ABSTRACT
Enteroendocrine cells (EECs) are a minor cell population in the intestine yet they play a major role in digestion, satiety and nutrient homeostasis. Recently developed human intestinal organoid models include EECs, but their rarity makes it difficult to study their formation and function. Here, we used the EEC-inducing property of the transcription factor NEUROG3 in human pluripotent stem cell-derived human intestinal organoids and colonic organoids to promote EEC development in vitro. An 8-h pulse of NEUROG3 expression induced expression of known target transcription factors and after 7 days organoids contained up to 25% EECs in the epithelium. EECs expressed a broad array of human hormones at the mRNA and/or protein level, including motilin, somatostatin, neurotensin, secretin, substance P, serotonin, vasoactive intestinal peptide, oxyntomodulin, GLP-1 and INSL5. EECs secreted several hormones including gastric inhibitory polypeptide (GIP), ghrelin, GLP-1 and oxyntomodulin. Injection of glucose into the lumen of organoids caused an increase in both GIP secretion and K-cell number. Lastly, we observed formation of all known small intestinal EEC subtypes following transplantation and growth of human intestinal organoids in mice.
KEY WORDS: Stem cell, Organoids, Neurogenin 3, Incretin, Satiety, Hormones
Summary: Manipulation of developmental signaling pathways and transcription factor expression allows differentiation of human pluripotent stem cells into nutrient-responsive, intestinal enteroendocrine cells.
INTRODUCTION
Enteroendocrine cells (EECs) occupy approximately 1% of the total epithelium in the intestine but secrete upwards of 15 different types of hormones and collectively make up the largest endocrine organ in the body. These hormones have a variety of roles that affect metabolism, the digestive process, satiety, hunger, and glucose homeostasis, among others. EECs express specific receptors that sense luminal nutrients, and in response, secrete peptides that serve paracrine, endocrine and neural functions within the body (Moran-Ramos et al., 2012).
The epithelium of the human intestine turns over approximately once every 5-7 days. All new epithelial cells derive from intestinal stem cells located at the base of the crypt. The differentiation of stem cells into secretory cells, including EECs, goblet cells and Paneth cells requires Notch inhibition and the subsequent expression of ATOH1 (Jensen et al., 2000; Shroyer et al., 2007; Yang et al., 2001). The specification of EECs requires an additional factor, the basic helix-loop-helix transcription factor neurogenin 3 (NEUROG3), which is required and sufficient for EEC differentiation in both mice and humans (Gradwohl et al., 2000; Jenny et al., 2002; López-Díaz et al., 2007; McGrath et al., 2015).
Though a few transcription factors (TFs) downstream of NEUROG3 have been implicated in EEC differentiation, the precise signaling mechanisms governing the differentiation of specific hormonal subtypes of EECs remains unclear. One of the direct targets of NEUROG3 is NEUROD1, and although expression of NEUROD1 has been found in most EEC subtypes, it is only required for differentiation of cholecystokinin (CCK)+ and secretin+ cells (Rindi et al., 1999). The TFs ARX, PDX1, NKX2-2, PAX4 and PAX6 have been shown to be involved in formation of multiple proximal and distal EEC subtypes (Beucher et al., 2012; Chen et al., 2009; Desai et al., 2008; Larsson et al., 1998). Additionally, there are several reports suggesting that EECs transcribe and secrete multiple hormones (Egerod et al., 2012; Sykaras et al., 2014). Whether these multi-hormonal EECs represent a differentiating or mature cell is currently unknown. In addition, owing to their high sensitivity to nutrients, alterations in diet composition can drastically affect the differentiation and function of these cells (Richards et al., 2016).
The main functions of EECs are to sense changes in luminal nutrients, secrete hormone and subsequently elicit a metabolic reaction. Hormones such as ghrelin are known to induce the hunger response and are upregulated in times of fasting. In contrast, GIP and GLP-1 have important roles in stimulation of pancreatic hormones (termed the incretin effect) and spike soon after a meal. Moreover, other hormones regulate the motility of the gut (motilin), promote pancreas enzyme secretion (CCK) and control the rate of gastric emptying (PYY) (reviewed by Posovszky and Wabitsch, 2015). As a whole, gastrointestinal hormone regulation is an intricately balanced process in which regional identity of the EEC (Table S1) and diet composition both influence secretion (Engelstoft et al., 2013). EECs subtypes are reactive to different macronutrients (Posovszky and Wabitsch, 2015), and the composition of a long-term diet not only influences the transcription of hormone within EECs, but also the number of specific EEC subtypes within the intestine (Ritze et al., 2015). Given the central role EECs play in regulating nutrient homeostasis, it is not surprising that misregulation of EEC hormones, including GLP-1, ghrelin and PYY, is associated with metabolic diseases such as obesity and type 2 diabetes (Ochner et al., 2010).
EEC formation and function has largely been studied in mice, and the molecular pathways that regulate EEC are believed to be conserved in humans. For example, the function of the TF Arx was compared side-by-side in mice and human intestinal organoids (HIOs) and found to have very similar functions in specifying EEC subtypes (Du et al., 2012). There are, however, significant differences between mouse and humans. For example, the hormone motilin is present in humans but absent in mice (Sanger et al., 2011). There are in vitro systems available to study human EECs, including transformed cell lines (Cao et al., 2003; Drucker et al., 1994; Le Nevé and Daniel, 2011; McLaughlin et al., 1998) and intestinal organoids derived either from human surgical samples of intestine (Mahe et al., 2015; Sato et al., 2011; Basak et al., 2017) or through the directed differentiation of pluripotent stem cells (PSCs) in vitro (Spence et al., 2011). For PSC-derived organoids, induced expression of exogenous NEUROG3 in organoids (McCracken et al., 2014; Múnera et al., 2017) resulted in increased number of EECs. However, the diversity and functionality of induced EECs was not determined.
Here, we utilized a NEUROG3-inducible approach in PSC-derived HIOs to (1) establish optimal conditions for the increased differentiation of EECs, (2) characterize the timing, formation and differentiation of specific EEC subtypes, and (3) assess the functionality of EECs by hormone secretion and nutrient responsiveness. Furthermore, we found that transplantation of HIOs into immune-compromised mice allowed maturation of the epithelium (Watson et al., 2014) and formation of all EEC subtypes. The ability to generate functional human intestinal EECs in vitro without the need of surgically derived human tissues represents a tractable new platform to study factors and drugs that can control EEC formation and function.
RESULTS
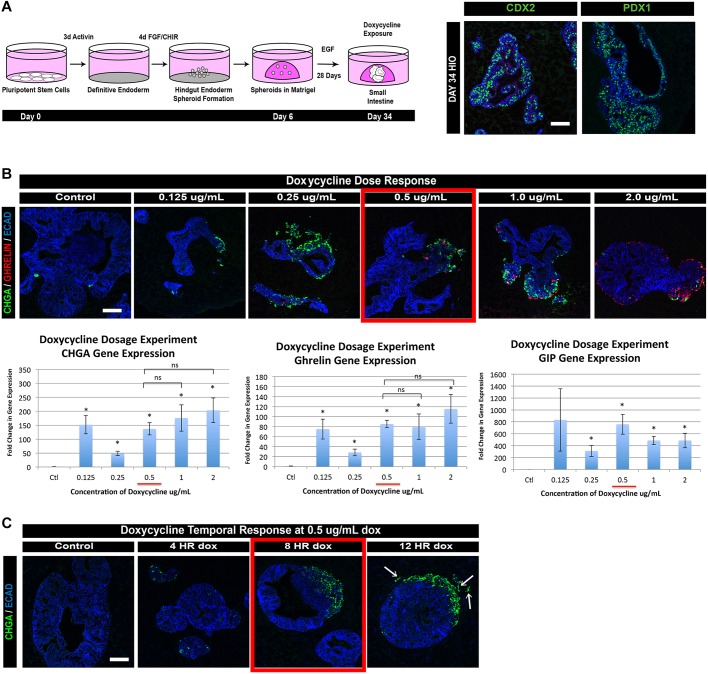
Because human EECs are exceedingly rare, it is often difficult to study their development and function. We therefore utilized human embryonic stem cell (hESC) and induced pluripotent stem cell (iPSC) lines in which we could induce expression of the pro-endocrine TF NEUROG3 by the addition of doxycycline to the culture media (McCracken et al., 2014). We then differentiated the NEUROG3-PSC lines into HIOs as previously described (Spence et al., 2011; Watson et al., 2014) (Fig. 1A) and at 34 days confirmed them to be completely composed of intestinal tissue by expression of CDX2. Moreover, analysis of ten separate experiments revealed that most of the epithelium was proximal small intestine as marked by PDX1 and CDX2. Although we could have used HIOs that were more uniformly composed of proximal small intestine cell types (CDX2+/PDX1+) by inhibition of BMP signaling with noggin (Múnera et al., 2017), we have observed that noggin also decreases EEC differentiation in HIO cultures (Múnera et al., 2017). We therefore used ‘unpatterned’ HIOs (cultured in epidermal growth factor alone), which contain a mix of CDX2+/PDX1+ and CDX2+/PDX1− epithelium.
