Abstract
Objective:
To assess the effects of both male and female body mass index (BMI), individually and combined, on IVF outcomes.
Design:
Prospective cohort study.
Setting:
University fertility center.
Patient(s):
All couples undergoing first fresh IVF cycles, 2005–2010, for whom male and female weight and height information were available (n=721 couples).
Intervention(s):
None.
Main Outcome Measure(s):
Embryologic parameters, clinical pregnancy, and live birth incidence.
Result(s):
The average male BMI among the study population was 27.5±4.8 kg/m2 (range, 17.3–49.3 kg/m2), while the average female BMI (n=721) was 25.2±5.9 kg/m2 (range, 16.2–50.7 kg/m2). Neither male nor female overweight (25–29.9 kg/m2), class I obese (30–34.9 kg/m2), or class II/III obese (≥35 kg/m2) status was significantly associated with fertilization rate, embryo score, or incidence of pregnancy or live birth compared with normal weight (18.5–24.9 kg/m2) status after adjusting for male and female age, partner BMI, and parity. Similar null findings were found between combined couple BMI categories and IVF success.
Conclusion(s):
Our findings support the notion that weight status does not influence fecundity among couples undergoing infertility treatment. Given the limited and conflicting research on BMI and pregnancy success among IVF couples, further research augmented to include other adiposity measures is needed.
Keywords: Body mass index, obesity, in vitro fertilization, pregnancy, live birth
The prevalence of obesity in the United States is at the highest level ever recorded, with approximately one-third of adults of peak reproductive age (20–39 year old) considered obese (body mass index [BMI] >30 kg/m2) (1). Although there has been substantial research pointing to overweight and obesity as major causes of chronic disease (2), other studies suggest that increased weight and adverse health do not necessarily follow a linear relationship, especially when taking cardiorespiratory fitness level into account (3). What remains to be determined, however, is the effect of male and female BMI on reproductive health outcomes, notably among couples seeking infertility treatment. Given that approximately 12% of couples seek out infertility services at some point during their reproductive years (4), the association between weight status and reproductive success among couples undergoing assisted reproductive technology (ART) warrants investigation.
There is general consensus that female obesity is associated with increased risk of infertility (5) and pregnancy complications (6, 7) among women attempting to conceive without medical assistance, but these associations are not as well agreed upon among women undergoing ART, specifically IVF (8, 9). Similarly, although excess body weight in men has been linked with infertility in several large, population-based studies (10–12), there have been no consistent findings regarding the effect of male obesity on IVF outcomes (13–18), with the exception of recent evidence linking lower clinical pregnancy rates among men undergoing testicular sperm extraction for nonobstructive azoospermia (19).
Given that reproductive success is dependent on the health of both members of the couple, there is a need to assess the combined effect of male and female BMI on pregnancy and live birth success among couples undergoing IVF. Of the two studies assessing the effect of both male and female BMI on incidences of pregnancy and live birth, one found no association (15), whereas the other concluded that male overweight/obesity was the biggest driver compared with female BMI for reduced pregnancy rates (16). Extrapolating from these studies is limited by the fact that BMI was dichotomized as either <25 or ≥25 kg/m2 in their analyses.
The primary objective of our study, therefore, was to assess the effects of both male and female BMI individually, and in combination, on fertilization rate, embryo score, and the incidences of clinical pregnancy and live births among couples undergoing IVF using prospectively collected clinical data.
MATERIALS AND METHODS
Study Sample
After obtaining approval through the University of Utah Institutional Review Board, we conducted a prospective cohort study of all couples undergoing their first fresh IVF cycles during 2005–2010 at the Utah Center for Reproductive Medicine, for whom male weight and height information were available. Men with nonobstructive azoospermia were excluded (n=135). Among the 735 men in the sample, 721 female partners (98%) had weight and height information available.
Body Mass Index Assessment
Data were abstracted from electronic medical records and patient medical charts. Upon presenting at the clinic for infertility evaluation, women’s height and weight were measured by medical assistants using standardized protocols as per clinical practice, whereas men self-reported their height and weight at this time.
