Abstract
Pediatric burn patients often have hypertension and tachycardia for several years post-injury. Propranolol has shown to be effective in treating the hypermetabolic state secondary to a major burn injury. This study was conducted to document a safe and effective dosing regimen for three different age groups. One hundred four burn-injured children with a 30% to 92% total body surface area burn were treated for 1 to 2 years with propranolol in the outpatient setting. Guardians of the patients were instructed on how to take and monitor the systolic blood pressure and heart rate, and document their vital signs several times a day. The documentation was reviewed with the guardian and patient, and based on age-specific vital sign parameters, propranolol dosing adjustment was done to measure at least 15% to 20% reduction in admission heart rate. Mean doses for the age groups were as follows: 0 to 3 years 5.2 ± 2.8 mg/kg/day, 4 to 10 years 4.2 ± 1.8 mg/kg/day, and 11 to 18 years 2.9 ± 1.4 mg/kg/day. The propranolol dose decreased as time post-burn increased. On selected patients, propranolol was stopped due to changes in the heart rate, but at all times, it was safe and effective. No adverse effects were noted. The dosing regimen was not affected by burn size or gender. Propranolol can be safely stopped abruptly with no rebound hypertension. Individuals older than 10 years required a lower dose per kilogram following the burn injury than prepubertal burn survivors. Propranolol proved to be both safe and effective in the management of cardiovascular changes occurring in the hypermetabolic state.
In 1986, Dr. Herndon and associates introduced the use of propranolol to pediatric patients admitted to Shrine Burn Hospital in Galveston, Texas who suffered from the hypermetabolic state that occurs after a major burn injury. Historically, this drug has been used in cardiac patients. But given the properties of propranolol, it was believed to be helpful in expediting the healing process of pediatric burn patients and preventing further damage on the body. Herndon et al1 demonstrated that propranolol reduces cardiac energy expenditure, improves scar healing, and improves glucose tolerance.
The hypermetabolic response encompasses the many changes that can occur in the body due to the body’s reaction to a major trauma, such as a burn. The following may be seen in this response: increase in heart rate and blood pressure, elevation in resting energy expenditure, catabolism (a destructive phase of metabolism resulting from a combination of elevated plasma catecholamines, cortisol and inflammatory cells), insulin resistance, total body protein loss, muscle wasting, increased infection risks, multiorgan failure, and/or death of the patient. Catecholamines initiate the hypermetabolic process and induce tachycardia, which increases oxygen demand.2 In the case of pediatric burn injury, catecholamines that trigger the hypermetabolic response can be present up to 24 months post-burn.1
Propranolol is a nonselective β-adrenergic receptor antagonist which affects the cardiovascular system. It blocks endogenous catecholamines from binding with β-adrenergic receptors.2 The immediate release propranolol takes effect after 1 to 2 hours and lasts 6 to 12 hours, whereas the extended release (ER) lasts about 24 to 27 hours.3 It is absorbed rapidly, but it undergoes a high first-pass effect and only 25 per cent of the drug reaches systemic circulation.3 Propranolol is used primarily in people with tremors, angina, hypertension, heart rhythm disorders, and other circulatory conditions and is also used to treat or prevent heart attacks and migraine headaches. People suffering from asthma, heart block, or decompensated heart failure should not be using this medication. Common and non-life-threatening adverse effects include hypotension, bradycardia, and hypoglycemia.4 Some of the life-threatening reactions to propranolol include pulmonary edema, complete heart block, and shock.5 The efficacy of propranolol has been proven in previous studies.1, 4, 6–9 The primary aim of this study was to document the safe and effective practice of taking propranolol at an age-appropriate dose.
