Abstract
Outcomes among people living with HIV (PLWH) in New York City (NYC) remain suboptimal. To assess the potential role of the city's sexual health clinics (SHCs) in improving HIV outcomes and reducing HIV transmission, we examined HIV care status and its correlates among HIV-positive SHC patients in NYC. Clinic electronic medical records were merged with longitudinal NYC HIV surveillance data to identify HIV-positive patients and derive their retrospective and prospective HIV care status. Evidence of HIV care and viral load suppression (VLS) after clinic visit were considered outcomes. Logistic regression models were used to assess their correlates. A third of the 1045 PLWH who visited NYC SHCs in 2012 were out of HIV care (OOC) in the 12 months preceding the clinic visit, and were less likely than those previously in HIV care (IC) to have subsequent evidence of HIV care (42% vs. 72%) or VLS in the 12 months after the visit (39% vs. 76%). VLS was particularly low among patients diagnosed with ≥2 sexually transmitted infections (46%). The odds of VLS were lowest among those OOC before the clinic visit [versus those IC, adjusted odds ratio (aOR): 0.21, 95% confidence interval (CI): 0.16–0.29], non-Hispanic blacks (versus non-Hispanic whites, aOR: 0.58, 95% CI: 0.37–0.90), and residents of high-poverty neighborhoods (>30% vs. <10%, aOR: 0.51, 95% CI: 0.29–0.89). Our findings suggest that SHCs could serve as an intervention point to (re-)link PLWH to HIV care. Real-time provider alerts about patients' OOC status could help achieve that goal.
Keywords: : sexual health clinic, HIV care continuum, linkage to HIV care, viral load suppression, implementation science
Introduction
Many HIV-positive New Yorkers encounter wide-ranging barriers to HIV care engagement and viral load suppression (VLS), including health system factors, social factors, and individual risk factors.1,2 City and state efforts to diagnose HIV-positive persons and engage them in care have improved HIV outcomes. Nevertheless, in 2016, only 72% of newly diagnosed New Yorkers were linked to HIV medical care within 3 months of diagnosis, and only 84% of those receiving care achieved viral suppression.3 Disparities in outcomes persist throughout the HIV care continuum, with particularly low viral suppression rates among black and young (≤24 years) New Yorkers (81% and 70%, respectively, among those in care).3 In addition to suboptimal health outcomes on the individual level, lack of viral suppression contributes to ongoing transmission of HIV, a key metric of interest in New York State's (NYS) “Ending the Epidemic” effort to decrease the number of new HIV infections and achieve a decline in HIV prevalence in the state by 2020.4
HIV-positive persons with unsuppressed viral load (VL) and with incident sexually transmitted infections (STIs) are an important group with regard to preventing further spread of HIV. High proportions of New York City (NYC) sexual health clinic (SHC) patients report risk behaviors, such as condomless sex.5 Coinfection with other STIs may also render HIV-positive patients more infectious by augmenting replication and shedding of the virus.6–8 Further, STI diagnosis and/or incidence rates in the networks of some people living with HIV (PLWH) may be increasing, possibly, in part, as a result of increased screening associated with preexposure prophylaxis (PrEP),9 an unintended result of HIV risk reduction strategies such as substituting oral sex for anal sex by men who have sex with men,10 and individual-11 and community-level9 risk compensation associated with increased use of PrEP.
Nationwide, the important role of SHCs has been recognized in HIV testing and diagnosis,12–16 including as part of STI partner services programs,17 as well as increasingly post- and preexposure HIV prophylaxis.15 Estimates of linkage to HIV care among newly diagnosed patients, often suboptimal, have also been published.13,18,19 However, little is known about the HIV care continuum outcomes and their correlates among persons previously diagnosed with HIV seeking care for other STIs. NYC SHCs may have an opportunity to improve HIV care outcomes, particularly among patients not engaged in HIV care, for whom the visits may constitute a rare opportunity to (re-)link to HIV care. This analysis explores HIV care receipt and viral suppression among 1045 HIV-positive patients who sought services from an NYC SHC in 2012.
Methods
Study setting
The NYC Department of Health and Mental Hygiene (DOHMH) operates eight SHCs, which offer services to patients aged 12 years or older, regardless of insurance or immigration status. Most commonly provided services include STI testing and treatment as well as HIV testing, treatment, and counseling. Patients receiving STI evaluation and/or treatment may also be offered biomedical HIV prevention interventions, behavioral health services, contraception services, and select vaccinations. HIV testing is conducted on an opt-out basis; patients with an earlier HIV diagnosis may also be tested if they do not disclose their HIV status. Patients newly diagnosed with HIV, and those known to be previously diagnosed but not receiving HIV care, are referred to an HIV care provider. Shortly after the clinic visit, SHC staff contact both the HIV provider and the patient to determine whether a linkage appointment was kept and whether the patient has remained in care and make additional referrals as needed.
