Abstract
Objective
Metabolomic is now widely used to characterize metabolic phenotypes associated with lifestyle risk factors such as obesity. The objective of the present study was to explore the associations of body mass index (BMI) with 145 metabolites measured in blood samples in the European Prospective Investigation into Cancer and Nutrition (EPIC) study.
Methods
Metabolites were measured in blood from 392 men from the Oxford (UK) cohort (EPIC-Oxford) and in 327 control subjects who were part of a nested case-control study on hepatobiliary carcinomas (EPIC-Hepatobiliary). Measured metabolites included amino acids, acylcarnitines, hexoses, biogenic amines, phosphatidylcholines, and sphingomyelins. Linear regression models controlled for potential confounders and multiple testing were run to evaluate the associations of metabolite concentrations with BMI.
Results
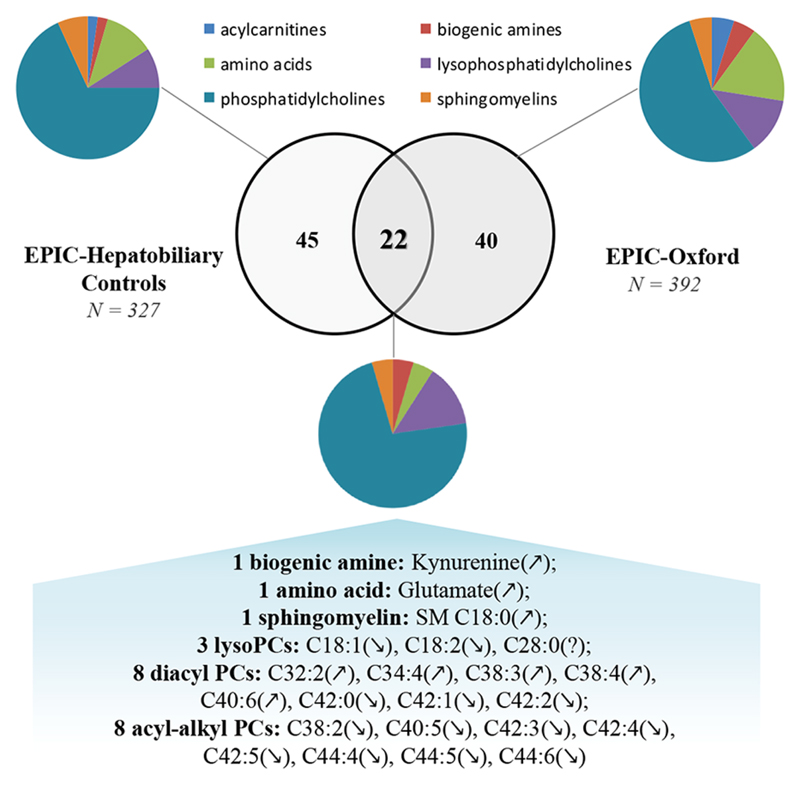
40 and 45 individual metabolites showed significant differences according to BMI variations, in the EPIC-Oxford and EPIC-Hepatobiliary sub-cohorts, respectively. Twenty two individual metabolites (kynurenine, one sphingomyelin, glutamate and 19 phosphatidylcholines) were associated with BMI in both sub-cohorts.
Conclusions
The present findings provide additional knowledge on blood metabolic signatures of BMI in European adults, which may help identifying mechanisms mediating the relationship of BMI with obesity-related diseases.
Keywords: Body mass index, Obesity, Targeted metabolome, Metabolic profiling, Blood
Introduction
Obesity is associated with increased morbidity and mortality from non-communicable diseases, such as diabetes, cardiovascular disease, and some cancers.1–3 The World Health Organization estimates that 2.8 and 3.2 million people worldwide die each year due to excess body weight and physical inactivity, respectively.4 In the US and Europe, 20 to 30% of all cancers could be prevented by the adoption of a healthy diet, normal body weight and appropriate physical activity habits. Epidemiologic studies suggest that obesity modifies sex hormone production, circulating inflammatory biomarkers, insulin resistance, oxidative stress, and lipid metabolism that have been associated with increased risk of major non-communicable diseases.6–8 However, further investigations are needed to understand how these factors may exert their effects.
