Abstract
Background
The incidence of squamous cell carcinoma (SCC) of the anal canal has been rising over the past decades, especially in patients infected with human immunodeficiency virus (HIV). Despite the advent of potent multidrug regimens to treat HIV-termed highly active antiretroviral therapy (HAART), anal SCC rates have not declined, and the impact of HAART on anal SCC remains controversial.
Aim
The purpose of this study was to define outcomes of anal SCC treatment in HIV-positive and HIV-negative patients.
Methods and materials
A retrospective single-institution analysis was performed on all patients with anal SCC treated at the Johns Hopkins Hospital between 1991 and 2010. The primary outcomes measured were 5-year overall survival (5-year OS), median survival, and relapse rates.
Results
Our search identified 93 patients with anal SCC. Patients had a mean age of 54 years; 37.6% were male, and 21.5% were HIV-positive. Median follow-up was 28 months. Relapse occurred in 16.1% of patients. Median time to relapse was 20 months. Relapse rates were slightly higher with HIV-positive versus negative patients (30.0 vs.12.3%) but did not reach statistical significance (p = 0.06). Among HIV-positive patients, those who relapsed were more likely to be on HAART than those who did not relapse (83.3 vs. 14.3%, p = 0.007). 5-year OS was 58.9% for the total group of patients with no significant difference between those who relapsed versus those who did not (76.2 vs. 54.5%, p = 0.20). No survival difference was seen between HIV-positive and negative patients. Survival was associated with AJCC stage in all patients.
Conclusion
In our small series, HIV infection was not associated with a significantly higher relapse rate or worse 5-year OS among patients with anal SCC. HAART was associated with a higher rate of relapse in HIV-positive patients. AJCC staging predicted survival in both relapsed and non-relapsed patients regardless of HIV status.
Introduction
It is estimated that about 7000 new cases of anal canal cancer are diagnosed annually in the USA, causing approximately 800 deaths [1]. Although a rare type of malignancy represents only 1–2% of all gastrointestinal cancers, the incidence of anal canal cancer has continued to rise over the past decades, both in the USA and elsewhere [2] . The most common histologic variant of anal canal cancer is squamous cell cancer (SCC anus or anal SCC) [3]. Treatment of SCC anus consists primarily of concurrent chemoradiation, with the most common concurrent regimens being 5-fluorouracil (5-FU) plus mitomycin (MMC) or 5-FU plus cisplatin [4].
Patients with human immunodeficiency virus (HIV) are at increased risk for SCC anus. Studies have shown that chronic immunosuppression, such as HIV infection, can play a significant role in the development of anal canal cancer and may accelerate the progression of precursor lesions [5]. In fact, the prevalence of precursor lesions leading to anal canal cancer is 40–50% in HIV-positive (HIV+) men compared to 10–20% in HIV-negative (HIV−) men [6]. Unlike other HIV-associated cancers, the introduction of potent multidrug regimens termed highly active antiretroviral therapy (HAART) in 1996 has not led to a decrease in the incidence of SCC anus [7]. HAART has been associated with decreased progression of HIV infection to AIDS and death, but at the same time, the risk of SCC anus has risen in this population [8–10].
Many reports of HIV+ populations with SCC anus have been published with variable results [11–21]. HIV+ patients have often been excluded from major randomized trials of SCC anus, and optimal treatment for this patient population remains to be defined. Moreover, it is unclear if compliance with HAART is associated with relapse of anal SCC, or better survival outcomes.
The aim of this single-institution analysis was to retrospectively investigate outcomes of patients with SCC anus, in an effort to identify clinicopathologic predictors of relapse, and prognostic factors of survival. Additionally, given the large HIV+ subpopulation at our institution, we investigated the effect of HAART on relapse and survival among HIV+ patients.
Patients and methods
Patients
The Institutional Review Board at the Johns Hopkins University School of Medicine approved this study. We reviewed the records of all patients treated for anal canal cancer at the Johns Hopkins Hospital from January 1991 to December 2010. Hospital records and tumor registry data were used to gather patient information. Clinical characteristics and outcomes were analyzed retrospectively. Pretreatment staging was performed according to the American Joint Committee on Cancer (AJCC) and included digital examination, transanal endoscopic ultrasound, chest X-rays, and computed tomography [3]. Post-treatment evaluation included digital rectal examination and anoscopy. Post-treatment biopsies, computed tomography, PET-CT, or magnetic resonance imaging were performed when a suspicious lesion was identified. Early-stage anal SCC patients were those defined as either AJCC stage I or II.
