Abstract
Non-native ruffe (Gymnocephalus cernua; family Percidae) were first detected in the Laurentian Great Lakes in 1986, and are not included in regional larval fish keys which were published several years prior to their discovery. In addition, subsequent scientific literature has inconsistently described ruffe larvae. As a result, identification of larval ruffe remains challenging. We used traditional morphology paired with DNA technology to develop diagnostics for ruffe larvae collected in the lower St. Louis River, and compared them to similar species. We found that ruffe < 6 mm total length phenotypically resemble centrarchids, like black crappie, bluegill, and pumpkinseed, but have myomere counts that are intermediate between values for both common percid and centrarchid species. We suggest that developmental and pigment patterns as well as morphometrics can be used to distinguish ruffe from similar species at this size. At larger sizes, ruffe increasingly resemble other percids such as yellow perch, but can be distinguished using myomere counts and morphological features. The findings presented here clarify conflicting descriptions in the scientific literature, and provide additional data to support more confident morphological identification of larval ruffe.
Keywords: Ruffe, Fish Larvae, Identification, St. Louis River, Great Lakes
Introduction
Fish larvae are collected in a variety of research and monitoring activities, including species composition studies, identification of nursery areas and dispersal patterns (Allen and Barker, 1990; King, 2004; Robinson et al., 1998; Schluderman et al., 2012), and habitat management (Humphries et al., 2002). However, taxonomic identification of field-collected fish larvae remains challenging because taxonomic keys for larval fish often lack descriptions for various stages of development, or for entire species. This is especially true for recently established non-native species. For example, ruffe (Gymnocephalus cernua; family Percidae) were introduced to the Laurentian Great Lakes in 1986 (Pratt, 1988), but are not included in the Great Lakes larval fish key (Auer, 1982a), which was published prior to their detection.
Ruffe larvae have been described in subsequent peer-reviewed literature, but aspects of these descriptions are inconsistent and even contradictory. In particular, myomere counts, a key meristic and diagnostic used in taxonomic identification, are inconsistently described in the scientific literature (French and Edsall, 1992; Simon and Vondruska, 1991). Descriptions of larval pigment patterns and morphometrics are also inconsistent among the different literature describing larval ruffe. Like meristics, these features can be useful for distinguishing and identifying larval fishes (Bani et al., 2015; Kendall et al., 1984), but unlike meristics, often change with developmental stage. Further, pigment and morphometrics may vary as a result of environmental conditions (Blaxter, 1988; Fuiman et al., 1998; Sfakianakis et al., 2011), as well as handling and preservation (Theilacker, 1980). Consequently, field-collected specimens may differ phenotypically from laboratory-reared specimens (Blaxter, 1984) used to generate morphological and taxonomic descriptions of ruffe larvae. All of these discrepancies can contribute to error when identifying field-collected ruffe larvae.
Recent advances in DNA barcoding and sequencing technologies allow for identification and confirmation of field collected fish larvae (Ko et al., 2013). The combination of morphological and genetic analysis can be used to confidently describe and develop diagnostics for field-collected larval fish. As part of an invasive species early detection study, we identified fish larvae collected from the lower St. Louis River (SLR), which included the Duluth-Superior Harbor, in 2012 and 2013. Here we present a description of ruffe larvae from those samples based on genetically-confirmed individuals. We quantify meristics and other morphological characteristics for ruffe larvae and compare them with similar species found in the lower SLR. These data are used to clarify inconsistencies among published descriptions and to develop diagnostic traits to improve stage-based ruffe larvae identification. These traits are compared to the family-level dichotomous keys for yolk sac and larval fish stages found in the Great Lakes larval fish key (Auer, 1982a) to determine if they would lead to a correct designation of Percidae.
Methods
In 2012 and 2013, we sampled fish larvae at over 350 sites by neuston net, tucker trawl, larval beach seine, larval tow sled, or light trap. Samples were preserved in > 90% ethanol to maintain DNA integrity. All fish larvae within each sample were individually identified to the lowest practical taxonomic level using Auer (1982), French and Edsall (1992), Leslie et al. (2002), and Simon and Vondruska (1991). In all, over 2,000 and 12,000 fish larvae were morphologically identified in 2012 and 2013, respectively.
For diagnostics development, individual ruffe (n = 10), black crappie (Pomoxis nigromaculatus, n = 10), pumpkinseed (Lepomis gibbosus, n = 9), and johnny darter (Etheostoma nigrum, n = 7) were selected and removed from various samples. These species were chosen because their morphological characteristics are most similar to ruffe. All individuals compared were of similar size and all were subject to DNA analysis to verify morphological identification. The number of larvae removed from samples was minimal to maintain bulk sample integrity required for other aspects of the study. DNA was extracted from fish larvae tissue samples using a DNeasy ® Blood and Tissue Kit (Qiagen, Hilden, Germany), following manufacturer’s guidelines. PCR using primers dgLC01490 and dgHC02198 (Folmer et al., 1994) amplified the 658 bp barcode region of the mitochondrial cytochrome c oxidase subunit 1 gene (CO1). The PCR product of each specimen was sequenced at USEPA Cincinnati, OH using Sanger Sequencing with BigDye v3.1 in an ABI 3730xl DNA Analyzer (Applied Biosystems, Foster City, California). DNA barcode results for each individual were queried against the sequences of known reference material in the Barcode of Life Data Systems (BOLD; Ratnasingham and Hebert, 2007) and to sequences we generated from fin clips of adult fish. A DNA barcode match to assign a species level identification to an individual was defined as >99% similarity to a unique reference species.
