Abstract
Objectives
According to one hypothesis, the popularity of levothyroxine (L-T4)/liothyronine (L-T3) combination therapy relates to weight loss. The purpose of this study was to detect a possible correlation between thyroid-related quality of life (QoL) and weight loss in hypothyroid patients switched from L-T4 monotherapy to L-T4/L-T3 combination therapy.
Methods
In an open-label cohort study, all hypothyroid patients referred to the University Hospital endocrine clinic due to persistent symptoms despite adequate L-T4 monotherapy (without other explanations for the symptoms) were switched from L-T4 monotherapy to L-T4/ L-T3 combination therapy at a ratio of approximately 17/1. At baseline and after 3 months of treatment we measured: QoL by the Thyroid Patient-Reported Outcome (ThyPRO-39) questionnaire, thyroid hormones, body weight, body composition by a DEXA-scan, and cognitive function by evaluating participants' reaction time as well as working memory by the California Computerized Assessment Package (CalCAP®). QoL was re-evaluated after 12 months.
Results
Twenty-three patients participated (91% women, median age 47 years). The ThyPRO-39 composite score decreased from a median of 54 (quartiles: 34, 74) to 15 (11, 28) after 3 months (p < 0.0001), and 20 (14, 26) after 12 months, indicating a better QoL. There was no change in body weight, and no correlations between QoL and weight. There was a slight improvement in cognitive function, whereas body composition, heart rate, and serum TSH did not change.
Conclusion
Our study on hypothyroid patients switched from L-T4 monotherapy to L-T4/L-T3 combination therapy showed a substantial improvement in QoL measured by the ThyPRO-39. This improvement could not be explained by weight loss.
Keywords: Hypothyroidism, Liothyronine, Levothyroxine, Quality of Life, Weight loss, Patient-reported-outcome
Introduction
Hypothyroidism is a common disease usually treated with levothyroxine (L-T4) substitution [1]. Still, 5–10% of individuals with hypothyroidism are not satisfied with L-T4 monotherapy, and do not consider their previous well-being to be restored [1]. An alternative treatment with L-T4/liothyronine (L-T3) combination therapy remains controversial, but is nonetheless often requested by patients. The European Thyroid Association concluded in its 2012 guidelines that a small group of hypothyroid patients may benefit from this therapy [1].
In a recent questionnaire-based study on patients receiving either desiccated thyroid or synthetic L-T4/L-T3 combination therapy, we found that 69% of participants recalled having 6 or more symptoms before the initiation of T4/T3 combination therapy, even though they were biochemically euthyroid during monotherapy with L-T4 [2]. Ninety-one percent reported that their main complaint was tiredness, whereas the corresponding numbers for untreated hypothyroid patients were 81 and 41% for healthy controls [3].
When comparing the data from the Thyroid Patient-Reported Outcome (ThyPRO-39) questionnaire with background data on the general population, untreated hypothyroid patients have a severely decreased quality of life (QoL). After the initiation of L-T4 monotherapy, QoL is greatly improved; however, it remains somewhat decreased compared to the general population [4].
QoL for patients receiving L-T4/L-T3 combination therapy has also been studied using various questionnaires, such as SF-36 [5]. However, none of these questionnaires were specific for hypothyroidism.
It has been suggested that the reason for an increase in QoL related to L-T4/L-T3 combination therapy is weight loss [1, 6]. Weight gain prior to starting L-T4 therapy could be an indication for initiating L-T4 therapy in subclinical hypothyroidism or even over-replacement with L-T4. A study on data from primary care, investigating trends in L-T4 therapy initiation and thyrotropin levels, found that weight gain or obesity was one of the symptoms prior to the initiation of L-T4 therapy; however, these symptoms were not associated with overtreatment after 5 years [7].
Overt hypothyroid patients mainly lose lean body mass when started on a subsequent L-T4 monotherapy [8]. However, whether the pattern is the same for patients switching from L-T4 monotherapy to L-T4/L-T3 combination therapy is unknown. The aim of this study was to test a potential correlation between weight loss and improvement of QoL by using the thyroid-specific ThyPRO-39 questionnaire [9, 10, 11] in a cohort of patients switched from L-T4 monotherapy to L-T4/L-T3 combination therapy due to persistent symptoms on L-T4 monotherapy.
Materials and Methods
We designed an open-label cohort study, conducted from December 2014 to June 2015. All applicable (see inclusion and exclusion criteria below) patients referred to our University Hospital endocrine clinic were included. The participants were seen at 2 study visits: one before the treatment (baseline), and one after 3 months of treatment. Each visit included a clinical interview and clinical testing. Furthermore, a follow-up online questionnaire on QoL was sent to the patients approximately 12 months after treatment initiation.
