Abstract
Along with advanced age and apolipoprotein E (APOE)-4 genotype, female sex is a major risk factor for developing late-onset Alzheimer’s disease (AD). Considering that AD pathology begins decades prior to clinical symptoms, the higher risk in women cannot simply be accounted for by their greater longevity as compared to men. Recent investigation into sex-specific pathophysiological mechanisms behind AD risk has implicated the menopause transition (MT), a midlife neuroendocrine transition state unique to females. Commonly characterized as ending in reproductive senescence, many symptoms of MT are neurological, including disruption of estrogen-regulated systems such as thermoregulation, sleep, and circadian rhythms, as well as depression and impairment in multiple cognitive domains. Preclinical studies have shown that, during MT, the estrogen network uncouples from the brain bioenergetic system. The resulting hypometabolic state could serve as the substrate for neurological dysfunction. Indeed, translational brain imaging studies demonstrate that 40-60 year-old perimenopausal and postmenopausal women exhibit an AD-endophenotype characterized by decreased metabolic activity and increased brain amyloid-beta deposition as compared to premenopausal women and to age-matched men. This review discusses the MT as a window of opportunity for therapeutic interventions to compensate for brain bioenergetic crisis and combat the subsequent increased risk for AD in women.
Keywords: Perimenopause, women, Alzheimer’s disease, early detection, brain imaging
Alzheimer’s Disease Risk and Female-Specific Hormonal Changes
Along with advanced age and apolipoprotein E (APOE)-4 genotype, female sex is one of the major risk factor for developing late-onset Alzheimer’s disease (AD) (1). Epidemiologic studies have for years consistently documented that women comprise two-thirds of people living with AD, regardless of age and ethnicity (1–3).
These findings cannot be fully accounted for by the fact that women tend to have greater longevity than men (4). Further, there is consensus that AD is not primarily a disease of the old but rather begins decades prior to clinical symptoms with changes in brain biochemistry (4), which has led investigation into gender differences that may explain the increased risk for females of developing AD independent of longer lifespan in the national effort to accelerate and improve early detection of AD (3).
One major sex difference heralded by pre-clinical and human studies as having early prognostic import for AD is the menopause transition (MT) (5). Though commonly thought of as a neuroendocrine transition state in females ending with reproductive senescence, MT also manifests a number of neurological symptoms, including disruption of estrogen-regulated systems such as thermoregulation, sleep and circadian rhythms, depression, and impairment in multiple cognitive domains (5). Preclinical studies demonstrate that uncoupling of estrogen-mediated brain energy metabolism both marks the onset of MT and induces a hypometabolic state associated with accompanying neurological symptoms (5).
Pathophysiological mechanisms of AD that are activated years to decades prior to clinically detectable symptoms form the basis of a prodromal (preclinical) stage of the disease (5, 6). While symptoms of late-onset AD typically arise in the early to mid-70s, AD pathology appears during the prodromal phase, which is proximate to MT (5, 6). Menopausal changes therefore coincide with the timespan between average age of menopause, in the early-to-mid-50s (7), and average age of AD diagnosis, in the mid-seventies.
Recent translational brain imaging studies have found that 40-60 year-old perimenopausal and postmenopausal women exhibit an AD-endophenotype characterized by decreased metabolic activity (including reduced glucose metabolism and mitochondrial function) and increased brain amyloid-beta deposition (Aβ, a hallmark of AD pathology) as compared to premenopausal women and age-matched men (8, 9). These findings substantiate the eclipsing effects of endocrine aging on chronological aging in the female’s brain several years, if not decades, before possible clinical symptoms emerge and the progressively increased risk of AD in women due to MT (6).
Increased risk for AD in females as initiated by brain modifications during the MT is the focus of this review. It also considers the importance of further investigation of the MT as a risk-stratifying method for early detection of AD progression and subsequent intervention, which will be increasingly needed to curb the growing AD epidemic (3, 5, 6).
Clinical Characterization of the MT
Menopause, by definition, is the final menstrual period a woman experiences and is diagnosed after 12 months of amenorrhea. This universal aging process of a woman’s reproductive system occurs at an average age of 51 years with a range of 40-58 years for 88% of women (5, 7). From a clinical perspective, premenopausal women have regular menstrual cycles that have less than 7 days variability in cycle length. Perimenopausal women have irregular menstrual cycles, with variation in cycle length greater than 7 days. Postmenopausal women have had no menstrual cycles for 12 months or longer (10).
