1. Background
Ovarian cancer carries the highest fatality to case ratio for all gynecologic malignancies. In 2016, it is estimated that 22,280 women will be diagnosed with ovarian cancer, and 14,240 will die of this life-threatening disease [1]. Due to the aggressive nature of the disease and the lack of effective screening, over 2/3 of women with ovarian cancer are diagnosed in stages III and IV (http://seer.cancer.gov), which is conventionally treated with a combination of aggressive surgical cytoreduction and cytotoxic chemotherapy. However, controversy over the most effective treatment for newly diagnosed stage III ovarian cancer persists, specifically evaluating whether a combination of intraperitoneal (IP) and intravenous (IV) chemotherapy is superior to IV alone [2–6]. Taken together, results from these clinical trials require clinicians and patients to carefully evaluate the risks and outcomes associated with IP or IV treatment, particularly since at this time there is no clear guidance on which treatment is better for a given patient. Adding to the complexity of this decision is the observation that ovarian cancer patients utilize progression free survival (PFS) time to drive the preference of type of chemotherapy, although they are willing to trade significant PFS time for reductions in treatment-related toxicity [7].
Very few studies have examined the use of decision aids within the setting of ovarian cancer, although several authors have explicitly argued that there is a need to practice shared decision-making in order to elicit preferences and incorporate this information into the ovarian cancer treatment discussion [8–10]. In fact, when assessing preferences, those ovarian cancer patients with more serious illness desired more shared decision-making; and in total, >80% of women wanted detailed information about their disease, treatment, and care [11].
Perceived involvement in decision-making about ovarian cancer treatment has been associated with better quality of life [12], although the approach for involving patients in decision making has been debated [13–17], particularly in life-threatening cancer diagnoses. A review of patient preferences for shared decisions, drawing inferences from 43 cancer studies analyses, concluded that 77% of the respondents wanted to participate in decisions, rather than delegating the treatment choice to the physician [18]. In fact, there has been a substantial increase in the percent of cancer patients who wish to participate in medical decision-making, reaching 85% over the last decade [18]
The purpose of this study was to develop and test the effectiveness of a Patient-Centered Outcome Aid (PCOA) compared to a control condition of usual care, as patients chose between IV or IP therapy for advanced ovarian cancer. We developed an internet-based decision aid to improve patient-centered outcomes and tested it within a randomized clinical trial. The purpose of this paper is to describe the clinical trial development and protocol.
2. Methods/design
2.1. Conceptual model
We used shared decision-making to frame the conceptual model, with the specification that PCOA supplements, rather than replaces, the physicians' counseling about treatment options. Shared decision-making, defined as a decision-making process jointly shared by patients and their health care providers [19], explicitly places the patient at the center of care [20], educating and facilitating knowledge and understanding of the best available evidence of the risks and benefits across all available options while ensuring that the patient's values are taken into account [21]. Shared decision-making practice is known to improve when healthcare professionals are provided with feedback, educational meetings and materials, and use of patient decision aids [22]. Decision aids increase the patient's involvement, and improve both knowledge and a realistic perception of outcomes [23]. In essence, the decision aid utilizes the patient's personal views and preferences over benefits and harms, and prepares them to participate with their health care practitioner in making a decision, thereby directly facilitating a shared decision-making approach.
Due to the severity of the disease, the poor prognosis, and the side effects associated with the treatment, advanced epithelial ovarian cancer is an excellent candidate for shared decision making. When these patients face difficult treatment decisions, shared decision-making can offer important advantages. These include education about treatment options, their potential consequences, and potential risks and benefits. Further, shared decision-making facilitates an understanding of preferences, possibly through a formal preference elicitation exercise and always through active patient participation in the treatment choice decision. It is hypothesized that patients who are given the opportunity to participate in this process will feel ownership of the decision, will be more satisfied with the decision, and will likely perceive themselves to have better treatment outcomes, irrespective of the treatment chosen.
2.1.1. Focus groups and cognitive interviews
Stakeholder focus groups and cognitive interviews were conducted to inform each component of PCOA development. We conducted three separate focus groups of two hours duration, during which clinical stakeholders provided advice on 1) customizing risk information, 2) educational module content regarding ovarian cancer treatment and treatment side effects, 3) side effects and their severity levels (e.g. mild pain, moderate pain, etc.) which might occur from chemotherapy delivered either intraperitoneally or intravenously, and 4) wording of personal tradeoffs between survival and adverse events associated with treatment. Clinical team stakeholders included physicians, research assistants and nurses who represented urban and rural perspectives across 9 national sites in each region of the United States. In addition, 16 cognitive interviews, varying in length between 1 and 1.5 h were conducted with women without cancer, and women who had a family member with cancer. They were asked to review each PCOA mock-up screen and respond to questions such as sensibility and meaning of the screen content, whether too much or too little information was on each screen, and whether the instructions for the preference elicitation exercise and the time tradeoff exercise were clear. Six ovarian cancer survivors also participated in interviews and provided feedback on the topics above, and a group of ‘advocate’ stakeholders of ovarian cancer survivors were convened in person for 2 h to critique each component of PCOA, including the ease with which the screens could be navigated, the graphic appeal, and the user-friendly nature of the tool. Pilot testing of PCOA occurred with three patients each from OSU, Duke and University of Oklahoma in order to test the functionality of the device.