METHODS
A group of 104 burn survivors were included in the study (Table 1), who suffered Total Body Surface Area (TBSA) burns from 30% to 92%. IRB approval was obtained for the study, and informed consent was discussed with each parent and, if applicable, patient. Once the parent and/or patient consented, the patient was started on his/her study drug. Propranolol was started shortly after admission to the burn unit and given throughout their acute hospitalization. The vital signs were closely monitored, especially the systolic blood pressure (SBP) and heart rate (HR). Adjustments in dosage were made accordingly to reduce the admission HR by 20 per cent. Once the patient was discharged from the acute unit and went into the care of the guardian, the patient was seen in the outpatient clinic weekly by the physician and/or physician assistant. Education for the guardian began immediately upon discharge. The guardian was taught how and when to administer the propranolol and how to take and document the vital signs. A portable blood pressure machine and red booklet for vital sign recording was provided to the guardian. Parameters were given for the HR and SBP as to provide guidance to the guardian when discerning whether or not to administer the propranolol (Table 2). These parameters were determined by subtracting approximately one standard deviation (SD) from the mean HR and two SDs from the mean SBP for the age range.10,11 Anything more than three SDs were considered hypotension and warranted closer monitoring. Propranolol was given every 6 hours (q6h) by mouth, or daily as an ER tablet. As opposed to the tablet, the q6h propranolol came in liquid form, which was easier to administer to the 0- to 3-year age group. In order to reinforce the education, guardians were encouraged to demonstrate what they learned during their educational instruction. The vital signs recorded in the red booklet given to the guardian were reviewed at each clinic visit. Depending on the HR at that time, the propranolol dose may have been titrated, or the parameters may have been adjusted for that particular patient. Based on the patient’s weight, the physician may have increased or decreased the propranolol to achieve the target HR for that patient’s age range. Education on how to take and document the vital signs or how and when to administer the medication may have been revisited at that time. Vital signs were monitored throughout the administration of the drug. If unable to give the dose due, then that dose was held until the next due time. Since many patients spoke Spanish, instructions were given in Spanish. No additional antihypertensive medications were given concurrently.
Table 1.
Demographics of the subject population
| 0–3 y | 4–10 y | 11–18 y | |
|---|---|---|---|
| Males | 25 | 21 | 26 |
| Females | 11 | 11 | 10 |
Table 2.
Parameters for propranolol administration based on heart rate (HR) and systolic blood pressure (SBP) per age group
| 0–3 y | 4–10 y | 11–18 y | |
|---|---|---|---|
| HR | ≥ 65 | ≥ 65 | ≥ 50 |
| SBP | > 70* | ≥ 70 | ≥ 80 |
*Individually based.
RESULTS
It was seen that propranolol dosing for the various age groups at the start and end of the outpatient treatment phase was slightly different (Table 3). Prior to stopping propranolol, the average dosing was reduced in all age groups, the older age group requiring less milligrams/kilogram/day (mg/kg/day). Patients less than or equal to 10 years old had an average 4.8 ± 2.4 mg/kg/day and patients greater than or equal to 11 years old had an average 2.9 ± 1.4 mg/kg/day.
Table 3.
Average dosing (mg/kg/day) ± SD for the different age groups over time
| 0–3 y | 4–10 y | 11–18 y | |
|---|---|---|---|
| Starting dose | 5.1 ± 2.7 | 4.6 ± 2.3 | 2.8 ± 1.4 |
| Ending dose | 3.5 ± 2.2 | 3.0 ± 1.5 | 1.8 ± 1.0 |
The dosing of the propranolol was determined by the HR of the patients. Compared with the admission and outpatient HR, a remarkable difference was noted once the propranolol was discontinued (Table 4). There was no evidence of significant change in the SBP time points (Table 5). Multiple comparisons were made among the HRs, SBPs, time points, ages, genders doses, and incidences of bradycardia and hypotension. A t-test and Mann–Whitney U test were used to analyze these data. No significance was observed.
Table 4.
Comparison of heart rate (HR) per age group over time (HR was measured in beats per minute. Data are represented as a mean ± SD)*
| 0–3 y | 4–10 y | 11–18 y | |
|---|---|---|---|
| Admission | 161 ± 16 | 149 ± 22 | 137 ± 25 |
| Acute | 151 ± 21 | 136 ± 23 | 127 ± 18 |
| Therapeutic | 117 ± 21 | 107 ± 17 | 99 ± 22 |
| Prior to stop | 101 ± 19 | 84 ± 14 | 76 ± 16 |
| Stop | 94 ± 18 | 88 ± 16 | 77 ± 16 |
| Off drug | 96 ± 14 | 88 ± 9 | 76 ± 12 |
*Heart rates were measured in beats per minute. Data are represented as a mean ± standard deviation.