Data sources
We matched SHC electronic medical records (EMR) of patients who sought care at the clinics in 2012, and were documented in the EMR as HIV-positive, with longitudinal data from the NYC HIV Surveillance Registry. The registry contains information on all HIV and AIDS diagnoses (since 2001 and 1981, respectively) in NYC, as well as the results of HIV-related laboratory tests (CD4 count, VL, genotype) ordered by NYC providers for persons living in NYC.20 The deterministic matching algorithm has been described previously.21 For this study, 2012 EMR data were matched with HIV registry data for the 12 months before and after SHC visits, to calculate study exposures and outcomes (see “Exposures and Correlates of Outcomes” and “Outcomes” sections). In a related analysis,22 we matched a sample of SHC patients with an unknown HIV status to the registry, and few (5%) were determined to be PLWH, supporting the validity of this approach.
Data from the 2009 to 2013 American Community Survey were used to derive a neighborhood-level poverty measure, matched to patient addresses in the SHC EMR. Neighborhood-level poverty was defined as the percent of the population in a given ZIP code whose household income was below the federal poverty level and was categorized using standard cutoffs.23
Study sample
In 2012, 67,359 patients sought care at the NYC SHCs. Among them, 1563 were documented in the EMR as HIV-positive before or on the day of their SHC visit, based on an HIV-positive test at the SHCs or self-report, and were matched to the HIV registry. Among these patients, we identified persons (1) diagnosed with HIV for at least 12 months at the time of their SHC visit in 2012 (“index visit”), using the earliest HIV diagnosis date available in the registry or in the clinic EMR; (2) aged 15 years or older; and (3) residing in NYC or the NYC Metropolitan Statistical Area (Metropolitan NYC24) at the time of the index visit, resulting in the final sample of 1045 persons.
Exposures and correlates of outcomes
The main exposure was HIV care status before the index visit at a SHC. Persons “in HIV care” were those with two or more VL or CD4 results reported to the HIV registry and with specimen collection dates ≥90 days apart in the 12 months preceding the index visit.25,26 In addition, STI diagnosis at the index visit was examined as a binary (yes/no) and ordinal (no. of STIs) variable.
Other characteristics of interest at the time of index visit included sociodemographic {sex assigned at birth, age, residence (NYC proper or Metropolitan NYC), race/ethnicity, primary language spoken, ZIP-code poverty level: low [<10% at or below the federal poverty level (FPL)], medium (10 to <20%), high (20 to <30%), very high (≥30%)}, and epidemiological/behavioral factors (time since HIV diagnosis, transmission risk factor, number of sex partners in the past 3 months, number of SHC visits in the past 12 months, and receipt of an HIV test during the index visit—an indicator of failure to disclose HIV status to the provider and opt out of testing). HIV diagnosis and transmission risk factor information was extracted from the HIV registry, and all other information was obtained from the SHC EMR.
Outcomes
Evidence of HIV care after the index visit was defined as one or more VL or CD4 results reported to the registry in the 3 months following index visit. Three VL-related outcomes were examined in the 12 months after index visit: (1) receipt of VL result, (2) viral suppression (defined as last VL value ≤200 copies/mL; absence of a VL result was treated as absence of viral suppression), and (3) VL below the transmission threshold (<1500 copies/mL;27 absence of a VL result was treated as above the threshold).
Statistical analysis
Descriptive statistics, including chi-square tests for categorical variables and Mann–Whitney tests for continuous variables, were used to compare the distribution of covariates by patients' HIV care status before index visit. Logistic regression models were developed to assess the correlates of subsequent: (1) evidence of HIV care, among those out of HIV care (OOC) before the SHC visit, and (2) viral suppression following the index clinic visit, among all HIV-positive patients regardless of care status. All analyses were completed in SAS 9.3 (SAS Institute, Cary, NC).
The NYC DOHMH Institutional Review Board determined this activity was surveillance and not human subjects research.
Results
The majority of the 1045 patients had been assigned male sex at birth (93.8%), and were non-Hispanic black (52.1%) or Hispanic (27.5%). Approximately 11.1% reported a main language other than English and 65.3% resided in high- or very high-poverty neighborhoods. For three-quarters of the patients, the index visit was their only SHC visit in a 12-month period. Among those assigned male sex at birth, 73.7% reported being men who have sex with men (Table 1).
Table 1.
