Abstract
Background:
Preeclampsia is characterized by alterations in angiogenic factors that may increase neonatal morbidity independent of preterm birth.
Methods:
We estimated the controlled direct effect of preeclampsia on neonatal outcomes independent of preterm birth among 200,103 normotensive and 10,507 preeclamptic singleton pregnancies in the Consortium on Safe Labor (2002–2008). Marginal structural models with stabilized inverse probability weights accounted for potential confounders in the pathway from preeclampsia to preterm birth to neonatal outcomes, including mediator-outcome confounders related to preeclampsia status, such as cesarean delivery. Controlled direct effects of preeclampsia on perinatal mortality, small for gestational age (SGA), neonatal intensive care unit (NICU) admission, respiratory distress syndrome, transient tachypnea of the newborn, anemia, apnea, asphyxia, peri- or intraventricular hemorrhage, and cardiomyopathy were estimated for the hypothesized intervention of term delivery for all infants.
Results:
When delivery was set at ≥37 weeks, preeclampsia increased the odds of perinatal mortality (odds ratio = 2.2 [95% confidence interval = 1.1–4.5], SGA = (1.9 [1.8–2.1]), NICU admission (1.9 [1.7–2.1]), respiratory distress syndrome (2.8 [2.0–3.7], transient tachypnea of the newborn (1.6 [1.3–1.9]), apnea (2.2 [1.6–3.1]), asphyxia (2.7 [1.5–4.9]), and peri- or intraventricular hemorrhage (3.2 [1.4–7.7]). No direct effect of preeclampsia at term was observed for anemia or cardiomyopathy. Our results appear robust in the presence of moderate confounding, and restriction to severe preeclampsia yielded similar findings.
Conclusion:
Preeclampsia was directly associated with adverse neonatal outcomes beyond morbidity mediated by preterm birth. Although severe neonatal outcomes were less common at later gestational ages, marginal structural models suggested elevated neonatal risk due to preeclampsia even if it was possible to deliver all infants at term.
Preeclampsia is a common hypertensive disorder of pregnancy characterized by a new onset of hypertension after the 20th week of pregnancy, typically with accompanying proteinuria.1 Mechanistically, the hallmark of preeclampsia is an increase in antiangiogenic factors.2,3 The placental changes associated with these antiangiogenic factors are thought to affect vascularization, which may, in turn, have an adverse impact on newborns independent of preterm delivery.4 For example, soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng) are antiangiogenic compounds secreted by the placenta that are elevated in preeclampsia even before disease onset.5 The interference of these compounds with placental growth factor and vascular endothelial growth factor (VEGF) likely underlie the observed endothelial cell dysfunction and alterations in vascular development associated with preeclampsia5 and may also directly affect neonatal health through these pathways.
Therefore, it is plausible that preeclampsia has a direct effect on neonatal outcomes. However, to estimate the direct effect of preeclampsia on neonatal morbidity, analyses need to take into account the fact that women with preeclampsia have an increased risk of preterm birth, which is itself associated with increased neonatal morbidity. This risk may also be related to disease severity because delivery is the only way to stop the progression of preeclampsia and preterm birth may be indicated to protect the health of the mother or to prevent stillbirth.6 In fact, more than 20% of medically indicated pre-term births are associated with preeclampsia.7 Others have attempted to resolve the methodological issue of mediation by prematurity by restricting their analyses to preterm infant subgroups, as such “controlling” or “adjusting” for the effect of preterm delivery.8,9 However, these methods for mediation analysis can lead to biased estimates when there are confounders of the preterm birth–outcome association that are also influenced by preeclampsia, such as placental abruption and cesarean delivery, and when there is a potential interaction between preeclampsia and preterm birth.10
For example, preeclampsia appears to increase the risk for cerebral palsy,11–13 and a direct effect of preeclampsia, not mediated by preterm birth, has been suggested.12,14 This direct effect is conceivable, given that preeclampsia might contribute to vascular changes in the developing brain. However, the controlled direct effects of preeclampsia on other neonatal health outcomes have not been previously estimated—an important data gap, given the broad vascular impact of preeclampsia on fetal development.
We used marginal structural models in a large, contemporary cohort of US deliveries to estimate the controlled direct effect15 of preeclampsia on neonatal outcomes under a hypothesized intervention that assumes it is possible to deliver all infants at term.
METHODS
The Consortium on Safe Labor (2002–2008) was a retrospective cohort with 12 clinical centers (19 hospitals) across 9 American College of Obstetricians and Gynecologists U.S. districts.16 Detailed information was obtained from maternal electronic medical records on the following: maternal demographic characteristics; medical, reproductive, and prenatal history; and labor and delivery summaries. International Classification of Diseases, version 9 (ICD-9) maternal discharge diagnosis codes were recorded for each pregnancy. Maternal records were linked to newborn electronic medical records, which included details of neonatal intensive care (NICU) admissions, as well as ICD-9 discharge codes for the infants. A total of 228,562 deliveries ≥23 weeks of gestation with 233,736 newborns were included in the main study. The majority (87%) of the cohort delivered during 2005–2007. Institutional review board approval was obtained by all participating institutions.
We excluded all multifetal pregnancies (n = 5,050) because the majority of these pregnancies deliver preterm, and the relation between preeclampsia and preterm birth is likely different than among singletons. We also excluded singleton pregnancies in which the mother had chronic hypertension (n = 4,366), superimposed preeclampsia (n = 1,893), gestational hypertension (n = 6,080), eclampsia (n = 240), and suspected eclampsia (mothers with seizures and hypertension; n = 28) to clarify our assessment of the relation between neonatal health and preeclampsia given that the concentration of antiangiogenic factors is likely different in women with preeclampsia compared with women with other hypertensive disorders of pregnancy. Finally, women with missing data on maternal age (n = 295) were also excluded, resulting in an analytic sample of 210,610 singleton pregnancies among 192,957 women. Most women (175,589, 91%) contributed only 1 pregnancy.
Preeclampsia was ascertained using both the maternal medical records and discharge summaries. Best obstetrical estimate of gestational age was obtained from the medical records. Neonatal complications studied included perinatal mortality ≥23 weeks of gestation (defined as antepartum fetal deaths, intrapartum deaths, and neonatal deaths within the first week of life), small for gestational age (SGA) infants (lowest 10% of birth weight for age and sex),17 NICU admission, respiratory distress syndrome, transient tachypnea of the newborn, anemia, apnea, asphyxia, peri- or intraventricular hemorrhage, and cardiomyopathy. Demographic characteristics of women with and without preeclampsia and prevalence of neonatal complications were tabulated using contingency tables. We performed no statistical comparisons for differences in demographic, clinical, or medical history variables or for crude outcome prevalence.