Prior to DNA analysis, fish larvae were photo-documented (Nikon SMZ-U microscope with Nikon digital sight DS-5M camera) and any observed patterns in pigmentation were noted. A suite of morphological measurements were made on digital images of individual larvae using NIS-Elements D4.30.01 (64-bit) software. Morphometrics included total length (TL)—anterior margin of snout (AS) to posterior margin of caudal fin (PC), pre-anal length—AS to posterior margin of vent (PV), head length—AS to origin of pectoral fin, swim bladder length—if present, maximum body depth—includes yolk sac if present, post-anal depth—behind (B) PV, head depth—B posterior margin of eye (PE), caudal peduncle depth, swim bladder depth—if present, maximum body width, and head width—BPE (Simon et al., 1987). In addition, we quantified each larva’s pre-anal and post-anal myomeres. Each larva was staged as yolk sac, preflexion, flexion, or postflexion according to Kendall et al. (1984). Descriptions of larval ruffe focus on < 6 mm TL yolk sac and preflexion stages, which made up over 95% of the approximately 800 ruffe captured among the five different gears used in our survey. These stages, therefore, represent a size likely to be collected in monitoring surveys. We also describe 10 to 20 mm TL (postflexion) ruffe larvae; however, our catches lacked ruffe between the sizes of 6 mm and 10 mm TL.
Results and Discussion
Meristics
Myomere counts of ruffe ranged from 13 to 15 pre-anal and 20 to 22 post-anal in our study (Table 1, Table 2). According to descriptions in the Great Lakes larval fish key (Auer, 1982b), these pre-anal counts are lower than common percids like yellow perch (Perca flavescens) and logperch (Percina caprodes) which are reported to have between 17 and 24 pre-anal myomeres, but overlap slightly with some Great Lakes species of darter (Etheostoma spp.), and johnny darter from the Ohio River drainage (15 preanal, 21 postanal; Simon, 2006). Myomere counts for ruffe also overlap with reported values for some common Great Lakes centrarchid species (Heang, 1982) including black crappie (10–14 pre-anal and 19–23 post-anal myomeres), and pumpkinseed (10–13 pre-anal and 16–22 post-anal). We observed pre-anal myomere counts of 11–12 and post-anal counts of 21–23 and 18–20, respectively, for black crappie and pumpkinseed (Table 1, Table 2). The percid species found in our samples that was most similar to ruffe with respect to myomere counts was johnny darter, which had 16–17 pre-anal and 20–22 post-anal myomeres (Table 1, Table 2). Thus, pre-anal myomere counts for ruffe are intermediate between centrarchids such as black crappie and pumpkinseed, and common percids such as johnny darter, yellow perch and logperch, the latter two we observed to have between 18 and 21 pre-anal (19–22 post-anal) myomeres.
Table 1.
Morphometry and myomere counts for yolk sac Ruffe (Gymnocephalus cernua), Black Crappie (Pomoxis nigromaculatus), Pumpkinseed (Lepomis gibbosus), and Johnny Darter (Etheostoma nigrum) from the St. Louis River (TL - total length).