Characteristics of HIV-Positive New York City (NYC) Sexual Health Clinic (SHC) Patients Aged ≥15 at the Time of Index Visit in 2012, by HIV Care Status Before Index Visit (N = 1045)
| In HIV care before visit (N = 715), n (%) | Out of HIV care before visit (N = 330), n (%) | p | |
|---|---|---|---|
| Sociodemographic | |||
| Sex assigned at birth | |||
| Male | 672 (94.0) | 308 (93.3) | 0.685 |
| Female | 43 (6.0) | 22 (6.7) | |
| Age | |||
| Median (IQR) | 40 (31–48) | 34 (28–43) | <0.001 |
| 15–24 | 45 (6.3) | 37 (11.2) | <0.001 |
| 25–34 | 196 (27.4) | 139 (42.1) | |
| 35–50 | 353 (49.4) | 119 (36.1) | |
| >50 | 121 (16.9) | 35 (10.6) | |
| Race/ethnicity | |||
| White | 101 (14.1) | 54 (16.4) | 0.182 |
| Non-Hispanic black | 362 (50.6) | 182 (55.2) | |
| Hispanic | 208 (29.1) | 79 (23.9) | |
| Other | 44 (6.2) | 15 (4.6) | |
| Primary language spoken | |||
| English | 624 (87.3) | 304 (92.1) | 0.021 |
| Other | 91 (12.7) | 26 (7.9) | |
| Residence at the time of index visit | |||
| NYC | 707 (98.9) | 309 (93.6) | <0.001 |
| Outside NYC, but within Metropolitan NYC | 8 (1.1) | 21 (6.4) | |
| Neighborhood poverty level (%) | <0.001 | ||
| <10 | 73 (10.2) | 34 (10.3) | |
| 10–20 | 152 (21.3) | 75 (22.7) | |
| 20–30 | 298 (41.7) | 125 (37.9) | |
| >30 | 183 (25.6) | 76 (23.0) | |
| Unknown (Metropolitan NYC) | 9 (1.3) | 20 (6.1) | |
| Sexual health-related | |||
| Time since first known HIV diagnosis (years) | |||
| 1–3 | 95 (13.3) | 74 (22.4) | <0.001 |
| 4–6 | 144 (20.1) | 89 (27.0) | |
| >6 | 476 (66.6) | 167 (50.6) | |
| Transmission risk | |||
| Men who have sex with men | 517 (72.3) | 254 (77.0) | 0.300 |
| Injection drug use history | 61 (8.5) | 19 (5.8) | |
| Heterosexual contact | 49 (6.9) | 18 (5.5) | |
| Unknown | 88 (12.3) | 39 (11.8) | |
| Number of sex partners in past 3 months | 0.156 | ||
| 0 | 11 (1.5) | 2 (0.6) | |
| 1 | 234 (32.7) | 111 (33.6) | |
| 2–4 | 291 (40.7) | 126 (38.2) | |
| ≥5 | 113 (15.8) | 46 (13.9) | |
| Missing | 66 (9.2) | 45 (13.6) | |
| No. of SHC visits within 12 months earlier (including index visit) | |||
| 1 | 553 (77.3) | 254 (77.0) | 0.894 |
| ≥2 | 162 (22.7) | 76 (23.0) | |
| Index visit characteristics | |||
| HIV test receipt | |||
| Yes | 33 (4.6) | 40 (12.1) | <0.001 |
| No | 682 (95.4) | 290 (87.9) | |
| STI diagnosis | |||
| No | 414 (57.9) | 157 (47.6) | 0.002 |
| Yes | 301 (42.1) | 173 (52.4) | |
| >1 STI diagnosis (of total) | 40 (5.6) | 38 (11.5) | <0.001 |
IQR, interquartile range; NYC, New York City; SHC, sexual health clinic; STI, sexually transmitted infection.
p < 0.05 in bold.
Almost a third of the sample (330/1045; 31.6%) were OOC in the 12 months before the index visit. Compared to those in care (IC), patients who were OOC were younger (median, 34 vs. 40 years; p < 0.001), and a higher proportion were diagnosed with HIV in the previous 1–3 years (22.4% vs. 13.3%, p < 0.001). On the day of index visit, OOC patients were more likely than those IC to receive an STI diagnosis (52.4% vs. 42.1%, p = 0.002), including multiple STI diagnoses (11.5% vs. 5.6%, p < 0.001) and an HIV test (12.1% vs. 4.6%, p < 0.001) (Table 1).
Receipt of an HIV test was also more common among patients assigned female sex at birth (27.7% vs. 5.6% among those assigned male sex, p < 0.0001), those diagnosed between 1 and 3 years ago (13.6% vs. 5.7% among those diagnosed for 4 years or longer, p = 0.0007), those with heterosexual contact as main HIV risk factor (17.9% vs. 4.2% among men who have sex with men and those with injection drug use history, p < 0.0001), and those for whom this was their only STI clinic visit in the year leading up to it (8.7% vs. 1.3% among those with two or more visits, p < 0.0001).
Evidence of HIV care within 3 months after index visit
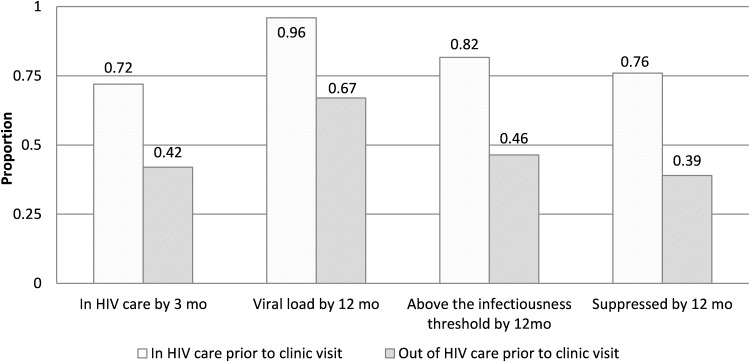
Overall, 654 of 1045 patients (62.6%) had evidence of HIV care within 3 months after the index visit. Compared to those who were engaged in HIV care before the index visit, patients who were previously OOC were less likely to have evidence of HIV care within 3 months after the index visit (72.3% [517/715] vs. 41.5% [137/330], p < 0.001) (Fig. 1). In multivariable analysis restricted to those who were OOC before the index visit, the odds of having subsequent evidence of care were higher among those assigned female versus male sex at birth [adjusted odds ratio (aOR): 2.9, 95% confidence interval (CI): 1.1–7.4] and lower among those with five of more sex partners in the preceding 3 months versus those with fewer than five partners (aOR: 0.42, 95% CI: 0.19–0.92) (Table 2).
