6 | 7; 2 | – | 70 | 14 (2-122) | 8 | – | CDDP | 13.7 | 4.6 | – | Radionecrosis: 3 |
| Shepherd et al 199721 | 33 | 37 (19-55) | 0/36 | 80 | 20-50; 5 | 4-10; daily | 2 | 80-90 | 24 (3-93) | 29 | – | – | 11 | – | – | Radionecrosis: 6 Reoperation: 2 |
| Hudes et al 199922 | 20 | 52 (26-77) | 19/1 | 80 | 24-35; 3-3.5 | 8-10; daily | 0 | 80-95 | 12.6 (0.9-47.5) | 3.1 | – | – | 10.5 | – | – | – |
| Lederman et al 200023 | 88 | 56 (21-82) | 88/0 | 70 | 24; 6 | 4; 1 | – | 80-90 | 32.7 (1.5-150) | 7.8 | – | Paclitaxel | 7 | – | Tumor volume | Radionecrosis: 7 Reoperation: 11 |
| Selch et al 200024 | 21 | 54 (14-72) | 14/7 | 80 | 20-35; 4-6 | 5; – | 0-3 | 70-90 | 11.6 (4.5-33.7) | 11 | 21% | – | 6 | 4 | – | – |
| Voynov et al 200225 | 10 | 48 (33-85) | 4/6 | 80 | 25-40; 5 | 5-8; – | 0 | 71-93 | 34.7 (4.3-75) | 19 | 5 | – | 10.1 | – | – | Reoperation: 2 |
| Vodermark et al 200526 | 19 | 50 (11-74) | 9/10 | 90 | 20-30; 4-10 | 2-6; – | 1-3 | 70-90 | 15 (4-70) | 19 | 12 | – | 9.3 | 4.9 | Tumor grade | Reoperation: 5 |
| Grosu et al 200527 | 44 | 50 (36-75) | 33/11 | 75 | 30; 5 | 6; daily | 3 | 100 | 15 (1-61) | 16 | – | TMZ | 13 | – | SPECT/CT/MRI, TMZ | – |
| Wurm et al 200628 | 25 | 46 (11-66 | 20/5 | 80 | 25-30; 5 | 5-6; daily | – | 80 | 16.5 (1-70.9) | – | – | Topotecan | 14.5 | 10.5 | – | – |
| Ernst-Stecken et al 200729 | 15 | 49 (31-69) | 11/4 | 80 | 35; 7 | 5; 3 | 3 | 90 | 5.7 (0.8-22) | 10 | – | – | 12 | 7 | – | – |
| Schwer et al 200830 | 15 | 47 (23-65) | 11/4 | 70 | 18-36; 6-12 | 3; 3 | 2 | 90 | 41.3 (5-150) | 12 | 7 | Gefitinib | 10 | 7 | – | – |
| Fokas et al 200931 | 53 | 53 (22-71) | 53/0 | – | 25-60; 2-5 | 5-30; – | 3 | 90 | 35.01 (3-204) | – | 23 | – | 9 | 12 | KPS | – |
| Patel et al 200932 | 10 | 44 (28-60) | 10/0 | 90 | 36; 6 | 6; 2 | 0 | 90 | 51.1 (16.1-123.3) | 14.9 | 7 | – | 7.5 | – | Radiographic responders | – |
| Gutin et al 200933 | 25 | 56 (30-80) | 20/5 | 80 | 30; 6 | 5; – | 5 | 100 | 34 (2-62) | 15 | – | Bevacizumab | GBM: 12.5 Grade III: 16.5 |
GBM: 7.3 Grade III: 7.5 |
– | Reoperation: 3 Hemorrhage: 1 Wound dehiscence: 1 |
| Henke et al 200934 | 31 | 50 (16-74) | 29/2 | 90 | 20-25; 4-5 | 4-5; – | 3-10 | – | – | 18 | 15 | – | 10.2 | – | – | – |
| Fogh et al 201035 | 147 | 53 (28-80) | 105/42 | – | 35; 3.5 | 10; daily | – | 85-90 | 22 (0.6-104) | 8 | 84 | Various agents | GBM: 8 Grade III: 11 |
– | Younger age Smaller GTV Shorter time between diagnosis and recurrence |
Steroids increase: 19 |
| Minniti et al 201336 | 54 | 52 (30-72) | 38/16 | 80 | 30; 6 | 5; daily | 3-5 | 90 | 9.7 (3.1-32.3) | 15.5 | 12 | TMZ | 12.4 | 6 | KPS Tumor grade |