Weight and height were used to calculate BMI according to the standard formula: BMI=weight/height2 (kg/m2). Men and women were divided into five groups according to the World Health Organization (WHO) classification cut-points: underweight (BMI <18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), class I obesity (30–34.9 kg/m2), and class II/III obesity (BMI R35 kg/m2). Because there was only one underweight man (BMI 17.3 kg/m2), we collapsed the lowest BMI category to <25 kg/m2. For analyses considering the association of couple BMI and pregnancy/live birth, we restricted the analyses to men and women with BMI ≥18.5 kg/m2 (n=688) and collapsed the class I with class II/III obesity categories (men, n=122 and 55, respectively; women, n=72 and 63, respectively), resulting in nine unique couple BMI combinations: [1] male normal weight/female normal weight, [2] male normal weight/female overweight, [3] male normal weight/female obese, [4] male overweight/female normal weight, [5] male overweight/female overweight, [6] male overweight/female obese, [7] male obese/female normal weight, [8] male obese/female overweight, and [9] male obese/female obese. A sensitivity analysis was done including underweight men (n=1) and women (n=32) and collapsing with respective normal-weight categories.
Covariate Assessment
Both members of the couple also completed questionnaires on reproductive history. These forms were supplemented by physician intake notes that recorded a comprehensive medical history, with information collected focusing on infertility and any previous treatments for infertility. Parity was defined as the number of deliveries for the female partner before treatment cycle that resulted in a birth at ≥20 weeks’ gestational age. Although lifestyle information was not collected in our electronic medical records, we randomly sampled 70 men and women to abstract data on alcohol (yes/no), daily exercise (yes/no), caffeine (caffeinated beverages per day), and smoking (yes/no) from patients’ paper-based health history forms.
After the initial consultation, women underwent an infertility evaluation that included a physical examination; a 1-month menstrual cycle diary with a urine ovulation predictor kit provided to detect the LH surge; uterine/oviduct imaging; and biochemical testing to document ovarian reserve and occurrence of ovulation and to rule out other endocrine and structural abnormalities. Men were evaluated for infertility by providing a fresh semen sample for measurement of sperm concentration, motility, morphology, and penetration. Information on male androgenic axis or testis size was not collected for our study sample. Utah Center for Reproductive Medicine physicians recorded reason(s) for IVF treatment using the following infertility diagnosis options: male factor, endometriosis, ovulation disorder, diminished ovarian reserve, tubal disease, or uterine disorder. More than one infertility diagnosis could be recorded for each couple.
IVF Outcome Assessment
Couples underwent IVF with conventional insemination or with intracytoplasmic sperm injection (ICSI), as clinically indicated. Embryologists classified oocytes as germinal vesicle, metaphase I, metaphase II, atretic, or empty zona. Oocytes exposed to sperm that subsequently showed two pronuclei were deemed fertilized. Embryos were then scored per standard protocol at the time of transfer (20), with the highest score reported. The majority were scored at day 5 (n=528, 72%) compared with day 3 (n=182, 25%); 3% of embryos (n=25) were missing information on day of transfer. Scores ranged from 3.5 to 24 (mean±SD, 14.7±3.2) for day-5 embryos and from 1 to 18 (mean±SD, 9.7±3.4)for day-3 embryos, with higher numbers reflecting improved embryo quality. Clinical pregnancy was defined by the presence of one or more gestational sacs confirmed on ultrasound, and/or clinical recording of fetal heart tones, or documentation of a birth, spontaneous abortion, or therapeutic abortion in cases of missing ultrasound data. Live birth was defined as the birth in which at least one fetus was live-born.
Statistical Analyses
Descriptive statistics of the study population, including age, reproductive history, and infertility diagnosis were compared among categories of male (n=735) and female BMI (n=721). We assessed differences using analysis of variance for continuous variables and Fisher’s exact tests for categorical variables. We examined the possibly nonlinear relationship between male and female BMI and clinical pregnancy and live birth success nonparametrically with restricted cubic splines, adjusting for male and female age, partner BMI, and parity (21). Tests for nonlinearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms.
We used generalized linear models for estimating the association between male BMI, female BMI, and combined couple BMI categories and continuous embryologic parameters. Relative risk (RR) by Poisson regression with robust standard error variance was used to estimate BMI category and pregnancy and live birth incidence (22). If there was no evidence of nonlinearity between either male or female BMI and incidence of clinical pregnancy or live birth in cubic spline analyses, we ran our RR models with BMI as a continuous variable.
