Abstract
Rapid, accurate, and precise measures of kidney function are essential for the daily management of patients. While plasma and urinary clearances provide the greatest accuracy for assessing glomerular filtration rate (GFR), they are often impractical particularly for the care of children. Serum creatinine, the most commonly used endogenous marker, is simple, convenient, and practical but less accurate because of the influence of non-GFR determinants such as muscle mass which increases with age in children making growth a confounding variable in the interpretation of kidney function from creatinine alone. GFR estimating equations have been developed for adults and children to improve the accuracy of endogenous biomarkers, such as creatinine and cystatin C, by accounting for some of the non-GFR determinants thus enhancing the practitioner’s ability to assess GFR. In the steady state, when height is used as a surrogate for growth, there is a strong correlation between height/SCr and GFR. Current national guidelines recommend that the estimated GFR routinely be reported alongside the creatinine value for adults using the CKD-EPI creatinine-based formula and the updated Schwartz “bedside” formula (CKiD 2009) for children.
Keywords: Glomerular filtration rate (GFR), Estimating equations, Children, Creatinine, Cystatin C
NEED FOR GLOMERULAR FILTRATION RATE MEASUREMENT AND ESTIMATION IN CHILDREN
The ability to accurately, precisely, and efficiently assess kidney function is essential in clinical medicine to facilitate the early detection of acute kidney injury (AKI), to monitor medication-related nephrotoxicity, to make dose adjustments of medications which are toxic to and/or excreted by the kidney, to perform risk assessments for contrast-enhanced imaging studies, as well as for staging CKD and monitoring its progression. The need for precision is underscored by the now well-accepted finding that increases in serum creatinine (Scr) as small as 0.3 mg/dL are associated with increased morbidity and mortality.1,2 Several tools are available to assess kidney function in the steady state but vary in their accuracy, precision, and practicality. Unfortunately, none possess the sensitivity and specificity to detect incipient AKI. The growing epidemic of CKD coupled with disappointing results from AKI therapeutic intervention trials and the recognition that AKI is a risk factor for future CKD has fueled the search for novel markers and methods to assess kidney function. This article will focus on known biomarkers of kidney function and how they are utilized to estimate glomerular filtration rate (GFR) in children.
ASSESSMENT OF GLOMERULAR FUNCTION
GFR is considered the best overall indicator of kidney function but remains challenging to accurately and efficiently measure in clinical practice. Conceptually, it represents the volume of plasma that can be completely cleared of a substance per unit of time. Functionally, the total kidney GFR is determined by the collective sum of nephrons and the GFR within each nephron (single nephron GFR [SNGFR]). A decrease in SNGFR (eg, hypoperfusion) and/or nephron number can thus lead to a decline in kidney function. The impact of early nephron loss may initially be unappreciated as the kidney compensates by increasing SNGFR in the remaining functioning nephrons. Loss of this compensatory ability or “kidney reserve” is one of the earliest manifestations of kidney injury and is even more difficult to measure than GFR.3-6
GFR is a dynamic variable which demonstrates both interindividual variability due to differences in age, gender, and body size as well as intraindividual variability based on hydration status, activity, and protein consumption.7,8 Facilitating comparison of GFR among children and adults requires the absolute GFR in children be normalized to body surface area, which correlates well with kidney weight, the most direct standard of reference.9 In children, there is also a maturational increase in GFR beginning at birth when it is very low (~20 mL/min/1.73 m2) and ultimately culminating in the adult level of ~120 mL/min/1.73 m2 by approximately 2 years of age.10
Although GFR itself cannot be directly measured, it can be assessed by measuring the clearance of an ideal filtration marker or estimated using predictive formulas. Properties of an ideal marker include being freely filtered by the glomerulus without being secreted, reabsorbed, or metabolized by the tubules; additionally, it must be exclusively eliminated by the kidney.11 Urinary and plasma clearances provide the greatest accuracy. The kidney clearance of inulin remains the gold standard for measuring GFR. Inulin, an inert, uncharged 5.2 kDa polymer of fructose fulfills many of the criteria for an ideal marker.11 In the steady state, the filtered load of inulin (GFR × Pin) equals its urinary excretion (Uin × V) where Pin and Uin are the plasma and urine concentrations of inulin (mg/dL), respectively, and V is the urine flow rate (mL/min). The kidney clearance of inulin (Cin) can be calculated: Cin = GFR = (Uin × V)/Pin.
Cin is usually scaled for BSA to facilitate comparison between individuals of differing size:
However, urinary clearance studies are cumbersome and the accuracy highly dependent on ensuring complete bladder drainage, which can be particularly challenging in pediatrics where some children may not be toilet trained or may have urologic disease (eg, vesicoureteral reflux, bladder dyssynergia) and not spontaneously empty their bladder. While placement of a bladder catheter can improve accuracy, it can produce discomfort and is associated with a small risk for infection. A bladder scanner can also be used to confirm voiding to completion but is of limited benefit with VUR.
Plasma disappearance techniques obviate the need for urine collections but still require injection of a filtration marker and generation of a plasma disappearance curve from which the kidney clearance can be calculated by dividing the delivered dose of the tracer by the entire area under the curve12 (Fig. 1). The curve can be well approximated by a double exponential curve characterized by an initial “fast” curve and a subsequent “slow” curve. The “fast” curve reflects the distribution phase during which the tracer diffuses from the intravascular space and early kidney elimination begins, whereas the “slow” curve depicts only kidney elimination. Use of a one-compartment model focusing on the kidney elimination phase simplifies the procedure. GFR can then be derived from the slope of the slow curve but requires incorporation of a correction factor to compensate for overestimation of the GFR, which results from exclusion of the area under the fast curve.12-16 Only 2 blood specimens are needed but must be timed appropriately; the first sample is obtained after the marker has equilibrated (~2 hours postinjection), whereas the second must be obtained at least 5 hours postinjection and potentially longer depending on the degree of CKD. The validity of this model may be compromised if the second sample is obtained too early as GFR can be overestimated from an inaccurate depiction of the lower slope of the plasma disappearance curve especially with low GFR.13
Figure 1.