FIG. 1.
Evidence of HIV care and viral load suppression after index visit among all patients, by HIV care status before visit.
Table 2.
Factors Associated with Evidence of HIV Care Within 3 Months After Index Visit, Among Those Out of HIV Care Before Index Visit (N = 330)
| Bivariate | Multivariable (n = 326) | ||
|---|---|---|---|
| N | OR (95% CI) | ||
| Sociodemographic | |||
| Sex assigned at birth | |||
| Male | 308 | Ref | Ref |
| Female | 22 | 3.38 (1.34–8.53)* | 2.86 (1.11–7.38)* |
| Age | |||
| 15–24 | 37 | 2.23 (1.07–4.65)* | 2.04 (0.96–4.33) |
| 25–34 | 139 | Ref | Ref |
| 35–50 | 119 | 1.60 (0.96–2.65) | 1.50 (0.89–2.52) |
| >50 | 35 | 0.99 (0.45–2.16) | 0.96 (0.43–2.13) |
| Race/ethnicity | |||
| White | 54 | Ref | |
| Non-Hispanic black | 182 | 1.29 (0.68–2.42) | |
| Hispanic | 79 | 1.62 (0.79–3.31) | |
| Other | 15 | 0.67 (0.19–2.39) | |
| Residence at the time of index visit | |||
| NYC | 309 | Ref | |
| Outside NYC, but within Metropolitan NYC | 21 | 0.56 (0.21–1.48) | |
| Sexual health-related | |||
| Time since first known HIV diagnosis (years) | |||
| 1–3 | 74 | Ref | |
| 4–6 | 89 | 0.69 (0.36–1.29) | |
| >6 | 167 | 0.69 (0.40–1.21) | |
| Transmission risk | |||
| Men who have sex with men | 254 | Ref | |
| Injection drug use history | 19 | 0.71 (0.26–1.95) | |
| Heterosexual contact | 18 | 3.20 (1.13–8.54)* | |
| Unknown | 39 | 1.20 (0.61–2.37) | |
| Number of sex partners in past 3 months | |||
| 0–1 | 113 | Ref | Ref |
| 2–4 | 126 | 0.71 (0.42–1.19) | 0.79 (0.47–1.35) |
| ≥5 | 46 | 0.36 (0.17–0.79)* | 0.42 (0.19–0.92)* |
| Missing | 45 | 1.27 (0.62–2.58) | 1.25 (0.61–2.58) |
| HIV test receipt at index visit | |||
| Yes | 40 | 2.53 (1.25–5.16)* | |
| No | 290 | ref | |
p < 0.05.
VL testing and suppression within 12 months after index visit
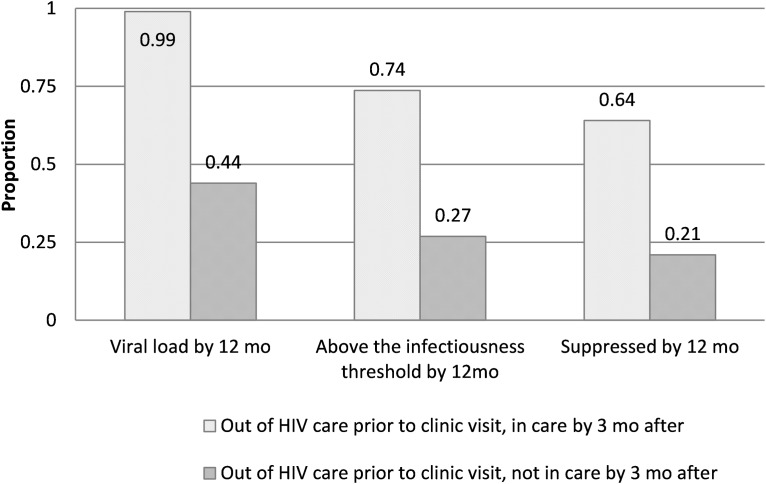
Within 12 months after the index visit, 86.8% of all patients (906/1045) had a VL measurement, 70.5% (737/1045) had a VL below the transmission threshold (<1500 copies/mL), and 64.0% (669/1045) were virally suppressed. A stark contrast in VLS was observed by HIV care status before the index visit: 75.5% of those IC (540/715), but only 39.1% of those OOC (129/330) in the 12 months before the index visit, were virally suppressed at last VL measurement in the 12 months after that visit (p < 0.001) (Fig. 1). Among those OOC before the index visit, those who also had no evidence of care within 3 months afterward were even less likely to have achieved viral suppression within 12 months after the visit than those with evidence of care within 3 months [21.2% (41/193) vs. 64.2% (88/137), p < 0.001] (Fig. 2). Viral suppression was lowest among those with two or more STIs diagnosed at the index visit [no STIs vs. ≥2 STIs: 67.8% (387/571) vs. 44.9% (35/78) suppressed, p = 0.001].
