Abstract
Tissue-engineered human blood vessels may enable in vitro disease modeling and drug screening to accelerate advances in vascular medicine. Existing methods for tissue-engineered blood vessel (TEBV) fabrication create homogenous tubes not conducive to modeling the focal pathologies characteristic of certain vascular diseases. We developed a system for generating self-assembled human smooth muscle cell (SMC) ring units, which were fused together into TEBVs. The goal of this study was to assess the feasibility of modular assembly and fusion of ring building units to fabricate spatially controlled, heterogeneous tissue tubes. We first aimed to enhance fusion and reduce total culture time, and determined that reducing ring preculture duration improved tube fusion. Next, we incorporated electrospun polymer ring units onto tube ends as reinforced extensions, which allowed us to cannulate tubes after only 7 days of fusion, and culture tubes with luminal flow in a custom bioreactor. To create focal heterogeneities, we incorporated gelatin microspheres into select ring units during self-assembly, and fused these rings between ring units without microspheres. Cells within rings maintained their spatial position along tissue tubes after fusion. Because tubes fabricated from primary SMCs did not express contractile proteins, we also fabricated tubes from human mesenchymal stem cells, which expressed smooth muscle alpha actin and SM22-α. This work describes a platform approach for creating modular TEBVs with spatially defined structural heterogeneities, which may ultimately be applied to mimic focal diseases such as intimal hyperplasia or aneurysm.
Keywords: : tissue-engineered blood vessel, gelatin microspheres, vascular disease model, bioreactor, modular tissue engineering, electrospun cannulation cuff
Introduction
Cardiovascular disease is the leading cause of death in the United States.1 By 2030, 43.9% of Americans will be living with some form of cardiovascular disease.1 Before clinical testing, new treatments for cardiovascular diseases are tested in 2D cell cultures and animal models. However, 2D cultures are not representative of the 3D mechanical environment and cell–matrix interactions found in vivo,2 and animal studies do not accurately predict the success of drugs in humans; many drugs that are successful in animals fail in human clinical trials.3,4 Thus, there is a strong need for 3D human tissues to model vascular diseases and serve as tools to screen potential therapies.5–10 Testing drugs on functional human tissues in vitro may allow researchers to eliminate ineffective drugs earlier in the testing process, and accelerate the development of new, lifesaving treatments.
Several approaches have been reported for fabricating functional 3D human vascular tissue for drug screening and disease modeling.10–14 For example, Fernandez et al. developed a functional smooth muscle cell (SMC)-endothelial cell co-culture tube for drug testing by seeding cells in collagen gels, which reacted to vasoactive stimuli.11 There are also many existing approaches for fabricating functional human tissue-engineered blood vessels (TEBVs) for implantation, including seeding cells on polymer scaffolds,15,16 seeding cells in hydrogels,11,17 and using scaffold-free cellular self-assembly approaches.10 However, most TEBV approaches use cells seeded on or within tubular scaffolds, or use rolled cell sheets, to create a homogenous tissue tube. In contrast, many vascular diseases, such as aneurysm and intimal hyperplasia, create focal changes in SMC phenotype or matrix composition in localized regions of the blood vessel wall, not the entire length of the vessel.18,19
Alternatively, bioprinting can be used to create complex, modular, tubular tissue structures, which is typically done by fusing spheroidal subunits.20–22 However, tissue spheroids do not fuse as effectively as other cell aggregate shapes, because even tightly packed spheroids have limited surface area where spheres are in contact with one another, compared to other shapes such as rings.20 Thus, TEBVs fabricated from spheroids often have distinct fusion boundaries or gaps where spheroids were not in close contact.20,23,24
While some existing homogenous TEBVs may be effective for screening drugs on healthy tissues, they are not conducive to creating focal regions of pathological tissue. Regardless of the tissue engineering method used, the creation of localized heterogeneities within human TEBVs has not been previously reported. Thus, the primary goal of this study was to establish a model system that achieves spatial control of cell position and tissue structure within human TEBVs to introduce focal heterogeneities. To achieve this goal, we utilized a unique modular system developed in our laboratory for fabricating self-assembled vascular tissue from individual ring units (shown schematically in Fig. 1).25 Human SMCs were seeded into ring-shaped agarose molds, where they aggregated in less than 24 h to form self-assembled tissue rings.25 Within 3 days, rings can be threaded onto silicone tubing, stacked together, and fused to form vascular tissue tubes. Unlike spheroids, rings can be pushed into close contact and fuse without gaps between tissue units.26 We have also shown that we can incorporate degradable gelatin microspheres within tissue rings during self-assembly, which can be used to deliver growth factors and modulate cell phenotype within individual ring subunits.27
FIG. 1.
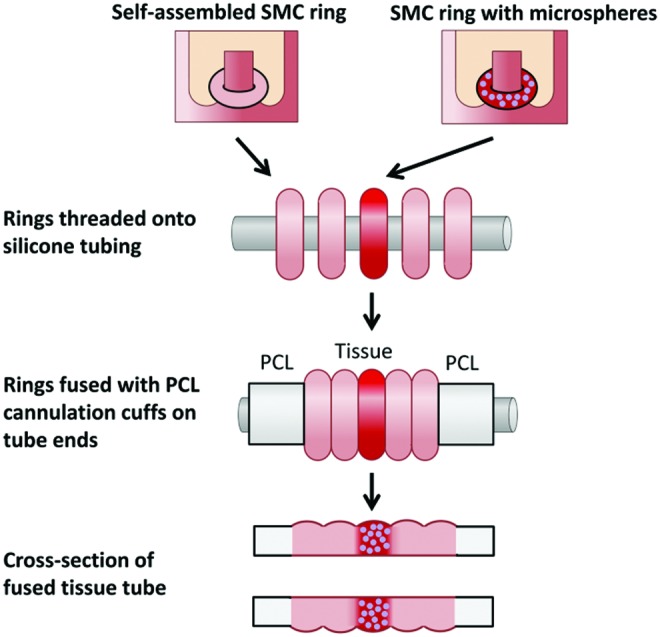
Fabrication of modular tissue tubes with focal heterogeneities. Rings with incorporated microspheres are fused between rings without microspheres, with PCL cuffs on either end. The resulting construct is a fused tissue tube with focal region of microsphere incorporation. PCL, polycaprolactone. Color images available online at www.liebertpub.com/tea
In this study, we present a novel approach for fabricating 3D vascular tissue from human cells with spatially controlled heterogeneities, which may ultimately serve as a platform technology to introduce focal regions of pathological tissue within TEBVs. We demonstrated, in principle, that modular building units comprising self-assembled primary human SMC rings (with or without incorporated gelatin microspheres) can be fused together into a contiguous and heterogeneous tissue tube with distinct structural regions. To aid in handling and cannulation, electrospun polycaprolactone (PCL) cannulation cuffs can be fused onto tube ends as reinforced extensions. We then validated that after 7 days of fusion culture, tubes with PCL cuffs could be cannulated and dynamically cultured on a custom luminal flow bioreactor. Finally, we tested human mesenchymal stem cells (MSCs) as an alternative source of SMCs and fabricated tubes that produce smooth muscle contractile proteins.
In summary, we demonstrated a technology for creating TEBVs that allows for customization of tissue structure and composition along the vessel length, through use of self-assembled cell-ring units, degradable gelatin microspheres, and PCL cannulation cuffs. In future studies, this system may be modified to model focal human vascular diseases.
Methods
The first goal of the study was to enhance ring fusion and reduce fusion time. This was accomplished by evaluating the effects of ring preculture time on ring fusion. The next goal was to evaluate if rings maintain spatial positioning during fusion, to determine the feasibility of creating focal heterogeneities. The third goal of this study was to demonstrate that tissue tubes can be cannulated and dynamically cultured. PCL cannulation cuffs were incorporated on tube ends as reinforced extensions for cannulation, and the use of a custom luminal flow bioreactor for dynamic tube culture was demonstrated. The final goal was to create focal heterogeneities within tubes, which was accomplished by creating localized regions of microsphere incorporation.