Models were adjusted for available potential confounders that were selected a priori and included factors known to be associated with BMI and clinical pregnancy or live birth success in the IVF population, and that were not along the causal pathway (23). We used the DAGitty program to determine the minimal sufficient adjustment set (24). Individual assessments of male or female BMI on outcomes were adjusted for male and female age, parity, and partner BMI, whereas for analyses considering the association of couple BMI categories and pregnancy/live birth, we only adjusted for male and female age and parity because partner BMI was incorporated into the couple BMI category. Our male BMI models did not stratify or adjust by fertilization method (conventional insemination vs. ICSI) or by infertility diagnosis (male factor) because we considered these factors to be along the causal pathway from BMI to pregnancy/live birth outcome. For our female BMI models, we did conduct a sensitivity analysis additionally adjusting for reason for infertility treatment (endometriosis, ovulation disorders, diminished ovarian reserve, tubal disease, uterine disorders, or unexplained infertility) because although these factors are also most likely on the causal pathway between BMI and pregnancy success, it is possible that they are additionally or alternatively common causes of both BMI and pregnancy success, and thus potential confounders. An additional sensitivity analysis was conducted on all models additionally adjusting for male and female alcohol (yes/no), daily exercise (yes/no), caffeine (caffeinated beverages per day), and smoking (yes/no) for the random sub-sample (n=70) containing lifestyle information. Analyses were performed using SAS version 9.4 (SAS Institute).
RESULTS
The average male BMI among the study population was 27.5±4.8 kg/m2 (median [interquartile range], 26.5 [24.4–29.8]; range, 17.3–49.3), whereas the average female BMI (n=721) was 25.2±5.9 kg/m2 (median [IQR], 23.4 [21.0–27.6]; range, 16.2–50.7). Among the random subsample of 70 men and women for whom we abstracted lifestyle information, 38% of men and 31% of women reported any alcohol consumption, 78% of men consumed caffeinated beverages with an average (SD) of 1.8 (1.1) cups per day, 49% of women consumed caffeinated beverages with an average of 1.7 (1.2) cups per day, 71% of men and 86% of women reported being daily exercisers, and 4% of men and 3% of women reported smoking.
Average female BMI across male BMI categories showed a positive association (P<.001), as did average male BMI across female BMI categories (P<.001) (Table 1). In addition, female BMI was also positively associated with male and female age (P=.05). In regard to infertility diagnoses, although there were no significant differences in the proportion of couples with an infertility diagnosis that included male factor infertility among male BMI categories (P=.63), higher female BMI category was associated with a higher proportion of ovulation disorders and tubal disease diagnoses (P<.001 and P=.02, respectively). Although female BMI was also associated with an idiopathic (P=.02) and “other diagnosis” (e.g., preimplantation genetic diagnosis) (P=.002), there were no clear linear trends across BMI categories.
TABLE 1.
Participant characateristics by male BMI (n=735) and female BMI (n=721).
| Male BMI (kg/m2) (n = 735) | Female BMI (kg/m2) (n = 721) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | ≤24.9, n = 224a (30.3%) | 25–29.9, n = 334 (45.4%) | 30–34.9, n = 122 (16.6%) | ≥35, n = 55 (7.5%) | P value | <18.5, n = 32 (4.4%) | 18.5–24.9, n = 407 (56.5%) | 25–29.9, n = 147 (20.4%) | 30–34.9, n = 72 (10.0%) | ≥35, n = 63 (8.7%) | P value |