FIG. 2.
Viral suppression after index visit among patients previously out of HIV care, by subsequent care status.
In multivariable analysis, OOC status before the index visit remained strongly associated with lower odds of viral suppression in the 12 months after the visit (aOR: 0.21, 95% CI: 0.16–0.29). The odds were also lower among non-Hispanic black patients (vs. non-Hispanic white, aOR: 0.58, 95% CI: 0.37–0.90), among those living in high-poverty neighborhoods (>30% vs. <10%, aOR: 0.51, 95% CI: 0.29–0.89), and among those who received an HIV test at the index visit (aOR: 0.58, 95% CI: 0.34–1.0). Patients assigned female sex at birth had higher odds of viral suppression than those assigned male sex (aOR: 2.2, 95% CI: 1.2–4.1) (Table 3).
Table 3.
Factors Associated with Viral Suppression Within 12 Months After Index Visit (N = 1045)
| Bivariate | Multivariable | |||
|---|---|---|---|---|
| N = 1045 | N not suppressed = 376 | OR (95% CI) | ||
| Sociodemographic | ||||
| Sex assigned at birth | ||||
| Male | 980 | 357 | Ref | Ref |
| Female | 65 | 19 | 1.39 (0.80–2.40) | 2.18 (1.16–4.10)* |
| Age | ||||
| 15–24 | 82 | 40 | 0.78 (0.48–1.27) | 0.86 (0.50–1.46) |
| 25–34 | 335 | 143 | Ref | Ref |
| 35–50 | 472 | 152 | 1.57 (1.17–2.10)** | 1.15 (0.84–1.59) |
| >50 | 156 | 41 | 2.09 (1.38–3.17)** | 1.55 (0.98–2.45) |
| Race/ethnicity | ||||
| White | 155 | 43 | Ref | Ref |
| Non-Hispanic black | 544 | 219 | 0.57 (0.39–0.84)** | 0.58 (0.37–0.90)** |
| Hispanic | 287 | 87 | 0.88 (0.57–1.36) | 0.88 (0.54–1.43) |
| Other | 59 | 27 | 0.46 (0.24–0.85)** | 0.36 (0.18–0.70)** |
| Residence at the time of index visit | ||||
| NYC | 1016 | 360 | Ref | |
| Metropolitan NYC | 29 | 16 | 0.45 (0.21–0.94)** | |
| Primary language spoken | ||||
| English | 928 | 341 | Ref | |
| Other | 117 | 35 | 1.36 (0.90–2.07) | |
| Neighborhood poverty level (%) | ||||
| <10 | 107 | 31 | Ref | Ref |
| 10–20 | 227 | 86 | 0.67 (0.41–1.10) | 0.66 (0.38–1.15) |
| >20–30 | 423 | 130 | 0.92 (0.58–1.47) | 1.00 (0.59–1.70) |
| >30 | 259 | 113 | 0.53 (0.32–0.86)** | 0.51 (0.29–0.89)* |
| Unknown (Metropolitan NYC) | 29 | 16 | 0.33 (0.14–0.77)** | 0.60 (0.24–1.52) |
| Sexual health-related | ||||
| Time since first known HIV diagnosis (years) | ||||
| 1–3 | 169 | 79 | Ref | |
| 4–6 | 233 | 86 | 1.50 (1.00–2.24)* | |
| >6 | 643 | 211 | 1.80 (1.27–2.54)** | |
| HIV care status before index visit | ||||
| In care | 715 | 175 | Ref | Ref |
| Out of care | 330 | 201 | 0.21 (0.16–0.27)*** | 0.21 (0.16–0.29)*** |
| Transmission risk | ||||
| Men who have sex with men | 771 | 280 | Ref | |
| Injection drug use history | 80 | 31 | 0.90 (0.56–1.45) | |
| Heterosexual contact | 67 | 22 | 1.17 (0.69–1.98) | |
| Unknown | 127 | 43 | 1.11 (0.75–1.65) | |
| HIV test receipt at index visit | ||||
| Yes | 73 | 39 | 0.46 (0.29–0.75)** | 0.58 (0.34–1.00)* |
| No | 972 | 337 | Ref | Ref |
| STI diagnosis at index visit | ||||
| Yes | 571 | 192 | 0.70 (0.54–0.90)** | |
| No | 474 | 184 | Ref | |
Number of sex partners in past 3 months, number of clinic visits within a year earlier, type of clinic visit—not statistically significant and not included in table.
p < 0.05, **p < 0.01, ***p < 0.001.