Table 5.
Comparison of systolic blood pressure (SBP) per age group over time (SBP was measured in mmHg. Data are represented as a mean ± SD)
| 0–3 y | 4–10 y | 11–18 y | |
|---|---|---|---|
| Admission | 101 ± 17 | 111 ± 18 | 119 ± 21 |
| Acute | 104 ± 16 | 111 ± 15 | 124 ± 20 |
| Therapeutic | 103 ± 18 | 109 ± 12 | 122 ± 11 |
| Prior to stop | 99 ± 13 | 104 ± 13 | 115 ± 10 |
| Stop | 95 ± 8 | 105 ± 12 | 116 ± 15 |
| Off drug | 98 ± 12 | 106 ± 14 | 110 ± 19 |
Each patient had at least one incidence of bradycardia. Patients documented at least one measurement per day, and patients on propranolol were found to have an average of 7.6 days (out of an average of 666 days of measurements) when bradycardia was noted (Table 7). Control patients had an average of 0.3 days of bradycardia (average days of measurements: 132). Each patient also had at least one incidence of hypotension. With the same number of days of measurements, the propranolol patients had an average of 24.2 days and the control patients had an average of 1.8 days (Table 8).
Table 7.
Number of days with some incidence of bradycardia (heart rate [HR] < 65 for ages 0–10 years, <50 for ages 11 and older. P-value < .0001)
| Control | Propranolol (combination of ER and q6h drug groups) | |
|---|---|---|
| Total number of patients | 136 | 104 |
| Average number of days with measurements | 132 | 666 |
| Average number of days with bradycardia | 0.3 | 7.6 |
Table 8.
Number of days with some incidence of hypotension (SBP < 70 for ages 0–10 years, <80 for ages 11 and older. P-value < .0001)
| Control | Propranolol (combination of ER and q6h drug groups) | |
|---|---|---|
| Total number of patients | 136 | 104 |
| Average number of days with measurements | 132 | 666 |
| Average number of days with hypotension | 1.8 | 24.2 |
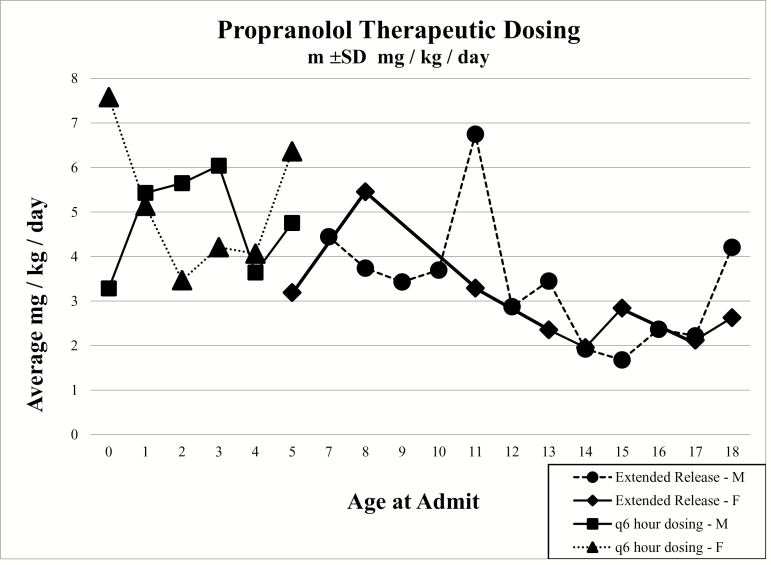
Comparing the genders at the different phases of propranolol dosing, it was found that males required slightly less mg/kg/day (Figure 1). The dose of the ER capsules was also found to be less than the q6h regimen (Table 6). In the q6h propranolol category, there was a total of 20 females and they had an average dose of 4.83 mg/kg/day. The males (n = 34) received an average of 4.8 mg/kg/day. A total of 54 patients took propranolol q6h. In the ER propranolol category, there was a total of 12 females and they had an average of 3.44 mg/kg/day. The males (n = 38) received an average of 3.41 mg/kg/day. A total of 50 patients took the ER propranolol.