Cell culture
Human aortic SMCs (Lifeline) were cultured in Lifeline VascuLife complete growth medium containing 10 mM L-glutamine, 5% FBS, 5 μg/mL insulin, 5 ng/mL fibroblast growth factor-basic, 50 μg/mL ascorbic acid, 5 ng/mL epidermal growth factor, 30 mg/mL gentamicin, and 15 μg/mL amphotericin B. Bone marrow-derived human MSCs (hMSCs) were purchased from RoosterBio, Inc. and expanded according to the manufacturer's instructions in a propriety growth medium (RoosterBio, Inc.).
Tissue ring fabrication
Agarose wells (2 mm post diameter) were prepared as described previously from 2% agarose (Lonza) dissolved in DMEM and autoclaved.28 Human aortic SMC rings were seeded into agarose molds designed to fit five rings in a well of a six-well plate,28 at a density of 400,000 cells/ring. Molds were equilibrated overnight in growth medium before use. All seeded rings were incubated overnight to allow cell aggregation, and then, wells were flooded with fresh growth medium.
Human MSCs were seeded in agarose molds at a concentration of 600,000 cells/ring. Wells were flooded after 2 h of cell aggregation, and then switched to a custom medium after 24 h containing DMEM, 5% FBS, 1% l-glutamine, 1% ITS, 1% penicillin-streptomycin, and 50 μg/mL ascorbic acid.
Tissue tube fusion with varying preculture time
To generate tissue tubes, rings fabricated from human aortic SMCs were removed from agarose molds at 3, 5, or 7 days in culture and threaded onto silicone tubing mandrels (Specialty Manufacturing Inc., O.D. 2 mm).26 Three rings per tube were gently pushed together on the mandrel to ensure rings were in contact with each other (Fig. 2B) and the mandrel was secured in custom polycarbonate holders, which were placed in a 10 cm dish with 45 mL medium.26 The tubes were then allowed to fuse for an additional 7 days of static culture on the silicone mandrels. The experiment was duplicated once more with the same human aortic SMCs, and once again with human coronary artery SMCs from a different donor (Supplementary Figs. S1–S3; Supplementary Data are available online at www.liebertpub.com/tea).
FIG. 2.
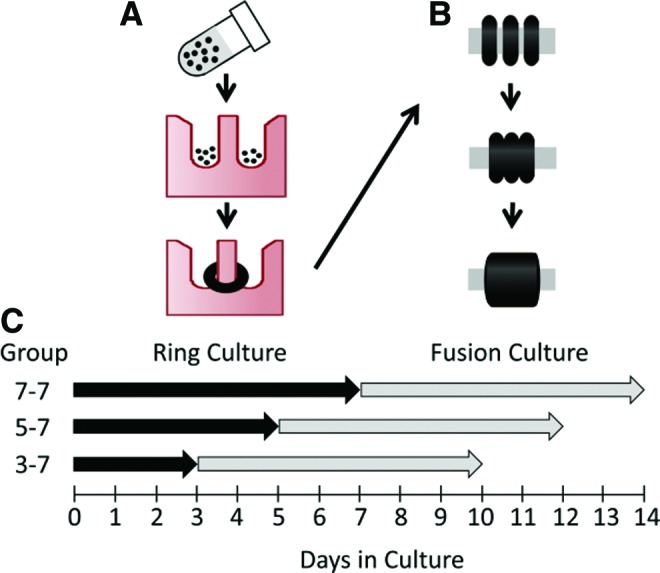
Schematic of tube fabrication process, and tissue tube culture experimental groups for the ring preculture duration experiment. Rings are formed by seeding SMCs into ring-shaped agarose molds, where cells aggregate around 2 mm diameter posts and form rings in less than 24 h (A). Rings are then removed from molds and threaded onto silicone tubing, where they are pushed together and cultured for 7 additional days to allow fusion (B). To test the effects of varying ring culture duration, rings were cultured for 3, 5, or 7 days (“ring culture”), followed by 7 days of “fusion culture” for all groups (C). Groups are labeled as follows: days in ring culture–days in fusion culture (ex. Group 3–7 = 3 days in ring culture followed by 7 days in fusion culture). Black dots = SMCs. SMC, smooth muscle cell. Color images available online at www.liebertpub.com/tea
Fusion angle, length, and thickness measurements
A Leica inverted microscope (DMIL) with a digital camera (Leica DFC 480) was used to take brightfield images of tubes daily for 1 week. Image J software (NIH) was used to measure the angle between rings (fusion angle, 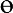 ), tube thickness (T), and tube length (L). Four fusion angle measurements, six thickness measurements, and two length measurements were obtained for each tube sample at each time point and averaged to yield a single mean for each parameter per tube per time point. Three independent tube samples were averaged for each of the three preculture conditions. After 7 days of culture, tissue samples were fixed for 1 h in 10% neutral buffered formalin for histological analysis. Data are represented as mean ± SD.
), tube thickness (T), and tube length (L). Four fusion angle measurements, six thickness measurements, and two length measurements were obtained for each tube sample at each time point and averaged to yield a single mean for each parameter per tube per time point. Three independent tube samples were averaged for each of the three preculture conditions. After 7 days of culture, tissue samples were fixed for 1 h in 10% neutral buffered formalin for histological analysis. Data are represented as mean ± SD.
CellTracker labeling
CellTracker red and green (CMTPX and CMFDA; Invitrogen) were reconstituted to 10 mM in DMSO and diluted to a final concentration of 5 μM in DMEM (Corning). Plates of human aortic SMCs were rinsed with PBS and incubated with either red or green CellTracker solution at 37°C for 45 min. The plates were rinsed with PBS, and growth medium was added for an additional 30 min at 37°C. Cells were then passaged and seeded into ring molds as described in the Tissue ring fabrication section. After 3 days of culture, tubes were fabricated with alternating red- and green-labeled rings and imaged with an inverted fluorescent microscope (Leica DMIL) daily for 7 days. The experiment was duplicated with human coronary artery SMCs from a different donor (Supplementary Methods). In a separate experiment examining cell proliferation, rings were loaded with CellTracker Red dye only, precultured for 3 days, and then fused into tubes with three rings per tube. Tubes were fixed after 1 or 2 days of fusion, to evaluate proliferation at earlier time points with Ki67 staining.
PCL cannulation cuff fabrication
Electrospun PCL cuffs were prepared as described previously.29 Briefly, PCL was dissolved in 2,2,2 tri-fluoro-ethanol (TFE, T63002; Sigma) to form a 12% solution. The solution was then electrospun onto a 2 mm diameter mandrel using a 5 mL/h flow rate, 15 cm collector distance, and voltage of 15–20 Kv.29 Cuffs were cut into segments ∼3–4 mm in length, sterilized with ethylene oxide, and allowed to de-gas for a minimum of 48 h before use.
Bioreactor culture
Rings were fabricated with human aortic SMCs (Lifeline) as described in the Tissue ring fabrication section, and threaded onto silicone tubing after 3 days of ring preculture, with PCL cuffs adjacent to rings on either end. Tubes were allowed to fuse for 7 days on silicone mandrels in static culture before removal from the silicone tubing mandrel and cannulation onto a custom bioreactor modified from Piola et al.30 Each bioreactor fits in its own individual 15 mL conical tube, which allows for multiple units to be cultured independently, with minimal culture medium (∼19 mL medium to fill each bioreactor unit and its tubing). The inner cannulas are adjustable, to accommodate tubes with lengths ranging from a few millimeters up to 3 cm. The medium flows from a peristaltic pump (Watson Marlow, Model 323Du), equipped with a multichannel pump head (Watson Marlow, Model 318MC). A syringe with 2 mL of medium and 3 mL of air is positioned between the pump and vessel, to dampen oscillations in medium flow between the pump and the tissue tube. The medium then flows back into the culture chamber, before returning to the pump. For these experiments, the bioreactor was set up to apply luminal flow, but not pressure. We verified that pressures between cannulas are approximately zero in benchtop experiments (not shown) before beginning these studies. Cannulated tubes (n = 5) were cultured with 35 mL/min applied luminal flow (corresponding to an estimated 12 dyne/cm2 wall shear stress) for 7 days before fixing for histology. Two control tubes were left on silicone mandrels in static conditions for a total of 14 days (same total culture time as tube exposed to 7 days of flow).