Fig. 1.
Establishing an inducible neurogenin 3 overexpression system in HIOs to generate EECs. (A) We utilized an inducible NEUROG3 construct, which has previously been reported, within PSC lines throughout this study. The construct allows doxycycline-regulated expression of human NEUROG3. Using a previously published protocol, PSCs were differentiated into definitive endoderm using 3 days of activin A, then posteriorized into hindgut intestinal spheroids by manipulation of FGF and WNT signaling. Intestinal spheroids were then plated in a 3D matrix of Matrigel. At 34 days, HIOs robustly expressed the pan-intestinal marker CDX2 and contained regions that expressed the proximal intestinal marker PDX1. (B) Day 34 HIOs were given a 24-h pulse of doxycycline at increasing concentrations to determine optimal response in HIOs. HIOs were collected and analyzed by immunofluorescence and qPCR 7 days after doxycycline induction. Treatment with 0.5 μg/ml doxycycline resulted in expression of the endocrine marker CHGA as well as the hormone markers GHRL and GIP (n=3; mean ±s.e.m.). *P<0.05 (compared with control; Student's t-test). (C) Day 34 HIOs were exposed to 0.5 μg/ml doxycycline for varying amounts of time to assess optimal length of exposure within HIO cultures. HIOs were collected and analyzed 3 days after doxycycline induction. An 8-h pulse of 0.5 μg/ml doxycycline allows efficient induction of CHGA+ cells without causing aberrant EECs within the mesenchymal compartment (12 HR, arrows). ECAD, E-cadherin (cadherin 1). Scale bars: 50 μm. See also Fig. S1.
We then identified the optimal time and doxycycline dosage for production of EECs. All concentrations of doxycycline led to an increase in EECs, as measured by the endocrine marker chromogranin A (CHGA) and expression of two hormone markers, ghrelin and GIP (Fig. 1B). However, we found that an 8-h pulse of 0.5 μg/ml of doxycycline resulted in optimal production of EECs without causing the off-target effect of EEC delamination away from the epithelium (Fig. 1C) as previously described in the endocrine pancreas (Gouzi et al., 2011; Grapin-Botton et al., 2001). Analysis of 20-, 34- and 62-day-old HIOs determined that day 34 HIOs were competent to form hormone-positive cells in response to NEUROG3 expression (Fig. S1). At day 34, we began to observe EEC formation in uninduced HIOs, which suggests that formation of endogenous EECs normally begins at this stage of HIO development (Spence et al., 2011; data not shown).
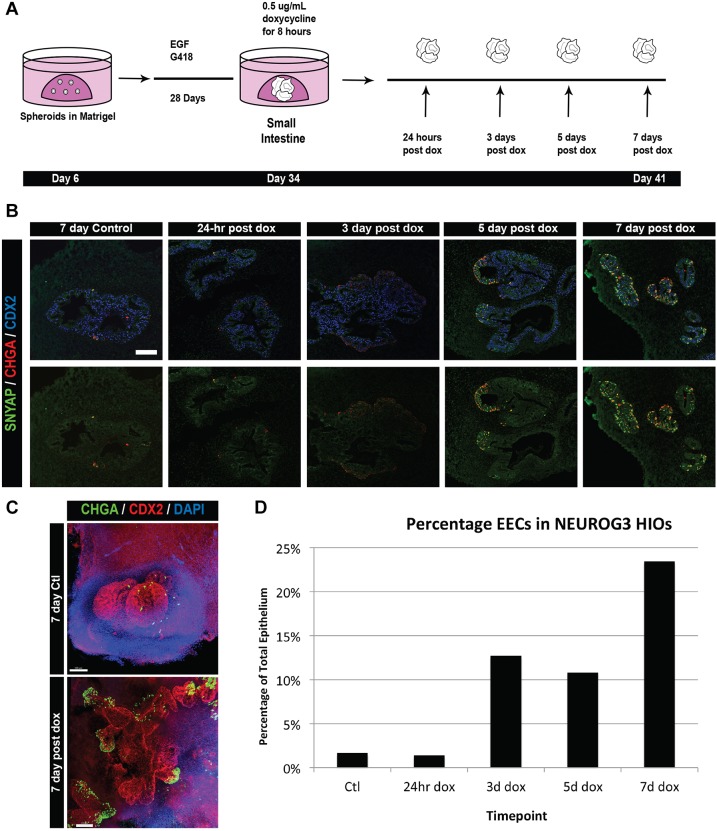
The intestinal epithelium, including EECs, is believed to turn over every 5-7 days in mice (reviewed by Noah et al., 2011). Beyond that, little is known about the kinetics of EEC differentiation either during development or postnatally. We used the dox-inducible system to study the timing of EEC differentiation after an 8-h pulse of NEUROG3 expression in day 34 HIOs and analyzed EEC differentiation at 24 h, 3 days, 5 days and 7 days after induction (Fig. 2A). We saw no increase in EECs at 24 h. However, at 3 and 5 days post-induction we observed that 11-13% of cells were EECs and at 7 days up to 23% of cells were EECs as marked by CHGA or synaptophysin (SYP) protein expression (Fig. 2). This observation suggests that the lifespan of EECs in HIOs is at least 7 days. NEUROG3-induced HIOs cultured longer than 7 days appeared necrotic (data not shown). In addition, whole-mount immunofluorescence staining indicated that the most robust EEC induction extended away from the core of the HIO epithelium (Fig. 2C). This highlighted either a differential responsiveness in the HIO epithelium or limitations to doxycycline accessibility.
Fig. 2.
EEC numbers in NEUROG3-induced HIOs increase over time. (A) Outline of the time-course experiment after NEUROG3 induction. Day 34 HIOs were induced with 0.5 μg/ml doxycycline for 8 h and collected at increasing lengths of time after exposure. (B) Immunofluorescence of EECs induced in HIOs. The endocrine markers CHGA and SYP label EECs. (C) Whole-mount imaging of control and doxycycline-treated HIOs reveal CHGA+ cells within distinct areas of the HIO by 7 days after doxycycline treatment. (D) Quantification of EECs within pooled HIOs after doxycycline treatment. Although increasing numbers of EECs were seen as early as 3 days after NEUROG3 induction, the greatest increase was observed 7 days after doxycycline treatment. Pooled HIOs from three different experiments were quantified. Scale bars: 50 μm (B); 100 μm (C).
