Abstract
Objective
Several recent reviews of published studies have shown that the eradication of H. pylori infection in patients with ITP improved thrombocytopenia in about half of the cases. However, most included studies were observational case series. We performed the first meta-analysis of randomized trials to gain a better insight into the effect of H. pylori eradication in ITP patients.
Methods
A systematic computerized search of the electronic databases including PubMed, EMBASE, Google Scholar, and Cochrane Library (up to December 2017) was conducted.
Results
From six studies, a total of 241 patients (125 in eradication group and 116 in control group) were included in the meta-analysis. Patients in the eradication group showed significantly higher overall platelet response rate than those in the control group (odds ratio = 1.93, 95% confidence interval: 1.01–3.71, P = 0.05). In the subgroup analysis, however, children in the eradication group failed to show statistically better response rate than those in the noneradication group (odds ratio = 1.80, 95% confidence interval: 0.88–3.65, P = 0.11).
Conclusions
This meta-analysis indicates that H. pylori eradication has a significant therapeutic effect in patients with ITP. Considering the intrinsic limits in the design and sample size of the included studies, however, large randomized controlled trials are warranted to validate the therapeutic impact of H. pylori eradication in adults as well as children with ITP.
1. Introduction
Idiopathic or immune thrombocytopenic purpura (ITP) is an autoimmune-mediated acquired bleeding disorder of children as well as adults. It is characterized by the destruction of host platelet caused by anti-platelet antibodies [1]. However, the mechanisms that trigger the development of platelet auto-antibodies remain poorly understood. Persistent thrombocytopenia for more than 6 or 12 months defines the chronic form of ITP [2, 3]. ITP is typically a diagnosis of exclusion, made by clinicians after ruling out other possible etiologies. ITP can be a primary disease or secondary to a variety of etiologies including bacterial or viral infection, autoimmune disease, or neoplasm [1–3].
Helicobacter pylori (H. pylori) is the most common microbial pathogen that colonizes in the mucosal layer of the stomach. It is causally associated with a variety of gastrointestinal disorders including chronic gastritis, gastric mucosal atrophy, peptic ulcer, gastric mucosa-associated lymphoid tissue lymphoma, and gastric adenocarcinoma [4, 5]. A pathophysiologic link between ITP and H. pylori infection was initially proposed in 1998 by Gasbarrini et al. who reported a significant increase of platelet count after bacterial eradication in 8 of 11 ITP patients infected with H. pylori [6]. Although the pathogenesis of H. pylori-associated ITP is still uncertain, several studies have suggested that H. pylori virulence factor, cytotoxin-associated gene A (CagA), stimulates the development of anti-CagA antibodies (Abs) that cross-react with platelet surface antigens (Ags), resulting in thrombocytopenia [7–9]. Many studies have reported that H. pylori eradication led to an increase of platelet counts and even a regression of ITP [10–18]. As other studies have failed to demonstrate the beneficial effect of bacterial eradication in ITP [19–21], however, there is a debate as to whether the eradication of H. pylori in chronic ITP is effective in increasing platelet counts or not.
Several recent reviews of previously published studies have shown that the eradication of H. pylori infection in patients with chronic ITP improved thrombocytopenia in about half of the cases [22–24]. The metaregression model revealed that the success of bacterium eradication was highly significant as an explanatory variable for increase of platelet count [22]. However, most studies included were observational case series [22–24], which might subject the results to possible bias. In addition, several recent randomized trials in adults or children showed the inconsistent effect of bacterium eradication on platelet recovery in ITP patients infected with H. pylori [16–21]. Therefore, we performed this meta-analysis of randomized trials to gain a better insight into the effect of H. pylori eradication in ITP patients.
2. Materials and Methods
2.1. Publication Searching Strategy
The current study was conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [25, 26]. A systematic computerized search of the electronic databases including PubMed, EMBASE, Google Scholar, and Cochrane Library (up to December 2017) was performed. The search used the following keywords variably combined: “Helicobacter pylori,” “H. pylori,” “thrombocytopenia,” “idiopathic thrombocytopenic purpura,” “immune thrombocytopenic purpura,” and “ITP.” The related article function in PubMed was used to identify all relevant articles. In addition, the bibliographic references of all retrieved studies and reviews were evaluated for additional eligible articles.
