ABSTRACT
The definitive endoderm (DE) is the embryonic germ layer that forms the gut tube and associated organs, including thymus, lungs, liver and pancreas. To understand how individual DE cells furnish gut organs, genetic fate mapping was performed using the Rosa26lacZ Cre-reporter paired with a tamoxifen-inducible DE-specific Cre-expressing transgene. We established a low tamoxifen dose that infrequently induced heritable lacZ expression in a single cell of individual E8.5 mouse embryos and identified clonal cell descendants at E16.5. As expected, only a fraction of the E16.5 embryos contained lacZ-positive clonal descendants and a subset of these contained descendants in multiple organs, revealing novel ontogeny. Furthermore, immunohistochemical analysis was used to identify lacZ-positive hepatocytes and biliary epithelial cells, which are the cholangiocyte precursors, in each clonally populated liver. Together, these data not only uncover novel and suspected lineage relationships between DE-derived organs, but also illustrate the bipotential nature of individual hepatoblasts by demonstrating that single hepatoblasts contribute to both the hepatocyte and the cholangiocyte lineage in vivo.
KEY WORDS: Lineage tracing, Liver, Definitive endoderm, Hepatoblast, Mouse
Summary: Single-cell genetic fate mapping reveals novel progenitor relationships between endoderm-derived organs and demonstrates that individual hepatoblasts contribute both hepatocytes and cholangiocytes to the mature liver.
INTRODUCTION
The definitive endoderm (DE), the embryonic germ layer that produces the gut tube as well as the parenchyma of the liver, pancreas, lung, thymus and thyroid, arises by embryonic day (E) 7.5 as an epithelial sheet on the ventral surface of the developing mouse that is broadly patterned by overlapping transcription factors. As development proceeds, the transcription factor network is further refined and by E9.5 distinct and non-overlapping transcriptional boundaries are apparent throughout the gut tube, representing adjacent organ progenitor populations (Sherwood et al., 2009). Murine fate mapping has demonstrated that hepatoblasts, the embryonic hepatic progenitors, arise from discrete non-contiguous populations of E8.25 DE (Tremblay and Zaret, 2005). Hepatoblasts can be distinguished from the remainder of the endoderm by the expression of liver-specific genes such as α-fetoprotein and albumin, starting at the 7-8 somite stage (7-8S; ∼E8.5) (Deutsch et al., 2001; Gualdi et al., 1996; Serls et al., 2005). By E9.0 the hepatoblasts are contained in a recognizable liver bud from which they delaminate and invade adjacent mesenchyme, initiating a period of rapid hepatic outgrowth and proliferation (Bort et al., 2006; Sosa-Pineda et al., 2000).
The liver comprises two functional cell types: hepatocytes and cholangiocytes. Hepatocytes interact with blood and produce bile, which is then shuttled through a biliary network composed of cholangiocytes. Defects in either of these cell types can have enormous clinical consequences and can result in liver fibrosis, cirrhosis, biliary atresia, cholangiocarcinoma, hepatitis and Alagille syndrome (Friedman, 2003; Rowe, 2017). Furthermore, the liver sustains injury and repairs itself through an unparalleled regenerative plasticity. A complete understanding of such capacity must include a developmental understanding of the hepatoblast population.
Although it is clear that hepatoblasts are the embryonic progenitors of adult cholangiocytes and hepatocytes, and that individual progenitors can initiate both differentiated cell types in vitro (Suzuki et al., 2002), lineage tracing of individual hepatoblasts in vivo has not yet been performed and the bipotential nature of individual hepatoblasts has been indirectly assumed despite some contradictory evidence (Koga, 1971; Shiojiri et al., 2001). To investigate the potency of individual DE cells, we used a genetic labeling strategy to perform retrospective fate mapping of single E8.5 DE cells and analyzed descendants at E16.5, when the gut tube has formed and differentiated tissues and cell types can be discerned. By analyzing the ontogeny of single E8.5 DE progenitors, we uncovered shared organ progenitors, linking a cell in the E8.5 endoderm to multiple endoderm-derived organs. Furthermore, we used this in vivo system to demonstrate that individual hepatoblasts contribute to both the hepatocyte and biliary epithelial cell (BEC) lineages of the E16.5 liver. Together, these data provide important insights into endoderm organogenesis that could link pathologies to defects during normal developmental processes and could guide in vitro differentiation strategies aimed at the production of endoderm-derived cell types.
RESULTS AND DISCUSSION
Establishing a protocol to produce a single genetically labeled cell per embryo
Despite in vitro evidence supporting the bi-potency of individual hepatoblasts, in vivo experiments are conspicuously absent. Furthermore, the existence of at least two spatially distinct liver bud populations with differential requirements for BMP and FGF suggest distinct hepatoblast subpopulations (Palaria et al., 2018; Wang et al., 2015). To probe this putative heterogeneity and to assess the potential of individual hepatoblasts we used an in vivo fate mapping strategy that pairs a tissue-specific TMX-inducible Cre-transgene and a lacZ reporter.
The Foxa2mcm allele, which is produced by insertion of a TMX-inducible Cre-recombinase into the 3′ UTR of the Foxa2 locus, recapitulates endogenous Foxa2 expression in the DE, notochord and floorplate of the neural tube (Park et al., 2008). When present in embryos also harboring the ubiquitously expressed Rosa26lacz Cre-reporter (Soriano, 1999), TMX administration activates lacZ expression specifically in Foxa2-expressing cells. lacZ recombination produces a chromogenically identifiable and genetically heritable cellular mark. Because the level of Cre-mediated recombination can be altered by dose, we hypothesized that we could identify a TMX concentration that reliably induces recombination in a single cell (Hayashi and McMahon, 2002; Park et al., 2008).