| Demographics/reproductive history | |||||||||||
| Male age (y) | 33.9 ± 6.0 | 34.8 ± 5.8 | 34.2 ± 5.2 | 34.7 ± 5.7 | .24 | 32.2 ± 5.9 | 34.4 ± 5.6 | 34.0 ± 6.5 | 35.4 ± 5.2 | 35.4 ± 5.7 | .05 |
| Female age (y) | 32.1 ± 5.0 | 32.5 ± 4.8 | 32.6 ± 4.7 | 32.6 ± 4.3 | .65 | 30.4 ± 4.8 | 32.3 ± 4.8 | 32.2 ± 4.7 | 33.2 ± 4.0 | 33.1 ± 4.8 | .05 |
| Female BMI (kg/m2) | 23.4 ± 4.2 | 24.6 ± 5.5 | 27.1 ± 6.0 | 31.5 ± 7.8 | < .001 | 26.0 ± 4.6 | 26.4 ± 3.7 | 27.8 ± 4.6 | 30.5 ± 6.1 | 31.3 ± 6.0 | < .001 |
| Prior ART, n (%) | 45 (20.1) | 52 (15.6) | 21 (17.2) | 8(14.6) | .53 | 5(15.6) | 69(17.0) | 23 (15.7) | 16 (22.2) | 8(12.7) | .65 |
| Parity, n (%) | 48 (21.5) | 91 (27.3) | 26(21.3) | 20 (36.4) | .07 | 3 (9.4) | 109 (26.8) | 32 (21.8) | 19 (26.4) | 18(28.6) | .19 |
| Reason for ART selectionb, n (%) | |||||||||||
| Male factor (n = 388, 52.8%) | 119(53.1) | 169 (50.6) | 68 (55.7) | 32 (58.2) | .63 | ||||||
| Endometriosis (n = 129, 17.9%) | 8 (25.0) | 76(18.7) | 27 (18.4) | 14(19.4) | 4 (6.4) | .13 | |||||
| Ovulation disorders (n = 109, 15.1%) | 1 (3.1) | 46(11.3) | 18 (12.2) | 23 (31.9) | 21 (33.3) | < .001 | |||||
| Diminished ovarian reserve (n = 87, 12.1%) | 5(15.6) | 52 (12.8) | 15 (10.2) | 10 (13.9) | 5 (7.9) | .68 | |||||
| Tubal disease (n = 79, 11.0%) | 1 (3.1) | 35 (8.6) | 20 (13.6) | 10 (13.9) | 13 (20.6) | .02 | |||||
| Uterine (n = 14, 1.9%) | 1 (3.1) | 6(1.5) | 3 (2.0) | 2 (2.8) | 2(3.2) | .83 | |||||
| Idiopathic/unexplained (n = 75, 10.4%) | 2 (6.3) | 56(13.8) | 11 (7.5) | 3 (4.2) | 3 (4.8) | .02 | |||||
| Otherc (n = 39, 5.4%) | 6 (18.8) | 22 (5.4) | 6(4.1) | 0 | 5 (8.7) | .002 | |||||
Note: Analyses conducted using analysis of variance and exact χ2 tests. Values reported as mean±SD unless indicated otherwise. There were 721 couples for whom we have both male and female BMI information.
Includes one mn with BMI <18.5 kg/m2.
Couples can have more than one infertility diagnosis. Three couples are missing infertility diagnosis.
Includes advanced maternal age, preimplantation genetic diagnosis, and other comorbidities, such as breast cancer.
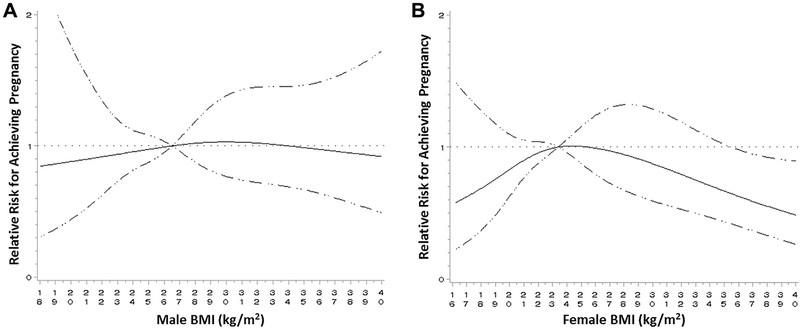
Using restricted cubic spline regression, there was no evidence of nonlinearity between male BMI and incidence of clinical pregnancy or live birth (P=.85 and .93, respectively) after adjusting for male and female age, parity, and partner BMI (Fig. 1). Female BMI, although also nonstatistically significant, trended toward nonlinearity in its relationship with incidence of pregnancy and live birth (P=.11 and .25, respectively), with lower pregnancy/live birth incidence among women with high or low BMI.
FIGURE 1.
Restricted cubic spline regression assessing nonlinear adjusted relationship between male or female BMI and IVF outcome. Adjusted for male and female age, partner BMI, and parity. Dotted line=null relationship, relative risk=1; solid line: spline graph with 4 knot points, reference value is median male (A) and female (B) BMI; dashed line: 95% confidence interval.
Neither male nor female BMI status alone was associated with fertilization rate, embryo score, or pregnancy/live birth outcomes after adjusting for male and female age, partner BMI, and parity (Table 2). Additional adjustment for reason for infertility treatment in the female BMI models did not appreciably alter findings (data not shown). Continuous assessment of male BMI on IVF outcomes mirrored the null categorical findings, with a 1.00 (95% confidence interval [CI] 0.99–1.02) and 0.99 (95% CI 0.98–1.01) adjusted RR (aRR) of pregnancy or live birth with each unit increase in BMI.
TABLE 2.