Inclusion Criteria
- age between 18 and 80 years
- being able to read and understand Danish
- diagnosis of overt hypothyroidism with serum TSH (S-TSH) above the normal range (> 4.0 mU/L) and serum T4 (S-T4) below the normal range (< 70 nmol/L) at the time of diagnosis
- stable and normal S-TSH on L-T4 monotherapy for at least 6 months prior to inclusion
- self-reported consistently reduced QoL after the diagnosis of hypothyroidism compared to before, despite biochemically adequate treatment with L-T4
Exclusion Criteria
- pregnancy or planned pregnancy within the next year
- comorbidity which could explain the symptoms, e.g., depression
- abnormal levels of vitamin D, hemoglobin, calcium, cobalamin, liver and renal function tests, blood glucose, or an insufficient corticotropin stimulation test
The participants were treated with L-T4/L-T3 combination therapy at a dose ratio of approximately 17/1, calculated for each participant, based on their L-T4 dose before the study. We used 5-µg breakable tablets of L-T3 (Glostrup Pharmacy, Glostrup, Denmark), administered twice a day: approximately one third of the L-T3 dose in the morning together with L-T4 and the remaining two-thirds of the L-T3 at bedtime.
Outcomes
The primary outcome was the change in thyroid-specific QoL and its correlation with change in body weight. Secondary outcomes included impacts on heart rate, body composition, and cognitive function.
Clinical Parameters
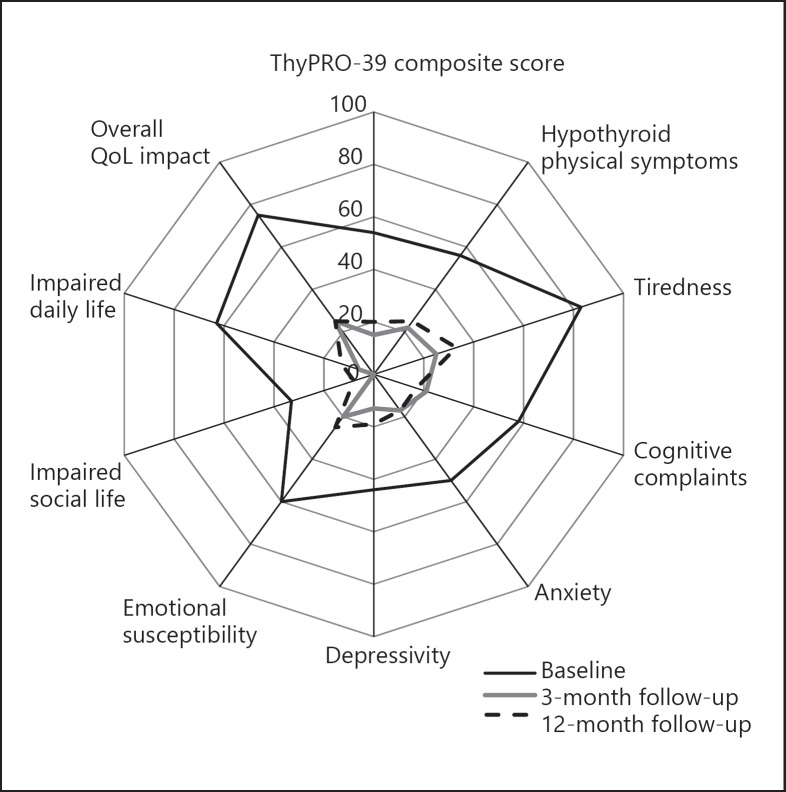
QoL was measured by an online version of ThyPRO-39 [9, 10, 11, 12]. The questionnaire was answered at home before each visit and 12 months after initiation of the treatment. We used the 9 subscales from ThyPRO-39 (Fig. 1). Each score was constructed by the summation of relevant items and linear transformation to a range of 0–100, where 100 indicated most symptoms/impact on QoL [11]. The scores, apart from the hypothyroid physical symptoms score, were summarized as the ThyPRO Composite Score.
Fig. 1.
Radar diagram presenting the median ThyPRO composite score of QoL of all 3 measurements of the study population.
Weight was measured in a nonfasting state at both visits. Heart rate was measured after 20–40 min of resting. Body composition was assessed by a whole-body dual-energy X-ray Absorptiometry scan (DEXA-scan; Hologic®, Marlborough, MA, USA) and analyzed as total body fat (%), body fat (kg), and lean body mass (kg).
A computer program was used to examine cognitive function: California Computerized Assessment Package (CalCAP®; Eric N. Miller, Palm Springs, CA, USA). The test battery consisted of 4 tasks, all 4 of which assessed simple reaction times, while tasks 3 and 4 also assessed working memory. We measured the mean reaction time (MRT) to a stimulus, in this case the appearance of a number on a screen. For tasks 2, 3, and 4, the error rate (ER = [false positive + false negative reactions]/total number of tasks [13]) was calculated in order to analyze the accuracy.
Nonfasting blood samples were drawn between 8 and 10 a.m., at baseline, after 1 month of treatment, and after 3 months of treatment. Participants were allowed to take their medications prior to each visit. Serum TSH, free T4 and T3 levels were measured through direct chemiluminescence (ADVIA Centaur XP® Immunoassay Systems; Siemens, Berlin, Germany). A change in S-TSH of more than 1.0 mU/L or S-TSH outside the range of 0.35–4.0 mU/L at the 1-month measurement led to an adjustment of the L-T4 dose.