Fabricating tubes with spatially defined regions of microsphere incorporation
Rings were fabricated from human aortic SMCs (Lifeline), with genipin-crosslinked Type A gelatin microspheres 59.3 ± 28.8 μm in diameter and with a 32.3% ± 15.3% crosslink density incorporated as described previously.27 Briefly, microspheres were hydrated in PBS (25 μL per mg microspheres) for 2 h at 37°C, resuspended at 9.6 mg/mL, and mixed 1:1 with CellTracker Red-labeled human aortic SMCs (16 million cells/mL suspension). Rings were seeded with 50 μL cell–microsphere suspension per ring, resulting in rings with 400,000 cells/ring and 0.6 mg microspheres/million cells. After 3 days of preculture, rings were threaded onto silicone tubing mandrels with a central region of three microsphere-incorporated rings, and two outer regions with eight rings without microspheres per side (Fig. 1). Cannulation cuffs were placed adjacent to rings on tube ends. Tubes were cultured for 4 days on silicone mandrels in static conditions before fixation and paraffin embedding.
hMSC tube fabrication
Rings fabricated from hMSCs were harvested on day 3 for tube fabrication. After 4 days of fusion culture (7 days total), 5 ng/mL TGF-β1 and 2.5 ng/mL BMP-4 were added to the medium to stimulate differentiation to an SMC phenotype. Tubes were fixed after 7 days of fusion culture (10 days total) for immunohistochemistry and histology.
Histology and immunohistochemistry
After fixing for 1 h in 10% neutral buffered formalin, samples were processed and embedded in paraffin. Longitudinal sections 5 μm thick were adhered to positively charged slides. Hematoxylin and eosin staining was used to examine tube morphology. Picrosirius red/fast green and orcein stains were used examine collagen and elastin deposition, respectively.
Antigen retrieval was performed on samples to be stained for Ki67, smooth muscle alpha actin (SMA), smooth muscle protein 22 alpha (SM22-α), and calponin by incubating slides in 10 mM Tris, 1 mM EDTA, and 0.05% Tween-20 (pH 9.0) in a pressure cooker for 5 min. Slides were blocked in 5% normal goat serum (Ki67) or 1.5% normal rabbit serum (SMA, SM22-α, and calponin) for 30 min, and were incubated overnight at 4°C in anti-Ki67 (Abcam Ab16667; 1:100), SMA (Dako; clone 1A4, 1:100), SM22-α (BioRad VPA00048; 1:100), or calponin (Dako; CALP, 1:100) antibodies. Negative control samples were incubated with rabbit, mouse, or goat immunoglobulin G (Vector). Samples were incubated in a secondary antibody (Invitrogen; Alexa Fluor 488 goat anti-rabbit, rabbit anti-mouse, or mouse anti-goat) at a 1:400 dilution for 1 h at room temperature. Samples with CellTracker labeling or antibody stains were stained with Hoechst dye to visualize nuclei (Invitrogen; 1:6000 in DI water for 6 min). Images were acquired using an epifluorescent microscope (Leica DMLB2) with a digital camera (Leica DFC 480).
Statistics
Statistical tests were performed using SigmaPlot software (Version 11.0 Systat Software, Inc.). A two-way analysis of variance (ANOVA) with Holm–Sidak post hoc analysis was used to compare fusion angles, thicknesses, and lengths of tissue tubes. A p-value of less than 0.05 was considered significant. A sample size of n = 3 was used in statistically analyzed ring preculture experiments.
Results
Effect of ring preculture time on human SMC tube fusion rate
The first goal of this study was to accelerate production of tissue tubes and enhance ring fusion by examining how ring “preculture” time before tube fabrication affects fusion. In previous studies, we observed cohesive tubes after fusion, but ring boundaries remained visible after a total 14-day culture period (7 days as rings and 7 as tubes).25 By decreasing ring preculture duration before fusion, we aimed to decrease the length of time required to generate tissue tubes, and generate a more seamless ring fusion. Other published studies also suggest that less mature cell aggregates fuse together more rapidly than more mature tissues.31,32 Therefore, we hypothesized that decreasing the ring preculture duration before fusion would decrease the length of time required to generate tissue tubes, and lead to more seamless ring fusion.
Human aortic SMC rings were removed from agarose molds after 3, 5, or 7 days of ring preculture and cultured as tubes for 7 days, resulting in groups 3–7, 5–7, and 7–7, respectively (shown schematically in Fig. 2). Fusion was measured daily as the angle between adjacent rings, to determine the time course for tissue fusion31,33 (Fig. 3A). When human aortic SMC tubes were fused, there was a significant difference in fusion angle only on day 2 between the 7–7 group versus 5–7 group, and on day 3 between the 5–7 group versus 7–7 and 3–7 groups (Fig. 3B). In all groups, the fusion angle appeared to plateau by day 3, with only slight increases after this point. Tube length (Fig. 3C) remained relatively constant over time, although tubes in the 3–7 group were significantly longer overall, and the 5–7 group was significantly shorter than the other 2 groups. Significant differences were not observed in tube thickness (Fig. 3D), although tubes appear to thin slightly over time. These results are consistent with duplicate studies performed both with human aortic SMCs and human coronary artery SMCs (Supplementary Figs. S1 and S2).
FIG. 3.
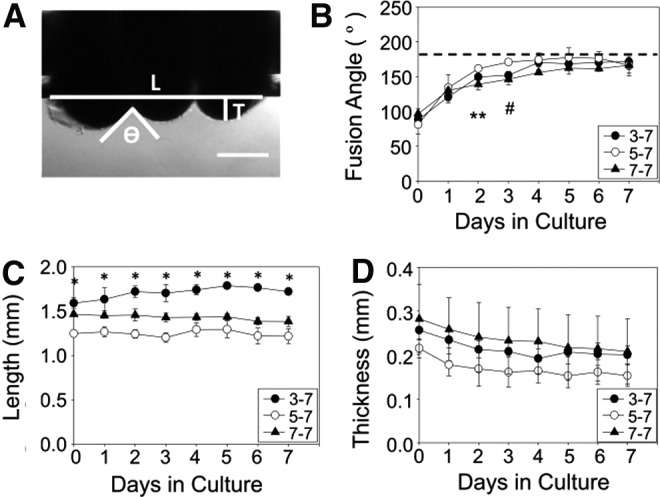
Fusion kinetics of human SMC rings. Three human SMC rings were threaded onto silicone tubing mandrels (A). The angle between rings (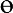 ), tube length (L), and thickness (T) was measured for each sample on each day of culture (A). Fusion angles (B), tube length (C), and thickness (D) as a function of time for tubes fabricated from rings cultured for 3 (3–7), 5 (5–7), or 7 (7–7) days before 7 days of fusion culture. N = 3 tubes per group. Data points are mean ± SD. #p < 0.05 for 5–7 versus 3–7 and 7–7, ** p < 0.05 for 5–7 versus 7–7, *p < 0.05. Scale = 0.5 mm.
), tube length (L), and thickness (T) was measured for each sample on each day of culture (A). Fusion angles (B), tube length (C), and thickness (D) as a function of time for tubes fabricated from rings cultured for 3 (3–7), 5 (5–7), or 7 (7–7) days before 7 days of fusion culture. N = 3 tubes per group. Data points are mean ± SD. #p < 0.05 for 5–7 versus 3–7 and 7–7, ** p < 0.05 for 5–7 versus 7–7, *p < 0.05. Scale = 0.5 mm.