2.1.2. Delphi survey of expert panel
Through a modified Delphi technique we sought to determine the degree of professional consensus regarding the probabilities of specific patient outcomes associated with IV and IP chemotherapy. Clinical experts estimated survival probabilities for two types of patients: a healthier (low comorbidities) and a less healthy (high comorbidities) patient, based on pre-specified scenarios. Through this iterative anonymous process consensus was ultimately achieved. This informed PCOA estimates for survival for both IV and IP [24]. This was particularly important since the survival results of GOG 252 were not publically available until after the PCOA RCT had completed recruitment.
2.1.3. GOG 252 Patient-reported outcome data analyses
A novel aspect of this study and PCOA was utilization of previous IP vs IV clinical trial information about probabilities of side effects by severity levels (which we denote for simplicity as side effects/severity dyads) conditional on the patient baseline and pre-treatment clinical risk factors. These allowed us to calculate expected marginal disutilities for side effects/severity dyads. To obtain the conditional probabilities we performed an analysis of the GOG 252 data, in order to identify specific patient/‘host’ characteristics or groups of characteristics that quantify the likelihood that an individual woman will experience a grade 3-4 adverse event. GOG 252 is a recently completed trial with one IV regimen, and two IP regimens. We analyzed specific GOG 252 patient-reported outcome data, comparing IV and IV/IP treatments in order to obtain the predicted probabilities of the side effects of pain, nausea and vomiting, neurotoxicity and fatigue at three levels: mild, moderate and severe, conditioned on the patient's personal and clinical characteristics before treatment began. These probabilities were then applied to the patient's elicited preferences with respect to side effects to obtain the expected disutility of the side effects for a patient conditional on her baseline health status.
2.1.4. Description of PCOA
PCOA includes four components. The first includes a customized information module that was individually relevant given the condition and the risks the patient was facing (see 2.1.d.i.). The second component is an educational module regarding ovarian cancer treatment and treatment side effects. The third guides the patient through a series of queries designed to elicit her preferences regarding the side effect/severity dyads (e.g. mild pain, moderate pain, etc.) which might occur from chemotherapy delivered either IP or IV. The fourth module assists the patient in discovering her personal tradeoff between survival and the adverse events associated with treatment. This information was presented graphically, giving her an opportunity to revisit her choices, and to test the sensitivity of the tradeoff of these choices. This was summarized on screen, and printed to enhance the follow-up discussion with the treatment team and support shared decision making.
2.1.4.1. Customized information module.
PCOA was constructed based on the assumption that side effects associated with IP therapy are worse than IV therapy during the 6 months of active treatment, but that survival is longer. Therefore, the decision between IP and IV treatments relied on the individual tradeoff between the marginal utility of survival and the marginal disutility of side effects due to IP. However, we personalized patients' risk of side effects based on their own characteristics. These data were used to develop regression models predicting for each patient her probability of experiencing each side effect with either IV or IP treatment, based on data available from GOG 252. Physicians rated the patients as either “healthy” or “less healthy” based on the patient's overall medical condition at the time of study enrollment, and the complexity of her cytoreduction and postoperative course. This rating of the patient's health status (0 or 1) was used to inform the toxicity and survival estimates that were imputed into the PCOA decision tool. This predictive algorithm was applied to the personal health and socio-demographic information of the patient. This provided PCOA with the predicted probabilities for each side effect and its severity that the patient might experience with either IV or IP.