The study was approved by the Regional Scientific Ethical Committee (No. H-3-2014-102) and The Danish Data Protection Agency (No. HEH-2014-03189, I-Suite No. 03169).
Statistical Analyses
We tested for normality using quantile-quantile plots (Q-Q plots). The t test was used for normally distributed variables and the Wilcoxon test was used for skewed data. Tests for correlation were performed through Spearman's rank order correlation test.
QoL in the study population was also compared to data from 2 previously published reference populations: the Danish general population [14] and a sample of newly diagnosed hypothyroid patients [4]. For these comparisons, the Student t test was applied. A 2-sided p value ≤0.05 was considered statistically significant.
Results
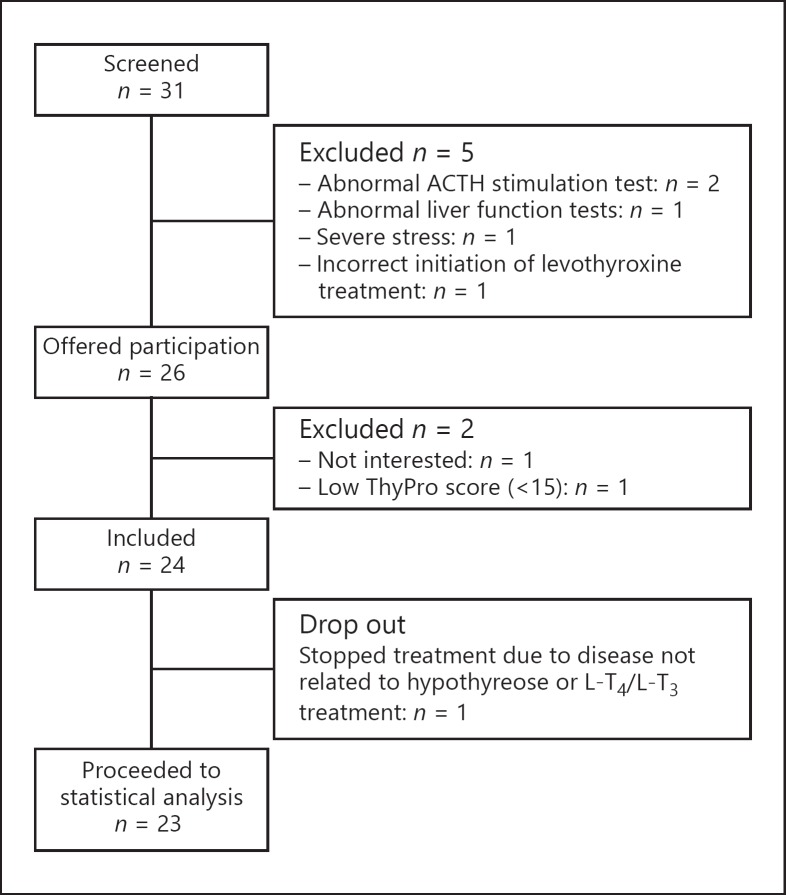
Thirty-one patients were screened. In total, 23 participants completed both study visits (Fig. 2). The baseline characteristics are presented in Table 1. After the 3-month study period, 4 participants did not experience any improvement in QoL, and the treatment was discontinued. However, all participants, including those out of treatment, were included in the 12-month follow-up analysis.
Fig. 2.
Flowchart of participants in the study of hypothyroid patients treated with L-T4/L-T3 combination therapy.
Table 1.
Baseline characteristics of hypothyroid patients treated with L-T4/L-T3 combination therapy
| Baseline (n = 23) | |
|---|---|
| Age, years | 47 [33–76] (40, 53) |
| Female | 21 (91.3) |
| Disposition to thyroid disease | 15 (65.2) |
| Etiology of hypothyroidism | |
| Autoimmunity | 17 (73.9) |
| Radioiodine therapy | 4 (17.4) |
| Thyroidectomy | 1 (4.3) |
| Congenital | 1 (4.3) |
| Self-reported duration of hypothyroidism | 9.0 (4.8, 21.0) |
Values are presented as median [range] (quartiles) or n (%).
Primary Outcome
The QoL scores for our study group (measured by ThyPRO-39) are shown in Table 2. Compared to baseline, the 3-month ThyPRO-39 composite scores improved substantially (p < 0.001) and remained significantly better at the 12-month follow-up (n = 21, p < 0.001; Table 2; Fig. 1). However, there was a slight worsening of the ThyPRO-39 composite scores from the 3-month to the 12-month measurements (p = 0.03).
Table 2.