Structure and morphology of fused human SMC tubes
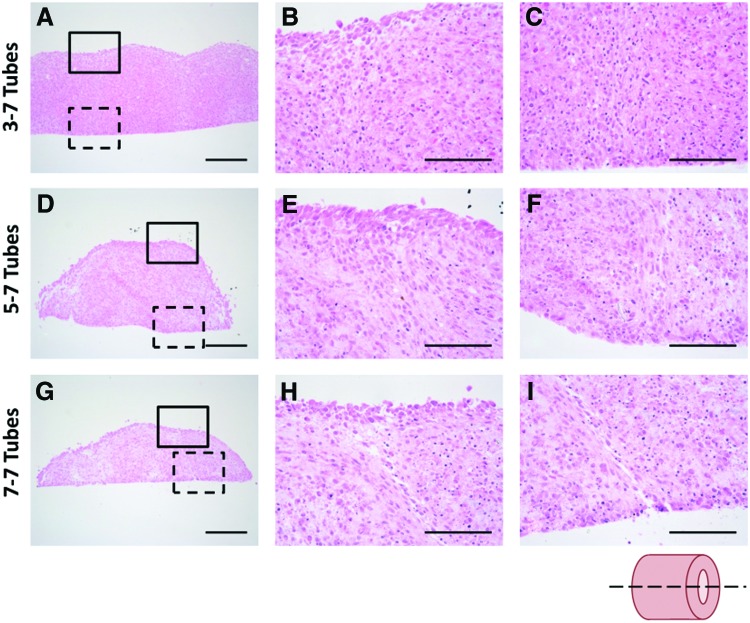
To evaluate fusion of SMC ring units, tissue tube sections were stained with hematoxylin and eosin to compare morphology of the 3–7, 5–7, and 7–7 tubes. Tubes appeared well fused after a 7-day fusion period, although ring boundaries remained detectable in all groups (Fig. 4). Ring boundaries are most distinct in the 7–7 group. Nearly seamless fusion was observed in the 3–7 group, although ridges at ring boundaries were still slightly visible on the tube exterior (Fig. 4). This suggests that rings precultured for a shorter duration before tube fabrication may allow for more complete tissue fusion.
FIG. 4.
Histological assessment of human SMC tubes. H&E-stained tissue tubes comprised rings precultured for 3 (A–C), 5 (D–F), or 7 (G–I) days before fusion. Low magnification longitudinal sections shown in (A, D, G). Higher magnification views show one fusion point at the outer surfaces (solid box; B, E, H) and at the inner surfaces (dashed box; C, F, I) of the tissue tubes. Lumen on bottom, scale bars = 250 μm (low magnification) or 100 μm (high magnification). Images representative from n = 3 samples/group. Sectioning schematic shown in lower right. Color images available online at www.liebertpub.com/tea
Spatial positioning of SMCs within rings during fusion
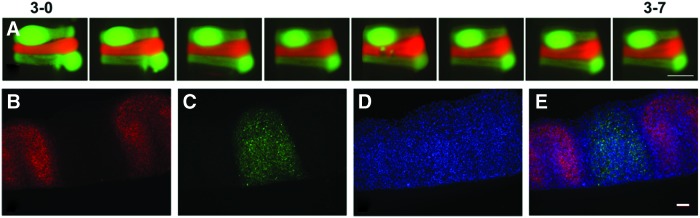
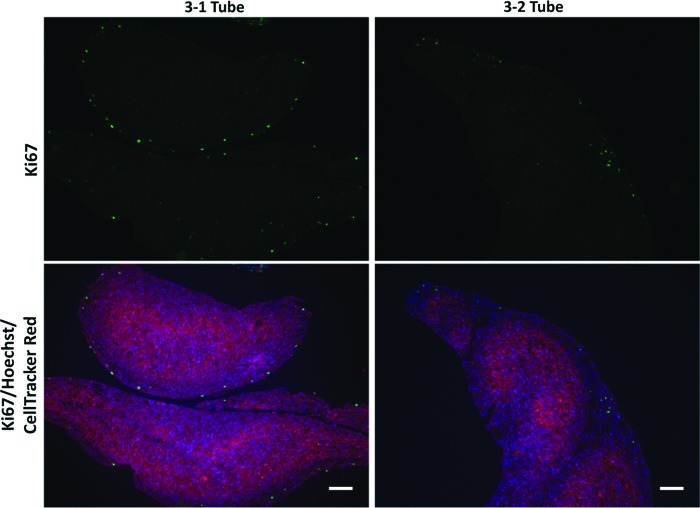
To assess the feasibility of creating tubes with distinct tissue regions along the tube length, we next evaluated whether cells within ring units maintain their spatial position along tissue tubes after ring fusion. Three-day-old human aortic SMC rings were created from green or red CellTracker-labeled cells. Alternating red and green fluorescently labeled rings were fused in culture for 7 days, and images were acquired daily. We did not observe “mixing” of cells at the ring borders over the culture period (Fig. 5A). This observation was confirmed when tubes fused for 7 days were examined histologically and stained with Hoechst (Fig. 5B–E). Similar results were observed when the experiment was repeated with coronary artery SMCs (Supplementary Fig. S3). Although some tissue compaction was visible, Hoechst-stained sections clearly show that cells within rings maintain their original spatial position after tube fusion. Some decrease in CellTracker signal was distinguishable at ring edges after fusion (Fig. 5E). We hypothesized that this was due to cellular proliferation at ring edges during fusion, which may dilute CellTracker signal. To test this, rings loaded with only CellTracker red dye were fused, and were fixed after either 1 or 2 days of fusion. Rings fused for only 1 day partially separated during processing, indicating they were not fully fused. Ki67 staining of these sections shows proliferating cells around the edges of individual rings (Fig. 6A, B). After 2 days of fusion, fewer proliferating cells are visible, and are predominately at the tube surfaces and not between individual rings (Fig. 6C, D). This suggests that cell proliferation at the edges of the rings may cause the decrease in CellTracker signal, and may play a role in initial ring fusion.
FIG. 5.
Spatial position of rings during fusion. Human aortic SMCs were preloaded with red or green CellTracker dye before ring seeding. Rings with alternating dyes were then stacked and allowed to fuse for 7 days (A). Tubes were then sectioned and stained with Hoechst dye. Red = CellTracker Red (B), green = CellTracker Green (C), and blue = nuclei (D). Merged image shown in (E). Scale = 1 mm (A) or 100 μm (B–E). Lumen on bottom (B–E). Images representative from n = 3 samples. Color images available online at www.liebertpub.com/tea
FIG. 6.
Cell proliferation during fusion. Human aortic SMCs were preloaded with red CellTracker dye before ring seeding. Rings were allowed to fuse for 1 (3-1 Tube) or 2 (3-2 Tube) days. Tubes were then sectioned and stained for Ki67 to examine proliferation. Green = Ki67, red = CellTracker Red, blue = nuclei. Scale = 100 μm. Images representative of n = 2 samples. Color images available online at www.liebertpub.com/tea
PCL cannulation cuffs and dynamic tube culture
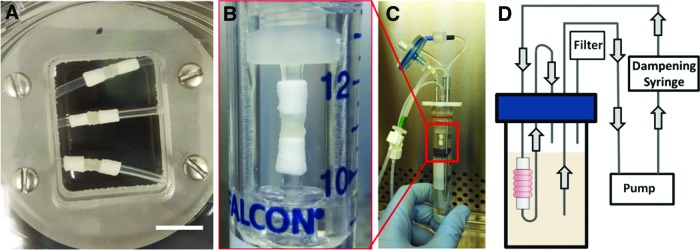
The next step in generating an in vitro TEBV model is applying luminal flow, which is critical for maintaining blood vessel function.34–36 However, self-assembled tissues can be fragile at early time points in culture, and may not withstand handling or suturing forces necessary to load the tissue into a flow bioreactor. Thus, our modular system for vascular tissue fabrication includes electrospun PCL cannulation cuffs incorporated onto each end of the tube by cellular attachment and infiltration from adjacent cell rings.29 Previously, we incorporated PCL cuffs into tubes made from rat aortic SMCs in static experiments.29 In this study, we assessed the feasibility of incorporating PCL cannulation cuffs into human aortic SMC tubes to serve as reinforced extensions to aid in cannulation and dynamic culture (Fig. 7A). After 7 days of fusion (3 days of ring preculture), we removed fused human tissue tubes from silicone tubing and mounted them onto a custom bioreactor to demonstrate that tubes are strong enough to withstand luminal flow. Cannulation cuffs fit snugly over bioreactor cannulas, and did not require additional suturing, as shown in Figure 7B.