IVF and ICSI treatment outcomes by male BMI (n=735) and female BMI (n=721).
| Male BMI (kg/m2) (n = 735) | Female BMI (kg/m2) (n = 721) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | ≤ 24.9, n = 224a (30.5) | 25–29.9, n = 334 (45.4) | 30–34.9, n = 122 (16.6) | ≥35, n = 55 (7.5) | <18.5, n = 32 (4.4) | 18.5–24.9, n = 407 (56.5) | 25–29.9, n = 147 (20.4) | 30–34.9, n = 72 (10.0) | ≥35, n = 63 (8.7) |
| Fertilization rate, mean (95% Cl) | 89(87–91) | 91 (90–93) | 94 (91–97) | 88 (84–92) | 88 (83–93) | 92 (90–93) | 91 (88–93) | 88 (84–92) | 90 (86–94) |
| Embryo score (day 5), mean (95% Cl) | 14.7 (14.2–15.2) | 14.6 (14.2–15.0) | 15.1 (14.4–15.8) | 15.0(14.0–16.1) | 15.5 (14.1–17.0) | 14.6(14.2–15.0) | 15.3(14.6–15.9) | 13.7 (12.8–14.6) | 15.0(13.9–16.0) |
| Pregnancy | |||||||||
| n (%) | 111 (49.6) | 189 (56.6) | 67 (54.9) | 28(50.9) | 17 (53.1) | 225 (55.3) | 89 (60.5) | 33 (45.8) | 27 (42.9) |
| aRR (95% Cl) | 1.0 | 1.15 (0.98–1.34) | 1.15 (0.94–1.42) | 1.08 (0.80–1.46) | 0.92 (0.66–1.28) | 1.0 | 1.10(0.94–1.28) | 0.86 (0.67–1.12) | 0.80(0.59–1.08) |
| Live birth | |||||||||
| n (%) | 103 (46.2) | 169 (50.6) | 58 (47.5) | 25 (46.3) | 17 (53.1) | 199 (49.0) | 83 (56.5) | 28 (39.4) | 24 (38.1) |
| aRR (95% Cl) | 1.0 | 1.11 (0.93–1.32) | 1.07 (0.85–1.36) | 1.07 (0.76–1.49) | 1.02 (0.73–1.43) | 1.0 | 1.16(0.98–1.38) | 0.87 (0.64–1.17) | 0.83 (0.59–1.17) |
Note: Analyses conducted using generalized linear models for fertilization rate and embryo score (reporting least-squares mean and 95% confidence interval) and Poisson regression with robust error variance for pregnancy and live birth (reporting frequency [%] and relative risk and 95% confidence interval). All models adjusted for male and female age, partner BMI, and parity. Missing one observation for fertility rate and two observations for live birth. Day-5 embryo score includes the 528 couples (72%) who had a day-5 embryo score.
Includes one man with BMI <18.5 kg/m2.
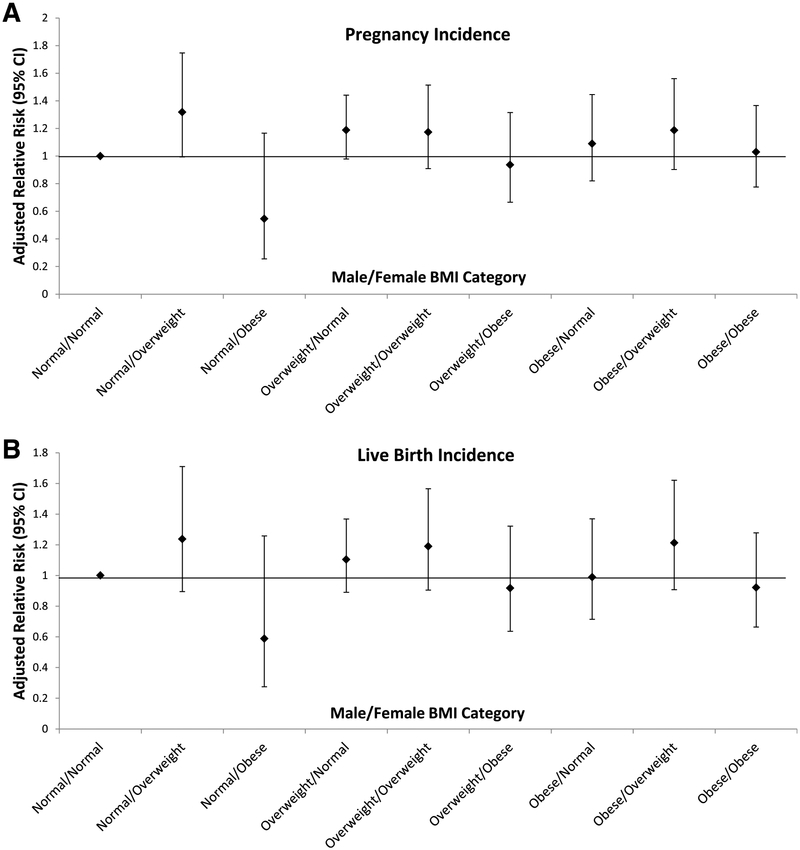
When examining couple BMI categories, we found no associations between couple BMI and pregnancy incidence or live birth after adjusting for male and female age and parity. There was a slight indication that normal-weight men partnered with overweight women had increased pregnancy incidences (aRR 1.32, 95% CI 0.99–1.75, and aRR 1.24, 95% CI 0.89–1.71, respectively) compared with the reference of normal-weight men partnered with normal-weight women, although the 95% CI included the null (Fig. 2). Expanding our inclusion of normal weight to include the 1 underweight male and 37 under-weight women did not appreciably change our estimates. Additionally, although female smoking was a significant covariate in the subsample final model (P=.02), adjusting for it or male and female exercise, caffeine and alcohol intake, or male smoking did not significantly alter findings.