2.1.4.2. Educational module.
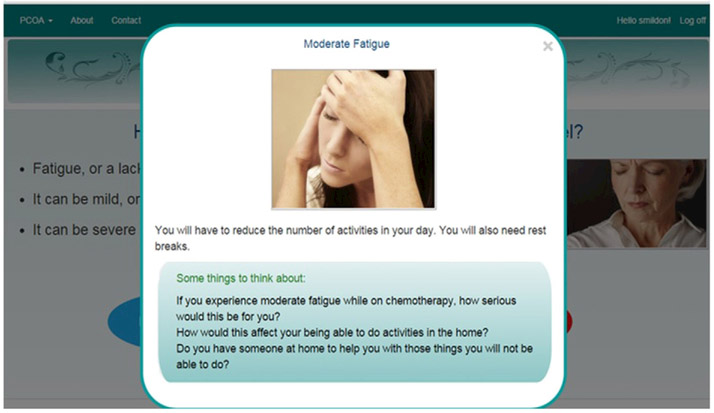
PCOA first takes users through a series of screens educating them about the cancer treatment options available to newly diagnosed ovarian cancer patients, in this case IV or IP therapy, and describes why this information might be important and useful when making a decision about cancer treatment. It then walks the patient through potential side effects. Side effects of treatment are defined first, and then the user can click on examples illustrating symptom severity of mild, moderate or severe (e.g., see Figs. 1 & 2) for each of the following: fatigue, peripheral neuropathy, pain, and nausea and vomiting. The symptom levels were based on NCI Common Toxicity Criteria Adverse Event version 3 (CTCAE) criteria. Users can swipe back to other screens to review, or swipe forward to continue.
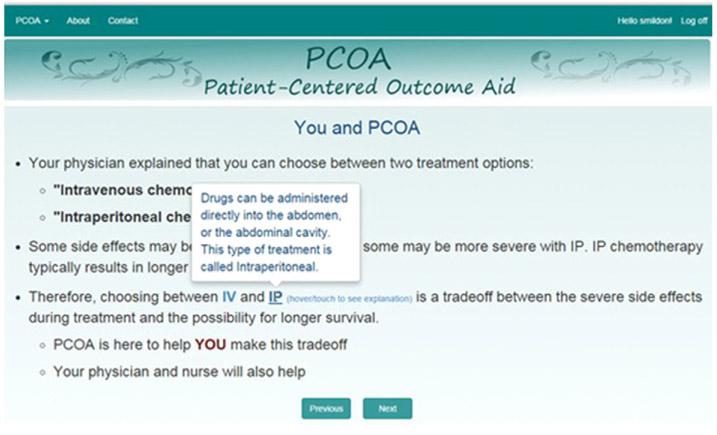
Fig. 1.
Definition of IP therapy.
Fig. 2.
Side effect explanation example.
2.1.4.3. Preference elicitation module.
This module was developed to elicit the patient's values and the relative importance she assigns to the different treatment attributes, in this case the treatment side effects and their level of severity. First, the user is presented with a pre-recorded audiovisual demonstrating the ranking exercise. Patients are presented with a list of the 12 side effects/severity dyads and asked to place them on a Visual Analog Scale, ranking them from 0 - defined as “not bothersome at all” to 100 - defined as “worst possible”. Ties are allowed and patients can change their mind and rearrange the dyads if they wish. They can also go back from the ranking screen back to the educational module to read again about any particular dyad if they need to refresh their memory, and then come back without losing the choices they have already made. Before proceeding, they see a summary of their ranking which allows them another opportunity to change their choices. In order to elicit preferences for survival and to link them to the preferences for the side effects, patients are asked to respond to time tradeoff questions comparing additional months of life in good health against 6 months with the worst side effect/severity dyad that they identified. They go through a series of titrating questions starting with five years of healthy life until they reach equivalency.
2.1.4.4. Outcomes module.
The patient's preference, or utility values, that were revealed in the previous module are combined with the predicted probabilities that the patient would experience from each side effect/severity dyad to calculate the expected marginal disutility of IP for this patient. Her revealed survival utility is combined with her survival probability per the Delphi survey to calculate her expected marginal utility of survival. A comparison of the two provides the patient with an indication if IP or IV might be better for her given her preferences and baseline clinical condition. This information is then provided both on the screen and is printed and the patient takes the information to the discussion with her physician regarding choice of cancer treatment (IP versus IV). This information was presented as the intended starting point for a true shared decision making process; where the patient is knowledgeable about the facts of her disease, her treatment options and her preferences.
2.2. Study design
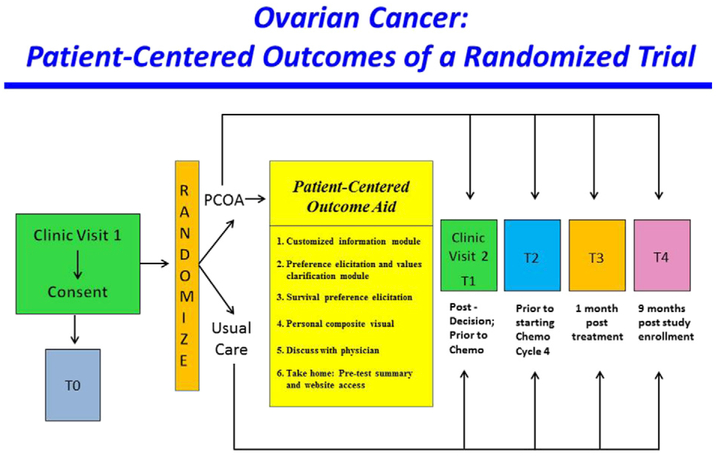
The PCOA intervention was evaluated using a two-arm randomized controlled trial (RCT; Fig. 3), NCT 02259699. Study comparisons were made between patients randomly assigned to the PCOA group versus those assigned to usual care. The design, conduct and reporting of this RCT adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines for trials. Human subjects' approval for this study was obtained from the Institutional Review Board of the University of California, Irvine and each of the study sites.