Total effects of preeclampsia on neonatal outcomes among all infants were estimated using logistic regression with generalized estimating equations. We adjusted analyses for study site, maternal age, maternal race/ethnicity, insurance status, marital status, parity, prepregnancy body mass index (BMI), and chronic diseases during pregnancy (asthma, thyroid disease, diabetes, depression and heart, renal or gastrointestinal disease). Singleton pregnancy was the unit of analysis for all statistical testing, and an autoregressive correlation matrix was used to account for correlation between pregnancies of the same woman. Neonates with missing outcome data were excluded from the analyses of the outcome in question (eg, 1 study site did not report infant apnea), but not from other analyses.
Marginal structure models with stabilized inverse probability weights were applied to estimate controlled direct effects of preeclampsia on neonatal complications, not mediated by preterm delivery.15 We used weighted logistic regression with generalized estimating equations to estimate the parameters of the marginal structure models and the controlled direct effect of preeclampsia on neonatal outcomes. The controlled direct effects conceive of hypothetical interventions on the mediator (here, preterm birth) that change its value so that controlled direct effects can be conceptualized with fixed values of the mediator (eg, delivery at term defined as ≥37 weeks of gestation). We estimated the controlled direct effects of preeclampsia for a hypothetical intervention where all infants were delivered at term. The controlled direct effect of preeclampsia was modeled as a function of preeclampsia (a), preterm birth (m), and the interaction between preeclampsia and preterm birth, as follows:
| (1) |
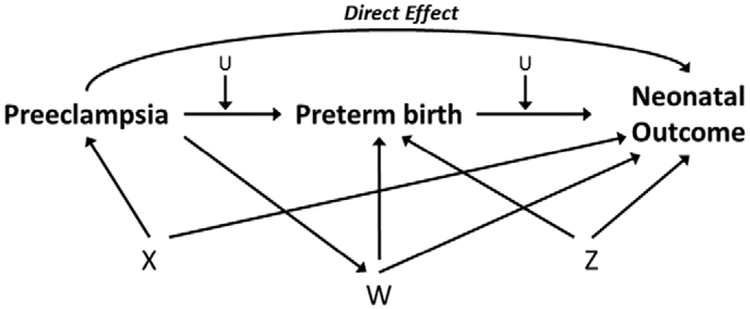
Stabilized inverse probability weights were obtained by estimating 2 sets of weights with logistic regression, 1 for dichotomous preeclampsia status and 1 for the categories of preterm status.15,18–20 The weights were multiplied together to form a single weight for each mother–infant pair, and weights at the 1st and 99th percentile were truncated. The preeclampsia weight models included hypothesized confounders of the preeclampsia–neonatal outcome and preeclampsia–preterm birth associations (eg, study site, maternal age, maternal race/ethnicity, insurance status, marital status, parity, prepregnancy BMI, and chronic diseases during pregnancy; Figure, factors included in “X”) and hypothesized confounders of the preterm birth–neonatal outcome association (including pregnancy complications such as chorioamnionitis and placenta previa, maternal and fetal indications for delivery [including oligoand polyhydramnios, active herpes, HIV and unspecified reasons], and major fetal anomalies; Figure, factors included in “Z”). The preterm weight models additionally included confounders of the preterm birth–neonatal outcome association that can be influenced by the presence of preeclampsia (such as cesarean delivery, placental abruption, and fetal compromise/distress; Figure, factors included in “W”), where risk of the confounder is increased by the presence of preeclampsia, as well as macrosomia, which is likely to be decreased given the relationship of preeclampsia and fetal growth restriction.6 We note that the Figure is relatively simplistic, given the complex relationships that exist for specific outcomes related to timing of delivery, but it serves to illustrate the assumptions behind our modeling approach.
FIGURE.
Potential confounders of the preeclampsia-preterm birth (<37 weeks)-neonatal health relationship included in the calculation of weights for the marginal structural model. X represents confounders of the preeclampsia-neonatal outcome association and preeclampsia-preterm birth association: study site, maternal age, race/ethnicity, insurance status, marital status, prepregnancy body mass index, parity, and maternal chronic diseases. W represents confounders of the preterm birth-neonatal outcome association that are affected by prior exposure to preeclampsia: fetal macrosomia, fetal distress/compromise, placental abruption, and cesarean delivery. Z represents confounders of the preterm birth-neonatal outcome association not included in X: chorioamnionitis, placenta previa, major fetal anomaly, maternal HIV positivity, maternal genital herpes, oligo- and polyhydramnios, other maternal or fetal reasons for delivery. U represents an unmeasured confounder.
Inverse probability weighting is used to consistently estimate the parameters of the marginal structure model under the assumptions of positivity (assumes that data are available in all conditions), no unmeasured confounding, and correct model specification.15,18–20 The mean of the stabilized weights for the models was 1.00 (range = 0.04–4.61).
To assess the effect of a possible unmeasured confounding factor (such as maternal infection) of the preterm birth–neonatal outcomes (mediator-outcome) pathway on estimates of direct effects, we performed a sensitivity analysis for direct effects under a range of potential bias conditions.21,22 Maternal infection was considered a potential unmeasured confounder because neonatal morbidity and preterm birth may both be affected by infection, and (with the exception of chorioaminionitis) infection data were not available in this study. In the sensitivity analyses, we allowed the prevalence of the binary unmeasured confounder to vary between 1% and 99% among normotensive term and preterm pregnancies, and the relationship between the unmeasured binary confounder and the outcome of interest to vary between odds of 2.0 and 10.0. We additionally estimated the constant effect size of the unmeasured confounder required to completely explain away the observed direct effect of preeclampsia (ie, leading to direct effects odds ratio [OR] = 1.0). These analyses were performed for the outcomes with the largest and smallest observed direct effects to cover the range of associations observed in this study.21,22
As timing of diagnosis was not available and we recognize there are likely to be differences in antiangiogenic factors in early versus late onset preeclampsia,23 we repeated our analyses restricting data to normotensive women and to women with severe preeclampsia. This analysis was intended to assess whether our findings were robust when considering severe preeclampsia cases that were most likely to deliver pre-term (eFigure; http://links.lww.com/EDE/A854) and potentially have greater neonatal health impact.
