Abstract
Context:
Despite support for active surveillance (AS) as a first treatment choice for men with low-risk prostate cancer (PC), this strategy is largely underutilized.
Objective:
To systematically review barriers and facilitators to selecting and adhering to AS for low-risk PC.
Evidence Acquisition:
We searched PsychINFO, PubMed, Medline 2000-now, Embase, CINAHL and Cochrane Central databases between 2002–2017 using the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement. The Purpose, Respondents, Explanation, Findings and Significance (PREFS) and STROBE quality criteria were applied. Forty-seven studies were identified.
Evidence Synthesis:
Key themes emerged as factors influencing both choice and adherence to AS: (1) patient- and tumour factors (age, co-morbidities, knowledge, education, socioeconomic status, family history, grade, tumour volume, fear of progression/side-effects); (2) family and social support; (3) provider (specialty, communication, attitudes); (4) healthcare organisation (geography, type of practice) and (5) health policy (guidelines, year, awareness).
Conclusion:
Many factors influence men’s choice and adherence to AS on multiple levels. It is important to learn from the experience of other chronic health conditions as well as from institutions/countries that are making significant headway in appropriately recruiting men to AS protocols; through standardised patient information, clinician education and nationally agreed guidelines, to ultimately decrease heterogeneity in AS practice.
Patient Summary:
We reviewed the scientific literature for factors affecting men’s choice and adherence to AS for low-risk PC. Our findings suggest that the use of AS could be increased by addressing a variety of factors such as information, psychosocial support, clinician education and standardised guidelines.
Keywords: active surveillance, treatment choice, adherence, prostate cancer, facilitators, barriers, treatment selection, chronic disease adherence
1.0. Introduction
Prostate cancer (PC) accounts for 400,000 new cancer cases in Europe [1] and 160,000 in the US [2] annually. Rapid uptake of prostate specific antigen (PSA) testing and better diagnostic procedures have led to a significant stage migration with earlier diagnosis of localized, low-risk PC (LRPC), ranging from 10% - 80% of all men diagnosed with PC worldwide [3–5]. A large proportion of these men do not require immediate radical treatment, but can be monitored using blood tests, digital rectal examination, prostate biopsy and/or multi-parametric magnetic resonance imaging (MRI) - an approach known as active surveillance (AS) [5].
While there are no universally agreed upon selection criteria for AS, the authors of a recent review of currently used guidelines worldwide agreed on the following criteria, consistent with the definition of very LRPC: clinical stage T1c-T2a; PSA <10 ng/mL; biopsy Gleason score 6; maximum 1 or 2 positive biopsy cores; and/or maximum 50% of cores with cancer [6].
Large cohort studies (appendix 1) reporting over the last 5 years have shown little physical morbidity and low PC specific mortality whilst on AS: 0.1% to 5.7% over 10 – 15 years [7, 8], observations which have recently contributed to an increased uptake of this management strategy [5, 9].
AS uptake continues to vary across countries, practices and amongst physicians [10]. This was most noticeable in the US CaPSURE database, which reported a sharp rise in the uptake of AS, from 10% over the past two decades to 40% in 2010–2013 [5] and the Swedish National Prostate Cancer Register which noted a rise from 40% to 74% between 2009 and 2014 [11]. In Australia, where the healthcare culture is fairly evenly split between private and public systems, a 25% overall recruitment to AS was recorded by the Victorian PC Registry during the period 2008–2012 [12]. However, in Sweden, where healthcare is delivered largely by the public sector, the proportion of men selecting AS was significantly higher (80–90% of eligible men) [11]. Understanding the drivers for this variation in practice is essential.
In cohort studies reporting on AS adherence, a large proportion of men continue to drop out of AS, despite no evidence of disease progression (Appendix 1). Much research has focused on the influence of anxiety and depression on adherence. Cancer Research UK describes depression as an established response to a diagnosis of cancer, unrelated to stage or severity [13]. However, in PC the risk of moderate to severe depression (requiring treatment) has been reported as relatively low in comparison to other tumour groups, at 5% [14].
There is thus a need to identify and understand the barriers and facilitators to AS. This would then provide means for future research themes to study interventions aimed at increasing both the uptake of and adherence to AS. The purpose of this paper is therefore to systematically evaluate the literature for factors affecting choice and adherence to AS as a PC management strategy for LRPC.
2.0. Evidence acquisition
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [15].
2.1. Search strategy
Studies published between 2002 (when AS was first described in the literature [16]and December 2017 were identified through a systematic search of electronic databases (PsychINFO, PubMed, Medline 2000-now, Embase, CINAHL and Cochrane Library) (Figure 1). The search strategy focused on the use of keyword search terms to identify studies based on PC AS: Prostate cancer OR prostatic neoplasm, active surveillance OR watchful waiting, facilitators OR barriers, treatment adherence OR treatment compliance, treatment OR therapy OR therapeutics and decision making. The full search strategy is identified in Figure 2. References were also searched for eligible publications.
Figure 1.
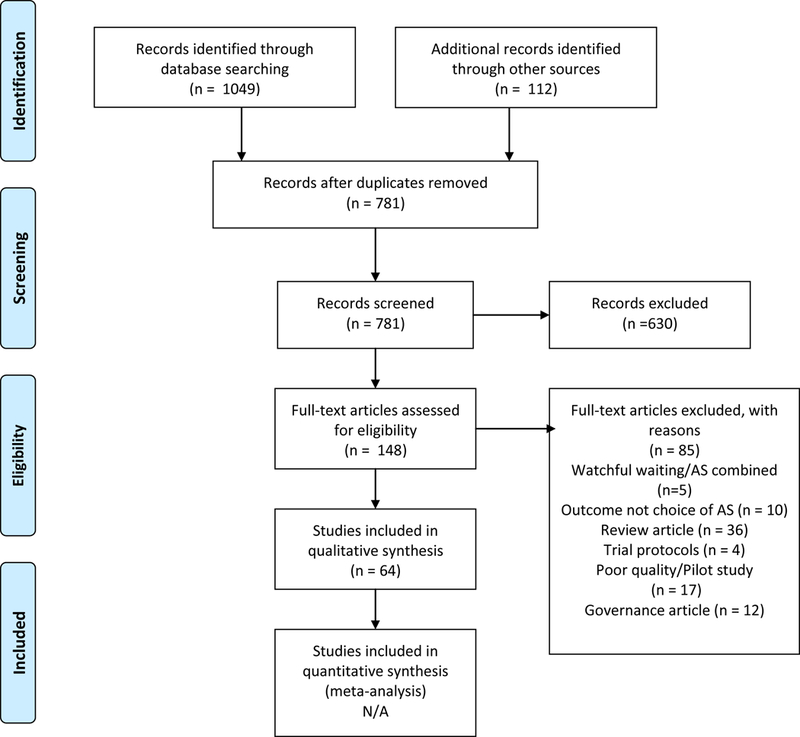
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram
Figure 2. –
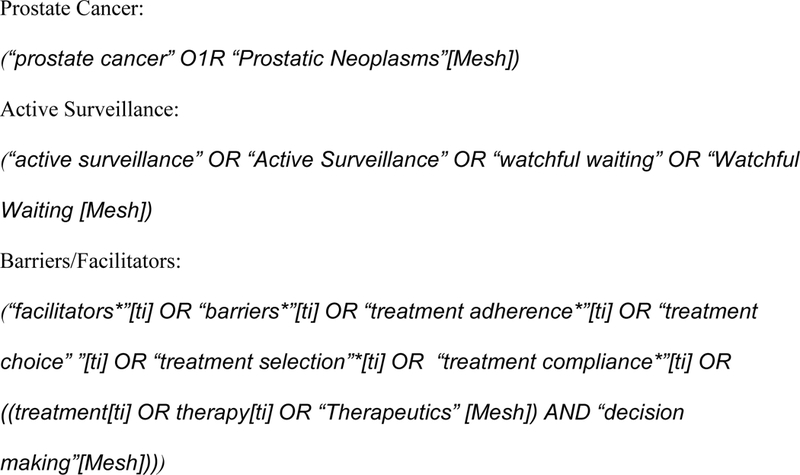
Search Strategy
2.2. Study eligibility and selection
Eligible studies for inclusion in the final analysis were those that evaluated choice and/or adherence to AS rather than watchful waiting (WW). Although there are similarities between choice of AS and WW, they are conceptually different management strategies (AS is a strategy employed to monitor a patient where there is intention to offer radical treatment with curative intent when/if required, WW implies no intention to offer curative treatment). Hence, studies where AS and WW sub-groups were combined were excluded to reduce bias.
We considered studies eligible if they were original articles with a qualitative or quantitative design generating data on decision-making in LRPC when AS was considered as a primary treatment option. Eleven studies were excluded on the basis of poor study quality or mixed WW/AS sub-group [17], as were qualitative studies that failed to state that saturation of information had been reached, usually ≥20 participants. Studies including at least 20 participants is a general guideline in qualitative research to reach data saturation [18]. One study that fell beneath this threshold was included as information saturation was demonstrated.
Cohort/Registry studies were included when they were multi-institutional and included >500 patients to reduce the associated risk of bias in small sample sizes and increase the external validity and generalisability. Studies reporting on AS adherence also included ≥2 years of follow-up.
2.3. Data quality
Qualitative and mixed methodology studies were evaluated for quality using The Purpose, Respondents, Explanation, Findings and Significance (PREFS) quality checklist [17]. This checklist was developed by Joy and Bridges [17]. for assessing quality of reports in systematic reviews of literature on patient preferences and comprises questions regarding five aspects of each study: purpose (P), respondents (R), explanation (E), findings (F), and significance (S). The complete PREFS checklist is shown in Table 1. The quality questions are outlined in [17]. A quality score was calculated by adding one point for each “yes” answer on the PREFS checklist, with a maximum potential score of 5. Papers were categorized in a similar way to that reported by Joy et al [17]: standard gamble (SG, i.e. HRQoL’s) – 17 papers; contingent evaluation (CO, i.e. survey) – 14 papers; stated preference other (SPO, i.e. monetary value or choices or ratings) – 3 papers; qualitative (i.e. interviews) – 13 papers and 3 papers with mixed methodology.