To assess the TMX dose response, Rosa26lacZ/lacZ females were mated with Foxa2mcm/mcm males, producing embryos heterozygous for both alleles. Pregnant dams received a single dose of TMX on E7.75 and embryos were X-gal stained on E8.5. A 50 mg kg−1 TMX dose, half the typical maximal dose, produced numerous labeled cells in all embryos (Fig. S1A). Decreasing the dose to 6 mg kg−1 resulted in notably fewer labeled cells [3±3 (mean±s.d.); Fig. S1B] while only 50% (n=16/32) of embryos underwent recombination. At 4 mg kg−1 TMX, 48% (n=10/21) of embryos demonstrated one or two labeled cells (±1; Fig. S1C). At 3 mg kg−1 TMX, 13.9% (n=11/79) of embryos were labeled and only a single lacZ-positive cell was present in each of those embryos (Fig. S1D). A final reduction to 2 mg kg−1 TMX did not produce any labeled embryos (n=0/30; Fig. S1E) nor did control animals receiving only peanut oil (n=0/49; data not shown). Statistical analysis of these data indicate that, at 6 mg kg−1 and below, there is a linear relationship between dose and the number of labeled cells (Fig. S1F; ANOVA ***P=2.23e−9). Furthermore, although the ventral neural tube is labeled at high TMX doses (Fig. S1G), section analysis of a subset of embryos provided with low TMX doses (n=4 at 4 mg kg−1 and n=9 at 3 mg kg−1) reveals that most labeled cells are confined to the DE layer, whereas a minority (1/15) are found in the notochord (Fig. S1H). These experiments demonstrate that, at 3 mg kg−1 TMX, if recombination occurs it is confined to a single cell per embryo, indicating the utility of this system for lineage tracing.
When establishing this retrospective lineage analysis, it was essential to confirm that Cre-mediated recombination did not occur outside of the expected parameters (Liu et al., 2010; Vooijs et al., 2001). We considered the possibility that recombination could be induced beyond E8.5 by lingering TMX. However the half-life of TMX in mouse is less than 12 h (Robinson et al., 1991), and thus in 12 h a single 3 mg kg−1 dose would decay to below 2 mg kg−1, a dose at which we have seen no recombination.
Assessing the potency of individual E8.5 DE cells at E16.5
With our single-cell labeling strategy defined, pregnant dams were administered 3 mg kg−1 TM on E7.75 and embryos collected at E16.5. The entire gut tube and visceral organs, including kidney and heart, were dissected from each embryo and then subject to X-gal staining to identify any labeled descendants in all gut derivatives. Because the vertebral areas were discarded, we would not expect to detect the few embryos harboring labeled notochord descendants, which are confined to the intervertebral disks at E16.5 (McCann et al., 2012). Importantly, all viscera containing lacZ-expressing clonal descendants were confined to DE-derived organs and were never found in attached mesoderm-derived tissues, further validating the specificity of the experimental system. Finally, the low frequency of recombination at E16.5 (11.8%; 57/472) ensures a low risk of independently labeling two cells in the same embryo (1.4%=0.118×0.118).
