Abstract
Carnations carrying a recessive I gene show accumulation of the yellow pigment chalcononaringenin 2′-glucoside (Ch2′G) in their flowers, whereas those with a dominant I gene do accumulation the red pigment, anthocyanin. Although this metabolic alternative at the I gene could explain yellow and red flower phenotypes, it does not explain the development of orange flower phenotypes which result from the simultaneous accumulation of both Ch2′G and anthocyanin. The carnation whole genome sequencing project recently revealed that two chalcone isomerase genes are present, one that is consistent with the I gene (Dca60979) and another (Dca60978) that had not been characterized. Here, we demonstrate that Dca60979 shows a high level of gene expression and strong enzyme activity in plants with a red flower phenotype; however, functional Dca60979 transcripts are not detected in plants with an orange flower phenotype because of a dTdic1 insertion event. Dca60978 was expressed at a low level and showed a low level of enzyme activity in plants, which could catalyze a part of chalcone to naringenin to advance anthocyanin synthesis but the other part remained to be catalyzed chalcone to Ch2′G by chalcone 2′-glucosyltransferase, resulting in accumulation of anthocyanin and Ch2′G simultaneously to give orange color.
Keywords: anthocyanin, chalcone isomerase, chalcononaringenin 2′-glucoside, I gene, orange carnation
Introduction
Over the past eighty years, many investigations into carnation (Dianthus caryophyllus L.) flower coloration have been conducted. Initially, these studies exploited the traditional genetic approach of cross-hybridization (Geissman and Mehlquist 1947, Geissman et al. 1956, Mehlquist 1939, Mehlquist and Geissman 1947); subsequently, progress in biochemical and molecular biological technologies have allowed identification of the genes responsible for the enzymes involved in anthocyanin synthesis (Tanaka and Brugliera 2013). Most studies have focused on red, white and yellow flowers that are composed of a single color (Okamura et al. 2013) and some carnation cultivars showed variegated flower colors which are caused by movement of a transposable element (Itoh et al. 2002). Although orange flower carnations have been established as ornamental cultivars and used as mutant sources by ion beam irradiation to generate novel color varieties (Okamura et al. 2006), a few studies have investigated to clarify mechanism of orange coloration. These previous studies have shown that red coloration is produced by anthocyanins of pelargonidin or cyanidin glucosides and malyl-glucosides and white is caused by the loss of anthocyanins and accumulation of kaempferol 3-neohesperidoside, kaempferol 3-sophoroside and kaempferol 3-glucosyl-(1→2)-[rhamnosyl-(1→6)-glucoside], and/ or naringenin glycosides (Iwashina et al. 2010, Mato et al. 2000, Onozaki et al. 1999). Loss of activity of anthocyanin synthesis is caused by disruption of structural genes for enzymes such as flavanone 3-hydroxylase (F3H) and dihydroflavonol 4-reductase (DFR) (Itoh et al. 2002, Mato et al. 2000) and by reduction of gene expression for a transcriptional regulatory factor of a member of the basic helix-loop-helix (bHLH) super-family which has an important role in the expression of genes, such as DFR, in the later part of the anthocyanin synthetic pathway (Totsuka et al. 2018). The yellow color is produced by accumulation of isosalipurposide (chalcononaringenin 2′-glucoside: Ch2′G), and the orange color arises through accumulation of both Ch2′G and anthocyanidin glycosides (Forkmann and Dangelmayr 1980, Iwashina et al. 2010, Mato et al. 2000, Nakayama et al. 2000, Stich et al. 1992, Yoshida et al. 2004). Thus, the orange color is generated by a mixture of yellow Ch2′G and red anthocyanins in petal cells. This means that the enzymes for the synthetic pathway, especially the later part of the pathway, are active to synthesize anthocyanin.