Table 1:
Purpose, Respondents, Explanation, Findings and Significance (PREFS) Checklist
| Study, publication date | Quality Score | Purpose (P) | Respondents (R) | Explanation (E) | Findings (F) | Significance (S) | Category of paper |
|---|---|---|---|---|---|---|---|
| Davison et al, 2012, Canada | 4 | Yes | Yes | Yes | No | Yes | CO |
| Goh et al, 2012, USA |
3 | Yes | Yes | Yes | No | No | CO/Q |
| Orom et al, 2014, USA |
4 | Yes | Yes | Yes | No | Yes | CO |
| Xu et al, 2016, USA |
4 | Yes | Yes | Yes | No | Yes | CO |
| Orom et al, 2017, USA |
4 | Yes | Yes | Yes | No | Yes | CO |
| Anandadas et al, 2011, UK |
3 | Yes | Yes | No | No | Yes | SG |
| Gorin et al, 2011, USA |
3 | Yes | No | Yes | Yes | No | CO |
| Loeb et al, 2016, USA |
3 | Yes | No | Yes | Yes | No | Q |
| Volk et al, 2014, USA |
3 | Yes | Yes | Yes | No | No | Q |
| Xu et al, 2012, USA |
3 | Yes | No | Yes | Yes | No | Q |
| O’Callaghan et al, 2014, Australia | 3 | Yes | No | Yes | Yes | No | Q |
| Mishra et al, 2013, USA |
3 | No | No | Yes | Yes | Yes | SPO |
| Ehdaie et al, 2017, USA |
4 | No | Yes | Yes | Yes | Yes | SPO |
| Venderbos et al, 2015, Netherlands |
4 | Yes | No | Yes | Yes | Yes | SG |
| Venderbos et al, 2017, Europe |
4 | Yes | No | Yes | Yes | Yes | SG |
| Lang et al, 2017, USA |
4 | Yes | No | Yes | Yes | Yes | CO |
| Kendel et al, 2016, Germany | 4 | Yes | Yes | Yes | No | Yes | CO |
| Vasarainen et al, 2012, Helsinki | 4 | Yes | No | Yes | Yes | Yes | SG |
| Bellardita et al, 2013,4 Italy 4 |
4 | Yes | No | Yes | Yes | Yes | SG |
| Vanagas, 2013, Lithuania |
3 | Yes | No | Yes | No | No | SG |
| Hegarty et al, 2008, USA and Ireland |
3 | Yes | No | Yes | Yes | No | SG |
| Smith et al, 2009, Australia |
3 | Yes | No | Yes | Yes | No | SG |
| Parker et al, 2016, USA |
4 | Yes | Yes | Yes | No | Yes | SG |
| Punnen, 2013, USA, |
4 | Yes | Yes | Yes | No | Yes | SG |
| Lane et al, 2016, UK |
4 | Yes | Yes | Yes | No | Yes | CO |
| Van den Bergh et al, 2010, Netherlands |
4 | Yes | No | Yes | Yes | Yes | SG |
| Xu et al, 2011, USA |
4 | Yes | No | Yes | Yes | Yes | Q |
| Xu et al, 2016, USA |
4 | Yes | Yes | Yes | No | Yes | CO |
| Wilcox et al, 2014, Australia |
4 | Yes | No | Yes | Yes | Yes | SG |
| Burnet et al, 2007, UK |
4 | Yes | No | Yes | Yes | Yes | SG |
| Davison et al, 2011, Canada |
3 | Yes | No | Yes | No | No | CO |
| Anderson et al, 2014, Australia | 3 | Yes | Yes | No | No | Yes | SG |
| Oliffe et al, 2009, Canada |
3 | Yes | Yes | No | yes | No | CO |
| Berger et al, 2015, USA |
3 | Yes | Yes | No | No | Yes | Q |
| Seiler et al, 2012, USA |
4 | Yes | No | Yes | Yes | Yes | CO |
| Kinsella et al, 2015, UK |
3 | No | Yes | No | Yes | Yes | SPO |
| Wade et al, 2015, UK |
3 | Yes | No | Yes | Yes | No | CO/Q |
| Kazer et al, 2011 USA |
4 | Yes | No | Yes | Yes | Yes | Q |
| Wade et al, 2013, UK |
3 | Yes | No | Yes | Yes | No | SG |
| Wade et al, 2015, UK |
3 | Yes | No | Yes | Yes | No | Q |
| Latini et al, 2007, USA |
4 | Yes | Yes | No | Yes | Yes | CO |
| Jeldres et al, 2015, USA |
3 | Yes | No | Yes | No | Yes | SG |
| Donovan et al, 2016, UK | 4 | Yes | No | Yes | Yes | Yes | SG |
| Loeb et al, 2017, USA | 33 | Yes | No | Yes | Yes | No | Q |
| Le et al, 2016, USA |
3 | Yes | No | Yes | Yes | No | Q |
| Scherr et al, 2017, USA | 3 | Yes | No | Yes | Yes | Yes | Q/SG/CO |
| Taylor et al, 2017, USA | 4 | Yes | No | Yes | Yes | Yes | Q |
| Mader et al, 2017, USA |
3 | Yes | No | Yes | Yes | No | Q |
| Lyons et al, 2017, USA | 2 | Yes | No | Yes | No | No | Q |
| Chen et al, 2017, USA |
4 | Yes | Yes | Yes | Yes | No | CO |
SG = standard gamble (HRQoL’s), CO = contingent evaluation (survey), SPO = stated preference (other) - (monetary value or choices or ratings), Q = qualitative (interviews).
The mean PREFS quality score was 3.46 (SD 0.54), and the scores ranged from 2 to 5. The mean quality scores were 3.15 (SD 0.55) in studies with qualitative methods, 3.33 (SD 0.57) in studies with SPO methods, 3.76 (SD 0.44) in studies with CO methods, 3.58 (SD 0.51) in studies with SG methods and 3 (SD 0) in studies with mixed methodology. 46 studies explicitly stated that the purpose (P domain) of the study was to evaluate factors effecting choice or adherence to AS, and 31 studies included all respondents in the evaluation of findings (F domain). There was more variability among the studies in satisfying the R, E, and S domains of the PREFS checklist, demonstrating that many reports lacked details regarding whether responders were similar to non-responders and failed to include statistical tests to evaluate results where possible.
The included cohort/registry epidemiology papers were assessed for strength of evidence. Although no quality assessment tool completely fitted the purpose of this review, assessments were made using a modified Strengthening The Reporting of Observational Studies in Epidemiology (STROBE) checklist [19] (Table 2). The following items were assessed: number of participants, variables (clear explanation of all outcomes, exposures, potential confounders and effect modifiers), data source (details given of measurement), bias (effort made to address potential sources of bias), statistical methodology (description of methods, missing data addressed, sensitivity analysis performed), descriptive data (characteristics of individuals given: demographic, clinical and social) and limitations (generalisability addressed, cautious interpretation). In each of the seven categories 1 point was assigned to each positive response, giving a possible total score of 12.
Table 2.
Strength of confidence in quality of quantitative Cohort/Registry studies (Modified STROBE checklist)
| Author and year |
Variables documented | Bias | Statistical methodology | Descriptive data | Limitations | Score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Exposure | Confounders and modifiers |
Documented | Addressed | Full description |
Missing Data |
Sensitivity analysis |
Demographic | Clinical | Social | |||
| Aizer, et al, 2012, USA | Yes | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | 10 |
| Loeb et al, 2013, Sweden |
Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
| Filson et al, 2014, USA |
Yes | Yes | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | 9 |
| Hoffman et al, 2014, USA |
Yes | Yes | No | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | 9 |
| Liu et al, 2015, USA |
Yes | No | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | 8 |
| Maurice et al, 2016, USA |
Yes | Yes | Yes | No | No | Yes | No | No | Yes | Yes | Yes | Yes | 8 |
| Womble et al, 2014, USA |
Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
| Loeb et al, 2016, Sweden |
Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
| Loeb et al, 2015, Sweden |
Yes | Yes | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | 9 |
| Hamdy et al, 2016, UK |
Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 10 |
| Bokhorst et al, 2015, Netherlands | Yes | Yes | Yes | No | No | Yes | No | No | No | Yes | No | Yes | 6 |
| Weerakoon et al, 2015 Australia. |
Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | No | Yes | 8 |
| Parikh et al, 2017, USA | Yes | No | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| Bokhorst et al, 2016, Worldwide |
Yes | Yes | No | No | No | Yes | No | No | No | Yes | No | Yes | 5 |
Notes: variables (clear explanation of all outcomes, exposures, potential confounders and effect modifiers) data source (details given of measurement), study size (≥500 patients), bias (effort made to address potential sources of bias), statistical methodology (description of methods, missing data addressed, sensitivity analysis performed), descriptive data (characteristics of individuals given: demographic, clinical and social) and limitations (generalisability addressed, cautious interpretation).
The mean quality score was 8.78 (SD 1.8). The scores ranged from 5 to 11. All studies included outcome variables, a full description of statistical methodology, clinical data and limitations. The highest level of variability was found in relation to bias, missing data and sensitivity analysis. Five papers scored ≥10.
3.0. Evidence synthesis and extraction
We identified 1,049 unique citations; of these 901 were excluded as review articles, commentaries or narratives. Full-text screening was carried out on 148 articles, of which 85 were excluded, rendering 64 articles included for synthesis. Given the heterogeneous study designs, no statistical comparisons were made.
This mixed-methods systematic review uses a modified version of The Joanna Briggs Institute – Methodology for JBI Mixed Methods Systematic reviews (Integrated approach) [20]. Joanna Briggs Institute integrated methodology combines both qualitative and quantitative date into a single mixed methods synthesis [20]. A meta-aggregation of data is presented using a Bayesian approach, whereby all data was codified into themes and then presented in a meta-aggregation This approach generates summative statements of the evidence to equally inform the topic in a mutually compatible format [21], as seen in Table 2. The six themes identified were: cancer characteristics, patient, family and social support, provider, healthcare organization/practice and health policy.
Three authors (NK, MVH and SC) independently screened all titles and abstracts. The resulting reference list was compiled for full-text screening and data extraction. The final reference list was screened and agreed by all authors. A data abstraction form was developed for both qualitative and quantitative studies, based on review of the first articles of each type from among the selected papers. One author (NK) extracted data onto a spreadsheet, which was checked by two other authors (SC and MVH). Data abstraction was performed separately by two reviewers (NK and SC or MVH). Data extraction included: publication year, authors, journal name, title, study design, setting, population, eligibility criteria for participants, data collection method, response rate, and outcomes (Table 1). The review of findings/themes was open-ended with no pre-specified coding system, with intent to describe the primary conclusions of the authors.
3.1. Study design of included studies
Table 3 summarises the 64 included studies. 37 studies took place in North America, 20 in Europe, 5 in Australia, one compared America to Ireland and one was a worldwide study. The total number of study participants was 452,4560, ranging from 7 to 189,768 in each study. The average age at diagnosis across the studies was 65 years.
Table 3.
Overview of the studies included in the systematic review on factors affecting choice and adherence to active surveillance in men with low-risk prostate cancer.