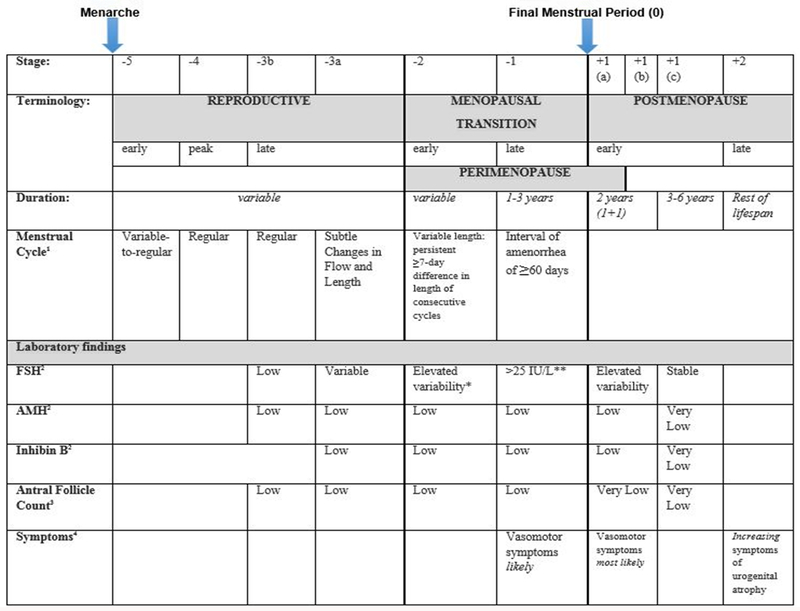
From an endocrinological perspective, perimenopause is characterized by increased variability in the length of the menstrual cycle and levels of circulating hormones. During perimenopause, decreased ovarian reserve results in fluctuations in levels of hypothalamic, pituitary and ovarian hormones, and, notably, a decrease in ovarian secretion of estrogen and progesterone (10). Hormonal changes and clinical symptoms occur over a period leading up to and immediately following menopause. This period is frequently termed climacteric, or perimenopause, but is increasingly referred to as the MT. Determination of these well-defined endocrine features has enabled a symptom and indicator classification system, known as The Stages of Reproductive Aging Workshop (STRAW) criteria (10), that describes the stages of reproductive aging across the transition from perimenopause to postmenopause (Figure 1).
Figure 1.
The Stages of Reproductive Aging Workshop + 10 staging system for reproductive aging in women
Neurological symptoms of MT include temperature dysregulation (hot flashes), disturbed sleep, insomnia, pain, depression, and cognitive dysfunction (5). Extensive epidemiological analyses designate these symptoms as risk factors for AD (5). Neurological symptoms tend to cluster together in occurrence and severity and, in many instances, persist into postmenopause (11). The breadth of neurological symptoms that can occur during the perimenopausal transition is indicative of disruption in multiple systems, whereas the simultaneous emergence of such disruptions is indicative of a common controlling factor: estrogen.
The Role of Estrogen in the Brain
One of the brain’s master regulatory systems is the estrogen receptor network. Under its influence, the brain effectively responds at proper timescales to regulate brain energy metabolism, such as in the ovarian-neural estrogen axis (5). Changes in either the availability of estrogen or its receptor network can affect intracellular signaling, neural circuit function, and energy availability (5).
Neural structures in the brain that control the multiple functions affected during MT are populated with estrogen receptors (12, 13). Such receptors are localized to plasma membranes, mitochondria, and in the nucleus of cells, and are particularly abundant in the hypothalamus, which is the primary thermoregulatory center and regulator of sleep and circadian rhythms (14). Brain regions that are crucial to learning and memory, including the prefrontal cortex, hippocampus, amygdala, and posterior cingulate cortex, also contain substantial estrogen receptors (14). Finally, the serotoninergic neurons of the raphe nucleus are positive for estrogen receptors, as are the adrenergic neurons of the locus coeruleus (14).