FIG. 7.
PCL cannulation cuff incorporation for bioreactor culture. Electrospun PCL cuffs were threaded onto silicone tubing and pushed into contact with cell rings at each end of the tube. Tubes were cultured for 7 days on silicone mandrels (A) to achieve ring fusion, and then mounted onto the cannulas in the chamber of a custom luminal flow bioreactor (B). Image of bioreactor with SMC tube is shown in (C), and a schematic of the medium flow loop is shown in (D). Scale = 1 cm. Color images available online at www.liebertpub.com/tea
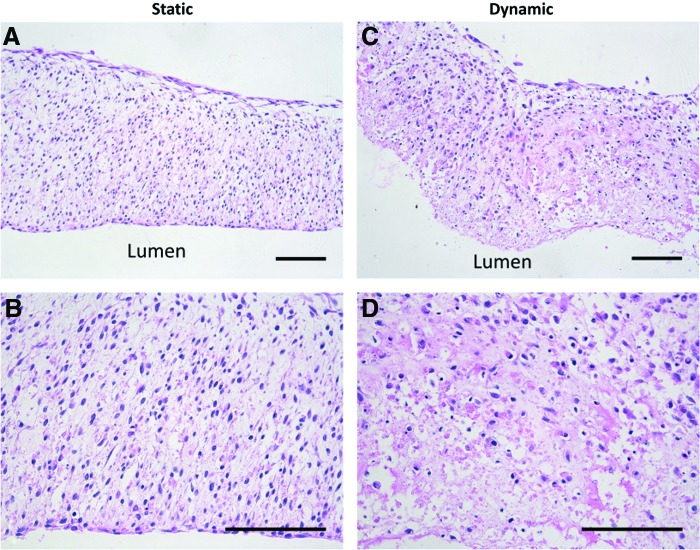
The bioreactor used in these studies was modified from Piola et al.30 An image of the bioreactor with a cannulated SMC tube inside is shown in Figure 7C, and a schematic of the bioreactor flow loop is shown in Figure 7D. Five tubes were successfully mounted onto bioreactors and cultured for an additional 7 days (17 days total culture) under luminal flow (12 dyne/cm2). Tubes fixed for histology are shown in Figure 8, which demonstrates that tubes remained intact and rings are fully fused. Ring boundaries are almost indistinguishable in both static (on silicone mandrels) and dynamically cultured tubes. Tubes exposed to flow appeared to have fewer cell nuclei on the luminal surface of the tube than static controls.
FIG. 8.
Histological images of tubes cultured in a luminal flow bioreactor. Hematoxylin and eosin stain of longitudinal section of tissue tubes cultured as rings for 3 days, fused as tubes for 7 days, and then cultured on silicone mandrels in static conditions (A, B) or with ∼12 dyne/cm2 shear stress (C, D) for an additional 7 days. Lumen at bottom of image. Scale = 100 μm. Color images available online at www.liebertpub.com/tea
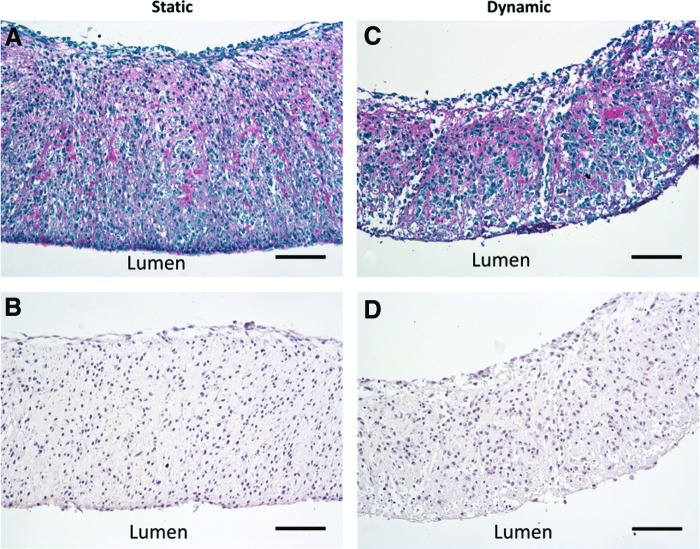
When this experiment was repeated, one tube out of six tore during loading, but the remainder of the tubes were cultured successfully for 7 days. Picrosirius red/fast green and orcein stains were used to examine extracellular matrix (ECM) deposition of fused tubes following static (Fig. 9A, B) or dynamic (Fig. 9C, D) culture. Collagen deposition is visible throughout tubes (Fig. 9A, C). Elastic fibers were not visible (Fig. 9B, D).
FIG. 9.
Matrix deposition in fused tissue tubes. Longitudinal sections of tubes cultured in static conditions for 14 days (A, B), or in static conditions for 7 days followed by 7 days of dynamic culture with ∼12 dyne/cm2 of applied shear (C, D). Picrosirius red fast green stain shown in (A, C; red = collagen, green = counterstain), orcein stain shown in (B, D; dark pink = elastic fibers, purple = nuclei). Lumen on bottom of image. Scale = 100 μm. Color images available online at www.liebertpub.com/tea
Incorporation of degradable gelatin microspheres to fabricate focal heterogeneities in fused tissue tubes
An important step toward modeling focal vascular diseases is the ability to create spatially controlled heterogeneities within engineered vessel walls. To do this, we incorporated degradable gelatin microspheres within three rings (with CellTracker Red dye) and positioned them in a central region of the tube, between rings without microspheres (eight per side, Fig. 10A). The region with incorporated microspheres is clearly visible due to CellTracker Red dye and genipin crosslinked microspheres, which both impart a purple hue to the tissue in these regions. Histological analysis demonstrated fully fused tubes with regions of microsphere incorporation within a localized region of the tube (Fig. 10B, C).
FIG. 10.