2.2. Inclusion Criteria
We only included randomized controlled trials in this meta-analysis. Retrospective or observational case control studies were excluded. Eligible studies should meet the following inclusion criteria: (i) patients with a diagnosis of chronic ITP according to the American Society of Hematology (ASH) guidelines [2]; (ii) H. pylori infection documented by reliable tests such as 13C-urea breath test (UBT), serologic test for antibody to H. pylori, stool antigen test, or histology of gastric mucosal biopsies; (iii) randomization of ITP patients infected with H. pylori to either bacterial eradication or noneradication; (iv) providing treatment outcomes (platelet counts or response rate) of these two groups. Reports published only in abstract form were not considered eligible.
2.3. Data Extraction
Two reviewers (BJK and HJJ) independently screened relevant studies and extracted the data from each eligible study. If these two authors did not agree, the other investigator (JHK) was consulted to resolve the disagreement through discussion.
The following data were extracted from the included studies: the first author, year of publication, country, number of patients, demographics (age, gender), detecting methods for H. pylori infection, duration of ITP, treatment, platelet counts (before and after treatment) or treatment outcomes, and relapse rate.
2.4. Quality Assessment
The methodological quality of the randomized trials was scored using the Jadad five-item scale, taking into account randomization, double-blinding process, and withdrawals [27]. The final score ranged from 0 to 5, with low-quality studies having a score ≤ 2 and high-quality studies having a score of ≥3.
2.5. Statistical Analyses
We chose to record the overall response rate (ORR) as primary assessment criteria. The odds ratios (ORs) and 95% confidence intervals (CIs) for ORR were calculated indirectly from original articles. The effect size of ORR was pooled through OR and its 95% CI. The heterogeneity across studies was estimated by the Q statistics and I2 inconsistency test. The fixed-effect model (Mantel–Haenszel method) was used for pooling homogeneous outcomes (P ≥ 0.1 and I2 ≤ 50%), and the random-effect model (DerSimonian–Laird method) was selected if significant heterogeneity was observed (P < 0.1 and I2 > 50%).
The RevMan version 5.3 was used to combine the data. The plots show a summary estimate of the results from all the studies combined. The size of the squares represents the estimate from each study, reflecting the statistical “weight” of the study. Outcomes are provided as forest plots with diamonds representing the estimate of the pooled effect and the width of diamond implying its precision. The line of no effect is number one for binary outcomes, which depicts statistical significance if not crossed by the diamond [28]. The OR > 1.0 implies better response for patients receiving the eradication treatment of H. pylori infection.
The possibility of publication bias was assessed with a visual inspection of the graphical funnel plot [29]. The statistical methods for detecting funnel plot asymmetry were the rank correlation tests of Begg and Mazumdar and Egger's regression asymmetry test [29, 30]. Statistical significance was considered for a P value of less than 0.05 for the summary estimate of OR and publication biases.
3. Results
3.1. Results of Search
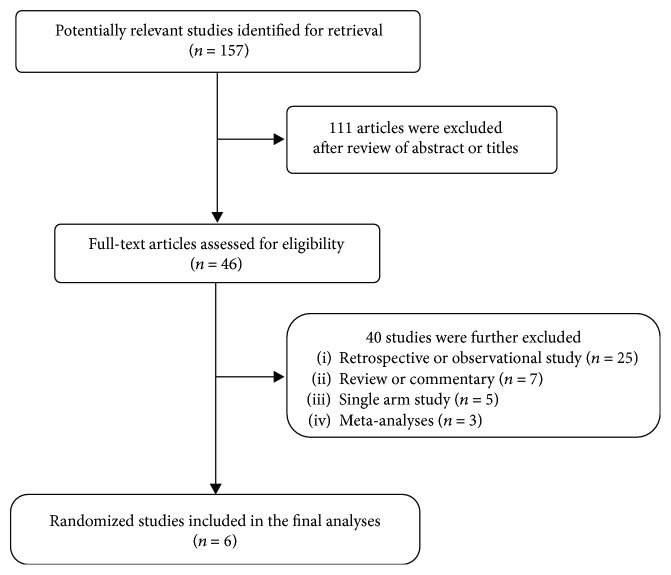
A total of 157 potentially relevant articles were initially found, but 111 of them were excluded after careful screening of the titles and abstracts. We retrieved 46 articles for full-text evaluation and further excluded 40 by the inclusion criteria. Finally, 6 studies were included in the meta-analysis [16–21]. Figure 1 shows the search flow diagram of this meta-analysis.
Figure 1.
Flow diagram of search process.