Neurological deterioration grade 3: 4 |
| Shapiro et al 201337 | 24 | 56 (30-80) | 20/4 | 80 | 30; 6 | 5; 2 | 5 | – | – | – | 2 | Bevacizumab | 32.1 (from diagnosis) | 7.5 | Radiographicresponse | – |
| Ogura et al 201338 | 30 | 52.5 (19-81) | 15/9 | – | 22.5-35; 4.5-7 | 5; daily | 1-2 | 70-80 | 3.02 (0-36.1) | 24.8 | – | Various agents | 10.4 | 3 | Morphology Tumor type Tumor volume |
Radionecrosis grade 3: 2 |
| Ciammela et al 201339 | 15 | 51.5 (41-73) | 91/0 | 90 | 25; 5 | 5; daily | 3–5 | 70 | – | 10.8 | – | – | 9.5 | – | Age Initial extent surgery RPA KPS MGMT status |
Neurological deterioration: 2 |
| Wuthrick et al 201440 | 11 | 51 (37-67) | 8/3 | – | 30-42; 2.5-3.75 | 10-15; daily | 0 | 85-90 | 16.75 (0.05-72.01) | 19.5 | – | Sunitinib | 11 | 5.8 | – | – |
| Miwa et al 201441 | 21 | 53.9 (22-76) | 21/0 | 80 | 25-35; 5-7 | 5; daily | 3 | 80-95 | – | 12 | – | TMZ | 11 | 6 | KPS | Radionecrosis: 2 |
| Dincoglan et al 201542 | 28 | 55.6 (38-76) | 28/0 | 80 | 25; 5 | 5; daily | 3 | 85-95 | – | 11.2 | – | – | 10.3 | 5.8 | KPS Age PTV size Time from diagnosis to recurrence |
– |
| Minniti et al 201543 | 54 | 54 (30-72) | 42/12 | 70 | 25; 5 | 5; daily | 1–2 | 90 | 12.4 (1.8-43.3) | 14 | – | Bevacizumab or fotemustine | Bevacizumab: 11 Fotemustine: 8.3 |
Bevacizumab: 6 Fotemustine: 4 |
KPS Tumor grade Bevacizumab |
Radionecrosis: 3 |
| Shi et al 201644 | 12 | 46 (33-66) | 8/4 | 80 | 30-35; 3-3.5 | 10; daily | 5 | – | 26.8 (2.7-143) | – | – | Panobinostat 10-20-30 mg | 7.8, 6.1, 16.1 | – | – | Radionecrosis grade 3: 1 |
| Antoni et al 201645 | 20 | 55.7 (33.9-82.9) | 13/7 | – | 18.75-37.5; 6.25 | 3-6; 3 | – | Isocenter | 0.91 (0.02-18.5) | 18.3 | – | TMZ Bevacizumab |
17.7 | 12 | Bevacizumab High-dose radiation |
– |
| Clarke et al 201746 | 15 | 63 (50-73) | 10/5 | 90 | 27-33; 9-11 | 3; – | 2-5 | – | – | – | – | Bevacizumab | 13 | 7 | – | Radionecrosis grade 3: 1 |
| Present study | 32 | 61.5 (33-77) | 18/14 | – | 27-30; 5-9 | 3-6; 2-3 | 2-5 | 80 | 6.1 (0.1-42.2) | 22.8 | 7 | – | 15.6 | 3.7 | ECOG status Tumor grade Dose |
Radionecrosis grade 1: 4 |
Abbreviations: CT, computed tomography, fr, fraction; GBM, glioblastoma; GTV, gross tumor volume; HFSRT, hypofractionated stereotactic radiotherapy; IDS, isodose surface; KPS, Karnofsky performance score; MGMT, O6-methylguanin-DNA-methyltransferase; MRI, magnetic resonance imaging; OS, overall survival; PFS, progression-free survival; PTV, planning target volume; RPA, recursive partitioning analysis of prognostic factors; SPECT, single-photon emission computed tomography; TMZ, temozolomide.