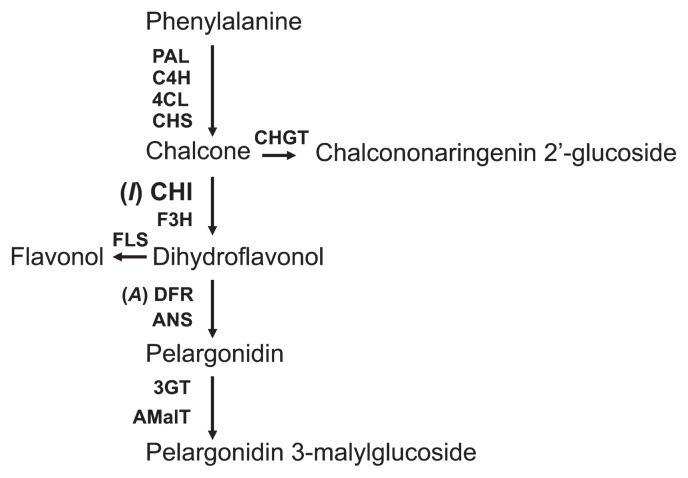
Genetic studies showed that recessive I locus was responsible for yellow and recessive A locus was for white colors in carnations (Geissman and Mehlquist 1947, Geissman et al. 1956, Mehlquist 1939, Mehlquist and Geissman 1947). Recent molecular biological studies reported that the I and A loci include genes encoding the enzymes chalcone isomerase (CHI) and dihydroflavonol 4-reductase (DFR), respectively (Fig. 1) (Forkmann and Dangelmayr 1980, Itoh et al. 2002, Larsen 1996, Stich et al. 1992). When A gene is recessive but I gene is dominant, flavonol synthesis occurs branching from anthocyanin synthetic pathway as shown in Fig. 1 to give white and creamy white flower colors (Itoh et al. 2002, Stich et al. 1992). Although production of the orange color of carnation requires both Ch2′G and anthocyanin, neither genetic studies nor the well-known anthocyanin biosynthetic pathway explain how both yellow and red pigments can be synthesized and accumulated simultaneously (Fig. 1). Stich et al. (1992) described spontaneous isomerization of some chalcone molecules to flavones in a carnation cultivar carrying a recessive I gene and dominant A gene resulting in formation of orange flowers; however, there was no convincing evidence to explain the details of this mechanism.
Fig. 1.
Metabolic pathway for the synthesis of Ch2′G, flavonols (kaempferol glycosides) and anthocyanin.
As a result of recent progress in the carnation genome project, three annotated genes for chalcone isomerases have been identified as Dca60977, Dca60978 and Dca60979 on scaffold 94 (Yagi et al. 2014, http://carnation.kazusa.or.jp/). In this report, we investigated the expression of CHI genes and the CHI enzymatic functions of these translated proteins and demonstrated how both yellow and red pigments can be synthesized and accumulated simultaneously to produce orange coloration in carnation flowers.
Materials and Methods
Plant materials and chemicals
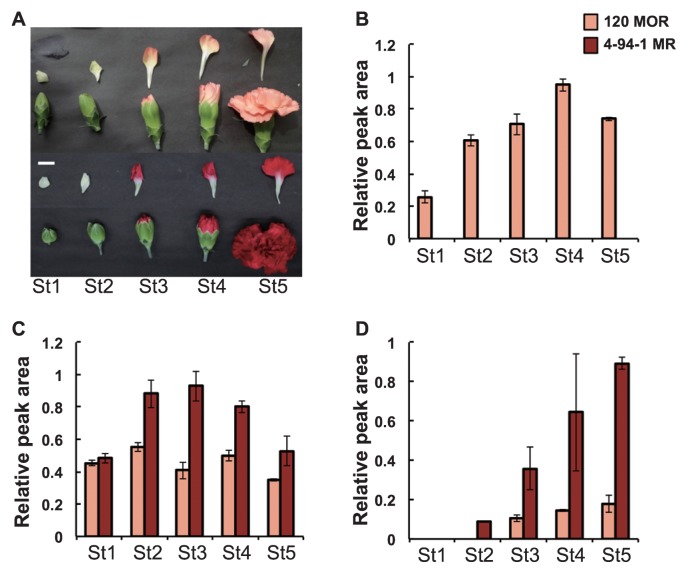
The spray carnation (D. caryophyllus) cultivars used here, namely, red-flowered ‘4-94-1 MR’, orange-flowered ‘120 MOR’, yellowish orange-flowered ‘129 MOR’ and reddish orange-flowered ‘129 MOR-MOR1’, and all of them showed solid colors (Figs. 2A, 5). All cultivars were a gift of the Japan Agribio Company. The definition of petal developmental stages has been described in our previous reports (Mato et al. 2000, Matsuba et al. 2010, Totsuka et al. 2018). Harvested petals were immediately frozen in liquid nitrogen and stored at −80°C until use.
Fig. 2.
The phenotypes of the carnation cultivars used in this experiment. (A) Flower developmental stages in ‘120 MOR’ and ‘4-94-1 MR’. White bar indicates 1 cm. (B) Relative accumulation of Ch2′G. In petals of ‘4-94-1 MR’ at all stages, Ch2′G was below detectable levels in the HPLC analysis. (C) Relative accumulation of flavonols (total of kaempferol 3-neohesperioside, kaempferol 3-sophoroside and kaempferol 3-glucosyl-(1→2)-[rhamnosyl-(1→6)-glucoside]). (D) Relative accumulation of anthocyanin (total of pelargonidin 3-glucoside and pelargonidin 3-malylglucoside). Error bars indicate ± SD for three biological replicates.
