Abstract
Background:
Personal protective equipment (PPE) is worn by workers in surgical settings to protect them and patients. Food and Drug Administration (FDA) clears some PPE (e.g., surgical masks (SM)) as class II medical devices, and regulates some (e.g. surgical head cover) as class I exempt devices. For respiratory protection, National Institute for Occupational Safety and Health (NIOSH)-approved N95 filtering facepiece respirators (FFRs), and powered air-purifying respirators (PAPRs) are used. One type of PPE, “surgical N95 respirators”, is a NIOSH-approved FFR that is also cleared by the FDA for use in medical settings. The surgical environment poses unique risks such as the potential for surgical fires. As part of its substantial equivalence determination process, FDA requests testing of flammability and other parameters for SM and surgical N95 respirators. A lack of data regarding flammability of PPE used in healthcare exists. We hypothesize that commonly used PPE, regardless of whether regulated and/or cleared by FDA or not, will pass an industry standard such as the 16 CFR 1610 flammability test.
Methods:
Eleven N95 FFR models, eight surgical N95 respirator models, seven SM models, five surgical head cover models, and five PAPR hood models were evaluated for flammability with a 45 degree flammability tester using the 16 CFR 1610 method. Three common fabrics were included for comparison.
Results:
All of the PPE samples regulated/and or cleared by FDA or not, passed the flammability test at class 1 (normal flammability), meaning they are less likely to burn. Only one of the three common fabrics, a cotton fabric at the lowest basis weight, was class 3 (high flammability).
Conclusions:
The results obtained in the study suggest that NIOSH-approved N95 FFRs would likely pass the 16 CFR 1610 flammability standard. Moreover, results suggest that NIOSH is capable of undertaking flammability testing using the 16 CFR 1610 standard as the flammability results NIOSH obtained for N95 FFRs were comparable to the results obtained by a third party independent laboratory.
Keywords: Flammability, N95 filtering facepiece, surgical N95 respirator, surgical mask, surgical head cover, powered air-purifying respirator (PAPR) hood, surgery, healthcare
INTRODUCTION
The focus of this study was on the flammability of surgical masks and surgical N95 respirators. However, flammability is just one of many device characteristics, which are used in evaluating the performance of these devices for FDA clearance. Because NIOSH approves N95 filtering facepiece respirators (FFRs) with no evaluation for and has not traditionally evaluated flammability. This study sought to understand the flammability of NIOSH-approved FFRs as well as NIOSH’s capability to undertake flammability testing.
While surgical fires occur infrequently in healthcare workplaces, serious patient or healthcare professional injuries may result when they happen. The Food and Drug Administration (FDA) describes many potential sources for fire in the operating room including surgical lasers, electrosurgical units, endoscopic fiber optic light sources, and electro-medical devices (FDA 2004). Surgical fire has been identified as one of the top ten technology hazards in operating rooms (ECRI Institute, 2009a). The Emergency Care Research Institute (ECRI) estimates the occurrence of about 550 to 650 surgical fires every year in the surgical environment. Healthcare workers wearing PPE including surgical gowns, respirators, and surgical head covers in an operating room (OR) may be exposed to surgical fires; it is important that these devices not contribute to further injuries or fatalities. Several studies have reported catastrophic incidents in OR fires (Smith and Roy, 2011; Weber et al., 2006).
A survey on OR fires experienced by otolaryngologists was advertised to 8,523 members of the American Academy of Otolaryngology-Head and Neck Surgery (Smith and Roy 2011). Of the 349 questionnaires completed, 88 surgeons witnessed at least one OR fire during their career. The most common sources of ignition were an electrosurgical unit (59%), a laser (32%), and a light cord (7%). Fire occurred during endoscopic airway surgery (27%), oropharyngeal surgery (24%), cutaneous or transcutaneous surgery of the head and (18%) and other procedures. Another study analyzed the reports of 65 surgical fires in Pennsylvania hospitals from 2004 to 2011 and identified ignition sources including, an electrosurgical unit (58%), a fiber optic light cord (38%), and a laser (3%) (Clarke et al., 2012).
Three major components of fire combustion process include, an oxidizer, an ignition source and a fuel, also described as a fire triangle, come together in the proper proportions at the right conditions to produce fire (ECRI Institute, 2009b; Fan and Lau, 2009; Wagner, 1996);. The commonly used ignition sources are electrosurgical units, lasers, electrocautery units and fiber optic sources. Oxidizers such as oxygen and nitrous oxide are used for surgical and anesthetic procedures. The major fuels involved in surgical procedures are endotracheal and tracheostomy tubes, surgical drapes, prepping agents, and gauze sponges.