Treatments
The primary combined modality therapy regimen delivered to SCC anus patients consisted of conventional radiation therapy with concurrent 5-fluorouracil (5-FU) and Mitomycin C (MMC), or 5-FU and Cisplatin. 5-FU was administered continuously during 4 days (1000 mg/m2), starting on day 1 and 29 of radiation therapy. MMC was given as a bolus on day 1 and 29 of radiation therapy (10 mg/m2). Cisplatin was administered intravenously during 1-hour infusion, in week 1 and 4 at a dose of 40 mg/m2 over 4 days. Radiation therapy was delivered over a 5–6 week period of time with 36–45 Grays (Gy) to the pelvic lymph nodes and a boost to gross nodal and/or primary disease to 50.4–59.40 Gy. Salvage surgery with an abdominoperineal resection (APR) was performed in patients with local failure.
Statistical methods
Median or mean values with standard deviations (SD) are reported. Differences between groups were tested using χ2-, or Fisher’s exact test for categorical variables, and t-tests for continuous variables. Survival and time to relapse were calculated from the beginning of therapy to the day of death and relapse respectively, or the date of last follow-up. Survival curves were plotted using the Kaplan–Meier method. Differences in survival across groups were tested using the Log-rank (Mantel–Cox) test. Confidence intervals (CI) were calculated using the formula 95% CI–M ± (SE × 1.96). Log-rank test and Cox proportional hazard models controlling for stage, age, and gender were used to analyze the effect of categoric and continuous data, respectively, on risk of relapse. STATA version 11 (StataCorp, 2009) was used.
Results
Patient population
We identified 105 patients with histologically proven anal canal cancer. Ninety-three (88.6%) patients had SCC anus, and twelve (11.4%) had adenocarcinomas (all adenocarcinoma patients were HIV-negative). Adenocarcinoma patients were excluded from subsequent statistical analyses. Of the 93 SCC patients, 35 were male (37.6%), and 58 were female (62.4%), with a mean age of 54.0 years (SD 12.6). Twenty (21.5%) were HIV-positive (HIV+), and 73 (78.5%) were HIV-negative (HIV−). Median follow-up from time of diagnosis was 28 months (range 2–186 months).
Relapse and treatment
Of all 93 patients with histologically proven SCC anus, relapse occurred in 15 patients (16.1%; Table 1). Overall median time to relapse was 20 months (range 5–59). Relapse was local in 8 (8.6% of total 93 SCC patients, 53.3% of those who relapsed) patients, distant in 3 (3.2% of total SCC patients, 20% of those who relapsed), and a combination of local and distant in 4 (4.3% of total SCC patients, 26.6% of relapsed patients). There was a trend toward more relapse in the HIV+ group than the HIV− group, but this was not statistically significant (30.0% vs. 12.3%, p = 0.06). Median time to relapse in HIV+ patients was 20 months (range 5–59), versus 21 months (range 6–58) in HIV− patients (p = 0.62). Median followup time was not significantly different in patients without versus with relapse (27 months, [range 2–186] vs. 70 months, [range 9–119]; p = 0.28). Mean tumor size was similar in both no-relapse and relapse groups (3.4 cm vs. 2.4 cm, p = 0.40).
Table 1.
Demographic data of the squamous cell anal canal cancer population in this study
| Total 93 anal SCC patients | No-relapse (n = 78) | Relapse (n = 15) | p value |
|---|---|---|---|
| Sex, % male (n) | 35.9% (28) | 46.6% (7) | 0.43 |
| Mean age ± SD (years) | 54.9 ± 13.1 | 49.7 ± 8.7 | 0.08 |
| Median follow-up in months (range) | 27 (range 2–186) | 70 (range 9–119) | 0.28 |
| HIV-positive patients (n) | 17.9% (14) | 40.0% (6) | 0.06 |
| Mean tumor size ± SD (cm) | 3.4 ± 1.6 | 2.4 ± 2.1 | 0.40 |
N absolute number of patients, HIV human immunodeficiency virus and SD standard deviation
Early-stage anal SCC was defined in the current study as AJCC stage I or II. Table 2 delineates the stage at initial diagnosis of SCC patients based on relapse. In the no-relapse group, 34/75 patients (45.3%) were early stage, and 41 (54.7%) were late stage. In the relapsed group, 9/14 patients (64.3%) were early stage, and 5 patients (35.7%) were late stage. There was no significant difference in stage by relapse status (p = 0.53). Of note, four patients had missing staging data.
Table 2.
