Abstract
Aortic aneurysm is a life-threatening disease due to the risk of aortic rupture. The only curative treatment available relies on surgical approaches; drug-based therapies are lacking, highlighting an unmet need for clinical practice. Abdominal aortic aneurysm (AAA) is frequently associated with atherosclerosis and cardiovascular risk factors including male sex, age, smoking, hypertension, and dyslipidaemia. Thoracic aortic aneurysm (TAA) is more often linked to genetic disorders of the extracellular matrix and the contractile apparatus but also share similar cardiovascular risk factors. Intriguingly, a large body of evidence points to an inverse association between diabetes and both AAA and TAA. A better understanding of the mechanisms underlying the negative association between diabetes and aortic aneurysm could help the development of innovative diagnostic and therapeutic approaches to tackle the disease. Here, we summarize current knowledge on the relationship between glycaemic parameters, diabetes, and the development of aortic aneurysm. Cellular and molecular pathways that underlie the protective effect of diabetes itself and its treatment are reviewed and discussed, along with their potential implications for clinical translation.
Keywords: Diabetes , Aneurysm , Pathophysiology
Introduction
Aortic aneurysm corresponds to a localized enlargement of the aorta resulting from a weakening of the aortic wall. The disease is often asymptomatic but is associated with high rates of mortality, especially when complications such as aortic rupture occur.1 Despite significant advances in our understanding of the disease mechanisms, the only curative treatment available today relies on endovascular or open surgical repair; drug-based therapies are still lacking.1 The disease may occur both in the thoracic and abdominal parts of the aorta, the most frequent localization being the abdominal infrarenal aorta. Thoracic and abdominal aortas differ in structure, biochemical composition, smooth muscle cell origin and biology, and even if abdominal and thoracic aneurysms share common mechanisms, they both have distinct features.2 The pathologic processes that lead to abdominal aortic aneurysm (AAA) formation are complex, and involve alteration and loss of vascular smooth muscle cells (VSMCs), in association with inflammatory cell infiltration, extracellular matrix (ECM) remodelling, and intraluminal thrombus (ILT) formation.3,4 Thoracic aortic aneurysms (TAA) frequently develop at younger ages, are often linked to genetic mutations such as Marfan syndrome or Loeys-Dietz syndrome, and are frequently associated with aortic dissection.5 Alterations of ECM and VSMC tone play important roles in TAA, but the inflammatory component and VSMC apoptosis are less prominent than in AAA.2,5,6 Despite these differences, epidemiological studies have paradoxically highlighted an inverse relationship between diabetes mellitus (DM) and both TAA and AAA.3,7 The aim of our review is to summarize the current knowledge on the pathophysiological mechanisms that underlie the inverse association between diabetes and aortic aneurysm.
Diabetes and the epidemiology of aortic aneurysm
Diabetes mellitus corresponds to a heterogeneous disease characterized by a chronic hyperglycaemia and is generally classified in several categories including mainly type 1 (T1D) and type 2 diabetes (T2D).8 Although DM represents a major cardiovascular risk factor,8,9 the vast majority of epidemiological studies have paradoxically identified an inverse association between DM and the prevalence and incidence of AAA.10–16 Indeed, several reports have shown that diabetic patients develop smaller AAA, as demonstrated by significantly lower aortic diameters compared to non-diabetic subjects.10,17 Besides, several studies highlighted lower growth rates of AAA in diabetic patients compared to controls.11,13,18,19 In practice, the decision to treat AAA takes into consideration the risk of rupture. As large aortic diameter and high growth rate represent major risk factors of rupture,7 it is not surprising that a negative association between DM and aneurysm rupture was identified.20
While DM appears as a protective factor of AAA formation and expansion, the prognosis and outcome after AAA treatment also differs between diabetics and non-diabetic patients. Heterogeneous results were found among different studies, some reports revealing increased operative mortality in diabetics,11,21 others showing no difference,12,22 and some reporting lower mortality.15 Besides, morbidity following AAA repair was analysed, and DM was identified either as a negative or protective factor of specific post-operative outcomes. Higher rates of complications such as myocardial infarction, infection, or pancreatitis were observed in diabetic patients after AAA open repair,22 and higher incidence of device-related complications following endovascular AAA repair were found.21 On the opposite, DM had a protective effect on AAA growth and re-interventions after endovascular repair,23 but no significant difference in the occurrence of neck dilatation or type 1 endoleaks was identified between diabetics and controls.24
Similar to AAA, a negative association was identified between DM and TAA.25,26 A meta-analysis combining four studies which included patients with ascending TAA, thoraco-abdominal aneurysms, and non-syndromic TAA confirmed the negative association between diabetes and TAA.25 However, the impact of diabetes on TAA growth is still unknown and its effect on post-operative outcome has been poorly investigated. It will be important to better explore the impact of diabetes in the different forms of TAA distinguishing ascending and descending TAA, thoraco-abdominal aneurysms, as well as TAA caused by genetic disorders in order to fully understand the link between these disease states.
Glycaemic parameters and aortic aneurysm
Glucose homeostasis results from a complex mechanism involving two main hormones secreted by the pancreas, insulin, and glucagon, which have opposite effects.8 The impairment of glucose regulation leads to chronic hyperglycaemia, which characterizes DM. In practice, diabetes is biologically defined as fasting plasma glucose (FPG) >7.0 mmol/L (>126 mg/dL) or as a 2-h plasma glucose value after a 75 g oral glucose tolerance test (OGTT) >11.1 mmol/L (>200 mg/dL)8 or as a glycated haemoglobin A1c (HbA1c) >6.5%.8
It is noteworthy that in the wide majority of epidemiological studies on AAA, DM was defined as a known history of the disease according to the patient’s declaration and to medical records. However, Hjellestad et al.27 found that according to OGTT and HbA1c values, half of the patients with AAA and DM were unaware of their DM diagnosis. Hence, the exclusive reliance on history of disease and disease records could lead to an underestimation of DM in the studied cohorts. Moreover, most of the studies did not distinguish between T1D and T2D diabetic patients. Given the distinct characteristics of pathophysiological patterns between these two forms of DM,8 it is possible that their effects and relationships with AAA differ. Also, the relationship between AAA development and the pre-diabetic state is still poorly understood, underlining the need for a better investigation of the association between AAA and the different forms and stages of DM.
Nevertheless, several studies investigated the relationship between glycaemic parameters and AAA characteristics. An inverse correlation was found between fasting glucose and aortic diameter, suggesting a relationship between glucose metabolism and AAA formation.17,28 However, no association was found between serum levels of glucose and aortic expansion in a cohort of 198 patients followed up to 3 years.19 While fasting blood glucose reflects short-term glucose regulation, HbA1c is representative of the glycaemic status over the past 3 months.8 In this regard, an inverse association between the growth rate of AAA and the level of HbA1c was found both in individuals with and without DM, suggesting a mechanistic relationship between long-lasting elevated blood glucose levels and AAA progression.18
Another study found no association between fasting blood glucose measured at mean age 33 and aortic diameter measured at age 65 years.29 Moreover, Taimour et al.30 revealed that AAA prevalence did not differ between 65-year-old men who were newly diagnosed as T2D patients and non-diabetic men, and HbA1c level did not correlate with AAA diameter in newly diagnosed T2D patients. These results suggest that mechanisms linking altered glucose metabolism and a decreased risk of AAA are not relevant until late in life or until they are sustained long after the diagnosis of DM.
While short- and long-term glucose levels have been investigated in the context of AAA, the relationships between AAA characteristics and parameters of glucose regulation, such as insulin or glucagon, have so far received little attention. One study did not identify any association between fasting insulin and AAA,28 whereas another reported a positive correlation between C-peptide and AAA diameter in T2D patients.30 C-peptide is a biomarker used in medical practice to assess endogenous insulin secretion and is also useful to indirectly evaluate insulin resistance. Nevertheless, the significance and direct role of C-peptide in AAA pathogenesis are still poorly understood. Moreover, the link between glycaemic parameters and TAA has not been investigated so far.
Animal models of diabetes and aortic aneurysm
Several experimental models have been developed to understand the impact of DM on aneurysm. Chemically induced AAA models are schematically categorized in non-dissecting and dissecting models and reproduce main human pathophysiological features even if they present some limits.31 Non-dissecting AAA models can be created through perfusion or application of porcine pancreatic elastase or calcium chloride.31 These models reproduce some of the main features of human AAA, including VSMC loss, inflammatory cell infiltration, ECM degradation, and aortic dilatation. However, they are self-contained and do not progress to rupture. Dissecting AAA models are characterized by intramural delamination leading to haematoma formation and aortic dilation and are often associated with dilatation and dissection of the thoracic aorta.31 They can be induced by infusion of angiotensin II, with or without inhibition of transforming growth factor (TGF)-β or lysyl-oxidase, or through administration of a mineralocorticoid-receptor agonist and high salt. Animal models of genetically triggered TAA (Marfan or Loeys-Dietz syndromes) have also been developed through mutation or deletion of the incriminated gene, such as fibrillin-1 or TGF-β ligands and receptors.32
In parallel, several animal models of DM have been developed, allowing to study the impact of the disease on AAA development. T1D can be induced by administration of streptozotocin or alloxan, which affects pancreatic beta cell survival and leads to chronic hyperglycaemia.33 Streptozotocin-induced T1D had a protective effect on elastase-induced AAA development, as demonstrated by decreased aortic diameter and decreased AAA growth.34,35 Similar results were obtained in calcium phosphate-induced aneurysm model and after angiotensin II perfusion in Apoe−/− mice.35,36
Models of T2D tend to reproduce human features of insulin resistance and/or beta cell failure and can be associated with obesity.33 Obese animal models of T2D include monogenic models such as mice deficient in leptin signalling pathway or polygenic models. Among them, KK-Ay mice develop severe hyperinsulinaemia and insulin resistance associated with impairment of pancreatic islet function. Non-obese models of T2D, such as Goto-Kakizaki (GK) rats, are also available allowing the study of disease mechanisms in a lean colony.33 Similarly to humans, T2D exerts a protective effect on aneurysm development in animal models, as demonstrated by a decrease of calcium phosphate-induced aneurysm formation in KK-Ay mice.36 The induction of aortic aneurysm in different models of diabetic mice has offered the opportunity to better understand the link between these two diseases (Table 1). Unfortunately however, among the previously described animal models of diabetes and AA, only a few were used to address that link, and most of them were restricted to chemically induced AAA and T1D models.