The liver is one of the first foregut organs specified (at 7-8S, E8.5), with the ventral and dorsal pancreas being specified shortly thereafter (Deutsch et al., 2001; Gannon and Wright, 1999; Gualdi et al., 1996). Thus, in accordance with our labeling strategy, the liver was the only foregut organ that produced descendants that were mainly confined to a single organ (n=22/25 or 88% of embryos with labeled livers were restricted to the liver). Of the embryos with clonal descendants in other foregut organs, 9/20 (45%) were confined to a single organ and seven of those were restricted to the pancreas (Table 1, Fig. S2).
Table 1.
Distribution of E16.5 extrahepatic clones
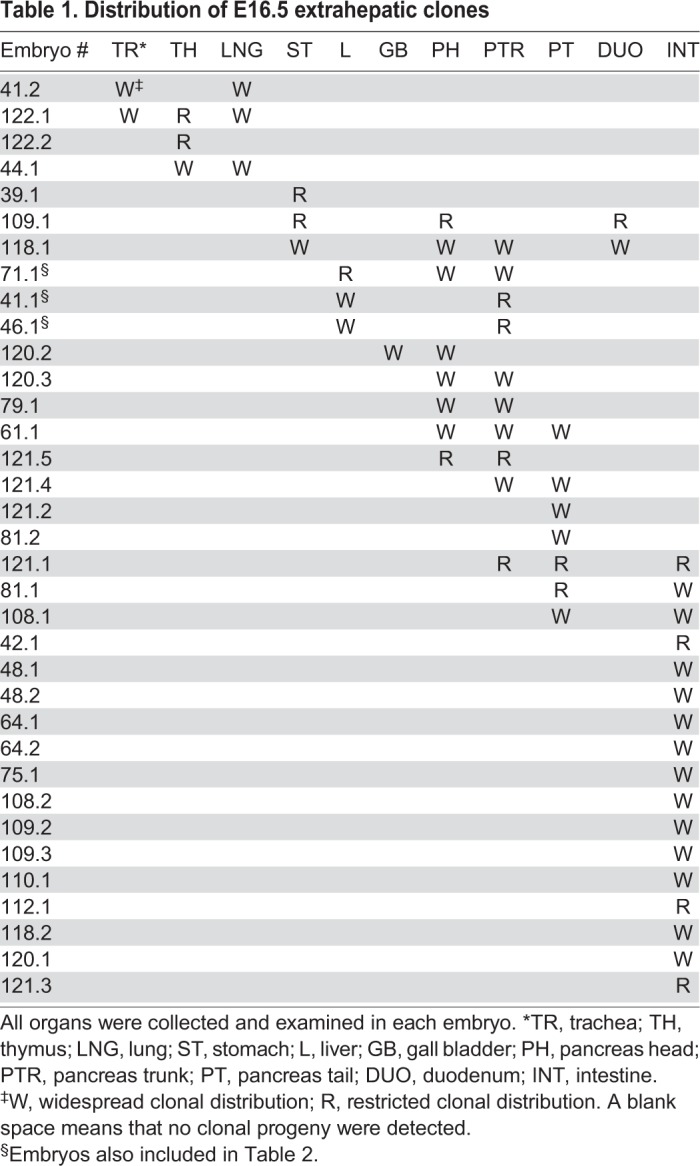
The presence of clonal cell descendants in multiple organs of individual embryos reveals the multipotentcy of the originally labeled E8.5 DE cell. Interestingly, of those embryos with clonal descendants in multiple organs, several patterns were apparent. These patterns highlight known or suspected ontogeny and also revealed novel progenitor relationships. For example, we previously used prospective DiI fate-mapping to demonstrate that at E8.25 a subset of hepatic progenitors contribute to the ventral pancreas bud (Angelo et al., 2012; Tremblay and Zaret, 2005). Indeed, of the limited number of embryos with multi-organ contribution that had descendants in liver, all three contributed to the pancreas (Table 1, Fig. 1C). Similarly, although only a single embryo produced labeled descendants in both the pancreas and in the gallbladder (Table 1), it too supports the previously described common ventral pancreas/gall bladder progenitor (Spence et al., 2009; Uemura et al., 2015).
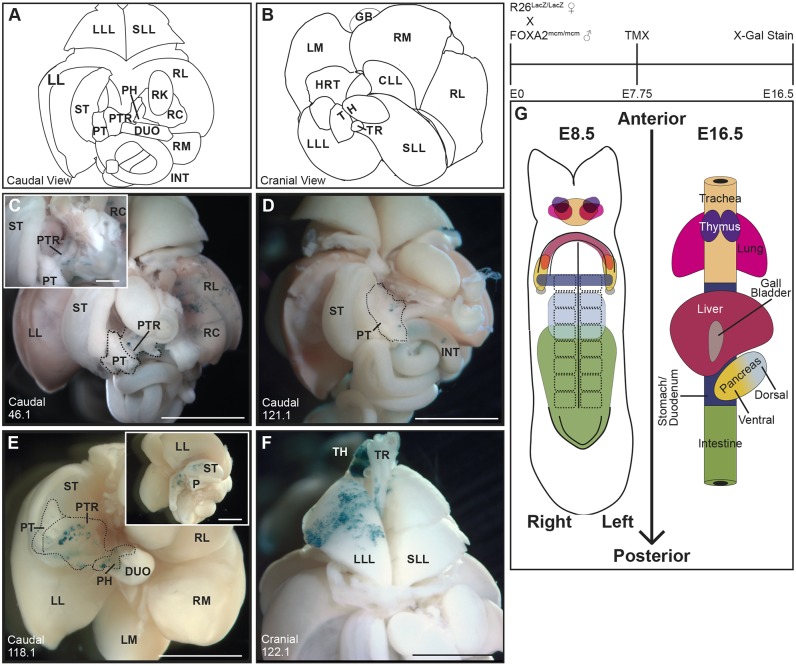
Fig. 1.
Lineage tracing reveals individual multi-organ progenitors in the E8.5 DE. Three mg kg−1 TMX was maternally administered at E7.75 to Foxa2mcm/+; R26R−/+ embryos and X-gal staining of dissected viscera performed at E16.5. (A,B) Illustrated and annotated caudal (A) and cranial (B) views of the dissected viscera are provided for reference. (C) A caudal view of embryo 46.1 reveals lacZ-expressing descendants in the right lateral (RL) and right medial (RM; not seen in this view) liver lobes, as well as in the pancreatic trunk (PTR; n=3 embryos with this pattern). (D) A caudal view of the viscera dissected from embryo 121.1 reveals blue lacZ-expressing cells in the pancreatic tail (PT) and intestine (INT; n=2 with this pattern). (E) A caudal view of the viscera of embryo 118.1 reveals lacZ-positive cell descendants in the stomach (ST) as well as in the PTR and pancreatic head (PH). The left/caudal view of this viscera in the inset reveals additional lacZ-positive descendants in the ST and duodenum (DUO; n=3 with this pattern). (F) A cranial view of embryo 122.1 demonstrates lacZ-expressing clonal descendants in the left lung lobe (LLL), left thymus (TH) and in the trachea (TR). (G) A fate map of the E8.5 DE, produced by combining previously published fate mapping with the novel progenitor relationships reported herein. The fate map is drawn on a linearized 8S embryo. The midline placed notochord, paired somites, and anterior and posterior intestinal portal are drawn on the embryo as landmarks. The shapes indicate organ progenitor domains and the color represents individual organs as identified on the simplified E16.5 gut tube. Progenitor domains that overlap represents endoderm that will contribute to each of those organs. CLL, cardiac lung lobe; GB, gall bladder; HRT, heart; LL, left lateral liver lobe; LM, left medial liver lobe; RC, right caudate liver lobe; RK, right kidney; SLL, superior lung lobe. Scale bars: 1 mm.