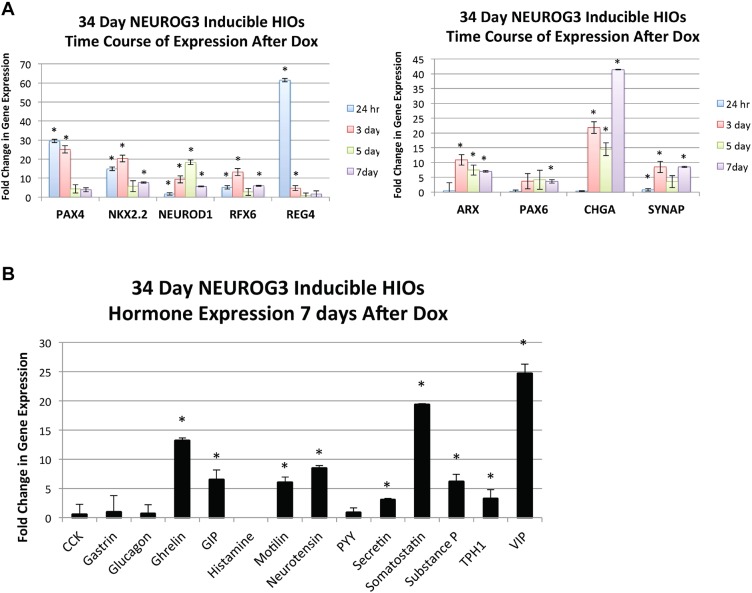
Several TFs that are known to act downstream of NEUROG3 have been identified in the developing mouse pancreas (reviewed by Pan and Wright, 2011). Less is known about whether these factors are also NEUROG3 targets in the mouse intestine and it is not known whether this is the case in the human intestine. We sought to determine whether these TFs were upregulated in response to induced NEUROG3 expression and identified two patterns of expression: early upregulated TFs and those that were upregulated at later time points (Fig. 3A, Fig. S2A). TF-encoding genes that were highly upregulated within 24 h included NKX2-2, PAX4 and RFX6, all of which are direct NEUROG3 targets (Smith et al., 2003, 2004, 2010; Watada et al., 2003). PAX6 and ARX peaked 3 days after NEUROG3 induction and NEUROD1, which is also a direct target of NEUROG3 in the pancreas (Huang et al., 2000), did not peak until 5 days post-induction. The fact that some NEUROG3 targets, such as NEUROD1, had slow kinetics of induction could be due to differences in expression of transcriptional co-factors between pancreatic and intestinal endocrine cells, or differences in the epigenetic state of the NEUROD1 gene. As expected from the immunofluorescence data, the EEC differentiation markers CHGA and SYP increased in expression over time and were most abundant in day 7 HIOs. Despite the fact that this NEUROG3-inducible system is non-physiological, induced NEUROG3 expression in HIOs results in expression of similar TFs as in the pancreas.
Fig. 3.
Patterns of hormone and TF expression in NEUROG3-induced HIOs. (A) Expression of TFs and vesicle markers known to influence EEC differentiation and subtype specification. As shown, some genes are expressed early and go on to decrease in expression as EECs presumably mature (left) whereas others come up primarily at 3 days after the doxycycline pulse and remain present at the 7-day time point (right). (B) qPCR expression of intestinal EEC hormones 7 days after NEUROG3 induction. Most proximal and pan-intestinal hormones are transcribed most highly at 7 days after a pulse of doxycycline. TPH1 encodes the enzyme catalyzing the production of serotonin. *P<0.05 (compared with uninduced control HIOs; Student's t-test; n=3 for each time point; mean±s.e.m.). See also Fig. S2.
There are over 12 different subtypes of EECs that are distributed along the intestinal tract that all originate from a NEUROG3+ progenitor (Table S1). It is not known how these different lineages are allocated, in part owing to the lack of a tractable model system. We therefore investigated the diversity of EEC subtypes that could be generated in response to NEUROG3 expression and their functionality within human intestinal epithelium. Analysis of HIOs 7 days after the 8-h pulse of NEUROG3 expression revealed an induction of ghrelin, GIP, motilin, neurotensin, secretin, somatostatin, substance P, serotonin and VIP transcript expression (Fig. 3B, Fig. S2B). We also observed glucagon expression but it was variable and often did not reach statistical significance above control HIOs that were uninduced. Many of these hormones, such as ghrelin and motilin, are highly enriched within the proximal duodenum, supporting the conclusion that HIOs are proximal in nature. In contrast, we have previously shown that distal/colonic organoids in which we express NEUROG3 produced distal-specific subtypes, such as PYY- and INSL5-expressing cells (Múnera et al., 2017). Surprisingly, we noted that one prominent proximal hormone, CCK, was not upregulated in our in vitro system, whereas other rare hormones, including motilin, were present in HIOs but are not found in rodent models (He et al., 2010).
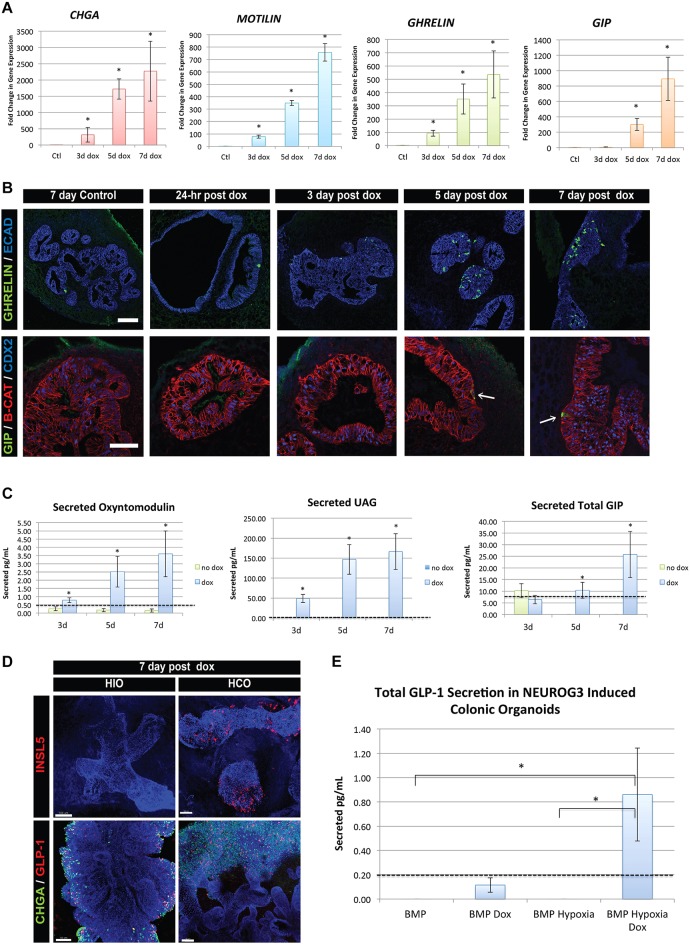
We next used this model system to study the kinetics of development of specific subtypes of EECs, focusing on X/A cells, which co-express ghrelin and motilin, and K cells, which produce GIP. Ghrelin is most commonly associated with the hunger response, whereas GIP is an incretin hormone known to potentiate insulin secretion. The kinetics of EEC formation were different between these cell types with ghrelin and motilin mRNA first appearing after 3 days following EEC specification, and GIP mRNA first appearing at 5 days (Fig. 4A). In addition, we noted that other subtypes, such as neurotensin-expressing cells, were also upregulated early, whereas the hormones somatostatin and serotonin (TPH1) followed the same later expression pattern as GIP (Fig. S2B).
Fig. 4.
EEC hormone subtypes display individualized timing for differentiation, maturation and hormone secretion. (A) qPCR data for the endocrine marker CHGA and EEC hormones motilin, ghrelin and GIP. Whereas ghrelin and motilin were significantly upregulated 3 days after a doxycycline pulse, GIP only began to be expressed at the 5-day time point. This is indicative of differential timing of maturation and subtype differentiation in EECs. Transcripts were normalized to control, uninduced HIO samples (n=3 for each time point). (B) Immunofluorescence for X/A and K cells as marked by the hormones ghrelin and GIP, respectively. As time after doxycycline increases, the number of ghrelin+ cells increases. However, GIP+ cells remain rare in the HIO epithelium even at 7 days (arrows). (C) The timing of secretion of hormones from EECs followed a similar trend, in which increased ghrelin and oxyntomodulin secretion can be detected in cultures at 3 days after doxycycline, whereas GIP is only increased at 7 days (n=6). (D) Expression of NEUROG3 in HCOs induced formation of distal EEC subtypes that express INSL5 and GLP-1 as shown by whole-mount immunofluorescence. DAPI (white) is used to stain all nuclei. (E) NEUROG3-induced HCOs secrete GLP-1 hormone when generated under optimized growth conditions that include 3 days of hypoxic growth on days 6-9 (n=3). Values in graphs represent mean±s.e.m. *P<0.05 (Student's t-test). Dashed lines represent detection limits for ELISA assays. iPSC lines were used for secretion studies. Scale bars: 50 μm (B); 100 μm (D). See also Figs S3 and S4.