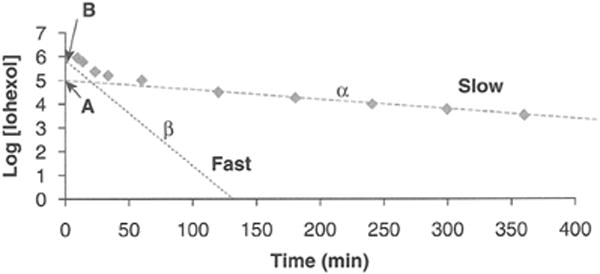
Plasma disappearance of filtration marker as a function of time after injection of marker into blood. The curve is comprised of 2 components: a slow curve with slope α and intercept A and a fast curve with slope β and intercept B. Modified from Schwartz GJ, Furth SL. Glomerular filtration rate measurement and estimation in chronic kidney disease, Pediatr Nephrol 22[11]:1839-1848, 2007.
Given the drawbacks of clearance studies, they are not routinely used in clinical practice but are reserved for research studies and select situations where an accurate assessment of kidney function is necessary (eg, chemotherapy dosing, kidney transplant donation). Scr and GFR estimating equations are used most commonly, as they are convenient, noninvasive, and inexpensive with timely reporting of results making them well suited for serial evaluation.
Creatinine Clearance
Ideally, an endogenous marker such as Scr can serve as a surrogate for GFR if it is produced at a constant rate and eliminated only via glomerular filtration at a rate equivalent to its production such that a steady state exists. Scr is produced at a relatively constant rate and eliminated predominantly by glomerular filtration, but it is also influenced by several factors other than glomerular filtration such as diet and illness, thereby making it a suboptimal biomarker (Table 2). Most notably, it is produced from the nonenzymatic dehydration of muscle creatine thus making it highly dependent on muscle mass. The generation of creatinine in children is therefore also affected by growth, making growth a confounder in the assessment of kidney function from creatinine alone (Fig. 2). At birth, Scr is elevated reflecting the mother’s kidney function, not the infant’s, due to fetal-maternal-placental equilibration; however, GFR is physiologically low (~20 mL/min/1.73 m2 for a full-term infant). Scr gradually declines over the first few weeks to become reflective of the infant’s kidney function, whereas GFR progressively increases after birth until adult levels are reached by approximately 1.5-2 years of age. The Scr level then remains relatively stable for the next 2 years as the infant accrues muscle mass proportionally to the GFR increase. Beyond this, once GFR per body surface area has fully matured, the ongoing accretion of muscle leads to a progressive rise in Scr, especially in boys, until adolescence when adult levels are achieved.17
Table 2.
Non-GFR Factors Affecting Creatinine Levels in Children (Modified From Mian AN, Schwartz GJ. Tests of Kidney Function in Children. In: Fuhrman BP, Zimmerman JJ, eds. Fuhrman and Zimmerman’s Pediatric Critical Care. Fifth ed. 2017:1026-1039)76
| Factor | The Estimated GFR Level
|
|
|---|---|---|
| Increase | Decrease | |
| Affecting creatinine generation | Age (infancy through adolescence) | Chronic illness, anorexia, malnutrition, neuromuscular disease, liver disease |
| Male gender (after puberty) | Body habitus (amputation) | |
| Body habitus (muscular) | Diet (vegetarian) | |
| Weight lifting | ||
| Diet: consumption of cooked meat | ||
| Creatine supplements | ||
| Affecting creatinine elimination | Impaired tubular secretion (trimethoprim, cimetidine) | Tubular secretion |
| Impaired extrarenal elimination (sterilization of gastrointestinal flora by antibiotics) | Extrarenal elimination (gastrointestinal degradation) | |
Abbreviation: GFR, glomerular filtration rate.
Figure 2.
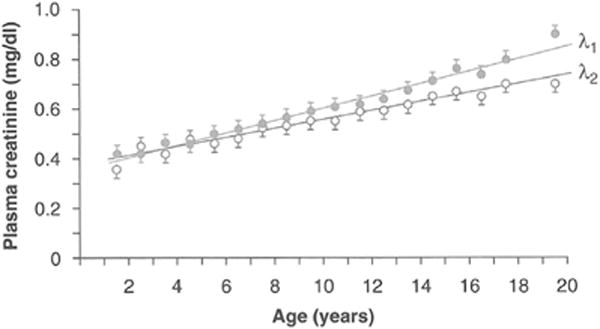
Growth-related change in the estimated GFR during childhood. Mean plasma creatinine vs age demonstrating gender difference in the estimated GFR beginning in adolescence. Standard error limits for estimate of the mean are shown. λ1 ~ males; λ2 ~ females. Reprinted with permission from Schwartz GJ, Haycock GB, Spitzer A. Plasma creatinine and urea concentration in children: normal values for age and sex. J Pediatr. 1976; 88:828-830.