Table 1.
Experimental models used to study aneurysm development in diabetic mice
| Diabetes model | Aneurysm model | Main results | References |
|---|---|---|---|
| T1D: streptozotocin administration | CaPO4-induced aneurysm in the carotid artery of C57BL/6 mice | Decreased AAA formation | Tanaka et al.36 |
| Decreased macrophage infiltration | |||
| Elastase infusion in the abdominal aorta of C57BL/6 mice | Decreased AAA formation | Dua et al.34 | |
| Decreased macrophage infiltration associated with decreased PAI-1 aortic gene expression | |||
| Decreased AAA formation | Miyama et al.35 | ||
| Decreased macrophage infiltration associated with a decrease of mural neovascularity and MMP9 activity and a better elastin preservation | |||
| Angiotensin II infusion in ApoE−/− mice | Decreased AAA formation | Miyama et al.35 | |
| Better elastin preservation | |||
| Angiotensin II infusion in CDA1/ ApoE−/− mice | Development of AAA in diabetic CDA1/ ApoE−/− mice, not observed in diabetic ApoE−/− or diabetic wild type mice | Li et al.37 | |
| Increased macrophage infiltration and MMP12 expression, reduced collagen expression and TGF-β signalling in diabetic CDA1/ ApoE−/− mice | |||
| T2D: KK-Ay mice | CaPO4-induced aneurysm in the carotid artery | Decreased aneurysm formation | Tanaka et al.36 |
| Decreased macrophage infiltration |
Mechanistic effects of diabetes on aortic aneurysm
ECM remodelling
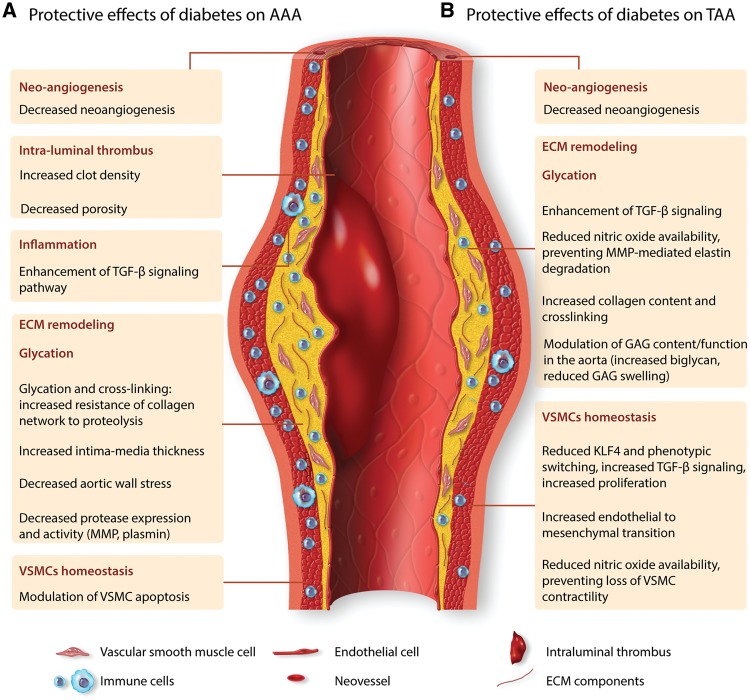
Several studies addressed the impact of DM on vascular wall structure (Figure 1). In the absence of difference in aortic lumen diameter, aortic wall stress of the abdominal aorta was shown to be 20% lower in T1D patients compared to controls, with 22% larger aortic intima-media thickness, which significantly correlated with the duration of DM.38 A study integrated protein–protein interaction and genetic interaction to examine biological pathways related to DM, aneurysm, and atherosclerosis.39 The study identified a set of 16 proteins with high brokerage values (i.e. essential for the interconnectedness of the network), and this network was enriched in proteins involved in cell matrix-adhesion, supporting a role for ECM alterations in the link between DM and reduced risk of aneurysm. ECM is a dynamic network composed of basement membrane, collagen, elastin, and proteoglycans that contribute to tissue organization and structure. Interestingly, proteome analysis of non-atherosclerotic arteries (free of adventitia) from T2D patients showed increased amounts of the basement membrane and ECM components including alpha1-type IV and alpha2-type IV collagen, gamma1, and beta2 laminin.40 These results suggest distinct properties of ECM in diabetic patients and could partly be involved in the protective effect on AAA development. In addition to the fibrous proteins, the background of the ECM is composed of glycosaminoglycans (GAG), which are complex polysaccharides covalently linked to ECM proteins in the form of proteoglycans. GAGs are categorized into four classes according to their disaccharide units, including chondroitin sulfate/dermatan sulfate, heparan sulfate, keratin sulfate, and hyaluronan. Biglycan, a member of chondroitin sulfate/dermatan sulfate, is abundant in normal human aorta and its expression is decreased in abdominal aortic aneurysmal tissue.41 Moreover, biglycan gene defects cause an X-linked syndromic form of severe TAA and dissection.42 To better understand the role of biglycan in aneurysmal pathology, experimental models were used. Biglycan deficiency in Ldlr−/− mice aggravated angiotensin II-induced aortic aneurysm, with increased rates of vascular mortality and rupture, and induced the development of descending TAA.43 This was associated with breaks in medial elastin as well as a decrease in dense collagen fibre network in the aortic wall. In addition, biglycan deficiency in BALB/cA mice induced mortality due to spontaneous aortic dissection and rupture.44 Transmission electron microscopy and biomechanical testing revealed abnormalities of collagen fibrils with marked variations in size and shape associated with reduced tensile strength. Other studies pointed to interactions between biglycan and cytokines, particularly TGF-β, which plays a central role in both TAA and AAA.45,46 Biglycan can regulate TGF-β signalling pathway47 and TGF-β can enhance biglycan expression,48 suggesting a mutual and positive feedback interaction to preserve the ECM and protects the arterial wall against aneurysm development and dissection. Although the direct impact of diabetes on biglycan expression in the aneurysmal aortic wall is still uncertain, diabetes is associated with an upregulation of Cell Division Autoantigen 1 (CDA1) which enhances TGF-β signalling pathway and contributes to the protective effect of diabetes on AAA formation.37 DM can also alter the production, degradation and deposition of other GAGs in the aorta, with additional consequences on ECM remodelling as well as the structural and physical properties of the arterial wall. Decorin, a small leucin-rich repeat proteoglycan, is increased in response to high-glucose concentration, interacts with TGF-β to regulate matrix organization and collagen matrices and may reduce aneurysm formation in part through modulation of ECM remodelling.49–51 On the other hand, substantial accumulation of (foci of) GAGs, mainly hyaluronan, aggrecan and versican, is frequently associated with the progression and severity of TAA.52–54 These GAG foci generate important osmotic pressures with localized increases in intramural stress, and are thought to initiate medial delamination.53 We speculate that an imbalance between GAGs and the connective tissue in the aorta of diabetic patients in favour of increased collagen deposition, may limit the susceptibility of the aorta to intralamellar Donnan swelling pressures caused by focal accumulation of GAGs,55 thereby limiting the risk of aneurysmal dilatation and dissection, particularly in the thoracic aorta.
Figure 1.
Hypothesized protective effects of diabetes mellitus in AAA and TAA. The main mechanisms that underlie the protective effect of diabetes in the pathogenesis of aortic aneurysm are mediated through effects on aortic mural neoangiogenesis, intraluminal thrombus formation, inflammation, glycation, extracellular matrix remodelling, and vascular smooth muscle homeostasis. Even though AAA and TAA share common pathophysiological mechanisms, they are characterized by distinct features. The main pathways underlying the protective effect of diabetes mellitus may differ between AAA and TAA.
ECM remodelling also results from a balance between the synthesis of ECM components and their degradation by proteases including cathepsins, metalloproteinases (MMPs), and serine proteases. In some studies, the protective effect of DM on experimental AAA was associated with a decrease of MMP9 expression and activity, and a relative elastin preservation compared to controls.34,35 This is corroborated by the observation of decreased MMP9 secretion by activated monocytes exposed to aortic media extracted from diabetic patients compared to non-diabetics.19 To further address the mechanisms involved, in vitro studies were performed using murine macrophage cell line RAW264.7 treated with tumour necrosis factor alpha (TNF alpha) and calcium phosphate (CaPO4) to mimic conditions of CaPO4-induced AAA formation.36 Hyperglycaemic conditions (incubation with glucose 15.5 mmol/L) attenuated TNF/CaPO4-induced elevation of MMP9 at the gene, protein and activity levels, in a Nr1h2-dependent mechanism.36 However, others reported an induction of MMP expression and activity, including MMP9 in human monocyte-derived macrophages, and MMP1/MMP2 in human endothelial cells, under higher glucose concentrations (25 mmol/L).56 MMP-2 and MMP-9 serum concentrations were lower in T2D patients compared to non-diabetics and hyperglycaemia during OGTT induced a decrease in MMP9. However, HbA1c paradoxically correlated with MMP9 in T2D patients.57 Hence, the effect of hyperglycaemia on MMP regulation is complex and would require further studies to fully understand it. It is also important to note the conflicting evidence regarding the role of MMP collagenases in aneurysm formation, and more particularly the increased susceptibility to angiotensin II-induced dissecting AAA in hypercholesterolaemic mice with MMP9 deficiency or genetically engineered resistance to collagenases,58,59 some of these effects being related to altered biomechanical properties of the aortic wall due to altered collagen deposition and fibre orientation. In addition to MMPs, serine proteases such as plasmin represent one of the major proteolytic enzymes involved in ECM remodelling. Plasmin activity results from the activation of plasminogen by plasminogen activators (PA), and this pathway is inhibited by plasminogen activator inhibitor-1 (PAI-1).60 Interestingly, AAA development was decreased in diabetic mice compared to controls, concomitantly with an increase of plasma PAI-1 level and aortic PAI-1 gene expression, and a decrease of plasmin generation.34 However, the mechanisms behind PAI-1 modulation in diabetic mice, and the direct link with reduced AAA development have not been explored.