Unlike other endoderm-derived organs, the pancreas is produced by the fusion of discrete and non-contiguous dorsal and ventral pancreas buds, which contribute by E11.5 to the pancreas tail and head, respectively (Spooner et al., 1970; Wessels and Cohen, 1967). Therefore, it is not surprising that, of the embryos with labeled descendants in the pancreas and other organs, the contribution to the pancreas reflects a dorsal or ventral origin. For example, of the three embryos with clonal descendants that spanned the pancreas and intestine (n=3; Fig. 1D, Fig. S1), all pancreatic descendants were confined to the trunk and tail of the pancreas, consistent with a dorsal pancreas origin. In other embryos, clonal descendants were observed in the ventral pancreas-derived pancreatic head, as well as in the duodenum and stomach (n=2; Fig. 1E).
Together, these data provide new insights into pancreatic, gastric and intestinal development, and point to the existence of a common dorsal pancreas/intestine progenitor as well as a common stomach/ventral pancreas/duodenum progenitor. This idea is also supported by the existence of multiple, yet transcriptionally distinct, endoderm domains in the posterior foregut endoderm at E9.5 (Sherwood et al., 2009), and serves to highlight the dynamic nature of the developing posterior foregut at E8.5 (McCracken and Wells, 2017). Furthermore, when the pancreas data are examined in total, they lend credence to the hypothesis that the dorsal and ventral pancreas each contribute to the pancreatic trunk (Table 1), a process that has been experimentally verified in Xenopus (Jarikji et al., 2009). Given the extensive efforts that have gone into recapitulating pancreatic development from pluripotent mammalian tissues in vitro, the novel developmental relationships uncovered herein are significant and may suggest new strategies for generating pancreatic tissues. One reason these relationships have not been uncovered is because much of the retrospective fate mapping performed in the pancreas has relied on genetic techniques that are restricted to already specified pancreatic cells (Gu et al., 2002; Kawaguchi et al., 2002; Kopinke et al., 2011; Larsen et al., 2017; Offield et al., 1996; Zhou et al., 2007). Similarly, in the case of prospective progenitor fate mapping, the experiments are terminated by E9.5, which is too early to easily distinguish gastric and intestinal fates (Angelo et al., 2012; Miki et al., 2012).
Finally, four embryos contained clones in distinct but overlapping combinations of trachea, thymus and lung: three organs that originate from the anterior ventral foregut endoderm (Fig. 1F, Table 1). These include independent embryos with descendants in each of four categories: trachea and lung; thymus and lung; trachea, thymus and lung; thymus alone. Despite the unique organ grouping in each of these embryos, the presence of the same organ descendants in at least two embryos supports the existence of a common E8.5 lung/thymus/trachea endoderm progenitor. These conclusions are further supported by the presence of thymus and lung descendants, when observed in the same embryo, on the same side of the embryo (left or right), consistent with the expectation for multi-lobed organs that originate from distinct left and right lateral progenitors. Although the existence of a common DE progenitor pool for trachea and lung has been assumed, a common trachea/thymus/lung E8.5 progenitor is less well established but not without precedent (Perl et al., 2002).
In this study, individual embryos with clonal descendants in more than one organ reveal the multi-organ fate of an individual E8.5 DE cell. The endoderm emerges during gastrulation in an orderly fashion, with the foregut emerging first, followed by the midgut and then the hindgut (Tam et al., 2007). This broad regionalization, although initially overlapping, becomes sharper as development proceeds (Tam et al., 2007). Furthermore, it is clear that coherent groups of endoderm contribute to contiguous domains of cell descendants within the gut tube and associated organs (Angelo et al., 2012; Franklin et al., 2008; Miki et al., 2012; Tremblay and Zaret, 2005). Armed with this information, the novel progenitor relationships described herein and published fate maps, we produced a fate map of the E8.5 DE (Fig. 1G). Each colored shape on the E8.5 map represents an organ progenitor domain and the color a particular organ, as identified on the E16.5 gut tube cartoon. The overlapping progenitor domains on the E8.5 map represent the multi-organ progenitors identified herein. The position of the liver, gallbladder and pancreas progenitor domains are based on published results (Angelo et al., 2012; Franklin et al., 2008; Miki et al., 2012; Tam et al., 2007; Tremblay and Zaret, 2005; Uemura et al., 2015). The remainder of the organ domains are positioned based on their ultimate arrangement in the embryo relative to the experimentally identified organ domains. In addition to providing a better understanding of normal organ ontogeny, the developmental relationships described herein may provide novel induction strategies for the parenchymal tissues of the thymus, lung, stomach, pancreas, duodenum and intestine.
Assessing hepatoblast potency
To begin a more in-depth analysis of the E16.5 livers obtained, we examined the distribution of clonal descendants between the five readily identifiable liver lobes (Table 2, Fig. 2A). Early prospective fate mapping and recent retrospective clonal analysis suggests a left/right asymmetry in descendant contribution to the lobes (Angelo and Tremblay, 2013; Weiss et al., 2016). In agreement with these studies, we find that clonal descendants mainly contribute to contiguous lobes (Fig. 2B, Table 2, Fig. S3). Given the large number of embryos with descendants in the liver in our study (n=25), we calculated that up to three may result from two independent recombination events. We believe that two independent recombination events could have produced the two embryos with unique and discontinuous hepatic lobe contribution (embryos 49.1 and 53.1; Table 2, Fig. S3).
Table 2.