MT is also associated with the emergence of estrogen receptor splice variants, changes in protein expression, alterations in receptor degradation, and possible epigenetic reconfigurations, all which lead to plummeting estrogen levels (5). The brain compensates for decreased estrogen and reduced receptor activity during the MT. But for some women this adaptive compensation is diminished, lacking, or only expressed in a sub-set of estrogen-regulated neural networks, giving rise to the complex neurological phenotype of menopause (7, 15).
In particular, estrogenic regulation of brain glucose metabolism is dismantled during perimenopause, inducing a hypometabolic state (12, 16, 17). Preclinical studies indicate that during perimenopause, when brain estrogen plummets, the systems required for estrogen activation of cerebral glucose metabolism rates (CMRglc) and suppression of the ketogenic pathways disassemble (18). Following the decline in CMRglc is induction of an adaptive starvation reaction to increase fatty acid metabolism for the generation and utilization of ketone bodies by mitochondria as an alternative fuel (5, 18–21). Hypometabolism, reduced mitochondrial function and subsequent oxidative damage are known to promote accumulation of Aß pathology and neuronal dysfunction (22), therefore increasing risk of developing AD later in life.
These findings collectively provide additional evidence for the contribution of declining estrogen levels to dysregulated glucose metabolism in brain regions that confer cognitive functioning.
Imaging the Menopausal Brain
Recent brain imaging studies have investigated AD risk during endocrine transition states in clinically and cognitively normal women and men to characterize sex-dependent emergence of an AD endophenotype, characterized by Aβ deposition (11C-PiB PET), glucose hypometabolism (18F-FDG PET) and brain atrophy (MRI) (9).
Both postmenopausal and perimenopausal groups exhibited AD biomarker abnormalities as compared to age-matched men (9). These abnormalities included reduced CMRglc, increased Aβ accumulation, and gray and white matter loss in key brain regions for AD, such as parieto-temporal, posterior cingulate, and frontal cortices. Biomarker deficits were most pronounced in postmenopausal women, intermediate in perimenopausal women, and lowest in premenopausal women (9).
Additionally, Aβ deposition was exacerbated in postmenopausal women positive for APOE-4 (9), a major genetic risk factor for AD that disproportionally affects AD risk in women (1, 23). Even in the absence of dementia, APOE-4 is known to significantly increase brain atrophy and decrease brain connectivity much more strongly in women than in men (24, 25). While it seems likely that loss of estrogen during perimenopause may be driving the observed AD biomarker shifts, other theories exist. For example, the drop in estrogen levels may act as a surrogate biomarker of other underlying molecular changes, including age-related declines in bioenergetic function. As mitochondria are the generators of ATP required for cognitive and other brain functions, and mitochondrial deficits have long been implicated in AD (26), a follow-up PET study investigated whether AD biomarker changes in postmenopausal women were related to altered mitochondrial activity (8).
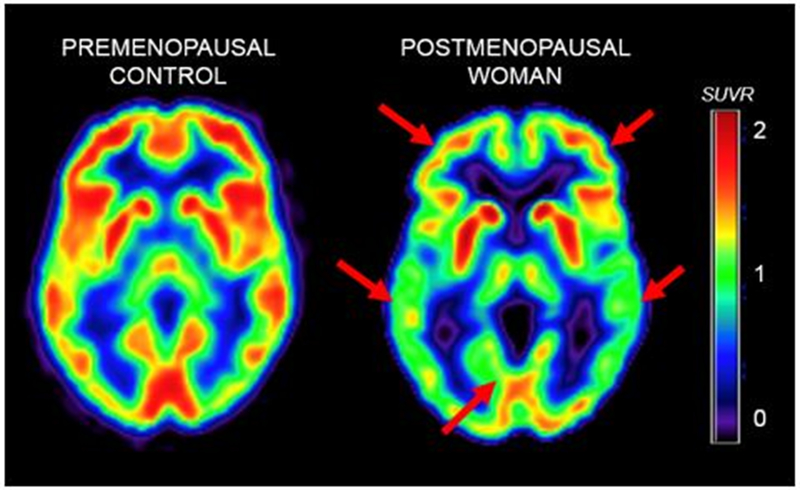
On 18F-FDG PET, as compared to premenopausal women, perimenopausal and postmenopausal women exhibited hypometabolism in the same brain regions as clinical AD patients, as well as correlated reductions in platelet mitochondrial cytochrome oxidase (COX, electron transport chain complex IV) activity, a rate limiting step in ATP production (8). A gradient effect was observed so that bioenergetic abnormalities were again most pronounced in postmenopausal, intermediate in perimenopausal, and lowest in premenopausal women (Figure 2) (8). Results were independent of age, education, and APOE genotype (8).