Figure 1.
Comparison of the average mg/kg/day for males (M) and females (F) in the q6h and extended release (ER) categories. The markers are based on the patient ages.
Table 6.
Dose of propranolol (mg/kg/day) ± SD per age group and gender
| Gender | 0–3 y | Avg. dose | 4–10 y | Avg. dose | 11–18 y | Avg. dose | |
|---|---|---|---|---|---|---|---|
| n | n | n | |||||
| Q6H | M | 25 | 5.46 ± 2.80 | 9 | 4.14 ± 1.92 | 0 | 0 |
| F | 11 | 4.70 ± 2.90 | 9 | 4.83 ± 2.29 | 0 | 0 | |
| ER | M | 0 | 0 | 12 | 3.84 ± 1.38 | 26 | 2.98 ± 1.56 |
| F | 0 | 0 | 2 | 4.32 ± 1.60 | 10 | 2.56 ± 0.86 |
DISCUSSION
Propranolol is a β blocker used to help reduce the cardiac workload. It has been used primarily in cardiac cases. Pediatric studies involving propranolol have mainly demonstrated the benefit of the medication in regards to burns and infantile hemangiomas. Korownyk et al12 showed that propranolol stops the growth of infantile hemangiomas when using 2 mg/kg dosing. No significant hypotension, bradycardia, or hypoglycemia were noted.12
Numerous studies have demonstrated the safety and efficacy of propranolol in the acute burn phase. Herndon et al9 demonstrated that the cardiac effort was significantly reduced by 20% to 36% when the patients were given 2 mg/kg every 24 hours for 5 days. They determined that an ideal dose of 0.5 to 1.0 mg/kg intravenously every 6 to 8 hours should be given to adequately reduce left ventricular work without adversely affecting oxygen delivery or other cardiac functions. In a study by Minifee et al8, IV propranolol showed decreased myocardial oxygen requirements without adversely affecting overall oxygen delivery and total body oxygen consumption. This study also noted that none of the patients suffered harmful side effects. Another study by Baron et al13 showed that prolonged administration of oral or IV propranolol does not have an adverse effect on morbidity or mortality. They also showed no hypotension, hypothermia, azotemia, hyperglycemia or hypoglycemia, arrhythmia, bronchospasm, or peripheral ischemia during or after treatment.13 Both oral and IV propranolol were tested, and even though the IV propranolol reduced the heart rate more per milligram per kilogram, both routes were effective and safe. In a study by Norbury et al14, propranolol demonstrated safe and efficacious use in modulating the sepsis response. During periods of stress and sepsis, the propranolol reduced energy expenditure and lipolysis, reversed catabolism, and restored glycemic control.14 Mohammadi et al7 showed propranolol aided in improving burn wound healing and decreased healing time and hospital stay.
Williams et al6 noted in terms of gender that there were no dosing or response differences between males and females in the acute setting. Both had significant decreases in HR as well as required 4 mg/kg/day to maintain these decreases. They also did not observe any clinically relevant hypotension among their study group. Herndon et al1 studied the efficacy of propranolol on a long-term basis with a set dosage of 4 mg/kg/day. They studied burn patients with greater than 30 per cent TBSA for 1 year post-burn and they found the heart rate decreased 110 per cent at 6 months post-burn with the 4 mg/kg/day regimen. Neither bradycardia nor significant decreases in blood pressure were observed.1 In regards to age, this study determined that an average dose of 4.6 mg/kg/day was effective in managing the blood pressure and heart rate of post-burn prepubertal patients. It was noted that the dosage decreased as post-burn time increased. Again, gender did not affect the regimen and no adverse effects were observed.