QoL measured by the ThyPRO-39 scores (0-100; higher scores represent worse QoL) in hypothyroid patients treated with L-T4/L-T3 combination therapy
| Baseline (n = 23) |
3-month follow-up (n = 22) |
12-month follow-up (n = 21) |
General population [14] (n = 754) | Hypothyroid patients [4] |
|||||
|---|---|---|---|---|---|---|---|---|---|
| median (quartile) | mean (SD) | median (quartile) | mean (SD) | median (quartile) | mean (SD) | Newly diagnosed (n = 78) | after 6 months (n = 63) | ||
| Median age, years | 47 (40, 53) | – | – | 50 (38, 64) | 47 [18–91] | – | |||
| Female, n (%) | 21 (91.3) | – | – | 602 (81) | 70 (90) | – | |||
| QoL | |||||||||
| ThyPRO-39 composite score | 54 (34, 74) | 56 (20) | 15 (11, 28)a | 20 (16) | 20 (14, 26)b | 22 (12) | na | na | na |
| ThyPRO-39 subscales | |||||||||
| Tiredness | 83 (67, 92) | 75 (20) | 25 (17, 42)a | 30 (19) | 33 (25, 50)b | 40 (21) | 35 (21)c, d | 58 (28)c, d | 43 (27)c |
| Cognitive complaints | 58 (33, 83) | 59 (31) | 21 (8, 25)a | 21 (16) | 17 (0, 25)b | 18 (17) | 14 (17)c, d | 27 (26)c, d | 22 (23)c |
| Anxiety | 50 (l7, 67) | 44 (31) | 17 (0, 25)a | 21 (25) | 17 (4, 25)b | 19 (22) | 13 (16)c, d | 21 (20)c | 11 (17)c, d |
| Depressivity | 44 (25, 75) | 48 (29) | 13 (6, 27)a | 18 (16) | 19 (6, 35)b | 24 (21) | 21 Q8)c, d | 32 (23)c, d | 25 (19)c |
| Emotional susceptibility | 60 (40, 80) | 63 (21) | 20 (9, 38)a | 25 (18) | 25 (18, 35)b | 28 (14) | 23 (19)c, d | 40 (25)c, d | 28 (22)c |
| Impaired social life* 1 | 33 (l7, 50) | 35 (23) | 0 (0, 25)a | 12 (17) | 8 (0, 8)b | 7 (11) | – | 13 Q9)c, d | 8 (15)c |
| Impaired daily life1 | 63 (44, 75) | 56 (26) | 6 (0, 15)a | 14 (18) | 13 (6, 25)b | 17 (14) | – | 22 (27)c, d | 12 (20)c, d |
| Overall QoL impact1 | 75 (50, 100) | 77 (24) | 25 (19, 31)a | 26 (20) | 25 (25, 25)b | 29 (24) | – | 37 (36)c, d | 19 (26)c, d |
| Hypothyroid physical | 2 | ||||||||
| symptoms2 | 56 (44, 75) | 59 (19) | 22 (13, 33)a | 26 (15) | 25 (19, 47)b | 35 (20) | 14 (16)c, d | 27 (24)c, d | 3 (21)c, d |
Values are presented as the median (quartiles), median [range], n (%), and mean (SD). For comparison and interpretability, previously published reference data are provided on the general population [14], as well as newly diagnosed hypothyroid patients in the untreated state and 6 months after initiation of monotherapy with L-T4 [4]. Our new data, however, are not corrected for age and gender.
Baseline versus 3 months, p < 0.05
Baseline versus 12 months, p < 0.05.
Compared to baseline, p < 0.05.
Compared to 12 months, p < 0.05. na, not available, since these data were not available in the previous studies.
The participants were asked how much hypothyroidism affected the particular aspect of their lives.
Including only physical symptoms, i.e. cold intolerance, swollen hands or feet, dry skin and itchy skin.
A similar pattern was observed for hypothyroid physical symptoms and other ThyPRO-39 scales (Table 2; Fig. 1). The largest improvement was found on the impaired daily life-scale from a median score of 63 to a median score of 6 after 3 months of treatment (p < 0.001).
The median change in body weight between baseline and the 3-month measurement was −0.45 kg (quartiles: −1.2, 0.7); however, the decrease was not significant (p = 0.245). Based on an SD of 1.8 kg in delta weight, power of 90% was present to detect a minimal relevant difference of 1.75 kg.
ThyPRO-39 composite scores and weight did not correlate at baseline (p = 0.536) or at the 3-month measurement (p = 0.620). Similarly, changes in ThyPRO-39 composite scores and in weight did not correlate (p = 0.490). Furthermore, there was no correlation between 3-month ThyPRO-39 composite scores and change in weight (p = 0.143).
Secondary Outcomes
From baseline to the 3-month visit, the S-TSH did not change significantly (p = 0.08). As expected, free T3 levels increased and free T4 levels decreased (Table 3).
Table 3.