Glycation and advanced glycation end products
DM and chronic hyperglycaemia lead to an increase of advanced glycation end products (AGEs), which result from non-enzymatic glycoxidation of proteins and peptides after contact with aldose sugar.61 These products include non-cross-linking AGEs such as carboxymethyllysine (CML) and carboxyethyllysine (CEL), and cross-linking AGEs such as pentosidine. Interestingly, concentrations of pentosidine, CML and CEL were in general lower in AAA biopsies compared to non-aneurysmal aortic tissues.62 When comparing diabetic patients to non-diabetics, an increase of pentosidine concentration was observed in AAA aortic samples and its concentration showed a tendency toward a negative correlation with AAA diameter, suggesting a protective effect of cross-linking AGEs. Besides, a significant decrease of carboxyterminal telopeptide of collagen type 1, and a tendency toward a decrease of collagen type 1C-telopeptide was observed in glycated AAA tissues compared to non-glycated AAA tissues. As those collagen telopeptides are products from the degradation of mature type 1 collagen by MMPs and cysteine proteases, and no difference was found in protease activity (including MMP2, MMP9, cathepsin A, B, and S) between AAA biopsies of diabetics compared to non-diabetics, the results suggest that glycation of AAA tissue protects the collagen network and increases its resistance to proteolysis. The precise effects of glycation and cross-linking on ECM remodelling were further investigated by in vitro studies on THP1 cell lines and peripheral blood mononuclear cells (PBMCs).19 Interestingly, glycated bovine serum albumin (BSA) and glycated monomer collagen enhanced the secretion of MMP9 by THP1 cells. In contrast, glycated collagen lattices and cross-linked collagen lattices reduced the secretion of MMP2 and MMP9 by both THP1 cells and PBMCs. These results underline the complexity of the effect of glycation on monocytes and ECM remodelling, which may differ depending on the type of glycated protein.
In addition to direct modification of protein structure, AGEs can lead to the activation of several signalling pathways through various cell surface receptors. The most known of those receptors is the multi-ligand receptor for advanced glycation end products (RAGE), which has been identified in various cells including monocytes/macrophages, endothelial, and VSMCs.63 Experimental studies unravelled the complex role of AGEs and their receptor RAGE in angiotensin II-induced AAA model using Apoe−/−RAGE−/− mice. RAGE deficiency decreased the incidence of AAA, and this was associated with a decrease of MMP9 activity and cellularity of the adventitia. Further in vitro studies revealed that stimulation with RAGE ligands increased MMP9 gene expression as well as MMP9 activity in murine macrophage cell line RAW 264.7.64 Another study using transgenic mice overexpressing the human S100A12 (a ligand of RAGE known to be increased in DM) reported progressive dilatation of the aorta with elastin fibre disruption, fibrosis, and loss of VSMCs within the medial layer, along with an increase of MMP2 protein expression.65 Besides, analysis of human samples revealed that S100A12 protein was expressed in all cases of thoracic aortic type A dissection, in 25% of stable TAA, but was not detected in non-aneurysmal control aortic tissue, consistent with a potential pathogenic role in human aortic disease.66 Interestingly, investigation of the association between RAGE gene polymorphisms and AAA revealed that the 82S allele of RAGE was a risk factor for AAA.67 Taken together, these results suggest that the impact of glycation on protein structure could partly account for the protective effect of DM on AAA formation (Figure 1), whereas its effect through RAGE signalling pathway may exert a pathogenic effect on the aortic wall and contribute to AAA development.
As glycation affects AAA development, investigators examined if measurement of AGEs could be of interest as a biomarker in AAA. Interestingly, skin accumulation of AGEs measured by auto fluorescence was found to be higher in patients with AAA compared to controls.68 However, this association was lost when adjusted for cardiovascular risk factors. No association was found between AAA progression and serum CML concentration.19 Besides, no correlation was found between pentosidine concentration and AAA diameter, and there was no correlation between plasma levels of pentosidine and its levels in AAA biopsies.62 It is reasonable to hypothesize that AGEs are formed in other tissues, making the systemic measurement less representative of the local effect in the AAA wall. This field of research is still in its infancy, and new clinical studies are currently investigating the types and locations of AGEs in aortic tissues and in several biological fluids of patients with AAA, and will probably provide a better understanding of the complex role of glycation in this disease.69
Inflammation
Over the past decades, several studies revealed a close link between metabolism and immunity; alterations of the immune system contribute to the pathogenesis of DM and conversely, metabolic alterations leading to DM modulate the inflammatory response.70 The enhanced state of immune activation in DM, whether chronic autoimmunity in T1D or chronic low-grade inflammation in T2D with enhanced production of inflammatory mediators, is not expected to protect against the development and/or progression of aortic aneurysm. Most of the studies point to a promoting role of DM on vascular inflammation through several mechanisms, including increased oxidative and endoplasmic reticulum stress responses, and impaired activation of protective anti-inflammatory pathways (reduced endothelial nitric oxide synthase, insulin resistance in vascular cells, etc.).71 It should be kept in mind, however, that this is not a black-and-white situation, and strong counter-regulatory mechanisms (e.g. TGF-β) may still operate and may even be promoted, systemically and locally, under diabetic conditions. Monocyte recruitment to the endothelium is finely regulated by glycaemic conditions. A study in Goto-Kakizaki rats (GK-rat), a genetic non-obese model of T2D, revealed that fluctuation of blood glucose enhanced monocyte adhesion to the thoracic aortic endothelium, an effect possibly due to increased gene expression of connecting segment (CS)-1 and very-late acting antigen (VLA)-4.72 On the other hand, administration of insulin and nateglinide (known to stimulate insulin secretion) in GK-rats reduced post-prandial hyperglycaemia, thus attenuating blood glucose fluctuation, and induced a decrease of monocyte adhesion to endothelial cells.73 The results suggest a promoting effect of DM on vascular inflammation. However, diabetes alters the differentiation of haematopoietic stem cells towards monocytes and macrophages, in part through upregulation of DNMT1 and hypermethylation of major transcription regulators PU.1, NOTCH1, and KLF4,74 which may limit the inflammatory process. Furthermore, increased monocyte recruitment may not favour AAA development unless monocytes differentiate into pro-inflammatory macrophages. However, DM or hyperglycaemic conditions have been shown to modulate macrophage phenotype towards either a pro-74–76 or anti-inflammatory state,77 through various mechanisms including upregulation of acyl-CoA synthetase 175 or regulation of AKT/mTOR and ERK pathways.77 The pro-inflammatory phenotype appears to be prevalent after short-term diabetes and in association with a hypercholesterolaemic setting,75 whereas the anti-inflammatory phenotype was observed after several months of diabetes induction.77 Thus, increased monocyte recruitment into the injured artery wall in DM may not lead invariably to increased accumulation of pro-inflammatory macrophages. Furthermore, glycation has been shown to alter monocyte/macrophage function toward an anti-inflammatory phenotype, reducing interleukin (IL)-6 production as shown in lipopolysaccharide-activated THP1 monocytes incubated with glycated collagen lattices and cross-linked collagen lattices.19 As IL-6 deficiency limits both TAA and AAA development in mice, and Mendelian randomization approaches supported the involvement of the IL6 receptor pathway in human AAA,78 the effect of glycation on IL-6 may contribute to the protective effect of DM in aortic aneurysm.
Studies in animal models of AAA revealed a decrease of macrophage infiltration in diabetic mice compared to controls.34–36 In contrast, a higher macrophage infiltration was observed in AAA wall of diabetic compared to non-diabetic patients who underwent AAA surgical repair and was correlated with serum glucose concentration.79 AAA diameter was similar between diabetics and non-diabetics, and no significant difference was observed for T-lymphocyte infiltration. The discrepancy between those human and animal studies underlines the complex impact of diabetes on inflammation and suggests that the protective effect of DM on AAA development and progression may not be linked to its impact on inflammatory pathways (Figure 1). Another plausible explanation is that a higher level of inflammation is required to overcome the resistance of the aorta of diabetic patients to aneurysm development.
Aortic mural neoangiogenesis
Aortic mural neoangiogenesis is involved in TAA and AAA development, and in the progression to aortic rupture.80 Interestingly, a decrease of mural neovascularity was observed in the aneurysmal wall of diabetic mice compared to controls.35 Studies in animal models revealed that DM inhibits neoangiogenesis in aortic tissue, impairs ischaemia-induced neovascularization,81 and impairs vascular endothelial growth factor pathway induction by hypoxia-inducible factor 1.82 Similar mechanisms may be involved during AAA. Angiogenesis inhibitors limit AAA progression in a dissecting angiotensin II model, possibly through reduced immune cell infiltration83 and reduced interstitial oedema,55 suggesting that the protective effect of DM on AAA and TAA could partly be attributed to a decrease of neovascularization (Figure 1).