An efficacious dose was determined based on previous studies. Williams et al6 started patients on 1 mg/kg/day and titrated based on the heart rate. The desired decrease was between 15% and 20% of admission heart rate. On this regimen, a decrease of 10 per cent was noted. The doses were increased to 4 mg/kg/day to achieve the desired 15 per cent decrease. Herndon et al9 suggested that 0.5 to 1.0 mg/kg IV q6-8h would be an ideal dose in the inpatient setting. Baron et al13 used 0.5 to 1.0 mg/kg orally or IV every 8 hours for 10 days to notice a beneficial decrease in tachycardia and myocardial oxygen demand. Mohammadi et al7 found a decrease in wound surface area needing grafting by administering 1 mg/kg/day orally. For this study, age played a major role in dosing and formulation. In determining a q6h vs ER dose, the q6h dose was multiplied by 4 (times a day), and the value just less than the result was the dose used, as long as it was safe (eg, propranolol 30 mg × 4 = 120 mg, ER dose would be around 100 mg). Based on historical results and the findings of this study, dosing recommendations can be made regarding q6h vs ER dosing.
The effects of propranolol are immediate in that they can be seen within hours. In checking the vital signs every 6 hours or before the next dosing interval, they were assessed as to whether or not they were within the set guidelines for their age and if the dose of propranolol was still effective. Also noting if they had decreased since medication was last administered. To assure the safety, the propranolol was started at a low dose for the patient’s weight. It was increased by small increments to decrease the HR by at least 20 per cent with the goal of arriving at the normal HR range for the patient’s age group. Over a period of days, it was seen that the blood pressure and HR decreased. If not, the dose was titrated in order to see a full antihypertensive response in the documentation. The study