Although qPCR data indicated that we were generating a variety of EEC subtypes, we also understood from previous studies that mRNA expression does not necessarily correlate with hormone protein expression (Beucher et al., 2012; Mortensen et al., 2003). It has been shown that single EECs that express one or two hormone proteins can express the mRNA for up to six different hormones (Egerod et al., 2012). Moreover, the normal proportion of hormone-expressing EEC subtypes varies based on the region of the intestine (Gunawardene et al., 2011; Sjölund et al., 1983). We therefore used immunofluorescence staining to determine whether mRNA levels correlated with cellular protein in this induced system. Ghrelin+ cells increased in accordance with mRNA levels (Fig. 4B), as did neurotensin and somatostatin (Fig. S3). However, despite robust induction of GIP mRNA between 5 and 7 days, GIP+ cells were not abundant at days 5 and 7 (Fig. 4B). This suggested that, as in vivo, expressed mRNA does not always get translated into protein.
The hallmark feature of a mature EEC is the ability to secrete hormones. We therefore measured the basal secretion of hormones in the media of NEUROG3-induced HIOs at 3, 5 and 7 days by ELISA. Much like the qPCR and protein data, hormone secretion of oxyntomodulin and un-acylated ghrelin (UAG) was detected as early as day 3 (Fig. 4C). Despite the relative rarity of GIP+ cells, we detected both total (Fig. 4C) and active (Fig. S4A) GIP at day 7. These data further indicated that different hormonal populations of human EECs form, differentiate, and functionally mature at different rates within our system. Although we did detect some GLP-1 secreted in culture media (Fig. S4B), we predicted that L-cell numbers in HIOs might be low, as they are in the proximal small intestine. In contrast, L-cells are found in higher numbers in the colon. Therefore, we generated human colonic organoids (HCOs) by distalizing organoids with a 3-day pulse of BMP2 between days 6 and 9 during the differentiation as described (Múnera et al., 2017), and inducing NEUROG3 at day 34. As predicted, we found that HCOs allowed for robust expression of the distal EEC hormones INSL5 and GLP-1 (Fig. 4D), as well as a trend of increased active secretion of GLP-1 at 7 days post doxycycline (Fig. 4E). We have also identified that 3 days of hypoxic growth during BMP2 induction (days 6-9) results in more efficient patterning and growth of HCOs. HCOs generated in this way produced significantly more GLP1 relative to uninduced controls (Fig. 4E). This study highlighted the reproducibility and feasibility of the system in patterned organoids as well as increasing our understanding of the functionality of EECs.
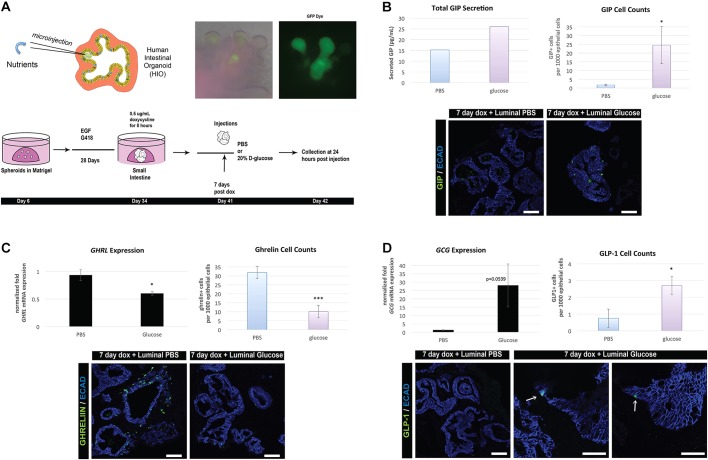
EECs are highly responsive to nutrients, and dietary changes have been shown to impact the differentiation of certain EEC cell types (Gniuli et al., 2010). Secretion of some hormones such as the incretin GIP is stimulated by nutrients. In contrast, other hormones, such as those that regulate satiety like ghrelin, are reduced in response to increased nutrients. To expose HIOs to luminal nutrients, we developed a system to microinject nutrients into the lumen of the HIOs (Fig. 5A, Fig. S5A). We first investigated whether exposure to luminal nutrients could promote EEC formation by injecting 15 nl nutrient-rich gut media, which contained serum, glucose and non-essential amino acids, into day 34 HIOs. Immediately following injection, we induced EEC specification with NEUROG3 and after 7 days observed a nutrient-stimulated increase in GIP transcription and the number of GIP-expressing cells, suggesting that HIOs are responsive to luminal nutrients (Fig. S5).
Fig. 5.
EECs functionally respond to luminal nutrients. (A) Schematic of injection-secretion protocol. Day 34 HIOs were induced with 0.5 μg/ml doxycycline for 8 h. At 41 days, NEUROG3-induced HIOs were then injected with either 20 nl of PBS or 20% D-glucose. Collection of tissue and media was performed 24 h after injection, at 42 days. To confirm efficient injection delivery of nutrients to the lumen, we added a GFP dye. (B) Luminal glucose injections increased secretion of GIP (left) as well as the number of GIP+ cells (right, n=6) in HIOs compared with PBS controls. (C) GHRL expression (n=3) and cell counts (n=6) both decreased as a result of luminal glucose exposure. (D) GCG expression increased modestly in glucose-injected HIOs (n=3) and a small increase in the number of GLP-1+ cells was observed (arrows) (n=6). Values in graphs represent mean±s.e.m. *P<0.05, ***P<0.0005 (Student's t-test). iPSC lines were used for the secretion studies. Scale bars: 50 µm. See also Fig. S5.
Specific nutrients are known to regulate production of EECs and secretion of hormones. For example, K cells secrete GIP in response to luminal sugars and fatty acids (Moran-Ramos et al., 2012). We therefore determined whether induced EECs that were allowed to differentiate for 7 days would secrete GIP in response to luminal glucose. We grew organoids overnight in low-glucose medium, then microinjected organoids with 20 nl of either PBS or 20% D-glucose (Fig. 5A). Twenty-four hours later, we detected an increase in secreted GIP in the organoids injected with glucose compared with those injected with saline, as well as a significant increase in GIP+ cell numbers (Fig. 5B). Interestingly, luminal exposure to glucose resulted in significant reduction of ghrelin mRNA and ghrelin+ cells compared with a PBS control (Fig. 5C), and the secreted hormone was below the detectable limits of the ELISA (50 pg/ml). Ghrelin is a satiety factor and ghrelin levels are known to decrease after ingestion of food (Steinert et al., 2017), and in response to oral glucose (Outeiriño-Blanco et al., 2011; Prodam et al., 2014). In addition, we noted a small increase in GLP-1 expression and cell number within the injected HIOs (Fig. 5D). Taken together, these data demonstrate that human EECs in this system are functional and respond to nutrients in the expected manner.