Additionally, although predominantly eliminated by glomerular filtration, a small but variable amount (~10%) of creatinine is excreted by tubular secretion and gastrointestinal degradation.18-20 While these latter two contributions are minimal when kidney function is normal, their impact proportionally increases as kidney function deteriorates resulting in an overestimation of kidney function based on Scr alone. It is well recognized that Scr is an insensitive marker for detection of small changes in kidney function, a shortcoming which becomes magnified at low levels of creatinine typical for infants/small children (normal creatinine 0.2-0.4 mg/dL). For example, if approximately 50% of remaining kidney function is lost for every doubling of Scr, significant kidney function will have been lost before this is reflected by any rise in Scr in young children. Finally, until recently, the assay for creatinine was not standardized and an IFCC (International Federation of Clinical Chemistry) reference standard against which the assay could be calibrated was not available, thereby making analytical factors another potential source of error in creatinine measurements. An international effort was launched in the mid-2000s requiring standardization of creatinine assays and calibration against the IFCC standard. Unfortunately, the lowest IFCC standard is 1 mg/dL which is significantly higher than the range of normal creatinine for infants and young children and those with decreased muscle mass.21,22
The challenge associated with assessing kidney function from Scr has driven the search for alternative biomarkers of kidney function. Of the candidate biomarkers, cystatin C, a 13 kDa cationic cysteine protease inhibitor produced at a constant rate by all nucleated cells, has shown the greatest promise and has been the most extensively studied.23,24 It is freely filtered by the glomerulus, but unlike creatinine, it is not secreted by the tubules, but instead, it is reabsorbed and metabolized by the proximal tubule epithelial cells.25,26 It is generally considered to not be significantly affected by age, gender, and muscle mass making it a potentially more useful biomarker of kidney function in growing children and those with atypical body composition/ reduced muscle mass (eg, malnourished, anorexia, spina bifida, neuromuscular disease).27-29 However, like creatinine, cystatin C also has its limitations and can be affected by other confounding factors such as glucocorticoids and thyroid dysfunction.30
GLOMERULAR FILTRATION RATE ESTIMATING EQUATIONS
Given the limitations of Scr, prediction formulas have been developed to enhance the practitioner’s ability to more accurately assess kidney function. These formulas incorporate many of the non-GFR determinants including clinical variables such as height, weight, and gender as surrogates for muscle mass. Prediction formulas are particularly indispensable in pediatrics where the level of Scr corresponding to normal kidney function/GFR varies with age.
The original pediatric GFR formulas were derived independently by Schwartz and Counahan-Barratt in the mid-1970s.31,32 (Table 3) The equations take the same form using height as a surrogate for growth and demonstrating a strong correlation between GFR and Ht/SCr.31-34 They differ in the proportionality constant which reflects the relationship between urinary creatinine excretion and units of body size; the discrepancy is attributed to differences in creatinine assay methodology. The original Schwartz formula yielding a constant at 0.55 was developed in children with CKD using a modified Jaffe assay and inulin clearance as the reference standard. In subsequent studies, the proportionality constant was adjusted to better account for age and gender-related differences.35 Both equations assume a steady state and normal body habitus. The Jaffe assay, based on an alkaline picrate colorimetric reaction, tends to falsely elevate the true Scr by up to 20% (for creatinine ~1 mg/dL) due to the presence of interfering, noncreatinine chromogens, the most significant of which is total protein.36 This effect is greatest at the lower levels of creatinine typically observed in infants and young children.35
Table 3.
GFR Estimating Equations
| Pediatric estimating equations | |
|---|---|
| Schwartz “bedside”31,35 (original—1976) | eGFR = k × L (cm)/PCr (mg/dL) where k ~ 0.33 (preterm infant), k ~ 0.45 (full term), k ~ 0.55 (children and adolescent females), k ~ 0.7 (adolescent males) |
| Counahan-Barratt32 | eGFR = 0.43 × L (cm)/PCr (mg/dL) |
| Updated Schwartz (CKiDCr)39 | eGFR = 0.413 × L (cm)/PCr (mg/dL) |
| CKiDCys-C (Schwartz “bedside” cystatin C)33 | eGFR = 70.69 × [cystatin C (mg/L) ]−0.931 |
| CKiDCr - Cys-C33 (Combined CKiD creatinine—cystatin C) | eGFR = 39.8 × [height (m)/SCr (mg/dL)]0.456 × [1.8/cystatin C (mg/L)]0.418 × [30/BUN (mg/dL)]0.079 × [1.076]gender × [height (m)/1.4]0.179 |
| Pottel and colleagues56 | eGFR = 107.3/(SCr/Q) where Q ~ population normalized the estimated GFR |
| Adult estimating equation CKD-EPICr77 | eGFR = 141 × min [SCr/κ, 1]α × max [SCr/κ, 1]−1·209 × 0.993age × 1.108 (if female) × 1.159 (if black) where κ = 0.7, α = −0.329 (females) and κ = 0.9, α = −0.411 (males); min = min of SCr/κ or 1; max = max of SCr/κ or 1 |
Abbreviations: GFR, glomerular filtration rate; eGFR, estimated GFR.
Where eGFR is expressed in mL/min/1.73 m2; L ~ length/height in cm; PCr and SCr ~ plasma or the estimated GFR in mg/dL, cystatin C in mg/L, BUN in mg/dL.