Among virally unsuppressed patients, those who were OOC before the index visit were more likely to have VL above the transmission threshold (≥1500 copies/mL) than those previously IC [88.1% (177/201) vs. 74.9% (131/175)] in the year following the index visit.
Discussion
Approximately one-third of HIV-positive patients attending NYC SHCs were found not to be engaged in HIV care in the 12 months leading up to the clinic visit, and these patients were significantly less likely to achieve viral suppression in the 12 months following their visit than those previously in care. This suggests that SHCs, already recognized for linking large numbers of newly diagnosed patients to care nationwide,13 also have an important opportunity to (re-)link previously diagnosed patients to care. In the absence of recent population-based estimates on the HIV care status of HIV-STI coinfected New Yorkers, the 330 OOC patients seen at the SHCs in a 12-month period can also provide insight into the characteristics of patients who are OOC.
An intervention at the point of the SHC visit to improve the staff's ability to identify OOC patients and thereby offer them available (re-)linkage services could lower the risk of onward HIV transmission. OOC patients were more likely than those IC to be diagnosed with STIs at the index visit, and less than half of patients positive for STIs achieved viral suppression after the clinic visit. Considering the potential intersection of active STIs and subsequent failure to achieve viral suppression among patients OOC at the time of SHC visit, facilitating their return to HIV care could support New York's goal of lowering the number of new HIV infections.4
Intervention in the SHC setting could also help decrease persistent racial/ethnic and socioeconomic disparities in HIV care outcomes among its patient population. Patients who were OOC before the clinic visit, of non-Hispanic black race, or residing in very high-poverty neighborhoods were less likely to have evidence of subsequent VLS. NYC SHCs see a substantial number of such patients and are poised to facilitate (re-)linkage to care for vulnerable populations that may face a variety of barriers to healthcare and for whom HIV care outcomes are particularly poor.3 Expanded focus on (re-)linkage for persons previously diagnosed with HIV may have the potential to reduce disparities in HIV care outcomes and incidence outside NYC as well, as SHCs nationwide are a high-volume provider of HIV testing, diagnosis, and linkage services to underserved populations with substantial HIV prevalence.13,19,28
Despite all patients in our study having received HIV diagnoses at least 12 months before their SHC visit, 7% received an HIV test, suggesting that they did not opt out of testing or inform the provider about a previous diagnosis. These patients were more likely to have been born female, exposed to HIV via heterosexual contact, diagnosed with HIV within the previous 1–3 years, and be accessing clinic services for the first time within a year. Together with our recent finding that 5% of patients whose HIV status was unknown to SHC staff at the time of visit had in fact been previously diagnosed with HIV, and largely OOC before and/or after the visit,22 this points to potential barriers to (re-)linkage to care among patients who fail to disclose their HIV status to clinic staff, perhaps as a result of their desire to confirm a diagnosis received in relatively recent past. It is also possible that some clinic attendees were not aware of their HIV diagnosis, not having returned to receive their positive test result from the diagnosing provider.
However, even when (re-)linkage opportunities arise after a positive HIV test during the clinic visit, previously OOC patients remain at risk of suboptimal outcomes. Following the positive test, they would have received intensive (re)linkage assistance from the clinic staff. Nonetheless, while previously OOC persons who received an HIV test during their index visit were more likely to have evidence of care in the 3 months afterward than OOC patients who were not retested for HIV, they were less likely than those not tested to be virally suppressed within 12 months following the clinic visit, suggesting ongoing barriers to engagement in HIV care and VLS. Understanding why some HIV-positive patients do not disclose their HIV status to SHC providers could inform the development of interventions to increase reengagement in care and subsequent VLS in this group.
Ongoing improvements in collection of patients' self-reported HIV status at various points throughout the clinic visit (e.g., at registration, during clinical evaluation) and documentation in the EMR may facilitate identification of patients in need of (re-)linkage to HIV care. Providing SHC clinic staff with real-time access to the NYC HIV registry would enable systematic and rigorous identification of patients not receiving HIV care at the time of the clinic visit. Louisiana has implemented a system (Louisiana Public Health Information Exchange—LaPHIE) feeding real-time data from the statewide HIV surveillance registry to EMR at a network of participating facilities. Notifications about patients who have been OOC for at least a year and clinical support recommendations give clinicians an opportunity to intervene, leading to reengagement in care of over 80% of identified patients, for whom clinicians documented a (re-)linkage attempt in response to the alert.29,30 The system has received positive feedback from patients and has been expanded to encompass individuals needing tuberculosis follow-up,29 underscoring the wide applicability of streamlined health data exchanges.
Even though NYS law was expanded in 2014 to permit sharing of limited HIV surveillance data with healthcare providers for purposes of linkage to and retention in care (NYS Public Health Law § 2135),31 development of systems such as LaPHIE entails myriad complex confidentiality and technical issues, which can be time-consuming and costly. Therefore, other interventions should also be considered by SHCs and other high-volume STI diagnosing providers. Since outcomes were suboptimal regardless of previous HIV care status, interventions potentially applicable to both OOC and IC patients, such as brief case management, patient navigation, care coordination, closer linkage monitoring, and motivational interviewing,1,32 might help (re-)link more OOC patients to HIV care or prevent disengagement among those IC. Patient navigation and closer linkage monitoring have been implemented in NYC SHCs in recent years.