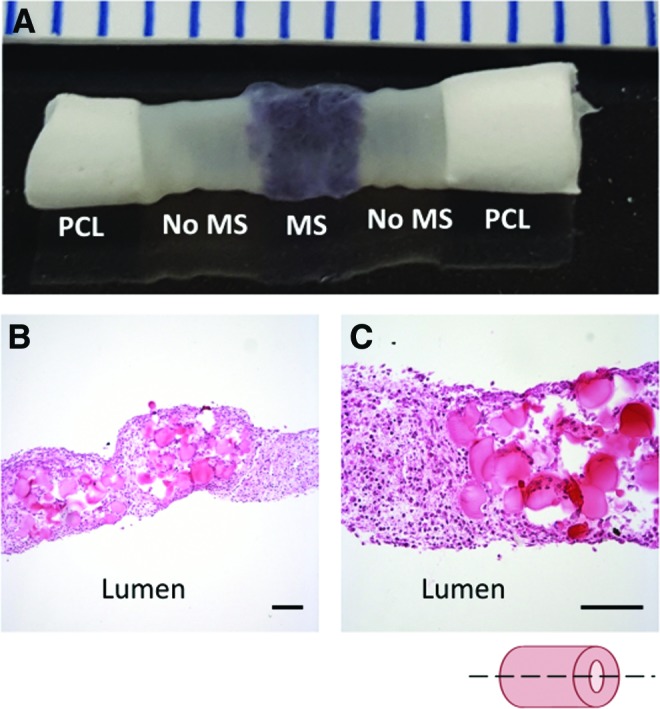
Human SMC tube with spatial heterogeneity. Human aortic SMC rings were either loaded with gelatin microspheres and red CellTracker dye, or without microspheres or dye. Rings with microspheres were placed in the central region of the tube, between outer regions without microspheres. Photograph of fused tissue tube shown in (A). PCL cuffs on either end reinforce the tube ends to aid in handling and cannulation. Low (B) and high (C) magnification H&E images of the vessel wall show that rings appear well fused, and microspheres maintain their spatial position in the center. Lumen on bottom of image (B, C). Scale in mm (A) or 100 μm (B, C). Images representative of two samples. Color images available online at www.liebertpub.com/tea
Fabrication of tissue tubes from human MSC rings
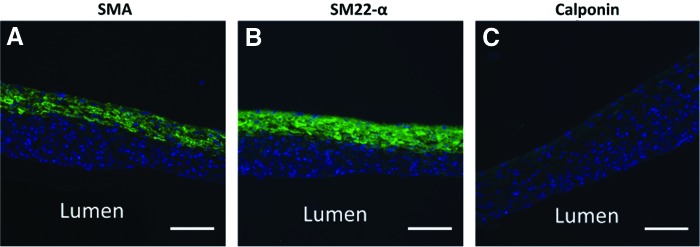
When fabricating engineered blood vessels, it is important that cells express smooth muscle proteins, indicative of a healthy, contractile SMC phenotype. We did not observe contractile protein expression in tubes fabricated from primary aortic SMCs (Supplementary Fig. S4). Therefore, we decided to explore alternative SMC sources. Human MSCs are highly proliferative and are well established to differentiate into contractile SMCs with treatment of TGF-β1 and BMP-4.37 In this study, we demonstrated that rings fabricated from bone marrow-derived hMSCs can be fused into tubes, which were strong enough to handle after 7 days of fusion. Figure 11 shows contractile protein expression of tubes after 7 days of fusion. SMA and SM22-α expression are clearly visible, although calponin appears limited. Contractile proteins are predominantly localized to the tube outer edge.
FIG. 11.
Contractile protein expression in fused hMSC tubes. Tubes were fabricated from 3-day-old hMSC rings, which were allowed to fuse for 7 days in static conditions. Green = SMA (A), SM22-α (B), or calponin (C), blue = nuclei. Lumen on bottom of image. Scale = 100 μm. hMSC, human mesenchymal stem cell. Color images available online at www.liebertpub.com/tea
Discussion
The long-term goal of these studies is to develop a platform for fabricating 3D human vascular tissue that may potentially be used for in vitro modeling of focal vascular diseases such as intimal hyperplasia or aneurysm. We previously described a method to rapidly generate vascular tissue tubes from individual self-assembled SMC ring units.25,26 In this study, we reduced the total time needed to create cohesive tissue tubes from self-assembled rings, and utilized the modular nature of this system to create focal heterogeneities within the tube wall. Furthermore, we incorporated PCL cannulation cuffs29 on each end, making the tubes amenable for cannulation and dynamic culture within a custom-designed luminal flow bioreactor. In future studies, this unique modular platform may be modified to model a variety of vascular diseases.
The first goal of this study was to improve and accelerate tissue fusion. In our studies, fusion is defined as an increase in fusion angle (angle between adjacent rings) to ∼180°.31,33 While we did not observe significant differences in fusion rate with varying preculture time, histological images suggest more complete fusion in the 3–7 group. This is consistent with other reports, which suggest tissues fuse more rapidly and more completely when precultured for less time before fusion.31 Others have suggested that tissues with increased ECM (such as collagen) are generally more cohesive and difficult to remodel.38 It is possible that increased matrix deposition at later time points may be a reason why more mature tissues fuse less completely than less mature tissues.
Additionally, fusion angle, parameters such as thickness and length of constructs can be used to assess fusion.31 We did not observe significant changes in length over time, which is contrary to previous reports of spheroid fusion.31,39 This may be due to differences in tissue size or geometry, which are known to affect fusion,20,31,33 or due to differences in cell type. Overall, 3–7 tubes were significantly longer than the 5–7 and 7–7 groups, and the 5–7 tubes were significantly shorter. This may be due to ring remodeling, compaction, and thinning over time during ring culture, resulting in the 5–7 and 7–7 groups being constructed from thinner rings.
A primary goal of varying preculture time was to determine a time course for ring fusion, and develop cohesive tissue tubes in a minimal amount of time. In all experiments, fusion angles plateaued after 3–4 days, which is consistent with other reports.20,23,33 In preliminary studies, we aimed to begin dynamic culture at this point, based on the observation that tubes are fully fused. However, when tubes were fused for only 4 days, 66% of the tubes tore during the cannulation procedure (data not shown). Thus, we increased fusion time to 7 days to allow for increased ECM deposition, which improved our ability to cannulate tubes and substantially reduced tube failure rates to an average of 8%. Importantly, this is still less time than described in most other published reports, where engineered vascular tissue is typically matured 2 weeks to several months in static culture before mounting on bioreactors for dynamic culture,12,40,41 compared to the 3 days of ring preculture and 7 days of fusion culture used in this study. This may be because cell-derived tissues can have enhanced ECM production and tissue strength compared to tissue fabricated using degradable scaffold materials.42–46 Additionally, PCL cannulation cuffs aided in handling and cannulation of tissue tubes at early time points.
To further evaluate fusion, we examined if cells maintain spatial positioning within rings during fusion. We observed that rings with red and green CellTracker dye are still spatially distinct after 7 days of fusion. This result is consistent with previous reports examining fusion of tissue sheets and spheroids, which showed that limited cellular migration or “mixing” is evident between most fusing tissues, despite some tissue remodeling and compaction.20,33,47–50 Because cells within ring units maintained their spatial positioning along tubes, we can customize individual rings and place them in distinct regions of the tube before fusion (Fig. 1). This feature may enable us to model focal disease pathologies in future studies, by engineering regions of tissue that contain a diseased cell phenotype in the middle of an otherwise healthy vascular tissue tube. CellTracker dye signal was less visible around individual ring edges, possibly due to the dye diluting as cells proliferate. We verified this by staining tubes for Ki67 after 1 or 2 days of fusion. Ki67 staining was visible predominantly around individual ring edges after 1 day of fusion. This was also evident at day 2, although fewer cells were Ki67 positive, likely due to contact inhibition of SMC proliferation. This suggests that proliferation may play a role in tissue fusion.
Shear forces created from fluid flow are important for the progression of many vascular diseases.51,52 Thus, it is critical to incorporate luminal flow during early culture of engineered vascular disease models. However, self-assembled, scaffold-free tissues may be too fragile at early time points in culture to be sutured onto cannulas for dynamic culture. The modular nature of our system is conducive to adding biomaterial units on either end of the tissue tube to serve as reinforced extensions, without affecting tissue structure. Previously, we evaluated the incorporation of PCL cannulation cuffs with tubes fabricated from rat aortic SMCs in static culture.29 In this study, we applied this technology to human TEBV constructs, enabling the successful cannulation of vascular tissue tubes in a custom bioreactor for dynamic culture within 10 days of cell seeding. In these studies, human aortic SMC tubes remained intact in a proof-of-concept experiment for 7 days of dynamic culture under physiologically relevant wall shear stress. It was not surprising that fewer nuclei were observed on the luminal surface of the dynamically cultured tube, as the endothelium typically prevents SMCs from being exposed directly to shear forces. Others have reported that direct exposure to shear stress can trigger SMC apoptosis.53 Future studies will focus on establishing a functional endothelial layer by developing a luminal cell seeding system for our custom bioreactor.