In surgical settings, many types of personal protective equipment (PPE) are worn on the head and face of workers to protect both them and the patients against the transfer of microorganisms, body fluids, particulate material, and surgical fires. Some types of the PPE (e.g., surgical masks (SMs)) are cleared by the FDA as class II medical devices and/or approved by National Institute for Occupational Safety and Health (NIOSH) for respiratory protection (e.g., N95 filtering facepiece respirators (FFRs) and powered air-purifying respirators (PAPRs)). SMs are loose-fitting devices used by healthcare personnel as a barrier for both the patient and the healthcare personnel from body fluid splashes and particulate material (FDA 2004). Surgical N95 respirators are FFRs that are approved by NIOSH and also cleared by FDA and are intended to protect against the transfer of microorganisms, body fluids and particulate material. When used in a complete respiratory protection program, surgical N95 respirators, FFRs, and PAPRs reduce inhalation of infectious aerosols. Loose-fitting PAPRs are NIOSH-approved respirators that cover the head and neck and sometimes portions of the shoulders but do not seal completely to the face or neck. Surgical head covers are commonly found in surgical settings to help maintain a sterile field and can also provide protection against fluid penetration. Surgical head covers are class I exempt devices and exempt from submission of a premarket notification. Surgical head covers are regulated by FDA under 21 CFR 878.4040. This regulation requires manufacturers to follow general controls of the Food, Drug, and Cosmetic Act, which includes, but is not limited to, good manufacturing practices, registration and listing, misbranding, and adulteration.
During the clearance process, FDA reviews the differences in technological characteristics of a device to determine whether performance data is necessary to establish substantial equivalence. For flammability testing, the FDA guidance document (FDA, 2004) recommends the Consumer Product Safety Commission (CPSC) CS-191-53 flammability method (16 CFR 1610), NFPA standard 702-1980 method, or Underwriters Laboratories’ UL2154 method. The CPSC 16 CFR 1610 method is commonly used to evaluate the flammability of respiratory devices and fabric materials using a 45° angle flammability tester (Federal Register, 2008; United States Consumer Product Safety Commission, 2008). The average burn time for five samples obtained in the test is used to assign the flammability class of the test material. Flammability class 1 and class 2 represent fabrics with average burn times >3.5 sec and 3.5 to 7.0 sec, respectively. Flammability class 3 represents materials with average burn times <3.5 sec. Class 1 fabric materials exhibit normal flammability, which are accepted for use in clothing. Class 2 fabrics refer to fabrics with an intentionally raised fiber or yarn surface, such as a pile, including a flocked pile, nap, or tufting. The flammability of these fabric materials is intermediate, and may be used in clothing. Flammability class 3 fabrics are dangerously flammable and cannot be used in clothing.
Surprisingly, little data is available in the published literature regarding flammability of PPE used in surgical settings. It was hypothesized that commonly used PPE, regardless of whether it was cleared by FDA or not, will pass the industry standard flammability test. To test this hypothesis, N95 FFRs, surgical N95 respirator, surgical mask (SM), surgical head cover, powered air-purifying respirator (PAPR) hood and common fabric materials were tested using the 16 CFR 1610 method with a 45 degree flammability tester (United States Consumer Product Safety Commission, 2008). The results of the study, potential implications for regulatory agencies, and future needs are discussed.
MATERIALS AND METHODS
Respirators
NIOSH-approved N95 FFR (11 models) and PAPR hood (5 models), and FDA cleared surgical N95 FFR (8 models), SM (6 models) and surgical head cover (5 models), were selected for the present study. Table I shows the types of respiratory devices, design, models, and manufacturers. Respiratory devices were selected based on the commonly used models in healthcare (Wizner et al., 2016), and the availability of the devices on the market.
Table I.