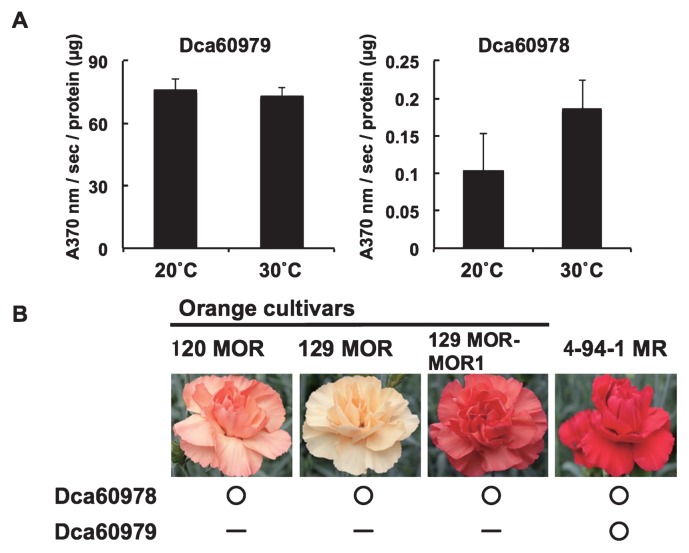
Fig. 5.
Dca60979 and Dca60978 recombinant enzyme assays and the CHI phenotypes of different cultivars. (A) Recombinant enzyme activities of Dca60979 (left) and Dca60978 (right) at 20°C and 30°C. Activity was measured as the decrease in absorbance at 370 nm over a 60 sec period per μg protein. (B) CHI genotypes of carnation cultivars. Orange flowered cultivars ‘120 MOR’, ‘129 MOR’ and ‘129 MOR-MOR1’, and red flowered cultivar ‘4-94-1 MR’. The colors were defined by Royal Horticultural Society (RHS) Colour Chart as; ‘120 MOR’, 24C; ‘129 MOR’, 21D; ‘129 MOR-MOR1’, 31B; ‘4-94-1 MR’, 41A. The circles indicate the presence of transcripts for undisrupted functional sequences and dashes indicate the absence of transcripts or undisrupted functional sequences.
Preparation of the standard reagents pelargonidin 3-glucoside and pelargonidin 3-malylglucoside was described previously (Abe et al. 2008). Flavonols (kaempferol 3-neohesperioside, kaempferol 3-sophoroside and kaempferol 3-glucosyl-(1→2)-[rhamnosyl-(1→6)-glucoside]) were extracted from the white carnation cultivar ‘Bridal White’ as standards (Iwashina et al. 2010). Ch2′G was extracted from ‘120 MOR’ petals. The identities of these compounds were confirmed by measurement of molecular ion peaks on mass spectrograms.
Measurement of Ch2′G, flavonols and anthocyanin accumulation in petals
Total accumulated compounds were extracted from 0.1 g of petals at five different developmental stages from early buds to fully-opened flowers (Fig. 2A) using 80% methanol containing 0.1% trifluoroacetic acid for anthocyanin, or 80% methanol for flavonols and Ch2′G. The extracted compounds were analyzed by high-performance liquid chromatography (HPLC) as Ch2′G, kaempferol 3-neohesperioside, kaempferol 3-sophoroside, kaempferol 3-glucosyl-(1→2)-[rhamnosyl-(1→6)-glucoside], pelargonidin 3-glucoside and pelargonidin 3-malylglucoside by comparison to the standards. Quantification of the compounds was performed through measurement of peak areas showing a peak at 370 nm for Ch2′G, 350 nm for kaempferol glycosides and 520 nm for pelargonidin glycosides on HPLC chromatographs produced using an HPLC-photodiode array detector system (LaChrome Elite; Hitachi High-Technologies) equipped with an ODS column (i.d. 4.6 × 250 mm; Wakopak Handy ODS; Wako Pure Chemical Industries). The following conditions were used: linear gradient elution (1.5 mL min−1) of 15–85% methanol in 1.5% aqueous phosphoric acid for 10 min (for anthocyanin) or 35–65% methanol in 1.5% aqueous phosphoric acid for 22 min (for flavonols and Ch2′G).