Distribution of E16.5 hepatic clones
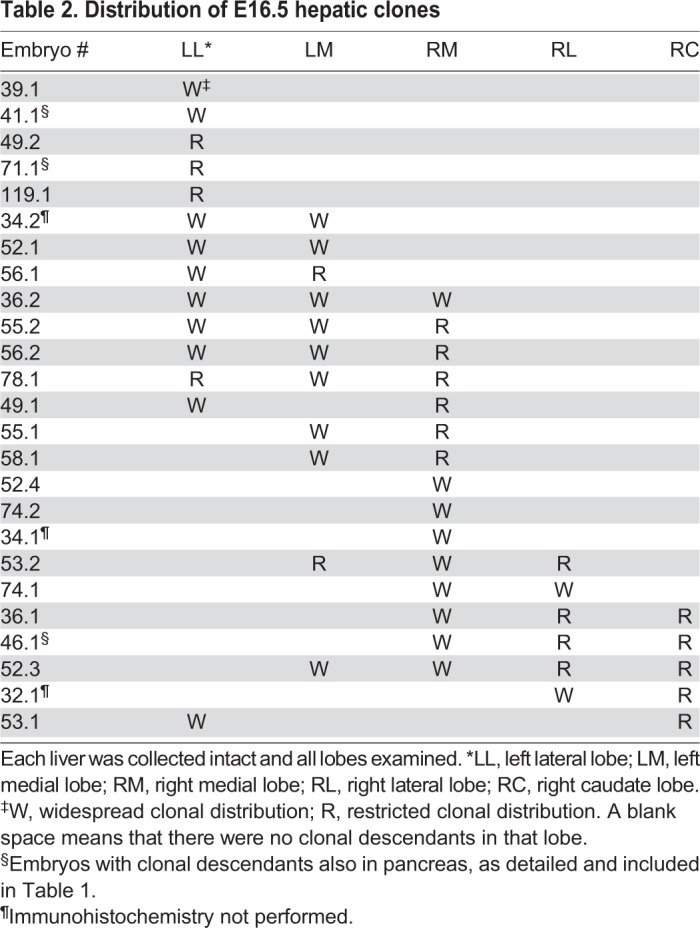
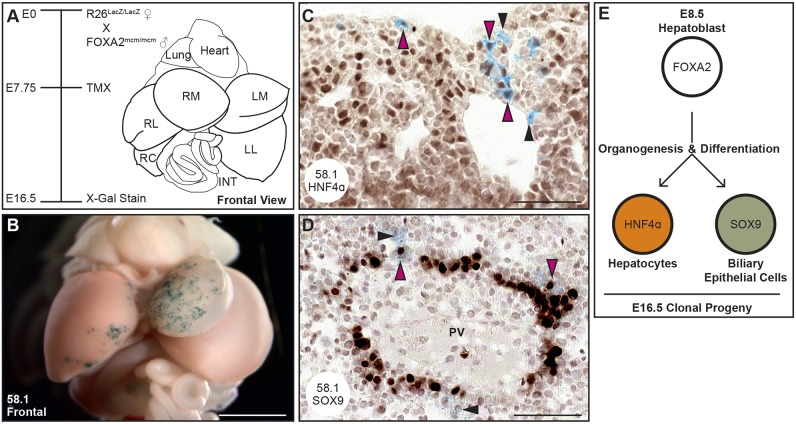
Fig. 2.
Individual hepatoblasts contribute hepatocytes and BECs to each E16.5 liver. Embryos were maternally administered 3 mg kg−1 TMX at E7.75 and then dissected and X-gal stained at E16.5. (A) An annotated illustration of a frontal view of dissected viscera at E16.5. (B) A frontal image of the viscera of embryo 58.1 reveals widespread lacZ expression in the left medial liver lobe (LM) and more restricted expression on the left edge of the right medial liver lobe (RM). (C,D) Immunohistochemistry, performed on sections from the embryo in B, reveals the colocalization of lacZ-expressing clonal descendants (blue) with the hepatocyte marker HNF4α (C, pink arrowheads) and the biliary epithelial cell marker SOX9 (D, pink arrowheads). The black arrowheads indicate lacZ-expressing descendants that are negative for the indicated immunohistochemistry marker. (E) Schematic illustrating that each clonally labeled E8.5 hepatoblast contributes to the hepatocyte and biliary epithelial cell lineage by E16.5 (n=22/22; χ2, ***P=9.11−4). Scale bars: 1 mm in B; 50 µm in C,D. INT, intestine; LL, left lateral liver lobe; RC, right caudate liver lobe; RL, right lateral liver lobe.
The hepatoblast-to-hepatocyte transition is completed by E15.5. By E16.5 hepatocytes and the cholangiocyte precursor, biliary epithelial cells (BECs), can be identified both molecularly and histologically (Yang et al., 2017). Hepatocytes express hepatocyte nuclear factor 4α (HNF4α) and reside throughout the liver parenchyma, whereas SOX9-expressing BECs are confined to the periportal epithelium (Antoniou et al., 2009; Parviz et al., 2003; Shiojiri, 1997). To analyze the differentiation state of the clonal descendants, each lacZ-expressing liver was sectioned and adjacent or near adjacent sections subjected to immunohistochemistry for HNF4α or SOX9. In each of the 22 livers analyzed, lacZ colocalized with both HNF4α and SOX9 (Fig. 2C,D; Table 2, Fig. S4), demonstrating for the first time that individual hepatoblasts differentiate into both hepatocytes and BECs in vivo by E16.5 (Fig. 2E; χ2, ***P=9.11−4). These data offer unequivocal results, as 100% of livers analyzed showed that both populations arise from a single cell.