List of Manufacturers of other PPE Models and Design
| Respirator and PPE Type | Manufacturer | Model | Design |
|---|---|---|---|
|
N95 |
3M | V-Flex 9105 | Cup (Flexible) |
| 3M | 9210 | Flat fold | |
| 3M | 8210 | Cup | |
| Condor | 22EL78 | Cup | |
| Drager | 1350 | Cup | |
| Drager | 1750 | Flat Bi Fold | |
| Jackson Safety | R-10 | Cup | |
| Kimberly-Clark | 62126 | Flat Bi Fold Pouch | |
| Moldex | 2200 | Cup | |
| Moldex | AirWave | Cup (Flex fit) | |
| Wilson | Saf-T-Fit | Cup | |
|
Surgical N95 |
3M | 1860 | Cup |
| 3M | 1870 | Flat fold | |
| AlphaProTech | Critical Cover 695 | Flat Pleated | |
| Gerson | 1730 | Cup | |
| Kimberly-Clark | 46727 | Flat Fold Pouch | |
| Moldex | 1512 | Cup | |
| Sperian | HC-NB095 | Cup | |
| Sperian | HC-NB295F | Flat fold Pouch | |
|
Surgical Mask |
3M | 1820 | Flat Pleated |
| AM-Touch | Patient Armor M2200B | Flat Pleated | |
| Cellucap | 1826 | Flat Pleated | |
| Condor | 4KMY1C | Flat Pleated | |
| Keystone | FM EL | Flat Pleated | |
| Precept | 15320 | Flat Pleated | |
|
Surgical Head Cover |
Action Chemical | A-2302W | - |
| AlphaProTech | Critical Cover GenPro | - | |
| Keystone | Polypropylene Hood | - | |
| Kimberly-Clark | KleenGuard 36860 | - | |
| Medline | Pro Series NONSH600 | - | |
|
PAPR Hood |
3M | Versaflo S403L20 | - |
| 3M | BE-103 | - | |
| Bullard | 20TIC | - | |
| Dover | Sentinel XL HP | - | |
| MSA | OptimAir TL | - |
Fabric materials
Non-FDA-cleared common fabric materials including five different types of cotton cloths, two linen fabrics, a hemp fabric, and a cheesecloth (Table II) were also evaluated for flammability. The fabric materials were obtained from local fabric stores or the manufacturers.
Table II.
Flammability Class of N95 Respirators, Surgical N95 Respirators, Surgical Masks, and other Fabric Materials
| Respirator, PPE and Fabric Material | Weight (g/m2) | Average Burn Time (sec) | Flammability Class |
|---|---|---|---|
| N95 FFRs (11) | 140-469 | DNI (11) | 1 |
| Surgical N95 FFRs (8) | 98 - 502 | DNI (7) | 1 |
| 7.8 (1) | |||
| Surgical Masks (7) | 59 – 86 | DNI (5) | 1 |
| 5.7-6.7 (2) | |||
| 100% Cotton | 81.8 | 14.2 | 1 |
| 100% Cotton Bubble Gauze | 52.2 | 11.53 | 1 |
| 100% Cotton Harem Cloth | 35.6 | 3.78 | 1 |
| 100% Cotton Voile | 33.2 | 5.14 | 1 |
| Double Cotton Fabric | 17.3 | 2.5 | 3 |
| 100% Linen | 130 | DNI | 1 |
| 100% Linen | 92.7 | 6.95 | 1 |
| 100% Hemp Fabric | 203 | DNI | 1 |
| Cheesecloth | 84.6 | 22.9 | 1 |
The number in parentheses represents the number of models tested in the study.
DNI - did not ignite
Flammability Tester
The flammability of respirators and other PPE was evaluated as per the Consumer Product Safety Commission (CPSC) CS-191-53 flammability (16 CFR 1610) method (Federal Register, 2008; United States Consumer Product Safety Commission, 2008) The flammability of respiratory devices and other fabrics were tested using a 45° Automatic Flammability Tester (Model M233G, SDL Atlas LLC, Rock Hill, SC) (Figures 1a, 1b, and 1c). The major components of the flammability tester include a test chamber, a specimen rack, a specimen holder, an ignition mechanism, and an automatic timing mechanism. The test chamber is a metallic draft-proof ventilated chamber (35.3 cm (H) × 36.8 cm (W) × 21.6 cm (D)). The front of the chamber has a glass door to permit observation of the entire test. The base of the door in the front of the apparatus has a ventilating strip.