All results are presented as ORs with 95% confidence intervals (CIs). SAS version 9.3 (SAS Institute Inc., Cary, NC) was used in all analyses.
RESULTS
Characteristics of normotensive and preeclamptic mothers are described in Table 1. Mean maternal age was 27.5 (standard deviation = 6.1) in normotensive women and 27.1 (6.7) in women with preeclampsia. As anticipated, preterm birth <37 weeks was substantially higher in pregnancies complicated by preeclampsia (34%) compared with normotensive pregnancies (10%). The crude prevalence of adverse neonatal outcomes (Table 2) was generally higher for preterm infants among both normotensive and preeclamptic pregnancies.
TABLE 1.
Maternal Demographic and Clinical Characteristicsa of Singleton Pregnancies by Preeclampsia Status in the Consortium on Safe Labor (2002–2008)
| Normotensive (n = 200,103) | Preeclampsia (n = 10,507) | |
|---|---|---|
| No. pregnancies per woman | ||
| 1 | 182,083 (91.1) | 9,944 (94.6) |
| 2 | 17,169 (8.6) | 525 (5.0) |
| ≥3 | 851 (0.4) | 38 (0.4) |
| Maternal age (years); mean (SD) | 27.5 (6.1) | 27.1 (6.7) |
| Race/ethnicity | ||
| Non-Hispanic White | 100,506 (50.2) | 4,593 (43.7) |
| Non-Hispanic Black | 42,529 (21.3) | 3,100 (29.5) |
| Hispanic | 35,432 (17.7) | 1,728 (16.5) |
| Asian/Pacific Islander | 8,619 (4.3) | 381 (3.6) |
| Other | 4,721 (2.4) | 273 (2.6) |
| Unknown | 8,296 (4.2) | 433 (4.1) |
| Insurance status | ||
| Private | 112,701 (56.3) | 5,850 (55.7) |
| Public/self-pay | 65,840 (32.9) | 3,737 (35.6) |
| Other | 267 (0.1) | 18 (0.2) |
| Unknown | 21,295 (10.6) | 902 (8.6) |
| Nulliparous | 77,490 (38.7) | 6,118 (58.2) |
| Prepregnancy BMIb | ||
| Underweight | 7,604 (3.8) | 208 (2.0) |
| Normal weight | 74,020 (37.0) | 2,562 (24.4) |
| Overweight | 29,775 (14.9) | 1,591 (15.1) |
| Obese | 13,205 (6.6) | 986 (9.4) |
| Severely obese | 9,264 (4.6) | 947 (9.0) |
| Unknown | 66,235 (33.1) | 4,213 (40.1) |
| Marital status | ||
| Married | 119,179 (59.6) | 5,400 (51.4) |
| Divorced/widowed | 3,102 (1.6) | 168 (1.6) |
| Single | 71,449 (35.7) | 4,628 (44.1) |
| Unknown | 6,373 (3.2) | 311 (3.0) |
| Chronic disease during pregnancyc | 33,256 (16.6) | 2,173 (20.7) |
| Maternal indication for deliveryd | 7,060 (3.5) | 772 (7.4) |
| Suspected fetal macrosomia | 21,013 (10.5) | 1,053 (10.0) |
| Fetal indication for deliverye | 17,470 (8.7) | 1,414 (13.5) |
| Chorioamnionitis | 6,238 (3.1) | 303 (2.9) |
| Placenta previa | 1,437 (0.7) | 63 (0.6) |
| Major fetal anomaly | 3,517 (1.8) | 350 (3.3) |
| Preterm birth <37 weeks | 19,546 (9.8) | 3,597 (34.2) |
All results are reported as No. (%), unless otherwise specified.
Prepregnancy BMI is categorized as: underweight, BMI <18.5 kg/m2; normal weight, BMI 18.5–24.9 kg/m2; overweight, BMI 25.0–29.9 kg/m2; obese, BMI 30.0–34.9 kg/m2; and severely obese, BMI ≥35 kg/m2.
Includes asthma, thyroid disease, diabetes, depression and heart, renal or gastrointestinal diseases.
Includes HIV positivity, thromboembolic disorders, active genital herpes, polyhydramnios, and unspecified maternal indications.
Includes fetal compromise or distress, oligohydramnios and blood type incompatibility.
TABLE 2.
Prevalence of Neonatal Outcomes by Maternal Preeclampsia Status in the Consortium on Safe Labor (2002–2008)
| Outcome | Normotensive (n = 200,103) | Preeclampsia (n = 10,507) | ||
|---|---|---|---|---|
| Preterm (n = 19,546) No. (%) |
Term (n = 180,557) No. (%) |
Preterm (n = 3,597) No. (%) |
Term (n = 6,910) No. (%) |
|
| Perinatal mortalitya | 916 (4.7) | 292 (0.2) | 99 (2.8) | 12 (0.2) |
| Small for gestational ageb | 1,429 (7.3) | 17,458 (9.7) | 745 (20.7) | 1,076 (15.6) |
| NICU admission | 9,530 (48.8) | 12,135 (6.7) | 2,212 (61.5) | 794 (11.5) |
| Respiratory distress syndrome | 4,255 (21.8) | 1,085 (0.6) | 977 (27.2) | 78 (1.1) |
| Transient tachypnea of the newborn | 2,059 (10.5) | 4,610 (2.6) | 426 (11.8) | 251 (3.6) |
| Neonatal anemia | 2,305 (11.8) | 792 (0.4) | 559 (15.5) | 37 (0.5) |
| Apneac | 2,639 (13.5) | 759 (0.5) | 678 (18.9) | 58 (1.1) |
| Asphyxia | 195 (1.0) | 279 (0.2) | 31 (0.9) | 16 (0.2) |
| Peri- or intraventricular hemorrhage | 861 (4.4) | 114 (0.1) | 169 (4.7) | 11 (0.2) |
| Cardiomyopathy | 81 (0.4) | 84 (0.1) | 16 (0.5) | 4 (0.1) |
Defined as fetal deaths ≥23 weeks, intrapartum deaths, and neonatal deaths during the first week of life.