Figure 2.
FDG-PET scans of two representative female participants at the premenopausal (left side of figure) and menopausal stages (right side of figure)
Further investigation is required to accurately determine the role of bioenergetic dysfunction in the pathogenesis of AD. The amyloid cascade hypothesis situates Aß dysmetabolism upstream of oxidative stress, a mechanism typically associated with the rare, early-onset familial AD cases arising from autosomal dominant genetic mutations (27). In early-onset familial AD cases, brain imaging demonstrates that amyloid deposition indeed occurs prior to hypometabolism (28). As an alternative to the amyloid cascade hypothesis, reduced metabolic activity in perimenopausal and postmenopausal women could potentially arise as a consequence of the hypothesized bioenergetic and mitochondrial crisis in the brain during MT (5). Further imaging studies are needed to confirm where bioenergetic dysfunction sits in the common, late-onset form of AD.
Finally, early age of menopause was found to influence cognitive decline and Alzheimer’s amyloid pathology in a large cohort of older women which all had surgically-induced menopause (29). Prospective studies are warranted to provide accurate determination of how age of menopause influences the observed biomarker changes in naturally-occurring as well as surgically-induced menopausal women.
Interventions
As noted, although the outcome of the MT is inevitable for all women, the experience of the MT and need for intervention can be highly variable. For a thorough discussion of available therapeutic options, we recommend (30). Below is a brief summary of the literature so far, with a focus on AD.
Hormone Replacement Therapy. Hormone Replacement Therapy (HRT) replaces estrogen and progesterone to treat common symptoms of menopause, such as vasomotor symptoms. HRT can help to provide relief of vasomotor symptoms (e.g., hot flashes), reduce the risk of unwanted pregnancy, avoid the irregularity of menstrual cycles, and preserve bone density. However, current FDA guidelines only approve HRT for severe vasomotor symptoms (31). The FDA also advises women to use HRT for the shortest time and at the lowest dose possible to control menopausal symptoms. The FDA lists blood clots, heart attacks, strokes, breast cancer, and gall bladder disease as potential risks of HRT (31).
Whether the risks of HRT outweigh its benefits is an area of active debate. The most comprehensive evidence about risks and benefits of HRT comes from two randomized clinical trials that were sponsored by the National Institutes of Health as part of the Women’s Health Initiative (WHI), a 15-year study tracking over 161,800 healthy, postmenopausal women (32). The clinical trials included the WHI Estrogen-plus-Progestin Study, in which women with a uterus were randomly assigned to receive either a hormone medication containing both estrogen and progestin (Prempro™) or a placebo; and the WHI Estrogen-Alone Study, in which women without a uterus were randomly assigned to receive either a hormone medication containing estrogen alone (Premarin™) or a placebo. More than 27,000 healthy women aged 50 to 79 years at the time of enrollment took part in the trials. Both trials were stopped early as it was determined that both types of therapy were associated with specific health risks, including cardiovascular disease and breast cancer (33).
The WHI trials have also shown some benefits related to use of HRT, including one-third fewer hip and vertebral fractures, and one-third lower risk of colorectal cancer relative to placebo (32, 34). However, a follow-up study found that neither benefit persisted after the study participants stopped taking combined hormone therapy medication (34). Longer-term follow-up of the participants continues to provide new information about the health effects of HRT. Specifically, HRT when instituted prior to the onset of menopause provides protective effects on cardiovascular health (35).
The impact of HRT on the risk of AD remains controversial. The WHI clinical trials reported that combined hormonal therapy doubled the risk of developing dementia among postmenopausal women age 65 and older (33). However, other clinical trials have shown that HRT is effective at preserving CMRglc in AD-regions and preventing cognitive decline, if initiated prior to menopause (36, 37). There is also evidence that, among women who undergo bilateral oophorectomy prior to menopause, those who do not use HRT may double their risk for AD compared to those who initiate HRT soon after the procedure and continue usage at least until the age of natural menopause (38). Altogether, research indicates that HRT may be protective against AD if initiated within a maximum of 5 years after menopause (39–41), whereas HRT can be deleterious if initiated later (42, 43) or in type 2 diabetic women (36, 44).