| # | Author | Year | Reference | Design | N or Response rate (%) |
Country | Study population/ setting |
Data collection method (instrument) |
Study period |
Age (mean) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Davison, et al | 2012 | [32] | Cross-sectional survey to AS patients | 180/258 (70%) | Canada | Clinical/ Hospital |
Survey (Likert scale) |
2009–2011 | 67 |
| 2 | Goh, et al | 2012 | [50] | Cross-sectional survey to AS patients | 34/34 (100%) | USA | Research/Support Group | Survey/Interviews | May-August 2011 | 63 |
| 3 | Orom, et al | 2014 | [51] | Cross-sectional survey to AS patients | 120/126 (79%) | USA | Clinical/Hospital | Survey | 2010–2012 | 65 |
| 4 | Xu, et al | 2016 | [27] | Cross-sectional survey to patients with localized PC | 260/559 (68%) | USA | Population Database | Survey | 2009–2010 | 61 |
| 5 | Orom, et al | 2017 | [33] | Prospective survey of patients undergoing AS/RP/RT | 1,531/3,337 (46%) | USA | Database/ Hospital |
Survey (Distress thermometer - 11 point analogue scale) |
2010–2014 | Unknown |
| 6 | Anandadas, et al | 2011 | [25] | Prospective survey to AS patients | 768/768 (100%) | UK | Clinical/Hospital | Survey (RAND-36 Item – SF-36 v.2, UCLA-PCI, study-specific survey) |
2000–2006 | 65 |
| 7 | Gorin, et al | 2011 | [41] | Prospective survey to AS patients | 105/185 (57%) | USA | Clinical/ Hospital |
Survey | Unknown | 66 |
| 8 | Loeb, et al | 2016 | [52] | Qualitative interviews with PC clinicians | 24 | USA | Hospital/ Community |
Interviews (thematically analysed) |
July to Dec 2015 | N/A |
| 9 | Volk, et al | 2014 | [40] | Qualitative interviews with AS patients and RP/RT patients | 30/36 (83%) | USA | Clinical/ Hospital |
Interviews (thematically analysed) |
2011 | 63 |
| 10 | Xu, et al | 2012 | [45] | Qualitative interviews with patients with LRPC | 21 | USA | Research/ Clinical community |
Interviews (thematically analysed) |
Unknown | 58 |
| 11 | O’Callaghan, et al | 2014 | [46] | Qualitative interviews with patients with LRPCand their partners | 21 men and 14 partners | Australia | Clinical/ Hospital and community |
Interviews (thematically analysed and inter-rated) | 2012–2013 | Range 61–70 |
| 12 | Aizer, et al | 2012 | [22] | Retrospective cohort/registry study | 701 | USA | Clinical/ Hospital |
Database | Unknown | 62 |
| 13 | Loeb, et al | 2013 | [24] | Retrospective cohort/registry study | 57,713 | Sweden | Population database. | Database | 1998–2011 | 65 |
| 14 | Filson, et al | 2014 | [37] | Retrospective cohort/registry study | 7,347 | USA | Population database | Database | 2004–2007 | 66+ |
| 15 | Hoffman, et al | 2014 | [34] | Retrospective cohort/registry study | 12,068 | USA | Population database. | Database | 2006–2009 | 66 |
| 16 | Liu, et al | 2015 | [26] | Retrospective cohort/registry study | 609 | USA | Population database. Hospital and community | Database | 2012–2013 | 65 |
| 17 | Maurice, et al | 2015 | [35] | Retrospective cohort/registry study | 189,768 | USA | Population database | Database | 2010–2011 | Unknown |
| 18 | Womble, et al | 2015 | [23] | Retrospective cohort/registry study | 682 | USA | Population database. Hospital and community | Database | 2012–2013 | 63 |
| 19 | Loeb, et al | 2016 | [11] | Retrospective cohort/registry study | 32,518 | Sweden | Population database. | Database | 2009–2014 | 67 |
| 20 | Mishra, et al | 2013 | [47] | Content analysis of patient’s internet conversations | 464 | USA | Internet conversations | Qualitative software – (Sentiment index) | 2002–2012 | Unknown |
| 21 | Ehdaie, et al | 2017 | [44] | Prospective counselling intervention to physicians | 5 surgeons; 1,003 patients | USA | Clinical/ Hospital |
Database and Survey | 2014–2015 | 60 |
| 22 | Venderbos, et al | 2015 | [82] | Retrospective survey comparing anxiety and distress at 0, 9 and 18 months on AS | 150 (86%, 90%, 96% at 0, 9 and 18 months) | Netherlands | Clinical/ Hospital |
Survey (CES-D Scale, MAX-PC, STAI-6, DCS) |
2007–2008 | Unknown |
| 23 | Venderbos, et al | 2017 | [67] | Retrospective survey comparing HRQoL in treatment groups: AS, RP, RT to matched patients | 879 (65–75%) |
Europe | Clinical/ Hospital |
Survey (EPIC, SF-12, STAI-6, EQ-VAS) |
2015 | 65 (AS), 66 (RT), 70 (RP) |
| 24 | Loeb, et al | 2015 | [29] | Retrospective cohort/registry study | 11,726 | Sweden | Population database | Database | 2003–2007 | 64 |
| 25 | Lang, et al | 2017 | [31] | Prospective survey to AS patients | 531 | USA | Clinical/ Hospital |
Survey | 2011–2012 | Unknown |
| 26 | Hamdy, et al | 2016 | [30] | Prospective cohort/registry study | 1643 | UK | Clinical/ Hospital |
Survey/Database | 1999–2009 | 61 |
| 27 | Kendel, et al | 2016 | [79] | Prospective survey study – men on AS and RP | 316 | Germany | Clinical/ Hospital |
Survey | 2008–2013 | 67 |
| 28 | Vasarainen, et | 2012 | [65] | Prospective cohort/registry study. HRQoL + drop-out |
75,124 | Helsinki | Clinical/ Hospital |
Survey (RAND-36, IIEF-5, IPSS) |
2006–2010 | 64 |
| 29 | Bellardita, et al | 2013 | [60] | Prospective cohort/registry study. HRQoL and database AS adjustment analysis | 103 | Italy | Clinical/ Hospital |
Survey/ Database (Mental health - symptom checklist-90, HRQoL – Prostate version) |
2007–2012 | 67 |
| 30 | Vanagas, et al | 2013 | [66] | Prospective survey study. HRQoL, anxiety and depression amongst AS, RP, RT, Chemo and HT |
61/650 | Lithuania | Clinical/ Hospital |
Survey (EORTC, QLQ-C30) |
2010–2011 | >64 |
| 31 | Hegarty, et al | 2008 | [61] | Retrospective survey of men on AS – to assess differences in HRQoL and anxiety and depression in men in the US and Ireland | 29 | USA and Ireland | Clinical/ Hospital |
Survey (MUIS-C, UCLA-PCI, QLI) |
Unknown | 76 |
| 32 | Smith, et al | 2009 | [70] | Retrospective cohort registry survey assessing AS HRQoL and cancer related data 3 years into an AS pathway | 200/1647 | Australia | Clinical/ Hospital |
Survey/ Database (SF-12, IPSS) |
2000–2002 | 63 |
| 33 | Parker, et al | 2016 | [63] | Prospective cohort survey assessing HRQoL and anxiety in AS | 180 | USA | Clinical/ Hospital |
Survey (EPIC, SF-12) |
2006–2012 | 67 |
| 34 | Punnen, et al | 2013 | [14] | Prospective cohort survey, assessing differences between RP and AS (at 1 and 3 years) in respect to HRQol, anxiety and depression. | 122/679 | USA | Clinical/ Hospital |
Survey (PHQ-9, GAD-7) |
2007–2010 | 60 |
| 35 | Lane, et al | 2016 | [75] | Prospective cohort survey study, assessing HRQoL in AS, RP, RT | 1438 | UK | Clinical/ Hospital |
Survey | 1999–2009 | 61 |
| 36 | van den Bergh, et al | 2010 | [71] | Prospective cohort survey study (PRIAS) assessing progression, HRQol, anxiety and depression and patient factors between men on AS, RP and RT. | 129/150 | Netherlands | Clinical/ Hospital |
Survey (CES-D. STAI, SF-12) |
2007–2008 | 65 |
| 37 | Xu, et al | 2011 | [81] | Qualitative interviews with patients with LRPC | 7 black men, 14 white men | USA | Clincal/ Hospital | Qualitative interview (thematically analysed) |
Unknown | Unknown |
| 38 | Xu, et al | 2016 | [78] | Retrospective cross-sectional survey | 266/391 (68%) | USA | Clinical/ Hospital |
Survey | 2009–2010 | 75 |
| 39 | Wilcox, et al | 2014 | [62] | Prospective survey of men on AS – assessing HRQoL and anxiety and depression | 47/61 | Australia | Clinical/ Hospital |
Survey (IIEF-5, IPSS, MAX-PC, UAS) |
2013 | 62 |
| 40 | Burnet, et al | 2007 | [72] | Prospective survey study assessing anxiety and depression in men on AS | 100/329 | UK | Clinical/ Hospital |
Survey (HADS) |
Unknown | 67 |
| 41 | Davison, et al | 2011 | [73] | Retrospective survey study to assess the support and information men require to sustain them while on AS. | 25 | Canada | Clinical/ Hospital |
Survey (The control preferences scale) |
2009–2010 | 64 |
| 42 | Anderson, et al | 2014 | [74] | Prospective cohort survey study to assess anxieties in men AS and determine which of these anxieties predicted health-related quality of life (HRQL) | 86 | Australia | Clinical/ Hospital |
Survey (HADS MAX-PC, FACT-P, STAI) |
Unknown | 66 |
| 43 | Oliffe, et al | 2009 | [59] | A prospective survey study to describe the range of men’s self-management strategies used to overcome AS-related uncertainty | 25 | Canada | Clinical/ Hospital |
Survey | Unknown | Unknown |
| 44 | Berger, et al | 2014 | [85] | Retrospective interview study to generate hypotheses about the factors that influence patients’ decisions to leave AS. | 14/1159 | USA | Clinical/ Hospital |
Qualitative interviews (thematically analysed) |
2010–2013 | Unknown |
| 45 | Seiler, et al | 2012 | [86] | Prospective cross-sectional survey study, to assess differences in anxiety and depression amongst couples | 133 | USA | Clinical/ Hospital |
Survey | Feb 2010 – August 2010 | 66 |
| 46 | Kinsella, et al | 2015 | [88] | Prospective education intervention to men on AS | 117/244 | UK | Clinical/ Hospital |
Survey/ Database |
2011–2012 | 63 |
| 47 | Bokhorst, et al | 2015 | [93] | Retrospective registry study to determine the number of non-compliers with the follow-up protocol of the (PRIAS) study | 4547 | Netherlands | Population database | Database/ Registry |
Unknown | 66 |
| 48 | Wade, et al | 2015 | [48] | A prospective cohort survey study assessing nurse-led AS clinics for accessibility, flexibility and level of support | 85 | UK | Clinical/ Hospital |
Survey and interviews (thematically analysed) |
2006–2008 | 64 |
| 49 | Weerakoon, et al | 2015 | [12] | Retrospective registry study | 1603 | Australia | Population | Registry | 2008–2012 | 66 |
| 50 | Kazer, et al | 2011 | [55] | Focus group analysis of the needs of men on AS | 7 | USA | Research | Focus group | 2009–2010 | 70 |
| 51 | Wade, et al | 2013 | [49] | Prospective/Retrospective survey study | 1144 (88–95%) at 7 and 35 days post biopsy | UK | Clinical/ Research |
Survey HRQoL, HADS. |
2006–2008 | 62 |
| 52 | Wade, et al | 2015 | [89] | In-depth semi-structured interviews at 10 and 18 weeks post biopsy | 85 | UK | Clinical/ Research | Interviews (thematically analysed) |
2006–2008 | 64 |
| 53 | Latini, et al | 2007 | [80] | Retrospective survey study | 105 | USA | Registry/ Database |
Survey 5-item fear of cancer re-occurrence questionnaire |
1997–2002 | 75 |
| 54 | Jeldres, et al | 2015 | [64] | A prospective cohort survey using validated HRQoL questionnaires |
305 | USA | Clinical/ Hospital |
Survey (EPIC, RAND Medical outcomes short form, SF-36) |
2007 | 65 (AS), 58 (RP) |
| 55 | Donovan, et al | 2016 | [68] | A prospective cohort survey using validated questionnaires to assess PROMs - HRQoL to compare men choosing surgery versus radiotherapy versus active monitoring. | 545/1643 | UK | Clinical/ Hospital |
Survey (EPIC, SF-12, HADS, EORTC QLQ-C3021, ICQ-16, ICSmaleSF) |
1999–2009 | 61 |
| 56 | Parikh, et al | 2017 | [36] | Retrospective cohort/registry study using the National Cancer Database to determine patterns of AS uptake | 40,839 | USA | Clinical/ Hospital |
Registry | 2010–2013 | 61 (CI) 63 (AS) |
| 57 | Loeb, et al | 2017 | [83] | A qualitative study with patient focus groups and semi-structured interviews with providers, to understand the informational needs of men on AS and influences on provider decision making. | 61 | USA | Clinical/ Hospital |
7 focus groups and 24 semi-structured interviews | July 2015-March 2016 | Unknown |
| 58 | Bokhorst, et al | 2016 | [92] | A retrospective cohort review of hospital charts to assess the impact of biopsy complications on re-biopsy | 1164 | Worldwide | Clinical/ Hospital |
Review of hospital charts | Unknown | Unknown |
| 59 | Le, et al | 2017 | [43] | In-depth close-ended questions and semi-structured telephone interviews, to assess decision making processes by men and their partners in respect to AS and CI | 30 | USA | Clinical/ Hospital |
Interviews | May-August 2011 | Range 49–72 |
| 60 | Scherr, et al | 2017 | [53] | A retrospective review of hospital charts, and structured interview questions and prospective likeart scale survey and HRQOL validated tools. | 92/211 | USA | Clinical/ Hospital |
Review of hospital charts, structured interview, Survey (MAX-PC) |
2008–2012 | 63 |
| 61 | Taylor, et al | 2016 | [28] | A prospective longitudinal cohort study of telephone interviews pre-treatment to assess influences on treatment decision preferences | 1140 | USA | Clinical/ Hospital | Interviews | 2012–2014 | 61.5 |
| 62 | Mader, et al | 2017 | [42] | Retrospective patient semi-structured interviews assessing choice factors in AS | 15 | USA | Clinical/ Hospital | Interviews | Unknown | 65 |
| 63 | Lyons, et al | 2017 | [56] | A retrospective interview study on cognitive and affective processing involved in treatment decision making with patients and clinicians | 18 (providers) and 19 (patients) | USA | Clinical/ Hospital | Interviews | Unknown | 65 |
| 64 | Chen, et al | 2017 | [69] | A prospective cohort survey using a validated questionnaire to assess HRQoL. | 314/1141 | USA | Clinical/ Hospital |
Survey - Prostate cancer symptom indicies |
2011–2013 | 67 |
The assessments were reported between 2007 and 2017. The studies consisted of 13 surveys, 12 qualitative interviews/focus groups, 12 cohort/registry studies, 2 content analysis and 1 intervention study and 7 mixed method studies. 17 studies reported Health related quality of life (HRQoL) and/or psychological assessment data using validated questionnaires/tools (Appendix 2).
Some overlap in studies was noted with 8 of 64 studies reporting on factors influencing both active surveillance choice and adherence.
4.0. Results
This review has been split into 2 sections: Barriers and facilitators to AS selection and to AS adherence. These have been further synthesised and demonstrate influence on six different levels: cancer characteristics, patient factors, family and social support, provider, organisation and practice, and health policy. The sections below discuss facilitators and barriers according to these levels.