Stage of squamous cell anal canal cancer patients at initial diagnosis
| Stage % (n) | No-relapse (n = 75*) | Relapse (n = 14) | Total (n = 89) |
|---|---|---|---|
| I (n) | 20.0% (15) | 28.6% (4) | 21.3% (19) |
| II (n) | 25.3% (19) | 35.7% (5) | 27.0% (24) |
| IIIA (n) | 21.3% (16) | 21.4% (3) | 21.3% (19) |
| IIIB (n) | 17.3% (13) | 14.3% (2) | 16.9% (15) |
| IV (n) | 16.0% (12) | 0% (0) | 13.5% (12) |
4 patients had missing staging data
n absolute number of patients
Of the 93 SCC patients, 56 (60.2%) received combined chemoradiation (Table 3). Average dose of radiation therapy was 53.4 Gy in the no-relapse and 53.1 Gy in the relapse patient group (p = 0.33). Cisplatin was administered in 10 of the 78 no-relapse patients (12.8%) and 2 of the 15 relapse patients (13.3%, p = 1.00). Initial surgery was a local excision of the lesion in ten patients and abdominoperineal resection (APR) in 13 patients. Sixteen of the 78 (20.5%) patients in the no-relapse group had missing treatment data.
Table 3.
Treatment characteristics of all patients treated with squamous cell anal canal cancer
| Treatment modality | No-relapse (n = 78) | Relapse (n = 15) | p value |
|---|---|---|---|
| Chemoradiation (n) | 57.7% (45) | 73.3% (11) | 0.39 |
| RT dose (Gy) | 53.4 ± 5.6 | 53.1 ± 5.6 | 0.33 |
| Cisplatin (n) | 12.8% (10) | 13.3% (2) | 1.00 |
| Mitomycin (n) | 53.8% (42) | 80.0% (12) | 0.08 |
| Surgery, initial | |||
| Local excision (n) | 11.5% (9) | 6.7% (1) | 0.52 |
| APR (n) | 10.3% (8) | 33.3% (5) | 0.08 |
| Surgery for relapse | |||
| Local excision (n) | n/a | 6.7% (1) | 0.32 |
| APR (n) | n/a | 13.3% (2) | 0.14 |
Not listed in table above—one hepatic resection and one pelvic exenteration in the relapse group. 16 of the 78 (20.5%) patients in the no-relapse group had missing treatment data
n absolute number of patients, RT radiation treatment and APR abdominoperineal resection
After diagnosis of relapse, three patients required reexcision of the area, two with APR, and one with a wide local excision. Distant metastases were treated with chemotherapy in all instances. One patient with local relapse was treated with a pelvic exenteration, and one patient with metastatic disease in the liver was treated with hepatectomy.
HIV+ population
A comparison of HIV+ versus HIV− patients is provided in Table 4. Mean age of HIV+ patients was 43.8 years ± 8.6 SD and not statistically different from the HIV− group (56.9 years ± 12.1 SD; p = 0.08). There were more males in the HIV+ group (n = 17; 85%) versus the HIV− group (n = 18; 24.7%; p < 0.01). In the HIV+ group, 10 patients (50.0%) were early stage, compared to 33 (45.2%) of early stage HIV− patients (p = 0.82). Mean total radiation dose did not differ between the two cohorts (p = 0.24). Fourteen HIV+ patients (14/20, 70%) received MMC, as compared to 40/73 (54.8%) of the HIV− patients (p = 0.19).
Table 4.
HIV-negative versus HIV-positive patients
| Prognostic factor | HIV-negative (n = 73) | HIV-positive (n = 20) | p value |
|---|---|---|---|
| Mean age ± SD (years) | 56.9 ± 12.1 | 43.8 ± 8.6 | 0.08 |
| Sex, % male (n) | 24.7% (18) | 85.0% (17) | <0.01 |
| Mean tumor size ± SD (cm) | 3.3 ± 1.6 | 3.3 ± 2.4 | 0.165 |
| Stage (n) | 0.70 | ||
| I, II | 45.2% (33) | 50.0% (10) | |
| III, IV | 54.8% (40) | 50.0% (10) | |
| Treatment-related factors | |||
| Mitomycin (n) | 54.8% (40) | 70% (14) | 0.19 |
| RT dose (Gy) | 53.7 ± 5.7 | 52.2 ± 4.3 | 0.24 |
| Colostomy (APR) (n) | 13.6% (10) | 15.0% (3) | 0.11 |
APR abdominoperineal resection
Among the 20 HIV+ patients, sex and age did not differ by relapse status (Table 5). At time of diagnosis, mean CD4 count was similar in the no-relapse (296 cells/μL ± 219 SD) and relapse groups (197 cells/μL ± 124 SD) (p = 0.18). Six (42.9%) no-relapse HIV+ patients had a CD4 > 200 cells/μL, and three (50.0%) relapsed patients had a CD4 > 200 cells/μL at time of initial diagnosis. One patient did not have a CD4 count available.
Table 5.