Weight and hormone measurements in hypothyroid patients treated with L-T4/L-T3 combination therapy
| Baseline (n = 23) | 3 months (n = 23) | p value | |
|---|---|---|---|
| Mean weight, kg | 79.6±13.2 | 79.1±12.2 | 0.25 |
| Median thyroid hormones1 | |||
| S-TSH, mU/L | 1.01 (0.56, 2.04) | 0.54 (0.21, 1.7) | 0.08 |
| Free S-T3, pmol/L | 4.7 (4.4, 5.0) | 5.2 (4.9, 5.5) | <0.001 |
| Free S-T4, pmol/L | 20.8 (l7.1, 22.2) | 16.6 (16.1, 19.1) | <0.001 |
Data are presented as the mean ± SD or median (quartiles). Bold p values are significant.
Reference range: S-TSH 0.35-4.0 mU/L; free S-T3 3.5-6.5 pmol/L; free S-T4 11.8-24 pmol/L.
ThyPRO-39 composite scores and S-TSH did not correlate, either at baseline (p = 0.471) or at the 3-month measurement (p = 0.199), or when looking at changes in these parameters over time (p = 0.879).
The baseline ThyPRO-39 composite score correlated to baseline free T3 (rs = 0.481; p = 0.02), indicating that higher free T3 levels were associated with higher values of ThyPRO, i.e., poorer QoL. There was no correlation for the 3-month measurement (p = 0.8). There was a negative correlation between the change in ThyPRO-39 composite score and change in free T3 levels (rs = −0.425; p = 0.049): the largest decrease in ThyPRO, i.e., largest improvement in QoL, was associated with the smallest increase in free T3 levels.
As for the cognitive function, some changes were observed. The median MRT was lower after 3 months (Table 4), though only significantly for task 3 (p = 0.03). With regards to the ER, there was a reduction, i.e., improvement, in tasks 2 and 3 (p = 0.01 and p = 0.02). ThyPRO-39 composite scores did not correlate with changes in either MRT or ER.
Table 4.
Clinical parameters in hypothyroid patients treated with L-T4/L-T3 combination therapy
| Baseline (n = 23) | 3 months (n = 23) | p value | |
|---|---|---|---|
| Heart rate, bpm | 67 (57, 73) | 64 (56, 73) | 0.60 |
| Body composition | |||
| Total body fat, % | 34.1±6.1 | 34.2±6.1 | 0.79 |
| Body fat, kg | 27.8 (21.2, 34.7) | 28.1 (20.7, 34.2) | 0.32 |
| Lean mass, kg | 49.8 (44.8, 53.9) | 50.2 (45.1, 52.8) | 0.36 |
| Cognitive function | |||
| MRT task 1, ms | 361 (347, 466) | 350 (317, 385) | 0.08 |
| MRT task 2, ms | 463 (433, 491) | 446 (420, 480) | 0.36 |
| MRT task 3, ms | 605 (565, 657) | 555 (513, 643) | 0.03 |
| MRT task 4, ms | 647 (574, 742) | 607 (553, 634) | 0.08 |
| ER 2 | 0.02 (0.01, 0.04) | 0.01 (0, 0.03) | 0.01 |
| ER 3 | 0.04 (0.02, 0.08) | 0.01 (0, 0.07) | 0.02 |
| ER 4 | 0.09 (0.05, 0.14) | 0.07 (0.05, 0.09) | 0.08 |
| Thyroid hormones | |||
| S-TSH, mU/L | 1.01 (0.56, 2.04) | 0.54 (0.21, 1.7) | 0.08 |
| Free T3, pmol/L | 4.7 (4.4, 5.0) | 5.2 (4.9, 5.5) | <0.001 |
| Free T4, pmol/L | 20.8 (17.1, 22.2) | 16.6 (16.1, 19.1) | <0.001 |
Data are presented as median (quartiles) or mean ± SD. Bold p values are significant. MRT, mean reaction time; ER, error rate.
Body composition and heart rate did not change over the 3-month combination therapy (Table 4). Furthermore, heart rate and S-TSH levels did not correlate, either at baseline or when comparing changes over time.
Discussion
In this study on 23 patients switching from L-T4 to L-T4/L-T3 combination therapy, we observed a substantially improved QoL as measured by ThyPRO-39 after 3 months, which remained present at the 12-month follow-up. We also saw an improvement in cognitive function after 3 months. There was no significant weight loss, and we found no correlation between weight and QoL, or between changes in thyroid hormone levels and QoL.
In a randomized controlled trial (RCT), Appelhof et al. [6] found a correlation between weight loss and satisfaction with treatment in an L-T4/L-T3 combination therapy group. However, the participants received higher doses of L-T3 (ratio 5: 1) than recommended (ratio 13: 1–20: 1) [1], and had a significant decrease in S-TSH (from 1.0 to 0.07, p < 0.01) and an increase in heart rate of 3.9 bpm compared to baseline, suggesting overtreatment. In comparison, we administered the physiological ratio of 17: 1 of L-T4:L-T3 and recorded a stable S-TSH and heart rate.