4.1. Barriers and facilitators to AS selection
4.1.1. Cancer characteristics
Facilitators:
Nine studies described how cancer characteristics such as PSA, number of positive cores, Gleason score and tumour volume, influenced the selection of patients for AS. A low Gleason score and a low PSA value were identified as facilitators of AS in five of them [11, 22–25]. Five studies [22, 23, 26–28] also suggested that tumour volume played an influential role in AS selection, with lower volume associated with higher levels of AS selection.
Barriers:
The UK-based Prostate Testing for Cancer and Treatment (ProtecT) study, US based CEASAR Study and Swedish NCPR (National Prostate Cancer Register) [29–31] found that men with higher PSA’s and tumour staging were disinclined to choose AS when diagnosed.
4.1.2. Patient level
Facilitators:
Seven studies [26, 28, 32–36] indicated that older men were more likely to choose AS than younger men, but were in general less engaged in the decision-making process than younger men [32]. Co-morbidity was featured in seven studies [11, 22, 24, 33–35, 37]. The SEER registry reviews [34, 37], the US National Cancer Database [38], Swedish studies [11, 24], a US Multi-disciplinary clinic study [22] based in Boston and the Michigan MUSIC Study [23] showed an association between a higher Charlson co-morbidity index and choice of AS. Orom et al. [33], however, found that the presence of cardiovascular disease was associated with a greater likelihood of choosing radiation over AS. Both Orom’s [39] and Parikh’s [36] studies suggested that men with more years of education were more willing to opt for AS, however another study suggested higher education was a barrier to AS choice [24].
Six studies [25, 32, 40–43] suggested that fear of side effects (in particular erectile dysfunction and incontinence) following radical treatment was a strong determinant of AS. Ehdaie [44] found that significantly more men opted for AS (12%) if during treatment counselling they had pro-actively been encouraged not to ignore potential harms of treatment.
Barriers:
Nine studies showed that younger men were less likely to choose AS. Three studies [32, 33, 45] found men younger than 60 years old were less likely to choose AS. One comment from a patient in a qualitative interview was “choosing AS would be irresponsible, ridiculous even - at my age (aged 53)”.
Other studies reported patient-related factors including ethnicity and family history of PC. Aizers et al [22] suggested that family history of PC increased the likelihood of an early drop out from AS; this is consistent with Volks’ [40] interview findings that twice as many patients who opted for primary curative treatment had a relative with PC, compared to patients who opted for AS. Xu’s study [27] showed that black men, as compared to white, were less likely to choose radical treatment, and specifically surgery, due to the high risk of adverse effects. In contrast, Oroms’ survey [33] suggested a racial difference in the use of AS, with a higher proportion of black men choosing radiotherapy over AS.
The association between the level of education and AS is inconsistent: the Prostate Cancer Database Sweden (PCBaSe) [24] suggested that a higher level of education precipitates lower use of AS in the low-risk PC group (26% of men versus, 39% with a mid-level education and 35% with a lower education level).
The psychological burden of AS in respect to the associated repeat testing during AS [46, 47], as well as the morbidity from repeat prostate biopsies [25], were also linked to reduced uptake of AS. Wade [48, 49] found that men who were well informed about prostate biopsy were less likely to refuse repeat biopsy. This is important to acknowledge in the context of AS where monitoring includes regular re-biopsy. However, several studies have described the process of repeat testing on AS as a reassuring process [40, 42, 46].
4.1.3. Family and social support
Facilitators:
In all of the interview studies, men on AS described justifying their decision to others as one of the most difficult aspects of the decision-making process. Both Xu and Goh [45, 50] reported that AS referred to as “no treatment” was often more challenging for the spouse and children to understand than for the patient. Successful reassurance and education of the family was highlighted as a key facilitator to patients choosing and adhering to AS [40, 45, 46].
Four interview studies from the US, UK and Canada [25, 32, 40, 46], showed that “avoidance of treatment side effects”, “more convenient for lifestyle” and a “combination of reasons” were recurring themes explaining the choice of AS. The UK study [25] also showed that men were equally as satisfied with their decision to undertake AS as those who had undergone radical treatment two years later.
Barriers:
Pressure from family and friends has been found to be high at the point of diagnosis, many of whom urged curative treatment [45, 47]. To give an example, in qualitative interviews performed by Xu et al [45] one man stated that he initially preferred AS plus nutrition supplements to avoid treatment-related side effects, but that his family pressured him to choose RP and, as a compromise, he finally chose radiation. In another example, one man stated that he felt unspoken pressure from his family to choose curative treatment.
Perception and acceptance of AS requires careful management. Orom’s 2017 survey [33] described that high levels of distress at the time of diagnosis and at the time of the treatment decision were a predictor for choosing radical prostatectomy over AS. In contrast, Xu et al [27] found that black men were more likely to report higher levels of “cancer worry”, but that their perception of the negative effects of radical treatment often led to fewer men choosing radical treatment.
4.1.4. Healthcare provider level
Facilitators:
Eleven of the reviewed studies suggested that the clinician heavily influences the decision-making process [26, 32, 33, 41, 44, 46, 47, 50–53]. Scherr, Liu and Davison all found that the diagnosing urologist was most influential clinician associated with AS choice [26, 32, 53]. Orom’s 2014 survey [51] found that a poor relationship between patient and clinician was mentioned by only 1% of men who were appropriately recommended AS for LRPC, but by as many as 53% of men who were recommended a definitive treatment only.
Loeb et al [52] found that clinicians were concerned about the burden of intensive monitoring and that they might miss disease progression. However, both Orom [51] and Loeb [52] found that shared decision-making would address this.
Health literacy is defined as an “individual’s capacity to access, understand, communicate, evaluate, utilize, and make decisions based on health information” [54]. Therefore, provision and access to relevant information is a consistent theme in both increasing the uptake of and adherence to AS. Mishra at al [47] described the views of patients and their families in internet conversations over a 10-year period; they found that access to unbiased information was associated with more patients opting for AS. Four other studies [32, 40, 42, 46] reported similar results. Goh et al [50] found that men who perceived they were receiving consistent information felt more in control of their decision-making and experienced a greater degree of satisfaction and certainty in choosing AS. Three other studies concluded that access to educational resources, in addition to professional and peer support (supported self-management), optimised the treatment selection process and empowered men “to take control” of their diagnosis and of their choices thereafter [42, 47, 55]. However, in contrast Taylor and colleagues [28] found that even when patients had access to information and support, men who choose AS were less likely to access these resources than those men choosing radical treatment.
A US multidisciplinary clinic study [22] found that AS selection was further facilitated through a multi-disciplinary provider approach, removing the bias of treatment recommendation which is often associated with clinicians that carry out a particular treatment. Patients receiving treatment-counselling from two or more specialist clinicians were twice as likely to opt for AS, than radical treatment (43% versus 22%). This was further confirmed and described during semi-structured interviews by Lyons et al [56].
Ehdaie [44] further found that a systematic approach for communicating the merits of AS using appropriate framing techniques, increased the proportion of patients selecting AS (69% pre- intervention to 81% afterwards). Equating to a 30% relative reduction in unnecessary curative treatment.
Several studies have also reported that by slowing down the treatment decision-making process providers might influence an increase in the proportion of men choosing AS [46, 47, 56]. Volk and colleagues [40] found significance in men viewing their cancer as “low-risk” as it offered an opportunity to postpone treatment allowing sufficient time for technological advances in treatment. When asked if men chose AS as a holding mechanism only or as a long-term solution, patients fitted into two groups: those who concluded selection of AS meant avoidance of side effects and those that felt AS gave them time to make a treatment decision.
Barriers:
Conversely, the clinician’s influence has also been listed as a barrier for choice of AS. Mishras review of the changing trends in internet conversations over a 10-year period suggested that patients were increasingly receptive to considering AS, but many questioned if physicians could provide an unbiased treatment recommendation [47]. This concern was also articulated in four of the interview studies [40, 45, 46, 50] where patients expressed concern about the possibility of clinician bias at the time of consultation, with many of them recalling that they were either not offered AS as an option or the patients perception of AS was “doing nothing”. Scherr and colleagues [53] reviewed patient-physician interactions over the period that treatment decisions were made and found that physicians were heavily influenced by age and Gleason score, but not by the value that patients put on treatment outcomes e.g. sexual dysfunction.
Both Parikh and colleagues [36] used the US National Cancer Database (NCDB) to review 40,839 men with low-risk prostate cancer in relation to a number of variables. They found that the barriers to AS included living a longer distance from the provider facility, and those men diagnosed by a physician working outside of an academic or research facility.
4.1.5. Healthcare organisation and practice level
Facilitators and Barriers:
Many narrative reviews of AS highlight differences in AS protocols among healthcare providers, health care systems and also within individual countries [11, 12, 23, 26]. For example, the MUSIC group found that the use of AS varied substantially between different urology practices in the state of Michigan, ranging from 27–80% of eligible patients [23, 26]. This variability could simply be attributed to the recommendation of the clinician, but two studies [40, 47] focused on access to clinical expertise and technology, suggesting that patients felt that they were only offered AS where there was availability of imaging facilities and expert clinicians to deliver the AS protocol. A similar diagnostic practice related influence was also observed in the Michigan MUSIC study [23, 26].
Type of healthcare system as a barrier to AS was mentioned in one study [52] which found that the US system placed limitations on a clinician’s ability to recommend AS (although did not find that this was due to financial incentives) and carry out the necessary regular re-testing associated with AS. However, Parikh and colleagues [36] found that analysis of the NCDB suggested higher uptake of AS in the uninsured.
4.1.6. Health policy level
Facilitators and barriers:
In 2006–2007, Mishra et al [47] reported that, patient and clinician attitudes towards AS (calculated numerically as a “sentiment-index” in the content in internet conversations) were at their lowest, with both judging AS “not safe” and “should only be offered to older patients”. By 2009, Mishra et al found the internet sentiment-index was high, meaning there was a more positive attitude towards AS. Reasons for this included a 2009 American Urology Association endorsement for AS [57] and a 2010 publication of a large scale AS cohort [58] reporting a very low mortality rate over intermediate follow-up. In Sweden, Loeb and colleagues [11] also found that AS uptake (2009–2014) significantly increased and hypothesised that this was largely due to National Guidelines recommending AS.
4.2. Barriers and facilitators to AS adherence
4.2.1. Cancer characteristics
Facilitators:
Several population-based studies reported on cancer characteristics in relation to AS adherence. The UK-based Prostate Testing for Cancer and Treatment (ProtecT) study, US based CEASAR Study and Swedish NCPR (National Prostate Cancer Register) [29–31] found that men with lower PSA’s and tumour staging were more inclined to adhere to AS.
Barriers:
The Swedish NPCR study [29] reported that the <65-year age group and those with higher education levels (<9 years full-time education) were significantly less likely to continue AS than the older and less educated groups. These findings have been replicated in qualitative studies such as those carried out by Lang and O’Callaghan [31, 46], which found that younger men with higher educational level found the notion of long-term AS, i.e. “doing nothing”, less tolerable than the associated morbidity of radical treatment. The Swedish National Prostate Cancer Registry (NPCR) study [29] also reported that out of those men who discontinued AS, 52% did so because of PSA progression and 24% due to biopsy progression.
4.2.2. Patient level
Facilitators:
Patient perceived experience of AS featured in 3 studies. Goh [50] analysed the experience of AS in 34 men and found that those who viewed their experience with cancer as having a positive impact on their lives were better able to manage the uncertainty of AS, and felt more in control of their decision-making, and during AS. Oliffe [59] found that men reporting a higher level of positivity in the face of their cancer diagnosis (attributed to consistency in information and support) were less likely to exhibit decision-making conflict related to the perceived effectiveness of their treatment plan and therefore may be more inclined to remain on AS. These studies suggest that careful patient selection is important for both treatment choice and adherence [50, 59]. Volk et al [40] found that men described the process of AS as an organized, supportive process of regular monitoring.
Four of the seven HRQoL studies had no control group [60–63]. Wilcox and Jeldres [62, 64] found no change in HRQoL before or after entry into an AS programme. Parker [63] found that as age and body mass index increased, HRQoL decreased over time. Both Parker and Bellardita [60, 63] found that >5 months after diagnosis and commencement of AS, HRQoL improved.
Bellardita [60] also noted that men with a partner and men who had >18 biopsy cores taken at diagnosis were more likely to have a good HRQoL on AS. Hegarty [61] compared HRQoL between men on AS in Ireland and the US: general HRQoL and vitality was lower in the US group – which they reported was likely due to differences in health-care expectations.