Figure 1:
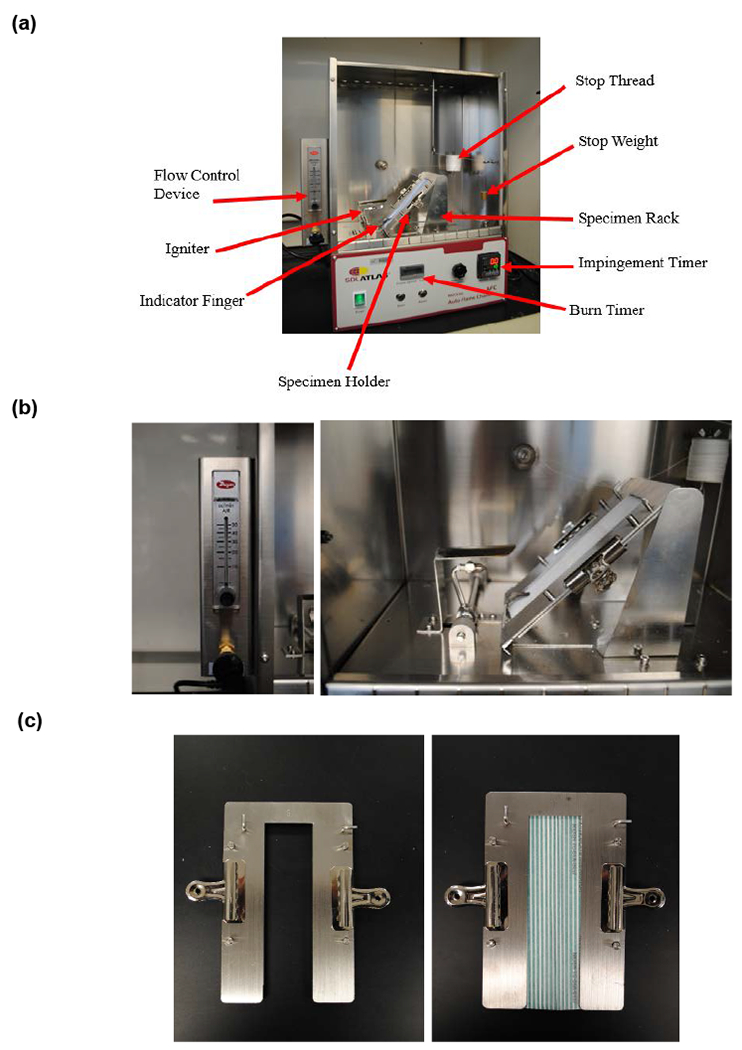
(a) Top Panel: A 45° angle Flammability tester used for testing the flammability of respirators and other head/facial PPE (16 CFR 1610 Test Method). (b) Middle Panel: Flammability tester components. The flow control meter (Left). The igniter and specimen rack with specimen holder mounted in place (Right). (c) Bottom Panel: The specimen holder without (Left) and with a specimen (Right).
The specimen rack (Figure 1a) provides support for the specimen holder in which a specimen is mounted. The specimen holder supports the test specimen. Each specimen is mounted in a specimen holder (Figure 1b). The specimen holder consists of two 2 mm (0.06 in) thick ‘U’ shaped matched metal plates. The plates are slotted and loosely pinned for alignment. The specimen is firmly sandwiched in between the metal plates with clamps mounted along the sides. The two plates of the holder cover all but 3.8 cm (1.5 in) of the width of the specimen for its full length. An indicator finger is located just in front of the ignition head to ensure consistent application of the flame from one sample to the next. The position of the specimen rack is adjusted so that the tip of the indicator finger just touches the surface of the specimen. The ignition mechanism consists of a motor-driven butane gas jet formed around a 26 gauge hypodermic needle, which creates the test flame. The specimen is tested using a 16 millimeter (5/8 in) length flame specified in the standard (Federal Register, 2008).
A stop thread (No. 50, 100% cotton mercerized thread) connected to a 30 g weight is set in place before conducting the test. The stop thread is fed by a spool and then laced through a series of hooks in the chamber as well as on the sample holder. The hooks on the sample holder ensure that the thread is positioned 127 mm (5 in) from the point where the flame impinges on the sample. Also, the hooks on the sample holder keep the thread 9.5 mm (0.37 in) above and parallel to the sample’s surface. The purpose of the hooks in the chamber is to direct the thread so that the stop weight hangs directly above the stop button for the burn timer.
The flammability tester has two timing devices. One controls the impingement time and the other measures the burn time. At the start of the test, a motor drive lowers the impingement head to apply the flame to the specimen. Upon impingement, the two timers begin counting. The first timer is the impingement timer, which will stop after one second and activate the motor to raise the impingement head from the specimen. The second timer is the burn timer and it continues counting until the flame travels the length of the specimen. When the flame burns through the stop thread, the weight will drop onto the switch stopping the burn timer. The burn time is defined as the time elapsed from the time of ignition until the stop thread is severed. The average burn time for five specimens of each test device or fabric material is calculated.
Flammability Test
The flammability for all respirator and other PPE specimens was evaluated following the test procedure for plain surface fabrics. For each test device or fabric material, five specimens were cut to size (5 × 15 cm). Each specimen was clamped in the holder and preconditioned in an oven at 105 ±3°C for 30 minutes. The specimen holder containing specimen was removed from the oven and placed in a desiccator. After cooling, the specimen holder was supported on the specimen rack at a 45° angle (Figure 1a). The position of the specimen holder was adjusted, so the tip of the indicator finger just touched the surface of the specimen.