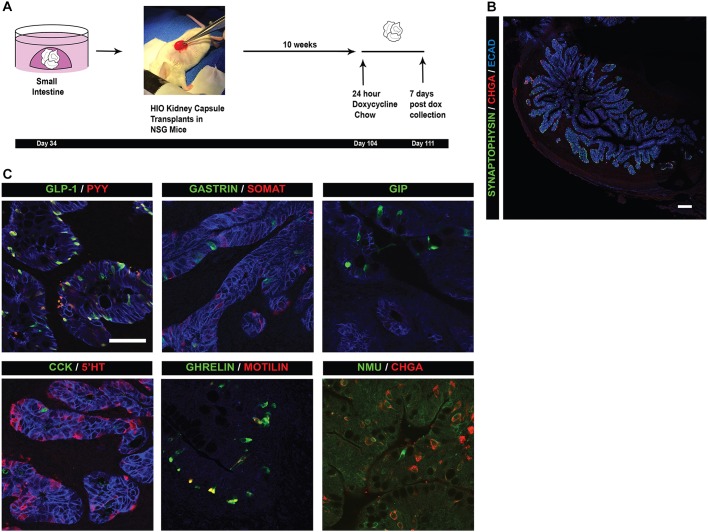
Although the inducible HIO system is tractable and rapid, certain cell types, such as CCK-expressing cells, did not form in vitro. This suggested that formation of CCK-expressing cells may occur later in development or require factors that were missing in vitro. To overcome the limitations of the in vitro system and to assess the potential for the epithelium to produce a wider variety of hormone-producing cells, we used a transplantation method developed previously (Watson et al., 2014) that allows for extended growth and maturation of HIOs in vivo. Day 34 HIOs were transplanted under the kidney capsule of NSG mice and allowed to grow for up to 10 weeks in the mouse, at which time mice were given doxycycline chow for 24 h to induce expression of NEUROG3 (Fig. 6A). Transplanted HIOs were collected 7 days later and analyzed. The epithelium of HIOs had undergone extensive maturation (Fig. 6B), as previously reported. Whereas induced HIOs in vitro lacked certain EEC subtypes, transplanted HIOs formed all known EEC subtypes of the proximal intestine (Fig. 6C), including CCK-expressing cells and a lesser understood EEC subtype expressing the hormone neuromedin U (NMU). Taken together, NEUROG3 inducible organoids represent a highly robust system in which to study a wide variety of EEC hormone subtypes, their development and their function.
Fig. 6.
Transplanted inducible HIOs are competent to form all EEC subtypes in vivo. (A) Schematic of transplantation methods. Day 34 HIOs are transplanted underneath the kidney capsule of immune-compromised NSG mice. HIOs were grown in vivo for 10 weeks. Mice were then fed doxycycline chow ad libitum for 24 h to induce the NEUROG3 construct in the HIOs. Organoids were collected from the mice 1 week after the doxycycline pulse. (B) EECs in transplanted HIOs as marked by the endocrine markers CHGA and SYP. HIOs induced 10 weeks after transplantation are competent to respond to a doxycycline pulse and increase the number of EECs. HIOs matured in vivo and formed crypt/villus-like structures, with EECs dispersed throughout the epithelium. (C) Immunofluorescence analysis of intestinal EEC subtypes that develop in transplanted HIOs. NEUROG3 HIOs are competent to give rise to all EEC subtypes in the proximal small intestine. Scale bars: 25 μm (C); 200 μm (B).
DISCUSSION
In this study, we developed and optimized an experimental system that is capable of generating vast amounts of EECs in the HIO model for the study of gastrointestinal hormone development and function. We identified specific timing and dosage requirements using the doxycycline-inducible system, which allowed us to control timing of EEC differentiation, expand numbers of EECs, and study factors associated with their maturation and function. The HIO system has the additional feature of allowing human intestinal developmentto be studied and we showed that certain hormones not present in mouse, such as motilin, were generated and can be studied in an unprecedented context in this system. Although transformed human endocrine cell lines have been utilized for studying functional aspects of EEC biology, our study is the first to utilize a 3D culture system that more readily mimics the normal intestinal epithelium. As the hormonal products of EECs exert their effects on neighboring epithelial and non-epithelial cell types, this HIO system provides a platform for studying paracrine interactions. This system is of great interest to the translational community in that it could be an invaluable tool for studying potential drug therapeutics for metabolic diseases such as diabetes and obesity.
Using the inducible NEUROG3 HIO system, we found that timing, dosage and maturation after NEUROG3 expression were all factors that influence the development of EECs. Our data correlate with many other studies, which highlight a transient need for NEUROG3 in specification of all EECs in the human intestine. The pulse of NEUROG3 expression led to the activation of a cascade of TFs known to influence the differentiation of subtypes of EECs as well as affect their maturation. As expected, direct targets of NEUROG3, such as PAX4 and NKX2-2, were upregulated 24 h after doxycycline exposure (Smith et al., 2003, 2004) and decreased expression as time went on. In contrast, certain vesicle markers, such as CHGA and SYP, were upregulated later, but maintained consistent expression. This inducible system provides a framework for a thorough examination of the TF networks involved in the differentiation of various hormone-expressing EECs from a common NEUROG3+ progenitor, a process that remains poorly understood.
We noted during our studies that individual subtypes of EECs developed and matured at independent rates. We consistently found that transcription and differentiation of ghrelin+ cells occurred sooner after NEUROG3 induction than that of other subtypes such as GIP+ cells. This observation also correlated with basal secretion of hormone; secreted UAG could be detected at 3 days after NEUROG3 induction, and GIP at 7 days. This observation could be a fundamental difference in the differentiation and maturation of EEC subtypes. Another possibility that remains uninvestigated is that EECs subtypes may turn over at different rates.
Despite generation of small intestinal HIOs, with the capacity to give rise to upwards of ten hormone-producing EECs, we did not observe all of these EEC subtypes in vitro. For example, we were never able to detect cells expressing CCK in vitro. It has been shown that in vitro HIOs have low GATA4 expression (Spence et al., 2011) and GATA4 intestinal knockout mice have decreased CCK (Kohlnhofer et al., 2016), possibly accounting for the lack of CCK within our NEUROG3 inducible system. Conversely, when HIOs are transplanted in vivo, the small intestinal epithelium matures to increase expression of GATA4 (Múnera et al., 2017). It is additionally possible that CCK+ cells take longer than 1 week to develop; however, owing to the necrotic nature of HIOs after 7 days, we could not fully examine this possibility. It is also possible that our in vitro culture system may be lacking in some external cue that ultimately gives rise to other subtypes we do not see in HIOs or that the HIOs are simply too immature at the time of induction to give rise to such subtypes. Both of these pitfalls were alleviated when we transplanted HIOs into mice (Watson et al., 2014). We found that maturation and growth of HIOs under the kidney capsule led to increased diversity in hormone subtypes within the organoid. Most notably, we were able to identify CCK+ cells present within the epithelium of transplanted HIOs. These data indicated that the HIO epithelium has the potential to give rise to all subtypes, but limitations in vitro halt the differentiation of certain EECs. Although beyond the scope of this study, using microfluidics to enhance the maturation of the intestinal epithelium would be a valuable tool to answer these questions. In addition, we note that an enteric nervous system and blood circulation are missing in this system. Although not well studied in the intestine, the enteric nervous system helps regulate pancreatic endocrine mass (Nekrep et al., 2008), suggesting that these components missing from this in vitro HIO culture system may play a key role in EEC differentiation.
EECs generated in vitro were capable of sensing and responding to luminal nutrient challenges. We tested this in two ways. First, we injected nutrient-rich gut media into the lumen, which resulted in an increase in GIP gene expression and GIP+ cell number. This has been noted in other systems (Martínez-Rodríguez and Gil, 2012; Koletzko et al., 2013), but had not been shown in humans. Second, induced EECs were functionally competent to respond to luminal glucose, resulting in stimulated GIP secretion analogous to the incretin response observed in vivo. In addition, luminal glucose caused a decrease in ghrelin production, suggesting that HIOs are mounting a satiety response as observed in vivo (Moran-Ramos et al., 2012; Outeiriño-Blanco et al., 2011). The ability of HIOs to mount an incretin and satiety response suggests that this new model system could be used to study the effects of nutrients, metabolites and microbiome components, and for screening small molecules for EEC modulatory effects designed to treat obesity and diabetes.
