Abstract
Backgrounds
SET and MYND domain-containing protein 2 (SMYD2), which is identified as a protein-lysine methyltransferase, plays a crucial role in the progression of some tumors such as bladder carcinoma. However, the clinical significance of SMYD2 in patients with papillary thyroid carcinoma (PTC) has not been elucidated. In the present study, we aimed to investigate the expression and role of SMYD2 in human PTC.
Material/Methods
Clinicopathological analysis was performed in 107 patients with PTC. Expression of SMYD2 was determined by immunohistochemistry staining, quantitative RT-PCR, or Western blotting in PTC tissues, adjacent normal tissues, and PTC cells (K1 and B-CPAP). The prognostic value of SMYD2 in PTC patients was assessed by univariate and multivariate analysis. Clinical outcomes were evaluated by Kaplan-Meier log-rank tests. Cell proliferation was examined in PTC cells following overexpression or knockdown of SMYD2.
Results
SMYD2 was highly expressed in PTC tissues compared to adjacent thyroid tissues. Additionally, high expression of SMYD2 was significantly related to tumor size, lymph node metastasis, and TNM stage. Moreover, SMYD2 was identified as an independent prognosis factor by multivariate analysis. Using 2 PTC cell lines, K1 and B-CPAP, we demonstrated that high expression of SMYD2 can promote tumor cell proliferation.
Conclusions
SMYD2 expression was upregulated in PTC tissues and significantly related to the poorer prognosis of PTC patients. Our studies suggested the potential role of SMYD2 as a new therapeutic target and prognostic biomarker in human PTC.
MeSH Keywords: Cell Proliferation, Prognosis, Thyroid Neoplasms
Background
Papillary thyroid carcinoma (PTC) is the most common type of human thyroid cancer and approximately 70% of PTC are malignant [1,2]. PTC was usually caused by exposure to radiation and the incidence rate is increasing all over the world [3]. Current treatment for PTC patients includes radiotherapy, chemotherapy, and surgical resection [4]. Although great improvement in PTC treatment has been achieved, PTC patients with curative therapy have worse prognosis, and recurrence occasionally occurs [5,6]. Therefore, it is crucial to study the pathogenesis of PTC and explore novel therapeutic strategies and new prognostic biomarkers for more effective treatment of PTC [7].
The SET and MYND domain-containing protein (SMYD) family contains 5 members SMYD 1–5, which all contain similar conserved domains [8]. Amino acid sequence shows that the SMYD family contains a SET domain that is spilt into 2 portions by an MYND domain [9]. Recently, the role of SMYD protein in progression of some tumors has been clarified. For example, SMYD1 has been previously demonstrated to be correlated with solid tumors such as breast cancer [10]. SMYD2 was found to be a potential oncogene in the development of various tumors [11]. It was also reported that SMYD3 is involved in the invasion of cancer cells and is overexpressed in hepatocellular carcinoma and colorectal cancer [12].
SMYD2 has been identified as a lysine methyltransferase that can methylate lys36 of histone H3 and some non-histone protein substrates such as p53 [13]. It was found that SMYD2 is extensively distributed in a variety of organs and is especially highly expressed in the brain, heart, liver, and kidneys [14]. Recent evidence shows that SMYD2 can downregulate the activity of tumor suppressor protein p53 by methylating the lys370 site and inhibited its tumor-suppressive function [15]. It was also reported that SMYD2 can enhance the activity of the PARP1, a known oncogenic protein, in various types of cancer cells [16]. Moreover, SMYD2 has been found to be highly expressed in glioma, bladder cancer, and gastric adenocarcinoma [17]. In addition, high expression of SMYD2 was reported to be closely related to worse prognosis of lymphoblastic leukemia patients, as well as patients with esophageal squamous cell carcinoma (ESCC) and HPV-unrelated head and neck squamous cell carcinoma (HNSCC) [18]. However, there has been no report about the potential role of SMYD2 in PTC. Therefore, we performed the present study to investigate the clinical and prognostic significance of SMYD2 in PTC patients.