was for a period of 1 to 2 years post-burn, monitoring the vital signs throughout that time, and at the end of that period the propranolol was withdrawn. Vital signs continued to be monitored for several days after the propranolol was stopped. The propranolol proved to be effective because the HR stayed within the age-appropriate range.
Herndon et al1 demonstrated that propranolol treatment for 12 months post-burn injury alleviates the hypermetabolic process. The long-term goal for these patients was to use propranolol to reduce cardiac strain, which is reflected as tachycardia and hypertension. Observing age-appropriate vital signs demonstrated the propranolol’s ability in providing cardiac relief and reduce the likelihood of cardiac dysfunction in the future. Throughout this 1 to 2 year time period that the study was conducted, it was seen that the propranolol was effective, safety was never an issue, and due to the close monitoring of the vital signs, two patients had to be removed from propranolol because their HRs were continually below their set parameters. One patient was a physically fit male and the other was a female who did not reach HRs that warranted propranolol. But no adverse effects were noted.
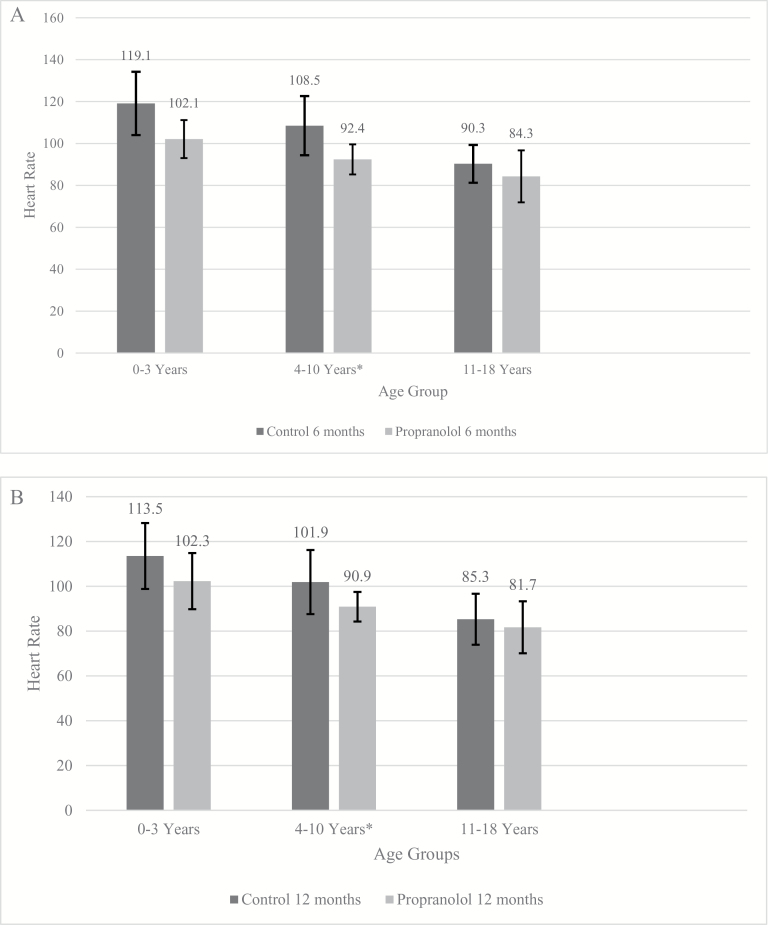
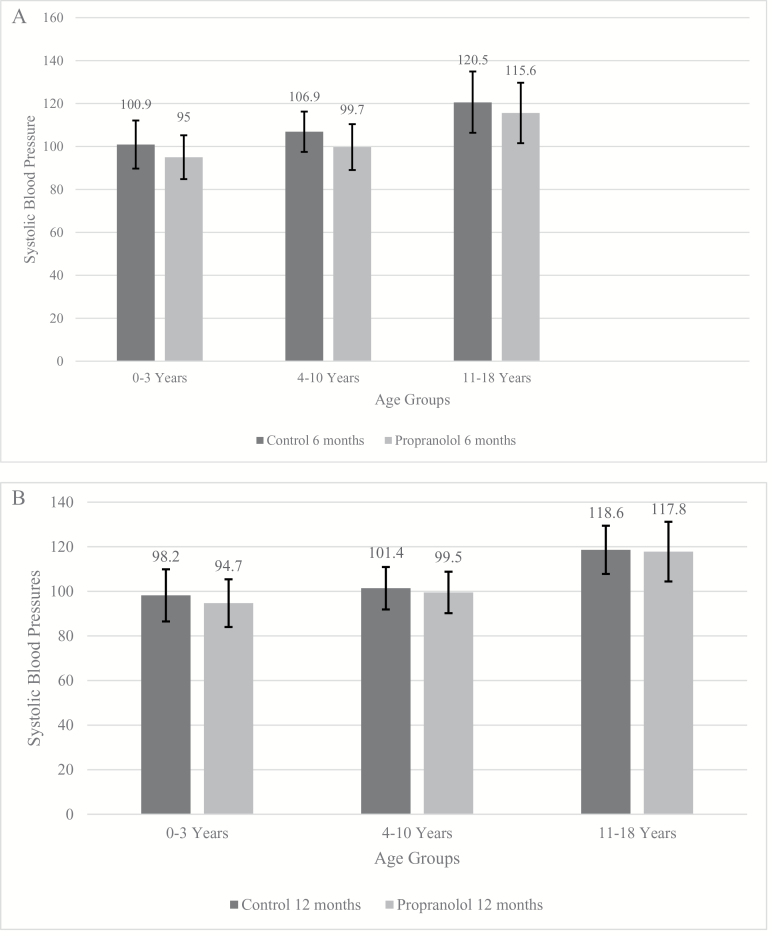
On average, the HRs and SBPs of the propranolol patients were lower than those in the control group. Measurements were collected at the 6-month (Figures 2A and 3A) and 12-month (Figures 2B and 3B) time points for the three age groups. Each patient improved with time; however, the propranolol patients were consistently lower at both time points. The HRs and SBPs for the control group dropped significantly over time, whereas the propranolol group had smaller, steadier changes. These results show that the hypermetabolism of a burn eventually resolves, but propranolol will bring a patient to a more normal state and sustain it.
Figure 2.