The six studies that included control subjects of either men with no cancer [65] or those undergoing radical treatment [66–69] or both [70], showed no statistical differences in HRQoL between the control subjects and men on AS. The Finnish section of the PC Research International: Active Surveillance (PRIAS) study [65] found better than average HRQoL (as defined by the RAND-36 Questionnaire) than in the general Finnish male population, both immediately following entry into AS and 1 year later. Vanagas [66] and Venderbos [67] both reported better HRQoL in men on AS than in men who had undergone radical treatment with respect to physical, emotional and social scales. Donovan and colleagues reviewed patient-reported outcomes comparing monitoring to surgery and radiotherapy as part of the ProtecT trial [68]. The monitoring cohort consistently did better in respect to three of the four domains; erectile, urinary and bowel function, however there was no significant difference in HRQoL amongst the three treatment groups. Smith [70] found similar HRQoL scores across AS and treatment groups. Chen and colleagues [69] reported similar results recently from the North Carolina Prostate Cancer Comparative Effectiveness and Survivorship Study (NC ProCESS) where contemporary PC treatments were compared to AS in respect to HRQoL. However, after 24 months it was notable that QoL scores were not clinically meaningfully different between the treatment and AS groups.
When measuring the anxiety associated with long-term surveillance adherence, the majority of studies included in this review suggested that anxiety reduced [65, 66] or remained the same over time [14, 60, 62, 71–74], with no observed impact on long-term adherence.
In the recently published ProtecT study, depression was reported in only 6% of the 545 men allocated to active monitoring over a period of 10 years, suggesting that depression and anxiety do not increase significantly whilst on AS [30, 75].
A number of studies have shown that emotional distress is relatively high in men at the time of their PC diagnosis [33, 76]. However, anxiety in men on long-term AS has been generally reported as favourably low. A 2015 systematic review [77] reported no overall difference between levels of anxiety following diagnosis of LRPC and during AS, which was reported as between 4–15%. In fact, more studies have suggested that anxiety in men on AS reduces [65, 66, 71] or remains the same over time [14, 60, 62, 71–74].
Barriers:
The Swedish National Prostate Cancer Registry study (NPCR) [29] reported that out of those men who discontinued AS, 20% stopped due to patient preference alone. The US based CEASAR Study [31], which included men from five Surveillance, Epidemiology and End Results (SEER) catchment areas, and the Cancer of the Prostate Strategic Urologic Research Endevour (CaPSURE) database [31] found that 8–23% of men converted to curative treatment for reasons of personal preference rather than disease progression.
These findings were further explored in two large qualitative studies exploring men’s survival expectations as a result of selecting treatment for low risk PC. Xu [78] reviewed men’s perceptions of the likely benefits and harms of radical treatment versus no treatment (AS). This was calculated in the form of patient perceived life expectancy (LE). At the time of the survey, two thirds of the 229 men had started or completed treatment. Of these, 30% in the AS group expected to live <5 years. This was in contrast to in the treatment group where >95% of patients estimated their LE to be >5 years. Kendel [79] also reviewed perceptions of the risk of death from PC between matched patients who had undergone AS or radical prostatectomy (RP). Men who had undergone RP estimated the risk of dying from PC associated with AS to an average of 51%, at least a 10-fold overestimation of the true risk. They believed that their 10-year risk of dying from PC after a RP was only one third of the risk of AS (18% - also a substantial overestimation). Both studies thus suggest that patients’ perception of survival is a barrier to both AS selection and long-term adherence.
Although some studies suggested that “fear of cancer progression” may be a limiting factor to choosing AS [59, 80–82], none have convincingly shown that this contributes to a significant number of men opting out of AS without documented clinical progression (Venderbos [82] found this was 5%). Both Davison [73] and Parker [63] found that the degree of “fear of progression” did not change significantly over a period of AS.
4.2.2. Family and social support
Facilitators:
Four studies highlighted that successful reassurance and education of the family was a key facilitator to patients both in choosing and adhering to AS [40, 45, 46]. Loeb’s [83] qualitative focus group and interview study found that social support and interaction (support groups, online forums, support for spouse and family) was of particular importance in AS adherence. An AS support group for both family and men with PC was a particularly strong recommendation. Mader [42] interviewed 15 men following their decision for AS and found strong correlation between AS adherence and social support, including spouse, extended family and experiences of others. Anandadas and colleagues [84] further demonstrated that men were equally as satisfied with their decision to undertake AS as those who had undergone radical treatment two years later.
Barriers:
Pressure from family and friends is reportedly high at the point of diagnosis, many of whom urge curative treatment [45, 47]. The experiences of friends and family members with cancer (often not PC) are consistently reported as a significant pressure in men on AS [45, 47]. Berger [85] noted men reported leaving AS and pursuing treatment to limit their loved ones’ worry, or in reaction to the fear of cancer expressed by their family. Partner anxiety was also documented by Seiler [86], who compared levels of anxiety and depression between men on AS and their partner. Anxiety scores were much lower in the men than in their partners. The CaPSURE database [31] found that 16% of men converted to curative treatment on the basis of “spousal encouragement”.
4.2.3. Healthcare provider
Facilitators:
Seven studies identified facilitators to AS adherence including; education, self-management techniques, healthcare professional and peer support in the furtherance of increasing AS adherence. Loebs [83] combination of focus groups and semi-structured interviews found six themes relating to the facilitation of AS adherence. Five of these were consistent with healthcare provider responsibilities in the form of informational needs; (1) information on PC (biopsy features, prognosis), (2) information on AS (testing protocol, difference between AS and WW), (3) information on complimentary options (diet, lifestyle), (4) variety of resources (source, format), (5) integrity of information (trusted source, secure, multidisciplinary). Oliffe [59] found that self-management strategies helped men cope with some of the long-term uncertainty of AS, whilst ‘The Prostate Cancer Lifestyle Trial’ based on lifestyle modifications, including exercise and attention to stress management, demonstrated an improved treatment-free survival for men on AS [87]. Goh [50] found that men who perceived that they were receiving useful, consistent information were more satisfied with AS and therefore more likely to continue on AS. Kinsella and colleagues [88] found that AS classes significantly improved long-term adherence to AS, as did consistency of personnel to support and inform patients as found in the UK based ProtecT Trial [89]. Interventions relating to peer support have also demonstrated a significant improvement in the quality of life of men with any stage of PC [88, 89].
Barriers:
The ability of healthcare providers to deliver long-term support to patients on AS was highlighted in several studies [85, 90, 91]. Men leaving AS describe a number un-met supportive care needs [85]. These include lack of education and clarity concerning the correct time to pursue treatment, triggers for treatment both from a patient and relative perspective.
Two studies [90, 91] specifically focused on the influence of support groups. These noted either no effect or even a negative effect on long-term AS adherence, possibly due to the group consisting of a mix of survivors, i.e. those who had undergone radical treatment and those on AS [91]. Chapple [90] found support groups were of no help to men on AS, with one man reporting that he felt he “had to defend himself “in the support group for choosing AS.
Mishra [47] concluded that there was a lack of consistent and reliable information available to men during long-term AS.
4.2.4. Healthcare organisation and practice level
Facilitators and Barriers:
Differences in surveillance strategies have also demonstrated an association with adherence both in a positive and negative respect (Appendix 1). These contemporary cohort studies all include a strategy of repeat biopsy as part of a robust AS protocol. Two studies found that prostate biopsy was associated with significant morbidity and AS adherence could therefore be influenced by frequency/requirement to re-biopsy. The ProBE (The effects of Prostate Biopsy) study nested within ProtecT, assessed patient response to prostate biopsy and indicated that 25% of men who had undergone prostate biopsy were dis-inclined to undergo subsequent biopsies [48], whilst a study by Bokhorst [92] as part of the PRIAS study suggested that complications following prostate biopsy were not uncommon (20% of men), and after complication men were less likely to accept repeat biopsy.
However, in two interview-based studies that took place in the US [40, 46], men gave a consistent description of AS, viewing it as a reassuring, organised and supportive process despite invasive testing.
4.2.5. Health policy level
Facilitators and Barriers:
AS protocols in respect to patient selection, safe monitoring and triggers for intervention vary between study cohorts, healthcare systems and countries, which makes it difficult to produce a coherent, consistent clinical guidance document. In Sweden, Loeb and colleagues [11] found that AS uptake and adherence (2009–2014) significantly increased and hypothesised that this was largely due to clear National Guidelines recommending AS. Likewise the PRIAS study [93] suggested improved compliance and adherence as part of an official AS programme.
4.3. Results synthesis
The barriers and facilitators to AS choice and adherence were categorised into six levels as part of this review: cancer characteristics, patient, family and tumour characteristics, patient factors, family and social support, provider, organisation and practice, and health policy, these are summarised in table 4. These barriers and facilitators varied in both strength of association and level of evidence, and are described in Figure 3. The barriers and facilitators identified as most influential over the six levels are symbolised as circles, with larger circles representing a greater degree of evidence as supported by this review.
Table 4.