VSMC homeostasis
VSMCs have the ability to switch from a contractile phenotype to a secretory phenotype and impact on vascular homeostasis in physiological and pathological states.84 Several studies addressed the impact of DM on VSMCs.84 First, the morphology of saphenous vein VSMCs collected from T2D patients differ from non-diabetics, with a rhomboid morphology suggesting a dedifferentiated phenotype.85 Focal adhesions characterized by vinculin immunostaining were increased in VSMCs from diabetic patients, with a disparate organization of the alpha actin network. Histologic analysis of tissues from patients with AAA suggested decreased staining for alpha-smooth muscle actin in diabetic patients compared with non-diabetics.62 The significance of this finding is unclear and may reflect either reduced VSMC number or increased VSMC dedifferentiation. Although some studies reported less proliferation in VSMCs from T2D compared to non-diabetics,85 most of the studies are in favour of a promoting effect of DM on VSMC proliferation through activation of ERK signalling pathways and the secretion of mitogenic factors.86,87 Note that VSMCs were harvested from the saphenous vein and from the internal mammary arteries, respectively, which may explain, at least in part, the discrepancy between the studies. Experimental studies in animal models revealed persistent phenotypic changes and altered inflammatory gene expression in aortic VSMCs cultured from diabetic mice.88 High glucose and palmitic acid induce epigenetic changes in aortic VSMCs that promote VSMC ‘dysfunction’. For example, miR-504 is upregulated in VSMC under those conditions, leading to inhibition of contractile gene expression, while increasing migration, proliferation, and inflammatory gene expression, through effects on Grb10 and Egr2.89 The study fell short however of establishing a direct link between diabetic conditions, epigenetic alterations, and VSMC dedifferentiation. Moreover, the term VSMC ‘dysfunction’ is too vague and may be misleading. For example, inflammatory changes in VSMCs under diabetic conditions may be expected to be conducive for aortic aneurysm development and progression. In contrast, acquisition of migratory and proliferative properties by VSMCs may have a protective healing and stabilizing role. Phenotypic switching of VSMC is controlled by the transcription factor KLF490 and is an important feature of aortic aneurysms.91 Selective deletion of KLF4 in VSMCs reduces the development and severity of aortic aneurysm in both dissecting and non-dissecting mouse models of the disease.91 Intriguingly, hyperglycaemia reduces KLF4 expression in VSMCs and arteries of mice and patients with diabetes, in part through Foxoa2-dependent upregulation of miR-29c.92 The results are consistent with the upregulation of miR-143/145 (a major regulator of KLF4 expression93) in VSMCs (from saphenous vein) of patients with diabetes, and suggest a potential protective role of diabetes against AAA and TAA through limitation of KLF4 induction. Along this line of thinking, increased activation of the TGF-β signalling pathway under diabetic conditions, both systemically and locally in aortic VSMCs,94,95 may also play an important protective role. TGF-β is required for the induction and maintenance of VSMC differentiation and homeostasis. Genetic mutations in the TGF-β signalling pathway predispose to TAA in humans,96 and selective deletion of TGF-β signalling pathway in VSMCs promotes TAA development and dissection in mice.97–99 Beyond a direct effect on VSMC differentiation, TGF-β signalling in VSMCs appears to limit their production of IL-1β,100 which further contributes to the protection against aneurysm formation. Another pathway involved in the protective effect of TGF-β may be related to the regulation of VSMC contractility.101 Genetic mutations in genes affecting VSMC contractility predispose to TAA in humans,102 and alteration of VSMCs contractility has been suggested to contribute to aortic dissection in mice with disrupted TGF-β signalling.103 In this regard, it is interesting to note that the reduction of KLF4 expression in VSMCs of diabetic mice and diabetic patients was associated with increased expression of contractile proteins.92 Others have also reported increased expression of contractile genes and proteins in VSMCs under elevated glucose levels, related in part to activation of protein kinase C, Rho/Rho-kinase and actin polymerization.104 Given the contribution of VSMC contractility to microvascular tone and ECM contraction, and the proposed roles of the latter physiological pathways in aortic wall homeostasis and its resistance to aneurysm formation and dissection,55 those changes in VSMCs induced by, or promoted under diabetic conditions may further account for the protection against aortic aneurysm.
Endoplasmic reticulum (ER) stress induction in aortic VSMCs is thought to promote cell death and aneurysm,105 a pathway that might be counter-regulated by induction of autophagy.106 High-glucose conditions may favour the induction of ER stress in aortic VSMCs.107 However, DM also highly promotes AGE-dependent VSMC proliferation through induction of autophagy,108 which may overcome its deleterious effect on ER stress.
Endothelial to mesenchymal transition, which corresponds to the process by which endothelial cells are able to switch to a myofibroblastic phenotype in response to injury, is impaired in TAA.109 As diabetes is associated with enhanced endothelial to mesenchymal transition in the vascular tissue, this process could potentially be involved in the inverse association between diabetes and aneurysm. Finally, a recent study unravelled a detrimental role of nitric oxide (as a result of NOS2 activation) in promoting medial aortic degeneration and dilatation in a mouse model of Marfan syndrome.110 The mechanisms may involve an impact of nitric oxide on VSMC contractility and MMP9-mediated elastin fragmentation. It is therefore tempting to hypothesize that reduced nitric oxide bioavailability in diabetic setting may limit this pathogenic process. In general, the impact of DM on VSMC phenotype during aneurysm has not been thoroughly investigated so far (Figure 1), and the available studies did not specifically distinguish between VSMCs derived from the thoracic and the abdominal aortic region. This is of major importance given the distinct embryonic origins of VSMCs in AAA and TAA, and the distinct pathological features of aortic aneurysm in those different locations.
Intraluminal thrombus formation
ILT formation is a factor contributing to AAA development. The wall underlying the thrombus is thinner and exhibits increased signs of degradation. The rate of thrombus growth also correlates with aneurysm growth and rupture.111 Interestingly, glucose concentration is associated with altered clot structure, with a higher clot density and decreased porosity reported in T2D patients compared to controls.112 This may confer increased clot resistance to fibrinolysis in diabetic patients. As ILT is a source of protease release and activation, it can be hypothesized that a more compact but less active clot could limit AAA expansion in diabetic patients (Figure 1). In TAA, thrombus may develop within the aortic wall after dissection but it is not considered as an important factor in the initiation of dissection.
Effects of antidiabetic treatments
Treatment of DM relies on lifestyle measures associated with blood glucose lowering drugs.9 Insulin administration is the main therapy for T1D patients. Pharmacological treatments mostly used in T2D include insulin sensitizers such as biguanides (metformin) or thiazolidinediones (rosiglitazone, pioglitazone), drugs stimulating insulin secretion such as sulfonylureas or meglitinides, and drugs with incretin effects such as glucagon-like-peptide-1 (GLP-1) receptor agonists (i.e. liraglutide, exenatide, lixisenatide) or dipeptidyl peptidase 4 (DPP-IV) inhibitors (i.e. alogliptin).
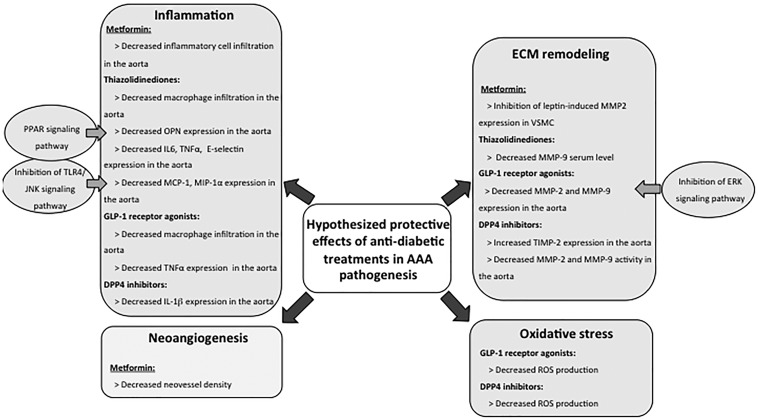
Intriguingly, epidemiological studies have revealed that mechanisms conferring a protective effect of DM on AAA are not only related to the pathophysiology of DM but also to antidiabetic treatments. A study including 1269 patients with AAA showed that the use of antidiabetic drugs was associated with a 56% reduction in AAA growth rate, and this association was independent of confounding factors including other therapeutic agents.113 In addition, a nested case–control analysis including 4468 patients with AAA and 4468 matched controls revealed that metformin, sulfonylurea, and thiazolidinedione use was associated with a lower risk of developing aneurysm.114 The negative association between metformin use and AAA enlargement and growth was confirmed in other studies.115,116 No significant association was found between metformin use and the risk of rupture.117
Experimental studies were performed to better understand the cellular and molecular pathways underlying the negative association between antidiabetic use and AAA development. Studies using the elastase-induced AAA model in normoglycaemic mice suggest a protective role of metformin, with preservation of VSMCs and aortic medial elastin, associated with decreased inflammatory cell infiltration115 (Figure 2).
Figure 2.
Hypothesized protective effects of antidiabetic treatments in AAA. Antidiabetic treatments may contribute to the negative association observed between diabetes and AAA through protective effects on inflammation, neoangiogenesis, extracellular matrix remodelling, and oxidative stress.
However, lower levels of ECM components or basement membrane proteins, including alpha-chains of type IV collagen, collagen type XVIII, laminin-gamma-1, laminin-beta-2, and nidogen-1 were observed in arteries from T2D patients treated by metformin compared to non-metformin users.39 Taken together, these results point to a complex effect of metformin on the pathophysiological events of AAA development and progression.