(A) Average daily heart rates (HRs) for the three age groups ± SD, control vs propranolol at 6 months (0–3: ±15.1, 4–10: ±14.1, 11–18: ±9.0). (B) Average daily HRs for the three age groups ± SD, control vs propranolol at 12 months (0–3: ±14.7, 4–10: ±14.3, 11–18: ±11.4).
Figure 3.
(A) Average daily systolic blood pressures (SBPs) for the three age groups ± SD, control vs propranolol at 6 months (0–3: ±11.2, 4–10: ±9.4, 11–18: ±14.4). (B) Average daily SBPs for the three age groups ± SD, control vs propranolol at 12 months (0–3: ±11.7, 4–10: ±9.5, 11–18: ±10.8).
The patients demonstrated an average of 18.6 days with bradycardia. Compared with the 666 days of measurements, this is a small incidence. Patients on ER medication measured at least once a day, maybe twice. Patients on q6h measured up to four times daily. This finding was based on having at least one incidence of bradycardia per day their HR was measured. The control group had a smaller average as a result of their constant tachycardic condition. Patients on propranolol were more likely to have episodes of bradycardia during the dosing process. The patient would hold the dose in an isolated instance, but if they consistently failed to meet parameters, then they may have been on an inappropriate dose, which was then adjusted in the clinic. The patient and guardian would also undergo re-education as needed, if the measurement was based on insufficient application or use of the blood pressure machine or misunderstanding of medication administration. Every propranolol patient has at least one incidence when the medication was held due to failure to meet the parameter. This would occur during the dosing process and tailoring the dose to fit the patient’s need. Most importantly, no symptomatic bradycardia was observed among these patients. No episodes of fainting or exhaustion were noted. Any symptoms relating to bradycardia and hypotension were self-reported, but in their clinic visits, none of the patients were so symptomatic as to require immediate intervention or follow-up with the physician.
It was also found that there was no rebound effect when propranolol was stopped abruptly vs tapering it. In previous adult studies, adverse effects were noted when using propranolol, such as bradycardia, bradypnea, hypotension, and ischemia.15 In an adult study by Brown et al4, 72.2 per cent of patients were hypotensive on propranolol and 14.8 per cent were bradycardic on at least one occasion. None of the common or the previously mentioned life-threatening adverse effects were observed in the pediatric patients of the study. However, if the patient is also on another antihypertensive agent, such as clonidine, it should be tapered.
LIMITATIONS
A limitation may be the accuracy in recording the vital signs and propranolol administration. The medication and blood pressure cuffs are entrusted to nonclinical individuals who self-report based on the education provided to them. If the cuff did not fit the patient, the guardian may not know that ill-fitting cuffs could skew the measurement. Another limitation may be how the baseline heart rates and blood pressures were determined. The first measurements of the day shift (7:00 AM) were selected to represent baseline. It may not have been an ideal depiction of the patient’s condition; however, it was selected in order to be consistent. By not controlling the times of vitals checks, there may be a bias, but it is not systemic. Patients were being woken up or doses were skipped in trying to adhere to a standardized medication schedule. As outpatients, compliance was promoted by allowing the guardian to check the vitals in accordance with the patient’s routine. Another limitation is the scope of these data. It may not be applicable to patients over the age of 18.
CONCLUSIONS
Propranolol is safe in treating tachycardia related to the hypermetabolic state following a pediatric burn injury. No adverse effects were noted in immediate discontinuation of the medication. Age had more of an impact than gender when determining the dose. Titration of the doses to appropriately control HR in the various age groups was determined by the documentation and monitoring of the vitals within their respective parameters. The goal was to reduce cardiac work with propranolol, which was determined by the HR as documented by the patient’s guardian. Since the study determined a safe and appropriate dosing for three different age groups in children who have sustained a major burn injury, this information can possibly be applied as a standard of care knowing a hypermetabolic state is bound to occur shortly after sustaining a major burn injury.