3.2. Characteristics of the Included Studies
Table 1 summarizes the main characteristics and treatment outcomes of the six studies. Four studies were conducted in children [18–21] and the remaining 2 in adults [16, 17]. The most common detection method for H. pylori infection was UBT [16, 18–21]. The prevalence of H. pylori infection ranged from 25.9% [21] to 73.9% [20]. Bacterium eradication consisted of standard triple therapy including clarithromycin, amoxicillin, and proton-pump inhibitor (PPI) (omeprazole or lansoprazole) for 7–14 days. Except for one study [16], patients in the control arms were usually treated with corticosteroid (prednisone or prednisolone) or PPI alone.
Table 1.
Summary of the six included studies.
| First author (year) Country [ref] |
Number of ITP pts | Detection of Hp infection | Number of Hp (+) pts | Randomization | Number of pts | M/F | Mean age (yr) (SD or range) | Duration of ITP (yr) | Platelet at enrollment (×109/L) | Platelet after 6 mo of Tx (×109/L) | Response‡ (CR + PR) | Relapse at 1 year | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suzuki (2005) Japan [16] |
36 | UBT or histology | 25 (69.4%) | Eradication | 13 | 5/8 | 57.4 ± 15.0 | 5.8 ± 7.2 | 54.7 ± 26.9 | 114.5 ± 90.5 | 6 (46.2%) | NA | 2 |
| Noneradication (observation) | 12 | 5/7 | 56.2 ± 7.8 | 4.6 ± 5.2 | 48.4 ± 22.1 | 48.1 ± 26.0 | 0 (0%) | NA | |||||
|
| |||||||||||||
| Tsutsumi (2005) Japan [17] |
25 | Anti-Hp antibody | 17 (68%) | Eradication | 9 | 2/7 | 60.3 | NA | NA | NA | 6 (66.7%) | 2 (33.3%) | 2 |
| Noneradication (PPI) | 8 | 3/5 | 63.3 | NA | NA | NA | 5 (62.5%) | 2 (40%) | |||||
|
| |||||||||||||
| Li (2009) China [18] |
NA | UBT | 93 | Eradication + PD | 51 | 27/24 | 6.7 ± 2.4 | NA | NA | NA | 45 (88.2%) | 11 (21.6%) | 1 |
| Noneradication (PD) | 42 | 22/20 | 5.8 ± 2.7 | NA | NA | NA | 37 (88.1%) | 17 (40.5%) | |||||
|
| |||||||||||||
| Treepongkaruna (2009) Thailand [19] |
55 | UBT | 16 (29.1%) | Eradication + PD | 7 | 3/4 | 11.0 | 3.4 (1.7–6.9) | 23.0 (3.0–84.0) | NA | 1 (14.3%) | NA | 2 |
| Noneradication (PD) | 9(8)∗ | 4/5 | 10.8 | 5.1 (1.2–9.5) | 34.0 (3.0–86.0) | NA | 1 (12.5%) | NA | |||||
|
| |||||||||||||
| Tang (2013) China [20] |
92 | UBT | 68 (73.9%) | Eradication ± PD | 34 | NA | NA (child) | NA | 14.8 ± 0.4 | 160.4 ± 1.0 | 26 (76.5%) | NA | 2 |
| Noneradication (±PD) | 34 | NA | NA (child) | NA | 15.1 ± 0.3 | 80.6 ± 1.1 | 20 (58.8%) | NA | |||||
|
| |||||||||||||
| Brito (2015) Brazil [21] |
85 | UBT or stool Ag test | 22 (25.9%) | Eradication ± PD | 11 | 6/5 | 12.7 (4.9–17.5) | 5 (1–8) | 35.0 (1–145) | 128 ± 73 | 7 (63.6%) | NA | 2 |
| Noneradication (±PD) | 11 | 5/6 | 10.5 (5.8–17.7) | 3 (0.7–11) | 47 (8–139) | 63 ± 44 | 4 (36.4%) | NA | |||||
ITP: idiopathic or immune thrombocytopenia purpura; Hp: Helicobacter pylori; UBT: 13C-urea breath test; pts: patients; yr: years; mo: months; SD: standard deviation; PPI: proton-pump inhibitor, PD: prednisone or prednisolone; Tx: treatment; CR: complete platelet response; PR: partial platelet response; NA: not available. ∗One patient was withdrawn due to massive gastrointestinal bleeding, requiring high-dose prednisolone. ‡Overall response criteria: Suzuki: platelet count increased by more than 50 × 109/L 6 months after eradication therapy; Tsutsumi: platelet count with a net increase greater than 30 × 109/L or a 50% increase in platelet count with a net increase of 10 × 109/L but less than 30 × 109/L; Li: platelet count with a net increase greater than 30 × 109/L; Treepongkaruna: platelet count more than 100 × 109/L sustaining for at least 3 months; Tang: platelet count more than 50 × 109/L or platelet count with a net increase greater than 30 × 109/L; Brito: platelet increase greater than 20–30 × 109/L.