Several experimental studies addressed the impact of thiazolidinediones on AAA development (Figure 2). This drug class improves insulin sensitivity through activation of the nuclear transcription factor peroxisome proliferator-activated receptor (PPAR)-gamma and plays important roles in regulating inflammation by antagonizing several inflammatory transcription factors AP-1, STAT, and NF-kappaB in various immune cells.118,119 Although treatment with thiazolidinediones has been associated with adverse hepatic, cardiovascular, osteogenic, and carcinogenic effects, their use in T2D is still debated.119 Treatment with pioglitazone as well as treatment with PPAR-alpha agonists (fenofibrate) reduced aortic diameter in the angiotensin II/Apoe−/− model, and this effect was associated with reduced osteopontin (OPN) staining in the aorta and reduced macrophage infiltration.120 As OPN is a chemotactic factor for macrophages and its deficiency improves AAA,121 the protective effect of pioglitazone could be partly due to downregulation of OPN. Treatment with rosiglitazone also reduced AAA formation and abolished death caused by rupture in the angiotensin II/Apoe−/− model.122,123 This selective agonist of PPAR-gamma decreased angiotensin II-induced expression of E-selectin, TNF-α and IL-6 in the aorta, which may in part explain the protective effect given the known roles of those pro-inflammatory mediators in experimental AAA.4 In addition, rosiglitazone inhibited angiotensin II-induced JNK activation, modulated the inflammatory process by downregulating TLR4, and suppressed the production of TLR4/JNK dependent chemokines Monocyte Chemoattractant Protein-1 and MIP1 alpha, which may further explain its protective effect on AAA development.122 At last, as rosiglitazone reduced serum levels of MMP9 in T2D patients, its protective effect on AAA development could potentially be linked with ECM preservation.124
The action of drugs with incretin effect including GLP-1 receptor agonists (i.e. lixisenatide, liraglutide) and DPP-IV inhibitors (i.e. alogliptin, sitagliptin, MKEY0620) has also been investigated (Figure 2). Subcutaneous administration of GLP-1 receptor agonist lixisenatide reduced CaCl2-induced AAA development through antioxidant, anti-inflammatory, and ECM preservation effects, as revealed by decreased reactive oxygen species (ROS), reduced macrophage infiltration and inhibition of TNF, MMP2, and MMP9 gene expression in the aortic wall.125 These effects were associated with a decrease of ERK expression in the AAA wall suggesting the involvement of this signalling pathway in the protective effect of GLP-1 analogues. The protective effect of this drug was confirmed in another study where administration of liraglutide reduced AAA formation in Apoe−/− mice infused with angiotensin II.126 The treatment increased circulating active GLP-1 and was associated with a preservation of elastin content, a decrease of macrophage infiltration, and a reduction of MMP2 and MMP9 expression. In vitro studies on monocytic U937 cells further confirmed the effect of the GLP-1 analogue, where pre-treatment with liraglutide decreased angiotensin II-induced ROS formation, MMP2 and MMP9 activities, and abolished angiotensin II-induced monocytic recruitment in a concentration dependent manner.126
Other studies investigated the effect of DPP-IV inhibitors (alogliptin, sitagliptin, MKEY0620) and revealed the protective effect of this class of drugs on AAA development126–128 (Figure 2). DPP-IV gene expression was increased in the aneurysmal aortic wall, and immunostaining for DPP-IV (also known as CD26) showed it was expressed in macrophages.127 Besides, plasma DPP-IV activity was increased, and plasma active GLP-1 concentration decreased in Apoe−/− mice after angiotensin II infusion compared to controls.126 As expected, the administration of DPP-IV inhibitors decreased DPP-IV activity and increased plasma active GLP-1 concentration.126,128 Intriguingly, DPP-IV inhibitors did not affect blood glucose concentration126,127 and plasma insulin levels,128 suggesting that the protective effect of DPP-IV inhibitors against AAA development may not be related to an incretin effect. DPP-IV inhibitors induced a dose dependent increase of elastin content, associated with a decrease in ROS production, and a reduction of the expression and gelatinolytic activities of MMP2 and MMP9 in the aneurysmal aortic wall.127 In another study, DPP-IV inhibitors reduced the incidence of AAA and the protective effect was associated with reduced aortic expression of IL1β and increased tissue inhibitor of metalloproteinase 2 (TIMP2).128 Additional treatment with an incretin receptor antagonist did not reverse the protective effect of DPP-IV inhibitors,128 confirming that DPP-IV inhibitors limit AAA formation in an incretin independent manner. In addition to GLP-1, DPP-IV has various substrates, at least in vitro, including gastro-intestinal hormones, neuropeptides, cytokines (IL-1β and IL-2), and chemokines (CCL5),129 which could potentially account for the protective effect of DPP-IV inhibitors.
While oral antidiabetic drugs were associated with a protective effect in experimental AAA, insulin therapy had an intriguing opposite effect, negating the protective effect of hyperglycaemia (induced by streptozotocin injection) on elastase-induced AAA formation.35
To conclude, the mechanism involved in the protective effect of antidiabetic drugs may not be directly related to an effect on blood glucose or insulin secretion. It may rather be due to improvement of insulin resistance, and modulation of other pathways, including inflammation, oxidative stress, and ECM remodelling (Figure 2). The impact of antidiabetic treatments on TAA development and progression is still unknown.
Conclusion and perspectives
Epidemiologic and experimental studies demonstrated a negative association between DM and aortic aneurysm formation and expansion. DM contributes to vascular tissue remodelling and has profound impact on several pathways relevant to aneurysm development, including ECM remodelling, inflammation, VSMC homeostasis, aortic mural neoangiogenesis, and ILT formation. Despite sharing a few common mechanisms, AAA and TAA display distinct features, and the main mechanisms underlying the protective effect of DM may slightly differ between AAA and TAA (Figure 1). The protective effect of diabetes on AAA may involve the role of glycation and cross-linking on ECM remodelling, the anti-inflammatory properties of TGF-β signalling pathway, and the impact on ILT formation, neoangiogenesis, and VSMC apoptosis. In addition to DM itself, antidiabetic drugs interfere with the pathophysiological mechanisms of aneurysm and may contribute partly to the protective effect observed in diabetic patients. In TAA, the protective effect of diabetes may involve the balance between collagen and GAG contents in the media and the resulting consequences on intramural stress distribution, the regulation of neoangiogenesis, the prevention of the deleterious effect of nitric oxide on aortic degeneration through reduced nitric oxide availability, and the promotion of protective TGF-β signalling in VSMCs, VSMC proliferation and endothelial to mesenchymal transition. Note, however, that the effect of antidiabetic drugs on TAA formation is still unknown, and merits appropriate investigation.
In the limelight of current knowledge, future directions can be proposed. First, DM is a complex disease classified in different types whose pathophysiological mechanisms differ. Future clinical studies should distinguish T1D from T2D patients to better understand the link of each type of DM with aortic aneurysm formation and progression. In parallel, the vast majority of experimental studies were performed in animal models mimicking T1D. More studies are needed in T2D animal models, with detailed investigation of the cellular and molecular mechanisms at play. Besides, the impact of intermediate states such as prediabetes is still unknown and remains to be elucidated. While the effect of hyperglycaemia itself has been investigated, the impact of major hormones involved in glucose homeostasis such as insulin and glucagon is poorly understood. Moreover, the molecular and cellular effects of DM in modulating the distinct pathophysiological mechanisms of AAA and TAA require further investigation. Given the availability of experimental models of DM and aortic aneurysm, and given the potential perspective to identify and develop new therapeutic tools based on modulation of glucose metabolism, the challenge is undoubtedly worth it.
Conflict of interest: none declared.
Funding
The authors are supported by the British Heart Foundation, and the European Research Council. We apologize for not being able to discuss all the relevant papers due to space limitations.
Footnotes
This manuscript was handled by a Consulting Editor, Professor Giuseppe Lembo.
References
- 1. Golledge J, Muller J, Daugherty A, Norman P.. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol 2006;26:2605–2613. [DOI] [PubMed] [Google Scholar]
- 2. Guo DC, Papke CL, He R, Milewicz DM.. Pathogenesis of thoracic and abdominal aortic aneurysms. Ann N Y Acad Sci 2006;1085:339–352. [DOI] [PubMed] [Google Scholar]
- 3. Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM.. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol 2011;8:92–102. [DOI] [PubMed] [Google Scholar]
- 4. Raffort J, Lareyre F, Clement M, Hassen-Khodja R, Chinetti G, Mallat Z.. Monocytes and macrophages in abdominal aortic aneurysm. Nat Rev Cardiol 2017;14:457–471. [DOI] [PubMed] [Google Scholar]
- 5. Davis FM, Rateri DL, Daugherty A.. Mechanisms of aortic aneurysm formation: translating preclinical studies into clinical therapies. Heart 2014;100:1498–1505. [DOI] [PubMed] [Google Scholar]
- 6. Humphrey JD, Milewicz DM.. Aging, smooth muscle vitality, and aortic integrity. Circ Res 2017;120:1849–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, van Herwaarden JA, Holt PJ, van Keulen JW, Rantner B, Schlosser FJ, Setacci F, Ricco JB.. European Society for Vascular S. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg 2011;41:S1–S58. [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37:S81–S90. [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2014;37:S14–S80. [DOI] [PubMed] [Google Scholar]