We have shown that carefully timed single-cell retrospective fate mapping is not only useful for confirming suspected ontogeny but can also be used to uncover novel progenitor relationships. Such studies also reveal how cell potential is gradually refined and provides insight into how individual cells physically contribute to mature organs. Furthermore as single-cell RNA-sequencing becomes more prevalent, its use as a predictive tool to infer developmental relationships will be strengthened and validated by single-cell lineage-tracing methods such as those presented herein.
MATERIALS AND METHODS
Mice
Homozygous Foxa2mcm/mcm males (C57BL/6J and 129S1 background; age 3-12 months) were crossed to homozygous Rosa26lacZ/lacZ reporter females (C57BL/6NJ; age 4-12 weeks) to generate heterozygous, Foxa2mcm/+; Rosa26lacZ/+ embryos (Park et al., 2008; Soriano, 1999). The morning the copulation plug was identified was defined as E0.5. All animal experiments were performed in accordance with guidelines issued by the Institutional Animal Care and Use Committee at the University of Massachusetts Amherst (IACUC #2015-45).
Tamoxifen administration
Tamoxifen (TMX; Sigma, #T5648) was administered on E7.75 (between 16:30 and 17:30) via oral gavage. To prepare the TMX solution, 100 μl of ethanol was aspirated with 5 mg of TMX and heated in a water bath until fully dissolved. The dose (2-50 mg kg−1) was calculated using the body weight at the time of administration and achieved by adding the appropriate volume of peanut oil (human consumption grade). A constant volume (100 µl) was administered to all animals, including the controls receiving only peanut oil.
X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) staining
Embryos were collected at either E8.5 or E16.5 in ice-cold PBS. E8.5 embryos were dissected intact, whereas E16.5 embryos were dissected to collect viscera only. X-gal staining of E8.5 embryos was performed as previously described (Tremblay et al., 2001). Although most were stored in ethanol, those presented herein were stored in glycerol for imaging. X-gal staining at E16.5 was performed similarly, except modifications to the protocol were made to reduce background, increase substrate penetration and ensure that the staining minimally interfered with subsequent immunohistochemistry. The 0.2% gluteraldehyde fixation step was limited to 10 min on ice. Although this modification diminished the intensity of the X-Gal stain, particularly on the innermost region of the livers, it allowed for reliable immunohistochemistry after X-gal staining. The X-gal buffer was adjusted to pH 8.0 and X-gal staining performed overnight on a nutator at room temperature, rather than at 37°C, which served to reduce the background that was otherwise prevalent in the liver (Nagy et al., 2003).
Immunohistochemistry
Samples were dehydrated in ethanol, cleared in xylenes, embedded in paraffin wax and sectioned at 7 μm. E8.5 embryos were sectioned onto a single slide, whereas only the X-gal positive regions of the E16.5 tissues were serially sectioned and distributed onto three slides. After dewaxing in xylene and rehydration, microwave antigen retrieval was performed in boiling 10 mM Tris buffer (pH 10) for 8 min. Slides were cooled to room temperature before incubating in block (0.5% milk powder in PBT) for 2 h at room temperature, and then incubated in primary antibody (diluted in block) at 4°C overnight. Slides were incubated with secondary antibodies diluted in block for 1 h at room temperature. ABC peroxidase staining (ThermoFisher, #32020) was followed by development with metal-enhanced DAB (ThermoFisher #34065). Finally, slides were mounted in Cytoseal-60 (ThermoScientific, #8310-16) for permanent storage.
The primary antibodies and dilutions used for immunohistochemistry were anti-HNF4α (SantaCruz, #sc6556, 1:200) and anti-SOX9 (Millipore, #ab5535, 1:1000). The secondary antibodies and dilutions used were biotinylated goat anti-rabbit (Vector Labs, #ba-1000, 1:500) and biotinylated rabbit anti-goat (Vector Labs, #ba-5000, 1:500).
Imaging
Whole-mount images were captured on a Nikon SMZ1500 dissecting microscope fitted with either a QImaging MicroPublisher 5.0 RTV or a Spot 29.2 color camera. Bright-field images were taken with a 3DHistech Panoramic MIDI slide scanner under a 20× objective. Images were cropped in Photoshop and arranged using InDesign.
Statistics
All embryos receiving TMX were collected and the number with recombined cells were reported herein. A one-way analysis of variance (ANOVA) was performed on the number of labeled cells observed in each E8.5 embryo collected at each TMX dose (***P=2.23−9), suggesting that the number of labeled cells observed is dependent on TMX dose. The frequency of labeling at E16.5 (11.8%) was slightly, but not significantly, lower than at E8.5 (13.9%; F-test, P=0.0523). lacZ-expressing cells within E16.5 livers consistently co-expressed both hepatocyte and biliary transcription factors (n=22/22) so hepatoblasts must be at least bipotential (χ2, ***P=9.11−4) to produce those cells. Statistics were performed in R v3.3.3 (Team, 2017).
Supplementary Material
Acknowledgements
We thank Dr Mary Weiss for her help in reducing background in our X-gal stained viscera, and members of the Tremblay and Mager labs for their discussion and advice during this project.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: K.D.T.; Methodology: G.K.E.S., J.M.M., M.-K.E.C., S.R., J.R.A., K.D.T.; Validation: G.K.E.S., K.D.T.; Formal analysis: G.K.E.S., K.D.T.; Investigation: G.K.E.S., J.M.M., M.-K.E.C., S.R., K.D.T.; Resources: J.M., K.D.T.; Writing - original draft: G.K.E.S., K.D.T.; Writing - review & editing: G.K.E.S., J.M., K.D.T.; Visualization: G.K.E.S., J.R.A., K.D.T.; Supervision: K.D.T.; Project administration: K.D.T.; Funding acquisition: K.D.T.