Summary table of determinants of choice and adherence to active surveillance among men with low-risk prostate cancer found in the systematic review
| Level | Factor | Potentially targetable interventions for future research and quality improvement initiatives | References |
|---|---|---|---|
| 1. Cancer characteristics | Cancer risk, stage, grade, PSA, tumour volume | Harmonizing national/local guidelines; Developing consensus-based appropriateness criteria | [11,22–31] |
| 2. Patient | Shared or collaborative role in decision-making; preferences; seeking information; feeling informed; knowledge | Shared decision-making; appropriate, reliable, and unbiased information; personal information; not contradictory and not stressful; increased availability of educational resources from trusted medical organizations for patients and families | [27,32,41,46,50] |
| Patient characteristics (age, comorbidities, race, family history of prostate cancer, education, employment, insurance, socioeconomic status) and | Physician judgment and recommendation in shared decision-making with patient preference. | [11,12,22–29,31–37,40,46,68] | |
| Impact of active treatment on: side-effects (urinary function, sexual function); preservation of HRQoL; time to accept diagnosis and to decide; “buying time” | Patient education and information | [25,27,28,32,40–43,46,47,53,60–62,64–67, 69,70,75] | |
| Self-management support; preference-style for diet, exercise and complimentary therapies; increased awareness and control of health; hope for prolonged and improved health; symptom monitoring; life-style | Patient education and information; self-management through diet and exercise, stress management, digital technology | [25,32,40,46,50,83] | |
| Preference for immediate cure; “cut it out”; desiring treatment efficacy/cure; avoid future regret | Patient education and information; supportive counselling | [27,31,40,43,45,80] | |
| Perceived cancer risk; cancer worry; fear of disease progression; illness uncertainty; anxiety and distress | Patient education and information; support; coping; manage anxiety; cognitive reframing; mindfulness; meditation; empowering; support groups; peer community; socialization; connect to others; shared activities; a sense of belonging; provide patients with a sense of meaning and control, robust monitoring processes, widespread agreement on monitoring process. | [14,25,27,30,32,33,40,41, 44–46,50,53,59,60,62, 63,65,66,71,72,74, 78–82,86] |
|
| Monitoring stressors; Coping with anxiety, frequent PSA testing and repeat biopsies | Patient education and information; support; coping | [25,27,32,40, 46–48,60,92,93] |
|
| Awareness and acceptance of AS; survival expectation on AS | Availability of AS “success stories” | [47,50,59,73,78] | |
| Unknown factors | Qualitative interview studies with physicians and patients | [40,45,46,52] | |
| 3. Family and social support | Advice/pressure from partner/spouse/children/friends; marital status; family member with PC | Supportive counselling and information; patient not having to justify decision to others; support; education; reassurance | [32,40–42,45,46,62,85] |
| Awareness and acceptance of AS | Public role models managed with AS and patient advocates | [40,42,45–47,85,86] | |
| Fear of progression; disagreement about safety; preference to “eradicate the cancer” | Counselling and information; Enhanced recognition with information sources, treatment support, medical consultations | [45–47,50] | |
| 4. Provider | Physician’s recommendation; consistency in medical/nursing personnel | Training specialists to use a systematic approach to counselling patients about treatment options; communicating clearly and with confidence; using nudging narratives and framing techniques from behavioural science theory; maintain a positive and hopeful attitude; provide support and reassurance; public reporting of physicians’ cancer management profiles | [26,30,32–34,40–42, 44–47,50,52,53,89] |
| Specialty of physician giving treatment information | Multi-disciplinary team of specialists | [22,26,32,56] | |
| Provision of information and support | Provide and direct patients to accurate and unbiased information rather than describing AS as “doing nothing” or “no treatment” or scaring patients to active treatment, access to AS support groups. Establish consistency of support through nurse specialist roles. | [26,28,32,40,43,46, 47,50,55,56,59,83,88,89] |
|
| Physician attitudes; reluctance; concern about disease progression; perceived lack of data | Raise awareness, ongoing discussions at national meetings, quality improvement initiatives; having clear plans and stopping rules; systematic counselling on AS | [40,44–47,51,52] | |
| Lack of availability of physicians recommending AS | Advocacy; subspecialty within urology | [40,47] | |
| Confidence and trust in health professionals; closeness with physician; share control over treatment decision making | Improved community and medical education about treatment options, prognosis, side-effects; raise awareness of AS; consistent, unbiased treatment information; decisional support information; building trust in physician; patient trusting the physician’s monitoring; patient feeling AS is an organized, supportive process; | [40,46,51,52,89] | |
| 5. Health care organization/practice | Urology practice site; hospital referral region; geographic region | Quality improvement initiatives to harmonize practice sites within networks | [26,35–37,46,47] |
| Degree to which physician shared control over treatment decision making | System-levels determinants of trust, closeness and shared decision-making; organizational changes, e.g. longer consultation times | [40,51,52] | |
| Consultation at a multidisciplinary clinic; University hospital setting; academic hospital or high volume of PC patients | Multidisciplinary clinic may reduce the bias that specialists prefer the modality of treatment they themselves deliver and patients receive a balance perspective of risks and benefits of options | [11,22,24,35–37] | |
| Differences in surveillance strategies | National/International consensus of safe AS. Selection, monitoring and progression, patient information on large AS cohorts | [11,12,23,26,88,93] | |
| 6. Health policy level | Guideline recommendations | Harmonizing national/local guidelines; developing appropriateness criteria; national guideline recommending AS; real-time feedback to units on adherence to national guideline in terms of annual report publicly available online | [11,47,52,53] |
| Trial/cohort data; year of diagnosis | Monitoring and future publications from ongoing prospective protocol-based AS cohorts and registries | [11,35,47,52] | |
| Awareness and acceptance | Guidelines; consensus; discussions at meetings; AS-specific billing code | [47,52] |
Figure 3:
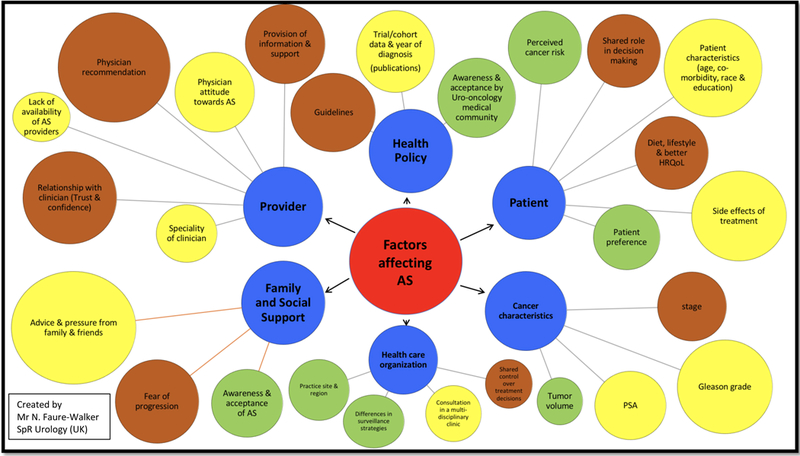
Barriers and facilitators to AS choice and adherence; size of circle signifies the strength of evidence for each influencing factor. Yellow circles = the evidence is strongest for active surveillance choice. Green circles = the evidence is strongest for active surveillance adherence. Brown circles = the evidence is relevant to both active surveillance choice and adherence.Circle size reflects the level of associated evidence. (Created by Mr N. Faure-Walker – SpR Urology)
5.0. Discussion
A demonstrable rise in the use of AS has been noted over a short timeframe, however there are significant differences between individual healthcare providers as seen in the US CaPSURE Database [5] and Swedish PcBaSe [11]. Local guidelines, national policy, patient education, supportive care, and medicolegal factors may be important factors driving this variation [10, 12] and therefore these issues require greater consideration and management if we are to better facilitate AS.
Studies focusing on men’s perception of risk as a barrier to choosing AS show that men continue to grossly overestimate the risk of dying from LRPC whilst on AS [25, 32, 45, 47]. Moreover, understanding the implications of HRQoL and psychological factors on the decision to both choose and adhere to AS requires further multi-dimensional assessment and interpretation. Clinicians and patient groups actively encourage the increasing responsibility that PC patients are taking for self-directed management and informed decision-making [87, 94]. Such patient empowerment has positive psychological effects on cancer patients in general and should be explored in the specific context of AS [87, 94].
These elements have been explored in the context of the chronic disease setting, which one could argue relates well to a diagnosis of LRPC that may never require curative treatment. A systematic review of general health screening and treatment decision making in the chronic disease setting [95] found that clinicians employing a process of ‘motivational interviewing’ consistently improved patients knowledge, perception of risk, and increased confidence in decision making. Motivational interviewing is specifically designed to help patients identify and resolve ambivalence about changing their behaviour by exploring personal perspectives and perceived barriers [96]. It employs a four-step guiding style (engaging, focusing, evoking, and planning) to foster a constructive clinician-patient relationship. Joosten [97] found that motivational interviewing improved the patients’ ability to self-manage and increased adherence to chronic-disease management plans.
Although there is currently no published experience of motivational interviewing in AS, a recent systematic review in other cancer patients [98] found that it was useful for eliciting lifestyle behavioural changes, decreasing cancer related anxiety and encouraging supported self-management. This also supports that argument that men on AS experience a similar physical and psychological burden to people living with other chronic conditions [99], such as asthma and diabetes [100–104], for whom quality of life relies on adherence to a treatment plan with the aim to optimize disease control, maintain quality of life, prevent unnecessary escalation of treatment.
Another systematic review [100] outlining the merits of supportive self-management in chronic disease adherence suggested that there are core components of support and that their implementation requires a holistic approach, which intervenes at the level of the patient, the health care professional and the organisation, much in the same way as identified in this review of AS:
provision of education about the long-term condition (LTC)
psychological strategies to support adjustment to the LTC
practical support tailored to the LTC (e.g. support around activities of daily living for disabling conditions and action plans in conditions subject to marked exacerbations)
social support
lifestyle modifications (e.g. diet and exercise)
Our current systematic review highlights the need for improved validated methods for patient and physician education to facilitate the uptake of AS among men with LRPC. Moreover, studies suggesting that clinician’s bias may influence the treatment decision-making process [26, 32, 33, 41, 46, 47, 50, 51] suggest that educational efforts aimed at clinicians and frameworks for how they deliver the information on treatment options [44] are important for increasing the acceptance of AS. Education, appropriate information and support aimed at both the patient and their family have long been recognised as important in the management of chronic conditions (77,78), with studies demonstrating an increase in adherence to treatment plans where these have been established.
In addition, many chronic disease studies have successfully explored strategies to reduce healthcare inequalities in chronic health conditions [103, 105, 106]. This has been achieved through the standardisation of education and training to both clinicians and patients, development of educational materials and decision aids, as well as creation of specialist centres for chronic conditions. To date, one study has replicated this in the context of AS. Formica and colleagues [107] reported that decision aids in combination with standardised patient education packages achieved a three-fold increase in AS acceptance.
Several AS papers suggested that National guidelines could have a significant impact on selection and adherence to AS [11, 47, 93]. This has been replicated in the chronic disease setting [104, 108]. In diabetes management, the introduction of the Dutch Guidelines increased clinician adherence by 60%, suggesting that guidelines are reassuring for both patients and clinicians [108]. The current discordance in AS management makes internationally ratified guidelines a priority, and efforts such as those employed by the GAP3 consortium (Movember) [109], which has established active communication and collaboration among research groups worldwide, are likely to change this. GAP3 aims to reach international consensus on the definitions and terms used in AS through analysis of a global database including >14,000 patients [109].
The requirement for continuous monitoring in AS has been described as both a barrier and facilitator. In chronic disease management, it gave rise to early developments in telehealth with some studies suggesting that easy-to-learn applications can improve adherence, lessen disease impact, accelerate behaviour change to improve outcomes and increase patient and partner confidence during remote monitoring [110, 111]. AS monitoring protocols are currently undergoing rationalisation, with more frequent imaging and less biopsies. Although we found no reports of the use of mobile health applications in the AS setting and only one small pilot of an internet based application specifically aimed at managing uncertainty in AS [112], the change in the way we survey patients could lend itself to the introduction of robust remote monitoring using this technology.
The role of social media in choice and adherence to AS has not yet been explored, however, on-line social networks have changed the way we communicate and provide new ways to engage patients. Twitter and Facebook groups established to engage cancer patients offer easy access to peer support and have been associated with less stress, anxiety and depression (86–87) and in the chronic disease setting, Kirwan [113] found that combining [114] on-line applications and social networking significantly increased diabetic glycaemic control in comparison to the control group.
A combination of these core elements is critical to ensuring positive experience and benefit of living with LRPC on AS.
Advances in PC management including the use of multi-parametric MRI, and more sophisticated prostate biopsy strategies in both diagnostics and surveillance programmes could also change the level of reassurance in AS. Alongside this, a recent systematic review focused on the development of genomic profiling suggests that combining genome-wide association studies (GWAS) data with gene expression and structural rearrangements and risk alleles could provide a new basis for developing a prognostication tool to guide therapy for men with LRPC [114].
6.0. Limitations
This review is limited as a mixed methodology paper. The included studies were heterogeneous and therefore a meta-analysis was not possible. This type of systematic review relies on a reasonable number of included studies for strength, however the weighting of individual studies needs to be adjusted based on the varying levels of evidence and methodological quality of the included studies. However, this has been represented using the PREFs quality checklist and a modified STROBE checklist, which was reviewed by the three reviewers independently.
Of the papers reviewed, some did not distinguish entirely between AS and WW and therefore older age and co-morbidity as facilitators to AS may be inaccurate in 2018. Another limitation concerns the generalizability. More than 50% of studies were North American, and this healthcare system may not be generalizable to other countries.
7.0. Conclusion
Many factors influence men’s choice and adherence to AS, such as the clinician’s attitudes, family and social support and patient education. The clear recommendations of this review include agreed international guidelines on AS and the introduction of a multidisciplinary management strategy with psychological support to facilitate the AS. Current clinical practice at centres with high AS uptake may provide insight of the changes required to ultimately decrease the overtreatment of PC worldwide, whilst experience gathered in the chronic disease setting such as the introduction of supportive self-management, social media interventions and motivational interviewing, could form the blueprint for future AS programmes to increase both choice of and adherence to AS in LRPC.
Supplementary Material
Acknowledgments
Financial disclosures: Sigrid Carlsson is supported in part by a Cancer Center Support Grant from the National Cancer Institute made to Memorial Sloan Kettering Cancer Center (P30-CA008748), a SPORE grant from the National Cancer Institute to Dr. H. Scher (P50-CA092629), a grant from the National Cancer Institute as part of the Cancer Intervention and Surveillance Modeling Network (U01CA199338–02) and the David H. Koch prostate cancer research fund. Ola Bratt is supported by the Swedish Cancer Foundation (2012/475).
Footnotes
No author has any conflict of interest to report.
References
- [1].Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European journal of cancer (Oxford, England : 1990). 2013;49:1374–403. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [3].Network NCI. Cancer incidence, males, ICD10 C61 : Prostate, 2008–2010. National Cancer Intelligence Network (NCIN), UK Cancer Information Service (UKCIS). on-line: UKCIS; 2010.