3.3. Platelet Response to Treatment
In most studies, complete response (CR) was defined as the achievement of a platelet count more than 150 × 109/L [16, 18–21]. However, the threshold for partial response (PR) varied among studies although platelet count with a net increase of greater than 30 × 109/L was most commonly adopted [17, 18, 20, 21] (Table 1). We defined the ORR by adding CR rate and PR rate. The ORR varied from 14.3% [19] to 88.2% [18] in the eradication arms and from 0% [16] to 88.1% [18] in the noneradication arms.
3.4. Quality of the Included Studies
The Jadad scores for the 6 randomized studies was 2 or less. The studies announced that patients were randomly assigned by concealed allocation, but no more information on the method to generate the sequence of randomization was available.
3.5. Therapeutic Effect of H. pylori Eradication: Meta-Analysis
From the six studies, a total of 241 patients (125 in the eradication group and 116 in the control group) were included in the meta-analysis of ORs for ORR. The fixed-effect model was selected because there was no significant heterogeneity among studies (X2 = 4.11, P = 0.53, I2 = 0%). Patients in the eradication group showed significantly higher ORR than those in the control group (OR = 1.93, 95% CI: 1.01–3.71, P = 0.05) (Figure 2(a)).
Figure 2.
Forest plots of odds ratios for overall platelet response rates in all patients (a) and in children (b).
In the subgroup analysis, children in the eradication group failed to show statistically higher ORR than those in the noneradication group (OR = 1.80, 95% CI: 0.88–3.65, P = 0.11) (Figure 2(b)). There was no significant heterogeneity among studies (X2 = 1.38, P = 0.71, I2 = 0%), and the fixed-effect model was used for pooling the data.
3.6. Publication Bias
Begg's funnel plot and Egger's test indicated no evidence of substantial publication bias for ORR (Begg's P = 0.452, Egger's P = 0.465) (Figure 3).
Figure 3.
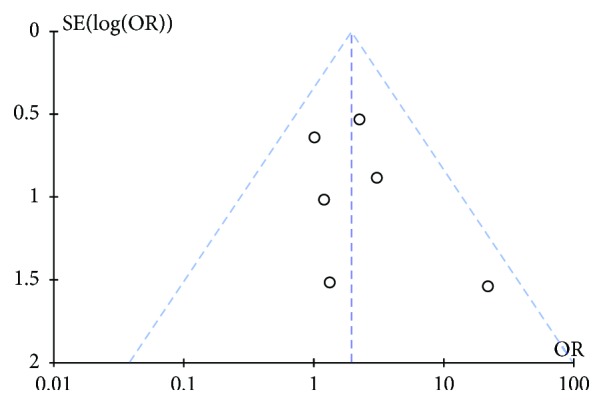
Funnel plot for publication bias regarding overall response rates.
4. Discussion
Despite the findings indicating that H. pylori infection plays an etiological role in ITP, several randomized trials to date have shown the inconsistent results in the effect of bacterium eradication. In this meta-analysis of 6 randomized trials, we evaluated the therapeutic effect of H. pylori eradication in patients with ITP. Our results indicate that bacterium eradication has a significant impact on platelet recovery in ITP.
ITP is considered an organ-specific autoimmune disease. It is mediated by anti-platelet Abs that bind to host platelets and megakaryocytes, accelerating platelet destruction by the reticuloendothelial system [31]. The auto-Abs primarily target platelet surface glycoproteins such as GP IIb/IIIa and GP Ib. Although the triggering factors for ITP are obscure, bacterial or viral infections are known to be associated with the development of ITP, indicating that infectious agents may play a critical role in the pathogenesis of a particular subset of ITP [32].