In our study we used ThyPRO, a well-validated questionnaire developed specifically for patients with thyroid disease, for the first time in a study focusing on QoL in L-T4-treated patients with persistent symptoms treated with L-T4/L-T3 combination therapy. We used the short form ThyrPRO-39, which is easy to apply in scientific settings. We did not find any weight loss and we could not reproduce the correlation between ThyPRO-39 composite scores and weight. Thus, the improvement in QoL cannot be attributed to weight loss. This is in accordance with our previous study comparing L-T4/L-T3 combination therapy to L-T4 monotherapy in a randomized crossover design [5] in which L-T4/L-T3 combination therapy resulted in an increase in QoL (SF-36 questionnaire) and no weight loss [5]. We did, however, find a correlation between baseline ThyPRO-39 composite score and baseline free T3 (rs = 0.481; p = 0.02), indicating that higher free T3 levels were associated with poorer QoL, and that those with the smallest increase had the largest increase in QoL. This could be a type 1 error, but could also be an argument to refute the claim that high levels of free T3 are essential for optimal QoL [15].
The existence of a subgroup of hypothyroid patients not performing well in QoL, and their potential positive response to L-T4/L-T3 combination therapy, may have an explanation in the presence of polymorphisms altering the metabolism of thyroid hormones. In a large population-based study including 552 participants from the UK, 16% of participants had a polymorphism in the deiodinase 2 (DIO2) gene coding for the enzyme, facilitating deiodination from T4 into T3. There was an association between the presence of the polymorphism and worse baseline QoL, measured through the General Health Questionnaire 12 (GHQ-12). Furthermore, there was a greater improvement for this group compared with those without the polymorphism [16]. Another recent study from our group of 44 hypothyroid patients also showed that patients with polymorphisms in both DIO2 and in the thyroid hormone cellular membrane transport-facilitating monocarboxylate transporter (MCT10) genes had a significantly higher preference for L-T4/L-T3 combination therapy [17]. No genetic testing was performed on the current patient group.
Due to the absence of a control group in this study, a potential placebo/nocebo effect cannot be ruled out. However, we did see an improvement of QoL on the ThyPRO-39 composite score by 72%. Such a degree of improvement is much higher than the estimated placebo effect of 35% reported in the literature [18]. Furthermore, the persistent improvement seen in the ThyPRO-39 score at the 12-month follow-up supports a true effect.
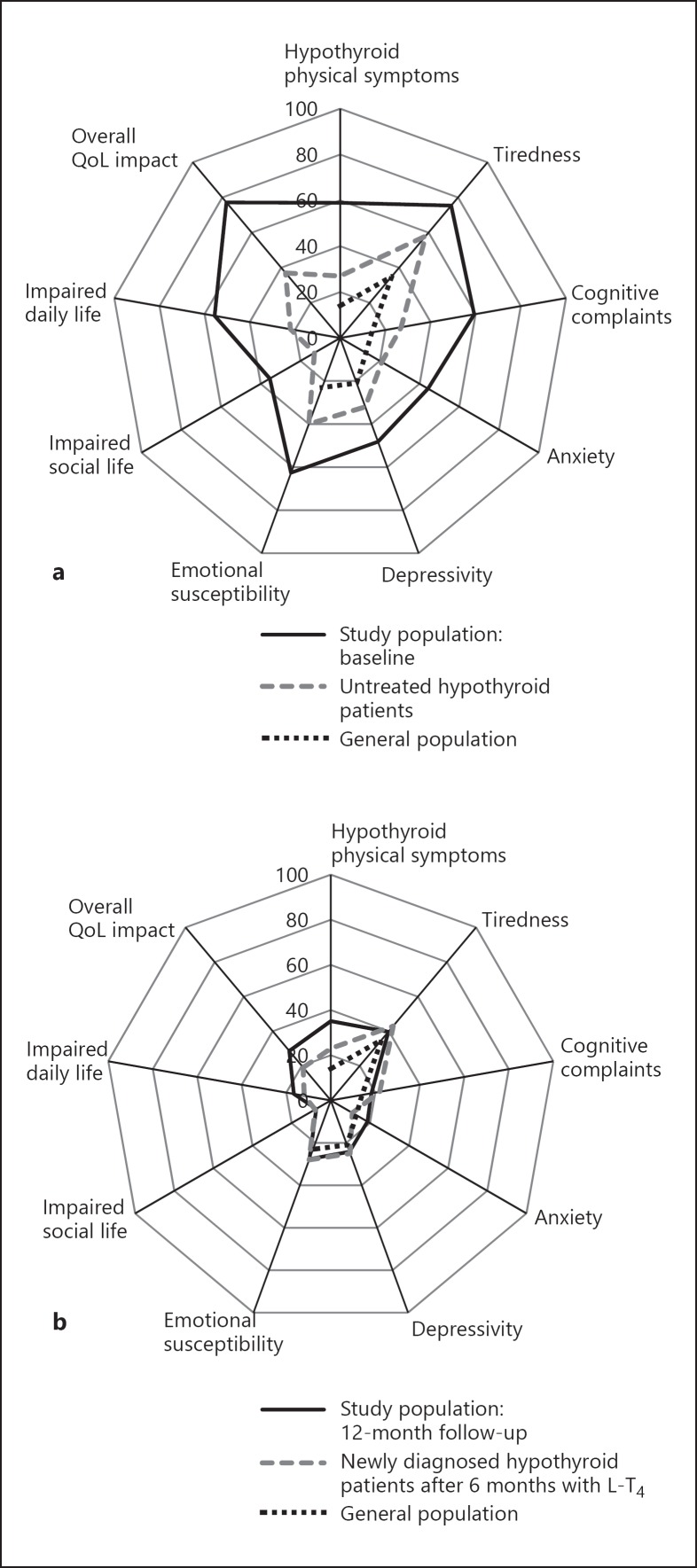
Compared to reference data from the general population [14] as well as newly diagnosed hypothyroid patients and hypothyroid patients treated with 6 months of L-T4 monotherapy [4], the current study population had substantially worse ThyPRO-39 scores at baseline on all available reference scales (see Table 2 and Fig. 3 for data from previous studies for comparison). This underscores that the population we are working with is highly burdened by symptoms, in accordance with findings in previous studies [2].