where gender = 1 if male and 0 if female
The Schwartz formula remains the most widely used prediction formula for assessing kidney function in children, as its simplicity facilitates application at the bedside. However, the changes in assay methodology have led to systematically lower creatinine values and subsequent overestimation of GFR when used with the original bedside formula.37 Many hospitals/laboratories, however, have now shifted to an enzymatic assay which has greater specificity than the conventional Jaffe assay resulting in 20-30% lower creatinine levels and which run closer to accurate HPLC-derived creatinines.22,38 For those laboratories which continue to use the modified Jaffe assay, as it is notably less expensive, Speeckaert and colleagues published a correction accounting for the contribution of serum protein to Jaffe creatinines. When applied, this correction yields a creatinine value more consistent with that obtained using an enzymatic creatinine assay.36
In 2009, using data from the CKiD Study (Chronic Kidney Disease in Children Study), Schwartz and colleagues39 developed an updated “bedside” formula based on a standardized creatinine method traceable to isotope dilution mass spectrometry (IDMS) and using the plasma disappearance of iohexol as the reference standard. Notably, this is the first multicenter study to generate an estimating formula in children with moderate CKD (GFR range 15-75 mL/min/1.73 m2; ages 1-16 years). Using the updated Schwartz formula, approximately 80% of estimated GFR (eGFR) values fell within 30% of the GFR measured by iohexol plasma disappearance and 37% fell within 10% (P30 ~80% and P10 37%).40
Given the limitations of creatinine, cystatin C–based GFR estimating equations have also been developed in an effort to improve accuracy. Several equations have been published both for use in adults as well as children.41-44 They vary in accuracy and precision, but appear at least as good as the creatinine-based equation42 for the general population. In high-risk populations with reduced muscle mass such as oncology patients, hematopoietic stem cell transplant recipients, spina bifida/muscular dystrophy, and spinal cord injury patients, cystatin C–based formulas seem to more accurately estimate the measured GFR than creatinine-based equations.45-49 The univariate cystatin C equation for children with CKD to facilitate use in the clinical setting33 is as follows:
However, cystatin C is not readily available in many hospital laboratories and is more expensive, and assays have not yet been standardized in the United States.30 As the cystatin C–based equations do not outperform the creatinine-based formulas in the general population, cystatin C is not recommended for routine assessment of kidney function but may be considered for special clinical situations.33 Formulas combining both creatinine and cystatin C provide a better estimate than either one alone.34,42 Using data from the CKiD study, Schwartz and colleagues33 developed a new multivariate eGFR equation incorporating both cystatin C and Scr in addition to height, BUN, and gender:
where gender = 1 for male and 0 for female. Using this formula, over 90% of the eGFRs were within 30% of the GFR measured by iohexol, the reference standard for this study, and 48% were within 10%.33,39 The equation is valid in the range of 15-75 mL/min/1.73 m2 and uses a standardized creatinine measurement and nephelometric assay for cystatin C (Siemens Dade Behring).33 The cystatin C values have not been referenced to an IFCC calibration yet, and cystatin C assays have not been appropriately standardized.50,51
Though several pediatric formulas are available for estimating GFR based on creatinine or cystatin C, current national guidelines recommend use of the updated “bedside” Schwartz formula as it is considered to perform better than the other equations.33 The equation, however, is only valid only with standardized Scr methods traceable to IDMS. For those in whom a more precise estimate is needed or in whom the use of a creatinine-based formula may be inaccurate, Grubb and CKiD34 suggest confirmation with a univariate cystatin C–based formula. If the two estimates are similar (within 10-15% agreement), use of the more complex multivariate cystatin C creatinine formula provides an estimated GFR which approximates the measured GFR. If, however, the univariate creatinine-based and cystatin C–based eGFRs are discrepant, a measured GFR is recommended.
CONSIDERATIONS FOR GROUPS NOT WELL REPRESENTED IN THE CKiD POPULATION
Healthy Children
The validity of Schwartz’s bedside equation when applied to children with mild CKD or normal kidney function is unclear given the formula was developed in children with CKD stages 2-4.39 Several studies have attempted to address this issue and have yielded conflicting results, with some demonstrating good performance, whereas others did not; the direction of bias was variable.52-55
Pottel’s group56 developed an estimating equation for children based on a population normalized Scr value Q (average creatinine for healthy children of a specific age) generated from a large Belgian Hospital database:
Using the concept of a population normalized Scr, they developed and validated 2 new eGFR equations for use in children, adolescents, and young adults.57 In one, Q is based on height (Qheight), whereas in the other, Q is height independent and based on age and gender (Qage). Though both equations have been validated in a Belgian cohort, the Qheight-based equation was more accurate across all ages and levels of kidney function and also performed better in underweight subjects further supporting the premise that height can be used as a good surrogate for muscle mass. The height independent Qage-based equation, however, may potentially allow development of a screening tool for kidney dysfunction in children as well as facilitate routine reporting by laboratories.34 Further studies are needed to assess the applicability of this formula and the validity of the technique in other diverse pediatric populations.58
Adolescents/Young Adults
Assessment of kidney function in this group can be particularly challenging as estimation of GFR using the CKiDCr and CKD-EPICr (Chronic Kidney Disease Epidemiology Collaboration) equations can yield significantly discrepant results. To help address this issue, Selistre and colleagues59 performed a retrospective cross-sectional study comparing use of the CKiDCr and CKD-EPICr equations in over 10,000 subjects ages 3-90 years with measured GFRs (inulin clearance) ranging 3-160 mL/min/1.73 m2. The investigators found that the CKiDCr equation performed slightly better than the CKD-EPICr equation in the 18-40 years old age group with mild-to-moderate kidney impairment; P30 accuracy for CKiDCr equation was 86.5% vs 81% for CKD-EPICr equation. It is hypothesized that CKD-EPICr may be less accurate in this age range because it incorporates a correction factor for the age-related decline in GFR, but this decline is not thought to occur until ~ age 40-50 years.59
Ng, Schwartz and colleagues specifically investigated this group by studying 219 young adults age 18-26 years with pediatric CKD using iohexol plasma clearance (iGFR) as a reference standard. Use of the CKiDCr formula resulted in an underestimation of iGFR, whereas CKD-EPIcr resulted in an overestimation. Use of the pediatric (CKiD) or adult (CKD-EPI) equations which combined creatinine and cystatin C yielded results comparable to iGFR. Working only with the creatinine-based formulas, as cystatin C is not always clinically available, the investigators found that averaging the eGFRs obtained using the Schwartz bedsidecr (CKiD cr) formula and the CKD-EPIcr formula also yielded results comparable to iGFR and CKiD Cr- Cys C with 80% of the estimates obtained using this technique falling within 30% of iGFR (P30 = 80%). (Manuscript submitted, Ng and colleagues).