A strength of this study is the combination of two longitudinal data sources with rich clinical and behavioral data. Among limitations, the analysis likely misclassifies some participants as OOC, especially those not receiving HIV care exclusively in NYC or those who moved out of NYC in the 12 months after their clinic visit, potentially leading to an underestimate of patients engaged in care before their SHC clinic visit, and linked to care and virally suppressed after the visit. A 2008–2010 tracing study of patients presumed to be OOC based on NYC HIV registry data found that 4% of patients had moved out of jurisdiction, though an additional 14% could not be located, suggesting that the proportion who move out could be higher.33 However, because all patients in this analysis had evidence of at least two NYC-based HIV and sexual health encounters at least 12 months apart (previous HIV diagnosis or laboratories in the HIV registry, and index SHC visit), the proportion receiving HIV care out of jurisdiction in the months following their SHC visit is likely low.
In addition, due to the low sample size, we were unable to examine transgender patients as a separate group, even though they may face unique and/or compounded barriers to healthcare.34,35 Finally, although patients in this analysis resemble other NYC PLWH with respect to distribution of race/ethnicity and neighborhood poverty level, the sample is younger, with a greater proportion of persons assigned male sex at birth, and men who have sex with men.3 Therefore, findings may only be generalizable to PLWH seeking sexual healthcare.
In summary, NYC SHCs serve a large population of HIV-positive patients, many of whom have suboptimal HIV care outcomes before and/or after their clinic visits. SHC providers are not always aware of their patients' HIV and/or HIV care status, limiting their ability to (re-)link patients to care through referrals and follow-up. Providing real-time, systematic HIV registry information to providers could allow them to (re-)link some patients to HIV care. Improved provider ability to identify patients OOC, and strengthened or diversified (re-)linkage interventions at the time of and shortly after clinic visit, could improve individual outcomes among vulnerable individuals, and help to limit further spread of the infection.
Acknowledgments
This work was supported by the HIV Center for Clinical and Behavioral Studies at the NYS Psychiatric Institute and Columbia University; P30 MH043520/MH/NIMH NIH HHS/United States.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bauman LJ, Braunstein S, Calderon Y, et al. . Barriers and facilitators of linkage to HIV primary care in New York City. J Acquir Immune Defic Syndr 2013;64 Suppl 1:S20–S26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remien RH, Bauman LJ, Mantell JE, et al. . Barriers and facilitators to engagement of vulnerable populations in HIV primary care in New York City. J Acquir Immune Defic Syndr 2015;69 Suppl 1:S16–S24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.HIV Epidemiology and Field Services Program. HIV Surveillance Annual Report, 2016. New York, NY: New York City Department of Health and Mental Hygiene, 2017 [Google Scholar]
- 4.New York State Department of Health. Ending the AIDS Epidemic in New York State. 2016. Available at: https://www.health.ny.gov/diseases/aids/ending_the_epidemic (Last accessed February5, 2017).
- 5.Pathela P, Jamison K, Braunstein SL, Schillinger JA, Varma JK, Blank S. Incidence and predictors of HIV infection among men who have sex with men attending public sexually transmitted disease clinics, New York City, 2007–2012. AIDS Behav 2017;21:1444–1451 [DOI] [PubMed] [Google Scholar]
- 6.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999;75:3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2004;2:33–42 [DOI] [PubMed] [Google Scholar]
- 8.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: A systematic review and meta-analysis. Sex Transm Dis 2008;35:946–959 [DOI] [PubMed] [Google Scholar]
- 9.Schillinger J. The intersection of PrEP and sexually transmitted infections. Conference on Retroviruses and Opportunistic Infections, March 4–7, 2018, Boston, MA [Google Scholar]
- 10.Glynn TR, Operario D, Montgomery M, Almonte A, Chan PA. The duality of oral sex for men who have sex with men: An examination into the increase of sexually transmitted infections amid the age of HIV prevention. AIDS Patient Care STDs 2017;31:261–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traeger MW, Schroeder SE, Wright EJ, et al. . Effects of pre-exposure prophylaxis for the prevention of HIV infection on sexual risk behavior in men who have sex with men: A systematic review and meta-analysis. Clin Infect Dis 2018. DOI: 10.1093/cid/ciy182 [DOI] [PubMed] [Google Scholar]