Defined as <10th percentile of birth weight for gestational age and infant sex after excluding missing and implausible values.
Data on apnea are missing from 1 study site (missing for 1874 pregnancies with preeclampsia and 18,508 normotensive pregnancies).
All adverse neonatal outcomes studied were associated with preeclampsia in adjusted total effects regression models (Table 3). When considering direct effects, we observed that preeclampsia remained associated with an approximately 2-fold increased odds of perinatal mortality, SGA, NICU admission, and apnea; a more than 2.5-fold increased odds of respiratory distress syndrome and asphyxia; and a 3-fold increased odds of peri- or intraventricular hemorrhage. Transient tachypnea of the newborn was also increased 1.6-fold at term. The evidence for a direct effect of preeclampsia at term was less clear for neonatal anemia (OR = 1.5; 95% CI = 1.0–2.4) and cardiomyopathy (OR=2.1; 95% CI: 0.6–7.4).
TABLE 3.
Association of Neonatal Outcomes with the Total Effects of Preeclampsia and Controlled Direct Effects Accounting for Mediation by Preterm Birth, Consortium on Safe Labor (2002–2008)
| Outcome | Total Effectsa | Controlled Direct Effectsb | ||
|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | |
| Perinatal mortalityc | 1.6 | (1.3–2.0) | 2.2 | (1.1–4.5) |
| Small for gestational aged | 1.9 | (1.8–2.0) | 1.9 | (1.8–2.1) |
| NICU admission | 3.1 | (2.9–3.2) | 1.9 | (1.7–2.1) |
| Respiratory distress syndrome | 4.1 | (3.8–4.4) | 2.8 | (2.0–3.7) |
| Transient tachypnea of the newborn | 1.9 | (1.7–2.0) | 1.6 | (1.3–1.9) |
| Neonatal anemia | 3.3 | (3.0–3.6) | 1.5 | (1.0–2.4) |
| Apneae | 4.2 | (3.8–4.6) | 2.2 | (1.6–3.1) |
| Asphyxia | 1.8 | (1.3–2.5) | 2.7 | (1.5–4.9) |
| Peri- or intraventricular hemorrhage | 2.9 | (2.4–3.4) | 3.2 | (1.4–7.7) |
| Cardiomyopathy | 2.9 | (1.8–4.8) | 2.1 | (0.6–7.4) |
The total effect odds of neonatal outcomes were estimated using logistic regression with generalized estimating equations. All analyses were adjusted for study site, maternal age, maternal race/ethnicity, insurance status, marital status, parity, prepregnancy body mass index and chronic diseases during pregnancy.
The controlled direct effect of preeclampsia not mediated by preterm was estimated using marginal structural models with inverse probability weights with delivery set at term (≥37 weeks). ORs were estimated using weighted logistic regression with generalized estimating equations.
Defined as fetal deaths ≥23 weeks, intrapartum deaths, and neonatal deaths during the first week of life.
Defined as <10th percentile of birth weight for gestational age and infant sex after excluding missing and implausible values.
Data on apnea are missing from 1 study site (missing for 1874 pregnancies with preeclampsia and 18,508 normotensive pregnancies).
Our sensitivity analysis across the range of direct effects demonstrated that the controlled direct effects we observed were generally robust in the presence of a moderate to strong binary confounder (Table 4). For example, when the unmeasured confounder was present in 1% of normotensive and 5% of preeclamptic pregnancies, the estimated direct effect of preeclampsia ranged from 2.4 to 3.1 for peri- or intraventricular hemorrhage, as compared with 3.2 in our main models, across a range of confounder–outcome associations from an OR of 2.0 to 10.0. In the same example, when the confounder prevalence is higher (20% for normotension, 40% for preeclampsia), the bias corrected odds are lower, ranging from 2.7 to 2.0. We additionally calculated the prevalence and strength of an unmeasured confounder needed to eliminate the direct effect we observed for peri- or intraventricular hemorrhage (OR = 3.2) and transient tachypnea of newborn (OR = 1.6), the largest and smallest effects we report. The observed direct effect would be explained by an unmeasured confounder only in scenarios such as when the confounder was rare (prevalence ≤5%) but had a very strong effect on the outcome (ORs >17); when the confounder was rare among normotensive term pregnancies (≤5%) but prevalent among preeclamptic term pregnancies and had at least a moderate effect on the outcome; or if the confounder was prevalent among normotensive term pregnancies and rare among preeclamptic term pregnancies with a strong protective effect on the outcome. When the observed effects are stronger, the scenarios need to be more extreme. For example, our smallest observed effect (OR = 1.6 for transient tachypnea of newborn) could be explained if our hypothesized confounder, maternal infection, was present in 5% of normotensive pregnancies and 40% of pregnancies with preeclampsia with moderately strong odds (OR = ≥2.8). Even in this potentially plausible scenario, pregnancies with preeclampsia would need to have an 8-fold increase in infection compared with normotensive pregnancies to account for the finding.
TABLE 4.