The average burn time was used to assign each test device or fabric material a flammability class. Flammability class 1 devices or fabric materials show a burn time of >3.5 sec and do not burn readily. The burn time for class 3 devices or fabric materials is < 3.5 sec. There are three alternative outcomes to the test. The first possible outcome is the specimen fails to ignite. The second possible outcome is the specimen ignites but extinguishes without spreading the length of the test specimen. The third possible outcome is the specimen ignites and burns the entire length of the specimen but the flame passes underneath the timing thread without breaking it. In this case, the timing device will not give a burn time. These specimens will be assigned Class 1 flammability level. RPDs and other fabric materials were only tested in the original state.
To confirm the flammability results obtained in our laboratory this study, five of the 11 N95 FFR models (3M V-Flex 9105, Drager1350, 3M 9210, Wilson Saf-T-Fit, and Kimberly-Clark 62126) were also tested using the 16 CFR 1610 flammability test by a third party independent (TPI) laboratory. The five N95 FFR models include four from the six N95 models tested in a previous study (Rengasamy et al., 2015) and one from the five additional N95 models selected in the present study.
RESULTS AND DISCUSSION
The flammability class for 11 N95 FFR models, eight surgical N95 respirator models, five SM models, and other head/facial PPE materials tested in the study are summarized in Table III. None of the eleven N95 FFR models ignited, and therefore were assigned flammability class 1. Similar results were obtained for all eight surgical N95 FFR models. Seven surgical N95 models did not ignite, while one model showed an average burn time of 7.8 sec. Based on the results, the surgical N95 respirators were designated flammability class 1. Of the five SM models tested, three models did not burn, and two models showed average burn times of 5.7 and 6.7 seconds. All five SM models were assigned flammability class 1. The results for five head cover models, and five PAPR hood models also showed flammability class 1. The fabric materials tested in the study were plain fabrics. None of them had raised fabrics. Therefore, fabric materials with an average burn time >3.5 sec were categorized as class 1. The results obtained for surgical N95 FFR models and SM models are expected, because these two categories of devices are FDA cleared for flammability (FDA, 2004). FDA recommends the use of flammability class 1 and class 2 SMs in the surgical environment. Flammability class 3 SMs with an average burn time <3.5 sec are also cleared based on a demonstration of substantial equivalence to a predicate. FDA recommends these devices to be labelled with a warning statement saying that “the device does not meet 16 CFR 1610, NFPA, or CPSC flammability standards” or “the device may burn when used in the presence of high intensity heat source or flammable gas.”
Table III.
Flammability Class of N95 Filtering Facepiece Respirator (N95 FFR), Surgical N95 Respirator (Surgical N95), Surgical Mask (SM), Head Cover and PAPR Hoods
| Respirator and other PPE | NIOSH | TPI | ||||
|---|---|---|---|---|---|---|
| Model | Burn Time (sec) | Flammability Class | Model | Flammability Test Result | Flammability Class | |
| N95 FFR | 11 | DNI | Class 1 | 5 | DNI | Class 1 |
| Surgical N95 | 7 1 |
DNI 7.8 |
Class 1 | ND | ND | ND |
| SM | 3 2 |
DNI 5.7 - 6.3 |
Class 1 | ND | ND | ND |
| Surgical Head Cover | 5 | DNI | Class 1 | ND | ND | ND |
| PAPR Hood | 1 3 1 |
DNI IBE 10.3 |
Class 1 | ND | ND | ND |
TPI – Third Party Independent Laboratory
DNI - did not ignite; IBE - ignited but extinguished; ND - not determined
To validate the results obtained in our laboratory, five of the 11 N95 FFR models were tested by a TPI laboratory and the results were compared (Table III). Results from the TPI laboratory showed that none of the five models ignited. The overall results showed flammability class 1 for all 5 N95 FFR models. The results obtained in the NIOSH laboratory and the TPI laboratory were comparable showing consistency between the two laboratories. The results indicate that NIOSH is capable of conducting the flammability test for respirators and other head/facial PPE consistent with the testing done by other laboratories.
All N95 FFRs, surgical N95 respirators, and other head/facial PPE tested in the study showed flammability class 1, indicating relatively poor flammability. The results raised uncertainty on whether the flammability test method can identify fabric materials of other flammability classes. To understand this, a variety of non-FDA cleared fabric materials including, cotton, linen, hemp and cheesecloth were evaluated for flammability (Table II). Of the five cotton fabrics tested, a double cotton thin lightweight fabric (17.30 g/m2) showed flammability class 3, whereas the four other cotton fabrics showed flammability class 1. Other fabrics including, two linen, a hemp, and a cheesecloth showed flammability class 1. The results obtained in the study indicate that the test method is capable of differentiating the flammability of different fabric materials.