| Ruffe (yolk sac) |
Black Crappie (yolk sac) |
Pumpkinseed (yolk sac) |
Johnny Darter (yolk sac) |
|||||
|---|---|---|---|---|---|---|---|---|
| n = 5 |
n = 5 |
n = 5 |
n = 4 |
|||||
| Character | Mean | Range | Mean | Range | Mean | Range | Mean | Range |
| TL (mm) | 4.7 | 4.5–5.1 | 4.4 | 4.2–4.5 | 4.8 | 4.6–5.0 | 6.0 | 5.7–6.2 |
| Body Length (% of TL) | ||||||||
| Pre-anal | 45.3 | 41.1–47.2 | 39.6 | 38.6–41.8 | 41.5 | 39.6–42.9 | 50.7 | 49.8–51.4 |
| Head | 17.9 | 15.7–20.0 | 15.9 | 15.0–17.3 | 17.1 | 16.6–18.4 | 16.1 | 15.0–17.9 |
| Body Depth (% of TL) | ||||||||
| Head depth | 13.7 | 12.8–16.1 | 15.1 | 13.9–16.1 | 13.3 | 12.5–14.4 | 12.2 | 9.4–15.0 |
| Posterior anus | 6.0 | 5.5–7.1 | 6.6 | 5.6–7.1 | 7.5 | 5.4–8.9 | 7.6 | 7.1–8.4 |
| Caudal peduncle | 3.3 | 2.9–3.6 | 3.5 | 3.0–4.0 | 3.5 | 3.3–3.7 | 3.1 | 2.8–3.7 |
| Maximum body depth | 13.8 | 12.0–16.8 | 12.8 | 11.4–13.7 | 13.0 | 11.5–14.2 | 13.5 | 13.1–13.8 |
| Body Width (% of TL) | ||||||||
| Head width | 12.4 | 11.0–14.1 | 10.6 | 9.1–11.8 | 11.2 | 10.6–11.7 | 12.4 | 11.4–13.8 |
| Maximum body width | 9.8 | 8.8–11.9 | 8.4 | 7.1–10.2 | 8.3 | 7.2–9.0 | 9.6 | 8.7–10.1 |
| Myomere Counts | ||||||||
| Pre-anal | 13–14 | 12 | 11–12 | 16 | ||||
| Post-anal | 20–22 | 22–23 | 19–20 | 20–22 | ||||
| Total | 33–36 | 34–35 | 30–32 | 36–38 | ||||
Table 2.
Morphometry and myomere counts for preflexion Ruffe (Gymnocephalus cernua), Black Crappie (Pomoxis nigromaculatus), Pumpkinseed (Lepomis gibbosus), and Johnny Darter (Etheostoma nigrum) from the St. Louis River (TL - total length).
| Ruffe (preflexion) |
Black Crappie (preflexion) |
Pumpkinseed (preflexion) |
Johnny Darter (preflexion) |
|||||
|---|---|---|---|---|---|---|---|---|
| n = 5 |
n = 5 |
n = 4 |
n = 3 |
|||||
| Character | Mean | Range | Mean | Range | Mean | Range | Mean | Range |
| TL (mm) | 5.7 | 5.0–7.6 | 4.8 | 4.4–5.1 | 5.4 | 4.7–6.2 | 7.5 | 7.2–7.8 |
| Body Length (% of TL) | ||||||||
| Pre-anal | 46.8 | 43.9–52.1 | 37.6 | 36.2–40.3 | 41.2 | 40.2–42.5 | 49.9 | 49.1–50.3 |
| Head | 21.2 | 17.1–24.9 | 16.5 | 15.4–17.1 | 16.6 | 15.9–17.3 | 18.6 | 18.1–19.3 |
| Body Depth (% of TL) | ||||||||
| Head depth | 15.2 | 14.0–16.3 | 14.3 | 13.3–15.8 | 13.2 | 12.0–14.8 | 13.1 | 12.4–14.2 |
| Posterior anus | 5.7 | 4.7–7.6 | 5.8 | 5.7–6.1 | 6.4 | 4.9–8.4 | 6.9 | 6.8–7.0 |
| Caudal peduncle | 2.9 | 1.6–4.1 | 3.4 | 2.5–4.1 | 3.4 | 2.8–3.9 | 3.8 | 3.4–4.1 |
| Maximum body depth | 14.8 | 12.5–17.8 | 12.5 | 10.9–15.0 | 12.1 | 11.2–13.2 | 11.1 | 10.5–11.8 |
| Body Width (% of TL) | ||||||||
| Head width | 11.7 | 9.1–13.5 | 10.1 | 8.7–11.8 | 10.1 | 7.2–11.6 | 11.7 | 11.2–12.3 |
| Maximum body width | 10.1 | 8.3–11.9 | 7.3 | 6.8–7.9 | 7.6 | 6.5–8.8 | 6.6 | 6.2–6.9 |
| Myomere Counts | ||||||||
| Pre-anal | 13–15 | 11–12 | 12 | 17 | ||||
| Post-anal | 20–22 | 21–22 | 18–19 | 22 | ||||
| Total | 33–37 | 33–34 | 30–31 | 39 | ||||
Our myomere counts for ruffe overlapped the range reported by French and Edsall (1992) of 14–15 pre-anal and 22–24 post-anal myomeres for larvae hatched from laboratory-fertilized ruffe eggs collected from the St. Louis River. These ranges are also consistent with Slovakian and European literature describing ruffe larvae (Kovác, 1993; Uhro, 1996), which report 13–16 pre-anal and 22–24 post-anal myomeres. However, our myomere counts differ from a description based on field-collected ruffe from the St. Louis River (Simon and Vondruska, 1991), which reports 17–20 pre-anal counts and 18–21 post-anal counts. While part of the variability in reported myomere counts may be due to differences in defining pre-anal versus post-anal myomeres (Auer, 1982a), the weight of evidence supports the conclusion that ruffe larvae have between 13–16 pre-anal and 20–24 post-anal myomeres. It is also important to recognize that in addition to natural variability, myomere counts are subject to observer interpretation and influenced by specimen condition.