Funding
This research is funded in part by the National Institutes of Health (R01DK087753 to K.D.T.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.168658.supplemental
References
- Angelo J. R. and Tremblay K. D. (2013). Laser-mediated cell ablation during post-implantation mouse development. Dev. Dyn. 242, 1202-1209. 10.1002/dvdy.24017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo J. R., Guerrero-Zayas M.-I. and Tremblay K. D. (2012). A fate map of the murine pancreas buds reveals a multipotent ventral foregut organ progenitor. PLoS ONE 7, e40707 10.1371/journal.pone.0040707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou A., Raynaud P., Cordi S., Zong Y., Tronche F., Stanger B. Z., Jacquemin P., Pierreux C. E., Clotman F. and Lemaigre F. P. (2009). Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology 136, 2325-2333. 10.1053/j.gastro.2009.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bort R., Signore M., Tremblay K., Martinez Barbera J. P. and Zaret K. S. (2006). Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. Dev. Biol. 290, 44-56. 10.1016/j.ydbio.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Deutsch G., Jung J., Zheng M., Lora J. and Zaret K. S. (2001). A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 128, 871-881. [DOI] [PubMed] [Google Scholar]
- Franklin V., Khoo P. L., Bildsoe H., Wong N., Lewis S. and Tam P. P. L. (2008). Regionalisation of the endoderm progenitors and morphogenesis of the gut portals of the mouse embryo. Mech. Dev. 125, 587-600. 10.1016/j.mod.2008.04.001 [DOI] [PubMed] [Google Scholar]
- Friedman S. L. (2003). Liver fibrosis--from bench to bedside. J. Hepatol. 38 Suppl. 1, S38-S53. 10.1016/S0168-8278(02)00429-4 [DOI] [PubMed] [Google Scholar]
- Gannon M. and Wright C. V. (1999). Endodermal patterning and organogenesis. In Cell Lineage And Fate Determination, pp. 583-614. Academic Press. [Google Scholar]
- Gu G., Dubauskaite J. and Melton D. A. (2002). Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447-2457. [DOI] [PubMed] [Google Scholar]
- Gualdi R., Bossard P., Zheng M., Hamada Y., Coleman J. R. and Zaret K. S. (1996). Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 10, 1670-1682. 10.1101/gad.10.13.1670 [DOI] [PubMed] [Google Scholar]
- Hayashi S. and McMahon A. P. (2002). Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 244, 305-318. 10.1006/dbio.2002.0597 [DOI] [PubMed] [Google Scholar]
- Jarikji Z., Horb L. D., Shariff F., Mandato C. A., Cho K. W. Y. and Horb M. E. (2009). The tetraspanin Tm4sf3 is localized to the ventral pancreas and regulates fusion of the dorsal and ventral pancreatic buds. Development 136, 1791-1800. 10.1242/dev.032235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., Cooper B., Gannon M., Ray M., MacDonald R. J. and Wright C. V. E. (2002). The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat. Genet. 32, 128-134. 10.1038/ng959 [DOI] [PubMed] [Google Scholar]
- Koga A. (1971). Morphogenesis of intrahepatic bile ducts of the human fetus. Light and electron microscopic study. Z. Anat. Entwicklungsgesch. 135, 156-184. 10.1007/BF00521108 [DOI] [PubMed] [Google Scholar]
- Kopinke D., Brailsford M., Shea J. E., Leavitt R., Scaife C. L. and Murtaugh L. C. (2011). Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development 138, 431-441. 10.1242/dev.053843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen H. L., Martin-Coll L., Nielsen A. V., Wright C. V. E., Trusina A., Kim Y. H. and Grapin-Botton A. (2017). Stochastic priming and spatial cues orchestrate heterogeneous clonal contribution to mouse pancreas organogenesis. Nat. Commun. 8, 605 10.1038/s41467-017-00258-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Suckale J., Masjkur J., Magro M. G., Steffen A., Anastassiadis K. and Solimena M. (2010). Tamoxifen-independent recombination in the RIP-CreER mouse. PLoS ONE 5, e13533 10.1371/journal.pone.0013533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann M. R., Tamplin O. J., Rossant J. and Seguin C. A. (2012). Tracing notochord-derived cells using a Noto-cre mouse: implications for intervertebral disc development. Dis. Model. Mech. 5, 73-82. 10.1242/dmm.008128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken K. W. and Wells J. M. (2017). Mechanisms of embryonic stomach development. Semin. Cell Dev. Biol. 66, 36-42. 10.1016/j.semcdb.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki R., Yoshida T., Murata K., Oki S., Kume K. and Kume S. (2012). Fate maps of ventral and dorsal pancreatic progenitor cells in early somite stage mouse embryos. Mech. Dev. 128, 597-609. 10.1016/j.mod.2011.12.004 [DOI] [PubMed] [Google Scholar]
- Nagy A., Gertsenstein M., Vintersten K. and Behringer R. (2003). Manipulating the Mouse Embryo: a Laboratory Manual, 3rd edn Cold Spring Harbor: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Offield M. F., Jetton T. L., Labosky P. A., Ray M., Stein R. W., Magnuson M. A., Hogan B. L. and Wright C. V. (1996). PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 122, 983-995. [DOI] [PubMed] [Google Scholar]
- Palaria A., Angelo J. R., Guertin T. M., Mager J. and Tremblay K. D. (2018). Patterning of the hepato-pancreatobiliary boundary by BMP reveals heterogeneity within the murine liver bud. Hepatology 68, 274-288. 10.1002/hep.29769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E. J., Sun X., Nichol P., Saijoh Y., Martin J. F. and Moon A. M. (2008). System for tamoxifen-inducible expression of cre-recombinase from the Foxa2 locus in mice. Dev. Dyn. 237, 447-453. 10.1002/dvdy.21415 [DOI] [PubMed] [Google Scholar]