We first examined the protein and RNA levels of SMYD2 in PTC tissues together with adjacent thyroid tissues. Then, we assessed the correlation between SMYD2 expression and poor clinical outcomes by statistical analysis. Furthermore, SMYD2 was identified as an independent prognostic factor for disease-free survival in PTC patients. Finally, we performed cell experiments to elucidate the potential effect of SMYD2 in PTC cells, which demonstrated that high expression of SMYD2 can promote the proliferation capacity in PTC cells.
Material and Methods
Patient and samples
PTC tissues and adjacent normal thyroid tissues were obtained from surgery in 107 randomly selected patients from 2005 to 2015 in Yidu Central Hospital of Weifang. All patients had follow-up ranging from 13 to 113 months and a total of 28 patients had recurrence at the end of follow-up. All specimens used in this study were confirmed based on pathology examination. This study was approved by the Ethics Committee of Yidu Central Hospital of Weifang. Written informed consent was obtained from all patients.
Immunohistochemistry staining and scoring
IHC staining for SMYD2 was performed as described previously [19]. Specimens were cut into 5-μm serial sections and then dried, deparaffinized, and rehydrated. Subsequently, sections were incubated with rabbit anti-human SMYD2 antibody (1: 300 dilution; PA5-51339; Thermo-Fisher Scientific) overnight. The next day, the sections were washed and detected by using peroxidase-labeled IgG and DAB substrate. SMYD2 antibody was replaced with PBS for the negative control. Whole sections were analyzed by 2 independent pathologists and at least 6 fields of each section were randomly selected. Patients were defined as high SMYD2 expression only when both the staining percentage was >25% and the staining intensity was dark yellow or brown. In this study, 42 patients had high SMYD2 expression and the other 65 patients had low SMYD2 expression in PTC tissues.
RNA extraction and real-time qPCR
Total RNA was extracted from 13 pairs of fresh-frozen PTC tissues together with normal adjacent tissues using Trizol reagent (Invitrogen, Carlsbad, US) according to a standard protocol. Then, the RNA was reverse-transcribed using the Primer-Script RT Enzyme Mix. Quantitative real-time PCR was performed using SYBR Premix Ex Tag (Takara, Japan) following the manual instructions. GAPDH was used as normalization control, and the primers were:
SMYD2-F: 5′-AATCCACCCAGAGAGAACAC-3′
SMYD2-R: 5′-AGTGATGGAG AGCAGCTATG-3′
GAPDH-F: 5′-CTCCTCCTGTTCGACAGTCAGC-3′
GAPDH-R: 5′-CCCAATACGACCAAATCCGTT-3′
Cell lines culture
The PTC cell line K1 was purchased from the European Collection of Cell Cultures and B-CPAP was obtained from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). All the cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. All cells were maintained at 37°C in a humidified atmosphere with 5% CO2.
Cell transfection and proliferation assay
The SMYD2 plasmid was purchased from GenScript Biotech Corp and siRNA (CAGGAACGACCGGTTAAGAGA) was purchased from Dharmacon. Cell transfection was performed using Lipofectamine2000 (Invitrogen; USA) according to the manufacturer’s instructions. After 24 h, transfected cells were partially harvested to test the transfection efficiency, and the other cells were expanded and subjected to proliferation assay. Cell proliferation assay was performed using the CCK-8 kit (Dojindo; Japan). Briefly, 100-μl cell suspension (5×103 cells per well) was inoculated in 96-well plates and cultured for preselected times. At the various time points, 10 μl of CCK-8 solution was added to each well of the plate and incubated at 37°C for 1 h. Then, we measured the absorbance at 450 nm wavelength by using the microplate reader. All experiments were repeated 3 times.