Pottel and colleagues58 suggested a different approach proposing a full age spectrum equation by extending use of the pediatric equation through age 40 years; beyond that age, the equation is modified by incorporating an adjustment factor to account for the age-related decline in GFR. The equation is appealing as it provides continuity in GFR estimates from childhood through adolescence and adulthood. The equation developed in a European Caucasian population needs to be validated in diverse populations.60
Neonates
Neonates, especially those born preterm, are particularly vulnerable to the development of AKI.61 Use of Scr to assess kidney function in this population poses some unique challenges. Notably, Scr at birth is elevated but not reflective of the infant’s kidney function because of placental equilibration of creatinine. Over the ensuing days/weeks, as GFR increases the creatinine gradually falls until it reflects the infant’s kidney function. Kidney dysfunction may therefore manifest as either a rise in creatinine or a failure to fall appropriately. In this regard, cystatin C may prove to be a better biomarker as it does not cross the placenta and therefore may more accurately reflect the infant’s kidney function at birth.62 Further, the observed maturational changes in cystatin C seem to follow those of GFR better. At birth, serum cystatin C levels are elevated presumably reflecting the lower physiologic GFR. As the GFR increases, cystatin C levels fall reaching a plateau by approximately 1.5-2 years of age corresponding to the full maturation of GFR.30,63 Cystatin C levels then remain constant until ~50 years of age as does GFR.27 Predictive formulas to estimate kidney function in neonates are greatly needed but sorely lacking. Schwartz has proposed adjusting the original height/Scr formula with a proportionality constant to account for the change in analytical factors (change in creatinine assay and standardization of creatinine) though this has not yet been validated. Using this approach, the new constants would be 0.34 for full-term infants and 0.25 for preterm infants (Schwartz GJ, unpublished observations).
Acute Kidney Injury
Timely estimates of kidney function are essential in the setting of AKI and/or clinical situations associated with a high risk of developing AKI (eg, sepsis). Although none of the GFR estimating equations is truly appropriate for assessing kidney function during AKI, as it is not a steady-state situation, suitable alternatives do not exist. Clearly, day-to-day estimates of GFR in the ICU are exceedingly inaccurate because of the rapid changes in function and lag in changes in Scr. However, the change in Scr probably gives the clinician some important information. For example, if Scr does not change, it suggests the function is stable. Should Scr increase by 0.5 mg/dL in 24 h, this would suggest very poor function and conversely, should Scr decrease by 0.5 mg/dL in 24 h, this would suggest rapidly improving function.
In summary, GFR prediction formulas enhance the clinician’s ability to assess kidney function. Current national guidelines recommend use of the CKiD creatinine-based equation (updated bedside Schwartz) for estimating kidney function in children and the 2009 CKD-EPI formula for adults. Although these formulas are creatinine-based and, therefore, subject to some of the same constraints as use of Scr alone (eg, effects of tubular secretion), they are practical and provide a more accurate estimate of kidney function than Scr alone. Several equations exist for both creatinine-based and cystatin C–based estimates. Proper use and interpretation of results from these equations require knowledge of the population in which the formula was derived (healthy vs CKD, age range, race), knowledge of analytical factors (assay used to measure creatinine or cystatin C and whether or not the measured analyte is traceable to an IDMS standard), and knowledge of the approach used for the reference standard (plasma vs urinary clearance, possible limitations of marker used). The ideal estimating equation would be simple to use at the bedside and developed in a diverse population (full spectrum of kidney function and age; multiracial/multiethnic) using a standardized assay for the analytes and an IDMS traceable biomarker. We have indeed made considerable progress in our ability to assess kidney function in children, but there is much work yet to be done.
Table 1.
Tests of Kidney Function (Modified From Levey AS, Inker LA. Assessment of Glomerular Filtration Rate in Health and Disease: a State of the Art Review. Clinical Pharmacology and Therapeutics. 2017;102:405-419)64
| Approach | Study Design | Advantages | Limitations |
|---|---|---|---|
| Urinary clearance + exogenous marker (inulin, EDTA, iothalamate) | Bladder catheter + continuous infusion of marker | Gold standard (inulin) | Timed blood/urine collections; vascular access; discomfort with catheter; small risk of infection; limited availability (inulin) |
| Spontaneous voiding ± bladder scanner | Comfort; scanner confirms bladder drainage | Timed urine collections: less accurate if incomplete bladder drainage | |
| Urinary clearance of creatinine | 24-hr creatinine clearance | No infusion | Secretion of Cr and incomplete collection decrease accuracy |
| Cimetidine creatinine clearance65 | ↑ accuracy by blocking Cr secretion; shorter test | Timed urine collections; less accurate if incomplete collection | |
| Plasma clearance | Accuracy; No urine collections | Vascular access/blood samples; longer study if advanced CKD | |
| Radiopharmaceuticals 51Cr-EDTA13 125I-Iothalamate66, 67 99mTc-DTPA67,68 |
Accuracy | Radiation exposure69 51Cr-EDTA: not available in US 125I-Iothalamate and 99mTc-DTPA: protein binding and Cr secretion |
|
| Nonradiolabeled tracer: iohexol15,70-75 | Accuracy comparable to inulin; low dose | Expensive assay if use low dose |
Abbreviation: Cr, creatinine.