The advancement of analytical technologies and metabolomics now allows the quantification of large numbers of low-molecular weight metabolites in human blood,9,10 the discrimination of metabolic signatures associated with individual phenotypes and the exploration of metabolic effects associated with lifestyle factors.11–13 Blood metabolic signatures of adiposity were characterized in multiple observation studies14–25 and associations between amino acids and body mass index (BMI), particularly isoleucine, glycine, and valine were consistently reported.14–16,18,20,21,24 However, findings related to other classes of compounds such as glycerophospholipids, sphingolipids, and acylcarnitines were largely inconsistent across studies.16–18,21–26
The aim of the present work was to examine the relationships between BMI and 145 endogenous metabolites, including amino acids, acylcarnitines, hexoses, biogenic amines, phosphatidylcholines (PCs), and sphingomyelins, measured in blood samples from the European Prospective Investigation into Cancer and Nutrition (EPIC) study.
Methods
Study design and population
EPIC is a large, multicenter prospective study aiming at investigating the associations between lifestyle, diet, and environmental factors and the incidence of cancers and other chronic, non-communicable diseases. Study population and data collection procedures have been described in details in previous publications.27 Diet and lifestyle variables were collected through questionnaires from about 520,000 men and women enrolled between 1992 and 2000 throughout 23 centers in 10 European countries.28 The present study involves two subsets of the EPIC population: the EPIC-Oxford and the EPIC-Hepatobiliary sub-cohorts.
EPIC-Oxford sub-cohort
Sixty-five thousand participants older than 20 years of age, of whom 14,606 (22%) were men, were recruited from across the UK into the EPIC-Oxford sub-cohort from 1993 to 2000.29 As one of the major aims of this cohort was to investigate associations of diet with cancer risk in individuals having contrasted habitual dietary habits, a large number of vegetarians and vegans were included.
In the present study, the eligibility criteria were: age 30 to 49 years; male gender; providing blood sample at study entry; known diet group (vegan, vegetarian, meat eater, fish eater); known smoking status; response to at least 80% of the food frequency questionnaire relevant questions with a daily energy intake ranging from 800 to 4,000 kcal; and no prior cancer (excluding non-melanoma skin cancer), cardiovascular disease, or treatment for any long-term illness or condition at recruitment. Of the 110 eligible vegans (who do not eat any animal products), we selected all those aged 30–39 years and randomly selected four out of every five aged 40–49 years. In addition, eligible meat-eaters (who eat meat), fish-eaters (who do not eat meat but do eat fish) and vegetarians (who do not eat meat or fish but do eat dairy products and/or eggs) were randomly selected in equal numbers within strata of age 30–39 and 40–49 years. A total of 392 men were included in this analysis and equally distributed in the four diet groups (n=98 per group)30,31. All individual participants included in the study provided signed informed consent and a multi-center research ethics committee approved the protocol for the EPIC-Oxford (MREC/02/0/90).
EPIC-Hepatobiliary control sub-cohort
Three hundred twenty seven controls (women and men) who provided blood samples at recruitment, were selected from a nested case-control study on hepatobiliary cancer within the EPIC study.32 The present analysis was carried out on healthy controls to avoid any risk of bias from metabolic changes due to hepatobiliary cancer development.
All individual participants included in the study provided signed informed consent. Approval for the metabolomics studies was obtained from the IARC Ethics Committee (Lyon, France) and the relevant ethical review boards of the institutions participating in the EPIC cohort.