MATERIALS AND METHODS
Generation and maintenance of neurogenin 3-inducible PSC lines
The doxycycline-inducible neurogenin 3 construct and H1 stem cell line have been previously reported (McCracken et al., 2014; Múnera et al., 2017). We additionally generated the doxycycline-inducible PSC lines 72.3 and 263.10 using the same methods. The construct introduced into the PSC lines contained neomycin selection, and stem cell cultures were maintained on G418 (200 μg/ml) to ensure the construct was active within all cells. ESCs and iPSCs were maintained in mTesR1 media (Stem Cell Technologies) as feeder-free colonies plated on ESC quality Matrigel (BD Biosciences) and split every 4 days using dispase (Invitrogen).
NEUROG3-inducible HIO generation
HIOs were generated using previously published protocols (Spence et al., 2011; Watson et al., 2014). The only modification was the addition of 5 ng/ml of BMP4 on day 1 of activin A treatment, in order to decrease neural contamination in PSC cultures. Spheriods were collected and plated in a 3D matrix of Matrigel (BD Biosciences). Organoids were split once every 2 weeks and embedded in a fresh drop of Matrigel. HIOs were fed every 3 days with standard gut media as previously reported.
As shown, optimal 8-h doxycycline exposure to induce NEUROG3 (0.5 μg/ml) was conducted at 34 days. HIOs were collected at 24 h, 3 days, 5 days and 7 days after NEUROG3 induction. Unless otherwise specified as pooled samples, replicates were carried out using individual HIOs.
Tissue processing, immunofluorescence and imaging
HIOs were washed with cold 1× PBS. Tissue was fixed in 4% paraformaldehyde overnight at 4°C and then washed three times with 1× PBS. Tissue was then placed in 30% sucrose overnight at 4°C. Samples were washed briefly with 1× PBS, embedded using O.C.T. Compound (Tissue-Tek), and frozen. Whole tissue blocks were serially sectioned at 8 μm.
A full list of antibodies can be found in Table S2. Slides were rehydrated in 1× PBS and then put in blocking buffer (10% normal donkey serum and 0.5% Triton X-100 in 1× PBS) for 1 h. Primary antibodies were diluted in blocking buffer and incubated overnight at 4°C. Slides were washed three times in 1× PBS. Secondary antibodies were diluted in blocking buffer and applied to the slides for 1 h at room temperature. For total EEC quantification, slides were stained with both CHGA and SYP antibodies, as it has been shown that CHGA, although used commonly as an endocrine marker, does not encompass all EECs (Engelstoft et al., 2015; Fothergill et al., 2017). Fluoromount-G (SouthernBiotech) was applied to slides and they were cover slipped. Tissue was imaged using a Nikon A1 inverted confocal microscope. Image analysis and quantification was performed using Nikon Elements and Imaris (Bitplane) software. Organoids from three separate experiments were pooled and were serial sectioned en masse. Nine serial sections from each condition were imaged and 192 images from all conditions were quantified. An average of 15,700 cells in each condition were analyzed to generate the percentage of induced EECs.
qPCR analysis
RNA was isolated from tissue samples using an RNAqueous-Micro Total RNA Isolation Kit (Life Technologies). cDNA was made using SuperScript Vilo cDNA synthesis kit (Invitrogen) and qPCR was performed using a predesigned TaqMan Array 96-well fast plate (Thermo Fisher Scientific; https://www.thermofisher.com/us/en/home/life-science/pcr/real-time-pcr/real-time-pcr-assays/taqman-gene-expression/real-time-pcr-taqman-arrays.html?SID=fr-taqmanarray-1). Additional in-house primers also used in experiments can be found in Table S3. Statistical analysis was performed using Student's t-test. All hormones and transcripts were normalized to the uninduced control HIO samples and are represented on a linear scale.
Secretion and injection studies
Injection then differentiation
Day 34 HIOs were given 3×5 nl microinjections of standard gut media and then exposed to doxycycline for 8 h to induce NEUROG3 expression and specification of EECs. To ensure confirm successful injection, a GFP dye was injected. HIOs were collected for qPCR and histology analysis 7 days after NEUROG3 induction.
Secretion
Media from individual wells was collected at 3 days, 5 days and 7 days post-doxycycline induction to assess hormone secretion. Media was pooled from four wells per sample (n=6). Media collected from the experiment was processed and analyzed using motilin (CEA575Hu, Cloud-Clone), ghrelin (also called acylghrelin) (Özcan et al., 2014), unacylated ghrelin (UAG) (Özcan et al., 2014), total GIP (EZHGIP-54K, Millipore), active GIP (Troutt et al., 2011) and oxyntomodulin (AL-139, AnshLabs) ELISA assays.
Injection/secretion studies
Day 34 HIOs were exposed to doxycycline for 8 h to induce NEUROG3 expression. At day 41, HIOs were starved overnight (16 h) by changing the medium to low-glucose DMEM. At day 35, HIOs were microinjected with either 20 nl of 1× PBS or 20% D-glucose (n=16). Media was collected at 24 h post-injection and organoids were processed for RNA expression analysis and sectioning as described above. Media from each condition was pooled for analysis of secreted hormone. Media was processed and analyzed using the above ELISA assays.
Mouse studies and in vivo transplantation of HIOs
All mouse work was preapproved by the Committee of Ethics of Animal Experiments at Cincinnati Children's Hospital Research Foundation (CCHRF) and was in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (IACUC2016-004). Euthanasia was performed as per protocol with a primary administration of isofluorane and a secondary method of cervical dislocation. Immune-deficient NOD-SCID IL-2γnull (NSG) mice were obtained from the Comprehensive Mouse and Cancer Core Facility, Cincinnati, OH, USA. Mice were housed in the CCHRF mouse facility and maintained on a 12-h light/dark cycle. Transplantation studies were carried out as described by Watson et al. (2014). Ten weeks after transplantation under the kidney capsule, mice received doxycycline chow for 24 h and then tissue was collected 7 days after induction. Tissue was analyzed by sectioning and staining.
Supplementary Material
Acknowledgements
We would like to thank the Wells and Zorn labs for feedback and reagents. In addition, we thank the CCHMC PSCF for cell lines, reagents and advice regarding stem cell maintenance/culture. We acknowledge CCHMC Vet Services, CCHMC Pathology Core, CCHMC Viral Vector Core, and the CCHMC Confocal Core.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: J.M.W., H.A.M., K.L.S.; Methodology: H.A.M., K.L.S., J.O.M., N.A.R., J.R.E., H.-C.Y., C.W., M.A.H.; Formal analysis: H.A.M., K.L.S., J.R.E., H.-C.Y.; Investigation: K.L.S., J.O.M.; Resources: J.M.W., J.O.M., N.A.R., H.-C.Y.; Writing - original draft: J.M.W., K.L.S., H.-C.Y.; Writing - review & editing: J.M.W., H.A.M., K.L.S., J.O.M., N.A.R., J.R.E.; Visualization: K.L.S.; Supervision: J.M.W.; Project administration: J.M.W.; Funding acquisition: J.M.W.