Statistical analysis
Statistical analysis was performed using SPSS version 19.0 (IBM, New York, USA). The correlations between the SMYD2 expression and clinical outcomes were analyzed by chi-square test. The overall survival analysis of PTC patients was conducted using the Kaplan-Meier test. Significant factors were identified by using the univariate models to test their independent roles. The t test was used to analyze the results of cell proliferation assay. P<0.05 was considered statistically significant.
Results
The expression of SMYD2 in patients with PTC
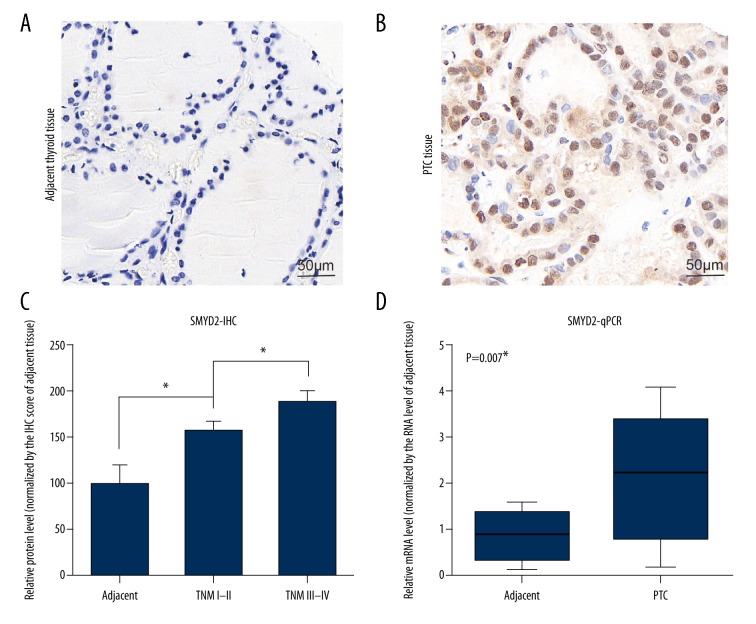
To investigate the role of SMYD2 in PTC patients, we first examined the protein level of SMYD2 in different tissues by IHC staining, showing that SMYD2 was highly expressed in PTC tissues compared to normal adjacent thyroid tissues (Figure 1A, 1B). We also found the correlation between the SMYD2 expression and TNM stage. The relative protein level of SMYD2 was upregulated in the TNM III–IV group compared to the I–II group (Figure 1C). Furthermore, we examined the RNA levels of SMYD2 in 13 pairs of flash-frozen PTC tissues together with adjacent thyroid tissues by real-time qRT-PCR (Figure 1D). The SMYD2 RNA level was also higher in PTC tissues than in normal adjacent tissues (P<0.01). Taken together, these data reveal that SMYD2 was highly expressed in tumor tissues and may be related to the worse clinical outcomes of PTC patients.
Figure 1.
Analysis of SMYD2 expression in patients with PTC. (A, B) IHC staining of SMYD2 in adjacent thyroid tissues (A) and PTC tissues (B). (C) Correlation between relative protein level of SMYD2 and different TNM stages. (D) RNA levels of SMYD2 were examined in PTC tissues and adjacent thyroid tissues. * P<0.05 by t test. Magnification: 400×.
Relationship between SMYD2 expression and clinicopathologic characteristics of PTC patients
For statistical analysis, we divided the 107 PTC patients into 2 group – the SMYD2 low-expression group (n=65) and the SMYD2 high-expression group (n=42) – based on the SMYD2 expression level. Then, we assessed the association between SMYD2 expression and the clinicopathological characteristics in PTC patients (Table 1). We found that SMYD2 expression was significantly related to tumor size (P<0.001), lymph node metastasis (P<0.005), and TNM stage (P<0.005). However, no correlations were found between SMYD2 expression and patient age, sex, thyroid capsular invasion (TCI), or extrathyroidal extension (all P>0.05).
Table 1.