RNA-seq analysis
Total RNAs were extracted from petals of ‘4-94-1 MR’ and ‘120 MOR’ at developmental stages 1 to 4 using an RNeasy Plant Mini Kit (Qiagen). RNA-sequencing and data analysis were performed as previously described (Totsuka et al. 2018). The sequence data were registered as DDBJ DRA Accession DRA006605. FPKM (fragments per kilobase of transcript per million mapped reads) values were obtained for genes involved in anthocyanin biosynthesis: CHI (Carnation DB gene ID: Dca60978 and Dca60979 (accession no. AF250367)), dihydroflavonol 4-reductase (DFR) (Dca4324), anthocyanidin synthase (ANS) (Dca23371), UDP-glucose: anthocyanin 3-glucosyltransferase (3GT) (Dic3GT1: accession no. AB191245, 3GT1: Dca47835 and 3GT2: Dca17573) (Ogata et al. 2004), glutathione S-transferase (GST) (DcGSTF2: AB688111: Dca57804) (Sasaki et al. 2013) (Fig. 3B, Supplemental Fig. 3).
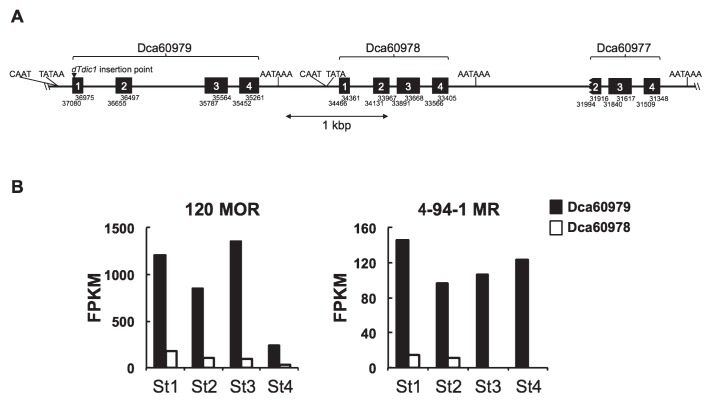
Fig. 3.
Genomic structures and expression analysis of Dca60978 and Dca60979. (A) The genomic structures of Dca60979, Dca60978 and Dca60977 in the cultivar ‘Francesco’ obtained from Carnation DB. CAAT-box and TATA-box motifs found at the putative promoter region upstream of the first exon of Dca60979 and Dca60978, and a polyadenylation signal motif, ‘AATAAA’, found at the proximal region at the end of the fourth exon of Dca60979, Dca60978 and Dca60977. (B) Expression profiles by FPKM values from RNA-seq analysis. First strand cDNAs prepared from petals of the orange cultivar ‘120 MOR’ and red cultivar ‘4-94-1 MR’ at stages 1, 2, 3 and 4 were used for RNA-seq analysis and FPKM values for Dca60978 and Dca60979 were obtained.
Cloning of chalcone isomerase genomic and cDNA sequences from orange and red flowered carnations
Genomic DNAs and total RNAs were isolated from ‘4-94-1 MR’ and ‘120 MOR’, ‘129 MOR’ and ‘129 MOR-MOR1’ petals at developmental stage 3 using a DNeasy Plant Mini Kit and a RNeasy Plant Mini Kit (Qiagen), respectively. First-strand cDNA was synthesized from 1 μg total RNA using oligo(dT)s and PrimeScript reverse transcriptase (Takara Bio). CHI (Dca60978 and Dca60979) cDNA fragments were obtained using specific primer sets (Dca60978 Fwd and Dca60978 Rv for Dca60978, Dca60979 Fwd and Dca60979 Rv for Dca60979) based on the nucleotide sequences of the cultivar ‘Francesco’ in the database Carnation DB (Yagi et al. 2014) (Supplemental Table 1). PCR was performed using Prime Star GXL polymerase (Takara Bio) under the following conditions: 2 min denaturation at 94°C, then 35 cycles of 10 s denaturation at 98°C, 15 s annealing at 55°C, 20 s extension at 68°C, and a final 1 min extension at 68°C. After an adenylation reaction, the amplified DNAs were introduced into the T-vector pMD20 (Takara Bio). The sequences of the genomic and cDNA fragments were determined using a BigDye terminator ver.3.1 cycle sequencing kit and an ABI PRISM 3130xl (Applied Biosystems). Nucleotide structures of Dca60978 and Dca60979, without the transposable element dTdic1, were confirmed by genomic and cDNA sequencing. Dca60979 with the dTdic1 insert was identified by its genomic sequence.