Fig. 3.
Mean ThyPRO composite score of QoL of the study population at baseline (a) and at the 12-month follow-up (b) in comparison with available reference scores for the general population (a, b) [14] as well as newly diagnosed hypothyroid patients in the untreated state (a) [4] and 6 months after the initiation of monotherapy with L-T4 (b) [4].
At the 12-month follow-up, patients had worse hypothyroid physical symptoms compared to the general population, but only small differences on all other comparable scales. As for hypothyroid patients treated with 6 months of L-T4 monotherapy, no differences were found for tiredness, cognitive complaints, depressivity, emotional susceptibility, or impaired social life; slightly worse scores for the study population were found on hypothyroid symptoms, anxiety, impaired daily life, and overall QoL impact. Our study population never achieves as good QoL scores as the general population [14]. However, at the 12-month measurement, for several scales, L-T4/L-T3 combination therapy succeeded in bringing our study population to the same level as hypothyroid patients treated with 6 months of L-T4 monotherapy [4], even though our study population had a baseline QoL worse than newly diagnosed hypothyroid patients [4].
A drawback of this study is the small selected sample size. Also, for an optimal RCT, a need for personalized treatment to avoid overtreatment, especially in older patients [19], and consideration regarding dietary and nutritional factors influencing the thyroid function (such as iodine, selenium, and vitamin D) should be (if possible) considered. Furthermore, a slow-release low-dose T3, optimal in combination with T4, would be the optimal drug to use instead of the complicated dose regimen used in this study.
This study was performed on a highly selected group with severe and persistent symptoms, thus only including patients potentially suitable for L-T4/L-T3 combination therapy. In a selected group like this, there can be a risk for bias. However, we may be able to detect an effect on QoL, which would disappear in a large heterogeneous study population.
Conclusions
Hypothyroid patients unsatisfied with their L-T4 monotherapy were switched from L-T4 monotherapy to L-T4/L-T3 combination therapy, resulting in a substantial improvement in QoL after the 12-month treatment period. Furthermore, participants achieved QoL scores similar to hypothyroid patients treated with 6 months of L-T4 monotherapy, though they did not achieve quite the same level as the general population. We found no significant weight loss during the study and no correlation between changes in weight and QoL. Thus, the improvement in QoL seemed not to be influenced by weight loss. An improvement in cognitive function was also seen, though only in a few of the tasks, and it did not correlate with QoL. Moreover, L-T4/L-T3 combination therapy does not seem to alter body composition during short-term therapy.
The large increase in QoL suggests that the topic of L-T4/L-T3 combination therapy remains highly relevant and calls for further RCT studies.
Disclosure Statement
None of the authors have any conflicts of interest to declare.