FIGURE 2.
Combined effect of male and female BMI category on pregnancy (A) and live birth (B) success (n=689 couples). Normal-weight men/normal-weight women (n=147); normal-weight men/overweight women (n=38); normal-weight men/obese women (n=20); overweight men/normal-weight women (n=200); overweight men/overweight women (n=67); overweight men/obese women (n=48); obese men/normal-weight women (n=60); obese men/overweight women (n=42); obese men/obese women (n=67). Adjusted for male and female age and parity.
DISCUSSION
This is the second-largest study to date, and the largest among a US population, to assess the individual and joint effects of male and female BMI on incidences of pregnancy and live birth after ART treatments. Overall we found no significant associations between male, female, or couple BMI and fertilization rate, embryo score, clinical pregnancy, or live birth after adjustment for relevant confounders. Although evidence has shown that obesity is associated with increased chronic disease risk (2), meta-analyses have reported a null if not protective effect for morbidity and mortality among overweight individuals (1). Findings from our study among couples seeking infertility treatment corroborate these null effects, with overweight status showing no adverse effects on fertility outcomes. Additionally, although male and female obesity has been linked to decreased fertility among couples not undergoing medical treatment for infertility (5, 10–12), our findings indicate no significant associations between obesity and pregnancy or live birth success.
Our finding of no significant associations with male obesity and success of pregnancy or live birth is in agreement with the largest population-based study to date (15). Petersen et al. found no significant associations with overweight (25.0–29.9 kg/m2) or obese (≥30 kg/m2) men compared with normal-weight men and odds of live birth, overall or stratified by fertilization method, among a population-based cohort study of 1906 couples after adjusting for male age and smoking status. Similarly, Colaci et al. (14) found no significant associations between overweight/obese men compared with normal-weight men in a prospective cohort study of 114 couples. In contrast, Keltz et al. (13) found that higher male BMI≥25 kg/m2 was associated with decreased odds of pregnancy after conventional insemination, but not ICSI, among 290 couples; Bakos et al. (18) found a linear reduction in both pregnancy rate and live birth rate with increasing BMI among 305 couples; and Umul et al. (17) found significantly decreased clinical pregnancy and live birth rates with increasing paternal BMI among 155 couples undergoing ICSI. Comparison between our study and these latter three studies is difficult given the influence of random error, different BMI categorization methods, lack of clarity as to whether males undergoing testicular sperm extraction for nonobstructive azoospermia were included, and/or these studies’ limited ability to adjust for confounding factors, including female BMI.
In support of our null findings on the effect of female BMI on pregnancy/live birth, a recent systematic review of 27 primarily retrospective studies found that pooled odds ratios for overweight vs. normal weight on clinical and ongoing pregnancy and live birth rates after ART were 0.94 (95% CI 0.69–1.30), 1.01 (95% CI 0.75–1.40), and 0.90 (95% CI 0.82–1.00), respectively (9). The authors concluded that higher female BMI only marginally reduces success rates, but further evidence, specifically via prospective studies that can account for potential confounding (such as male BMI), is needed. Although overweight women conceiving without medical intervention have been shown to be at increased risk for pregnancy complications (6, 7), women undergoing IVF are more closely monitored for pregnancy complications and thus not directly comparable.
Assessment of both male and female factors thought to have an important role in achieving pregnancy success, such as BMI, is particularly relevant for IVF research, in which the unit of treatment is the couple rather than the individual. Our study was able to fill an important gap in the current literature by looking at the combined effect of male and female BMI in a clinic population with 177 (24%) class I/II obese men and 135 (19%) class I/II obese women. In agreement with the largest study to date of 1906 Danish couples (15), we found no significant associations between couple BMI and pregnancy or live birth incidence after adjustment for important confounders, such as male and female age. Our and Petersen et al.’s findings are in contrast to those of Anfandis et al., who found increased pregnancy incidence among normal-weight men, regardless of the BMI of their partner, compared with overweight men (16); however, this study of 301 Greek couples did not account for any important potential confounding factors, such as male and female age or parity.