- [4].Van Hemelrijck M, Garmo H, Wigertz A, Nilsson P, Stattin P. Cohort profile update: the National Prostate Cancer Register of Sweden and Prostate Cancer data Base—a refined prostate cancer trajectory. International journal of epidemiology. 2015:dyv305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA. 2015;314:80–2. [DOI] [PubMed] [Google Scholar]
- [6].Bruinsma SM, Bangma CH, Carroll PR, Leapman MS, Rannikko A, Petrides N, et al. Active surveillance for prostate cancer: a narrative review of clinical guidelines. Nat Rev Urol. 2016;13:151–67. [DOI] [PubMed] [Google Scholar]
- [7].Tosoian JJ, Carter HB, Lepor A, Loeb S. Active surveillance for prostate cancer: current evidence and contemporary state of practice. Nat Rev Urol. 2016;13:205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kinsella N HJ, Bruinsma S, Carlsson S, Cahill D, Brown C, Van Hemelrijck M. Active surveillance for prostate cancer: a systematic review of contemporary worldwide practices. Transl Andr and Urol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Parker C Active surveillance: towards a new paradigm in the management of early prostate cancer. Lancet Oncol. 2004;5:101–6. [DOI] [PubMed] [Google Scholar]
- [10].Auffenberg GB, Lane BR, Linsell S, Cher ML, Miller DC. Practice- vs physician-level variation in use of active surveillance for men with low-risk prostate cancer: Implications for collaborative quality improvement. JAMA Surgery. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Loeb S, Folkvaljon Y, Curnyn C, Robinson D, Bratt O, Stattin P. Uptake of Active Surveillance for Very-Low-Risk Prostate Cancer in Sweden. JAMA Oncol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Weerakoon M, Papa N, Lawrentschuk N, Evans S, Millar J, Frydenberg M, et al. The current use of active surveillance in an Australian cohort of men: a pattern of care analysis from the Victorian Prostate Cancer Registry. BJU international. 2015;115 Suppl 5:50–6. [DOI] [PubMed] [Google Scholar]
- [13].UK CR. Prostate Cancer Statistics. In: CRUK, editor. on-line2012. [Google Scholar]
- [14].Punnen S, Cowan JE, Dunn LB, Shumay DM, Carroll PR, Cooperberg MR. A longitudinal study of anxiety, depression and distress as predictors of sexual and urinary quality of life in men with prostate cancer. BJU International. 2013;112:E67–E75. [DOI] [PubMed] [Google Scholar]
- [15].Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Choo R, Klotz L, Danjoux C, Morton GC, DeBoer G, Szumacher E, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol. 2002;167:1664–9. [PubMed] [Google Scholar]
- [17].Joy SM, Little E, Maruthur NM, Purnell TS, Bridges JF. Patient preferences for the treatment of type 2 diabetes: a scoping review. PharmacoEconomics. 2013;31:877–92. [DOI] [PubMed] [Google Scholar]
- [18].Guest G, Bunce A, Johnson L. How Many Interviews Are Enough? Field Methods. 2006;18:59–82. [Google Scholar]
- [19].Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Journal of clinical epidemiology. 2008;61:344–9. [DOI] [PubMed] [Google Scholar]
- [20].Institute JB. Methodology for JBI Mixed Methods Systematic Reviews. 2014. [Google Scholar]
- [21].Voils CI, Hassselblad V, Crandell JL, Chang Y, Lee E, Sandelowski M. A Bayesian method for the synthesis of evidence from qualitative and quantitative reports: the example of antiretroviral medication adherence. Journal of health services research & policy. 2009;14:226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Aizer AA, Paly JJ, Zietman AL, Nguyen PL, Beard CJ, Rao SK, et al. Multidisciplinary care and pursuit of active surveillance in low-risk prostate cancer. J Clin Oncol. 2012;30:3071–6. [DOI] [PubMed] [Google Scholar]
- [23].Womble PR, Montie JE, Ye Z, Linsell SM, Lane BR, Miller DC, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. European urology. 2015;67:44–50. [DOI] [PubMed] [Google Scholar]
- [24].Loeb S, Berglund A, Stattin P. Population based study of use and determinants of active surveillance and watchful waiting for low and intermediate risk prostate cancer. J Urol. 2013;190:1742–9. [DOI] [PubMed] [Google Scholar]
- [25].Anandadas CN, Clarke NW, Davidson SE, O’Reilly PH, Logue JP, Gilmore L, et al. Early prostate cancer--which treatment do men prefer and why? BJU Int. 2011;107:1762–8. [DOI] [PubMed] [Google Scholar]
- [26].Liu J, Womble PR, Merdan S, Miller DC, Montie JE, Denton BT, et al. Factors Influencing Selection of Active Surveillance for Localized Prostate Cancer. Urology. 2015;86:901–5. [DOI] [PubMed] [Google Scholar]
- [27].Xu JP, Janisse J, Ruterbusch J, Ager J, Schwartz KL. Racial Differences in Treatment Decision-Making for Men with Clinically Localized Prostate Cancer: a Population-Based Study. Journal of Racial and Ethnic Health Disparities. 2016;3:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Taylor KL, Hoffman RM, Davis KM, Luta G, Leimpeter A, Lobo T, et al. Treatment Preferences for Active Surveillance versus Active Treatment among Men with Low-Risk Prostate Cancer. Cancer Epidemiol Biomarkers Prev. 2016;25:1240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Loeb S, Folkvaljon Y, Makarov DV, Bratt O, Bill-Axelson A, Stattin P. Five-year nationwide follow-up study of active surveillance for prostate cancer. Eur Urol. 2015;67:233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016. [DOI] [PubMed] [Google Scholar]
- [31].Lang MF, Tyson MD, Alvarez JR, Koyama T, Hoffman KE, Resnick MJ, et al. The Influence of Psychosocial Constructs on the Adherence to Active Surveillance for Localized Prostate Cancer in a Prospective, Population-based Cohort. Urology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Davison BJ, Breckon E. Factors influencing treatment decision making and information preferences of prostate cancer patients on active surveillance. Patient Educ Couns. 2012;87:369–74. [DOI] [PubMed] [Google Scholar]
- [33].Orom H, Underwood W III, Biddle C . Emotional Distress Increases the Likelihood of Undergoing Surgery among Men with Localized Prostate Cancer. The Journal of Urology. 2017;197:350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hoffman KE, Niu J, Shen Y, Jiang J, Davis JW, Kim J, et al. Physician Variation in Management of Low-Risk Prostate Cancer: a Population-based Cohort Study. JAMA internal medicine. 2014;174:1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Maurice MJ, Abouassaly R, Kim SP, Zhu H. Contemporary Nationwide Patterns of Active Surveillance Use for Prostate Cancer. JAMA Intern Med. 2015;175:1569–71. [DOI] [PubMed] [Google Scholar]
- [36].Parikh RR, Kim S, Stein MN, Haffty BG, Kim IY, Goyal S. Trends in active surveillance for very low‐risk prostate cancer: do guidelines influence modern practice? Cancer medicine. 2017;6:2410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Filson CP, Schroeck FR, Ye Z, Wei JT, Hollenbeck BK, Miller DC. Variation in use of active surveillance among men undergoing expectant treatment for early stage prostate cancer. J Urol. 2014;192:75–80. [DOI] [PubMed] [Google Scholar]
- [38].Hoffman KE, Niu J, Shen Y, Jiang J, Davis JW, Kim J, et al. Physician variation in management of low-risk prostate cancer: a population-based cohort study. JAMA Intern Med. 2014;174:1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Orom H, Underwood W, Homish DL, Kapoor D, Nelson C, Schiffman Z, et al. Emotional distress predicts choosing surgery over active surveillance in clinically localized prostate cancer patients. Journal of Urology. 2015;193:e759. [Google Scholar]
- [40].Volk RJ, McFall SL, Cantor SB, Byrd TL, Le YC, Kuban DA, et al. ‘It’s not like you just had a heart attack’: decision-making about active surveillance by men with localized prostate cancer. Psychooncology. 2014;23:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gorin MA, Soloway CT, Eldefrawy A, Soloway MS. Factors that influence patient enrollment in active surveillance for low-risk prostate cancer. Urology. 2011;77:588–91. [DOI] [PubMed] [Google Scholar]
- [42].Mader EM, Li HH, Lyons KD, Morley CP, Formica MK, Perrapato SD, et al. Qualitative insights into how men with low-risk prostate cancer choosing active surveillance negotiate stress and uncertainty. BMC urology. 2017;17:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Le YL, McFall SL, Byrd TL, Volk RJ, Cantor SB, Kuban DA, et al. Is “Active Surveillance” an Acceptable Alternative?: A Qualitative Study of Couples’ Decision Making about Early-Stage, Localized Prostate Cancer. Narrat Inq Bioeth. 2016;6:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ehdaie B, Assel M, Benfante N, Malhotra D, Vickers A. A Systematic Approach to Discussing Active Surveillance with Patients with Low-risk Prostate Cancer. Eur Urol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xu J, Neale AV, Dailey RK, Eggly S, Schwartz KL. Patient perspective on watchful waiting/active surveillance for localized prostate cancer. Journal of the American Board of Family Medicine. 2012;25:763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].O’Callaghan C, Dryden T, Hyatt A, Brooker J, Burney S, Wootten AC, et al. ‘What is this active surveillance thing?’ Men’s and partners’ reactions to treatment decision making for prostate cancer when active surveillance is the recommended treatment option. Psychooncology. 2014;23:1391–8. [DOI] [PubMed] [Google Scholar]
- [47].Mishra MV, Bennett M, Vincent A, Lee OT, Lallas CD, Trabulsi EJ, et al. Identifying barriers to patient acceptance of active surveillance: content analysis of online patient communications. PLoS One. 2013;8:e68563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wade J, Rosario DJ, Howson J, Avery KNL, Salter CE, Goodwin ML, et al. Role of information in preparing men for transrectal ultrasound guided prostate biopsy: a qualitative study embedded in the ProtecT trial. BMC Health Services Research. 2015;15:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wade J, Rosario DJ, Macefield RC, Avery KNL, Salter CE, Goodwin ML. Psychological impact of prostate biopsy: physical symptoms, anxiety and depression. JCO. 2013;31. [DOI] [PubMed] [Google Scholar]
- [50].Goh AC, Kowalkowski MA, Bailey DE Jr., Kazer MW, Knight SJ, Latini DM. Perception of cancer and inconsistency in medical information are associated with decisional conflict: a pilot study of men with prostate cancer who undergo active surveillance. BJU Int. 2012;110:E50–6. [DOI] [PubMed] [Google Scholar]
- [51].Orom H, Homish DL, Homish GG, Underwood W 3rd. Quality of physician-patient relationships is associated with the influence of physician treatment recommendations among patients with prostate cancer who chose active surveillance. Urol Oncol. 2014;32:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Loeb S, Curnyn C, Fagerlin A, Braithwaite RS, Schwartz MD, Lepor H, et al. Qualitative study on decision-making by prostate cancer physicians during active surveillance. BJU Int. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Scherr KA, Fagerlin A, Hofer T, Scherer LD, Holmes-Rovner M, Williamson LD, et al. Physician recommendations trump patient preferences in prostate cancer treatment decisions. Medical Decision Making. 2017;37:56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nutbeam D The evolving concept of health literacy. Soc Sci Med. 2008;67. [DOI] [PubMed] [Google Scholar]
- [55].Kazer MW, Bailey DE Jr., Colberg J, Kelly WK, Carroll P. The needs for men undergoing active surveillance (AS) for prostate cancer: results of a focus group study. J Clin Nurs. 2011;20:581–6. [DOI] [PubMed] [Google Scholar]
- [56].Lyons KD, Li HH, Mader EM, Stewart TM, Morley CP, Formica MK, et al. Cognitive and affective representations of active surveillance as a treatment option for low-risk prostate cancer. American journal of men’s health. 2017;11:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Association AU. New Active Surveillance parameters allow for individualised care. April 26th 2009.