HIV+ patient characteristics at initial diagnosis
| No-relapse (n = 14) | Relapse (n = 6) | p value | |
|---|---|---|---|
| Mean tumor size ± SD (cm) | 3 ± 2.3 | 4.3 ± 3.2 | 0.721 |
| Mean CD4 count ± SD at diagnosis (cells/μL) | 296 ± 219 | 197 ± 124 | 0.18 |
| Percentage of patients with CD4 < 200 (cells/μL) (n) | 42.9% (6) | 50.0% (3) | 1.00 |
| Percentage of patients on HAART therapy (n) | 14.3% (2) | 83.3% (5) | 0.007 |
One patient in the no-relapse group did not have a CD4 count available n absolute number of patients
Seven of the 20 HIV+ patients (35%) were on HAART. The mean viral load of the 7 patients on HAART was <50 copies/mL in 6 (85.7%) and <400 copies/mL in 1 (14.7%) patient, suggesting compliance with therapy. The mean viral load of the 13 HIV+ patients not on HAART was 9907 copies/mL ± 4259 SD.
All 7 patients on HAART received a four-drug regimen in different combinations. Protease inhibitors were used in 7 (100%) of patients on HAART, non-nucleoside reverse transcriptase inhibitors in 6 (85.7%), nucleoside reverse transcriptase inhibitors in 4 (57.1%), and integrase inhibitors in 2 (28.5%).
There was an association between HAART and relapse: only 2 of the 14 (14.3%) HIV+ no-relapse patients were on HAART, whereas 5 of 6 (83.3%) HIV+ relapsed patients were on HAART (p = 0.007).
Among HIV+ patients, CD4 count <200 cells/μL (CD < 200) was not associated with need for APR (p = 0.58) or relapse (p = 1.00). CD4 < 200 was associated with decreased survival (p = 0.02; 5 deaths in the <200 group of 9 patients versus 1 in the >200 group of 11 patients).
Survival
After a median follow-up of 28 months, 5-year overall survival (5-year OS) of patients with anal SCC was 58.9% (95% CI, 45.2–70.3%; Fig. 1). The 5-year OS for patients with relapse and no-relapse patients were 76.2 (95% CI 42.1–91.2) and 54.5% (95% CI 39.0–67.7%), respectively (Fig. 2; p = 0.20).
Fig. 1.
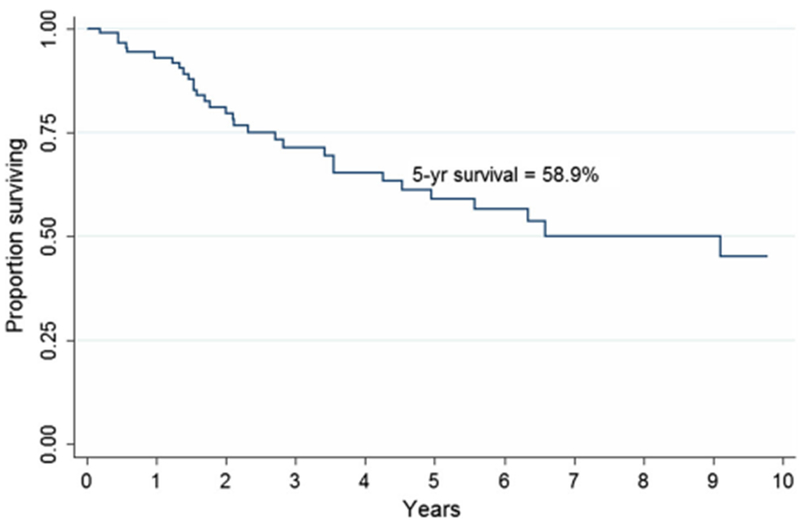
Overall survival of patients diagnosed with squamous cell anal canal carcinoma (n = 93). 44 patients were known to be alive at the end of follow-up
Fig. 2.
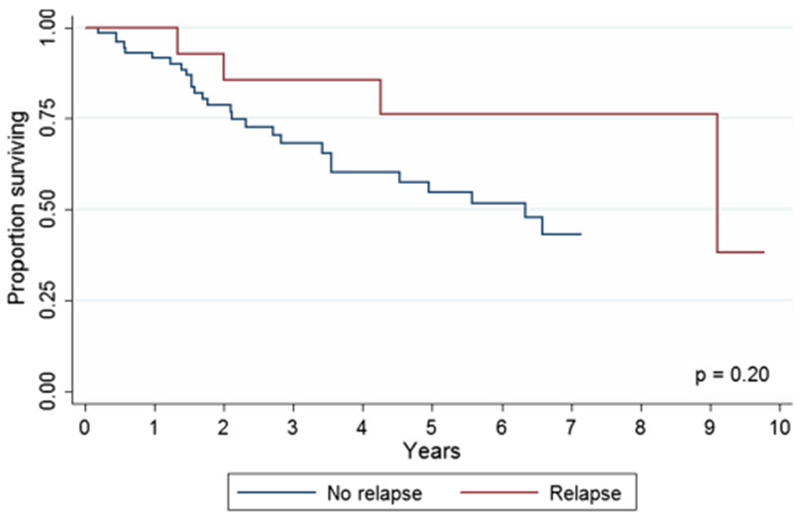
Overall survival of patients with anal squamous cell carcinoma with and without relapse (p = 0.2). 38 of the 78 patients with no-relapse were alive at the end of follow-up, whereas 7 of the 16 patients with relapse were alive at the end of the follow-up period
AJCC staging of patients with squamous cell cancer reliably predicted survival (Fig. 3): 5-year overall survival was 88.2% (95% CI, 60.2–96.9%) for stage 1, 71.7% (95% CI, 44.4–87.2) for stage 2, 55.7% (95% CI, 22.4–79.6%) for stage 3A, 46.7 (95% CI, 8.1–79.4%) for stage 3B, and 9.4% (95% CI, 0.6–34.0%) for stage 4 (p < 0.0001).