Since the first report in 1998 [6], the accumulating data have indicated that H. pylori may contribute to the pathogenesis of chronic ITP [7, 9, 31–34]. In addition, numerous clinical studies have reported that H. pylori eradication resulted in an increase of platelet counts in ITP [10–18]. Various mechanisms have been proposed for the role of H. pylori in chronic ITP: the production of Ag-specific Abs that are cross-reactive with platelet surface glycoproteins (GP IIb/IIIa or GP Ib) due to molecular mimicry increased plasmacytoid dendritic cell numbers and variable host immune response to virulence factors such as vacuolating-associated cytotoxin gene A (VacA) and CagA [7, 9, 33, 34]. A recent study in China reported that the FcγRIIB expression on circulating monocytes is downregulated in H. pylori-infected ITP patients [35]. Therefore, H. pylori infection may play an important role in the development of ITP by activating the Fcγ receptor of monocytes/macrophages through downregulation of the inhibitory receptor FcγRIIB. Despite mounting evidence of the association of H. pylori and ITP, published studies are inconclusive due to the scarcity of controlled clinical trials to date and some reports presenting conflicting results [16–21]. Moreover, empirical treatment of H. pylori in children with ITP remains controversial [36, 37]. Therefore, validating the therapeutic effects of H. pylori eradication has a critical impact on clinical diagnosis and treatment of ITP.
In the previous systemic reviews, the pooled prevalence of H. pylori infection in ITP ranged from 58% [38] to 65% [23]. The first meta-analysis by Franchini et al. reviewed 17 studies with 788 ITP patients, including 494 infected with H. pylori [22]. There was a statistically significant difference in the increase of platelet count in patients for whom H. pylori eradication was successful, compared with untreated patients (weighted mean difference (WMD) of 40.77 × 109/L, 95% CI: 20.92–60.63) and those who failed eradication (WMD of 52.16 × 109/L, 95% CI: 27.79–64.91). Another systemic review with a meta-analysis of 1555 patients with ITP from 25 studies reported the weighted mean CR (platelet count more than 100 × 109/L) of 42.7% and ORR (platelet count ≥ 30 × 109/L and at least doubling of basal platelet count) of 50.3% after successful eradication of H. pylori [23]. In addition, the most recent systemic review by Frydman et al. confirmed that eradication treatment in H. pylori-positive adult ITP patients resulted in about a 50% CR rate [24]. These findings indicate the beneficial effect of bacterium eradication in ITP patients infected with H. pylori. Except for one [16], however, almost all articles included in those reviews were retrospective or observational studies [22–24]. Thereafter, five more randomized trials were conducted in adult or children ITP patients [17–21]. While three studies observed a significant difference in the platelet response between the eradication group and noneradication group [16, 20, 21], there was no beneficial effect of H. pylori eradication in the other studies [17–19].
In this meta-analysis of 6 randomized trials [16–21], the prevalence of H. pylori infection ranged from 30% [21] to 74% [20]. Patients who received bacterium eradication therapy showed significantly higher ORR than those in the control group (OR = 1.93, 95% CI: 1.01–3.71, P = 0.05). This result suggests that H. pylori eradication in patients with ITP is effective in increasing platelet count. The recent ASH guidelines have recommended the examination and treatment of H. pylori infection in adult patients with ITP [2]. However, routine testing for H. pylori in children is not recommended. The prevalence of H. pylori infection is much lower in children than in adults with chronic ITP, suggesting that H. pylori play only a minor role in the development of childhood ITP [39]. Platelet responses after H. pylori eradication in children were highly variable and inconsistent among studies [18–21, 39–41]. In the subgroup analysis of the current study, children in the eradication group failed to reach statistical significance to show higher ORR than those in the control group (OR = 1.80, 95% CI: 0.88–3.65, P = 0.11). However, the subgroup analysis included only 4 studies with a small number of patients. Therefore, this finding seems not sufficient to determine the therapeutic efficacy of H. pylori eradication in childhood ITP. Larger randomized trials are necessary to confirm the beneficial role of H. pylori eradication in childhood ITP.
A number of questions regarding the eradication of H. pylori infection in ITP still remain to be resolved, including the difference of efficacy among countries, factors predicting the platelet response, and mechanisms responsible for therapeutic effect associated with H. pylori eradication [39]. There was a great variability in the platelet responses of H. pylori eradication between Western and Eastern series [23]. Studies from Japan tended toward better response rates (28%–100%) than those from USA, Spain, and Mexico (<20%). The reason for such variability among countries is not clear, but geographic differences in the epidemic H. pylori strains may, at least in part, account for the difference in the platelet responses. The frequency of CagA-positive strains varies among geographic locations. Eastern Asian H. pylori strains have been found as more pathogenic, correlating with an increased development of gastric adenocarcinoma in H. pylori-positive patients in East Asia [42, 43]. The majority of H. pylori strains detected in East Asia express CagA, whereas the proportion of CagA-positive H. pylori strains in Western countries was much lower [44]. Immune response to CagA protein may be associated with improved platelet count after H. pylori eradication in patients with ITP [7–9]. Takahashi et al. observed that the level of anti-CagA Ab in the platelet eluates declined in three patients showing CR after eradication [7]. Kodama et al. reported that reduction in the anti-CagA antibody titer after eradication therapy was significantly greater in responders than in nonresponders [9]. These findings suggest that anti-CagA Ab titer may be a biomarker to determine who is indicated for bacterium eradication among patients with ITP.