Several studies assessing the impact of obesity on adverse health outcomes, including stroke, myocardial infarction, heart failure, renal disease, and diabetes, have shown a nonlinear vs. linear relationship with overweight status conferring a protective effect in normal populations (3, 25). Although our restricted cubic spline regression failed to show a nonlinear relationship with male BMI and pregnancy/live birth success, a suggested inverted J-shape curve was observed for female BMI. Although previous research on relatively large cohorts has suggested that female BMI and IVF outcome is of an inverted U or J shape (26, 27), this has not been consistently found (28). Future appropriately powered research should be conducted, looking at the individual and joint effects of adiposity on IVF outcomes, expanded to other body composition assessment tools beyong BMI, and that take into account cardiorespiratory fitness level (3).
Our study had several strengths, including a relatively large sample size, prospective assessment, with both male and female BMI obtained before IVF treatment, and the evaluation of fertilization rate and embryo score in addition to pregnancy and live birth rates. However, our study had a number of limitations, including not being able to take into account lifestyle factors for our entire sample, such as smoking status, that could influence overall fitness. With such low smoking prevalence found in our subsample, however, we do not believe confounding by smoking is a major source of bias in our study; our study population was drawn from a relatively healthy population compared with the national average (29). Regardless, given the association between smoking and BMI and the body of evidence showing its adverse effects on IVF outcomes (30), future studies are advised to include valid and reliable measurements of smoking in addition to other confounding lifestyle factors when assessing the effect of BMI on IVF outcomes.
Additionally, similar to the other previous studies conducted to date on couples’ overweight status and IVF success, we were restricted in our assessment of adioposity to BMI. Furthermore, whereas female BMI was measured using standardized instruments for height and weight, male BMI was self-reported. The use of BMI to assess adiposity, vs. waist-to-hip ratio or percent body fat, has been challenged (31), and epidemiologic studies assessing the impact of obesity on mortality that do not take into account fitness have been criticized (3). Although we were limited in the number of WHO class II/III obese men and women (i.e., BMI >35 kg/m2), only one other (18) of the previous seven studies (12–17) assessing male BMI and IVF outcomes distinguished between WHO class I and class II/III obese categories. Future work appropriately powered to assess the impact of morbid obesity on IVF outcomes is needed. Finally, given that Utah is predominately non-Hispanic white (76.5%), the generalizability of our findings among other races/ethnicities is limited, particularly because recent evidence indicates significant racial/ethnic disparities in IVF outcomes (32).
In summary, we found no overall association of male and female BMI, individually or in combination, with IVF success after taking into account several important confounding factors, such as male and female age, partner BMI, and parity. Our finding of the marginal improvement in pregnancy success with a normal-weight male partnered with an overweight female should be interpreted with caution but suggests that future research is needed to assess the relationship between male and female adiposity, augmented to include other adiposity measures in addition to BMI, and reproductive success among infertile couples.
Acknowledgments:
The authors thank Radhika Deshmukh, M.B.B.S., and Becky Crockett, B.S., for their assistance with patient chart abstraction and database management.
Supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health, Rockville, Maryland (K.C.S., S.L.M., K.A.A., S.N.H., and K.K.).
Footnotes
K.C.S. has nothing to disclose. S.L.M. has nothing to disclose. K.A.A. has nothing to disclose. J.M.H. has nothing to disclose. D.T.C. has nothing to disclose. M.L. has nothing to disclose. S.N.H. has nothing to disclose. K.K. has nothing to disclose. C.A.P. has nothing to disclose. A.O.H. has nothing to disclose.