Morphometrics
Means and ranges of ruffe morphometry in our study were generally similar to, and overlapping those of black crappie, pumpkinseed, and johnny darter (Table 1, Table 2). A notable exception, pre-anal length of yolk sac ruffe, fell generally between the ranges of the centrarchids and johnny darter. Mean pre-anal length for ruffe was 3.8% and 5.7% greater than pumpkinseed and black crappie respectively, and 5.4% smaller than johnny darter. At the preflexion stage, both pre-anal length and head length for ruffe were greater than that of the two centrarchids, and more similar to johnny darter. This stage based pattern in ruffe pre-anal length may indicate the start of an ontogenetic progression toward a more percid like phenotype which we observe in larger ruffe larvae at the postflexion stage. Overall, the increased proportion of pre-anal length relative to centrarchids is consistent with other percids in the Laurentian Great Lakes. Yellow perch and logperch had about equal pre-anal and post-anal myomere counts, with the anus located at approximately 50% or greater of the fish’s total length (Auer, 1982b). Maximum body depth range for preflexion ruffe, however, is more similar to the centrarchids, and maximum body width is greater than all three species. Thus, ruffe does not consistently fit the yolk sac or preflexion morphometric profile of the other species. As a result, some stage based measures, most notably pre-anal length, may provide diagnostic support to help distinguish early life stage ruffe from pumpkinseed, black crappie and johnny darter.
Pigmentation
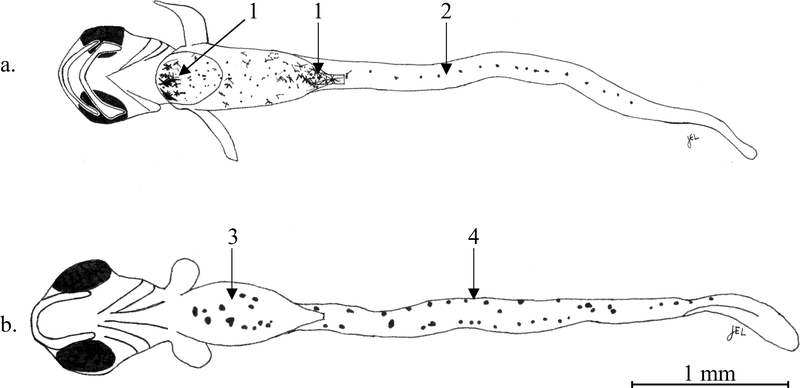
While pigment can be highly variable, we found some general patterns to aid morphological identifications. Similar to other percids, we observed ruffe to have a single row of post-anal melanophores along the ventral midline of the caudal peduncle from anus to caudal fin (Fig. 1a). In comparison, black crappie (Fig. 2b), pumpkinseed (also noted in Wallus, 2008b) and bluegill (Lepomis macrochirus), had two widely spaced rows of melanophores that converge near the caudal fin. These post-anal ventral patterns were sometimes faint, particularly for centrarchids, nontheless, we found this to be a key diagnostic character for distinguishing ruffe larvae from these three morphologically similar centrarchids at all larval stages. Compared with other percids, we observed individual melanophores in ruffe post-anal series to be similar to yellow perch, not elongated (extending lengthwise beyond a single myomere) as with johnny darter or logperch. Simon and Vondruska (1991) describe the post-anal series for ruffe of similar size as “8–10 midventral melanophores”, but we observed the number in this series to be more variable, and consistently greater than 10 (see example, Fig 1a).
Fig. 1.
(a) Ventral image of a yolk sac Ruffe (Gymnocephalus cernua), 4.5 mm TL. (a.1) Concentration of pigment anterior and posterior of the gut and yolk sac. (a.2) Postanal single row of melanophores. (b) Ventral image of a preflexion Black Crappie (Pomoxis nigromaculatus), 4.91 mm TL. (b.3) Concentration of multiple prominent melanophores scattered along gut venter. (b.4) Postanal double row of melanophores.
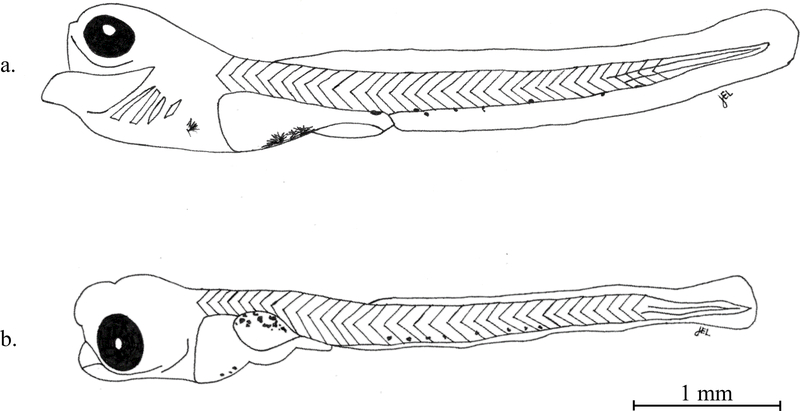
Fig. 2.