Heterologous production of chalcone isomerase protein in Escherichia coli
The full-length coding regions of CHI cDNAs were amplified by PCR: for Dca60978, first strand cDNA from ‘120 MOR’ was used as the template with the primer set Dca60978 + BamHI and Dca60978 + NotI; for Dca60979, first strand cDNA from ‘4-94-1 MR’ was used as the template with the primer set Dca60979 + BamHI and Dca60979 + NotI (Supplemental Table 1). The amplicons were digested with the appropriate restriction enzymes and introduced into pGEX6p-2 vector (GE Healthcare) to fuse a glutathione S-transferase (GST) tag. E. coli BL21(DE3) competent cells were transformed with the recombinant plasmid vectors. The GST-CHI supernatant was further purified using a pre-packed GSTrap HP column (GE Healthcare) and the purity of the protein solutions was analyzed by SDS-PAGE as described previously (Miyagawa et al. 2015).
Recombinant CHI activity assay for the purified recombinant proteins
The conditions for the enzymatic reactions were as follows: a total volume of 490 μL 10 mM KPi buffer (pH 7.5) containing purified recombinant protein and 50 mM potassium cyanide. The enzyme reaction was started by adding 10 μL chalcononaringenin (1 mg/mL). Reaction kinetics over a 60 sec period at 20 and 30°C were measured as the decrease in absorbance at 370 nm as the chalcononaringenin was converted to naringenin by CHI activity (Forkmann and Dangelmayr 1980). The concentrations of purified recombinant protein were quantified using a Coomassie brilliant blue (CBB) protein assay kit (Thermo Fisher Scientific Inc., MA, USA) with bovine serum albumin as the standard.
Results
Compounds accumulating in orange and red petals at different developmental stages
A high level of Ch2′G was produced and accumulated in the orange cultivar ‘120 MOR’; the amount of Ch2′G increased as petal development progressed and was maximum at stage 4 (Fig. 2B). By contrast, Ch2′G was difficult to detect at any stage of petal development in ‘4-94-1 MR’. Total flavonols (kaempferol 3-neohesperioside, kaempferol 3-sophoroside and kaempferol 3-glucosyl-(1→2)-[rhamnosyl-(1→6)-glucoside]) were relatively constant at most developmental stages in ‘120 MOR’, although they increased up to stage 3 and then fell at following stages in the red flowers of ‘4-94-1 MR’ (Fig. 2C). The accumulation of anthocyanin (pelargonidin 3-glucoside and pelargonidin 3-malylglucoside) increased to stage 5 in both cultivars although anthocyanin content of ‘4-94-1 MR’ at stage 5 was about 5-fold higher than that of ‘120 MOR’ (Fig. 2D).
Genomic structure and expression of CHI Dca60979 and Dca60978
The genomic sequence of carnation includes three CHI sequences that have been annotated as Dca60979, Dca60978 and Dca60977 (Yagi et al. 2014, http://carnation.kazusa.or.jp). The detailed relationship of these genes is summarized in Fig. 3A. The three genes are tandemly located within an approximately 6 kbp region with forward orientation in scaffold 94. Dca60979 and Dca60978 have been suggested to have four exons and three introns. In the database annotation predicted by the Augustus program, Dca60977 is suggested to have two exons and one intron; however, a detailed analysis by the GENETYX homology search program indicated that one more partial exonic sequence may be located at the front of the exons predicted by Augustus (Fig. 3A). The nucleotide sequences of the predicted exons and introns in Dca60977 and Dca60978 show over 90% identity (Supplemental Fig. 1), suggesting that Dca60977 might be the result of gene duplication of Dca60978 during evolution. In contrast to the high level of identity between Dca60978 and Dca60977, a lower level of identity is present in Dca60979 (Supplemental Fig. 1), suggesting that the latter was present in the genome prior to the duplication event generating Dca60978 and Dca60977.
The CHI sequence tagged with dTdic1 reported in an earlier study (Itoh et al. 2002) is identical to Dca60979 and has been registered with the accession number AF250367; Larsen (1966) identified this as the I gene. Dca60978 encodes a predicted protein with 54% identity and 91% amino acid sequence similarity to that of Dca60979 (Supplemental Fig. 2). A comparison of expression using FPKM values from the RNA-seq analysis showed transcripts from both Dca60979 and Dca60978 were detected. Dca60979 had high FPKM values compared with Dca60978 in both the ‘120 MOR’ and ‘4-94-1 MR’ cultivars; Dca60978 had low FPKM values especially in the ‘4-94-1 MR’ cultivar, and was below the detectable level at stages 3 and 4 (Fig. 3B). The expression of other genes in ‘120 MOR’ for anthocyanin biosynthesis, i.e., DFR, ANS, 3GT1, 3GT2, GST, were confirmed and their transcript sequences corresponded to those in the red cultivar ‘4-94-1 MR’ (Supplemental Fig. 3).