In this current study, HFSRT was delivered without chemotherapy, except for one patient, who was treated with concomitant bevacizumab. In 1997, Glass et al 20 tested this combined approach of stereotactic radiotherapy and chemotherapy with cisplatin. Since then, several studies that combined HFSRT with various drugs (paclitaxel, TMZ, topotecan, gefitinib, sunitinib, fotemustine, panobinostat, or bevacizumab)20,23,27,28,30,33,35-38,40,41,43-46 have been conducted. According to these studies, combined modality management appears to be feasible and well tolerated, and the results are encouraging especially with bevacizumab.33,37,43,45,46 The RTOG 1205 randomized phase II trial could shed new light on the efficacy of this strategy and clarify the role of bevacizumab in the management of HGG recurrence.
In the literature, hypofractionated stereotactic regimens varied from one study to another and sometimes within the same study. The reported doses ranged from 18 to 50 Gy with different rules for prescription, fractionations, and staggering. As a result, it is difficult to compare the radiobiological effects of these regimens. To date, no phase III trials have been conducted to compare the different stereotactic regimens and the vast majority of studies have been retrospective. Thus, no scheme has shown a benefit with respect to others.
In our study, the main scheme used was 30 Gy in 6 fractions of 5 Gy, the treatment plans were normalized to 100% at the isocenter and prescribed to the 80% isodose line. Also, the dose was delivered with a variable dose distribution: Dmax, Dmin, and Dmean were ranged from 32.7 to 42.0, 14.0 to 32.4, and 31.5 to 37.5 Gy, respectively. For our HSFRT scheme, a Dmean >35 Gy appeared to significantly prolong OS and Dmax >38 Gy significantly prolonged PFS. These results suggested that the dose distribution had a positive impact on tumor control and therefore that dose escalation might be beneficial.
A trend toward a beneficial effect on survival was suggested by Vordermark et al.26 In a study of 19 patients treated for HGG recurrence with a dose of 20 to 30 Gy in different fractionations (2-6 fractions), prescribed to a median isodose of 80%, OS was better for dose over 30 Gy.
Fogh et al 35 suggested the benefit of a dose over 35 Gy. In a study of 147 patients treated for HGG relapse by HFSRT at a median dose of 35 Gy in daily fractions of 3.5 Gy, prescribed for an isodose of 85% to 90%, survival seemed to be increased (P = .07). However, Laing et al 19 and Shepherd et al 21 reported that a dose >40 Gy was a major predictor of toxicity (especially major consumption of corticosteroids) in patients treated with doses of 20 to 50 Gy in 5 fractions (prescription: isodose 80% or 90%), thus highlighting the small therapeutic windows.
Recently, Clarke et al 46 evaluated a dose-escalation strategy for the management of recurrent HGG treated with HFSRT in a phase I study. Their scheme was based on a previous study (Gutin et al 33), which reported the feasibility of HFSRT with a scheme of 30 Gy in 5 fractions, prescribed to the 100% isodose line. The dose-escalation study evaluated tolerance of 3 dose steps: 3 × 9 Gy, 3 × 10 Gy, and 3 × 11 Gy in combination with bevacizumab. The results attested the feasibility of the strategy at doses up to 33 Gy in 3 fractions.
In the literature, the reported OS is in the range of 6 (Selche et al 24) to 17.7 months (Antoni et al 45), and PFS ranged from 3 (Ogura et al 38) to 12 months (Fokas et al 31, Antoni et al 45). In our data, OS was 15.6 months; this good result could have been explicated by the high proportion (43.75%) of patients treated for grade III glioma. Our results suggest that grade III glioma was a significant prognostic factor for longer OS; the specific OS for grade III glioma was 19.5 months versus 8.2 months for GBM. Indeed, these different pathologies have different courses and prognoses; survival was better in patients with grade III gliomas especially since these gliomas develop from low-grade gliomas.
Equally, a high proportion (71.9%) of patients had gross or subtotal initial surgery, which may have had an impact on patient survival.48
Although our patient population was in keeping with populations in the literature with respect to the characteristics of patients, tumor recurrences, and the stereotactic technique, PFS in our study was low.
The first progressions suspected on MRI after HFSRT were inside the PTV for majority of patients. Niyazi et al 49 reported a similar recurrence pattern after fractionated reirradiation with bevacizumab in a study of 31 patients treated for recurrent HGG. Altogether, 61.3% of progressions were in-field and 38.7% at the margin or ex-field. Similarly, Shapiro et al 37 used a reirradiation regimen of 30 Gy in 5 fractions with concomitant bevacizumab to treat 24 patients with HGG relapse and studied recurrence patterns: 52.4% progressions were in field, 23.8% were marginal, and 23.8% were outside the field.