Fig. 3.
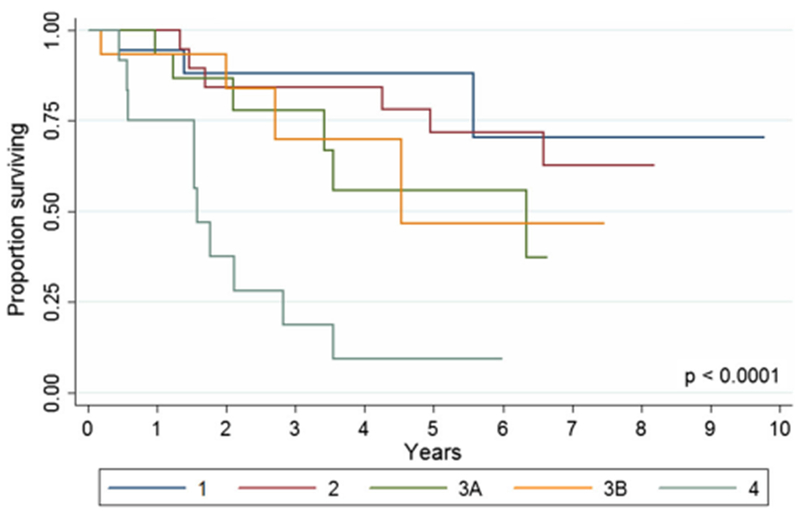
Overall survival of patients with squamous cell anal canal cancer by stage (p < 0.0001)
HIV status did not impact survival; 5-year OS survival of HIV− and HIV+ patients was 59.0% (95% CI, 42.9–72.0%) and 65.8 (95% CI, 38.7–83.2), respectively (p = 0.78; Fig. 4). HIV+ patients receiving versus not receiving HAART therapy had a median OS of 89.6 versus 32.4 months (p = 0.174).
Fig. 4.
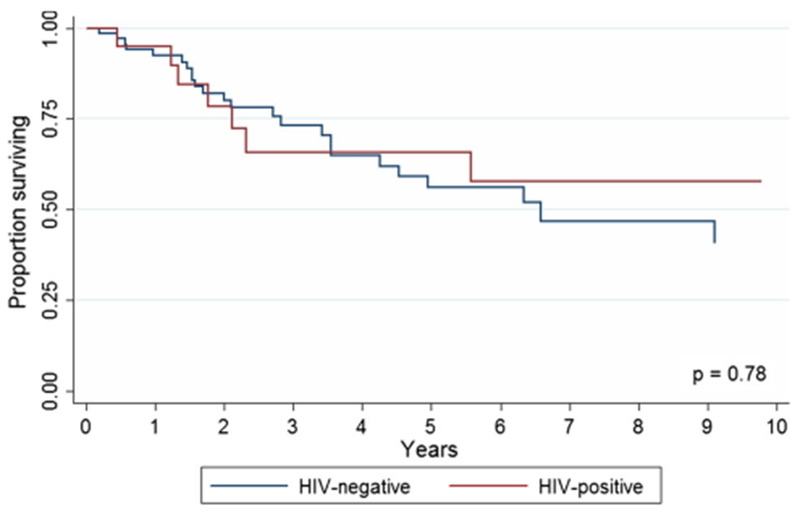
Overall 5-year survival of HIV-negative versus HIV-positive patients with squamous cell carcinoma of the anal canal (p = 0.78). 31 of the 71 HIV-negative patients were alive at the end of follow-up, and 12 of the 20 HIV-positive patients were alive at the end of the follow-up period
Discussion
This is a single-institution retrospective study assessing relapse and survival of SCC anus both overall and within HIV+ versus HIV− patients. In our small series, HIV infection was not associated with a worse OS in patients with SCC anus (5-year OS 65.8% for HIV+ vs. 59.0% for HIV− vs. p = 0.78). Relapse rates were higher among HIV+ versus HIV− patients (30.0 vs. 12.3%) but did not reach statistical significance (p = 0.06). Within the HIV+ group, relapse of squamous cell carcinoma was associated with use of HAART. No significant survival difference between relapse and no-relapse patients was observed. Finally, AJCC staging reliably predicted survival in both relapse and no-relapse SCC anus patients.