Clinicopathologic characteristics.
| Variables | Cases | SMYD2 expression | P value | |
|---|---|---|---|---|
| (n=107) | Low (n=65) | High (n=42) | ||
| Age (years) | 0.083 | |||
| ≤40 years | 50 | 26 | 24 | |
| >40 years | 57 | 39 | 18 | |
| Gender | 0.396 | |||
| Female | 82 | 48 | 34 | |
| Male | 25 | 17 | 8 | |
| Tumor size | <0.001* | |||
| ≤2.0 cm | 68 | 50 | 18 | |
| >2.0 cm | 39 | 15 | 24 | |
| TCI | 0.654 | |||
| Negative | 69 | 43 | 26 | |
| Positive | 38 | 22 | 16 | |
| ETE | 0.576 | |||
| Negative | 55 | 32 | 23 | |
| Positive | 52 | 33 | 19 | |
| LN metastasis | 0.003* | |||
| Negative | 74 | 52 | 22 | |
| Positive | 33 | 13 | 20 | |
| TNM stage | 0.004* | |||
| I–II | 84 | 57 | 27 | |
| III–IV | 23 | 8 | 15 | |
P<0.05 was considered as statistically significant.
ETE – extrathyroidal extension; LN – lymph node; TCI – thyroid capsular invasion; SMYD2 – SET and MYND domain-containing protein 2.
Correlation between SMYD2-positive expression and disease-free survival in PTC patients
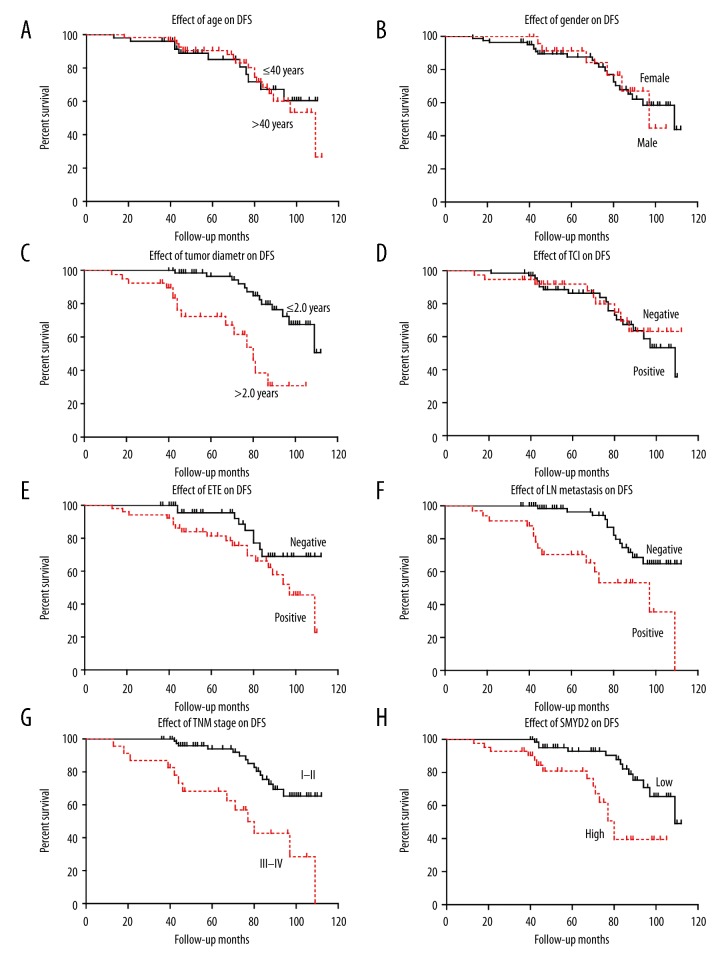
The role of SMYD2 expression in disease-free survival of patients was tested by using the Kaplan-Meier method and compared by the log-rank test. PTC patients who expressed high protein levels of SMYD2 had a poorer disease-free survival (DFS) time (78.8±4.9) compared to patients with low expression levels of SMYD2 (100.7±2.8; P<0.05; Figure 2H, Table 2). Other prognostic factors were also found to correlate with DFS, such as tumor size, extrathyroidal extension (ETE), lymph node metastasis, and TNM stage (all P<0.005) (Figure 2, Table 2).