The Effect of a Potential Unmeasured Binary Confounder in the Preterm Birth-to-Outcome Pathway, Considering Varying Relationships Between the Confounder and Outcome
| Prevalence of Binary Unmeasured Confounder | Bias-Corrected Odds Ratio | Odds Ratio Required to Explain Away the Observed Direct Effecta | ||||
|---|---|---|---|---|---|---|
| Normotensive Term Pregnancies (%) | Preeclampsia Term Pregnancies (%) | The Effect Size of the Unmeasured Confounder in Odds Ratio | ||||
| 2.0 | 4.0 | 6.0 | 10.0 | |||
| Peri- or intraventricular hemorrhage (direct effect OR = 3.2) | ||||||
| 1 | 5 | 3.1 | 2.9 | 2.7 | 2.4 | 124.5 |
| 10 | 3.0 | 2.5 | 2.3 | 1.8 | 33.5 | |
| 20 | 2.7 | 2.1 | 1.7 | 1.3 | 14.2 | |
| 40 | 2.3 | 1.5 | 1.1 | 0.8 | 7.0 | |
| 60 | 2.0 | 1.2 | 0.8 | 0.6 | 4.9 | |
| 80 | 1.8 | 1.0 | 0.7 | 0.4 | 3.9 | |
| 99 | 1.6 | 0.8 | 0.6 | 0.4 | 3.3 | |
| 5 | 5 | 3.2 | 3.2 | 3.2 | 3.2 | —b |
| 10 | 3.1 | 2.8 | 2.7 | 2.5 | — | |
| 20 | 2.8 | 2.3 | 2.0 | 1.7 | 56.9 | |
| 40 | 2.4 | 1.7 | 1.3 | 1.0 | 10.2 | |
| 60 | 2.1 | 1.3 | 1.0 | 0.7 | 6.0 | |
| 80 | 1.9 | 1.1 | 0.8 | 0.6 | 4.5 | |
| 99 | 1.7 | 0.9 | 0.7 | 0.5 | 3.7 | |
| 10 | 5 | 3.4 | 3.6 | 3.9 | 4.2 | — |
| 10 | 3.2 | 3.2 | 3.2 | 3.2 | — | |
| 20 | 2.9 | 2.6 | 2.4 | 2.2 | — | |
| 40 | 2.5 | 1.9 | 1.6 | 1.3 | 29.0 | |
| 60 | 2.2 | 1.5 | 1.2 | 1.0 | 8.9 | |
| 80 | 2.0 | 1.2 | 1.0 | 0.7 | 5.6 | |
| 99 | 1.8 | 1.1 | 0.8 | 0.6 | 4.3 | |
| 20 | 5 | 3.7 | 4.5 | 5.1 | 6.2 | — |
| 10 | 3.5 | 4.0 | 4.3 | 4.7 | — | |
| 20 | 3.2 | 3.2 | 3.2 | 3.2 | — | |
| 40 | 2.7 | 2.3 | 2.1 | 2.0 | — | |
| 60 | 2.4 | 1.8 | 1.6 | 1.4 | — | |
| 80 | 2.1 | 1.5 | 1.3 | 1.1 | 15.0 | |
| 99 | 1.9 | 1.3 | 1.1 | 0.9 | 7.4 | |
| 40 | 5 | 4.3 | 6.1 | 7.7 | 10.2 | — |
| 10 | 4.1 | 5.4 | 6.4 | 7.8 | — | |
| 20 | 3.8 | 4.4 | 4.8 | 5.3 | — | |
| 40 | 3.5 | 3.7 | 3.9 | 4.0 | — | |
| 60 | 3.2 | 3.2 | 3.2 | 3.2 | — | |
| 80 | 3.0 | 2.8 | 2.8 | 2.7 | — | |
| 99 | 2.8 | 2.5 | 2.4 | 2.3 | — | |
| 60 | 5 | 4.9 | 7.8 | 10.3 | 14.2 | — |
| 10 | 4.7 | 6.9 | 8.6 | 10.8 | — | |
| 20 | 4.3 | 5.6 | 6.4 | 7.3 | — | |
| 40 | 4.0 | 4.7 | 5.1 | 5.6 | — | |
| 60 | 3.7 | 4.1 | 4.3 | 4.5 | — | |
| 80 | 3.4 | 3.6 | 3.7 | 3.7 | — | |
| 99 | 3.2 | 3.2 | 3.2 | 3.2 | — | |
| 80 | 5 | 5.5 | 9.5 | 12.8 | 18.2 | 0.1 |
| 10 | 5.3 | 8.4 | 10.7 | 13.9 | 0.1 | |
| 20 | 4.8 | 6.8 | 8.0 | 9.4 | 0.1 | |
| 40 | 4.5 | 5.7 | 6.4 | 7.1 | — | |
| 60 | 4.1 | 5.0 | 5.4 | 5.7 | — | |
| 80 | 3.9 | 4.4 | 4.6 | 4.8 | — | |
| 99 | 3.6 | 3.9 | 4.0 | 4.1 | — | |
| Transient tachypnea of the newborn (direct effect OR = 1.6) | ||||||
| 1 | 5 | 1.5 | 1.4 | 1.3 | 1.2 | 17.6 |
| 10 | 1.4 | 1.2 | 1.1 | 0.9 | 7.8 | |
| 20 | 1.3 | 1.0 | 0.8 | 0.6 | 4.1 | |
| 40 | 1.1 | 0.7 | 0.6 | 0.4 | 2.5 | |
| 60 | 1.0 | 0.6 | 0.4 | 0.3 | 2.0 | |
| 80 | 0.9 | 0.5 | 0.3 | 0.2 | 1.7 | |
| 99 | 0.8 | 0.4 | 0.3 | 0.2 | 1.6 | |
| 5 | 5 | 1.6 | 1.6 | 1.6 | 1.6 | — |
| 10 | 1.5 | 1.4 | 1.3 | 1.2 | 27.5 | |
| 20 | 1.4 | 1.1 | 1.0 | 0.8 | 5.7 | |
| 40 | 1.3 | 1.0 | 0.8 | 0.6 | 2.8 | |
| 60 | 1.2 | 0.8 | 0.7 | 0.5 | 2.1 | |
| 80 | 1.1 | 0.7 | 0.6 | 0.4 | 1.8 | |
| 99 | 1.0 | 0.7 | 0.5 | 0.4 | 1.6 | |
| 10 | 5 | 1.7 | 1.8 | 1.9 | 2.1 | — |
| 10 | 1.6 | 1.6 | 1.6 | 1.6 | — | |
| 20 | 1.4 | 1.3 | 1.2 | 1.1 | 14.3 | |
| 40 | 1.3 | 1.1 | 0.9 | 0.8 | 3.3 | |
| 60 | 1.2 | 0.9 | 0.8 | 0.7 | 2.3 | |
| 80 | 1.2 | 0.8 | 0.7 | 0.5 | 1.9 | |
| 99 | 1.1 | 0.7 | 0.6 | 0.5 | 1.7 | |
| 20 | 5 | 1.8 | 2.2 | 2.5 | 3.0 | — |
| 10 | 1.7 | 1.9 | 2.1 | 2.3 | — | |
| 20 | 1.6 | 1.6 | 1.6 | 1.6 | — | |
| 40 | 1.5 | 1.3 | 1.3 | 1.2 | 7.6 | |
| 60 | 1.4 | 1.1 | 1.1 | 1.0 | 3.0 | |
| 80 | 1.3 | 1.0 | 0.9 | 0.8 | 2.2 | |
| 99 | 1.2 | 0.9 | 0.8 | 0.7 | 1.8 | |
| 40 | 5 | 2.1 | 3.0 | 3.8 | 5.0 | — |
| 10 | 2.0 | 2.7 | 3.1 | 3.8 | — | |
| 20 | 1.8 | 2.2 | 2.4 | 2.6 | — | |
| 40 | 1.7 | 1.8 | 1.9 | 2.0 | — | |
| 60 | 1.6 | 1.6 | 1.6 | 1.6 | — | |
| 80 | 1.5 | 1.4 | 1.4 | 1.3 | — | |
| 99 | 1.4 | 1.2 | 1.2 | 1.1 | — | |
| 60 | 5 | 4.9 | 7.8 | 10.3 | 14.2 | 0.4 |
| 10 | 4.7 | 6.9 | 8.6 | 10.8 | 0.3 | |
| 20 | 4.3 | 5.6 | 6.4 | 7.3 | 0.2 | |
| 40 | 4.0 | 4.7 | 5.1 | 5.6 | — | |
| 60 | 3.7 | 4.1 | 4.3 | 4.5 | — | |
| 80 | 3.4 | 3.6 | 3.7 | 3.7 | — | |
| 99 | 3.2 | 3.2 | 3.2 | 3.2 | — | |
| 80 | 5 | 5.5 | 9.5 | 12.8 | 18.2 | 0.5 |
| 10 | 5.3 | 8.4 | 10.7 | 13.9 | 0.5 | |
| 20 | 4.8 | 6.8 | 8.0 | 9.4 | 0.5 | |
| 40 | 4.5 | 5.7 | 6.4 | 7.1 | 0.3 | |
| 60 | 4.1 | 5.0 | 5.4 | 5.7 | 0.1 | |
| 80 | 3.9 | 4.4 | 4.6 | 4.8 | — | |
| 99 | 3.6 | 3.9 | 4.0 | 4.1 | — | |
The effect size required to explain away the direct effect of the outcome is calculated from the equation γ=(π1m+B-Bπ0m-1)/(π1m-Bπ0m), where B is the amount of bias necessary to render the association null (B = 3.21 and B = 1.57 for peri- or intraventricular hemorrhage and transient tachypnea of the newborn, respectively) and π1m and π0m are the prevalence of the unmeasured confounder in preeclamptic and normotensive term pregnancies, respectively.
The calculation results in odds <0 which are implausible.