Fig. 3.
Study Design.
2.3. Study setting
This RCT enrolled advanced optimally debulked ovarian cancer patients from 9 gynecologic cancer practices, located in urban and rural areas of the US. Institutions were invited to participate if they provided both IV and IP therapies to their eligible patients. The study recruitment period was December 2014 to March 2016.
2.4. Eligibility screening and recruitment
Patients who had been optimally cytoreduced with stages II to IV ovarian cancer, and were determined post-operatively (after primary or interval cytoreduction) to be candidates for either IV or IP treatment, were identified. At the time of a typical discussion regarding adjuvant chemotherapy for ovarian cancer (in the hospital after surgery or at an outpatient clinic visit, typically 2–4 weeks following cytoreductive surgery), study-eligible patients were identified. After introducing the study to the patient, those who were interested were consented. Patients who had received three courses of neoadjuvant chemotherapy prior to the cytoreductive surgery were also considered study eligible if they were still considered a candidate for either IV or IP treatment postoperatively, although we hypothesized that there may be important patient-reported outcome or disease differences based on their prior chemotherapy experience compared to those who were chemotherapy naive.
2.5. Randomization
Randomization occurred after the patient was consented and registered. Patients were randomized using a 1:1 ratio to either the PCOA intervention or usual care. Randomization was stratified by site to ensure balance between arms at all participating sites.
2.6. Enrollment and study population
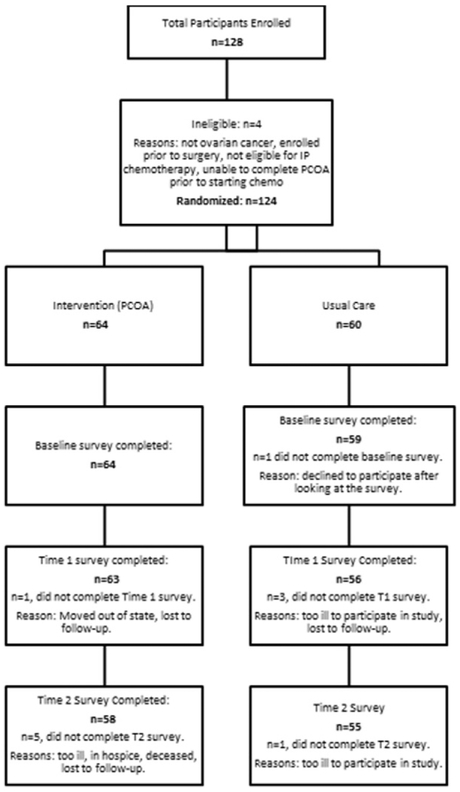
128 patients were consented to the study. With a sample size including 64 patients per arm, the study had 80% power to detect a difference between groups in the primary outcomes of interest satisfaction with decision and decisional regret of 0.5 SD using a two-sided two-sample t-test. Four subjects were subsequently deemed ineligible. Therefore, 124 were randomized into either the PCOA intervention (N = 64) or usual care (N = 60). The Fig. 4 CONSORT diagram reports on survey completion rates prior to chemotherapy cycle 4 (T2), which is an assessment conducted during active treatment. Primary reasons for uncompleted surveys included “patient too ill/hospice” and “patient deceased.”
Fig. 4.
CONSORT diagram.
2.7. Intervention
Patients randomized to the intervention group were given access to PCOA on an iPad that was provided to them during the first postoperative clinic visit. The research assistant enabled the device and assisted the patient in obtaining her unique password that ‘unlocked’ the program. Before leaving the clinic patients were given a password that allowed them to access it from home on an internet connected computer if they wished (Fig. 5).
Fig. 5.
PCOA at home.
2.8. Control group
Patients assigned to the control group received usual care only, which was identical to that provided in the PCOA group except that the patients were not exposed to the PCOA decision aid.