Our study has several inherent limitations that need to be discussed. First, this meta-analysis included a limited number of studies with a small sample size. Moreover, there were only two trials conducted in adults with ITP. Second, five studies were carried out in Asia and the remaining trial in South Africa. So the results might not be transferred to Caucasian populations. Third, the studies showed heterogeneity in the detection methods of H. pylori infection. Especially one study [17] adopted the serologic test which showed inferior sensitivity and specificity compared with other diagnostic methods [45]. Fourth, most studies had a low quality with Jadad score ≤ 2. Fifth, because of the paucity of data, we could not evaluate the difference in the increase of platelet count between the eradication group and control group. Finally, although the definition of CR was similar among studies, the threshold for PR varied a little.
In conclusion, our meta-analysis indicates that H. pylori eradication has a significant therapeutic effect in patients with ITP. This result suggests that the detection and eradication of H. pylori infection need to be considered in patients with chronic ITP. Taking into account the intrinsic limits in the design and sample size of the included studies, however, large randomized controlled trials are warranted to validate the therapeutic impact of H. pylori eradication in adults as well as children with chronic ITP.
Contributor Information
Hyun Joo Jang, Email: jhj1229@hallym.or.kr.
Jung Han Kim, Email: harricil@hallym.or.kr.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Neunert C. E. Current management of immune thrombocytopenia. Hematology. 2013;2013:276–282. doi: 10.1182/asheducation-2013.1.276. [DOI] [PubMed] [Google Scholar]
- 2.George J. N., Woolf S. H., Raskob G. E., et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88(1):3–40. [PubMed] [Google Scholar]
- 3.Neunert C., Lim W., Crowther M., et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 4.Suerbaum S., Michetti P. Helicobacter pylori infection. The New England Journal of Medicine. 2002;347(15):1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y.-I., Cho S.-J., Lee J. Y., et al. Effect of Helicobacter pylori eradication on long-term survival after distal gastrectomy for gastric cancer. Cancer Research and Treatment. 2016;48(3):1020–1029. doi: 10.4143/crt.2015.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasbarrini A., Franceschi F., Tartaglione R., Landolfi R., Pola P., Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. The Lancet. 1998;352(9131):p. 878. doi: 10.1016/S0140-6736(05)60004-9. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T., Yujiri T., Shinohara K., et al. Molecular mimicry by Helicobacter pylori CagA protein may be involved in the pathogenesis of H. pylori-associated chronic idiopathic thrombocytopenic purpura. British Journal of Haematology. 2004;124(1):91–96. doi: 10.1046/j.1365-2141.2003.04735.x. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi F., Christodoulides N., Kroll M. H., Genta R. M. Helicobacter pylori and idiopathic thrombocytopenic purpura. Annals of Internal Medicine. 2004;140(9):766–767. doi: 10.7326/0003-4819-140-9-200405040-00028. [DOI] [PubMed] [Google Scholar]
- 9.Kodama M., Kitadai Y., Ito M., et al. Immune response to CagA protein is associated with improved platelet count after Helicobacter pylori eradication in patients with idiopathic thrombocytopenic purpura. Helicobacter. 2007;12(1):36–42. doi: 10.1111/j.1523-5378.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 10.Stasi R., Rossi Z., Stipa E., Amadori S., Newland A. C., Provan D. Helicobacter pylori eradication in the management of patients with idiopathic thrombocytopenic purpura. The American Journal of Medicine. 2005;118(4):414–419. doi: 10.1016/j.amjmed.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Inaba T., Mizuno M., Take S., et al. Eradication of Helicobacter pylori increases platelet count in patients with idiopathic thrombocytopenic purpura in Japan. European Journal of Clinical Investigation. 2005;35(3):214–219. doi: 10.1111/j.1365-2362.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- 12.Veneri D., Krampera M., Franchini M. High prevalence of sustained remission of idiopathic thrombocytopenic purpura afterHelicobacter pylorieradication: A long-term follow-up study. Platelets. 2009;16(2):117–119. doi: 10.1080/09537100400015153. [DOI] [PubMed] [Google Scholar]