Two ongoing hypotheses offer explanations for the positive effects of early HRT on AD risk. The “window of opportunity” hypothesis proposes that long-term estrogen depletion (due to MT) may cause decreased levels of estrogen-receptor-alpha in the hippocampus, rendering it irresponsive to HRT (45). The other hypothesis, termed “healthy cell bias of estrogen benefit,” suggests that age-related damage to mitochondria may blunder the potentially positive effects of estrogen, or even negatively suffer from them (46). It is likely that both mechanisms contribute to differential benefits of HRT when initiated at different times during the MT.
Non-hormonal therapy. In June 2013, the FDA approved paroxetine mesylate (Brisdelle™) as the first non-hormonal therapy for vasomotor symptoms (hot flashes) associated with menopause (47). In women who suffer from hot flashes but either cannot undergo estrogen or hormone therapy because of a history of breast cancer and/or history of deep vein thrombosis, or choose not to do so, some FDA-approved anti-depressants (SSRIs and SNRIs—in particular, venlafaxine) have also been shown to alleviate vasomotor symptoms (48). More work is needed to evaluate these antidepressants for AD prevention in menopausal women.
Alternative products and lifestyle interventions. Phytoestrogens, estrogen-like compounds derived from sources such as soy products, oil seeds (primarily flaxseed), whole-grain cereals, legumes like chickpeas, or the plant black cohosh, are reputed to ease menopausal symptoms (49). A double-blind, randomized controlled trial reported mild but favorable effects of soy protein supplementation on body composition (e.g., weight, body mass index [BMI], and percent body fat) in treated postmenopausal women as compared with those who received milk protein, over a 6 month period (50). Each group also received isoflavones (50). However, other randomized, double-blind trials have not shown that any of these remedies is superior to a placebo in relieving hot flashes (51). Trials of other herbal remedies, such as evening primrose oil, ginseng, and wild yam, have also not shown efficacy (52).
Clinical trials are needed to test whether dietary patterns rich in these foods, rather than isolated food categories, are protective against AD in women. Two large cohort studies have hypothesized an association but reported conflicting findings (53, 54). The European Prospective Investigation into Cancer and Nutrition (EPIC) study found that women with a higher intake of total fat, protein, and meat experienced a delayed onset of natural menopause (53). The Shanghai Women’s Health Study found that, in women aged 40-70 who experienced natural menopause during the study, intake of protein, fruit and tea influenced later age at menopause, whereas fat intake was inversely correlated (54). It is possible that conflicting results may depend on the type of fat in the diet. Recently, the UK Women’s Cohort Study showed that a higher intake of oily fish and fresh legumes was associated with delayed onset of natural menopause by 3.3 and 0.9 years per portion/day, respectively, whereas consumption of refined grains was associated with an earlier age of menopause (−1.5 years per portion/day) (55). A higher intake of vitamin B6 and zinc was also associated with later age at menopause (55). By further establishing a predictive relationship between diet and age at natural menopause, and given findings that AD pathology starts to accumulate during the MT in women, dietary interventions would thus have a valuable impact on etiological research and prevention of AD.
In addition to diet, some evidence suggests that exercise and cardiorespiratory fitness are associated with a reduced risk of cognitive decline and AD in women (56, 57), though not all studies agree (58). These conflicting results may be due to the stage of life in which exercise is measured. In a very large cohort study, women who were physically active at age 30 and 50 had a reduced risk for AD as compared to more sedentary women (59). This advantage was significantly stronger in those women who were also physically active as teenagers (59).
Further Directions
Beyond statistics that expose this greater global burden of AD for females, empirical data about sex differences in AD pathogenesis is warranted to guide clinical and scientific approaches to AD for the benefit of both women and men. Since AD disproportionately affects women, clinical trials may ensure greater progress by focusing on the effects of sex differences on therapy response rates.