- 10. Pafili K, Gouni-Berthold I, Papanas N, Mikhailidis DP.. Abdominal aortic aneurysms and diabetes mellitus. J Diabetes Complications 2015;29:1330–1336. [DOI] [PubMed] [Google Scholar]
- 11. De Rango P, Farchioni L, Fiorucci B, Lenti M.. Diabetes and abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2014;47:243–261. [DOI] [PubMed] [Google Scholar]
- 12. Weiss JS, Sumpio BE.. Review of prevalence and outcome of vascular disease in patients with diabetes mellitus. Eur J Vasc Endovasc Surg 2006;31:143–150. [DOI] [PubMed] [Google Scholar]
- 13. Xiong J, Wu Z, Chen C, Wei Y, Guo W.. Association between diabetes and prevalence and growth rate of abdominal aortic aneurysms: a meta-analysis. Int J Cardiol 2016;221:484–495. [DOI] [PubMed] [Google Scholar]
- 14. Dinesh Shah A, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H.. Type 2 diabetes and incidence of a wide range of cardiovascular diseases: a cohort study in 1.9 million people. Lancet 2015;3:105.. [DOI] [PubMed] [Google Scholar]
- 15. Lopez-de-Andrés A, Jiménez-Trujillo I, Jiménez-García R, Hernández-Barrera V, de Miguel-Yanes J, Méndez-Bailón M, Perez-Farinos N, Salinero-Fort M, Carrasco-Garrido P.. National trends in incidence and outcomes of abdominal aortic aneurysm among elderly type 2 diabetic and non-diabetic patients in Spain (2003-2012). Cardiovasc Diabetol 2015;14:48.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Avdic T, Franzen S, Zarrouk M, Acosta S, Nilsson P, Gottsater A, Svensson AM, Gudbjornsdottir S, Eliasson B.. Reduced long-term risk of aortic aneurysm and aortic dissection among individuals with type 2 diabetes mellitus: a Nationwide Observational Study. J Am Heart Assoc 2018;7:e007618.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Le MT, Jamrozik K, Davis TM, Norman PE.. Negative association between infra-renal aortic diameter and glycaemia: the Health in Men Study. Eur J Vasc Endovasc Surg 2007;33:599–604. [DOI] [PubMed] [Google Scholar]
- 18. Kristensen KL, Dahl M, Rasmussen LM, Lindholt JS.. Glycated hemoglobin is associated with the growth rate of abdominal aortic aneurysms: a substudy from the VIVA (Viborg Vascular) Randomized Screening Trial. Arterioscler Thromb Vasc Biol 2017;37:730–736. [DOI] [PubMed] [Google Scholar]
- 19. Golledge J, Karan M, Moran CS, Muller J, Clancy P, Dear AE, Norman PE.. Reduced expansion rate of abdominal aortic aneurysms in patients with diabetes may be related to aberrant monocyte-matrix interactions. Eur Heart J 2008;29:665–672. [DOI] [PubMed] [Google Scholar]
- 20. Takagi H, Umemoto T.. ALICE Group. Negative association of diabetes with rupture of abdominal aortic aneurysm. Diab Vasc Dis Res 2016;13:341–347. [DOI] [PubMed] [Google Scholar]
- 21. Leurs LJ, Laheij RJ, Buth J.. EUROSTAR Collaborators. Influence of diabetes mellitus on the endovascular treatment of abdominal aortic aneurysms. J Endovasc Ther 2005;12:288–296. [DOI] [PubMed] [Google Scholar]
- 22. Hughes K, Jackson JD, Prendergast TI, Rose DA, Bolorunduro O, Obirieze A, Cornwell EE, Greene WR, Obisesan T.. Diabetes mellitus is not associated with major morbidity following open abdominal aortic aneurysm repair. J Surg Res 2013;184:751–754. [DOI] [PubMed] [Google Scholar]
- 23. Png CY, Tadros RO, Kang M, Beckerman WE, Tardiff ML, Vouyouka AG, Marin ML, Faries PL.. The protective effects of diabetes mellitus on post-EVAR AAA growth and reinterventions. Ann Vasc Surg 2017;43:65–72. [DOI] [PubMed] [Google Scholar]
- 24. Diehm N, Hobo R, Baumgartner I, Do DD, Keo HH, Kalka C, Dick F, Buth J, Schmidli J; EUROSTAR Investigators. Influence of pulmonary status and diabetes mellitus on aortic neck dilatation following endovascular repair of abdominal aortic aneurysms: a EUROSTAR report. J Endovasc Ther 2007;14:122–129. [DOI] [PubMed] [Google Scholar]
- 25. Takagi H, Umemoto T; ALICE Group. Negative association of diabetes with thoracic aortic dissection and aneurysm. Angiology 2017;68:216–224. [DOI] [PubMed] [Google Scholar]
- 26. Prakash SK, Pedroza C, Khalil YA, Milewicz DM.. Diabetes and reduced risk for thoracic aortic aneurysms and dissections: a nationwide case-control study. J Am Heart Assoc 2012;1:jah3-e000323.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hjellestad ID, Softeland E, Nilsen RM, Husebye ES, Jonung T.. Abdominal aortic aneurysms—glycaemic status and mortality. J Diabetes Complications 2016;30:438–443. [DOI] [PubMed] [Google Scholar]
- 28. Kubota Y, Folsom AR, Pankow JS, Wagenknecht LE, Tang W.. Diabetes-related factors and abdominal aortic aneurysm events: the Atherosclerotic Risk in Communities Study. Ann Epidemiol 2018;28:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Persson M, Zarrouk M, Holst J, Nilsson PM, Gottsater A.. No association between glucose at age 30 and aortic diameter at age 65 in men: a population-based study. Scand Cardiovasc J 2016;50:119–122. [DOI] [PubMed] [Google Scholar]
- 30. Taimour S, Zarrouk M, Holst J, Rosengren AH, Groop L, Nilsson PM, Gottsater A.. Aortic diameter at age 65 in men with newly diagnosed type 2 diabetes. Scand Cardiovasc J 2017;51:202–206. [DOI] [PubMed] [Google Scholar]
- 31. Senemaud J, Caligiuri G, Etienne H, Delbosc S, Michel JB, Coscas R.. Translational relevance and recent advances of animal models of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 2017;37:401–410. [DOI] [PubMed] [Google Scholar]
- 32. Wilson NK, Gould RA, Gallo MacFarlane E, Consortium ML.. Pathophysiology of aortic aneurysm: insights from human genetics and mouse models. Pharmacogenomics 2016;17:2071–2080. [DOI] [PubMed] [Google Scholar]
- 33. King AJ. The use of animal models in diabetes research. Br J Pharmacol 2012;166:877–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dua MM, Miyama N, Azuma J, Schultz GM, Sho M, Morser J, Dalman RL.. Hyperglycemia modulates plasminogen activator inhibitor-1 expression and aortic diameter in experimental aortic aneurysm disease. Surgery 2010;148:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miyama N, Dua MM, Yeung JJ, Schultz GM, Asagami T, Sho E, Sho M, Dalman RL.. Hyperglycemia limits experimental aortic aneurysm progression. J Vasc Surg 2010;52:975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tanaka T, Takei Y, Yamanouchi D.. Hyperglycemia suppresses calcium phosphate-induced aneurysm formation through inhibition of macrophage activation. J Am Heart Assoc 2016;5:e003062.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li J, Huynh P, Dai A, Wu T, Tu Y, Chow B, Kiriazis H, Du XJ, Bach LA, Wilkinson-Berka JL, Biros E, Walker P, Nataatmadja M, West M, Golledge J, Allen TJ, Cooper ME, Chai Z.. Diabetes reduces severity of aortic aneurysms depending on the presence of cell division autoantigen 1 (CDA1). Diabetes 2018;67:755–768. [DOI] [PubMed] [Google Scholar]
- 38. Astrand H, Rydén-Ahlgren A, Sundkvist G, Sandgren T, Länne T.. Reduced aortic wall stress in diabetes mellitus. Eur J Vasc Endovasc Surg 2007;33:592–598. [DOI] [PubMed] [Google Scholar]
- 39. Sarajlic A, Gligorijevic V, Radak D, Przulj N.. Network wiring of pleiotropic kinases yields insight into protective role of diabetes on aneurysm. Integr Biol (Camb) 2014;6:1049–1057. [DOI] [PubMed] [Google Scholar]
- 40. Preil SA, Kristensen LP, Beck HC, Jensen PS, Nielsen PS, Steiniche T, Bjorling-Poulsen M, Larsen MR, Hansen ML, Rasmussen LM.. Quantitative proteome analysis reveals increased content of basement membrane proteins in arteries from patients with type 2 diabetes mellitus and lower levels among metformin users. Circ Cardiovasc Genet 2015;8:727–735. [DOI] [PubMed] [Google Scholar]
- 41. Tamarina NA, Grassi MA, Johnson DA, Pearce WH.. Proteoglycan gene expression is decreased in abdominal aortic aneurysms. J Surg Res 1998;74:76–80. [DOI] [PubMed] [Google Scholar]
- 42. Meester JA, Vandeweyer G, Pintelon I, Lammens M, Van Hoorick L, De Belder S, Waitzman K, Young L, Markham LW, Vogt J, Richer J, Beauchesne LM, Unger S, Superti-Furga A, Prsa M, Dhillon R, Reyniers E, Dietz HC, Wuyts W, Mortier G, Verstraeten A, Van Laer L, Loeys BL.. Loss-of-function mutations in the X-linked biglycan gene cause a severe syndromic form of thoracic aortic aneurysms and dissections. Genet Med 2017;19:386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tang T, Thompson JC, Wilson PG, Yoder MH, Mueller J, Fischer JW, Williams KJ, Tannock LR.. Biglycan deficiency: increased aortic aneurysm formation and lack of atheroprotection. J Mol Cell Cardiol 2014;75:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heegaard AM, Corsi A, Danielsen CC, Nielsen KL, Jorgensen HL, Riminucci M, Young MF, Bianco P.. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation 2007;115:2731–2738. [DOI] [PubMed] [Google Scholar]
- 45. Mallat Z, Ait-Oufella H, Tedgui A.. The pathogenic transforming growth factor-beta overdrive hypothesis in aortic aneurysms and dissections: a mirage? Circ Res 2017;120:1718–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lareyre F, Clement M, Raffort J, Pohlod S, Patel M, Esposito B, Master L, Finigan A, Vandestienne M, Stergiopulos N, Taleb S, Trachet B, Mallat Z.. TGFbeta (Transforming Growth Factor-beta) blockade induces a human-like disease in a nondissecting mouse model of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 2017;37:2171–2181. [DOI] [PubMed] [Google Scholar]
- 47. Hara T, Yoshida E, Shinkai Y, Yamamoto C, Fujiwara Y, Kumagai Y, Kaji T.. Biglycan intensifies ALK5-Smad2/3 signaling by TGF-beta1 and downregulates Syndecan-4 in cultured vascular endothelial cells. J Cell Biochem 2017;118:1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Burch ML, Yang SN, Ballinger ML, Getachew R, Osman N, Little PJ.. TGF-beta stimulates biglycan synthesis via p38 and ERK phosphorylation of the linker region of Smad2. Cell Mol Life Sci 2010;67:2077–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wahab NA, Parker S, Sraer JD, Mason RM.. The decorin high glucose response element and mechanism of its activation in human mesangial cells. J Am Soc Nephrol 2000;11:1607–1619. [DOI] [PubMed] [Google Scholar]