Laboratory analysis
Blood metabolites from the two sub-cohorts were assayed with the same procedure, by tandem mass spectrometry at IARC, Lyon, France, using the AbsoluteIDQ™ p180 Kit (BIOCRATES Life Sciences AG, Innsbruck, Austria). Amino acids and biogenic amines were separated by liquid chromatography before injection into the mass spectrometer, while flow injection analysis was used for PCs, hexose, acylcarnitines, and sphingolipids. In both datasets, metabolites with coefficients of variation larger than 20%, those with more than 30% measurements outside the measurable range, and those with missing values for more than 30% of participants were excluded from the analyses. When measurements were outside the measurable range, values were imputed as follows: concentrations below the detection limit (LOD), when applicable, were set to half of the lowest measured concentrations in the EPIC-Oxford sub-cohort, and to half the LOD in the EPIC-Hepatobiliary control sub-cohort, respectively. In both datasets, concentrations below the limit of quantification (LOQ), when applicable, were set to half of the LOQ. In addition, measurements higher than the highest calibration standard concentration were set to the highest standard concentrations.
In the EPIC-Oxford sub-cohort, a total of 145 metabolites were quantified in citrated plasma. Five of these metabolites had coefficients of variation greater than 20%, 11 had more than 30% of the concentrations outside the measurable range and one metabolite had missing values for more than 30% of participants; these 17 metabolites were excluded. Thus, 128 metabolites (10 acylcarnitines, 21 amino acids, four biogenic amines, 78 PCs, hexose and 14 sphingolipids) were included in the study. The median coefficients of variation of quality control samples (calculated based on the 128 included metabolites) were 5.6% for acylcarnitines, 8.2% for amino acids, 8.1% for biogenic amines, 6.8% for PCs, 5.1% for hexose, and 6.7% for sphingolipids.
In the EPIC-Hepatobiliary controls sub-cohort, 145 metabolites were quantified in serum. Two of these metabolites had coefficients of variation greater than 20%, 13 metabolites had more than 30% of the measured concentrations outside the measurable range, and were therefore excluded. Thus, 130 metabolites (11 acylcarnitines, 20 amino acids, five biogenic amines, 79 PCs, hexose and 14 sphingolipids) were included in the study. Samples from different EPIC centers were randomly distributed between analytical batches. The median coefficients of variation of quality control samples (calculated based on the 130 included metabolites) were 3.3% for acylcarnitines, 7.2% for amino acids, 6.6% for biogenic amines, 4.1% for PCs, 2.6% for hexose, and 5.4% for sphingolipids.
The nomenclature of the metabolites is detailed in Supporting Information Appendix S1, and has been published previously.33
Assessment of BMI
Height and weight were used to calculate BMI in kg/m2, based on self-reported data in the EPIC-Oxford sub-cohort and standardized measurements in the EPIC-Hepatobiliary control sub-cohort. In addition to self-reported measurements in the EPIC-Oxford, height and weight were measured in a cohort subsample. Self-reported values showed very good agreement with those measured (correlation coefficient (r)>0.9).34
Statistical analysis
Selected characteristics of the study populations were described and compared according to BMI (<25; ≥25 kg/m2) in each sub-cohort. All metabolite concentrations were logarithmically transformed, and were Z-standardized for better comparison of variables with different blood concentrations.