Structures of Dca60979 and Dca60978 sequences in ‘120 MOR’
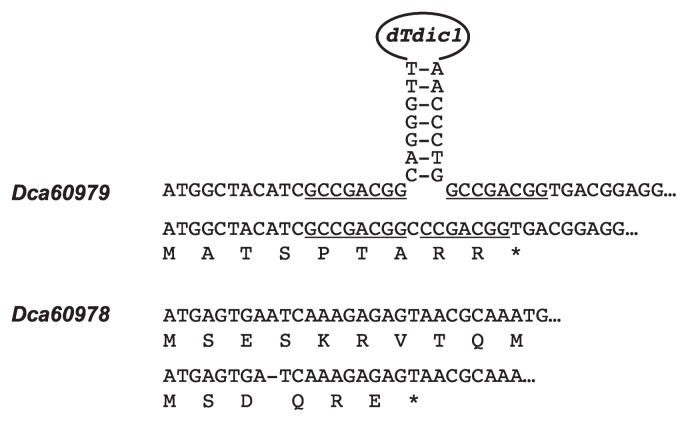
Two different nucleotide sequences were identified for Dca60979 and Dca60978 in ‘120 MOR’. For Dca60979, one variant included a dTdic1 inserted sequence, which was consistent with the registered CHI sequence AF250367 (Itoh et al. 2002, Larsen 1996). The second variant made a footprint sequence derived from a target site duplication ‘GCCGACGG’ at the insertion event, which caused an accidental stop codon (Fig. 4). For Dca60978, one variant was an undisrupted translatable nucleotide sequence; the second variant had a single nucleotide deletion 9 bp downstream of the first methionine and resulted in an accidental stop codon (Fig. 4). The genomes of the red cultivars ‘Francesco’ and ‘4-94-1 MR’ contained undisrupted Dca60979 and Dca60978 nucleotide sequences that could be translated to give presumably active CHI enzyme proteins.
Fig. 4.
The nucleotide sequences of Dca60979 and Dca60978 at the proximal region of the first methionine in the orange flowered cultivar ‘120 MOR’. Dca60979 has two variant sequences: in one, a dTdic1 transposable element insertion is present; in the other, a target site duplication sequence is present as a footprint that causes an accidental stop codon. Dca60978 has two variant sequences: one has an undisrupted and translatable sequence; the other has a single nucleotide deletion 9 bp downstream from the first methionine that causes an accidental stop codon. The underlines indicate the target site duplication sequence occurring at the dTdic1 insertion event. Asterisks indicate the change causing an accidental stop codon.
CHI enzyme activity of the recombinant proteins of Dca60979 and Dca60978 and the CHI phenotypes of orange flowered cultivars
Purified recombinant GST fusion proteins of Dca60979 and Dca60978 were produced and applied to a CHI enzyme assay. Both proteins showed CHI enzyme activity, but that of Dca60978 was much lower than that of Dca60979 (Fig. 5A). No significant difference was detected in enzyme activity at 20°C and 30°C for either Dca60979 or Dca60978. The nucleotide sequences of the cDNAs corresponding to Dca60979 and Dca60978 showed that three orange flowered cultivars, ‘120 MOR’, ‘129 MOR’ and ‘129 MOR-MOR1’, had an undisrupted Dca60978 sequence but a defective Dca60979 sequence including dTdic1 and a footprint sequence. The red flowered cultivar ‘4-94-1 MR’ had undisrupted sequences of both Dca60978 and Dca60979 (Fig. 5B).
Discussion
A series of genetic, biochemical and molecular biological studies have shown that the recessive I gene has a loss of CHI function and causes the accumulation of Ch2′G; the dominant I gene causes anthocyanin accumulation (Forkmann and Dangelmayr 1980, Geissman and Mehlquist 1947, Itoh et al. 2002, Stich et al. 1992). However, in orange flowered carnations, both Ch2′G and anthocyanin are synthesized and accumulated simultaneously. As shown in this report, two genes, Dca60979 and Dca60978, are located adjacent to each other in the red cultivar ‘Francesco’ (Yagi et al. 2014) (Fig. 3A). Transcripts of the two CHI genes had functional sequences encoding CHI proteins in red flowered cultivars; however, in orange flowered cultivars, only Dca60978 transcripts were functional as Dca60979 was disrupted (Figs. 4, 5B). The Dca60978 recombinant enzyme showed very weak activity compared to Dca60979 (Fig. 5A). Furthermore, an expression analysis showed high levels of Dca60979 transcripts in ‘4-94-1 MR’, but quite low levels of Dca60978 transcripts in both ‘4-94-1 MR’ and ‘120 MOR’ (Fig. 3B). These results indicated that there was another functional CHI gene, Dca60978, in addition to the previously known I gene (Dca60979) in the carnation genome. In red flowered cultivars, Dca60979 was strongly expressed and catalyzed the conversion of chalcononaringenin to naringenin to advance anthocyanin biosynthesis; the extremely low expression of Dca60978 suggests that it probably does not function in anthocyanin synthesis. For this reason, the recessive I gene (Dca60979) gives yellow coloration and the dominant I gene produces red coloration. In orange flowered cultivars, high levels of transcripts derived from Dca60979 were detected, but were not functional due to disruption by the inserted sequence dTdic1 or by a footprint sequence. Dca60978 was expressed as a functional CHI coding gene but its low expression and the weak activity of the translated protein probably catalyzed a part of chalcononaringenin to naringenin to advance anthocyanin synthesis, but the other part of that was catalyzed by chalcone 2′-glucosyltransferase, resulting in simultaneous accumulation of both red anthocyanin and yellow Ch2′G to generate orange coloration.