- 12.Hoover KW, Parsell BW, Leichliter JS, et al. . Continuing need for sexually transmitted disease clinics after the Affordable Care Act. Am J Public Health 2015;105 Suppl 5:S690–S695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seth P, Wang G, Sizemore E, Hogben M. HIV Testing and HIV service delivery to populations at high risk attending sexually transmitted disease clinics in the United States, 2011–2013. Am J Public Health 2015;105:2374–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llata E, Braxton J, Asbel L, et al. . New human immunodeficiency virus diagnoses among men who have sex with men attending STD clinics, STD Surveillance Network, January 2010 to June 2013. Sex Trans Dis 2018. DOI: 10.1097/OLQ.0000000000000802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoover KW, Ham DC, Peters PJ, Smith DK, Bernstein KT. Human immunodeficiency virus prevention with preexposure prophylaxis in sexually transmitted disease clinics. Sex Trans Dis 2016;43:277–282 [DOI] [PubMed] [Google Scholar]
- 16.Pathela P, Klingler EJ, Guerry SL, et al. . Sexually transmitted infection clinics as safety net providers: Exploring the role of categorical sexually transmitted infection clinics in an era of health care reform. Sex Trans Dis 2015;42:286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz DA, Dombrowski JC, Kerani RP, et al. . Integrating HIV testing as an outcome of STD partner services for men who have sex with men. AIDS Patient Care STDs 2016;30:208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yehia BR, Ketner E, Momplaisir F, et al. . Location of HIV diagnosis impacts linkage to medical care. J Acquir Immune Defic Syndr 2015;68:304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheffler-Collins S. Evaluating linkage to care for individuals with newly diagnosed HIV in the Philadelphia Department of Public Health STD Clinic. STD Prevention Conference, Alanta, GA, 2014 [Google Scholar]
- 20.New York City Department of Health and Mental Hygiene. HIV/AIDS Surveillance Data. 2017. Available at: www1.nyc.gov/site/doh/data/data-sets/hiv-aids-surveillance.page (Last accessed July18, 2017).
- 21.Drobnik A, Pinchoff J, Bushnell G, et al. . Matching HIV, tuberculosis, viral hepatitis, and sexually transmitted diseases surveillance data, 2000–2010: Identification of infectious disease syndemics in New York City. J Public Health Manage Pract 2014;20:506–512 [DOI] [PubMed] [Google Scholar]
- 22.Pathela P, Jamison K, Braunstein SL, Schillinger JA, Tymejczyk O, Nash D. Gaps along the HIV Care Continuum: Findings among a population seeking sexual health care services in New York City. J Acquir Immune Defic Syndr 2018;78:314–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toprani A, Hadler J. Selecting and Applying a Standard Area-Based Socioeconomic Status Measure for Public Health Data: Analysis for New York City. New York City Department of Health and Mental Hygiene: Epi Research Report, May 2013 [Google Scholar]
- 24.United States Census Bureau. Metropolitan Statistical Areas and Components. 2003; Available at: https://www.census.gov/population/estimates/metro-city/0312msa.txt (Last accessed January10, 2015)
- 25.Sabharwal CJ, Braunstein SL, Robbins RS, Shepard CW. Optimizing the use of surveillance data for monitoring the care status of persons recently diagnosed with HIV in NYC. J Acquir Immune Defic Syndr 2014;65:571–578 [DOI] [PubMed] [Google Scholar]
- 26.Health Resources and Services Administration. HAB HIV Core Clinical Performance Measures Group 1. Adult/Adolescent Clients: Group 1. July 2008
- 27.Quinn TC, Wawer MJ, Sewankambo N, et al. . Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000;342:921–929 [DOI] [PubMed] [Google Scholar]
- 28.Begley E, VanHandel M. Provision of test results and posttest counseling at STD clinics in 24 health departments: U.S., 2007. Public Health Rep 2012;127:432–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herwehe J, Wilbright W, Abrams A, et al. . Implementation of an innovative, integrated electronic medical record (EMR) and public health information exchange for HIV/AIDS. J Am Med Inf Assoc 2012;19:448–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnus M, Herwehe J, Gruber D, et al. . Improved HIV-related outcomes associated with implementation of a novel public health information exchange. Int J Med Inf 2012;81:e30–e38 [DOI] [PubMed] [Google Scholar]
- 31.New York State Department of Health. Security and confidentiality of HIV surveillance data—October 27, 2014. Available at: https://www.health.ny.gov/diseases/aids/ending_the_epidemic/docs/key_resources/data_committee_resources/nys_surveillance_security.pdf (Last accessed June11, 2018).
- 32.Brennan A, Browne JP, Horgan M. A systematic review of health service interventions to improve linkage with or retention in HIV care. AIDS Care 2014;26:804–812 [DOI] [PubMed] [Google Scholar]
- 33.Udeagu CC, Webster TR, Bocour A, Michel P, Shepard CW. Lost or just not following up?: Public health effort to re-engage HIV-infected persons lost to follow-up into HIV medical care: 108 (120). AIDS 2013;27:2271–2279 [DOI] [PubMed] [Google Scholar]
- 34.Sevelius JM, Patouhas E, Keatley JG, Johnson MO. Barriers and facilitators to engagement and retention in care among transgender women living with human immunodeficiency virus. Ann Beh Med 2014;47:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Safer JD, Coleman E, Feldman J, et al. . Barriers to healthcare for transgender individuals. Curr Opin Endocrinol Diab Obes 2016;23:168–171 [DOI] [PMC free article] [PubMed] [Google Scholar]