(a) Lateral image of a preflexion 4.6 mm TL Ruffe (Gymnocephalus cernua) with no visible sign of swim bladder development. (b) Lateral image of a preflexion 4.8 mm TL Black Crappie (Pomoxis nigromaculatus) with a well-developed and dorsally pigmented swim bladder.
Although not as well defined as post-anal patterns, we also observed differences in the pre-anal ventral, and dorsal pigment patterns between ruffe and similar species at the yolk sac and preflexion stages. Ruffe exhibited a variable pattern of pre-anal ventral melanophores (often stellate) scattered across the gut venter, but typically had a distinct, prominent mid-ventral melanophore or concentration of melanophores, occurring at the anterior and posterior terminus of the yolk sac (Fig. 1a), which remained after yolk absorption. The centrarchid species generally lacked distinct ventral pigment at these locations. Black crappie exhibited only a few non-stellate melanophores scattered lengthwise along the center of the gut venter (Fig. 1b). Pumpkinseed typically had more non-stellate melanophores on the gut venter, which sometimes included a prominent melanophore or two at the extreme anterior, similar to ruffe, however, none of the centrarchid species we observed had prominent ventral pigment at the posterior yolk sac terminus as described for ruffe. For johnny darter, gut venter pigment typically consisted of densely scattered melanophores, the outermost of which often formed a ring outlining the yolk sac. As the yolk sac absorbed, these melanophores converged to form a central elongated patch or line of prominent melanophores. Dorsal pigment of ruffe also differed from johnny darter as well as pumpkinseed, which typically had several to many melanophores on the head. Johnny darter also showed evidence of a single mid-dorsal series forming, which by late preflexion stage, extended the entire body length from nape to caudal fin. Ruffe, along with black crappie and bluegill, lacked significant dorsal pigment at the yolk sac and preflexion stages.
Postflexion (10–20mm TL) ruffe larvae pigmentation was relatively sparse compared to the three centrarchids and johnny darter of similar size, and occurred primarily as a post-anal ventral series (described above), and on the operculum and head dorsum. The general lack of body pigmentation at these sizes most closely resembled that of yellow perch in our samples.
Development
Ruffe were observed as yolk sac stage in our study at total lengths up to 5.1 mm (Table 1) and swim bladder development was not observed to begin until larvae were about 5 mm TL. Similarly, French and Edsall (1992) described yolk sac absorption as nearly complete at 4.9–5.6 mm TL. Simon and Vondruska (1991) described these stages of ruffe development occurring later, with yolk absorption complete at 6.2–6.9 mm TL and swim bladder development beginning between 5.9 and 6.4 mm TL. These developmental changes occurred earlier in black crappie compared with ruffe (Fig. 2), with yolk fully absorbed and a well-developed swim bladder evident by 4.0 mm TL. These findings are consistent with other studies that report yolk sac of black crappie absorbed at 3.5–3.9 mm TL (Heang, 1982; Wallus, 2008a). In contrast, length at hatch for johnny darter is reported as 5.0 mm TL (Auer, 1982b) and we observed them still as yolk sac at 6.2 mm TL (Table 1), which, based on our observations, suggests minimal overlap with ruffe development at these early stages.
While environmental conditions such as temperature can affect the rate of developmental changes in fish larvae, the size at which they occur has been shown to be less variable (Fuiman et. al., 1998). Our catch data corroborate that ruffe spawn over a prolonged period from late April to late June (Brown et. al., 1998), across a variety of habitats and conditions (Ogle, 1998), which overlap with those of black crappie (Wallus, 2008a). In 2012 and 2013 ruffe larvae were captured throughout our sampling window from early to mid-May through mid-July while black crappie appeared in samples from early to mid-June through mid-July. Pumpkinseed and bluegill larvae were also captured beginning in early July. Larvae of ruffe and black crappie were each captured in at least four different gears which sampled different habitats. We observed the length specific developmental patterns described above in both species consistently across all sampling habitats and temporal periods where they were captured.