Figure 2.
Analysis of disease-free survival in PTC patients. The disease-free survival curve was assessed by Kaplan-Meier and log-rank test, based on age (A), sex (B), tumor diameter (C), thyroid capsular invasion (D), extrathyroidal extension (E), lymph node metastasis (F), TNM stage (G), and SMYD2 expression level (H). * P<0.05 by log-rank test.
Table 2.
Univariate analysis.
| Variables | Cases | Disease-free survival | P value | |
|---|---|---|---|---|
| (n=107) | Mean ±SD (months) | 5-year (%) | ||
| Age (years) | 0.792 | |||
| ≤40 years | 50 | 92.6±4.4 | 85.1% | |
| >40 years | 57 | 93.5±3.6 | 90.4% | |
| Gender | 0.886 | |||
| Female | 82 | 93.3±3.3 | 89.4% | |
| Male | 25 | 91.2±4.6 | 91.3% | |
| Tumor size | <0.001* | |||
| ≤2.0 cm | 68 | 101.4±2.5 | 96.4% | |
| >2.0 cm | 39 | 74.7±5.3 | 72.3% | |
| TCI | 0.734 | |||
| Negative | 69 | 92.4±3.3 | 86.4% | |
| Positive | 38 | 95.1±4.9 | 91.9% | |
| ETE | 0.049* | |||
| Negative | 55 | 100.0±3.4 | 95.5% | |
| Positive | 52 | 81.2±4.3 | 81.4% | |
| LN metastasis | <0.001* | |||
| Negative | 74 | 100.5±2.6 | 96.3% | |
| Positive | 33 | 78.4±6.5 | 70.4% | |
| TNM stage | <0.001* | |||
| I–II | 84 | 99.7±2.7 | 94.0% | |
| III–IV | 23 | 74.1±7.4 | 68.3% | |
| SMYD2 expression | 0.001* | |||
| Low | 65 | 100.7±2.8 | 92.9% | |
| High | 42 | 78.8±4.9 | 80.9% | |
P<0.05 was considered as statistically significant.
ETE – extrathyroidal extension; LN – lymph node; TCI – thyroid capsular invasion; SMYD2 – SET and MYND domain-containing protein 2.
Moreover, SMYD2 expression, tumor size, lymph node metastasis, and TNM stage (all P<0.05) were identified as independent factors related to decreased DFS in PTC patients by multivariate analysis of survival curves (Table 3).
Table 3.
Multivariate analysis.
| Variables | Hazard ratio | 95% Confidence Interval | P value |
|---|---|---|---|
| Tumor size | 2.48 | 1.03–5.48 | 0.042* |
| ETE | 1.59 | 0.68–3.74 | 0.284 |
| LN metastasis | 2.66 | 1.07–6.58 | 0.035* |
| TNM stage | 4.66 | 1.89–11.47 | 0.001* |
| SMYD2 expression | 2.51 | 1.05–4.82 | 0.040* |
P<0.05 was considered as statistically significant.
ETE – extrathyroidal extension; LN – lymph node; SMYD2 – SET and MYND domain-containing protein 2.