Actually, it is quite challenging to interpret radiological evaluation imaging after stereotactic radiotherapy because it is difficult to distinguish between progression, pseudoprogression, and radionecrosis. Thus, the short PFS could be explained by an overestimation of progression and an underestimation of radionecrosis.
Furthermore, it would be interesting to evaluate the effect of cumulative BED and the time between irradiations on the occurrence of radionecrosis. However, due to the small number of events and the limited number of patients, a relevant statistical analysis is not feasible.
The limitations of this study were its retrospective design, selection bias, and of various treatment factors, including surgery and chemotherapy before and after HFSRT. In addition, molecular biology information was only available for a minority of patients and specific statistical analyses were not available. However, our data were similar to those in the literature especially for the sample size.
Conclusion
The HFSRT appears to be a feasible and effective salvage treatment option for recurrent grade III glioma or GBM, with OS of 15.6 months. Prognostic factors associated with longer OS were a good general state of health and grade III glioma. Dosimetric data suggested that the dose distribution had an impact on tumor control and indicate that a study with dose-escalation is warranted. These results need to be confirmed in a prospective study with a greater number of patients.
Acknowledgments
Philip Bastable for correcting the manuscript.
Abbreviations
- BED
biologically effective dose
- CI
confidence interval
- CTV
clinical target volume
- CT
computed tomography
- Dmax
maximum dose
- Dmean
mean dose
- Dmin
minimum dose
- GBM
glioblastoma
- ECOG
Eastern Cooperative Oncology Group
- HFSRT
hypofractionated stereotactic radiotherapy
- HGG
high-grade glioma
- MRI
magnetic resonance imaging
- PCV
lomustine, procarbazine and vincristine
- PFS
progression-free survival
- PTV
planning target volume
- OS
overall survival
- TMZ
temozolomide.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Thomas Reynaud, MD  http://orcid.org/0000-0002-4376-0697
http://orcid.org/0000-0002-4376-0697
References
- 1. Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. doi:10.1016/S1470-2045(17)30194-8. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol (Berl). 2016;131(6):803–820. doi:10.1007/s00401-016-1545 -1. [DOI] [PubMed] [Google Scholar]
- 4. van den Bent MJ, Brandes AA, Taphoorn MJB, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-Up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. doi:10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 5. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. doi:10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bent MJVD, Erridge S, Vogelbaum MA, et al. Results of the interim analysis of the EORTC randomized phase III CATNON trial on concurrent and adjuvant temozolomide in anaplastic glioma without 1p/19q co-deletion: an Intergroup trial. J Clin Oncol. 2016;34(suppl; abstr LBA2000). [Google Scholar]
- 7. Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE. 3. Lancet Oncol. 2008;9(1):29–38. [DOI] [PubMed] [Google Scholar]
- 8. Easaw JC, Mason WP, Perry J, et al. Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol. 2011;18(3):126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma – are we there yet? Neuro Oncol. 2013;15(1):4–27. doi:10.1093/neuonc/nos273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Niyazi M, Siefert A, Schwarz SB, et al. Therapeutic options for recurrent malignant glioma. Radiother Oncol. 2011;98(1):1–14. doi:10.1016/j.radonc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 11. Amelio D, Amichetti M. Radiation therapy for the treatment of recurrent glioblastoma: an overview. Cancers. 2012;4(4):257–280. doi:10.3390/cancers4010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nieder C, Andratschke NH, Grosu AL. Re-irradiation for recurrent primary brain tumors. Anticancer Res. 2016;36(10):4985–4996. doi:10.21873/anticanres.11067. [DOI] [PubMed] [Google Scholar]