To evaluate how much of the total variability in the metabolomics data was explained separately by BMI and confounders such as subjects’ characteristics and technical aspects of the sample analysis, the Principal Component Partial R-squared method was performed.35 A principal component analysis was performed on the concentrations of the metabolites. The first 16 and 17 principal components explaining more than 80% of the total variability were retained in the EPIC-Oxford subjects and the EPIC-Hepatobiliary controls, respectively. The principal components scores were regressed in multivariable models on covariates expressing participant’ and blood sample’ characteristics, including BMI. The Rpartial2 statistic was calculated for each principal component for all covariates and the overall Rpartial2 statistic was determined as a weighted average for each covariate. R software version 3.1.2 was used to run the Principal Component Partial R-squared analysis.36
Associations of individual metabolite concentrations with BMI (continuous) were tested using multivariable linear regression. In the EPIC-Oxford sub-cohort, adjustment variables were included as continuous variables i.e., age (years), time since last food/drink at blood collection (min); and indicator variables i.e., alcohol intake (<1; 1-7; 8-15; ≥16 g/d), smoking status (never; former; current), the Cambridge index of physical activity (inactive; moderately inactive; moderately active; active), diet group (meat eater; fish eater; vegetarian; vegan), batch (categorical), time between blood collection and processing (<25; 25-41; 41-72; ≥72h; unknown). In the EPIC-Hepatobiliary control sub-cohort, adjustment variables were included as continuous variable i.e., age (years); and indicator variables i.e., sex (female; male); smoking status (never; former; current; unknown), alcohol intake (<1; 1-7; 8-15; ≥16 g/d), the Cambridge index of physical activity, meat intake (quartiles in g/d: <68.5; 68.5-99.6; 99.6-144.3; ≥144.3), fish intake (quartiles in g/d: <16.1; 16.1-28.3; 28.3-46.3; ≥46.3), batch (categorical), time since last food or drink at blood collection (<3; 3 to 6; ≥6 hours; unknown), and country (United Kingdom; Germany; Sweden; Greece; Spain; The Netherlands; Italy; Denmark).
Pearson correlation coefficients (r) were estimated based on multivariable models to quantify magnitude of effects. Associations with r>|0.20| were considered as the most relevant regarding their magnitude.
Due to the many tests performed in these analyses, all p-values were adjusted for multiple testing as q-values by using false discovery rates with the Simes method,37 given that Bonferroni correction could be overly conservative.38 Significant threshold for Q-values39,40 was set at 0.05. The statistical analyses were run in Stata 12 (Stata Corp., Texas, USA), except otherwise specified.
Results
Participant and blood sample characteristics
Characteristics of participants and blood samples according to BMI are presented in Table 1, and the description of metabolites concentrations according to BMI is presented in Supporting Information Table S1.
Table 1. Population, blood sample characteristics and nutrient intakes of participants according to body mass index (BMI).
| EPIC-Oxford (N=392) | EPIC-Hepatobiliary controls (N=327) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMIb < 25 (N=280) | BMIb ≥ 25 (N=92) | Pa | BMI < 25 (N=100) | BMI ≥ 25 (N=227) | Pa | ||||||||||
| N (%) | |||||||||||||||
| Men | 280 | (100) | 92 | (100) | 1.0 | 52 | (52) | 131 | (57.7) | 0.34 | |||||
| UK samples | 280 | (100.0) | 92 | (100) | 1.0 | 17 | (17) | 19 | (8.37) | 0.022 | |||||
| Current smokingc | 25 | (8.9) | 9 | (9.8) | 0.35 | 24 | (24.0) | 43 | (18.9) | 0.14 | |||||