Additionally, the bioreactor chamber can also be easily modified to separate the luminal vessel compartment from the external medium compartment.54 In future studies, this will allow endothelialization of cannulated tissue tubes, and enable us to flow vasoactive substances through the tube lumen for endothelial and SMC functional testing. Alternatively, this bioreactor can also be modified to apply cyclic stretch to tissue tubes30 to enable mechanical conditioning during tissue tube culture and maturation.
We reported previously that incorporated gelatin microspheres can be used to locally deliver growth factors within SMC rings, for the purpose of controlling SMC phenotype.27 The gelatin microspheres degrade within ∼2 weeks, and do not adversely affect ring mechanical strength.27 In this study, we demonstrated that rings containing microspheres can be localized to a central region of the tissue tubes and successfully fuse with unmodified rings to create a focal heterogeneity (Fig. 10). This ability to create focal changes is a unique attribute of our system, as other methods for fabricating self-assembled TEBVs only create homogenous tubes.
In studies with primary aortic SMCs, we did not observe contractile smooth muscle markers such as SMA (Supplementary Fig. S4), or elastin deposition (Fig. 9). This is not surprising, since cells were cultured in a growth medium designed to support SMC proliferation and ECM deposition consistent with a synthetic SMC phenotype. We have previously observed that TGF-β1 treatment can stimulate contractile protein expression in 2D SMC cultures, but not in 3D rings or tubes (not shown). Creating a healthy, contractile SMC phenotype will be critical for fabricating functional vascular tissue in the future. Thus, we began exploring alternative cell sources, including hMSCs. We demonstrated in preliminary studies that tubes fabricated from hMSCs express the markers SMA and SM22-α (Fig. 11A, B), although calponin is not visible. The absence of late-stage differentiation markers is unsurprising, due to the short duration of the experiment. In an earlier study, we found that when TGF-β1 and BMP-4 were added at the beginning of ring and tube culture, rings did not fuse successfully (not shown). Thus, in this study, the differentiation factors (TGF-β1 and BMP-4) were added after 4 days of fusion (3 days total of growth factor treatment), which may also explain the absence of calponin (Fig. 11C, D).
Cell alignment is also important for creating functional vessels, as radially aligned cells give tubes the ability to constrict and dilate to regulate blood flow in vivo. In an hMSC tube cross-section, it is apparent that nuclei are radially aligned around tube outer edges, but not the middle region of the tissues (Fig. 12). Alignment may improve in future studies with mechanical stimulation.55 Human MSCs are highly proliferative and reliably differentiate to an SMC phenotype in 3D cultures, and hMSC rings fuse into tubes strong enough to handle after 4 days of fusion, making them an ideal cell type for future experiments. Additionally, hMSCs may allow for the fabrication of patient-specific tissue tubes to model vascular disease, as shown in principle in previous work with induced pluripotent stem cell-derived vascular SMCs (iPSC-VSMCs).13
FIG. 12.
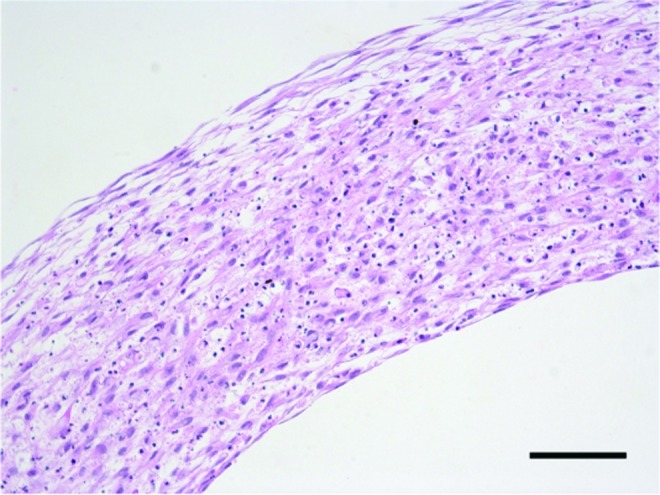
Alignment of hMSCs within hMSC tubes. Radial cross-section of hMSC tube after 4 days of fusion. Hematoxylin and eosin stain. Lumen on bottom of image. Scale = 100 μm. Color images available online at www.liebertpub.com/tea
In future studies, we will use growth factor-loaded microspheres, different cell types, or genetically modified cells to generate vessels with regions of cells that are compositionally distinct from adjacent regions. For example, growth factor-loaded microspheres may be able to create a hyperproliferative region for modeling intimal hyperplasia. Our ability to fabricate rings and tubes from a variety of SMC sources demonstrates the potential of this system to model focal diseases that manifest in various types of vessels. We have shown that we can produce rings using iPSC-VSMCs both from healthy patients (that produce elastin) and from patients with genetic disorders leading to elastin deficiencies.13 Using iPSC-VSMC rings to create a localized region of elastin deficiency may allow us to model aneurysm. We have also applied this modular tube fabrication system to create engineered cartilage rings and connective tissue rings with immature vascular structures, and fused these rings to engineer tracheal tissue that anastomosed with host vasculature upon subcutaneous implantation in a mouse model.49 The controlled release of growth factors through microsphere incorporation within individual rings may be ideal for such multitissue tubular structures, where different tissue regions may require different biochemical stimuli to maintain their differentiation and function. The ability to create high fidelity in vitro human tissue models may provide an invaluable tool for high-throughput drug screening, and potentially accelerate the development of new therapeutics.
In this study, we developed a modular system for fabricating focal heterogeneities within vascular tissue tubes, which may be modified in the future to model focal pathologies. Rings maintained their spatial position within tubes, which allowed us to generate localized regions of microsphere incorporation. After only 7 days of fusion, tubes were cohesive enough to be cannulated and cultured on a luminal flow bioreactor. Overall, this work will serve as a platform technology for fabricating engineered blood vessels with localized vascular diseases such as intimal hyperplasia, aneurysm, and atherosclerosis. Such in vitro disease models may serve as tools for high-throughput drug screening, and accelerate the development of new treatments for vascular diseases.
Supplementary Material
Acknowledgments
We gratefully acknowledge Jung-Youn Shin for fabricating gelatin microspheres, Jyotsna Patel for her assistance with histology, Allyson Mills, Kathy Suqui, Leonela Vega, and Hanna Gru Sihman for their assistance with image acquisition and analysis, and Emily Caron for optimizing hMSC medium. This work was supported by NSF IGERT DGE 1144804 (MWR, HAS), NIH R15 HL097332 (MWR, TH), NIH R01 AR063194 (EA), 1R01 EB023907-01 (EA, MWR, HAS), and NIH R15 HL137197 (MWR, HAS).
Disclosure Statement
The authors have no competing financial interests.