The difference in fabric flammability class can be explained partially by the burning mechanisms of fabrics. Of the three components of the fire triangle, oxidizer and fuel are essential where fabrics can influence the flammability and burning behavior. Several characteristics including, limiting oxygen index (LOI), weight and weave pattern of the fabric, fiber composition, and thermal transition temperatures are critical to control the burning behavior of fabrics. The LOI represents the minimum concentration of oxygen, expressed as a percentage that will support combustion of a polymer (Nelson et al., 2001). In general, fabrics with LOIs <21% burn readily because the atmospheric oxygen concentration (~21%) is large enough to easily support the burning process. On the other hand, fabrics with LOIs >21% do not readily burn because they require higher than 21% oxygen concentration for burning. The LOI of synthetic fiber materials is relatively larger than 21% and the values for natural fiber materials including cotton, linen and silk are less than 21% (Fan and Lau, 2009; Goodwin, 2006). The LOI is not the only parameter that limits the flammability of the fabrics, but other characteristics also play a significant role. For example, naturally occurring fiber materials such as cotton and linen tend to burn easily whereas synthetic fiber materials do not burn readily (Speece, 1974; Stone, 2003).
The weight and weave pattern of the fabrics considerably influence the flammability of fabrics. Lightweight fabrics with shorter average burn time burn more easily than heavier fabrics with longer average burn time. Plain surface fabrics weighing 88.2 g/m2 or more, regardless of fiber content, are considered not easily flammable, and are exempted from 16 CFR 1610 flammability testing (Federal Register, 2008). Table II shows the weight, average burn time and flammability class for the different types of materials tested in the study. For example, respirators and other PPE devices or fabrics tested in the study did not ignite or burn easily compared to some plain cotton and linen fabrics. The weight of respiratory devices per given area (98 - 502 g/m2) is higher than the weight of the loose natural fiber materials such as cotton (17.3 - 81.78 g/m2). The weight of the fabric was directly related to the average burn time. The relationship can be seen between the lightweight (17.3 g/m2) double cotton fabric having a shorter burn time (2.5 sec) than the other cotton fabrics (33.2 - 81.78 g/m2) with longer burn times (5.14 – 14.2 sec). The lightweight cotton fabric was highly flammable (flammability class 3) than the heavier weight cotton fabric (flammability class 1) showing the significance of weight on flammability. Although, cotton and linen fabrics have been described to burn readily (Speece, 1974; Stone, 2003) the two linen (92.7 - 130 g/m2), one hemp (203 g/m2) and one cheesecloth (84.62 g/m2) materials failed to burn easily. The results obtained in the study validate that the weight of the fabrics influence the flammability of fabrics. The loose, lightweight fabrics allow higher concentration of oxygen between the fabric fibers and support the flammability of the material. On the other hand, the tight heavier fabrics may not readily burn because of the lack of sufficient oxygen between the fibers to support flammability.7,15 It is somewhat surprising why the loose cheesecloth burned slowly with an average burn time 22.9 sec, similar to class 1 materials. One possible reason is that the weight of the cheesecloth (84.62 g/m2) is larger than the cotton fabrics (17.3 and 81.78 g/m2). Also, fabrics treated with fire resistant chemicals such as ammonium polyphosphate have been shown to release gases which deprive oxygen and prevent burning (Chang et al., 2014). It is not known if the tested cheesecloth was treated with chemicals for fire-resistance. Regarding the weave pattern of the fibers, respirators and other PPE are made of multilayered synthetic fibers bonded very close to each other in a complex manner to form a tight structure compared to the fiber arrangement in some cotton and other fabrics.
The respiratory devices and fabric materials were tested in the study using the methods described in 16 CFR 1610 method. The standard excludes flammability testing for specific fabrics and their products. As per the 16 CFR Part 1610 standard, “the experience gained from years of testing in accordance with the standard demonstrates that certain fabrics consistently yield acceptable results when tested in accordance with the Standard. Therefore, persons and firms issuing an initial guaranty of any of the following types of fabrics, or of products made entirely from one or more of these fabrics, are exempt from any requirement for testing to support guaranties of those fabrics: (1) Plain surface fabrics, regardless of fiber content, weighing 2.6 ounces per square yard or more; and (2) All fabrics, both plain surface and raised-fiber surface textiles, regardless of weight, made entirely from any of the following fibers or entirely from combination of the following fibers: acrylic, modacrylic, nylon, olefin, polyester, wool.”