- 50. Ferdous Z, Wei VM, Iozzo R, Hook M, Grande-Allen KJ.. Decorin-transforming growth factor- interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J Biol Chem 2007;282:35887–35898. [DOI] [PubMed] [Google Scholar]
- 51. Ueda K, Yoshimura K, Yamashita O, Harada T, Morikage N, Hamano K.. Possible dual role of decorin in abdominal aortic aneurysm. PLoS One 2015;10:e0120689.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jondeau G, Michel JB, Boileau C.. The translational science of Marfan syndrome. Heart 2011;97:1206–1214. [DOI] [PubMed] [Google Scholar]
- 53. Roccabianca S, Ateshian GA, Humphrey JD.. Biomechanical roles of medial pooling of glycosaminoglycans in thoracic aortic dissection. Biomech Model Mechanobiol 2014;13:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cikach FS, Koch CD, Mead TJ, Galatioto J, Willard BB, Emerton KB, Eagleton MJ, Blackstone EH, Ramirez F, Roselli EE, Apte SS.. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight 2018;3. doi: 10.1172/jci.insight.97167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mallat Z, Tedgui A, Henrion D.. Role of microvascular tone and extracellular matrix contraction in the regulation of interstitial fluid: implications for aortic dissection. Arterioscler Thromb Vasc Biol 2016;36:1742–1747. [DOI] [PubMed] [Google Scholar]
- 56. Death AK, Fisher EJ, McGrath KC, Yue DK.. High glucose alters matrix metalloproteinase expression in two key vascular cells: potential impact on atherosclerosis in diabetes. Atherosclerosis 2003;168:263–269. [DOI] [PubMed] [Google Scholar]
- 57. Lewandowski KC, Banach E, Bieńkiewicz M, Lewiński A.. Matrix metalloproteinases in type 2 diabetes and non-diabetic controls: effects of short-term and chronic hyperglycaemia. Arch Med Sci 2011;2:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Deguchi JO, Huang H, Libby P, Aikawa E, Whittaker P, Sylvan J, Lee RT, Aikawa M.. Genetically engineered resistance for MMP collagenases promotes abdominal aortic aneurysm formation in mice infused with angiotensin II. Lab Invest 2009;89:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Howatt DA, Dajee M, Xie X, Moorleghen J, Rateri DL, Balakrishnan A, Da Cunha V, Johns DG, Gutstein DE, Daugherty A, Lu H.. Relaxin and matrix metalloproteinase-9 in angiotensin II-induced abdominal aortic aneurysms. Circ J 2017;81:888–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost 2001;86:324–333. [PubMed] [Google Scholar]
- 61. Zieman S, Kass D.. Advanced glycation end product cross-linking: pathophysiologic role and therapeutic target in cardiovascular disease. Congest Heart Fail 2004;10:144–149. [DOI] [PubMed] [Google Scholar]
- 62. Koole D, van Herwaarden JA, Schalkwijk CG, Lafeber F, Vink A, Smeets MB, Pasterkamp G, Moll FL.. A potential role for glycated cross-links in abdominal aortic aneurysm disease. J Vasc Surg 2017;65:1493–1503. [DOI] [PubMed] [Google Scholar]
- 63. Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A.. Role of advanced glycation end products in cellular signaling. Redox Biol 2014;2:411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang F, Kent KC, Yamanouchi D, Zhang Y, Kato K, Tsai S, Nowygrod R, Schmidt AM, Liu B.. Anti-receptor for advanced glycation end products therapies as novel treatment for abdominal aortic aneurysm. Ann Surg 2009;250:416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hofmann Bowman M, Wilk J, Heydemann A, Kim G, Rehman J, Lodato JA, Raman J, McNally EM.. S100A12 mediates aortic wall remodeling and aortic aneurysm. Circ Res 2010;106:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Das D, Gawdzik J, Dellefave-Castillo L, McNally EM, Husain A, Raman J, Hofmann Bowman MA.. S100A12 expression in thoracic aortic aneurysm is associated with increased risk of dissection and perioperative complications. J Am Coll Cardiol 2012;60:775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yao Y, Zhuang J, Li Y, Jing B, Li H, Li J, Shao C, Li K, Wang H.. Association of polymorphisms of the receptor for advanced glycation end products gene and susceptibility to sporadic abdominal aortic aneurysm. Biomed Res Int 2015;2015:394126.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Boersema J, de Vos LC, Links TP, Mulder DJ, Smit AJ, Zeebregts CJ, Lefrandt JD.. Skin accumulation of advanced glycation end products is increased in patients with an abdominal aortic aneurysm. J Vasc Surg 2017;66:1696–1703. [DOI] [PubMed] [Google Scholar]
- 69. de Vos LC, Boersema J, Hillebrands JL, Schalkwijk CG, Meerwaldt R, Breek JC, Smit AJ, Zeebregts CJ, Lefrandt JD.. Diverging effects of diabetes mellitus in patients with peripheral artery disease and abdominal aortic aneurysm and the role of advanced glycation end-products: aRTERY study—protocol for a multicentre cross-sectional study. BMJ Open 2017;7:e012584.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Donath MY, Shoelson SE.. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107. [DOI] [PubMed] [Google Scholar]
- 71. Rask-Madsen C, King GL.. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab 2013;17:20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Azuma K, Kawamori R, Toyofuku Y, Kitahara Y, Sato F, Shimizu T, Miura K, Mine T, Tanaka Y, Mitsumata M, Watada H.. Repetitive fluctuations in blood glucose enhance monocyte adhesion to the endothelium of rat thoracic aorta. Arterioscler Thromb Vasc Biol 2006;26:2275–2280. [DOI] [PubMed] [Google Scholar]
- 73. Tanaka A, Azuma K, Toyofuku Y, Kurokawa A, Otsuka A, Mita T, Hirosea T, Fujitani Y, Miyauchi K, Daida H, Kawamori R, Watada H.. Insulin and nateglinide reduce monocyte adhesion to endothelial cells in Goto-Kakizaki rats exhibiting repetitive blood glucose fluctuation. Biochem Biophys Res Commun 2006;350:195–201. [DOI] [PubMed] [Google Scholar]
- 74. Yan J, Tie G, Wang S, Tutto A, DeMarco N, Khair L, Fazzio TG, Messina LM.. Diabetes impairs wound healing by Dnmt1-dependent dysregulation of hematopoietic stem cells differentiation towards macrophages. Nat Commun 2018;9:33.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kanter JE, Kramer F, Barnhart S, Averill MM, Vivekanandan-Giri A, Vickery T, Li LO, Becker L, Yuan W, Chait A, Braun KR, Potter-Perigo S, Sanda S, Wight TN, Pennathur S, Serhan CN, Heinecke JW, Coleman RA, Bornfeldt KE.. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci USA 2012;109:E715–E724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fadini GP, de Kreutzenberg SV, Boscaro E, Albiero M, Cappellari R, Krankel N, Landmesser U, Toniolo A, Bolego C, Cignarella A, Seeger F, Dimmeler S, Zeiher A, Agostini C, Avogaro A.. An unbalanced monocyte polarisation in peripheral blood and bone marrow of patients with type 2 diabetes has an impact on microangiopathy. Diabetologia 2013;56:1856–1866. [DOI] [PubMed] [Google Scholar]
- 77. Sun C, Sun L, Ma H, Peng J, Zhen Y, Duan K, Liu G, Ding W, Zhao Y.. The phenotype and functional alterations of macrophages in mice with hyperglycemia for long term. J Cell Physiol 2012;227:1670–1679. [DOI] [PubMed] [Google Scholar]
- 78. Harrison SC, Smith AJ, Jones GT, Swerdlow DI, Rampuri R, Bown MJ, Aneurysm C, Folkersen L, Baas AF, de Borst GJ, Blankensteijn JD, Price JF, van der Graaf Y, McLachlan S, Agu O, Hofman A, Uitterlinden AG, Franco-Cereceda A, Ruigrok YM, van't Hof FN, Powell JT, van Rij AM, Casas JP, Eriksson P, Holmes MV, Asselbergs FW, Hingorani AD, Humphries SE.. Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur Heart J 2013;34:3707–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Arapoglou V, Kondi-Pafiti A, Rizos D, Carvounis E, Frangou-Plemenou M, Kotsis T, Katsenis K.. The influence of diabetes on degree of abdominal aortic aneurysm tissue inflammation. Vasc Endovascular Surg 2010;44:454–459. [DOI] [PubMed] [Google Scholar]
- 80. Choke E, Thompson MM, Dawson J, Wilson WR, Sayed S, Loftus IM, Cockerill GW.. Abdominal aortic aneurysm rupture is associated with increased medial neovascularization and overexpression of proangiogenic cytokines. Arterioscler Thromb Vasc Biol 2006;26:2077–2082. [DOI] [PubMed] [Google Scholar]
- 81. Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, Gurtner GC.. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem 2008;283:10930–10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Thangarajah H, Yao D, Chang EI, Shi Y, Jazayeri L, Vial IN, Galiano RD, Du XL, Grogan R, Galvez MG, Januszyk M, Brownlee M, Gurtner GC.. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci USA 2009;106:13505–13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tedesco MM, Terashima M, Blankenberg FG, Levashova Z, Spin JM, Backer MV, Backer JM, Sho M, Sho E, McConnell MV, Dalman RL.. Analysis of in situ and ex vivo vascular endothelial growth factor receptor expression during experimental aortic aneurysm progression. Arterioscler Thromb Vasc Biol 2009;29:1452–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Porter KE, Riches K.. The vascular smooth muscle cell: a therapeutic target in Type 2 diabetes? Clin Sci 2013;125:167–182. [DOI] [PubMed] [Google Scholar]
- 85. Madi HA, Riches K, Warburton P, O'Regan DJ, Turner NA, Porter KE.. Inherent differences in morphology, proliferation, and migration in saphenous vein smooth muscle cells cultured from nondiabetic and type 2 diabetic patients. Am J Physiol Cell Physiol 2009;297:C1307–C1317. [DOI] [PubMed] [Google Scholar]
- 86. Oikawa S, Hayasaka K, Hashizume E, Kotake H, Midorikawa H, Sekikawa A, Kikuchi A, Toyota T.. Human arterial smooth muscle cell proliferation in diabetes. Diabetes 1996;45:S114–S116. [DOI] [PubMed] [Google Scholar]
- 87. Chung AW, Luo H, Tejerina T, van Breemen C, Okon EB.. Enhanced cell cycle entry and mitogen-activated protein kinase-signaling and downregulation of matrix metalloproteinase-1 and -3 in human diabetic arterial vasculature. Atherosclerosis 2007;195:e1–e8. [DOI] [PubMed] [Google Scholar]