Our preliminary results by RNA-seq analysis showed significantly high expression levels of Dca60978 and GST genes in ‘129 MOR-MOR1’ at St3 petals than that in ‘129 MOR’ (data not shown). The gene involved in flower color intensity in cyanic carnations was identified as S gene (Mehlquist and Geissman 1947). The S gene playing a part in anthocyanin accumulation in vacuoles was responsible to GST gene corresponding to DcGSTF2 (AB688111: Dca57804) in carnation flower colors (Momose et al. 2013, Sasaki et al. 2013). That could be concerned CHI (Dca60978) and GST for S gene might play an important role in accumulation of anthocyanin in orange carnation. As an orange-flowered phenotype possibly results from increased Dca60978 and GST expression, further investigation of Dca60978 and GST expression in flowers harboring color tones from pale orange to scarlet, as a result of different amounts of accumulated anthocyanins, will provide flower color varieties of benefit to carnation breeding.
Supplementary Information
Acknowledgements
This work was supported by “Breeding of floricultural plants adapted for high practical needs and development of low cost cultivation technique” of Ministry of Agriculture, Forestry and Fisheries, project number 15653424.
Literature Cited
- Abe, Y., Tera, M., Sasaki, N., Okamura, M., Umemoto, N., Momose, M., Kawahara, N., Kamakura, H., Goda, Y., Nagasawa, K.et al. (2008) Detection of 1-O-malylglucose: pelargonidin 3-O-glucose-6″-O- malyltransferase activity in carnation (Dianthus caryophyllus). Biochem. Biophys. Res. Commun. 373: 473–477. [DOI] [PubMed] [Google Scholar]
- Forkmann, G. and Dangelmayr, B. (1980) Genetic control of chalcone isomerase activity in flowers of Dianthus caryophyllus. Biochem. Genet. 18: 519–527. [DOI] [PubMed] [Google Scholar]
- Geissman, T.A. and Mehlquist, G.A.L. (1947) Inheritance in the carnation, Dianthus caryophyllus. IV. The chemistry of flower color variation, I. Genetics 32: 410–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissman, T.A., Hinreiner, E.H. and Jorgensen, E.C. (1956) Inheritance in the carnation, Dianthus caryophyllus. V. The chemistry of flower color variation, II. Genetics 41: 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, Y., Higeta, D., Suzuki, A., Yoshida, H. and Ozeki, Y. (2002) Excision of transposable elements from the chalcone isomerase and dihydroflavonol 4-reductase genes may contribute to the variegation of the yellow-flowered carnation (Dianthus caryophyllus). Plant Cell Physiol. 43: 578–585. [DOI] [PubMed] [Google Scholar]
- Iwashina, T., Yamaguchi, M.A., Nakayama, M., Onozaki, T., Yoshida, H., Kawanobu, S., Onoe, H. and Okamura, M. (2010) Kaempferol glycosides in the flowers of carnation and their contribution to the creamy white flower color. Nat. Prod. Commun. 5: 1903–1906. [PubMed] [Google Scholar]
- Larsen, E.S. (1996) Characterization of unstable anthocyanin loci in Dianthus caryophyllus L.: a new higher-plant transposable element system. PhD thesis of Stanford University, California. [Google Scholar]
- Mato, M., Onozaki, T., Ozeki, Y., Higeta, D., Itoh, Y., Yoshimoto, Y., Ikeda, H., Yoshida, H. and Shibata, M. (2000) Flavonoid biosynthesis in white-flowered Sim carnations (Dianthus caryophyllus). Sci. Hortic. 84: 333–347. [Google Scholar]
- Matsuba, Y., Sasaki, N., Tera, M., Okamura, M., Abe, Y., Okamoto, E., Nakamura, H., Funabashi, H., Takatsu, M., Saito, M.et al. (2010) A novel glucosylation reaction on anthocyanins catalyzed by acyl-glucose-dependent glucosyltransferase in the petals of carnation and delphinium. Plant Cell 22: 3374–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlquist, G.A.L. (1939) Inheritance in the carnation, Dianthus caryophyllus. I. Inheritance of flower color. Proc. Am. Soc. Hortic. Sci. 37: 1019–1021. [Google Scholar]