2.9. Patient-reported data sources and collection
We utilized well-validated measures with sound psychometric properties to assess the primary and secondary patient-reported outcomes. Satisfaction with decision [25]and decisional regret [26] were primary outcomes based on the hypothesis that women in the intervention arm would be more satisfied with the decision process, and would experience less decisional regret after treatment ensued. Secondary patient-reported outcomes included measures of quality of life [27], neuropathy [28], abdominal discomfort [29], fatigue [30], shared decision-making [31], and satisfaction with care and treatment [32,33] selected to further evaluate the potential effectiveness of the PCOA intervention. Usability and acceptability of PCOA were assessed from the intervention arm participants through a brief self-report survey at the second clinic visit (T1). QOL and fatigue were measured at the first clinic visit, considered T0 (Fig. 1), which was subsequent to the study consent and randomization. After the patient and physician had discussed adjuvant therapy for her ovarian cancer and a route of administration (IP versus IV) was agreed upon, the satisfaction with decision and shared decision-making measures were collected, which was prior to initiation of treatment following cytoreduction. Assessments of satisfaction with decision, decisional regret, and satisfaction with care and treatment occurred during active treatment (T2) and follow-up (T3-T4), representing time points in which patients would have benefitted and/or had toxicities from their chosen treatment. Assessments T2–T4 were obtained electronically, typically in conjunction with a clinic visit.
2.10. Functionality of PCOA
We collected information about all key strokes and mouse clicks by users of PCOA and the time in which they occurred. This information allowed us to observe how patients used PCOA. For example, we could observe if the user did their preference elicitation more than once and what their choices were in each iteration, and, if they read any of the educational screens more than once, and which educational screen they spent more time on.
3. Baseline results
The majority of participants were White (92%), with private insurance (66%), and a mean age of 58 years. Enrolled patients most commonly had Stage IIIc (66%), and Grade 3 disease (91%). Table 1 displays disease and treatment characteristics at study enrollment, comparing those with and without neoadjuvant treatment. Patients who received neoadjuvant therapy were more likely to have higher stage disease (p = 0.004), less likely to have had a colon resection (p = 0.010), and more likely to have had residual disease (p = 0.021). Among all participants, performance status (PS) was judged as roughly equivalent between PS 0 (50%) and PS 1 (48%), with physician ratings of the patients' health status as generally healthy at study entry (85%), as defined by criteria in section 2.9. Patient-reported outcomes on quality of life, abdominal discomfort, fatigue and neurotoxicity (Table 2), comparing those with and without neoadjuvant treatment did not differ at baseline between PCOA and Control study arms. Although patients who enrolled subsequent to three courses of neoadjuvant chemotherapy reported significantly more neurotoxicity (p = 0.020), they also reported significantly better quality of life (p= 0.020).
Table 1.
Patient characteristics at baseline.
| Patient characteristic | All |
Neoadjuvant = No |
Neoadjuvant = Yes |
p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |||
| Age | 124 | 58.1 (10.0) | 93 | 57.6 (10.2) | 30 | 60.1 (9.1) | 0.237 | |
| BMI | 124 | 27.1 (6.4) | 93 | 27.6 (6.5) | 30 | 25.6 (5.9) | 0.135 | |
| N | % | N | % | N | % | p-Value | ||
| Study arm | PCOA | 64 | 52 | 54 | 58 | 10 | 33 | 0.018 |
| Control | 59 | 48 | 39 | 42 | 20 | 67 | ||
| Ethnicity | Hispanic or Latino | 2 | 2 | 2 | 2 | 0 | 0 | 0.508 |
| Not Hispanic or Latino | 119 | 97 | 90 | 97 | 29 | 97 | ||
| Not reported | 2 | 2 | 1 | 1 | 1 | 3 | ||
| Race | Non-white | 10 | 8 | 6 | 6 | 4 | 13 | 0.230 |
| White | 113 | 92 | 87 | 94 | 26 | 87 | ||
| Insurance | Medicaid | 5 | 4 | 4 | 4 | 1 | 3 | 0.504 |