- [58].Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–31. [DOI] [PubMed] [Google Scholar]
- [59].Oliffe JL, Davison BJ, Pickles T, Mroz L. The self-management of uncertainty among men undertaking active surveillance for low-risk prostate cancer. Qual Health Res. 2009;19:432–43. [DOI] [PubMed] [Google Scholar]
- [60].Bellardita L, Rancati T, Alvisi MF, Villani D, Magnani T, Marenghi C, et al. Predictors of health-related quality of life and adjustment to prostate cancer during active surveillance. Eur Urol. 2013;64:30–6. [DOI] [PubMed] [Google Scholar]
- [61].Hegarty JM, Wallace M, Comber H. Uncertainty and quality of life among men undergoing active surveillance for prostate cancer in the United States and Ireland. Am J Mens Health. 2008;2:133–42. [DOI] [PubMed] [Google Scholar]
- [62].Wilcox CGD, Louie-Johnsun Anxiety and health related quality of life (HRQL) in patients undergoing active surveillance of prostate cancer in an Australian centre. BJU Int. 2014;113:64–8. [DOI] [PubMed] [Google Scholar]
- [63].Parker PA, Davis JW, Latini DM, Baum G, Wang X, Ward JF, et al. Relationship between illness uncertainty, anxiety, fear of progression and quality of life in men with favourable-risk prostate cancer undergoing active surveillance. BJU Int. 2016;117:469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jeldres C, Cullen J, Hurwitz LM, Wolff EM, Levie KE, Odem-Davis K, et al. Prospective quality-of-life outcomes for low-risk prostate cancer: Active surveillance versus radical prostatectomy. Cancer. 2015;121:2465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Vasarainen H, Lokman U, Ruutu M, Taari K, Rannikko A. Prostate cancer active surveillance and health-related quality of life: results of the Finnish arm of the prospective trial. BJU Int. 2012;109:1614–9. [DOI] [PubMed] [Google Scholar]
- [66].Vanagas G, Mickevičienė A, Ulys A. Does quality of life of prostate cancer patients differ by stage and treatment? Scandinavian Journal of Public Health. 2013;41:58–64. [DOI] [PubMed] [Google Scholar]
- [67].Venderbos LD, Aluwini S, Roobol MJ, Bokhorst LP, Oomens EH, Bangma CH, et al. Long-term follow-up after active surveillance or curative treatment: quality-of-life outcomes of men with low-risk prostate cancer. Qual Life Res. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. New England Journal of Medicine. 2016;375:1425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chen RC, Basak R, Meyer A- M, Kuo T- M, Carpenter WR, Agans RP, et al. Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. Jama. 2017;317:1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Smith DP, King MT, Egger S, Berry MP, Stricker PD, Cozzi P, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ. 2009;339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].van den Bergh RC, Essink-Bot ML, Roobol MJ, Schroder FH, Bangma CH, Steyerberg EW. Do anxiety and distress increase during active surveillance for low risk prostate cancer? J Urol. 2010;183:1786–91. [DOI] [PubMed] [Google Scholar]
- [72].Burnet KL, Parker C, Dearnaley D, Brewin CR, Watson M. Does active surveillance for men with localized prostate cancer carry psychological morbidity? BJU Int. 2007;100:540–3. [DOI] [PubMed] [Google Scholar]
- [73].Davison BJ, Goldenberg SL. Patient acceptance of active surveillance as a treatment option for low-risk prostate cancer. BJU Int. 2011;108:1787–93. [DOI] [PubMed] [Google Scholar]
- [74].Anderson J, Burney S, Brooker JE, Ricciardelli LA, Fletcher JM, Satasivam P, et al. Anxiety in the management of localised prostate cancer by active surveillance. BJU Int. 2014;114 Suppl 1:55–61. [DOI] [PubMed] [Google Scholar]
- [75].Lane A, Metcalfe C, Young GJ, Peters TJ, Blazeby J, Avery KNL, et al. Patient-reported outcomes in the ProtecT randomized trial of clinically localized prostate cancer treatments: study design, and baseline urinary, bowel and sexual function and quality of life. BJU International. 2016;118:869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Korfage I, Essink-Bot M-L, Janssens A, Schröder F, De Koning H. Anxiety and depression after prostate cancer diagnosis and treatment: 5-year follow-up. British journal of cancer. 2006;94:1093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Carter G, Clover K, Britton B, Mitchell AJ, White M, McLeod N, et al. Wellbeing during Active Surveillance for localised prostate cancer: a systematic review of psychological morbidity and quality of life. Cancer Treat Rev. 2015;41:46–60. [DOI] [PubMed] [Google Scholar]
- [78].Xu J, Janisse J, Ruterbusch JJ, Ager J, Liu J, Holmes-Rovner M, et al. Patients’ survival expectations with and without their chosen treatment for prostate cancer. The Annals of Family Medicine. 2016;14:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kendel F, Helbig L, Neumann K, Herden J, Stephan C, Schrader M, et al. Patients’ perceptions of mortality risk for localized prostate cancer vary markedly depending on their treatment strategy. International journal of cancer. 2016;139:749–53. [DOI] [PubMed] [Google Scholar]
- [80].Latini DM, Hart SL, Knight SJ, Cowan JE, Ross PL, Duchane J, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178:826–31; discussion 31–2. [DOI] [PubMed] [Google Scholar]
- [81].Xu J, Dailey RK, Eggly S, Neale AV, Schwartz KL. Men’s perspectives on selecting their prostate cancer treatment. J Natl Med Assoc. 2011;103:468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Venderbos LD, van den Bergh RC, Roobol MJ, Schroder FH, Essink-Bot ML, Bangma CH, et al. A longitudinal study on the impact of active surveillance for prostate cancer on anxiety and distress levels. Psychooncology. 2015;24:348–54. [DOI] [PubMed] [Google Scholar]
- [83].Loeb S, Curnyn C, Fagerlin A, Braithwaite RS, Schwartz MD, Lepor H, et al. Informational needs during active surveillance for prostate cancer: A qualitative study. Patient education and counseling. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Anandadas CN, Clarke NW, Davidson SE, O’Reilly PH, Logue JP, Gilmore L, et al. Early prostate cancer--which treatment do men prefer and why? BJU Int. 2011;107:1762–8. [DOI] [PubMed] [Google Scholar]
- [85].Berger ZD, Yeh JC, Carter HB, Pollack CE. Characteristics and experiences of patients with localized prostate cancer who left an active surveillance program. Patient. 2014;7:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Seiler D, Randazzo M, Leupold U, Zeh N, Isbarn H, Chun FK, et al. Protocol-based active surveillance for low-risk prostate cancer: anxiety levels in both men and their partners. Urology. 2012;80:564–9. [DOI] [PubMed] [Google Scholar]
- [87].Kronenwetter C, Weidner G, Pettengill E, Marlin R, Crutchfield L, McCormac P, et al. A Qualitative Analysis of Interviews of Men With Early Stage Prostate Cancer: The Prostate Cancer Lifestyle Trial. Cancer Nursing. 2005;28:99–107. [DOI] [PubMed] [Google Scholar]
- [88].Kinsella JE, Van Hemelrijck M, Allchorne P, Cahill D. 363 Educational seminars increase confidence and decreases dropout from active surveillance. European Urology Supplements. 2015;14:e363–ea. [DOI] [PubMed] [Google Scholar]
- [89].Wade J, Holding PN, Bonnington S, Rooshenas L, Lane JA, Salter CE, et al. Establishing nurse-led active surveillance for men with localised prostate cancer: development and formative evaluation of a model of care in the ProtecT trial. BMJ Open. 2015;5:e008953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chapple A, Ziebland S, Herxheimer A, McPherson A, Shepperd S, Miller R. Is ‘watchful waiting’ a real choice for men with prostate cancer? A qualitative study. BJU Int. 2002;90:257–64. [DOI] [PubMed] [Google Scholar]
- [91].Katz D, Koppie TM, Wu D, Meng MV, Grossfeld GD, Sadesky N, et al. Sociodemographic characteristics and health related quality of life in men attending prostate cancer support groups. The Journal of urology. 2002;168:2092–6. [DOI] [PubMed] [Google Scholar]
- [92].Bokhorst LP, Lepisto I, Kakehi Y, Bangma CH, Pickles T, Valdagni R, et al. Complications after prostate biopsies in men on active surveillance and its effects on receiving further biopsies in the Prostate cancer Research International: Active Surveillance (PRIAS) study. BJU Int. 2016;118:366–71. [DOI] [PubMed] [Google Scholar]
- [93].Bokhorst LP, Alberts AR, Rannikko A, Valdagni R, Pickles T, Kakehi Y, et al. Compliance Rates with the Prostate Cancer Research International Active Surveillance (PRIAS) Protocol and Disease Reclassification in Noncompliers. Eur Urol. 2015;68:814–21. [DOI] [PubMed] [Google Scholar]
- [94].Sommers BD, Beard CJ, D’Amico AV, Dahl D, Kaplan I, Richie JP, et al. Decision analysis using individual patient preferences to determine optimal treatment for localized prostate cancer. Cancer. 2007;110:2210–7. [DOI] [PubMed] [Google Scholar]
- [95].Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;10. [DOI] [PubMed] [Google Scholar]
- [96].Rollnick S, Miller WR, Butler CC, Aloia MS. Motivational Interviewing in Health Care: Helping Patients Change Behavior. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2008;5:203-. [Google Scholar]
- [97].Joosten EA, DeFuentes-Merillas L, De Weert G, Sensky T, Van Der Staak C, de Jong CA. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychotherapy and psychosomatics. 2008;77:219–26. [DOI] [PubMed] [Google Scholar]
- [98].Spencer JC, Wheeler SB. A systematic review of Motivational Interviewing interventions in cancer patients and survivors. Patient education and counseling. 2016;99:1099–105. [DOI] [PubMed] [Google Scholar]
- [99].Hedestig O, Sandman PO, Widmark A. Living with untreated localized prostate cancer: a qualitative analysis of patient narratives. Cancer Nurs. 2003;26:55–60. [DOI] [PubMed] [Google Scholar]
- [100].Warner G, Packer T, Villeneuve M, Audulv A, Versnel J. A systematic review of the effectiveness of stroke self-management programs for improving function and participation outcomes: self-management programs for stroke survivors. Disability and rehabilitation. 2015;37:2141–63. [DOI] [PubMed] [Google Scholar]
- [101].Rosser BA, Eccleston C. Smartphone applications for pain management. J Telemed Telecare. 2011;17:308–12. [DOI] [PubMed] [Google Scholar]
- [102].Rao A, Hou P, Golnik T, Flaherty J, Vu S. Evolution of data management tools for managing self-monitoring of blood glucose results: a survey of iPhone applications. J Diabetes Sci Technol. 2010;4:949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Frontiers in Pharmacology. 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ahmad A, Sorensen K. Enabling and hindering factors influencing adherence to asthma treatment among adolescents: A systematic literature review. J Asthma. 2016;53:862–78. [DOI] [PubMed] [Google Scholar]
- [105].Dalcin Pde T, Grutcki DM, Laporte PP, Lima PB, Viana VP, Konzen GL, et al. Impact of a short-term educational intervention on adherence to asthma treatment and on asthma control. J Bras Pneumol. 2011;37:19–27. [DOI] [PubMed] [Google Scholar]
- [106].Chapman SC, Barnes N, Barnes M, Wilkinson A, Hartley J, Piddock C, et al. Changing adherence-related beliefs about ICS maintenance treatment for asthma: feasibility study of an intervention delivered by asthma nurse specialists. BMJ Open. 2015;5:e007354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Formica MK, Wason S, Seigne JD, Stewart TM. Impact of a decision aid on newly diagnosed prostate cancer patients’ understanding of the rationale for active surveillance. Patient Educ Couns. 2016. [DOI] [PubMed] [Google Scholar]
- [108].Oude Wesselink SF, Lingsma HF, Robben PB, Mackenbach JP. Guideline adherence and health outcomes in diabetes mellitus type 2 patients: a cross-sectional study. BMC Health Serv Res. 2015;15:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bruinsma SM ZL, Bangma C, Roobol MJ, Steyerberg EW, Nieboer D, Van Hemelrijck M, on behalf ot he Movember Foundation’s Global Actional Plan Prostate Cancer Active Surveillance (GAP3) Consortium. . Worldwide variation in determinants for inclusion and follow-up in active surveillance for low-risk prostate cancer: results of the Movember Foundation’s Global Action Plan Prostate Cancer Active Surveillance (GAP3) Initiative
- [110].Abroms LC, Padmanabhan N, Thaweethai L, Phillips T. iPhone apps for smoking cessation: a content analysis. Am J Prev Med. 2011;40:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cohn RC. A review of the effects of medication delivery systems on treatment adherence in children with asthma. Curr Ther Res Clin Exp. 2003;64:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kazer MW, Bailey DE Jr., Sanda M, Colberg J, Kelly WK. An Internet intervention for management of uncertainty during active surveillance for prostate cancer. Oncol Nurs Forum. 2011;38:561–8. [DOI] [PubMed] [Google Scholar]
- [113].Kirwan M, Vandelanotte C, Fenning A, Duncan MJ. Diabetes self-management smartphone application for adults with type 1 diabetes: randomized controlled trial. Journal of medical Internet research. 2013;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Nowinski SSA, O’Leary B, Loda M, Mirchandani A, Emberton M, Van Hemelrijck M, Grigoriadis A. Systematic identification of functionally relevant risk alleles to stratify aggressive versus indolent prostate cancer. Oncotarget. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