The FDA recommends the use of flammability class 1 and 2 SMs for operating room purposes (FDA, 2004). The class 3 SMs with an average burn time <3.5 sec is highly flammable compared to class 1 and class 2 devices. FDA clearance of class 3 SMs with a warning statement such as “the device does not meet 16 CFR 1610, NFPA, or CPSC flammability standards” or “the device may burn when used in the presence of high intensity heat source or flammable gas” is important to ensure protection against fire hazards in healthcare. On the other hand, it should be recognized that class 1 and class 2 devices are also likely to burn in the presence of high intensity heat source or flammable gas such as oxygen. Though, the FDA recommendation of a warning statement on the flammability of respirators and SMs may be sufficient to address healthcare workers exposure to surgical fires, it raises a question of whether respirators, SMs, and other head/facial PPE should meet very high flammability standards as an added safety factor. Although surgical fires can occur and harm patients (Burgess III and LeJeune Jr, 1979; Moskowitz, 2009; Weber et al., 2006), preventive measures could reduce the risk. For example, the use of oxygen entrained into the surgical field through open pulmonary blebs caused a surgical fire (Burgess III and LeJeune Jr, 1979). A lack of communication between the workers and the surgeon resulted in the fire. Reducing the oxygen concentration is another factor that could have prevented a fire. The use of a surgical prep solution on a hirsute patient caused fire because the body hair interfered with the drying of prep solution (Weber et al., 2006). The results from the studies indicate that continued surgical fire training on prevention strategies, persistent vigilance, reducing the oxygen concentration in surgical procedures can reduce and prevent surgical fires. In the case of PPE, users should know the occurrence of surgical fires, and adhere to the warning on the use of the devices, and participate in fire prevention trainings.
The use of surgical N95 FFRs in surgical and non-surgical environments increases with outbreaks of transmissible diseases around the world. Previous studies have reported a shortage of respirators during the spread of severe acute respiratory syndrome (SARS) (Srinivasan et al., 2004) and influenza (Beckman et al., 2013). Large numbers of surgical N95 FFRs are needed for future pandemic events. Healthcare workers already use non-FDA cleared NIOSH-approved N95 FFR according to a recent survey (Wizner, 2016); the prevalence of this practice would likely increase during a pandemic when there are concerns about respirator shortages. Many N95 FFR models are not submitted for FDA clearance as surgical N95 respirators because they are not considered by the manufacturers to medical devices and/or because of the time and expense involved in obtaining multiple approvals. The process can be simplified by the incorporation of fluid resistance, flammability and biocompatibility in 42 CFR Part 84 respirator approval process as described recently (D’Alessandro and Surren, 2017; Federal Register, 2018). Streamlining the regulatory oversight of N95s by including flammability, fluid resistance and biocompatibility into the NIOSH certification process is expected to help ensure the availability of safe and effective devices for healthcare use, particularly during times of increased demand (e.g. during emergencies and pandemics.)
Limitations of this study include only five types of PPE were tested and within PPE type between five and eleven models were included. Further studies with additional models of the PPE are needed. Market data was available to guide selection of respiratory devices so that models known to be commonly used in healthcare were included. In addition, the other respiratory devices should also be selected randomly based on an internet search of available options similar to the selection of SMs and surgical head covers. Flammability test results were compared with the data from only one independent third party laboratory. Additional third party independent laboratories should be considered for the confirmation of NIOSH test results.
CONCLUSIONS
All of the PPE samples showed class 1 flammability level. All 11 non-FDA cleared NIOSH- approved N95 FFR models met the FDA recommendation for flammability indicating that these models could be used as surgical N95 respirators provided they met fluid resistance and other parameters required by FDA. Currently, these devices cannot be marketed as surgical N95 respirators, because manufacturers have not submitted these models for FDA clearance. A unified process between NIOSH and FDA is under development to improve the efficiency of the current processes that require both FDA clearance and NIOSH approval. The process can be simplified by incorporating testing for flammability, fluid resistance and other parameters in the NIOSH respirator approval process, which would identify the N95 FFR models that meet the FDA recommendations for surgical N95 respirators. The simplified process would ensure that all N95s used in healthcare meet the same performance recommendations/standards.
Acknowledgements
The authors acknowledge Lew Radonovich, Dana Rottach and James Harris of NIOSH, and Bryan Christensen of CDC, and Aftin Ross, Lauren Lilly, Joshua Silverstein, Karoll Cortez, Elizabeth Claverie of FDA for their useful suggestions and critical review of the manuscript. This research work was supported by NIOSH funding. The authors declare there are no conflicts of interest in relation to this paper.
Footnotes
Publisher's Disclaimer: Disclaimer
The findings and conclusions of this article are those of the authors and do not necessarily represent the views of the National Institute for Occupational Health and Safety. Mention of a commercial product or trade name does not constitute endorsement by the National Institute for Occupational Safety and Health.