To date, though no disease-modifying drug has been proven effective, there is consensus that interventions aimed at preventing or delaying AD progression would be more successful if instituted earlier in the disease course (3, 6). The MT might well represent a window of opportunity to prevent age-related neurological diseases in women.
Translational brain imaging findings indicate a progressively increased risk of AD in women undergoing the MT, and suggest that endocrine aging outweighs the effects of chronological aging in the female brain by several years, if not decades, before possible clinical AD symptoms emerges (8, 9). Imaging the menopausal brain is of relevance given the current understanding of AD as a progressive disorder characterized by an extended preclinical phase during which the disease is underway but has not led to any recognizable clinical or cognitive symptoms (6). Imaging data suggesting that bioenergetic deficits may be driving amyloid deposition in at least some women (8) may serve to revive zeal in pursuit of new treatments and preventative sex-targeted interventions for AD.
It is important to highlight the fact that not all menopausal women develop AD. Just as importantly, only approximately 80% of women experience neurological symptoms during the MT, while 20% do not (5). It is possible that estrogen-mediated brain networks of those women who do not experience symptoms are not dismantled to the same extent observed in those who experience those symptoms, therefore reducing risk of AD. Alternatively, it is possible that postmenopausal women who do not develop AD may be compensating against menopausal brain changes thanks to known AD-protective factors including healthy diet, exercise, intellectual stimulation, and lack or management of vascular risk factors such as hypertension, diabetes, obesity and cardiovascular disease (60). More research is needed to identify female-specific AD-protective factors.
Biomarker results support further investigation into the potential efficacy of estrogen-based and other therapies in preventing decline in brain bioenergetic capacity in women at the perimenopausal and postmenopausal stage. While it is currently unknown as to the most optimal time to begin potential risk reducing interventions, primary prevention and secondary prevention have received growing attention toward determining which stage of the MT may have the greatest potential for success. Many factors contribute to the confounding HRT reports, including unresolved issues regarding the timing of intervention, hormone formulation, dose, route of administration, metabolic comorbidities, and genetic factors, among other considerations. Optimizing and personalizing hormone therapy remains an unmet need in women’s health and a central issue for precision medicine to treat neurological symptoms that develop during, and persist beyond, the perimenopause.
We propose that, given the complexity of the MT, clinical trials examining the effectiveness of estrogen-based and other pharmacological interventions and lifestyle interventions would benefit from stratifying female participants into categories according to their symptoms so as to target specific MT phenotypes rather than enrolling a heterogeneous female population.
Acknowledgements:
This study was supported by NIH/NIA 2P01AG026572;
Weill Cornell Clinical and Translational Science Center UL1TR002384; the Women’s Alzheimer’s Movement; funding from the Department of Neurology at Weill Cornell Medicine and NewYork-Presbyterian; the Zuckerman Family Foundation; and philanthropic support from grateful patients of the Alzheimer’s Prevention Clinic, Weill Cornell Memory Disorders Program.
Funding: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
Footnotes
Conflicts of Interest: The authors declare no disclosures.
Ethical standards: The clinical and education research trials described have been reviewed and approved by the IRB.
References
- 1.Farrer LA, et al. , Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA, 1997. 278(16): p. 1349–56. [PubMed] [Google Scholar]
- 2.Alzheimer’s A, 2016 Alzheimer’s disease facts and figures. Alzheimers Dement, 2016. 12(4): p. 459–509. [DOI] [PubMed] [Google Scholar]
- 3.Stopping Alzheimer’s Disease and Related Dementias: Advancing Our Nation’s Research AgendaNIH Bypass Budget Proposal for Fiscal Year 2018 N.I.o. Health, Editor. 2018.