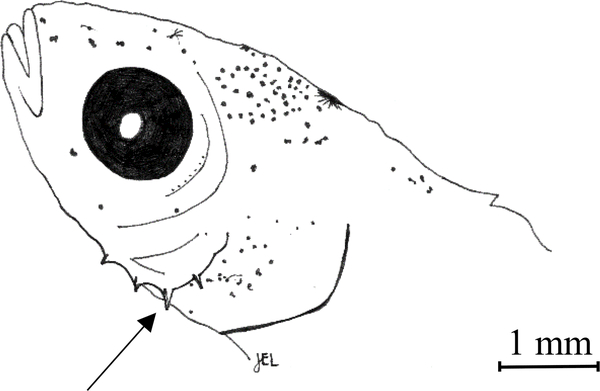
Our observations of ruffe from 10–20 mm TL agree with those of Simon and Vondruska (1991), suggesting that ruffe <10mm TL develop a more percid-like phenotype, but also take on characteristic body aspects of their own, including an arched nape. Of the percids collected in our study, ruffe were readily differentiated from johnny darter at this size because the latter have developed more prominent pectoral fins as well as a distinct pigment pattern and body shape. Ruffe morphology can however resemble yellow perch at this size, but we found they can be distinguished using pre-anal myomere counts and because ruffe develop a series of prominent preopercular spines (Fig. 3). This trait first appeared in our study when ruffe were about 10 mm TL. Simon and Vondruska (1991) note first observing this trait between 11.5–11.8 mm TL, but did not describe ruffe between 7.4 mm and 11.5 mm TL. Descriptions of ruffe from 6 mm to 10 mm TL are needed to complete our description and determine when ruffe first develop these spines. It should be noted that yellow perch are described elsewhere as also developing preopercular spines for larvae ≥ 14 mm (Mansueti, 1964), however we did not observe them on yellow perch in our study up to 20 mm TL.
Fig. 3.
Flexion 12.5 mm TL Ruffe (Gymnocephalus cernua). The arrow points to the series of preopercular spines.
Methodological Implications
As in our study, the use of DNA barcoding in addition to morphologically-based taxonomy to improve and confirm identification of larval fishes is being recommended (Puncher et. al., 2015; Overdyk et. al., 2016). To maintain genetic integrity for DNA taxonomy, ethanol is generally used as an alternative to formalin preservation. The relative effects of different preservation methods on morphometrics such as total length of larval fish, however, have been inconsistent (Neave et. al., 2006). Some have found greater shrinkage in ethanol relative to formalin (Fisher et. al., 1998). These potential differences should be acknowledged when applying or comparing our morphometric and developmental data to formalin preserved larvae, but should not affect key diagnostics used to differentiate between ruffe and other species. For example, myomere counts from our ethanol preserved larvae agree with other studies that used formalin to fix or preserve larvae of ruffe (French and Edsall, 1992; Urho, 1996), as well as black crappie and pumpkinseed (Heang, 1982; Wallus, 2008a, 2008b), and johnny darter (Simon, 2006). Likewise, post anal ventral pigment patterns observed in our ethanol preserved specimens would likely be similar to or more intense in formalin preserved specimens (Neave et. al., 2006).
Conclusion
Ruffe are unusual among percids currently occurring in the Laurentian Great Lakes because during their early development, they share many morphological characteristics with common centrarchid species. This superficial similarity to centrarchids presents a potential for misidentification because our catch data show that ruffe larvae overlap both temporally and spatially with larval centrarchids. We observed more than a month long period where both were present. Further, in samples where either ruffe or centrarchids were found during this period, nearly 50% (14 of 30) contained early stage larvae of both ruffe and centrarchids. To avoid misidentification of yolk sac and preflexion ruffe and centrarchid larvae, we found the post-anal ventral pigment to be a useful diagnostic. If a single midventral series of melanophores is present, the fish larva is likely ruffe and should be further examined using a variety of diagnostics including morphometrics, pigments, and developmental patterns. Of the percids we encountered, johnny darter has the most similar myomere counts, but we found ruffe to be readily distinguished using morphology, pigment and developmental patterns.
For postflexion ruffe larvae from 10 to 20 mm TL, we lack morphometric data, but observed an ontogenetic progression from a preflexion centrarchid phenotype to a postflexion percid phenotype most closely resembling yellow perch. Our observations suggest that pre-anal myomere counts as well as the presence or absence of prominent preopercular spines can be useful for distinguishing ruffe from morphologically similar percids, such as yellow perch, at these sizes.
These results provide diagnostic information that may serve as a cautionary check when identifying centrarchid or percid larvae based on the widely used Great Lakes larval fish key (Auer, 1982a). The key is organized to family using a provisional dichotomous key for either yolk sac stage or larval stage. The critical decision point for ruffe in these provisional keys occurs at step 6 (yolk sac key) or step 14 (larval key), in which pre-anal myomere counts are either (a) “greater than or equal to post-anal myomeres” or (b) “significantly less than post-anal myomere (difference greater than 5 myomeres)”. Path (a) would assign ruffe to the percid family, while path (b) would result in a centrarchid family designation. Given the myomere counts described here, there is a high probability that ruffe would follow the centrarchid path (b) in these provisional keys.
Because ruffe can overlap spatially with native species like yellow perch, johnny darter, black crappie, pumpkinseed, and bluegill, there is a high potential to encounter morphologically similar fish larvae in monitoring and assessment studies. Our findings support more confident morphological differentiation of these taxa, thus leading to an enhanced ability to assess larval fish composition and ecology, as well as contributing useful information for development of new taxonomic keys (e.g., www.larvalfishid.com). More comprehensive studies comparing the effects of preservation method or thermal regime may further improve taxonomic descriptions of ruffe larvae.