REFERENCES
- Beckman S, Materna B, Goldmacher S, Zipprich J, D’Alessandro M, Novak D, Harrison R (2013) Evaluation of respiratory protection programs and practices in California hospitals during the 2009-2010 H1N1 influenza pandemic Am J Infect Cont 41:1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess GE III, LeJeune FE Jr (1979) Endotracheal tube ignition during laser surgery of the larynx. Arch Otolaryngol 105:561–562. [DOI] [PubMed] [Google Scholar]
- Chang S-C, Slopek RP, Condon B, Grunlan JC (2014) Surface Coating for Flame-Retardant Behavior of Cotton Fabric Using a Continuous Layer-by-Layer Process. Ind Eng Chem Res, 2014 53:3805–3812. [Google Scholar]
- Clarke JR, Bruley ME, Editorial Advisory Board (2012) Surgical fires: Trends associated with prevention efforts. Pennsylvania Patient Safety Advisory 9:130–135. [Google Scholar]
- D’Alessandro D, Surren J (2017) Memorandum of understanding between the Food & Drug Administration/Centers for Devices & Radiological Health and the Centers for Disease Control & Prevention/National Institute for Occupational Safety & Health/National Personal Protective Technology Laboratory. MOU 225-18-006.
- ECRI Institute (2009a) 2010 top 10 technology hazards. In: Health Devices, pp 364–373. [PubMed] [Google Scholar]
- ECRI Institute (2009b) New clinical guide to surgical fire prevention. In: Health Devices, pp 314–332. [PubMed] [Google Scholar]
- Fan J, Lau L (2009) Flammability of fabrics and garments. in Engineering apparel fabrics and garments. Woodhead Publishing Limited and CRC Press, LLC, Boca Raton, FL. [Google Scholar]
- FDA (2004) Guidance for industry and FDA staff Surgical masks - premarket notification [510(k)] submissions; Guidance for industry and FDA. Washington, D.C. US Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health; http://wwwfdagov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm072549htm. [Google Scholar]
- Federal Register (2008) Standard for the flammability of clothing textiles. Consumer Product Safety Commission. 16 CFR Part 1610. US Government Printing Office, Office of Federal Register, Washington, DC: 73:15636–15661. [Google Scholar]
- Federal Register (2018) Medical devices; Exemption from pre-market notification: Class II devices; Surgical apparel. US Government Printing Office, Office of Federal Register, Washington, DC: 83:22846–26848. [PubMed] [Google Scholar]
- Goodwin P (2006) Flammability testing - a burning issue for technical textiles. Technical Textiles International (TTI) 2006 International Newsletters Ltd:11–14. [Google Scholar]
- Moskowitz M (2009) Fire in the operating room during open heart surgery: a case report. AANA J 77:261–264. [PubMed] [Google Scholar]
- Nelson MI, Sidhu HS, Weber RO, Mercer GN (2001) A dynamical systems model of the limiting oxygen index test. ANZIAM Journal 43:105–117. [Google Scholar]
- Rengasamy S, Sbarra D, Nwoko J, Shaffer RE (2015) Resistance to synthetic blood penetration of National Institute for Occupational Safety and Health-approved N95 filtering facepiece respirators and surgical N95 respirators. Am J Infect Cont 43:1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LP, Roy S (2011) Operating room fires in otolaryngology: risk factors and prevention. Am J Otolaryngol 32:109–114. [DOI] [PubMed] [Google Scholar]
- Speece J (1974) EC74-492 Fabric flammability and clothing. Historical Materials from University of Nebraska-Lincoln Extension, Paper 4245. [Google Scholar]
- Srinivasan A, Jernign DB, Liedtke L, Strausbaugh L (2004) Hospital preparedness for severe acute respiratory syndrome in the United States: Views from a national survey of infectious diseases consultants. Clin Infect Dis 39:272–274. [DOI] [PubMed] [Google Scholar]
- Stone J (2003) Facts about fabric flammability. North Central Regional Extension Publication; 174, Revised July 2003. [Google Scholar]
- United States Consumer Product Safety Commission (2008) Laboratory Test Manual for 16 CFR Part 1610: Standard for the flammability of clothing textiles.
- Wagner DH (1996) Fire Protection - A Guide for Facility Managers. Upward Publishing, Inc., New York, NY. [Google Scholar]
- Weber SM, Hargunani CA, Wax MK (2006) Duraprep and the risk of fire during tracheostomy. Head Neck 28:649–652. [DOI] [PubMed] [Google Scholar]
- Wizner K, Stradtman L, Novak D, Shaffer R (2016) Prevalence of respiratory protective devices in U.S. Health Care Facilities: Implications for emergence preparedness. Workplace Health and Safety 64:359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]