We also restricted the analyses to pregnancies complicated by severe preeclampsia (Table 5). Generally, the controlled direct effect estimates of neonatal outcomes in the subset of severe preeclampsia with delivery set at term were somewhat stronger than in the full group that included mild preeclampsia, but with a notable loss of precision.
TABLE 5.
Odds Ratios (OR) and 95% Confidence Intervals (CIs) for Neonatal Outcomes Associated with the Total Effects of Severe Preeclampsia and Controlled Direct Effects Accounting for Mediation by Preterm Birth in the Consortium on Safe Labor (2002–2008)
| Outcome | Total Effectsa | Controlled Direct Effectsb | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Perinatal mortalityc | 2.7 | 2.1–3.5 | 4.5 | 0.9–22.1 |
| SGAd | 2.6 | 2.4–2.9 | 3.5 | 2.9–4.3 |
| NICU admission | 7.6 | 7.0–8.1 | 2.9 | 2.2–3.7 |
| Respiratory distress syndrome | 9.5 | 8.7–10.4 | 8.1 | 4.4–14.9 |
| Transient tachypnea of the newborn | 2.7 | 2.4–3.0 | 1.8 | 1.1–2.8 |
| Neonatal anemia | 7.3 | 6.5–8.2 | 0.8 | 0.3–1.7 |
| Apneae | 8.9 | 8.0–9.9 | 3.0 | 1.4–6.3 |
| Asphyxia | 2.8 | 1.9–4.2 | 7.1 | 2.5–20.4 |
| Peri- or intraventricular hemorrhage | 5.6 | 4.7–6.8 | 16.9 | 4.9–58.4 |
| Cardiomyopathy | 5.3 | 2.8–9.9 | 3.3 | 0.5–23.4 |
The total effect odds of neonatal outcomes were estimated using logistic regression with generalized estimating equations. All analyses were adjusted for study site, maternal age, maternal race/ethnicity, insurance status, marital status, parity, prepregnancy body mass index, and chronic diseases during pregnancy. Statistically significant results are bolded.
The controlled direct effect of preeclampsia not mediated by preterm was estimated using marginal structural models with inverse probability weights with delivery set at term (≥37 weeks). ORs were estimated using weighted logistic regression with generalized estimating equations. Statistically significant results are bolded.
Defined as fetal deaths ≥23 weeks, intrapartum deaths, and neonatal deaths during the first week of life.
Defined as <10th percentile of birth weight for gestational age and infant sex after excluding missing and implausible values
Data on apnea are missing from one study site (missing for 113 pregnancies with severe preeclampsia and 18,508 normotensive pregnancies).
CI indicates confidence interval; NICU, neonatal intensive care unit; OR, odds ratio; SGA, small for gestational age.
DISCUSSION
Our analyses suggest that preeclampsia was associated with many serious neonatal complications through pathways not mediated by preterm birth. Using marginal structure models to adjust for cesarean delivery and placental abruption (important confounding factors of the mediator outcome association that are affected by preeclampsia), as well as estimating the direct effect of preeclampsia,24 uncovered novel associations that may be related to the underlying disease process. Even in a hypothetical intervention in which all infants could have been delivered at term (≥37 weeks), there was substantial morbidity associated with preeclampsia, including an increased odds of perinatal mortality, SGA, NICU admission, and respiratory distress syndrome. We also observed increased odds at term for transient tachypnea of newborn, peri- or intraventricular hemorrhage, apnea, and asphyxia. Interestingly, marginal structure models restricted to severe preeclampsia yielded similar results, suggesting that our findings were robust even among cases more likely to deliver pre-term and to have more severe pathology.
The total effect models include infants of all gestational ages and reflect the increased risk of preeclampsia on several neonatal outcomes due to preterm birth as well as preeclampsia itself. Consequently, the total effect estimates were in general larger than the direct effects at term, which are independent of the preterm birth mediator. When we restricted the total effects model to preterm infants, ignoring the potential interaction between preterm birth and preeclampsia, we found the odds were attenuated. However, marginal structure models allowed us to estimate the direct effects, while considering potential interactions and factors that were consequences of preeclampsia that confound the preterm birth and neonatal outcomes relationship, such as cesarean delivery and placental abruption. Although the adverse outcomes we studied were more common at earlier gestational ages, significant risks associated with preeclampsia remained when delivery was set at term. Theoretically, the marginal structure models provide us with a causal response to this hypothetical intervention, but we acknowledge the limitations of current methods and the necessary assumptions required for these analyses. Although we pose a relevant intervention for an important clinical question, our results are subject to those limitations and need to be interpreted within that context.