Discuss: You can discuss this article with its authors and with other ASRM members at http://fertstertforum.com/schliepk-male-female-bmi-pregnancy-live-birth-ivf/
REFERENCES
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307:491–7. [DOI] [PubMed] [Google Scholar]
- 2.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAuley PA, Blair SN. Obesity paradoxes. J Sports Sci 2011;29:773–82. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. American Society for Reproductive Medicine, Society for Assisted Reproductive Technology 2011 Assisted reproductive technology fertility clinic success rates report. Atlanta, GA: US Dept of Health and Human Services; 2013. [Google Scholar]
- 5.Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction 2010;140:347–64. [DOI] [PubMed] [Google Scholar]
- 6.Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA 2009;301:636–50. [DOI] [PubMed] [Google Scholar]
- 7.Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA 2014;311:1536–46. [DOI] [PubMed] [Google Scholar]
- 8.Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online 2011;23:421–39. [DOI] [PubMed] [Google Scholar]
- 9.Koning AM, Mutsaerts MA, Kuchenbecker WK, Kuchenbecher WK, Broekmans FJ, Land JA, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women. Hum Reprod 2012; 27:457–67. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen RH, Wilcox AJ, Skjaerven R, Baird DD. Men’s body mass index and infertility. Hum Reprod 2007;22:2488–93. [DOI] [PubMed] [Google Scholar]
- 11.Sallmén M, Sandler DP, Hoppin JA, Blair A, Baird DD. Reduced fertility among overweight and obese men. Epidemiology 2006;17:520–3. [DOI] [PubMed] [Google Scholar]
- 12.Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sørensen TI, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod 2007;22: 1634–7. [DOI] [PubMed] [Google Scholar]
- 13.Keltz J, Zapantis A, Jindal SK, Lieman HJ, Santoro N, Polotsky AJ. Overweight men: clinical pregnancy after ART is decreased in IVF but not in ICSI cycles. J Assist Reprod Genet 2010;27:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colaci DS, Afeiche M, Gaskins AJ, Wright DL, Toth TL, Tanrikut C, et al. Men’s body mass index in relation to embryo quality and clinical outcomes in couples undergoing in vitro fertilization. Fertil Steril 2012;98:1193–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen GL, Schmidt L, Pinborg A, Kamper-Jørgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril 2013;99:1654–62. [DOI] [PubMed] [Google Scholar]
- 16.Anifandis G, Dafopoulos K, Messini CI, Polyzos N, Messinis IE. The BMI of men and not sperm parameters impact on embryo quality and the IVF outcome. Andrology 2013;1:85–9. [DOI] [PubMed] [Google Scholar]
- 17.Umul M, Köse SA, Bilen E, Altuncu AG, Oksay T, Güney M. Effect of increasing paternal body mass index on pregnancy and live birth rates in couples undergoing intracytoplasmic sperm injection. Andrologia. 2014. April 9 10.1111/and.12272. [DOI] [PubMed] [Google Scholar]
- 18.Bakos HW, Henshaw RC, Mitchell M, Lane M. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil Steril 2011;95: 1700–4. [DOI] [PubMed] [Google Scholar]
- 19.Ramasamy R, Bryson C, Reifsnyder JE, Neri Q, Palermo GD, Schlegel PN. Overweight men with nonobstructive azoospermia have worse pregnancy outcomes after microdissection testicular sperm extraction. Fertil Steril 2013;99:372–6. [DOI] [PubMed] [Google Scholar]
- 20.Aoki VW, Wilcox AL, Peterson CM, Parker-Jones K, Hatasaka HH, Gibson M, et al. Comparison of four media types during 3-day human IVF embryo culture. Reprod Biomed Online 2005;10:600–6. [DOI] [PubMed] [Google Scholar]
- 21.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 22.Zou G A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 23.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia: Lippencott Williams & Wilkins; 2008. [Google Scholar]
- 24.Textor J, Hardt J, Knüppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology 2011;22:745. [DOI] [PubMed] [Google Scholar]
- 25.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JX, Davies MJ, Norman RJ. Obesity increases the risk of spontaneous abortion during infertility treatment. Obes Res 2002;10:551–4. [DOI] [PubMed] [Google Scholar]
- 27.Winter E, Wang J, Davies MJ, Norman R. Early pregnancy loss following assisted reproductive technology treatment. Hum Reprod 2002;17:3220–3. [DOI] [PubMed] [Google Scholar]
- 28.Fedorcsák P, Dale PO, Storeng R, Ertzeid G, Bjercke S, Oldereid N, et al. Impact of overweight and underweight on assisted reproduction treatment. Hum Reprod 2004;19:2523–8. [DOI] [PubMed] [Google Scholar]
- 29.United Health Foundation. America’s health rankings. 2014. Available at: www.americashealthrankings.org. Accessed August 27, 2014.
- 30.Homan GF, Davies M, Norman R. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: a review. Hum Reprod Update 2007;13:209–23. [DOI] [PubMed] [Google Scholar]
- 31.Ashwell M, Hsieh SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr 2005;56:303–7. [DOI] [PubMed] [Google Scholar]
- 32.Wellons MF, Fujimoto VY, Baker VL, Barrington DS, Broomfield D, Catherino WH, et al. Race matters: a systematic review of racial/ethnic disparity in Society for Assisted Reproductive Technology reported outcomes. Fertil Steril 2012;98:406–9. [DOI] [PMC free article] [PubMed] [Google Scholar]