- 4.Vina J and Lloret A, Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J Alzheimers Dis, 2010. 20 Suppl 2: p. S527–33. [DOI] [PubMed] [Google Scholar]
- 5.Brinton RD, et al. , Perimenopause as a neurological transition state. Nat Rev Endocrinol, 2015. 11(7): p. 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperling RA, Karlawish J, and Johnson KA, Preclinical Alzheimer disease-the challenges ahead. Nat Rev Neurol, 2013. 9(1): p. 54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson HD, Menopause. Lancet, 2008. 371(9614): p. 760–70. [DOI] [PubMed] [Google Scholar]
- 8.Mosconi L, et al. , Perimenopause and emergence of an Alzheimer’s bioenergetic phenotype in brain and periphery PLoS One, 2017. in press: p. e0193314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosconi L, et al. , Sex differences in Alzheimer risk Brain imaging of endocrine vs chronologic aging. Neurology, 2017. 89(13): p. 1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harlow SD, et al. , Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause, 2012. 19(4): p. 387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cray L, Woods NF, and Mitchell ES, Symptom clusters during the late menopausal transition stage: observations from the Seattle Midlife Women’s Health Study. Menopause, 2010. 17(5): p. 972–7. [DOI] [PubMed] [Google Scholar]
- 12.Brinton RD, Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci, 2009. 30(4): p. 212–22.19299024 [Google Scholar]
- 13.Nilsson S, Koehler KF, and Gustafsson JA, Development of subtype-selective oestrogen receptor-based therapeutics. Nat Rev Drug Discov, 2011. 10(10): p. 778–92. [DOI] [PubMed] [Google Scholar]
- 14.McEwen BS, et al. , Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci, 2012. 126(1): p. 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maki PM, The timing of estrogen therapy after ovariectomy--implications for neurocognitive function. Nat Clin Pract Endocrinol Metab, 2008. 4(9): p. 494–5. [DOI] [PubMed] [Google Scholar]
- 16.Yao J and Brinton RD, Estrogen regulation of mitochondrial bioenergetics: implications for prevention of Alzheimer’s disease. Adv Pharmacol, 2012. 64: p. 327–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu F, et al. , Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci, 2008. 11(3): p. 334–43. [DOI] [PubMed] [Google Scholar]
- 18.Yin F, et al. , The perimenopausal aging transition in the female rat brain: decline in bioenergetic systems and synaptic plasticity. Neurobiol Aging, 2015. 36(7): p. 2282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao J, et al. , Ovarian hormone loss induces bioenergetic deficits and mitochondrial beta-amyloid. Neurobiol Aging, 2012. 33(8): p. 1507–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding F, et al. , Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer’s mouse brain: implication for bioenergetic intervention. PLoS One, 2013. 8(11): p. e79977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rettberg JR, et al. , Identifying postmenopausal women at risk for cognitive decline within a healthy cohort using a panel of clinical metabolic indicators: potential for detecting an at-Alzheimer’s risk metabolic phenotype. Neurobiol Aging, 2016. 40: p. 155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattson MP and Magnus T, Ageing and neuronal vulnerability. Nat Rev Neurosci, 2006. 7(4): p. 278–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ungar L, Altmann A, and Greicius MD, Apolipoprotein E, gender, and Alzheimer’s disease: an overlooked, but potent and promising interaction. Brain Imaging Behav, 2014. 8(2): p. 262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleisher A, et al. , Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol, 2005. 62(6): p. 953–7. [DOI] [PubMed] [Google Scholar]
- 25.Damoiseaux JS, et al. , Gender modulates the APOE epsilon4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci, 2012. 32(24): p. 8254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swerdlow RH, Burns JM, and Khan SM, The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis, 2010. 20 Suppl 2: p. S265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh DM and Selkoe DJ, A critical appraisal of the pathogenic protein spread hypothesis of neurodegeneration. Nature Reviews Neuroscience, 2016. 17(4): p. 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benzinger TL, et al. , Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci U S A, 2013. 110(47): p. E4502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bove R, et al. , Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology, 2014. 82(3): p. 222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craig MC, Maki PM, and Murphy DG, The Women’s Health Initiative Memory Study: findings and implications for treatment. The Lancet Neurology, 2005. 4(3): p. 190–194. [DOI] [PubMed] [Google Scholar]
- 31.Menopause & Hormones, FDA, Editor. 2014. [Google Scholar]
- 32.Investigators W.G.f.t.W.s.H.I., Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. Jama, 2002. 288(3): p. 321–333. [DOI] [PubMed] [Google Scholar]
- 33.Shumaker SA, et al. , Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. Jama, 2003. 289(20): p. 2651–2662. [DOI] [PubMed] [Google Scholar]