- 13.Rostami N., Keshtkar-Jahromi M., Rahnavardi M., Keshtkar-Jahromi M., Esfahani F. S. Effect of eradication of Helicobacter pylori on platelet recovery in patients with chronic idiopathic thrombocytopenic purpura: a controlled trial. American Journal of Hematology. 2008;83(5):376–381. doi: 10.1002/ajh.21125. [DOI] [PubMed] [Google Scholar]
- 14.Emilia G., Luppi M., Zucchini P., et al. Helicobacter pylori infection and chronic immune thrombocytopenic purpura: long-term results of bacterium eradication and association with bacterium virulence profiles. Blood. 2007;110(12):3833–3841. doi: 10.1182/blood-2006-12-063222. [DOI] [PubMed] [Google Scholar]
- 15.Kim H., CoOperative Study Group A for Hematology (COSAH), Lee W.-S., et al. Efficacy of Helicobacter pylori eradication for the 1st line treatment of immune thrombocytopenia patients with moderate thrombocytopenia. Annals of Hematology. 2015;94(5):739–746. doi: 10.1007/s00277-014-2268-9. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T., Matsushima M., Masui A., et al. Effect of Helicobacter pylori eradication in patients with chronic idiopathic thrombocytopenic purpura-a randomized controlled trial. The American Journal of Gastroenterology. 2005;100(6):1265–1270. doi: 10.1111/j.1572-0241.2005.41641.x. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsumi Y., Kanamori H., Yamato H., et al. Randomized study of Helicobacter pylori eradication therapy and proton pump inhibitor monotherapy for idiopathic thrombocytopenic purpura. Annals of Hematology. 2005;84(12):807–811. doi: 10.1007/s00277-005-1071-z. [DOI] [PubMed] [Google Scholar]
- 18.Li C. X., Liu D. J., Pan C. Q., Sang X. F., Li X. Effect of Helicobacter pylori eradication on childhood acute idiopathic thrombocytopenic purpura. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29(6):1243–1244. [PubMed] [Google Scholar]
- 19.Treepongkaruna S., Sirachainan N., Kanjanapongkul S., et al. Absence of platelet recovery following Helicobacter pylori eradication in childhood chronic idiopathic thrombocytopenic purpura: a multi-center randomized controlled trial. Pediatric Blood & Cancer. 2009;53(1):72–77. doi: 10.1002/pbc.21991. [DOI] [PubMed] [Google Scholar]
- 20.Tang Y., Wang S. C., Wang L. J., Liu Y., Wang H. Y., Wang Z. J. Clinical significance of Helicobacter pylori in children with idiopathic thrombocytopenic purpura. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21(2):419–421. doi: 10.7534/j.issn.1009-2137.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 21.Brito H. S. H., Braga J. A. P., Loggetto S. R., Machado R. S., Granato C. F. H., Kawakami E. Helicobacter pyloriinfection & immune thrombocytopenic purpura in children and adolescents: a randomized controlled trial. Platelets. 2015;26(4):336–341. doi: 10.3109/09537104.2014.911836. [DOI] [PubMed] [Google Scholar]
- 22.Franchini M., Cruciani M., Mengoli C., Pizzolo G., Veneri D. Effect of Helicobacter pylori eradication on platelet count in idiopathic thrombocytopenic purpura: a systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy. 2007;60(2):237–246. doi: 10.1093/jac/dkm195. [DOI] [PubMed] [Google Scholar]
- 23.Stasi R., Sarpatwari A., Segal J. B., et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood. 2009;113(6):1231–1240. doi: 10.1182/blood-2008-07-167155. [DOI] [PubMed] [Google Scholar]
- 24.Frydman G. H., Davis N., Beck P. L., Fox J. G. Helicobacter pylori eradication in patients with immune thrombocytopenic purpura: a review and the role of biogeography. Helicobacter. 2015;20(4):239–251. doi: 10.1111/hel.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D., Liberati A., Tetzlaff J., Altman D. G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine. 2009;151(4):264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 26.Panic N., Leoncini E., de Belvis G., Ricciardi W., Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One. 2013;8(12, article e83138) doi: 10.1371/journal.pone.0083138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jadad A. R., Moore R. A., Carroll D., et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 28.Wald N. J., Bestwick J. P. Presentation of meta-analysis plots. Journal of Medical Screening. 2015;22(1):49–51. doi: 10.1177/0969141314556490. [DOI] [PubMed] [Google Scholar]