| Medicare | 30 | 24 | 24 | 26 | 6 | 20 | ||
| Private | 81 | 66 | 58 | 62 | 23 | 77 | ||
| Other | 5 | 4 | 50 | 5 | 0 | 0 | ||
| None | 2 | 2 | 2 | 2 | 0 | 0 | ||
| Primary site | Fallopian tube | 9 | 7 | 7 | 8 | 2 | 7 | 0.864 |
| Ovary | 103 | 84 | 77 | 83 | 26 | 87 | ||
| Peritoneum | 11 | 9 | 9 | 10 | 2 | 7 | ||
| Stage | II | 7 | 6 | 7 | 8 | 0 | 0 | 0.004 |
| III | 102 | 83 | 80 | 86 | 22 | 73 | ||
| IV | 14 | 11 | 6 | 6 | 8 | 27 | ||
| Grade (N = 98) | 1 | 4 | 4 | 4 | 6 | 0 | 0 | 0.383 |
| 2 | 5 | 5 | 3 | 4 | 2 | 8 | ||
| 3 | 89 | 91 | 65 | 90 | 24 | 92 | ||
| Surgical complications | No | 106 | 86 | 79 | 85 | 27 | 90 | 0.486 |
| Yes | 17 | 14 | 14 | 15 | 3 | 10 | ||
| Colon resection | No | 88 | 72 | 61 | 66 | 27 | 90 | 0.010 |
| Yes | 35 | 28 | 32 | 34 | 3 | 10 | ||
| IP port at surgery | No | 50 | 41 | 36 | 39 | 14 | 47 | 0.440 |
| Yes | 73 | 59 | 57 | 61 | 16 | 53 | ||
| Residual disease (%) | 0 | 82 | 67 | 63 | 68 | 19 | 63 | 0.021 |
| 1–2 | 28 | 23 | 24 | 26 | 4 | 13 | ||
| ≥ 5 | 13 | 11 | 6 | 6 | 7 | 23 | ||
| Performance status | 0 | 62 | 50 | 50 | 54 | 12 | 40 | 0.190 |
| 1 | 59 | 48 | 41 | 44 | 18 | 60 | ||
| 2 | 2 | 2 | 2 | 2 | 0 | 0 | ||
| BRCA | Negative | 9 | 7 | 3 | 3 | 6 | 20 | n/a |
| Positive | 7 | 6 | 2 | 2 | 5 | 17 | ||
| Unknown | 107 | 87 | 88 | 95 | 19 | 63 | ||
| Health resemblance | Healthier | 104 | 85 | 80 | 86 | 24 | 80 | 0.427 |
| Less healthy | 19 | 15 | 13 | 14 | 6 | 20 | ||
| MD recommendation | IP | 87 | 72 | 66 | 73 | 21 | 70 | 0.789 |
| IV | 34 | 28 | 25 | 27 | 9 | 30 | ||
| Treatment decision | IP | 80 | 66 | 60 | 65 | 20 | 67 | 0.885 |
| IV | 42 | 34 | 32 | 35 | 10 | 33 | ||
Table 2.
Patient QOL and symptom scores at baseline by neoadjuvant treatment status.
| All |
Neoadjuvant = No |
Neoadjuvant = Yes |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | p-Value | |
| Physical well-being | 123 | 19.7 | 5.6 | 93 | 19.3 | 5.7 | 30 | 20.9 | 5.0 | 0.184 |
| Functional well-being | 123 | 14.3 | 6.1 | 93 | 13.7 | 6.1 | 30 | 16.2 | 6.0 | 0.048 |
| Additional concerns | 123 | 31.6 | 6.6 | 93 | 30.7 | 6.5 | 30 | 34.2 | 6.4 | 0.012 |
| FACT-O-TOI | 123 | 65.6 | 15.6 | 93 | 63.8 | 15.5 | 30 | 71.3 | 14.7 | 0.020 |
| Abdominal discomfort | 123 | 11.1 | 3.7 | 93 | 10.9 | 3.8 | 30 | 11.6 | 3.4 | 0.376 |
| Neurotoxicity/ | 123 | 14.8 | 2.0 | 93 | 15.1 | 1.8 | 30 | 14.1 | 2.5 | 0.020 |
| neuropathy (high is good) | ||||||||||
| Fatigue (high = bad) | 123 | 19.2 | 8.9 | 93 | 19.6 | 9.1 | 30 | 18.0 | 8.3 | 0.396 |
4. Discussion
Shared decision-making has become a cornerstone of patient-centered care. In fact, the Patient-Centered Outcomes Research Institute (PCORI) has a mandate to improve the quality and relevance of evidence available to help patients, caregivers, clinicians, employers, insurers, and policy makers make informed health decisions. Broadly, this patient-centered outcomes research has a vision “to provide the patient and the public with information they can use to make decisions that reflect their desired health outcomes.” Indeed, given the aggressive nature of ovarian cancer, it is not surprising that many with advanced disease have expressed the need for more shared decision-making [11].
The purpose of our study was to develop and test a new ovarian cancer-specific Patient Centered Outcome decision Aid (PCOA). During the initial design of this study, recommendations for IP therapy continued to be controversial, despite the fact that IP therapy was widely acknowledged to provide survival gains, albeit with additional treatment toxicities. It was the belief of our study team that patients should share in decisions that may affect their longevity on the one hand, and their quality of life on the other. Therefore, our intent was to educate patients and allow them to assimilate information about the differences in outcomes between IP and IV therapies, and help them make the difficult trade-offs between these two treatment options. PCOA was specifically designed to incorporate each patient's personal preferences and clinical characteristics, together with the most contemporary science available for newly diagnosed advanced ovarian cancer patients, to support shared decision-making between the patient and her treatment team.