CLINICAL SUMMARY.
Glomerular filtration rate (GFR) estimating equations provide more accurate estimates of kidney function in children than the serum creatinine alone.
National guidelines recommend use of the creatinine-based updated Schwartz formula (CKiD) for estimating GFR in children and the CKD-EPI formula for estimating GFR in adults.
For young adults, CKiD formula underestimates estimated GFR (eGFR), whereas CKD-EPI overestimates eGFR. Averaging results from the 2 formulas provided an eGFR similar to an iohexol GFR.
Cystatin C–based equations do not outperform the creatinine-based formulas in the general population and are therefore not recommended for routine assessment of kidney function but may be considered for special clinical situations in which patients have reduced muscle mass.
Acknowledgments
This study was supported by NIDDK U01-DK82194.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Akcan-Arikan A, Zappitelli M, Loftis LL, et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 3.Bosch JP. Renal reserve: a functional view of glomerular filtration rate. Semin Nephrol. 1995;15:381–385. [PubMed] [Google Scholar]
- 4.Bosch JP, Saccaggi A, Lauer A, et al. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am J Med. 1983;75:943–950. doi: 10.1016/0002-9343(83)90873-2. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Iturbe B, Herrera J, Garcia R. Response to acute protein load in kidney donors and in apparently normal postacute glomerulonephritis patients: evidence for glomerular hyperfiltration. Lancet. 1985;2:461–464. doi: 10.1016/s0140-6736(85)90399-x. [DOI] [PubMed] [Google Scholar]
- 6.Rodenbach KE, Fuhrman DY, Maier PS, et al. Renal Response to a protein load in healthy young adults as determined by iohexol Infusion clearance, cimetidine-Inhibited creatinine clearance, and cystatin C estimated glomerular filtration rate. J Ren. 2017;27:275–281. doi: 10.1053/j.jrn.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Smith H. The Kidney: Structure and Function in Health and Disease. NY: Oxford University Press; 1951. Measurement of the filtration rate; pp. 39–62. [Google Scholar]
- 8.Smith H. The Kidney: Structure and Function in Health and Disease. NY: Oxford University Press; 1951. Trophic and other factors related to kidney function; pp. 461–491. [Google Scholar]
- 9.Smith H. The Kidney: Structure and Function in Health and Disease. NY: Oxford University Press; 1951. Renal function in infancy and childhood; pp. 492–519. [Google Scholar]
- 10.Heilbron DC, Holliday MA, al-Dahwi A, et al. Expressing glomerular filtration rate in children. Pediatr Nephrol. 1991;5:5–11. doi: 10.1007/BF00852829. [DOI] [PubMed] [Google Scholar]
- 11.Smith H. The Kidney: Structure and Function in Health and Disease. NY: Oxford University Press; 1951. The reliability of inulin as a measure of glomerular filtration; pp. 231–238. [Google Scholar]
- 12.Brochner-Mortensen J. Current status on assessment and measurement of glomerular filtration rate. Clin Physiol. 1985;5:1–17. doi: 10.1111/j.1475-097x.1985.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 13.Blaufox MD, Aurell M, Bubeck B, et al. Report of the Radionuclides in Nephrourology Committee on renal clearance. J Nucl Med. 1996;37:1883–1890. [PubMed] [Google Scholar]
- 14.Piepsz A, Colarinha P, Gordon I, et al. Guidelines for glomerular filtration rate determination in children. Eur J Nucl Med. 2001;28:BP31–BP36. [PubMed] [Google Scholar]
- 15.Schwartz GJ, Abraham AG, Furth SL, et al. Optimizing iohexol plasma disappearance curves to measure the glomerular filtration rate in children with chronic kidney disease. Kidney Int. 2010;77:65–71. doi: 10.1038/ki.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng DK, Schwartz GJ, Jacobson LP, et al. Universal GFR determination based on two time points during plasma iohexol disappearance. Kidney Int. 2011;80:423–430. doi: 10.1038/ki.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Haycock GB, Spitzer A. Plasma creatinine and urea concentration in children: normal values for age and sex. J Pediatr. 1976;88:828–830. doi: 10.1016/s0022-3476(76)81125-0. [DOI] [PubMed] [Google Scholar]
- 18.Bauer JH, Brooks CS, Burch RN. Clinical appraisal of creatinine clearance as a measurement of glomerular filtration rate. Am J Kidney Dis. 1982;2:337–346. doi: 10.1016/s0272-6386(82)80091-7. [DOI] [PubMed] [Google Scholar]
- 19.Mitch WE, Walser M. A proposed mechanism for reduced creatinine excretion in severe chronic renal failure. Nephron. 1978;21:248–254. doi: 10.1159/000181400. [DOI] [PubMed] [Google Scholar]
- 20.Dunn SR, Gabuzda GM, Superdock KR, et al. Induction of creatininase activity in chronic renal failure: timing of creatinine degradation and effect of antibiotics. Am J Kidney Dis. 1997;29:72–77. doi: 10.1016/s0272-6386(97)90010-x. [DOI] [PubMed] [Google Scholar]
- 21.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the laboratory working group of the national kidney disease Education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz GJ, Kwong T, Erway B, et al. Validation of creatinine assays utilizing HPLC and IDMS traceable standards in sera of children. Pediatr Nephrol. 2009;24:113–119. doi: 10.1007/s00467-008-0957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 24.Roos JF, Doust J, Tett SE, et al. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children–a meta-analysis. Clin Biochem. 2007;40:383–391. doi: 10.1016/j.clinbiochem.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Tenstad O, Roald AB, Grubb A, et al. Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest. 1996;56:409–414. doi: 10.3109/00365519609088795. [DOI] [PubMed] [Google Scholar]