Funding
This work was supported by grants from the National Institutes of Health (R01DK092456 and U19AI116491 to J.M.W.) as well as a research award from Eli Lilly and Company (to J.M.W.). We also acknowledge core support from the Pluripotent Stem Cell Facility of Cincinnati Children's Hospital Medical Center and from the Cincinnati Digestive Disease Center (P30DK0789392). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.165795.supplemental
References
- Basak O., Beumer J., Wiebrands K., Seno H., van Oudenaarden A. and Clevers H. (2017). Induced quiescence of Lgr5+ stem cells in intestinal organoids enables differentiation of hormone-producing enteroendocrine cells. Cell Stem Cell 20, 177-190.e4. 10.1016/j.stem.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Beucher A., Gjernes E., Collin C., Courtney M., Meunier A., Collombat P. and Gradwohl G. (2012). The homeodomain-containing transcription factors Arx and Pax4 control enteroendocrine subtype specification in mice. PLoS ONE 7, e36449 10.1371/journal.pone.0036449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Flock G., Choi C., Irwin D. M. and Drucker D. J. (2003). Aberrant regulation of human intestinal proglucagon gene expression in the NCI-H716 cell line. Endocrinology 144, 2025-2033. 10.1210/en.2002-0049 [DOI] [PubMed] [Google Scholar]
- Chen C., Fang R., Davis C., Maravelias C. and Sibley E. (2009). Pdx1 inactivation restricted to the intestinal epithelium in mice alters duodenal gene expression in enterocytes and enteroendocrine cells. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G1126-G1137. 10.1152/ajpgi.90586.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S., Loomis Z., Pugh-Bernard A., Schrunk J., Doyle M. J., Minic A., McCoy E. and Sussel L. (2008). Nkx2.2 regulates cell fate choice in the enteroendocrine cell lineages of the intestine. Dev. Biol. 313, 58-66. 10.1016/j.ydbio.2007.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. J., Jin T., Asa S. L., Young T. A. and Brubaker P. L. (1994). Activation of proglucagon gene transcription by protein kinase-A in a novel mouse enteroendocrine cell line. Mol. Endocrinol. 8, 1646-1655. [DOI] [PubMed] [Google Scholar]
- Du A., McCracken K. W., Walp E. R., Terry N. A., Klein T. J., Han A., Wells J. M. and May C. L. (2012). Arx is required for normal enteroendocrine cell development in mice and humans. Dev. Biol. 365, 175-188. 10.1016/j.ydbio.2012.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerod K. L., Engelstoft M. S., Grunddal K. V., Nøhr M. K., Secher A., Sakata I., Pedersen J., Windeløv J. A., Füchtbauer E.-M., Olsen J. et al. (2012). A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153, 5782-5795. 10.1210/en.2012-1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelstoft M. S., Egerod K. L., Lund M. L. and Schwartz T. W. (2013). Enteroendocrine cell types revisited. Curr. Opin. Pharmacol. 13, 912-921. 10.1016/j.coph.2013.09.018 [DOI] [PubMed] [Google Scholar]
- Engelstoft M. S., Lund M. L., Grunddal K. V., Egerod K. L., Osborne-Lawrence S., Poulsen S. S., Zigman J. M. and Schwartz T. W. (2015). Research resource: a chromogranin a reporter for serotonin and histamine secreting enteroendocrine cells. Mol. Endocrinol. 29, 1658-1671. 10.1210/me.2015-1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill L. J., Callaghan B., Hunne B., Bravo D. M. and Furness J. B. (2017). Costorage of enteroendocrine hormones evaluated at the cell and subcellular levels in male mice. Endocrinology 158, 2113-2123. 10.1210/en.2017-00243 [DOI] [PubMed] [Google Scholar]
- Gniuli D., Calcagno A., Dalla Libera L., Calvani R., Leccesi L., Caristo M. E., Vettor R., Castagneto M., Ghirlanda G. and Mingrone G. (2010). High-fat feeding stimulates endocrine, glucose-dependent insulinotropic polypeptide (GIP)-expressing cell hyperplasia in the duodenum of Wistar rats. Diabetologia 53, 2233-2240. 10.1007/s00125-010-1830-9 [DOI] [PubMed] [Google Scholar]
- Gouzi M., Kim Y. H., Katsumoto K., Johansson K. and Grapin-Botton A. (2011). Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development. Dev. Dyn. 240, 589-604. 10.1002/dvdy.22544 [DOI] [PubMed] [Google Scholar]
- Gradwohl G., Dierich A., LeMeur M. and Guillemot F. (2000). neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 97, 1607-1611. 10.1073/pnas.97.4.1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapin-Botton A., Majithia A. R. and Melton D. A. (2001). Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev. 15, 444-454. 10.1101/gad.846001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardene A. R., Corfe B. M. and Staton C. A. (2011). Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int. J. Exp. Pathol. 92, 219-231. 10.1111/j.1365-2613.2011.00767.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Irwin D. M., Chen R. and Zhang Y.-P. (2010). Stepwise loss of motilin and its specific receptor genes in rodents. J. Mol. Endocrinol. 44, 37-44. 10.1677/JME-09-0095 [DOI] [PubMed] [Google Scholar]
- Huang H.-P., Liu M., El-Hodiri H. M., Chu K., Jamrich M. and Tsai M.-J. (2000). Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol. Cell. Biol. 20, 3292-3307. 10.1128/MCB.20.9.3292-3307.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny M., Uhl C., Roche C., Duluc I., Guillermin V., Guillemot F., Jensen J., Kedinger M. and Gradwohl G. (2002). Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 21, 6338-6347. 10.1093/emboj/cdf649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J., Pedersen E. E., Galante P., Hald J., Heller R. S., Ishibashi M., Kageyama R., Guillemot F., Serup P. and Madsen O. D. (2000). Control of endodermal endocrine development by Hes-1. Nat. Genet. 24, 36-44. 10.1038/71657 [DOI] [PubMed] [Google Scholar]
- Kohlnhofer B. M., Thompson C. A., Walker E. M. and Battle M. A. (2016). GATA4 regulates epithelial cell proliferation to control intestinal growth and development in mice. Cell. Mol. Gastroenterol. Hepatol. 2, 189-209. 10.1016/j.jcmgh.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koletzko B., Beyer J., Brands B., Demmelmair H., Grote V., Haile G., Gruszfeld D., Rzehak P., Socha P., Weber M. et al. (2013). Early influences of nutrition on postnatal growth. Nestle Nutr. Inst. Workshop Ser. 71, 11-27. 10.1159/000342533 [DOI] [PubMed] [Google Scholar]
- Larsson L.-I., St-Onge L., Hougaard D. M., Sosa-Pineda B. and Gruss P. (1998). Pax 4 and 6 regulate gastrointestinal endocrine cell development. Mech. Dev. 79, 153-159. 10.1016/S0925-4773(98)00182-8 [DOI] [PubMed] [Google Scholar]
- Le Nevé B. and Daniel H. (2011). Selected tetrapeptides lead to a GLP-1 release from the human enteroendocrine cell line NCI-H716. Regul. Pept. 167, 14-20. 10.1016/j.regpep.2010.10.010 [DOI] [PubMed] [Google Scholar]
- López-Díaz L., Jain R. N., Keeley T. M., VanDussen K. L., Brunkan C. S., Gumucio D. L. and Samuelson L. C. (2007). Intestinal Neurogenin 3 directs differentiation of a bipotential secretory progenitor to endocrine cell rather than goblet cell fate. Dev. Biol. 309, 298-305. 10.1016/j.ydbio.2007.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahe M. M., Sundaram N., Watson C. L., Shroyer N. F. and Helmrath M. A. (2015). Establishment of human epithelial enteroids and colonoids from whole tissue and biopsy. J. Vis. Exp. 97, e52483 10.3791/52483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Rodríguez R. and Gil A. (2012). Nutrient-mediated modulation of incretin gene expression: a systematic review. Nutr. Hosp. 27, 46-53. 10.1590/S0212-16112012000100006 [DOI] [PubMed] [Google Scholar]