ACKNOWLEDGMENTS
The authors would like to note that this study was approved by the Institutional Review Board of the University of Texas Medical Branch in Galveston (No. 04-157). The authors would also like to acknowledge the following grants for their support: National Institutes of Health: P50GM060388, T32GM008256, R01HD049471; Shriners Hospitals for Children: 84080 and 79141. The contents of this paper were also developed under a grant from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR grant number 90DP0043-01-00). NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this paper do not necessarily represent the policy of NIDILRR, ACL, or HHS, and you should not assume endorsement by the Federal Government.
This work was presented in part at the 46th Annual Meeting of the American Burn Association (March 25–28, 2014, Boston, MA) and 47th Annual Meeting of the American Burn Association (April 21–24, 2015, Chicago, IL).
Funding
This study was funded by National Institutes of Health: P50GM060388, T32GM008256, R01HD049471; National Institute on Disability, Independent Living and Rehabilitation Research: 90DP0043-01-00; and Shriners Hospitals for Children: 84080 and 79141.
REFERENCES
- 1. Herndon DN, Rodriguez NA, Diaz EC, et al. Long-term propranolol use in severely burned pediatric patients: a randomized controlled study. Ann Surg 2012;256:402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bible LE, Pasupuleti LV, Alzate WD, et al. Early propranolol administration to severely injured patients can improve bone marrow dysfunction. J Trauma Acute Care Surg 2014;77:54–60; discussion 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lexicomp Online, Hudson. Ohio: Lexi-Comp, Inc; 2017. [Google Scholar]
- 4. Brown DA, Gibbons J, Honari S, Klein MB, Pham TN, Gibran NS. Propranolol dosing practices in adult burn patients: implications for safety and efficacy. J Burn Care Res 2016;37:e218–26. [DOI] [PubMed] [Google Scholar]
- 5. Greenblatt DJ, Koch-Weser J. Adverse reactions to propranolol in hospitalized medical patients: a report from the Boston collaborative drug surveillance program. Am Heart J 1973;86:478–84. [DOI] [PubMed] [Google Scholar]
- 6. Williams FN, Herndon DN, Kulp GA, Jeschke MG. Propranolol decreases cardiac work in a dose-dependent manner in severely burned children. Surgery 2011;149:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohammadi AA, Bakhshaeekia A, Alibeigi P, et al. Efficacy of propranolol in wound healing for hospitalized burn patients. J Burn Care Res 2009;30:1013–7. [DOI] [PubMed] [Google Scholar]
- 8. Minifee PK, Barrow RE, Abston S, Desai M, Herndon DN. Improved myocardial oxygen utilization following propranolol infusion in adolescents with postburn hypermetabolism. J Pediatr Surg 1989;24:806–10; discussion 810. [DOI] [PubMed] [Google Scholar]
- 9. Herndon DN, Barrow RE, Rutan TC, Minifee P, Jahoor F, Wolfe RR. Effect of propranolol administration on hemodynamic and metabolic responses of burned pediatric patients. Ann Surg 1988;208:484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moss AJ, Adams FH. Problems of blood pressure in childhood. Springfield, IL: Charles C Thoms; 1962. [Google Scholar]
- 11. Landtman B. Sinus arrhythmia. Acta Paediatrica 1947;34:16–26. [Google Scholar]
- 12. Korownyk C, Ross D, Fiorillo L. Novel treatment for infantile hemangiomas. Can Fam Physician 2014;60:e590. [PMC free article] [PubMed] [Google Scholar]
- 13. Baron PW, Barrow RE, Pierre EJ, Herndon DN. Prolonged use of propranolol safely decreases cardiac work in burned children. J Burn Care Rehabil 1997;18:223–7. [DOI] [PubMed] [Google Scholar]
- 14. Norbury WB, Jeschke MG, Herndon DN. Metabolism modulators in sepsis: propranolol. Crit Care Med 2007;35(9 Suppl):S616–20. [DOI] [PubMed] [Google Scholar]
- 15. Ali A, Herndon DN, Mamachen A, et al. Propranolol attenuates hemorrhage and accelerates wound healing in severely burned adults. Crit Care 2015;19:217. [DOI] [PMC free article] [PubMed] [Google Scholar]