- 13. Dong Y, Fu C, Guan H, et al. Re-irradiation alternatives for recurrent high-grade glioma (review). Oncol Lett. 2016;12(4):2261–2270. doi:10.3892/ol.2016.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amichetti M, Amelio D. A review of the role of re-irradiation in recurrent high-grade glioma (HGG). Cancers. 2011;3(4):4061–4089. doi:10.3390/cancers3044061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seystahl K, Wick W, Weller M. Therapeutic options in recurrent glioblastoma—an update. Crit Rev Oncol Hematol. 2016;99:389–408. doi:10.1016/j.critrevonc.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 16. Ryu S, Buatti JM, Morris A, Kalkanis SN, Ryken TC, Olson JJ. The role of radiotherapy in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118(3):489–499. doi:10.1007/s11060-013-1337-6. [DOI] [PubMed] [Google Scholar]
- 17. Clavier J-B, Voirin J, Kehrli P, Noël G. Radiothérapie en conditions stéréotaxiques des gliomes de haut grade: une revue de la littérature [in French]. Cancer Radiothérap. 2010;14(8):739–754. doi:10.1016/j.canrad.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 18. Romanelli P, Conti A, Pontoriero A, et al. Role of stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of recurrent glioblastoma multiforme. Neurosurg Focus. 2009;27(6):E8. [DOI] [PubMed] [Google Scholar]
- 19. Laing RW, Warrington AP, Graham J, Britton J, Hines F, Brada M. Efficacy and toxicity of fractionated stereotactic radiotherapy in the treatment of recurrent gliomas (phase I/II study). Radiother Oncol. 1993;27(1):22–29. [DOI] [PubMed] [Google Scholar]
- 20. Glass J, Silverman CL, Axelrod R, Corn BW, Andrews DW. Fractionated stereotactic radiotherapy with cis-platinum radiosensitization in the treatment of recurrent, progressive, or persistent malignant astrocytoma. Am J Clin Oncol. 1997;20(3):226–229. [DOI] [PubMed] [Google Scholar]
- 21. Shepherd SF, Laing RW, Cosgrove VP, et al. Hypofractionated stereotactic radiotherapy in the management of recurrent glioma. Int J Radiat Oncol Biol Phys. 1997;37(2):393–398. [DOI] [PubMed] [Google Scholar]
- 22. Hudes RS, Corn BW, Werner-Wasik M, et al. A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 1999;43(2):293–298. [DOI] [PubMed] [Google Scholar]
- 23. Lederman G, Wronski M, Arbit E, et al. Treatment of recurrent glioblastoma multiforme using fractionated stereotactic radiosurgery and concurrent paclitaxel. Am J Clin Oncol. 2000;23(2):155–159. [DOI] [PubMed] [Google Scholar]
- 24. Selch MT, DeSalles AA, Solberg TD, et al. Hypofractionated stereotactic radiotherapy for recurrent malignant gliomas. J Radiosurg. 2000;3(1):3–12. [Google Scholar]
- 25. Voynov G, Kaufman S, Hong T, Pinkerton A, Simon R, Dowsett R. Treatment of recurrent malignant gliomas with stereotactic intensity modulated radiation therapy. Am J Clin Oncol. 2002;25(6):606–611. [DOI] [PubMed] [Google Scholar]
- 26. Vordermark D, Kölbl O, Ruprecht K, Vince GH, Bratengeier K, Flentje M. Hypofractionated stereotactic re-irradiation: treatment option in recurrent malignant glioma. BMC Cancer. 2005;5(1):55 doi:10.1186/1471-2407-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grosu AL, Weber WA, Franz M, et al. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol. 2005;63(2):511–519. doi:10.1016/j.ijrobp.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 28. Wurm RE, Kuczer DA, Schlenger L, et al. Hypofractionated stereotactic radiotherapy combined with topotecan in recurrent malignant glioma. Int J Radiat Oncol. 2006;66(4):S26–S32. doi:10.1016/j.ijrobp.2006.04.062. [Google Scholar]
- 29. Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Survival and quality of life after hypofractionated stereotactic radiotherapy for recurrent malignant glioma. J Neurooncol. 2007;81(3):287–294. doi:10.1007/s11060-006-9231-0. [DOI] [PubMed] [Google Scholar]
- 30. Schwer AL, Damek DM, Kavanagh BD, et al. A Phase I dose-escalation study of fractionated stereotactic radiosurgery in combination with gefitinib in patients with recurrent malignant gliomas. Int J Radiat Oncol. 2008;70(4):993–1001. doi:10.1016/j.ijrobp.2007.07.2382. [DOI] [PubMed] [Google Scholar]
- 31. Fokas E, Wacker U, Gross MW, Henzel M, Encheva E, Engenhart-Cabillic R. Hypofractionated stereotactic reirradiation of recurrent glioblastomas: a beneficial treatment option after high-dose radiotherapy? Strahlenther Onkol. 2009;185(4):235–240. doi:10.1007/s00066-009-1753-x. [DOI] [PubMed] [Google Scholar]
- 32. Patel M, Siddiqui F, Jin JY, et al. Salvage reirradiation for recurrent glioblastoma with radiosurgery: radiographic response and improved survival. J Neurooncol. 2009;92(2):185–191. doi:10.1007/s11060-008-9752-9. [DOI] [PubMed] [Google Scholar]