- McCracken K. W., Catá E. M., Crawford C. M., Sinagoga K. L., Schumacher M., Rockich B. E., Tsai Y.-H., Mayhew C. N., Spence J. R., Zavros Y. et al. (2014). Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400-404. 10.1038/nature13863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath P. S., Watson C. L., Ingram C., Helmrath M. A. and Wells J. M. (2015). The basic helix-loop-helix transcription factor NEUROG3 is required for development of the human endocrine pancreas. Diabetes 64, 2497-2505. 10.2337/db14-1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J. T., Lomax R. B., Hall L., Dockray G. J., Thompson D. G. and Warhurst G. (1998). Fatty acids stimulate cholecystokinin secretion via an acyl chain length-specific, Ca2+-dependent mechanism in the enteroendocrine cell line STC-1. J. Physiol. 513, 11-18. 10.1111/j.1469-7793.1998.011by.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Ramos S., Tovar A. R. and Torres N. (2012). Diet: friend or foe of enteroendocrine cells: how it interacts with enteroendocrine cells. Adv. Nutr. 3, 8-20. 10.3945/an.111.000976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen K., Christensen L. L., Holst J. J. and Orskov C. (2003). GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul. Pept. 114, 189-196. 10.1016/S0167-0115(03)00125-3 [DOI] [PubMed] [Google Scholar]
- Múnera J. O., Sundaram N., Rankin S. A., Hill D., Watson C., Mahe M., Vallance J. E., Shroyer N. F., Sinagoga K. L., Zarzoso-Lacoste A. et al. (2017). Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell 21, 51-64. 10.1016/j.stem.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrep N., Wang J., Miyatsuka T., German M. S., Pedersen R. A., Rubenstein J. L. and German M. S. (2008). Signals from the neural crest regulate beta-cell mass in the pancreas. Development 135, 2151-2160. 10.1242/dev.015859 [DOI] [PubMed] [Google Scholar]
- Noah T. K., Donahue B. and Shroyer N. F. (2011). Intestinal development and differentiation. Exp. Cell Res. 317, 2702-2710. 10.1016/j.yexcr.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochner C. N., Gibson C., Carnell S., Dambkowski C. and Geliebter A. (2010). The neurohormonal regulation of energy intake in relation to bariatric surgery for obesity. Physiol. Behav. 100, 549-559. 10.1016/j.physbeh.2010.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiriño-Blanco E., Garcia-Buela J., Sangiao-Alvarellos S., Pertega-Diaz S., Martinez-Ramonde T. and Cordido F. (2011). Growth hormone, ghrelin and peptide YY secretion after oral glucose administration in healthy and obese women. Horm. Metab. Res. 43, 580-586. 10.1055/s-0031-1279779 [DOI] [PubMed] [Google Scholar]
- Özcan B., Neggers S. J. C. M. M., Miller A. R., Yang H.-C., Lucaites V., Abribat T., Allas S., Huisman M., Visser J. A., Themmen A. P. N. et al. (2014). Does des-acyl ghrelin improve glycemic control in obese diabetic subjects by decreasing acylated ghrelin levels? Eur. J. Endocrinol. 170, 799-807. 10.1530/EJE-13-0347 [DOI] [PubMed] [Google Scholar]
- Pan F. C. and Wright C. (2011). Pancreas organogenesis: from bud to plexus to gland. Dev. Dyn. 240, 530-565. 10.1002/dvdy.22584 [DOI] [PubMed] [Google Scholar]
- Posovszky C. and Wabitsch M. (2015). Regulation of appetite, satiation, and body weight by enteroendocrine cells. Part 1: characteristics of enteroendocrine cells and their capability of weight regulation. Horm. Res. Paediatr. 83, 1-10. 10.1159/000368898 [DOI] [PubMed] [Google Scholar]
- Prodam F., Monzani A., Ricotti R., Marolda A., Bellone S., Aimaretti G., Roccio M. and Bona G. (2014). Systematic review of ghrelin response to food intake in pediatric age, from neonates to adolescents. J. Clin. Endocrinol. Metab. 99, 1556-1568. 10.1210/jc.2013-4010 [DOI] [PubMed] [Google Scholar]
- Richards P., Pais R., Habib A. M., Brighton C. A., Yeo G. S. H., Reimann F. and Gribble F. M. (2016). High fat diet impairs the function of glucagon-like peptide-1 producing L-cells. Peptides 77, 21-27. 10.1016/j.peptides.2015.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindi G., Ratineau C., Ronco A., Candusso M. E., Tsai M. and Leiter A. B. (1999). Targeted ablation of secretin-producing cells in transgenic mice reveals a common differentiation pathway with multiple enteroendocrine cell lineages in the small intestine. Development 126, 4149-4156. [DOI] [PubMed] [Google Scholar]
- Ritze Y., Hengelhaupt C., Bárdos G., Ernst B., Thurnheer M., D'Haese J. G., Bischoff S. C. and Schultes B. (2015). Altered intestinal neuroendocrine gene expression in humans with obesity. Obesity 23, 2278-2285. 10.1002/oby.21253 [DOI] [PubMed] [Google Scholar]
- Sanger G. J., Holbrook J. D. and Andrews P. L. R. (2011). The translational value of rodent gastrointestinal functions: a cautionary tale. Trends Pharmacol. Sci. 32, 402-409. 10.1016/j.tips.2011.03.009 [DOI] [PubMed] [Google Scholar]
- Sato T., Stange D. E., Ferrante M., Vries R. G. J., Van Es J. H., Van den Brink S., Van Houdt W. J., Pronk A., Van Gorp J., Siersema P. D. et al. (2011). Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762-1772. 10.1053/j.gastro.2011.07.050 [DOI] [PubMed] [Google Scholar]
- Shroyer N. F., Helmrath M. A., Wang V. Y.-C., Antalffy B., Henning S. J. and Zoghbi H. Y. (2007). Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology 132, 2478-2488. 10.1053/j.gastro.2007.03.047 [DOI] [PubMed] [Google Scholar]
- Sjölund K., Sandén G., Håkanson R. and Sundler F. (1983). Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology 85, 1120-1130. [PubMed] [Google Scholar]
- Smith S. B., Gasa R., Watada H., Wang J., Griffen S. C. and German M. S. (2003). Neurogenin3 and hepatic nuclear factor 1 cooperate in activating pancreatic expression of Pax4. J. Biol. Chem. 278, 38254-38259. 10.1074/jbc.M302229200 [DOI] [PubMed] [Google Scholar]
- Smith S. B., Watada H. and German M. S. (2004). Neurogenin3 activates the islet differentiation program while repressing its own expression. Mol. Endocrinol. 18, 142-149. 10.1210/me.2003-0037 [DOI] [PubMed] [Google Scholar]
- Smith S. B., Qu H.-Q., Taleb N., Kishimoto N. Y., Scheel D. W., Lu Y., Patch A.-M., Grabs R., Wang J., Lynn F. C. et al. (2010). Rfx6 directs islet formation and insulin production in mice and humans. Nature 463, 775-780. 10.1038/nature08748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J. R., Mayhew C. N., Rankin S. A., Kuhar M. F., Vallance J. E., Tolle K., Hoskins E. E., Kalinichenko V. V., Wells S. I., Zorn A. M. et al. (2011). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105-109. 10.1038/nature09691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert R. E., Feinle-Bisset C., Asarian L., Horowitz M., Beglinger C. and Geary N. (2017). Ghrelin, CCK, GLP-1, and PYY(3–36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 97, 411-463. 10.1152/physrev.00031.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykaras A., Demenis C., Cheng L., Pisitkun T., McLaughlin J., Fenton R. and Smith C. P. (2014). Duodenal CCK cells from male mice express multiple hormones including ghrelin. Endocrinology 155, 3339-3351. 10.1210/en.2013-2165 [DOI] [PubMed] [Google Scholar]
- Troutt J. S., Siegel R. W., Chen J., Sloan J. H., Deeg M. A., Cao G. and Konrad R. J. (2011). Dual-monoclonal, sandwich immunoassay specific for glucose-dependent insulinotropic peptide1-42, the active form of the incretin hormone. Clin. Chem. 57, 849-855. 10.1373/clinchem.2010.159954 [DOI] [PubMed] [Google Scholar]
- Watada H., Scheel D. W., Leung J. and German M. S. (2003). Distinct gene expression programs function in progenitor and mature islet cells. J. Biol. Chem. 278, 17130-17140. 10.1074/jbc.M213196200 [DOI] [PubMed] [Google Scholar]
- Watson C. L., Mahe M. M., Múnera J., Howell J. C., Sundaram N., Poling H. M., Schweitzer J. I., Vallance J. E., Mayhew C. N., Sun Y. et al. (2014). An in vivo model of human small intestine using pluripotent stem cells. Nat. Med. 20, 1310-1314. 10.1038/nm.3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Bermingham N. A., Finegold M. J. and Zoghbi H. Y. (2001). Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294, 2155-2158. 10.1126/science.1065718 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.