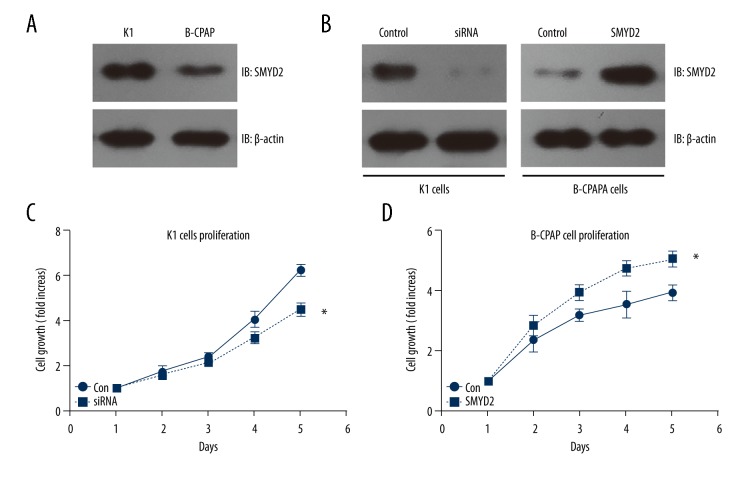
High expression of SMYD2 promotes proliferation of PTC cell lines
We performed cellular experiments to detect the potential effect of SMYD2 in PTC. The expression patterns of SMYD2 in 2 different PTC cell lines – K1 and B-CPAP – were examined by Western blot (Figure 3A). We found that SMYD2 expression was higher in K1 cells than in B-CPAP cells. Then, we knocked down SMYD2 in K1 cells and overexpressed SMYD2 in B-CPAP cells. The transfection efficiency was tested using Western blot and normalized with β-actin (Figure 3B). We assessed the transfected cell characteristics to investigate the role of SMYD2 in tumor progression and the results revealed that SMYD2 high expression can enhance the proliferation compacity of PTC cells (Figure 3C, 3D). These results suggest that HOXD4 plays a role in the progression of human PTC.
Figure 3.
The function of SMYD2 in PTC cell lines. (A) Expression level of SMYD2 in 2 different PTC cell lines: K1 and B-CPAP. (B) Knockdown of endogenous SMYD2 in K1 cell and overexpression of SMYD2 in B-CPAP cells. Transfection efficiency was examined by Western blot. (C, D) Knockdown of SMYD2 suppressed K1 cell proliferation, while overexpression of SMYD2 promoted B-CPAP cell proliferation. * P<0.05 by t test compared to the control group.
Discussion
Methylation plays multiple roles in cell signaling, which can affect gene stability, modulate transcription, and regulate protein activation [20]. Lysine methyltransferases involved in a variety of oncoproteins and tumor suppressor proteins are known to play a crucial role in the progression of different tumors [21]. There are intensive ongoing efforts to illustrate the underlying molecular mechanisms and related clinicopathologic characteristics in different cancers. Modulation of these methylations has been considered promising for the development of new anticancer drugs. The human SMYD family has been confirmed to play an important role in development of carcinoma [22]. For example, it has been found that disruption of SMYD1 leads to deranged cardiac morphogenesis and embryonic death [23]. Some studies have shown that high expression of SMYD3 was correlated with cancer cell invasion and can serve as a predictor of poor prognosis in hepatocellular carcinogenesis and gastric adenocarcinoma [24]. Additionally, it was also reported that SMYD4 plays a crucial role in progression of breast carcinoma [25]. Despite this evidence, the potential effect of SMYD2 in the progression and prognosis of PTC has not been clarified.
In the present study, we first examined expression level of SMYD2 in PTC patients by IHC staining and real-time qPCR. Our results showed that SMYD2 was expressed at significantly higher levels in PTC tissues compared to the adjacent normal thyroid tissues. The expression pattern of SMYD2 in patients at various TNM stages was also tested, and the results indicated that the role of SMYD2 may be related to the progression of PTC. Furthermore, we found an association between high expression of SMYD2 and clinicopathologic features of PTC patients. The high expression of SMYD2 was closely related to tumor size, lymph node metastasis, and TNM stage. Importantly, we demonstrated that high expression of SMYD2 was correlated with shorter disease-free survival time in PTC patients. In addition, we identified SMYD2 as an independent prognostic factor for PTC patients by using multivariate analysis. Furthermore, we conducted cellular experiments to investigate the potential effect of SMYD2 in PTC cells. Knockdown of SMYD2 in K1 cells suppressed the proliferation capacity of cancer cells, while overexpression of SMYD2 in B-CPAP cells promoted cell proliferation. Taken together, these results suggest that high expression of SMYD2 promotes the proliferation of tumor cells and is related to poor prognosis of PTC patients.