- 88. Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R.. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci USA 2008;105:9047–9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Reddy MA, Das S, Zhuo C, Jin W, Wang M, Lanting L, Natarajan R.. Regulation of vascular smooth muscle cell dysfunction under diabetic conditions by miR-504. Arterioscler Thromb Vasc Biol 2016;36:864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK.. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 2015;21:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Salmon M, Johnston WF, Woo A, Pope NH, Su G, Upchurch GR Jr, Owens GK, Ailawadi G.. KLF4 regulates abdominal aortic aneurysm morphology and deletion attenuates aneurysm formation. Circulation 2013;128:S163–S174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hien TT, Garcia-Vaz E, Stenkula KG, Sjogren J, Nilsson J, Gomez MF, Albinsson S.. MicroRNA-dependent regulation of KLF4 by glucose in vascular smooth muscle. J Cell Physiol 2018;233:7195–7205. [DOI] [PubMed] [Google Scholar]
- 93. Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D.. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009;460:705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kanzaki T, Shiina R, Saito Y, Zardi L, Morisaki N.. Transforming growth factor-beta receptor and fibronectin expressions in aortic smooth muscle cells in diabetic rats. Diabetologia 1997;40:383–391. [DOI] [PubMed] [Google Scholar]
- 95. Sharma K, Deelman L, Madesh M, Kurz B, Ciccone E, Siva S, Hu T, Zhu Y, Wang L, Henning R, Ma X, Hajnoczky G.. Involvement of transforming growth factor-beta in regulation of calcium transients in diabetic vascular smooth muscle cells. Am J Physiol Renal Physiol 2003;285:F1258–F1270. [DOI] [PubMed] [Google Scholar]
- 96. Gillis E, Van Laer L, Loeys BL.. Genetics of thoracic aortic aneurysm: at the crossroad of transforming growth factor-beta signaling and vascular smooth muscle cell contractility. Circ Res 2013;113:327–340. [DOI] [PubMed] [Google Scholar]
- 97. Li W, Li Q, Jiao Y, Qin L, Ali R, Zhou J, Ferruzzi J, Kim RW, Geirsson A, Dietz HC, Offermanns S, Humphrey JD, Tellides G.. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J Clin Invest 2014;124:755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Angelov SN, Hu JH, Wei H, Airhart N, Shi M, Dichek DA.. TGF-beta (Transforming Growth Factor-beta) signaling protects the thoracic and abdominal aorta from angiotensin ii-induced pathology by distinct mechanisms. Arterioscler Thromb Vasc Biol 2017;37:2102–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang P, Hou S, Chen J, Zhang J, Lin F, Ju R, Cheng X, Ma X, Song Y, Zhang Y, Zhu M, Du J, Lan Y, Yang X.. Smad4 deficiency in smooth muscle cells initiates the formation of aortic aneurysm. Circ Res 2016;118:388–399. [DOI] [PubMed] [Google Scholar]
- 100. Da Ros F, Carnevale R, Cifelli G, Bizzotto D, Casaburo M, Perrotta M, Carnevale L, Vinciguerra I, Fardella S, Iacobucci R, Bressan GM, Braghetta P, Lembo G, Carnevale D.. Targeting interleukin-1beta protects from aortic aneurysms induced by disrupted transforming growth factor beta signaling. Immunity 2017;47:959–973. [DOI] [PubMed] [Google Scholar]
- 101. Litteri G, Carnevale D, D'Urso A, Cifelli G, Braghetta P, Damato A, Bizzotto D, Landolfi A, Ros FD, Sabatelli P, Facchinello N, Maffei A, Volpin D, Colombatti A, Bressan GM, Lembo G.. Vascular smooth muscle Emilin-1 is a regulator of arteriolar myogenic response and blood pressure. Arterioscler Thromb Vasc Biol 2012;32:2178–2184. [DOI] [PubMed] [Google Scholar]
- 102. Milewicz DM, Trybus KM, Guo DC, Sweeney HL, Regalado E, Kamm K, Stull JT.. Altered smooth muscle cell force generation as a driver of thoracic aortic aneurysms and dissections. Arterioscler Thromb Vasc Biol 2017;37:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ferruzzi J, Murtada SI, Li G, Jiao Y, Uman S, Ting MY, Tellides G, Humphrey JD.. Pharmacologically improved contractility protects against aortic dissection in mice with disrupted transforming growth factor-beta signaling despite compromised extracellular matrix properties. Arterioscler Thromb Vasc Biol 2016;36:919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hien TT, Turczyńska KM, Dahan D, Ekman M, Grossi M, Sjögren J, Nilsson J, Braun T, Boettger T, Garcia-Vaz E, Stenkula K, Swärd K, Gomez MF, Albinsson S.. Elevated glucose levels promote contractile and cytoskeletal gene expression in vascular smooth muscle via Rho/protein kinase C and actin polymerization. J Biol Chem 2016;291:3552–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jia LX, Zhang WM, Zhang HJ, Li TT, Wang YL, Qin YW, Gu H, Du J.. Mechanical stretch-induced endoplasmic reticulum stress, apoptosis and inflammation contribute to thoracic aortic aneurysm and dissection. J Pathol 2015;236:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ramadan A, Al-Omran M, Verma S.. The putative role of autophagy in the pathogenesis of abdominal aortic aneurysms. Atherosclerosis 2017;257:288–296. [DOI] [PubMed] [Google Scholar]
- 107. Zhu Q, Guo R, Liu C, Fu D, Liu F, Hu J, Jiang H.. Endoplasmic reticulum stress-mediated apoptosis contributing to high glucose-induced vascular smooth muscle cell calcification. J Vasc Res 2015;52:291–298. [DOI] [PubMed] [Google Scholar]
- 108. Hu P, Lai D, Lu P, Gao J, He H.. ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int J Mol Med 2012;29:613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kostina AS, Uspensky VЕ, Irtyuga OB, Ignatieva EV, Freylikhman O, Gavriliuk ND, Moiseeva OM, Zhuk S, Tomilin A, Kostareva АА, Malashicheva AB.. Notch-dependent EMT is attenuated in patients with aortic aneurysm and bicuspid aortic valve. Biochim Biophys Acta 2016;1862:733–740. [DOI] [PubMed] [Google Scholar]
- 110. Oller J, Méndez-Barbero N, Ruiz EJ, Villahoz S, Renard M, Canelas LI, Briones AM, Alberca R, Lozano-Vidal N, Hurlé MA, Milewicz D, Evangelista A, Salaices M, Nistal JF, Jiménez-Borreguero LJ, De Backer J, Campanero MR, Redondo JM.. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat Med 2017;23:200–212. [DOI] [PubMed] [Google Scholar]
- 111. Swedenborg J, Eriksson P.. The intraluminal thrombus as a source of proteolytic activity. Ann N Y Acad Sci 2006;1085:133–138. [DOI] [PubMed] [Google Scholar]
- 112. Dunn EJ, Ariens RA, Grant PJ.. The influence of type 2 diabetes on fibrin structure and function. Diabetologia 2005;48:1198–1206. [DOI] [PubMed] [Google Scholar]
- 113. Thompson A, Cooper JA, Fabricius M, Humphries SE, Ashton HA, Hafez H.. An analysis of drug modulation of abdominal aortic aneurysm growth through 25 years of surveillance. J Vasc Surg 2010;52:55–61. [DOI] [PubMed] [Google Scholar]
- 114. Hsu CY, Su YW, Chen YT, Tsai SH, Chang CC, Li SY, Huang PH, Chen JW, Lin SJ.. Association between use of oral-antidiabetic drugs and the risk of aortic aneurysm: a nested case-control analysis. Cardiovasc Diabetol 2016;15:125.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fujimura N, Xiong J, Kettler EB, Xuan H, Glover KJ, Mell MW, Xu B, Dalman RL.. Metformin treatment status and abdominal aortic aneurysm disease progression. J Vasc Surg 2016;64:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Golledge J, Moxon J, Pinchbeck J, Anderson G, Rowbotham S, Jenkins J, Bourke M, Bourke B, Dear A, Buckenham T, Jones R, Norman PE.. Association between metformin prescription and growth rates of abdominal aortic aneurysms. Br J Surg 2017;104:1486–1493. [DOI] [PubMed] [Google Scholar]
- 117. Kristensen KL, Pottegard A, Hallas J, Rasmussen LM, Lindholt JS.. Metformin treatment does not affect the risk of ruptured abdominal aortic aneurysms. J Vasc Surg 2017;66:768–774. [DOI] [PubMed] [Google Scholar]
- 118. Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM.. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med 2013;19:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Davidson MA, Mattison DR, Azoulay L, Krewski D.. Thiazolidinedione drugs in the treatment of type 2 diabetes mellitus: past, present and future. Crit Rev Toxicol 2018;48:52–108. [DOI] [PubMed] [Google Scholar]
- 120. Golledge J, Cullen B, Rush C, Moran CS, Secomb E, Wood F, Daugherty A, Campbell JH, Norman PE.. Peroxisome proliferator-activated receptor ligands reduce aortic dilatation in a mouse model of aortic aneurysm. Atherosclerosis 2010;210:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U, Graf K, Law RE, Hsueh WA.. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest 2003;112:1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pirianov G, Torsney E, Howe F, Cockerill GW.. Rosiglitazone negatively regulates c-Jun N-terminal kinase and toll-like receptor 4 proinflammatory signalling during initiation of experimental aortic aneurysms. Atherosclerosis 2012;225:69–75. [DOI] [PubMed] [Google Scholar]
- 123. Jones A, Deb R, Torsney E, Howe F, Dunkley M, Gnaneswaran Y, Gaze D, Nasr H, Loftus IM, Thompson MM, Cockerill GW.. Rosiglitazone reduces the development and rupture of experimental aortic aneurysms. Circulation 2009;119:3125–3132. [DOI] [PubMed] [Google Scholar]
- 124. Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI.. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation 2002;106:679–684. [DOI] [PubMed] [Google Scholar]
- 125. Yu J, Morimoto K, Bao W, Yu Z, Okita Y, Okada K.. Glucagon-like peptide-1 prevented abdominal aortic aneurysm development in rats. Surg Today 2016;46:1099–1107. [DOI] [PubMed] [Google Scholar]
- 126. Lu HY, Huang CY, Shih CM, Chang WH, Tsai CS, Lin FY, Shih CC.. Dipeptidyl peptidase-4 inhibitor decreases abdominal aortic aneurysm formation through GLP-1-dependent monocytic activity in mice. PLoS One 2015;10:e0121077.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bao W, Morimoto K, Hasegawa T, Sasaki N, Yamashita T, Hirata K, Okita Y, Okada K.. Orally administered dipeptidyl peptidase-4 inhibitor (alogliptin) prevents abdominal aortic aneurysm formation through an antioxidant effect in rats. J Vasc Surg 2014;59:1098–1108. [DOI] [PubMed] [Google Scholar]
- 128. Kohashi K, Hiromura M, Mori Y, Terasaki M, Watanabe T, Kushima H, Shinmura K, Tomoyasu M, Nagashima M, Hirano T.. A dipeptidyl peptidase-4 inhibitor but not incretins suppresses abdominal aortic aneurysms in angiotensin II-infused apolipoprotein E-null mice. J Atheroscler Thromb 2016;23:441–454. [DOI] [PubMed] [Google Scholar]
- 129. Verspohl EJ. Novel therapeutics for type 2 diabetes: incretin hormone mimetics (glucagon-like peptide-1 receptor agonists) and dipeptidyl peptidase-4 inhibitors. Pharmacol Ther 2009;124:113–138. [DOI] [PubMed] [Google Scholar]