Acknowledgements
We thank scientists at the U.S. EPA Office of Research and Development, Mid-continent Ecology Division (Duluth)—specifically, Joel Hoffman and Anett Trebitz for offering advisement, review comments, and fieldwork support. In addition, Tim Corry, Sam Miller, Jill Scharold, Anne Cotter, Will Bartsch (ORISE), Tyler Billehus, Chelsea Hatzenbuhler (ORISE), Shane Zavodnik, Zach Polaske (Badger Technical Services), Hannah Coe (GRO Intern), George Grant (GRO Intern), and Mikayla Haynes (Volunteer), were all involved in aspects of sample collection and processing. We thank EPA’s Ecosystem Integrity Branch (EIB) and Internal Exposure Indicator Branch (IEIB), National Exposure Research Laboratory (Cincinnati), specifically Erik Pilgrim (EIB), John Martinson (IEIB), and Sara Okum (EIB, ORISE) for the work to complete the larval fish DNA-based identification. Julie Lietz prepared all illustrations. Thanks to David Jude and JGLR anonymous reviewers for constructive comments. Julie Lietz was an ORISE participant at EPA, during which she helped conduct this research.
This work was supported in part by an appointment to the ORISE participant research program supported by an interagency agreement between EPA and DOA (IA 92298301); views expressed in this paper are those of the authors and do not necessarily reflect the view or policies of the U.S. EPA.
References
- Allen DM, Barker DL, 1990. Interannual variations in larval fish recruitment to estuarine epibenthic habitats. Mar. Ecol. Prog. Ser 63, 113–125. [Google Scholar]
- Auer NA(Ed.), 1982a. Identification of larval fishes of the Great Lakes Basin with emphasis onthe Lake Michigan drainage. Great Lakes Fish. Comm. Spec Publ. No. 82–3. [Google Scholar]
- Auer NA, 1982b. Percidae, in: Auer NA (Ed.), Identification of larval fishes of the Great Lakes basin, with emphasis on the Lake Michigan drainage. Great Lakes Fish. Comm. Spec Publ. No. 82–3, pp. 581–648. [Google Scholar]
- Bani A, Toorchi M, Norouizi N, 2015. Morphometric and meristic variations in bream (Abramis brama orientalis, Berg, 1949) during larval development. Caspian J. Env. Sci 13 (2), 89–97. [Google Scholar]
- Blaxter JHS, 1984. Ontogeny, systematics and fisheries, in: Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AW Jr., Richardson SL (Eds.), Ontogeny and systematic of fishes Allen Press, Lawrence, Kansas, Am. Soc. Icthyol. Herpetol. Spec Publ. No. 1, pp. 1–6. [Google Scholar]
- Blaxter JHS, 1988. Pattern and variety in development, in: Hoar WS, Randall DJ (Eds.), Fish physiology, volume XI, the physiology of developing fish, part A, eggs and larvae. Academic Press Inc., San Diego, pp. 1–58. [Google Scholar]
- Brown WP, Selgeby JH, Collins HL, 1998. Reproduction and early life history of ruffe (Gymnocephalus cernuus) in the St. Louis River, a Lake Superior tributary. J. Great Lakes Res 24 (2), 217–227. [Google Scholar]
- Fisher SJ, Anderson MR, Willis DW, 1998. Total length reduction in preserved yellow perch larvae. N. Am. J. Fish. Manag 18, 739–742. [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R, 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol 3, 294–299. [PubMed] [Google Scholar]
- French JRP III, Edsall TP, 1992. Morphology of Ruffe (Gymnocephalus cernuus) protolarvae from the St. Louis River, Lake Superior. J. Fresh. Ecol 7, 59–68. [Google Scholar]
- Fuiman LA, Poling KR, Higgs DM, 1998. Quantifying developmental progress for comparative studies of larval fishes. Copeia, 3, 602–611. [Google Scholar]
- Heang TT, 1982. Centrarchidae, in: Auer NA (Ed.), Identification of larval fishes of the Great Lakes basin, with emphasis on the Lake Michigan drainage. Great Lakes Fish. Comm. Spec Publ. No. 82–3, pp. 525–580. [Google Scholar]
- Humphries P, Serafini LG, King AJ, 2002. River regulation and fish larvae: variation through space and time. Fresh. Biol 47, 1307–1331. [Google Scholar]
- Kendall AW Jr., Ahlstron EH, Moser HG, 1984. Early life history stages of fishes and their characters, in: Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AW Jr., Richardson SL (Eds.), Ontogeny and systematic of fishes Allen Press, Lawrence, Kansas, Am. Soc. Icthyol. Herpetol. Spec Publ. No. 1, pp. 11–22. [Google Scholar]