5-year OS of patients with SCC anus in our study was 59%. This is similar to the survival reported in most recent series [15, 22, 23]. Relapse did not seem to influence 5-year OS in our series (76.2 relapse vs. 54.5% no-relapse, p = 0.20). It is important to note that median time to relapse was 20 months, and that no-relapse patients in our study were followed for a median of 27 months. Both no-relapse and relapse patient groups in our analysis had similar demographics and stage at initial presentation. There was no difference in radiation dose or the percentage of patients receiving MMC versus cisplatin among these two groups. We could not identify any clinicopathologic predictors of relapse in HIV− patients.
During the last 20 years, many reports of HIV+ populations with SCC anus have been published with variable results [11–25]. As the treatment of SCC anus has evolved, more is becoming known about how to effectively treat HIV+ patients with this disease. Earlier reports from previous eras advised practitioners to carefully select patients for therapy [20, 24, 25]. In our small study, HIV+ patients had similar demographics and disease stage upon initial presentation as HIV− patients. Our HIV+ patients also received standard therapy with 5-FU, MMC, or cisplatin, and radiation therapy similarly to HIV-patients. Within the HIV+ SCC group of 20 patients, the use of HAART therapy appeared to increase the rate of subsequent disease relapse with uncertain impact on OS. Specifically, among HIV+ patients, the rate of relapse was almost six times higher on HAART as compared to no antiretroviral therapy (83.3 vs. 14.3%, p = 0.007) after a median follow-up of 33 months. It appeared that despite similar AJCC staging at initial presentation, HIV+ patients on HAART were at a high risk for treatment failure despite standard treatment and good initial tumor response. HIV status alone did not seem to influence relapse, or overall survival. In our small series, CD4 count had no significant influence on stage of presentation, relapse, or need for colostomy.
Our study comprises a small number of patients, but is the first to show that HAART could be associated with a significantly higher relapse rate of SCC anus in HIV+ patients. Two previous studies reporting on this topic so far have concluded that HIV status may be associated with a higher relapse rate [17, 23]. In both studies, all HIV+ patients were on HAART. Although HAART has reduced the incidence of opportunistic infections and acquired immunodeficiency syndrome (AIDS)-defining malignancies, such as Kaposi sarcoma and non-Hodgkin’s lymphoma, it has not been associated with reduction in the incidence of anal SCC or regression of anal intraepithelial neoplasia, the precursor of invasive cancer [26, 27].
The potential of HAART to cause drug interactions with antineoplastic agents has been raised in recent studies [28, 29]. The likelihood of drug interactions of HAART with chemotherapy agents such as mitomycin is high, since protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) are substrates and potent inhibitors of the cytochrome P450 (CYP) system, which is involved in bioactivation of mitomycin C [29]. Coadministration of HAART with chemotherapy agents could result in decreased efficacy. Nevertheless, formal pharmacokinetic studies are not available, and further studies are needed to clarify potential interactions and influence of HAART on the efficacy of various chemotherapy agents.
Our study had several limitations. These include the retrospective data collection and the limited number of HIV+ patients. Thus, the analysis should be interpreted with caution because small changes in the number of events can lead to a significant change in the results. Our study comprised a small number of HIV+ patients (20 in total) who developed SCC anus. Confirmation of these results in a multicenter retrospective series, or prospective trial, is important before any changes in the treatment of HIV+ patients with SCC anus are made.
Another limitation of our study is that we did not address treatment-related toxicity. Cutaneous, hematologic (pancytopenia), genitourinary (erectile dysfunction), and gastrointestinal (diarrhea) toxicity can occur with combined chemoradiation therapy. Protease inhibitors used for HAART can act as radio and chemosensitizers thereby increasing toxicity. Series of combined chemoradiation for SCC anus in HIV+ patients have reported conflicting results. Several retrospective studies have reported similar tolerability in HIV+ patients compared to HIV− patients [11, 21, 22, 30]. Others have observed enhanced treatment-related toxicity for HIV+ patients, resulting in lower dose chemoradiotherapy [23, 31]. In our series, the radiation dose between the HIV+ and HIV− groups was not statistically different.
Another limitation of our study is the variability of HAART therapy used among our patients with HIV+ disease, as well as the fact that drug development in HAART therapy has advanced significantly over the past three decades. Despite this limitation, it is important to note that all patients with HIV+ disease in this study were on a four-drug combination, including a protease inhibitor, similar to current treatment regimens. Also, the vast majority (85%) of HIV+ patients who were on HAART in our study had a low viral load (<50 copies/mL), suggesting compliance with HAART therapy.