References
- 1.Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyroid J. 2012;1:55–71. doi: 10.1159/000339444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michaelsson LF, Medici BB, la Cour JL, Selmer C, Roder M, Perrild H, Knudsen N, Faber J, Nygaard B. Treating hypothyroidism with thyroxine/triiodothyronine combination therapy in Denmark: following guidelines or following trends? Eur Thyroid J. 2015;4:174–180. doi: 10.1159/000437262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carle A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Laurberg P. Hypothyroid symptoms and the likelihood of overt thyroid failure: a population-based case-control study. Eur J Endocrinol. 2014;171:593–602. doi: 10.1530/EJE-14-0481. [DOI] [PubMed] [Google Scholar]
- 4.Winther KH, Cramon P, Watt T, Bjorner JB, Ekholm O, Feldt-Rasmussen U, Groenvold M, Rasmussen AK, Hegedus L, Bonnema SJ. Disease-specific as well as generic quality of life is widely impacted in autoimmune hypothyroidism and improves during the first six months of levothyroxine therapy. PLoS One. 2016;11:e0156925. doi: 10.1371/journal.pone.0156925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nygaard B, Jensen EW, Kvetny J, Jarlov A, Faber J. Effect of combination therapy with thyroxine (T4) and 3,5,3′-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. Eur J Endocrinol. 2009;161:895–902. doi: 10.1530/EJE-09-0542. [DOI] [PubMed] [Google Scholar]
- 6.Appelhof BC, Fliers E, Wekking EM, Schene AH, Huyser J, Tijssen JG, Endert E, van Weert HC, Wiersinga WM. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005;90:2666–2674. doi: 10.1210/jc.2004-2111. [DOI] [PubMed] [Google Scholar]
- 7.Taylor PN, Iqbal A, Minassian C, Sayers A, Draman MS, Greenwood R, Hamilton W, Okosieme O, Panicker V, Thomas SL, Dayan C. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med. 2014;174:32–39. doi: 10.1001/jamainternmed.2013.11312. [DOI] [PubMed] [Google Scholar]
- 8.Karmisholt J, Andersen S, Laurberg P. Weight loss after therapy of hypothyroidism is mainly caused by excretion of excess body water associated with myxoedema. J Clin Endocrinol Metab. 2011;96:E99–E103. doi: 10.1210/jc.2010-1521. [DOI] [PubMed] [Google Scholar]
- 9.Watt T, Hegedus L, Groenvold M, Bjorner JB, Rasmussen AK, Bonnema SJ, Feldt-Rasmussen U. Validity and reliability of the novel thyroid-specific quality of life questionnaire, ThyPRO. Eur J Endocrinol. 2010;162:161–167. doi: 10.1530/EJE-09-0521. [DOI] [PubMed] [Google Scholar]
- 10.Watt T, Cramon P, Hegedus L, Bjorner JB, Bonnema SJ, Rasmussen AK, Feldt-Rasmussen U, Groenvold M. The thyroid-related quality of life measure ThyPRO has good responsiveness and ability to detect relevant treatment effects. J Clin Endocrinol Metab. 2014;99:3708–3717. doi: 10.1210/jc.2014-1322. [DOI] [PubMed] [Google Scholar]
- 11.Watt T, Bjorner JB, Groenvold M, Cramon P, Winther KH, Hegedus L, Bonnema SJ, Rasmussen AK, Ware JE, Jr, Feldt-Rasmussen U. Development of a short version of the thyroid-related patient-reported outcome ThyPRO. Thyroid. 2015;25:1069–1079. doi: 10.1089/thy.2015.0209. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen SL, Rejnmark L, Ebbehoj E, Feldt-Rasmussen U, Rasmussen AK, Bjorner JB, Watt T. High level of agreement between electronic and paper mode of administration of a thyroid-specific patient-reported outcome, ThyPRO. Eur Thyroid J. 2016;5:65–72. doi: 10.1159/000443609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoi-Hansen T, Pedersen-Bjergaard U, Andersen RD, Kristensen PL, Thomsen C, Kjaer T, Hogenhaven H, Smed A, Holst JJ, Dela F, Boomsma F, Thorsteinsson B. Cognitive performance, symptoms and counter-regulation during hypoglycaemia in patients with type 1 diabetes and high or low renin-angiotensin system activity. J Renin Angiotensin Aldosterone Syst. 2009;10:216–229. doi: 10.1177/1470320309343007. [DOI] [PubMed] [Google Scholar]
- 14.Cramon P, Bonnema SJ, Bjorner JB, Ekholm O, Feldt-Rasmussen U, Frendl DM, Groenvold M, Hegedus L, Rasmussen AK, Watt T. Quality of life in patients with benign nontoxic goiter: impact of disease and treatment response, and comparison with the general population. Thyroid. 2015;25:284–291. doi: 10.1089/thy.2014.0433. [DOI] [PubMed] [Google Scholar]
- 15.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Recent advances in thyroid hormone regulation: toward a new paradigm for optimal diagnosis and treatment. Front Endocrinol. 2017;8:364. doi: 10.3389/fendo.2017.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, Dayan CM. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009;94:1623–1629. doi: 10.1210/jc.2008-1301. [DOI] [PubMed] [Google Scholar]
- 17.Carle A, Laurberg P, Steffensen R, Faber J, Nygaard B. Combination of DIO2 and MCT10 gene polymorphisms predicts the preference for T4+T3 therapy in hypothyroidism – a blinded randomized clinical study (abstract) Eur Thyroid J. 2016;5((suppl 1)):65–66. [Google Scholar]
- 18.Beecher HK. The powerful placebo. J Am Med Assoc. 1955;159:1602–1606. doi: 10.1001/jama.1955.02960340022006. [DOI] [PubMed] [Google Scholar]
- 19.Duntas LH, Wartofsky L. There is no ‘universal fit’: reflections on the use of L-triiodothyronine in the treatment of hypothyroidism. Metabolism. 2016;65:428–431. doi: 10.1016/j.metabol.2015.11.005. [DOI] [PubMed] [Google Scholar]