It has been previously reported that SMYD2 can promote the expression of cyclin D1, which is a regulator of cell proliferation, in hepatocellular carcinoma [26]. It was also reported that SMYD2 can methylate lys370 of p53, and consequently promotes tumor progression [24]. In addition, SMYD2 was shown to methylate lys313 of PTEN in breast cancer cells, and methylate Rb at lys860 in gastric cancer [21]. Therefore, it seems that SMYD2 may function through different signaling pathways in different types of cancer. Intensive studies focused on the underlying mechanisms of SMYD2 in progression of PTC are needed.
Conclusions
Our study demonstrates that the expression level of SMYD2 was significantly elevated in PTC tissues and closely related to poor prognosis in PTC patients. Furthermore, SMYD2 was identified as a potential independent prognostic factor by multivariate analysis, suggesting it may be a novel therapeutic target and prognostic biomarker for PTC to improve clinical prognosis of these patients.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Subha ST, Bakri MA, Salleh H, et al. Papillary thyroid carcinoma presenting as a cystic neck lesion: Case series. Iran J Otorhinolaryngol. 2018;30(96):49–54. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu X-C, Zhou K, Xu S-Q, Ma Y-B. Diagnostic value of semiquantitative analysis of 99mTechnetium-Methoxyisobutylisonitrile (99mTc-MIBI imaging in predicting early-stage cervical lymph node metastasis of thyroid carcinoma. Med Sci Monit. 2017;23:1552–58. doi: 10.12659/MSM.899966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaha AR. Central lymph node metastasis in papillary thyroid carcinoma. World J Surg. 2018;42(3):630–31. doi: 10.1007/s00268-017-4459-8. [DOI] [PubMed] [Google Scholar]
- 4.Rathod JK, Rathod SJ, Kadam V. Papillary carcinoma of thyroid in a thyroglossal cyst. J Oral Maxillofac Pathol. 2018;22(Suppl 1):S98–S101. doi: 10.4103/jomfp.JOMFP_173_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pourseirafi S, Shishehgar M, Ashraf MJ, Faramarzi M. Papillary carcinoma of thyroid with nasal cavity metastases: A case report. Iran J Med Sci. 2018;43(1):90–93. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou D, Li Z, Bai X. BRAFV600E and RET/PTC promote proliferation and migration of papillary thyroid carcinoma cells in vitro by regulating nuclear factor-κB. Med Sci Monit. 2017;23:5321–29. doi: 10.12659/MSM.904928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Güney G, Şahiner İT. Malignancy rates of thyroid cytology: cyst fluid benign or non-diagnostic? Med Sci Monit. 2017;23:3556–61. doi: 10.12659/MSM.905718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tracy C, Warren JS, Szulik M, et al. The Smyd family of methyltransferases: Role in cardiac and skeletal muscle physiology and pathology. Curr Opin Physiol. 2018;1:140–52. doi: 10.1016/j.cophys.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu S, Zhong C, Zhang T, Ding J. Structure of human lysine methyltransferase Smyd2 reveals insights into the substrate divergence in Smyd proteins. J Mol Cell Biol. 2011;3(5):293–300. doi: 10.1093/jmcb/mjr015. [DOI] [PubMed] [Google Scholar]
- 10.Li LX, Zhou JX, Calvet JP, et al. Lysine methyltransferase SMYD2 promotes triple negative breast cancer progression. Cell Death Dis. 2018;9(3):326. doi: 10.1038/s41419-018-0347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuo SR, Zuo XC, He Y, et al. Positive expression of SMYD2 is associated with poor prognosis in patients with primary hepatocellular carcinoma. J Cancer. 2018;9(2):321–30. doi: 10.7150/jca.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zipin-Roitman A, Aqaqe N, Yassin M, et al. SMYD2 lysine methyltransferase regulates leukemia cell growth and regeneration after genotoxic stress. Oncotarget. 2017;8(10):16712–27. doi: 10.18632/oncotarget.15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Perez-Burgos L, Placek BJ, et al. Repression of p53 activity by Smyd2-mediated methylation. Nature. 2006;444(7119):629–32. doi: 10.1038/nature05287. [DOI] [PubMed] [Google Scholar]