Unfortunately, very limited clinical data are available to guide concurrent use of antiretroviral drugs with antineoplastic agents. The narrow therapeutic window of anti-cancer chemotherapy warrants additional monitoring of its concurrent use with antiretroviral therapy in prospective clinical trials, in order to ensure adequate efficacy. Integration of alternative radiation therapy regimens, targeted chemotherapy regimens, or use of new classes of antiretrovirals might reduce the interaction potential with antineoplastic agents in the future. Alternatively, in patients with normal CD4 counts at diagnosis, clinicians might opt to initiate concurrent chemoradiation before initiating HAART. Finally, the increasing use of HPV vaccination in HIV+ men could potentially reduce the risk of anal canal cancer in this population, and surveillance with high-resolution anoscopy could lead to early detection and treatment of the disease in patients at risk [32].
Conclusions
Standard chemoradiotherapy appears to be safe and effective in HIV+ patients with SCC anus who are both on and off HAART. Our study is limited due to its retrospective nature and the limited patient number. In this small retrospective series, despite a good initial tumor response, HIV+ patients on HAART appeared to be at higher risk of disease relapse, and thus, continued surveillance is recommended, even after good initial local control. As the life expectancy of patients with HIV increases, the incidence of malignancies in the HIV+ population will similarly increase. Therefore, a multidisciplinary team consisting of infectious disease specialists and oncologists in the management of HIV+ patients receiving concurrent chemoradiation is strongly encouraged.
Acknowledgements
The authors thank Theresa Sanlorenzo-Caswell and the Johns Hopkins Cancer Registry for assistance with the primary cancer database. This work was completed without financial support.
Footnotes
Compliance with ethical standards
Conflict of interest The authors have no conflicts of interest to disclose.
Poster presentation at the Annual Meeting of the American Society of Colon and Rectal Surgeons, San Antonio, Tx, June 2–6, 2012; Presented at the Annual Spring Meeting of the Chesapeake Colorectal Society April 21, National Harbor, MD, April 21, 2012.
References
- 1.Siegel R, Naishadham D, Jemal A (2013) Cancer Statistics, 2013. CA Cancer J Clin 63:11–30 [DOI] [PubMed] [Google Scholar]
- 2.Johnson LG, Madeleine MM, Newcomer LM et al. (2004) Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer 101:281–288 [DOI] [PubMed] [Google Scholar]
- 3.Engstrom PF, Arnoletti JP, Benson AB 3rd et al. (2010) NCCN clinical practice guidelines in oncology. Anal carcinoma. J Natl Compr Canc Netw 8:106–120 [DOI] [PubMed] [Google Scholar]
- 4.Gunderson Ll, Winter KA Ajani JA et al. (2012) Long-term update of US GI intergroup RTOG 98–11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol 30:4344–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiao EY, Krown SE, Stier EA et al. (2005) A population-based analysis of temporal trends in the incidence of squamous anal canal cancer in relation to the HIV epidemic. J Acquir Immune Defic Syndr 40(451–45):5. [DOI] [PubMed] [Google Scholar]
- 6. Palefsky JM, Holly EA, Ralston Ml et al. (1998) High incidence of anal high-grade squamous intra-epithelial lesions among HIVpositive and HIV-negative homosexual and bisexual men. Aids 12(495–50):3. [DOI] [PubMed] [Google Scholar]
- 7.Heard I, Palefsky JM, Kazatchkine MD (2004) The impact of HIV antiviral therapy on human papillomavirus (Hpv) infections and Hpv-related diseases. Antivir Ther 9:13–22 [PubMed] [Google Scholar]
- 8.Crum-Cianflone NF, Hullsiek KH, Marconi VC et al. (2010) Anal cancers among HIV-infected persons: haart is not slowing rising incidence. Aids 24(535–54):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnet F, Lewden C, May T et al. (2004) Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer 101(317–32):4. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet F, Burty C, Lewden C et al. (2009) Changes in cancer mortality among HIV-infected patients: the mortalite 2005 survey. Clin Infect Dis 48:633–639 [DOI] [PubMed] [Google Scholar]
- 11.Blazy A, Hennequin C, Gornet JM et al. (2005) Anal carcinomas in HIV-positive patients: high-dose chemoradiotherapy is feasible in the era of highly active antiretroviral therapy. Dis Colon Rectum 48:1176–1181 [DOI] [PubMed] [Google Scholar]