During the course of our trial, results of GOG 252 revealed that the IP regimens evaluated in this trial did not prolong progression free survival, and by extension would not be likely to improve overall survival. Despite the results of GOG 252 and based on RCT data on older IP regimens, many experts continue to prefer IP treatment for select patients with optimally cytoreduced epithelial ovarian cancer. As ovarian cancer treatments evolve in the front-line and recurrent setting, we believe that the majority of content and functionality of PCOA can remain with minimal revisions. These revisions would include, for example, adjusting the different probabilities of toxicities and the different probabilities of survival to accommodate the contemporary cancer treatments. Further, PCOA would continue to be applicable to educate the patient about her disease and treatment options, and just as importantly her preferences and concerns related to treatment toxicities.
The primary change to PCOA would likely be in the survival time trade-off (TTO) exercise, which required patients to link side effect preferences to additional months of life in good health i.e., what type and severity of side effect would you tolerate for # of months of life in good health. Despite multiple iterations and piloting, the TTO exercise was challenging to develop, and difficult for many of the patients to complete. This exercise likely posed psychological challenges or obstacles often impeding the ability to accurately process the TTO questions. This may be especially true for this cancer patient population since they had only recently received the news of a life threatening diagnosis, and were confronted with numerous physical and emotional changes simultaneously. Therefore, the use of a TTO exercise with this population deserves further examination.
The baseline results allowed us to determine, to our surprise, that despite three cycles of neoadjuvant chemotherapy, this group of patients reported significantly better quality of life compared to the chemo-naïive enrollees. There are several potential explanations for this finding. It is likely that the three cycles of treatment reduced tumor burden for this population, which improved QOL. In short, a less aggressive surgery and chemotherapy benefit may explain the significant QOL difference at a post-operative baseline. This is an important finding because this difference may help to explain if neoadjuvant patients experience PCOA similarly to the chemotherapy naive population exposed to PCOA. In addition, the patients selected for upfront neoadjuvant treatment had a higher probability of residual disease, may have been more frail and would therefore have had a less aggressive surgery, compared to those who had aggressive upfront debulking surgery. They may, initially, therefore feel better.
Several methodologic considerations deserve attention. First, we do not have a true denominator that would allow calculation of the actual uptake for this randomized clinical trial. While several clinical sites were very enthusiastic about enrollment, the capacity to integrate this trial into a busy clinic setting was variable. Some of the enrollment sites also voiced concern that our study, which addressed choices between upfront IV or IP therapy might impede enrollment onto other clinical trials, thereby dampening enthusiasm for our study. In addition, the treatments for upfront chemotherapy were changing while this study was enrolling, so our pool of potential participants was smaller than anticipated. Further, both during PCOA development and subsequently, many patients and clinicians found the survival time trade off exercise to be too provocative. This sensitive issue was difficult to explore for the newly diagnosed, since they bring hope of longevity to their initial experience. Nevertheless, collaborators have recommended that a similar study be offered for women with recurrent ovarian cancer, where treatment options are few, efficacy is variable, and individual preferences are critical. This may also serve as a prototype for other cancers and chronic diseases where patients face similar tradeoffs.
Acknowledgements
Research reported in this publication was funded by a Patient-Centered Outcomes Research Institute (PCORI) award number CE-12-11-4755, and the National Cancer Institute of the National Institutes of Health under award number P30CA062203. The content is solely the responsibility of the authors and does not necessarily represent the official views of PCORI or the National Institutes of Health.
References
- [1].American Cancer Society, Cancer Facts & Figures 2016, American Cancer Society, Atlanta, 2016. [Google Scholar]
- [2].Alberts DS, et al. , Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer, N. Engl. J. Med 335 (26) (1996) 1950–1955. [DOI] [PubMed] [Google Scholar]
- [3].Armstrong DK, et al. , Intraperitoneal cisplatin and paclitaxel in ovarian cancer, N. Engl. J. Med 354(1) (2006) 34–43. [DOI] [PubMed] [Google Scholar]
- [4].Markman M, et al. , Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group, J. Clin. Oncol 19 (4) (2001) 1001–1007. [DOI] [PubMed] [Google Scholar]
- [5].Walker J, Brady M, DiSilvestro P, A phase III clinical trial of bevacizumab with IV versus IP chemotherapy in ovarian, fallopian tube and primary peritoneal carcinoma NCI-supplied agent (s): bevacizumab (NSC# 704865, IND# 7921) NCT01167712 a GOG/NRG trial (GOG 252), SGO Annual Meeting, 2016. [Google Scholar]