- 26.Jacobsson B, Lignelid H, Bergerheim US. Transthyretin and cystatin C are catabolized in proximal tubular epithelial cells and the proteins are not useful as markers for renal cell carcinomas. Histopathology. 1995;26:559–564. doi: 10.1111/j.1365-2559.1995.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 27.Finney H, Newman DJ, Thakkar H, et al. Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Child. 2000;82:71–75. doi: 10.1136/adc.82.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bokenkamp A, Domanetzki M, Zinck R, et al. Reference values for cystatin C serum concentrations in children. Pediatr Nephrol. 1998;12:125–129. doi: 10.1007/s004670050419. [DOI] [PubMed] [Google Scholar]
- 29.Bokenkamp A, Domanetzki M, Zinck R, et al. Cystatin C–a new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101:875–881. doi: 10.1542/peds.101.5.875. [DOI] [PubMed] [Google Scholar]
- 30.Andersen TB, Eskild-Jensen A, Frokiaer J, et al. Measuring glomerular filtration rate in children; can cystatin C replace established methods? A review. Pediatr Nephrol. 2009;24:929–941. doi: 10.1007/s00467-008-0991-y. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz GJ, Haycock GB, Edelmann CM, Jr, et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 32.Counahan R, Chantler C, Ghazali S, et al. Estimation of glomerular filtration rate from plasma creatinine concentration in children. Arch Dis Child. 1976;51:875–878. doi: 10.1136/adc.51.11.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82:445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz GJ. Height: the missing link in estimating glomerular filtration rate in children and adolescents. Nephrol Dial Transplant. 2014;29:944–947. doi: 10.1093/ndt/gft530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North America. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 36.Speeckaert MM, Wuyts B, Stove V, et al. Compensating for the influence of total serum protein in the Schwartz formula. Clin Chem Lab Med. 2012;50:1597–1600. doi: 10.1515/cclm-2012-0033. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz GJ, Furth S, Cole SR, et al. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 38.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 39.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832–1843. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 41.Levey AS, Fan L, Eckfeldt JH, et al. Cystatin C for glomerular filtration rate estimation: coming of age. Clin Chem. 2014;60:916–919. doi: 10.1373/clinchem.2014.225383. [DOI] [PubMed] [Google Scholar]
- 42.Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis. 2014;63:820–834. doi: 10.1053/j.ajkd.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng F, Finer G, Haymond S, et al. Applicability of estimating glomerular filtration rate equations in pediatric patients: comparison with a measured glomerular filtration rate by iohexol clearance. Transl Res. 2015;165:437–445. doi: 10.1016/j.trsl.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fadrowski JJ, Neu AM, Schwartz GJ, et al. Pediatric GFR estimating equations applied to adolescents in the general population. Clin J Am Soc Nephrol. 2011;6:1427–1435. doi: 10.2215/CJN.06460710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan C, Senthilselvan A, Bamforth F, et al. Correlation between cystatin C- and renal scan-determined glomerular filtration rate in children with spina bifida. Pediatr Nephrol. 2008;23:329–332. doi: 10.1007/s00467-007-0613-0. [DOI] [PubMed] [Google Scholar]
- 46.Nehus EJ, Laskin BL, Kathman TI, et al. Performance of cystatin C-based equations in a pediatric cohort at high risk of kidney injury. Pediatr Nephrol. 2013;28:453–461. doi: 10.1007/s00467-012-2341-3. [DOI] [PubMed] [Google Scholar]
- 47.Erlandsen EJ, Hansen RM, Randers E, et al. Estimating the glomerular filtration rate using serum cystatin C levels in patients with spinal cord injuries. Spinal Cord. 2012;50:778–783. doi: 10.1038/sc.2012.52. [DOI] [PubMed] [Google Scholar]
- 48.Laskin BL, Nehus E, Goebel J, et al. Estimated versus measured glomerular filtration rate in children before hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:2056–2061. doi: 10.1016/j.bbmt.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braat E, Hoste L, De Waele L, et al. Renal function in children and adolescents with Duchenne muscular dystrophy. Neuromuscul Disord. 2015;25:381–387. doi: 10.1016/j.nmd.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Eckfeldt JH, Karger AB, Miller WG, et al. Performance in measurement of serum cystatin C by laboratories Participating in the College of American Pathologists 2014 CYS Survey. Arch Pathol Lab Med. 2015;139:888–893. doi: 10.5858/arpa.2014-0427-CP. [DOI] [PubMed] [Google Scholar]
- 51.Bargnoux AS, Pieroni L, Cristol JP, et al. Multicenter evaluation of cystatin C measurement after assay standardization. Clin Chem. 2017;63:833–841. doi: 10.1373/clinchem.2016.264325. [DOI] [PubMed] [Google Scholar]
- 52.Selistre L, De Souza V, Cochat P, et al. GFR estimation in adolescents and young adults. J Am Soc Nephrol. 2012;23:989–996. doi: 10.1681/ASN.2011070705. [DOI] [PubMed] [Google Scholar]
- 53.Gao A, Cachat F, Faouzi M, et al. Comparison of the glomerular filtration rate in children by the new revised Schwartz formula and a new generalized formula. Kidney Int. 2013;83:524–530. doi: 10.1038/ki.2012.388. [DOI] [PubMed] [Google Scholar]