- King AJ, 2004. Ontogenetic patterns of habitat use by fishes within the main channel of an Australian floodplain river. J. Fish Biol 65, 1582–1603. [Google Scholar]
- Ko H-L, Wang Y-T, Chiu T-S, Lee M-A, Leu M-Y, Chang K-Z, Chen W-Y, Shao KT, 2013. Evaluating the accuracy of morphological identification of larval fishes by applying DNA barcoding. PLoS ONE 8, e5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovác V, 1993. Early development of ruff, Gymnocephalus cernuus. Folia Zool. 42, 269–280. [Google Scholar]
- Leslie JK, Timmins CA, Bonnell RG, 2002. Postembryonic development of the tubenose goby Proterorhinus marmoratus Pallas (Gobiidae) in the St. Clair River/Lake system, Ontario. Arch. Hydrobiol 154, 341–352. [Google Scholar]
- Mansueti AJ, 1964. Early development of the Yellow Perch, Perca flavescens. Coast. Estuar. Res. Fed 5(1/2), 46–66. [Google Scholar]
- Neave FB, Mandrak NE, Docker MF, Noakes DL, 2006. Effects of preservation on pigmentation and length measurements in larval lampreys. J. Fish Biol 68, 991–1001. [Google Scholar]
- Ogle DH, 1998. A synopsis of the biology and life history of ruffe. J. Great Lakes Res 24 (2), 170–185. [Google Scholar]
- Overdyk LM, Holm E, Crawford SS, Hanner RH, 2016. Increased taxonomic resolution of Laurentian Great Lakes ichthyoplankton through DNA barcoding: A case study comparison against visual identification of larval fishes from Stokes Bay, Lake Huron. J. Great Lakes Res 42, 812–818. [Google Scholar]
- Pratt D, 1998. Distribution and population status of the ruffe (Gymnocephalus cernua) in the St. Louis Estuary and Lake Superior. Great Lakes Fishery Commission, Research Completion Report. [Google Scholar]
- Puncher GN, Alemany F, Arrizabalaga H, Cariani A, Tinti F, 2015. Misidentification of bluefin tuna larvae: a call for caution and taxonomic reform. Rev. Fish Biol. Fisheries 25, 485–502. [Google Scholar]
- Ratnasingham S, Hebert PD, 2007. BARCODING BOLD: The Barcode of Life Data System (www.barcodinglife.org). Molec. Ecol. Notes [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AT, Clarkson RW, Forrest RE, 1998. Dispersal of larval fishes in a regulated tributary. Trans. Am. Fish. Soc, 127, 722–786. [Google Scholar]
- Schluderman E, Tritthart M, Humphries P, Keckeis H, 2012. Dispersal and retention of larval fish in a potential nursery habitat of a large temperate river: an experimental study. Can. J. Fish. Aquat. Sci 69 (8), 1302–1315. [Google Scholar]
- Sfakianakis DG, Leris I, Laggis A, Kentouri M, 2011. The effect of rearing temperature on body shape and meristic characters in zebrafish (Danio rerio) juveniles. Environ. Biol. Fish 92 (2), 197–205. [Google Scholar]
- Simon TP, Wallus R, Floyd KB, 1987. Descriptions of protolarvae of seven species of the subgenus Nothonotus (Percidae: Etheostomatini) with comments on intrasubgeneric characteristics. Am. Fish. Soc. Symp 2, 179–190. [Google Scholar]
- Simon TP, Vondruska JT, 1991. Larval identification of the ruffe, Gymnocephalus cernuus (Linnaeus) (Percidae: Percini), in the St. Louis River Estuary, Lake Superior drainage basin, Minnesota. Can. J. Zool 69, 436–442. [Google Scholar]
- Simon TP, 2006. Johnny darter complex, in: Simon TP, Wallus R(Eds), Reproductive Biology and Early Life History of Fishes in the Ohio River Drainage. Vol. 4 Percidae. CRC Press; Boca Raton, Florida: pp. 267–275. [Google Scholar]
- Theilacker GH, 1980. Changes in body measurements of larval northern anchovy, Engraulis mordax, and other fishes due to handling and preservation. Fish. Bull 78 (3), 685–692. [Google Scholar]
- Urho L, 1996. Identification of perch (Perca fluviatilis), pikeperch (Stizostedion lucioperca) and ruffe (Gymnocephalus cernuus) larvae. Ann. Zool. Fenn 33, 659–667. [Google Scholar]
- Wallus R, 2008a. Black crappie Pomoxis nigromaculatus (Lesueur), in: Wallus R, Simon TP (Eds.), Reproductive biology and early life history of fishes in the Ohio river drainage. Elassomatidae and Centrarchidae Vol. 6 Boca Raton, CRC Press, pp. 378–398. [Google Scholar]
- Wallus R, 2008b. Pumpkinseed Lepomis (Eupomotis) gibbosus (Linneaus), in: Wallus R, Simon TP (Eds.), Reproductive biology and early life history of fishes in the Ohio river drainage. Elassomatidae and Centrarchidae Vol. 6 Boca Raton, CRC Press, pp. 123–140. [Google Scholar]