- 34.LaCroix AZ, et al. , Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. Jama, 2011. 305(13): p. 1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shufelt CL, et al. , Timing of hormone therapy, type of menopause, and coronary disease in women: data from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Menopause (New York, NY), 2011. 18(9): p. 943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasgon NL, et al. , Prospective randomized trial to assess effects of continuing hormone therapy on cerebral function in postmenopausal women at risk for dementia. PLoS One, 2014. 9(3): p. e89095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maki PM and Resnick SM, Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging, 2000. 21(2): p. 373–83. [DOI] [PubMed] [Google Scholar]
- 38.Rocca WA, et al. , Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology, 2007. 69(11): p. 1074–1083. [DOI] [PubMed] [Google Scholar]
- 39.Mielke MM, Vemuri P, and Rocca WA, Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clinical epidemiology, 2014. 6: p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao H, et al. , Hormone therapy and Alzheimer disease dementia New findings from the Cache County Study. Neurology, 2012. 79(18): p. 1846–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitmer RA, et al. , Timing of hormone therapy and dementia: the critical window theory revisited. Annals of neurology, 2011. 69(1): p. 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodis HN, et al. , Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause, 2015. 22(4): p. 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Espeland MA, et al. , Postmenopausal hormone therapy, type 2 diabetes mellitus, and brain volumes. Neurology, 2015. 85(13): p. 1131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rasgon NL, et al. , Insulin resistance and hippocampal volume in women at risk for Alzheimer’s disease. Neurobiol Aging, 2011. 32(11): p. 1942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Q.-g., et al. , C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-α and the critical period hypothesis of estrogen neuroprotection. Proceedings of the National Academy of Sciences, 2011. 108(35): p. E617–E624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brinton RD, The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends in neurosciences, 2008. 31(10): p. 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.FDA approves first nonhormonal hot flash treatment. 2013, The North American Menopause Society. [Google Scholar]
- 48.Freeman EW, et al. , Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. Jama, 2011. 305(3): p. 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alexandersen P, et al. , Ipriflavone in the treatment of postmenopausal osteoporosis: a randomized controlled trial. Jama, 2001. 285(11): p. 1482–1488. [DOI] [PubMed] [Google Scholar]
- 50.Liu Z, et al. , A mild favorable effect of soy protein with isoflavones on body composition—a 6-month double-blind randomized placebo-controlled trial among Chinese postmenopausal women. International journal of obesity, 2010. 34(2): p. 309. [DOI] [PubMed] [Google Scholar]
- 51.Levis S, et al. , Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial. Archives of internal medicine, 2011. 171(15): p. 1363–1369. [DOI] [PubMed] [Google Scholar]
- 52.Nedrow A, et al. , Complementary and alternative therapies for the management of menopause-related symptoms: a systematic evidence review. Archives of internal medicine, 2006. 166(14): p. 1453–1465. [DOI] [PubMed] [Google Scholar]
- 53.Nagel G, et al. , Reproductive and dietary determinants of the age at menopause in EPIC-Heidelberg. Maturitas, 2005. 52(3): p. 337–347. [DOI] [PubMed] [Google Scholar]
- 54.Dorjgochoo T, et al. , Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women’s Health Study. Menopause (New York, NY), 2008. 15(5): p. 924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunneram Y, et al. , Dietary intake and age at natural menopause: results from the UK Women’s Cohort Study. J Epidemiol Community Health, 2018: p. jech-2017–209887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamer M and Chida Y, Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychological medicine, 2009. 39(1): p. 3–11. [DOI] [PubMed] [Google Scholar]
- 57.Hogervorst E, et al. , Exercise to prevent cognitive decline and Alzheimer’s disease: for whom, when, what, and (most importantly) how much. J Alzheimers Dis Parkinsonism, 2012. 2: p. e117. [Google Scholar]
- 58.Fallah N, et al. , Modeling the impact of sex on how exercise is associated with cognitive changes and death in older Canadians. Neuroepidemiology, 2009. 33(1): p. 47–54. [DOI] [PubMed] [Google Scholar]
- 59.Middleton LE, et al. , Physical activity over the life course and its association with cognitive performance and impairment in old age. Journal of the American Geriatrics Society, 2010. 58(7): p. 1322–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Norton S, et al. , Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. The Lancet Neurology, 2014. 13(8): p. 788–794. [DOI] [PubMed] [Google Scholar]