Prior studies of neonatal complications support pathways between preeclampsia and complications independent of preterm birth. This leaves the question—if not due to preterm delivery, how does preeclampsia impact neonatal morbidity? Possible mechanisms include a direct effect from placental transmission of antiangiogenic factors or a result of altered placental function due to an imbalance in maternal angiogenesis. Alterations in maternal angiogenic factors such as sFlt-1, placental growth factor, and sEng have been associated with maternal and neonatal complications, including NICU admission and preterm delivery, particularly for pregnancies presenting before 37 weeks.25 SGA and NICU admission have also been reported to be increased in infants born to mothers with hypertensive pregnancies (gestational hypertension combined with preeclampsia) delivered between 35 and 37 weeks,26 and at early term (37 weeks) NICU length of stay was also increased. Placental transmission of antiangiogenic factors in preeclampsia is not well understood. Amniotic fluid levels of sEng and sFlt-1 can be elevated in women with preeclampsia, as early as 17 weeks gestation,27,28 while other studies show no such difference.29 In preeclampsia, sFlt-1 is elevated in fetal circulation,28 suggesting that placental transmission occurs. In contrast, preeclampsia did not appear to have an important effect on VEGF or sFlt-1 in cord blood of preterm newborns with respiratory distress syndrome or in tracheal aspirate fluid of infants with severe respiratory distress syndrome.30 Given that we do not have any biomarker data to address this issue directly, we note that our finding of increased risk for peri- or intraventricular hemorrhage at term is inconsistent with the notion of restricted fetal vascularization. A protective effect of preeclampsia in reducing cerebral hemorrhage has been seen in other cohorts and this issue warrants further investigation.31,32 A direct causal pathway for other morbidities examined such as apnea, transient tachypnea of the newborn, and asphyxia, is less clear and further investigation is necessary.
The strengths of this study include the large, clinically rich database that provided a wealth of information on many potential factors in the mediation pathway between preeclampsia, preterm birth, and neonatal health. The use of marginal structure models to estimate controlled direct effects in this study offers several important advantages over standard approaches. Importantly, this approach accommodates potential interactions between preeclampsia and preterm birth, and accounted for confounders of the preterm birth and neonatal outcomes association that are affected by preeclampsia. In this situation, standard approaches are inadequate and controlled direct effects can be estimated using marginal structure models.10,15,33 However, our analysis was restricted by the assumptions of marginal structure models. In particular, when estimating controlled direct effects, we hypothesized interventions to deliver at term with the notion that this intervention would minimize adverse neonatal outcomes. Although intervention is theoretically possible, spontaneous preterm labor and delivery are not amenable to intervention, and clinical decision making and evaluation of individual cases would determine whether earlier or later delivery is appropriate—particularly because maternal health could be compromised and stillbirth risk could be increased by delaying delivery in some cases.34,35 Although natural direct and indirect effects would likely be of more interest in this case, these effects are generally not identifiable when time-dependent confounding is present.15 In addition, marginal structure models are based on several strong assumptions, such as no unmeasured confounding. However, we performed sensitivity analyses across a range of scenarios for unmeasured confounding to assess the robustness of the findings. Although our sensitivity analyses identified scenarios where an unmeasured confounder in the preterm birth and neonatal outcome pathway could explain the observed association, most scenarios required either a very strong harmful or protective effect on the outcome by the confounder or a very high prevalence of the confounder among pregnant women. The assumption of positivity was also assessed by evaluating whether there was a positive probability of preeclampsia at each level of the confounders, and by evaluating the distribution of the weight models. The distribution of the weight models was not indicative of nonpositivity, and the results were robust to different model specifications for the weights, as well as the final marginal structure models.
A limitation of the intrapartum medical records as a source of case ascertainment in our study is the lack of data on timing of preeclampsia diagnosis and lack of prenatal record data on proteinuria, blood pressure, or measurement of angiogenic factors. The large, clinically rich database provides a wealth of data on potential factors in the mediation pathway between preeclampsia, preterm birth, and neonatal health but not all data of interest are included in the hospital admission records. However, restricting the preeclampsia cases to those classified in the medical record as severe resulted in similar risks for infants hypothetically delivered at term. We also note that the sensitivity analyses we conducted assess the impact of a single unmeasured confounder (although multiple unmeasured confounders could be present) and use methods based on the situation where there is no mediator-outcome confounder that could be affected by prior exposure. Considering these methods as the best available strategies to assess unmeasured confounding, we find that our results were generally robust in the presence of an unmeasured confounder within most exposure scenarios.
Preterm birth is a major risk factor in neonatal complications, and preeclampsia substantially increases the risk of preterm delivery. Our novel analysis investigated the effects of preeclampsia while accounting for the mediating effect of preterm birth. We found increased direct effects for many serious neonatal complications at term including perinatal mortality, respiratory distress syndrome, transient tachypnea of the newborn, peri- or intraventricular hemorrhage, apnea, and asphyxia. Findings were similar among cases of severe preeclampsia and appeared stable in the presence of a strong unmeasured confounder. Applying marginal structure models to this important research question suggests that preeclampsia was associated with many serious neonatal complications through pathways not mediated by preterm birth, perhaps due to the antiangiogenic factors that are a hallmark of the disease. A hypothetical intervention where all infants were delivered at term did not protect against adverse neonatal outcomes associated with preeclampsia. This suggests further research on strategies to protect term infants from adverse effects related to preeclampsia is warranted.
ACKNOWLEDGMENTS
Our colleague and esteemed preeclampsia researcher, Richard J. Levine, proposed the idea for this manuscript and led some of the preliminary analyses before his death in 2011. The study team is honored to have carried his idea to fruition and we dedicate this effort to his memory.
We also thank Maeve Wallace for her assistance with analyses and graphics.