- Mehlquist, G.A.L. and Geissman, T.A. (1947) Inheritance in the carnation, Dianthus caryophyllus III. Inheritance of flower colour. Ann. Mo. Bot. Gard. 34: 39–75. [Google Scholar]
- Miyagawa, N., Miyahara, T., Okamoto, M., Hirose, Y., Sakaguchi, K., Hatano, S. and Ozeki, Y. (2015) Dihydroflavonol 4-reductase activity is associated with the intensity of flower colors in delphinium. Plant Biotechnol. 32: 249–255. [Google Scholar]
- Momose, M., Itoh, Y., Umemoto, N., Nakayama, M. and Ozeki, Y. (2013) Reverted glutathione S-transferase-like genes that influence flower color intensity of carnation (Dianthus caryophyllus L.) originated from excision of a transposable element. Breed. Sci. 63: 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama, M., Koshioka, M., Yoshida, H., Kan, Y., Fukui, Y., Koike, A. and Yamaguchi, M. (2000) Cyclic malyl anthocyanins in Dianthus caryophyllus. Phytochemistry 55: 937–939. [DOI] [PubMed] [Google Scholar]
- Ogata, J., Itoh, Y., Ishida, M., Yoshida, H. and Ozeki, Y. (2004) Cloning and heterologous expression of cDNAs encoding flavonoid glucosyltransferases from Dianthus caryophyllus. Plant Biotechnol. 21: 367–375. [Google Scholar]
- Okamura, M., Tanaka, A., Momose, M., Umemoto, N., Teixeira da Silva, J.A. and Toguri, T. (2006) Advances of mutagenesis in flowers and their industrialization. In: Teixeira da Silva, J.A. (ed.) Floriculture, Ornamental and Plant Biotechnology, Volume 1, Advances and Topical Issues, Global Science Books, Isleworth, UK, pp. 619–628. [Google Scholar]
- Okamura, M., Nakayama, M., Umemoto, N., Cano, E.A., Hase, Y., Nishizaki, Y., Sasaki, N. and Ozeki, Y. (2013) Crossbreeding of a metallic color carnation and diversification of the peculiar coloration by ion-beam irradiation. Euphytica 191: 45–56. [Google Scholar]
- Onozaki, T., Mato, M., Shibata, M. and Ikeda, H. (1999) Differences in flower color and pigment composition among white carnation (Dianthus caryophyllus L.). Sci. Hortic. 82: 103–111. [Google Scholar]
- Sasaki, N., Nishizaki, Y., Uchida, Y., Wakamatsu, E., Umemoto, N., Momose, M., Okamura, M., Yoshida, H., Yamaguchi, M., Nakayama, M.et al. (2013) Identification of the glutathione S-transferase gene responsible for flower color intensity in carnations. Plant Biotechnol. 29: 223–227. [Google Scholar]
- Stich, K., Eidenberger, T., Wurst, F. and Forkmann, G. (1992) Enzymatic conversion of dihydroflavonols to flavan-3,4-diols using flower extracts of Dianthus caryophyllus L. (carnation). Planta 187: 103–108. [DOI] [PubMed] [Google Scholar]
- Tanaka, Y. and Brugliera, F. (2013) Flower colour and cytochromes P450. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 368: 20120432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsuka, A., Okamoto, E., Miyahara, T., Kouno, T., Cano, E.A., Sasaki, N., Watanabe, A., Tasaki, K., Nishihara, M. and Ozeki, Y. (2018) Repressed expression of a gene for a basic helix-loop-helix protein causes a white flower phenotype in carnation. Breed. Sci. 68: 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi, M., Kosugi, S., Hirakawa, H., Ohmiya, A., Tanase, K., Harada, T., Kishimoto, K., Nakayama, M., Ichimura, K., Onozaki, T.et al. (2014) Sequence analysis of the genome of carnation (Dianthus caryophyllus L.). DNA Res. 21: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, H., Akimoto, H., Yamaguchi, M., Shibata, M., Habu, Y., Iida, S. and Ozeki, Y. (2004) Alteration of methylation profiles in distinct cell lineages of the layers during vegetative propagation in carnation (Dianthus caryophyllus). Euphytica 135: 247–253. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.