- Parviz F., Matullo C., Garrison W. D., Savatski L., Adamson J. W., Ning G., Kaestner K. H., Rossi J. M., Zaret K. S. and Duncan S. A. (2003). Hepatocyte nuclear factor 4alpha controls the development of a hepatic epithelium and liver morphogenesis. Nat. Genet. 34, 292-296. 10.1038/ng1175 [DOI] [PubMed] [Google Scholar]
- Perl A. K., Wert S. E., Nagy A., Lobe C. G. and Whitsett J. A. (2002). Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc. Natl. Acad. Sci. USA 99, 10482-10487. 10.1073/pnas.152238499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Langan-Fahey S. M., Johnson D. A. and Jordan V. C. (1991). Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metab. Dispos. 19, 36-43. [PubMed] [Google Scholar]
- Rowe I. A. (2017). Lessons from epidemiology: the burden of liver disease. Dig. Dis. 35, 304-309. 10.1159/000456580 [DOI] [PubMed] [Google Scholar]
- Serls A. E., Doherty S., Parvatiyar P., Wells J. M. and Deutsch G. H. (2005). Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development 132, 35-47. 10.1242/dev.01570 [DOI] [PubMed] [Google Scholar]
- Sherwood R. I., Chen T. Y. and Melton D. A. (2009). Transcriptional dynamics of endodermal organ formation. Dev. Dyn. 238, 29-42. 10.1002/dvdy.21810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojiri N. (1997). Development and differentiation of bile ducts in the mammalian liver. Microsc. Res. Tech. 39, 328-335. [DOI] [PubMed] [Google Scholar]
- Shiojiri N., Inujima S., Ishikawa K., Terada K. and Mori M. (2001). Cell lineage analysis during liver development using the spf(ash)-heterozygous mouse. Lab. Invest. 81, 17-25. 10.1038/labinvest.3780208 [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71. 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B., Wigle J. T. and Oliver G. (2000). Hepatocyte migration during liver development requires Prox1. Nat. Genet. 25, 254-255. 10.1038/76996 [DOI] [PubMed] [Google Scholar]
- Spence J. R., Lange A. W., Lin S.-C. J., Kaestner K. H., Lowy A. M., Kim I., Whitsett J. A. and Wells J. M. (2009). Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev. Cell 17, 62-74. 10.1016/j.devcel.2009.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner B. S., Walther B. T. and Rutter W. J. (1970). The development of the dorsal and ventral mammalian pancreas in vivo and in vitro. J. Cell Biol. 47, 235-246. 10.1083/jcb.47.1.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Zheng Y.-W., Kaneko S., Onodera M., Fukao K., Nakauchi H. and Taniguchi H. (2002). Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J. Cell Biol. 156, 173-184. 10.1083/jcb.200108066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam P. P., Khoo P. L., Lewis S. L., Bildsoe H., Wong N., Tsang T. E., Gad J. M. and Robb L. (2007). Sequential allocation and global pattern of movement of the definitive endoderm in the mouse embryo during gastrulation. Development 134, 251-260. 10.1242/dev.02724 [DOI] [PubMed] [Google Scholar]
- Team R. C. (2017). R: A Language and Environment for Statistical Computing. Vienna, Austria: The R Foundation for Statistical Computing. [Google Scholar]
- Tremblay K. D. and Zaret K. S. (2005). Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev. Biol. 280, 87-99. 10.1016/j.ydbio.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Tremblay K. D., Dunn N. R. and Robertson E. J. (2001). Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development 128, 3609-3621. [DOI] [PubMed] [Google Scholar]
- Uemura M., Igarashi H., Ozawa A., Tsunekawa N., Kurohmaru M., Kanai-Azuma M. and Kanai Y. (2015). Fate mapping of gallbladder progenitors in posteroventral foregut endoderm of mouse early somite-stage embryos. J. Vet. Med. Sci. 77, 587-591. 10.1292/jvms.14-0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vooijs M., Jonkers J. and Berns A. (2001). A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep. 2, 292-297. 10.1093/embo-reports/kve064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Rhee S., Palaria A. and Tremblay K. D. (2015). FGF signaling is required for anterior but not posterior specification of the murine liver bud. Dev. Dyn. 244, 431-443. 10.1002/dvdy.24215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. C., Le Garrec J.-F., Coqueran S., Strick-Marchand H. and Buckingham M. (2016). Progressive developmental restriction, acquisition of left-right identity and cell growth behavior during lobe formation in mouse liver development. Development 143, 1149-1159. 10.1242/dev.132886 [DOI] [PubMed] [Google Scholar]
- Wessels N. K. and Cohen J. H. (1967). Early pancreas organogenesis: Morphogenesis. tissue interactions and mass effects. Dev. Biol. 15, 271-287. 10.1016/0012-1606(67)90042-5 [DOI] [PubMed] [Google Scholar]
- Yang L., Wang W.-H., Qiu W.-L., Guo Z., Bi E. and Xu C.-R. (2017). A single-cell transcriptomic analysis reveals precise pathways and regulatory mechanisms underlying hepatoblast differentiation. Hepatology 66, 1387-1401. 10.1002/hep.29353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Law A. C., Rajagopal J., Anderson W. J., Gray P. A. and Melton D. A. (2007). A multipotent progenitor domain guides pancreatic organogenesis. Dev. Cell 13, 103-114. 10.1016/j.devcel.2007.06.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.