- 54.Staples A, LeBlond R, Watkins S, et al. Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol. 2010;25:2321–2326. doi: 10.1007/s00467-010-1598-7. [DOI] [PubMed] [Google Scholar]
- 55.Bacchetta J, Cochat P, Rognant N, et al. Which creatinine and cystatin C equations can be reliably used in children? Clin J Am Soc Nephrol. 2011;6:552–560. doi: 10.2215/CJN.04180510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pottel H, Hoste L, Martens F. A simple height-independent equation for estimating glomerular filtration rate in children. Pediatr Nephrol. 2012;27:973–979. doi: 10.1007/s00467-011-2081-9. [DOI] [PubMed] [Google Scholar]
- 57.Hoste L, Dubourg L, Selistre L, et al. A new equation to estimate the glomerular filtration rate in children, adolescents and young adults. Nephrol Dial Transplant. 2014;29:1082–1091. doi: 10.1093/ndt/gft277. [DOI] [PubMed] [Google Scholar]
- 58.Pottel H, Hoste L, Dubourg L, et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transpl. 2016;31:798–806. doi: 10.1093/ndt/gfv454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Selistre L, Rabilloud M, Cochat P, et al. Comparison of the Schwartz and CKD-EPI equations for estimating glomerular filtration rate in children, adolescents, and adults: a retrospective cross-sectional study. PLoS Med. 2016;13:e1001979. doi: 10.1371/journal.pmed.1001979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delanaye P, Ebert N, Pottel H. Newer GFR estimating equations require validation in different populations. Am J Kidney Dis. 2017;70:586. doi: 10.1053/j.ajkd.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 61.Jetton JG, Boohaker LJ, Sethi SK, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. The Lancet Child & Adolescent Health. doi: 10.1016/S2352-4642(17)30069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bokenkamp A, Dieterich C, Dressler F, et al. Fetal serum concentrations of cystatin C and beta2-microglobulin as predictors of postnatal kidney function. Am J Obstetrics Gynecol. 2001;185:468–475. doi: 10.1067/mob.2001.115283. [DOI] [PubMed] [Google Scholar]
- 63.Nakashima T, Inoue H, Fujiyoshi J, et al. Longitudinal analysis of serum cystatin C for estimating the glomerular filtration rate in pre-term infants. Pediatr Nephrol. 2016;31:983–989. doi: 10.1007/s00467-015-3309-x. [DOI] [PubMed] [Google Scholar]
- 64.Levey AS, Inker LA. Assessment of glomerular filtration rate in health and disease: a state of the Art review. Clin Pharmacol Ther. 2017;102:405–419. doi: 10.1002/cpt.729. [DOI] [PubMed] [Google Scholar]
- 65.Hellerstein S, Berenbom M, Alon US, et al. Creatinine clearance following cimetidine for estimation of glomerular filtration rate. Pediatr Nephrol. 1998;12:49–54. doi: 10.1007/s004670050402. [DOI] [PubMed] [Google Scholar]
- 66.Odlind B, Hallgren R, Sohtell M, et al. Is 125I iothalamate an ideal marker for glomerular filtration? Kidney Int. 1985;27:9–16. doi: 10.1038/ki.1985.3. [DOI] [PubMed] [Google Scholar]
- 67.Perrone RD, Steinman TI, Beck GJ, et al. Utility of radioisotopic filtration markers in chronic renal insufficiency: simultaneous com-parison of 125I-iothalamate, 169Yb-DTPA, 99mTc-DTPA, and inulin. The Modification of Diet in Renal Disease Study. Am J Kidney Dis. 1990;16:224–235. doi: 10.1016/s0272-6386(12)81022-5. [DOI] [PubMed] [Google Scholar]
- 68.Carlsen JE, Moller ML, Lund JO, et al. Comparison of four commercial Tc-99m(Sn)DTPA preparations used for the measurement of glomerular filtration rate: concise communication. J Nucl Med. 1980;21:126–129. [PubMed] [Google Scholar]
- 69.Stabin M, Taylor A, Jr, Eshima D, et al. Radiation dosimetry for technetium-99mMAG3, technetium-99m-DTPA, and iodine-131-OIH based on human biodistribution studies. J Nucl Med. 1992;33:33–40. [PubMed] [Google Scholar]
- 70.Back SE, Krutzen E, Nilsson-Ehle P. Contrast media as markers for glomerular filtration: a pharmacokinetic comparison of four agents. Scand J Clin Lab Invest. 1988;48:247–253. doi: 10.3109/00365518809167491. [DOI] [PubMed] [Google Scholar]
- 71.Gaspari F, Perico N, Ruggenenti P, et al. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol. 1995;6:257–263. doi: 10.1681/ASN.V62257. [DOI] [PubMed] [Google Scholar]
- 72.Nilsson-Ehle P, Grubb A. New markers for the determination of GFR: iohexol clearance and cystatin C serum concentration. Kidney International Suppl. 1994;47:S17–S19. [PubMed] [Google Scholar]
- 73.Soveri I, Berg UB, Bjork J, et al. Measuring GFR: a systematic review. Am J Kidney Dis. 2014;64:411–424. doi: 10.1053/j.ajkd.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 74.Stake G, Monn E, Rootwelt K, et al. The clearance of iohexol as a measure of the glomerular filtration rate in children with chronic renal failure. Scand J Clin Lab Invest. 1991;51:729–734. doi: 10.3109/00365519109104587. [DOI] [PubMed] [Google Scholar]
- 75.Vicente FB, Vespa GK, Carrara F, et al. Determination of iohexol in human serum by a semi-automated liquid chromatography tandem mass spectrometry method. Clin Biochem. 2015;48:679–685. doi: 10.1016/j.clinbiochem.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 76.Mian AN, Schwartz GJ. Tests of kidney function in children. In: Fuhrman BP, Zimmerman JJ, editors. Fuhrman and Zimmerman’s Pediatric Critical Care. 5th. 2017. pp. 1026–1039. [Google Scholar]
- 77.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]