- 14.Hamamoto R, Toyokawa G, Nakakido M, et al. SMYD2-dependent HSP90 methylation promotes cancer cell proliferation by regulating the chaperone complex formation. Cancer Lett. 2014;351(1):126–33. doi: 10.1016/j.canlet.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Saddic LA, West LE, Aslanian A, et al. Methylation of the retinoblastoma tumor suppressor by SMYD2. J Biol Chem. 2010;285(48):37733–40. doi: 10.1074/jbc.M110.137612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, Deng X, Yoshioka Y, et al. Effects of SMYD2-mediated EML4-ALK methylation on the signaling pathway and growth in non-small-cell lung cancer cells. Cancer Sci. 2017;108(6):1203–9. doi: 10.1111/cas.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng X, Hamamoto R, Vougiouklakis T, et al. Critical roles of SMYD2-mediated beta-catenin methylation for nuclear translocation and activation of Wnt signaling. Oncotarget. 2017;8(34):55837–47. doi: 10.18632/oncotarget.19646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguilar A. Polycystic kidney disease: SMYD2 is a novel epigenetic regulator of cyst growth. Nat Rev Nephrol. 2017;13(9):513. doi: 10.1038/nrneph.2017.99. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Xu Y, Zhang Q, et al. Prognostic significance of TBL1XR1 in predicting liver metastasis for early stage colorectal cancer. Surg Oncol. 2017;26(1):13–20. doi: 10.1016/j.suronc.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Tanaka K, Yan J, et al. Regulation of estrogen receptor alpha by histone methyltransferase SMYD2-mediated protein methylation. Proc Natl Acad Sci USA. 2013;110(43):17284–89. doi: 10.1073/pnas.1307959110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komatsu S, Ichikawa D, Hirajima S, et al. Overexpression of SMYD2 contributes to malignant outcome in gastric cancer. Br J Cancer. 2015;112(2):357–64. doi: 10.1038/bjc.2014.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donlin LT, Andresen C, Just S, et al. Smyd2 controls cytoplasmic lysine methylation of Hsp90 and myofilament organization. Genes Dev. 2012;26(2):114–19. doi: 10.1101/gad.177758.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y, Holcomb J, Spellmon N, Yang Z. Purification of histone lysine methyltransferase SMYD2 and co-crystallization with a target peptide from estrogen receptor alpha. Methods Mol Biol. 2016;1366:207–17. doi: 10.1007/978-1-4939-3127-9_16. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira-Santos W, Rabello DA, Lucena-Araujo AR, et al. Residual expression of SMYD2 and SMYD3 is associated with the acquisition of complex karyotype in chronic lymphocytic leukemia. Tumour Biol. 2016;37(7):9473–81. doi: 10.1007/s13277-016-4846-z. [DOI] [PubMed] [Google Scholar]
- 25.Reynoird N, Mazur PK, Stellfeld T, et al. Coordination of stress signals by the lysine methyltransferase SMYD2 promotes pancreatic cancer. Genes Dev. 2016;30(7):772–85. doi: 10.1101/gad.275529.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toghill BJ, Saratzis A, Freeman PJ, et al. SMYD2 promoter DNA methylation is associated with abdominal aortic aneurysm (AAA) and SMYD2 expression in vascular smooth muscle cells. Clin Epigenetics. 2018;10:29. doi: 10.1186/s13148-018-0460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]