- [6].Wright AA, et al. , Use and effectiveness of intraperitoneal chemotherapy for treatment of ovarian cancer, J. Clin. Oncol 33 (26) (2015) 2841–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Havrilesky LJ, et al. , Patient preferences in advanced or recurrent ovarian cancer, Cancer 120 (23) (2014) 3651–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shepherd HL, Butow PN, Tattersall MH, Factors which motivate cancer doctors to involve their patients in reaching treatment decisions, Patient Educ. Couns 84 (2) (2011) 229–235. [DOI] [PubMed] [Google Scholar]
- [9].Elit L, et al. , Women's perceptions about treatment decision making for ovarian cancer, Gynecol. Oncol 88 (2) (2003) 89–95. [DOI] [PubMed] [Google Scholar]
- [10].Brown R, et al. , Meeting the decision-making preferences of patients with breast cancer in oncology consultations: impact on decision-related outcomes, J. Clin. Oncol 30 (8) (2012) 857–862. [DOI] [PubMed] [Google Scholar]
- [11].Stewart DE, et al. , Information needs and decisional preferences among women with ovarian cancer, Gynecol. Oncol 77 (3) (2000) 357–361. [DOI] [PubMed] [Google Scholar]
- [12].Andersen MR, et al. , Involvement in decision-making about treatment and ovarian cancer survivor quality of life, Gynecol. Oncol 124(3) (2012) 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Beaver K, Bogg J, Luker KA, Decision-making role preferences and information needs: a comparison of colorectal and breast cancer, Health Expect. 2 (4) (1999) 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Degner LF, Sloan JA, Decision making during serious illness: what role do patients really want to play? J. Clin. Epidemiol 45(9) (1992) 941–950. [DOI] [PubMed] [Google Scholar]
- [15].Leydon GM, et al. , Cancer patients' information needs and information seeking behaviour: in depth interview study, BMJ 320 (7239) (2000) 909–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sutherland HJ, et al. , Cancer patients: their desire for information and participation in treatment decisions, J. R. Soc. Med 82 (5) (1989) 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gaston CM, Mitchell G, Information giving and decision-making in patients with advanced cancer: a systematic review, Soc. Sci. Med 61 (10) (2005) 2252–2264. [DOI] [PubMed] [Google Scholar]
- [18].Chewning B, et al. , Patient preferences for shared decisions: a systematic review, Patient Educ. Couns 86 (1) (2012) 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Towle A, Godolphin W, Framework for teaching and learning informed shared decision making, Br. Med. J 319 (7212) (1999) 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Weston WW, Informed and shared decision-making: the crux of patient-centred care, Can. Med. Assoc. J 165 (4) (2001) 438–439. [PMC free article] [PubMed] [Google Scholar]
- [21].Makoul G, Clayman ML, An integrative model of shared decision making in medical encounters, Patient Educ. Couns 60 (3) (2006) 301–312. [DOI] [PubMed] [Google Scholar]
- [22].Légaré F, et al. , Interventions for improving the adoption of shared decision making by healthcare professionals, Cochrane Database Syst. Rev 5 (2010) 1–47. [DOI] [PubMed] [Google Scholar]
- [23].Stacey D, et al. , Decision aids for people facing health treatment or screening decisions, Cochrane Database Syst. Rev 10 (10) (2011). [DOI] [PubMed] [Google Scholar]
- [24].Cohn DE, et al. , Consensus in controversy: the modified Delphi method applied to Gynecologic Oncology practice, Gynecol. Oncol 138 (3) (2015) 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Holmes-Rovner M, et al. , Patient satisfaction with health care decisions the satisfaction with decision scale, Med. Decis. Mak 16 (1) (1996) 58–64. [DOI] [PubMed] [Google Scholar]
- [26].Brehaut JC, et al. , Validation of a decision regret scale, Med. Decis. Mak 23 (4) (2003) 281–292. [DOI] [PubMed] [Google Scholar]
- [27].Basen-Engquist K, et al. , Reliability and validity of the functional assessment of cancertherapy-ovarian, J. Clin. Oncol 19 (6) (2001) 1809–1817. [DOI] [PubMed] [Google Scholar]
- [28].Huang H, et al. , Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a gynecologic oncology group study, Int. J. Gynecol. Cancer 17 (2) (2007) 387–393. [DOI] [PubMed] [Google Scholar]
- [29].Wenzel L, et al. , Validation of FACT/GOG-AD subscale for ovarian cancer-related abdominal discomfort: a Gynecologic Oncology Group study, Gynecol. Oncol 110 (1) (2008) 60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cella D, et al. , Fatigue in cancer patients compared with fatigue in the general United States population, Cancer 94 (2) (2002) 528–538. [DOI] [PubMed] [Google Scholar]
- [31].Kriston L, et al. , The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample, Patient Educ. Couns 80 (1) (2010) 94–99. [DOI] [PubMed] [Google Scholar]
- [32].Bredart A, et al. , An international prospective study of the EORTC cancer in-patient satisfaction with care measure (EORTC IN-PATSAT32), Eur. J. Cancer 41 (14) (2005) 2120–2131. [DOI] [PubMed] [Google Scholar]
- [33].Abetz L, et al. , Development of the cancer therapy satisfaction questionnaire: item generation and content validity testing, Value Health 8 (2005) S41–S53. [DOI] [PubMed] [Google Scholar]