- 29.Sterne J. A. C., Sutton A. J., Ioannidis J. P. A., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343(jul22 1, article d4002) doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 30.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stasi R. Immune thrombocytopenia: pathophysiologic and clinical update. Seminars in Thrombosis and Hemostasis. 2012;38(5):454–462. doi: 10.1055/s-0032-1305780. [DOI] [PubMed] [Google Scholar]
- 32.Stasi R., Willis F., Shannon M. S., Gordon-Smith E. C. Infectious causes of chronic immune thrombocytopenia. Hematology/Oncology Clinics of North America. 2009;23(6):1275–1297. doi: 10.1016/j.hoc.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Saito A., Yokohama A., Osaki Y., et al. Circulating plasmacytoid dendritic cells in patients with primary and Helicobacter pylori-associated immune thrombocytopenia. European Journal of Haematology. 2012;88(4):340–349. doi: 10.1111/j.1600-0609.2011.01745.x. [DOI] [PubMed] [Google Scholar]
- 34.Satoh K., Hirayama T., Takano K., et al. VacA, the vacuolating cytotoxin of Helicobacter pylori, binds to multimerin 1 on human platelets. Thrombosis Journal. 2013;11(1):p. 23. doi: 10.1186/1477-9560-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Z., Zhou J., Prsoon P., Wei X., Liu X., Peng B. Low expression of FCGRIIB in macrophages of immune thrombocytopenia-affected individuals. International Journal of Hematology. 2012;96(5):588–593. doi: 10.1007/s12185-012-1187-6. [DOI] [PubMed] [Google Scholar]
- 36.Kühne T., Imbach P. Management of children and adolescents with primary immune thrombocytopenia: controversies and solutions. Vox Sanguinis. 2013;104(1):55–66. doi: 10.1111/j.1423-0410.2012.01636.x. [DOI] [PubMed] [Google Scholar]
- 37.Ferrara M., Capozzi L., Russo R. Effect ofHelicobacter pylorieradication on platelet count in children with chronic idiopathic thrombocytopenic purpura. Hematology. 2013;14(5):282–285. doi: 10.1179/102453309X12473408860181. [DOI] [PubMed] [Google Scholar]
- 38.Franchini M., Veneri D. Helicobacter pylori infection and immune thrombocytopenic purpura: an update. Helicobacter. 2004;9(4):342–346. doi: 10.1111/j.1083-4389.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 39.Kuwana M. Helicobacter pylori-associated immune thrombocytopenia: clinical features and pathogenic mechanisms. World Journal of Gastroenterology. 2014;20(3):714–723. doi: 10.3748/wjg.v20.i3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russo G., Miraglia V., Branciforte F., et al. Effect of eradication of Helicobacter pylori in children with chronic immune thrombocytopenia: a prospective, controlled, multicenter study. Pediatric Blood & Cancer. 2011;56(2):273–278. doi: 10.1002/pbc.22770. [DOI] [PubMed] [Google Scholar]
- 41.Loffredo G., Marzano M. G., Migliorati R., et al. The relationship between immune thrombocytopenic purpura and Helicobacter pylori infection in children: where is the truth? European Journal of Pediatrics. 2007;166(10):1067–1068. doi: 10.1007/s00431-006-0344-4. [DOI] [PubMed] [Google Scholar]
- 42.Matsunari O., Shiota S., Suzuki R., et al. Association between Helicobacter pylori virulence factors and gastroduodenal diseases in Okinawa, Japan. Journal of Clinical Microbiology. 2012;50(3):876–883. doi: 10.1128/JCM.05562-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nature Reviews Cancer. 2004;4(9):688–694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki R., Shiota S., Yamaoka Y. Molecular epidemiology, population genetics, and pathogenic role of Helicobacter pylori. Infection, Genetics and Evolution. 2012;12(2):203–213. doi: 10.1016/j.meegid.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Logan R. P. H., Walker M. M. ABC of the upper gastrointestinal tract: epidemiology and diagnosis of Helicobacter pylori infection. BMJ. 2001;323(7318):920–922. doi: 10.1136/bmj.323.7318.920. [DOI] [PMC free article] [PubMed] [Google Scholar]