- 12.Svensson C, Kaigas M, Lidbrink E et al. (1991) Carcinoma of the anal canal in a patient with aids. Acta Oncol 30(986–98):7. [DOI] [PubMed] [Google Scholar]
- 13.Edelman S, Johnstone PA (2006) Combined modality therapy for HIV-infected patients with squamous cell carcinoma of the anus: outcomes and toxicities. Int J Radiat Oncol Biol Phys 66(206–21):1. [DOI] [PubMed] [Google Scholar]
- 14.Bottomley DM, Aqel N, Selvaratnam G et al. (1996) Epidermoid anal cancer in HIV infected patients. Clin Oncol (R Coll Radiol) 8:319–322 [DOI] [PubMed] [Google Scholar]
- 15.Hogg ME, Popowich DA, Wang EC et al. (2009) HIV and anal cancer outcomes: a single institution’s experience. Dis Colon Rectum 52(891–89):7. [DOI] [PubMed] [Google Scholar]
- 16.Bower M, Powles T, Newsom-Davis T et al. (2004) HlV-associated anal cancer: has highly active antiretroviral therapy reduced the incidence or improved the outcome? J Acquir Immune Defic Syndr 37:1563–1565 [DOI] [PubMed] [Google Scholar]
- 17.Wexler A, Berson AM, Goldstone SE et al. (2008) Invasive anal squamous-cell carcinoma in the HIV-positive patient: outcome in the era of highly active antiretroviral therapy. Dis Colon Rectum 51:73–81 [DOI] [PubMed] [Google Scholar]
- 18.Stadler RF, Gregorcyk SG, Euhus DM et al. (2004) Outcome of HIV-infected patients with invasive squamous-cell carcinoma of the anal canal in the era of highly active antiretroviral therapy. Dis Colon Rectum 47(1305–130):9. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman R, Welton ML, Klencke B et al. (1999) The significance of pretreatment Cd4 count on the outcome and treatment tolerance of HIV-positive patients with anal cancer. Int J Radiat Oncol Biol Phys 44:127–131 [DOI] [PubMed] [Google Scholar]
- 20.Lorenz HP, Wilson W, Leigh B et al. (1991) Squamous cell carcinoma of the anus and HIV infection. Dis Colon Rectum 34:336–338 [DOI] [PubMed] [Google Scholar]
- 21.Cleator S, Fife K, Nelson M et al. (2000) Treatment of HIV-associated invasive anal cancer with combined chemoradiation. Eur J Cancer 36(754–75):8. [DOI] [PubMed] [Google Scholar]
- 22. Fraunholz I, Weiss C, Eberlein K et al. (2010) Concurrent chemoradiotherapy with 5-fluorouracil and mitomycin C for invasive anal carcinoma in human immunodeficiency virus-positive patients receiving highly active antiretroviral therapy. Int J Radiat Oncol Biol Phys 76:1425–1432 [DOI] [PubMed] [Google Scholar]
- 23.Oehler-Janne C, Huguet F, Provencher S et al. (2008) HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: a multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol 26(2550–255):7. [DOI] [PubMed] [Google Scholar]
- 24.Chadha M, Rosenblatt EA, Malamud S et al. (1994) Squamous-cell carcinoma of the anus in HIV-positive patients. Dis Colon Rectum 37:861–865 [DOI] [PubMed] [Google Scholar]
- 25.Holland JM, Swift PS (1994) Tolerance of patients with human immunodeficiency virus and anal carcinoma to treatment with combined chemotherapy and radiation therapy. Radiology 193(251–25):4. [DOI] [PubMed] [Google Scholar]
- 26.Fox P, Stebbing J, Portsmouth S et al. (2003) Lack of response of anal intra-epithelial neoplasia to highly active antiretroviral therapy. Aids 17:279–280 [DOI] [PubMed] [Google Scholar]
- 27.Palefsky JM, Holly EA, Ralston ML et al. (1998) Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. J Infect Dis 177:361–367 [DOI] [PubMed] [Google Scholar]
- 28.Antoniou T, Tseng AL (2005) Interactions between antiretrovirals and antineoplastic drug therapy. Clin Pharmacokinet 44(111–14):5. [DOI] [PubMed] [Google Scholar]
- 29.Rudek MA, Flexner C, Ambinder RF (2011) Use of antineoplastic agents in patients with cancer who have HIV/AIDS. Lancet Oncol 12:905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo Y, Kinsella MT, Reynolds HL et al. (2009) Outcomes of chemoradiotherapy with 5-fluorouracil and mitomycin C for anal cancer in immunocompetent versus immunodeficient patients. Int J Radiat Oncol Biol Phys 75:143–149 [DOI] [PubMed] [Google Scholar]
- 31.Hammad N, Heilbrun LK, Gupta S et al. (2011) Squamous cell cancer of the anal canal in HIV-infected patients receiving highly active antiretroviral therapy: a single institution experience. Am J Clin Oncol 34:135–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benson AB 3rd, Arnoletti JP, Bekaii-Saab T et al. (2012) Anal carcinoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 10:449–454 [DOI] [PubMed] [Google Scholar]