Institutions involved in the Consortium on Safe Labor include, in alphabetical order: Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, Utah; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH.; Summa Health System, Akron City Hospital, Akron, OH; The EMMES Corporation, Rockville MD (Data Coordinating Center); University of Illinois at Chicago, Chicago, IL; University of Miami, Miami, FL; and University of Texas Health Science Center at Houston, Houston, Texas.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Defense, Department of Health and Human Services or the United States Government.
Supported by the Intramural Research Program of the National Institutes Health, Eunice Kennedy Shriver National Institute of C Human Development. The Consortium on Safe Labor the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Health through Contract No. HHSN267200603425C.
Footnotes
Supplemental digital content is available through direct URL in the HTML and PDF versions of this article (www.epidem.com). This content is not peer-reviewed or copy-edited; it is the sole respon sibility of the authors.
REFERENCES
- 1.Lindheimer MD, Taler SJ, Cunningham FG. ASH position paper: hyper-tension in pregnancy. J Clin Hypertens (Greenwich). 2009;11:214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. [DOI] [PubMed] [Google Scholar]
- 3.Levine RJ, Lam C, Qian C et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355: 992–1005. [DOI] [PubMed] [Google Scholar]
- 4.Wang A, Holston AM, Yu KF, et al. Circulating anti-angiogenic factors during hypertensive pregnancy and increased risk of respiratory distress syndrome in preterm neonates. J Matern Fetal Neonatal Med. 2012;25:1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Semin Nephrol. 2011;31:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. [DOI] [PubMed] [Google Scholar]
- 7.Auger N, Le TU, Park AL, Luo ZC. Association between maternal comorbidity and preterm birth by severity and clinical subtype: retrospective cohort study. BMC Preg Childbirth. 2011;11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu XD, Branch DW, Karumanchi SA, Zhang J. Preeclampsia and retinopathy of prematurity in preterm births. Pediatrics. 2012;130:e101–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortes Filho JB, Costa MC, Eckert GU, Santos PG, Silveira RC, Procianoy RS. Maternal preeclampsia protects preterm infants against severe retinopathy of prematurity. J Pediatr. 2011;158:372–376. [DOI] [PubMed] [Google Scholar]
- 10.VanderWeele TJ, Mumford SL, Schisterman EF. Conditioning on intermediates in perinatal epidemiology. Epidemiology. 2012;23:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strand KM, Heimstad R, Iversen AC, et al. Mediators of the association between pre-eclampsia and cerebral palsy: population based cohort study. BMJ. 2013;347:f4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann JR, McDermott S, Griffith MI, Hardin J, Gregg A. Uncovering the complex relationship between pre-eclampsia, preterm birth and cerebral palsy. Paediatr Perinat Epidemiol. 2011;25:100–110. [DOI] [PubMed] [Google Scholar]
- 13.Trønnes H, Wilcox AJ, Lie RT, Markestad T, Moster D. Risk of cerebral palsy in relation to pregnancy disorders and preterm birth: a national cohort study. Dev Med Child Neurol. 2014;56:779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.VanderWeele TJ, Hernández-Diaz S. Is there a direct effect of pre-eclampsia on cerebral palsy not through preterm birth? Paediatr Perinat Epidemiol. 2011;25:111–115. [DOI] [PubMed] [Google Scholar]
- 15.VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology. 2009;20:18–26. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Troendle J, Reddy UM et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Männistö T, Mendola P, Reddy U, Laughon SK. Neonatal outcomes and birth weight in pregnancies complicated by maternal thyroid disease. Am J Epidemiol. 2013;178:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- 19.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3:143–155. [DOI] [PubMed] [Google Scholar]
- 20.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VanderWeele TJ. Bias formulas for sensitivity analysis for direct and indirect effects. Epidemiology. 2010;21:540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLehose RF, Kaufman JS. Commentary: the wizard of odds. Epidemiology 2012;23:10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv. 2011;66:497–506. [DOI] [PubMed] [Google Scholar]
- 24.VanderWeele TJ. A three-way decomposition of a total effect into direct, indirect, and interactive effects. Epidemiology. 2013;24:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore AG, Young H, Keller JM, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2651–2657. [DOI] [PubMed] [Google Scholar]
- 26.Habli M, Levine RJ, Qian C, Sibai B. Neonatal outcomes in pregnancies with preeclampsia or gestational hypertension and in normotensive preg nancies that delivered at 35, 36, or 37 weeks of gestation. Am J Obstet Gynecol. 2007;197:406.e1–7. [DOI] [PubMed] [Google Scholar]
- 27.Wang CN, Chang SD, Peng HH, et al. Change in amniotic fluid levels of multiple anti-angiogenic proteins before development of preeclampsia and intrauterine growth restriction. J Clin Endocrinol Metab. 2010;95:1431–1441. [DOI] [PubMed] [Google Scholar]
- 28.Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;122:33–39. [DOI] [PubMed] [Google Scholar]
- 29.Park CW, Park JS, Shim SS, Jun JK, Yoon BH, Romero R. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol. 2005;193(3 Pt 2):984–989. [DOI] [PubMed] [Google Scholar]
- 30.Kalay S, Cakcak B, Oztekin O, et al. The role of VEGF and its soluble receptor VEGFR-1 in preterm newborns of preeclamptic mothers with RDS. J Matern Fetal Neonatal Med. 2013;26:978–983. [DOI] [PubMed] [Google Scholar]
- 31.Ancel PY, Marret S, Larroque B, et al. Are maternal hypertension and small-for-gestational age risk factors for severe intraventricular hemorrhage and cystic periventricular leukomalacia? Results of the EPIPAGE cohort study. Am J Obstet Gynecol. 2005;193:178–184. [DOI] [PubMed] [Google Scholar]
- 32.McElrath TF, Allred EN, Boggess KA, et al. Maternal antenatal complications and the risk of neonatal cerebral white matter damage and later cerebral palsy in children born at an extremely low gestational age. Am J Epidemiol. 2009;170:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufman JS, Maclehose RF, Kaufman S. A further critique of the analytic strategy of adjusting for covariates to identify biologic mediation. Epidemiol Perspect Innov. 2004;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205:191–198. [DOI] [PubMed] [Google Scholar]
- 35.Sibai BM. Management of late preterm and early-term pregnancies complicated by mild gestational hypertension/pre-eclampsia. Semin Perinatol. 2011;35:292–296. [DOI] [PubMed] [Google Scholar]