- 33. Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol. 2009;75(1):156–163. doi:10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Henke G, Paulsen F, Steinbach JP, et al. Hypofractionated reirradiation for recurrent malignant glioma. Strahlenther Onkol. 2009;185(2):113–119. doi:10.1007/s00066-009-1969-9. [DOI] [PubMed] [Google Scholar]
- 35. Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28(18):3048–3053. doi:10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Minniti G, Scaringi C, De Sanctis V, et al. Hypofractionated stereotactic radiotherapy and continuous low-dose temozolomide in patients with recurrent or progressive malignant gliomas. J Neurooncol. 2013;111(2):187–194. doi:10.1007/s11060-012-0999-9. [DOI] [PubMed] [Google Scholar]
- 37. Shapiro LQ, Beal K, Goenka A, et al. Patterns of failure after concurrent bevacizumab and hypofractionated stereotactic radiation therapy for recurrent high-grade glioma. Int J Radiat Oncol. 2013;85(3):636–642. doi:10.1016/j.ijrobp.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 38. Ogura K, Mizowaki T, Arakawa Y, Sakanaka K, Miyamoto S, Hiraoka M. Efficacy of salvage stereotactic radiotherapy for recurrent glioma: impact of tumor morphology and method of target delineation on local control. Cancer Med. 2013;2(6):942–949. doi:10.1002/cam4.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ciammella P, Podgornii A, Galeandro M, et al. Hypofractionated stereotactic radiation therapy for recurrent glioblastoma: single institutional experience. Radiat Oncol. 2013;8(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wuthrick EJ, Curran WJ, Camphausen K, et al. A pilot study of hypofractionated stereotactic radiation therapy and sunitinib in previously irradiated patients with recurrent high-grade glioma. Int J Radiat Oncol. 2014;90(2):369–375. doi:10.1016/j.ijrobp.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miwa K, Matsuo M, Ogawa S, et al. Re-irradiation of recurrent glioblastoma multiforme using 11C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity modulated radiation therapy. Radiat Oncol. 2014;9(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dincoglan F, Beyzadeoglu M, Sager O, et al. Management of patients with recurrent glioblastoma using hypofractionated stereotactic radiotherapy. Tumori J. 2015;101(2):179–184. doi:10.5301/tj.5000236. [DOI] [PubMed] [Google Scholar]
- 43. Minniti G, Agolli L, Falco T, et al. Hypofractionated stereotactic radiotherapy in combination with bevacizumab or fotemustine for patients with progressive malignant gliomas. J Neurooncol. 2015;122(3):559–566. doi:10.1007/s11060-015-1745-x. [DOI] [PubMed] [Google Scholar]
- 44. Shi W, Palmer JD, Werner-Wasik M, et al. Phase I trial of panobinostat and fractionated stereotactic re-irradiation therapy for recurrent high grade gliomas. J Neurooncol. 2016;127(3):535–539. doi:10.1007/s11060-016-2059-3. [DOI] [PubMed] [Google Scholar]
- 45. Antoni D, Jastaniah Z, Haoming QC, et al. Patterns of relapse in patients with high grade glioma receiving combined treatments including stereotactic re-irradiation for a first relapse. Cancer/Radiothérapie. 2016;20(4):282–291. doi:10.1016/j.canrad.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 46. Clarke J, Neil E, Terziev R, et al. Multicenter phase I dose escalation study of hypofractionated stereotactic radiotherapy with bevacizumab for recurrent glioblastoma and anaplastic astrocytoma. Int J Radiat Oncol. 2017;99(4):797–804. doi:10.1016/j.ijrobp.2017.06.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hess CF, Schaaf JC, Kortmann RD, Schabet M, Bamberg M. Malignant glioma: patterns of failure following individually tailored limited volume irradiation. Radiother Oncol J Eur Soc Ther Radiol Oncol. 1994;30(2):146–149. [DOI] [PubMed] [Google Scholar]
- 48. Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016;130(2):269–282. doi:10.1007/s11060-016-2110-4. [DOI] [PubMed] [Google Scholar]
- 49. Niyazi M, Jansen NL, Rottler M, Ganswindt U, Belka C. Recurrence pattern analysis after re-irradiation with bevacizumab in recurrent malignant glioma patients. Radiat Oncol. 2014;9(1). doi:10.1186/s13014-014-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]