References
- 1.Go A.S., Mozaffarian D., Roger V.L., et al. . Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129, e28, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stegemann J.P., and Nerem R.M. Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two- and three-dimensional culture. Exp Cell Res 283, 146, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Alexander J.H., Hafley G., Harrington R.A., et al. . Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: A randomized controlled trial. JAMA 294, 2446, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Kim F.Y., Marhefka G., Ruggiero N.J., Adams S., and Whellan D.J. Saphenous vein graft disease: review of pathophysiology, prevention, and treatment. Cardiol Rev 21, 101, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Wystrychowski W., McAllister T.N., Zagalski K., Dusserre N., Cierpka L., and L'Heureux N. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J Vasc Surg 60, 1353, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Hibino N., McGillicuddy E., Matsumura G., et al. . Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 139, 431, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Lawson J., Dahl S., Prichard H., et al. . Human tissue-engineered grafts for hemodialysis: development, preclinical Data, and early investigational human implant experience. J Vasc Surg 59, 32S, 2014 [Google Scholar]
- 8.McAllister T.N., Maruszewski M., Garrido S.A., et al. . Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet 373, 1440, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Truskey G.A., and Fernandez C.E. Tissue-engineered blood vessels as promising tools for testing drug toxicity. Expert Opin Drug Metab Toxicol 11, 1021, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.L'Heureux N., Stoclet J.-C., Auger F.A., Lagaud G.J.-L., Germain L., and Andriantsitohaina R. A human tissue-engineered vascular media: a new model for pharmacological studies of contractile responses. FASEB J 15, 515, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Fernandez C.E., Yen R.W., Perez S.M., et al. . Human vascular microphysiological system for in vitro drug screening. Sci Rep 6, 21579, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung Y., Ji H., Chen Z., et al. . Scaffold-free, human mesenchymal stem cell-based tissue engineered blood vessels. Sci Rep 5, 15116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dash B.C., Levi K., Schwan J., et al. . Tissue-engineered vascular rings from human iPSC-derived smooth muscle cells. Stem Cell Rep 7, 19, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert J., Weber B., Frese L., et al. . A three-dimensional engineered artery model for in vitro atherosclerosis research. PLoS One 8, e79821, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niklason L.E., Gao J., Abbott , et al. . Functional arteries grown in vitro. Science 284, 489, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Roh J.D., Sawh-Martinez R., Brennan M.P., et al. . Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A 107, 4669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swartz D.D., Russell J.A., and Andreadis S.T. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol 288, H1451, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Homme J.L., Aubry M.C., Edwards W.D., et al. . Surgical pathology of the ascending aorta: a clinicopathologic study of 513 cases. Am J Surg Pathol 30, 1159, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Nesi G., Anichini C., Tozzini S., Boddi V., Calamai G., and Gori F. Pathology of the thoracic aorta: a morphologic review of 338 surgical specimens over a 7-year period. Cardiovasc Pathol 18, 134, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Norotte C., Marga F.S., Niklason L.E., and Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30, 5910, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mironov V., Visconti R.P., Kasyanov V., Forgacs G., Drake C.J., and Markwald R.R. Organ printing: tissue spheroids as building blocks. Biomaterials 30, 2164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakab K., Norotte C., Marga F., Murphy K., Vunjak-Novakovic G., and Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2, 022001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan Y., Richards D.J., Trusk T.C., et al. . 3D printing facilitated scaffold-free tissue unit fabrication. Biofabrication 6, 1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twal W.O., Klatt S.C., Harikrishnan K., et al. . Cellularized microcarriers as adhesive building blocks for fabrication of tubular tissue constructs. Ann Biomed Eng 42, 1470, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gwyther T.A., Hu J.Z., Christakis A.G., et al. . Engineered vascular tissue fabricated from aggregated smooth muscle cells. Cells Tissues Organs 194, 13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gwyther T.A., Hu J.Z., Billiar K.L., and Rolle M.W. Directed cellular self-assembly to fabricate cell-derived tissue rings for biomechanical analysis and tissue engineering. J Vis Exp 57, e3366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strobel H.A., Dikina A.D., Levi K., Solorio L.D., Alsberg E., and Rolle M.W. Cellular self-assembly with microsphere incorporation for growth factor delivery within engineered vascular tissue rings. Tissue Eng A 23, 143, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strobel H.A., Calamari E.L., Alphonse B., Hookway T.A., and Rolle M.W. Fabrication of Custom Agarose Wells for Cell Seeding and Tissue Ring Self-Assembly Using 3D-Printed Molds. J Vis Exp 2018. [Epub ahead of print]; DOI: 10.3791/56618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strobel H.A., Calamari E.L., Beliveau A., Jain A., and Rolle M.W. Fabrication and characterization of electrospun polycaprolactone and gelatin composite cuffs for tissue engineered blood vessels. J Biomed Mater Res B Appl Biomater 106, 817, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Piola M., Prandi F., Bono N., et al. . A compact and automated ex vivo vessel culture system for the pulsatile pressure conditioning of human saphenous veins. J Tissue Eng Regen Med 10, E204, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Rago A.P., Dean D.M., and Morgan J.R. Controlling cell position in complex heterotypic 3D microtissues by tissue fusion. Biotechnol Bioeng 102, 1231, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Bhumiratana S., Eton R.E., Oungoulian S.R., Wan L.Q., Ateshian G.A., and Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. PNAS 111, 6940, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livoti C.M., and Morgan J.R. Self-assembly and tissue fusion of toroid-shaped minimal building units. Tissue Eng A 16, 2051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatzizisis Y.S., Coskun A.U., Jonas M., Edelman E.R., Feldman C.L., and Stone P.H. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 49, 2379, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Rzucidlo E.M., Martin K.A., and Powell R.J. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg 45 (Suppl. A), A25, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Hirschi K.K., Rohovsky S.A., and D'Amore P.A. PDGF, TGF-beta, and heterotypic cell–cell interactions mediate endothelial cell–induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 141, 805, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C., Yin S., Cen L., et al. . Differentiation of adipose-derived stem cells into contractile smooth muscle cells induced by transforming growth factor-β1 and bone morphogenetic Protein-4. Tissue Eng A 16, 1201, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Hajdu Z., Mironov V., Mehesz A.N., Norris R.A., Markwald R.R., and Visconti R.P. Tissue spheroid fusion-based in vitro screening assays for analysis of tissue maturation. J Tissue Eng Regen Med 4, 659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dean D.M., Napolitano A.P., Youssef J., and Morgan J.R. Rods, tori, and honeycombs: the directed self-assembly of microtissues with prescribed microscale geometries. FASEB J 21, 4005, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Syedain Z.H., Meier L.A., Bjork J.W., Lee A., and Tranquillo R.T. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials 32, 714, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong Z., and Niklason L.E. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J 22, 1635, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.L'Heureux N., Paquet S., Labbe R., Germain L., and Auger F.A. A completely biological tissue-engineered human blood vessel. FASEB J 12, 47, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Adebayo O., Hookway T.A., Hu J.Z., Billiar K.L., and Rolle M.W. Self-assembled smooth muscle cell tissue rings exhibit greater tensile strength than cell-seeded fibrin or collagen gel rings. J Biomed Mater Res A 101, 428, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashi K., and Tabata Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater 7, 2797, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Kelm J.M., Lorber V., Snedeker J.G., et al. . A novel concept for scaffold-free vessel tissue engineering: self-assembly of microtissue building blocks. J Biotechnol 148, 46, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Ahlfors J.E., and Billiar K.L. Biomechanical and biochemical characteristics of a human fibroblast-produced and remodeled matrix. Biomaterials 28, 2183, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Sasagawa T., Shimizu T., Sekiya S., et al. . Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials 31, 1646, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Shimizu T., Yamato M., Akutsu T., et al. . Electrically communicating three-dimensional cardiac tissue mimic fabricated by layered cultured cardiomyocyte sheets. J Biomed Mater Res 60, 110, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Dikina A., Alt D., Herberg S., et al. . A modular strategy to engineer complex tissues and organs. Adv Sci (Weinh), 2018. [Epub ahead of print]; doi: 10.1002/advs.201700402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solorio L.D., Phillips L.M., McMillan A., et al. . Spatially organized differentiation of mesenchymal stem cells within biphasic microparticle-incorporated high cell density osteochondral tissues. Adv Healthcare Mater 4, 2306, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samady H., Eshtehardi P., McDaniel M.C., et al. . Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation 124, 779, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Gusic R.J., Myung R., Petko M., Gaynor J.W., and Gooch K.J. Shear stress and pressure modulate saphenous vein remodeling ex vivo. J Biomech 38, 1760, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Apenberg S., Freyberg M.A., and Friedl P. Shear stress induces apoptosis in vascular smooth muscle cells via an autocrine Fas/FasL pathway. Biochem Biophys Res Commun 310, 355, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Piola M., Prandi F., Fiore G.B., et al. . Human saphenous vein response to trans-wall oxygen gradients in a novel ex vivo conditioning platform. Ann Biomed Eng 44, 1449, 2016 [DOI] [PubMed] [Google Scholar]
- 55.Standley P.R., Camaratta A., Nolan B.P., Purgason C.T., and Stanley M.A. Cyclic stretch induces vascular smooth muscle cell alignment via NO signaling. Am J Physiol Heart Circ Physiol 283, H1907, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.