| Physical activity (Cambridge index)c | 0.56 | 0.47 | |||||||||||||
| Inactive | 37 | (13.2) | 10 | (10.9) | 27 | (27.0) | 83 | (36.6) | |||||||
| Moderately inactive | 75 | (26.8) | 30 | (32.6) | 34 | (34.0) | 70 | (30.8) | |||||||
| Moderately active | 87 | (31.1) | 25 | (27.2) | 22 | (22.0) | 38 | (16.7) | |||||||
| Active | 80 | (28.6) | 27 | (29.4) | 16 | (16.0) | 35 | (15.4) | |||||||
| Meat eatersc | 55 | (19.6) | 39 | (42.4) | <0.0001 | 99 | (99) | 227 | (100) | 0.99 | |||||
| Time since last meal at blood collectionc | 0.056 | 0.076 | |||||||||||||
| Less than 3 hours | 159 | (56.8) | 23 | (57.6) | 19 | (19.0) | 69 | (30.4) | |||||||
| 3 to 6 hours | 86 | (30.7) | 20 | (21.7) | 15 | (15.0) | 42 | (18.5) | |||||||
| More than 6 hours | 31 | (11.1) | 14 | (15.2) | 50 | (50.0) | 84 | (37.0) | |||||||
| Median (IQR: P25 – P75) | |||||||||||||||
| Age at blood collection (years) | 40 | (36 - 44) | 43 | (36 - 44) | 0.198 | 61 | (55 - 65) | 60 | (54 - 64) | 0.33 | |||||
| BMI (kg/m2)b | 22.1 | (20.7 - 23.4) | 26.3 | (25.7 - 28.4) | 0.0001 | 22.9 | (21.5 - 24.0) | 28.3 | (26.4 - 31.0) | 0.0001 | |||||
| Waist-hip ratiob | 0.87 | (0.84 - 0.89) | 0.91 | (0.89 - 0.94) | 0.0001 | 0.84 | (0.76 - 0.91) | 0.91 | (0.84 - 0.97) | 0.0001 | |||||
| Energy (Kcal) | 2047 | (1702 - 2441) | 2118 | (1760 - 2608) | 0.098 | 2171 | (1752 - 2590) | 2079 | (1717 - 2509) | 0.47 | |||||
| Protein (% of energy) | 13.48 | (12.12 - 14.99) | 14.09 | (12.43 - 15.86) | 0.031 | 15.89 | (14.40 - 17.82) | 16.58 | (14.13 - 18.48) | 0.23 | |||||
| Carbohydrates (% of energy) | 53.72 | (48.86 - 58.16) | 53.22 | (48.60 - 57.99) | 0.63 | 44.92 | (39.23 - 48.60) | 43.05 | (38.61 - 47.63) | 0.090 | |||||
| Fat (% of energy) | 31.76 | (27.55 - 34.95) | 30.18 | (26.58 - 34.17) | 0.14 | 35.16 | (30.59 - 39.74) | 35.72 | (32.39 - 39.45) | 0.38 | |||||
| Saturated fat (% of energy) | 9.51 | (7.06 - 12.22) | 10.21 | (8.39 - 11.96) | 0.22 | 13.84 | (11.99 - 15.77) | 12.84 | (10.69 - 15.24) | 0.044 | |||||
| Monounsaturated fat (% of energy) | 10.34 | (8.77 - 11.82) | 9.80 | (8.56 - 11.50) | 0.19 | 12.36 | (10.70 - 14.66) | 13.41 | (11.41 - 15.62) | 0.007 | |||||
| Polyunsaturated fat (% of energy) | 7.37 | (5.71 - 9.44) | 6.94 | (5.14 - 8.21) | 0.047 | 5.26 | (4.07 - 6.65) | 5.40 | (4.35 - 6.89) | 0.42 | |||||
| Alcohol (g/d) | 9.69 | (1.84 - 18.32) | 9.44 | (2.61 - 28.90) | 0.44 | 7.06 | (1.41 - 19.48) | 7.57 | (1.48 - 20.94) | 0.76 | |||||
Differences between BMI groups were tested using the Kruskal-Wallis one-way analysis of variance and χ2 test for continuous and categorical variables, respectively.
Information on BMI was missing for 20 EPIC-Oxford participants.
Information was missing for some EPIC-Hepatobiliary control participants.
In the EPIC-Oxford sub-cohort (N=392), 80 men (20%) were overweight (defined as 25≤BMI<30), and 12 (3%) were obese (defined as BMI≥30).
The EPIC-Hepatobiliary controls sub-cohort (N=327) included 183 men (56%) and 144 women (44%). Among them, 157 individuals (48%) were overweight, and 70 (21%) were obese.
In both sub-cohorts, the intake of nutrients varied according to BMI, but the BMI groups were not different in smoking, alcohol intake, and physical activity practice.
Contributors to metabolome variability
In the EPIC-Oxford sub-cohort, lifestyle, dietary and blood sample characteristics combined explained 31.9% of the total variability in the metabolomics data (Supporting Information Fig. S1). BMI explained 1.4% of the total variability. The major contributor to the observed variation was diet group, explaining 20.7% of the total variability.
In the EPIC-Hepatobiliary controls sub-cohort, lifestyle, and personal and blood sample characteristics combined explained 37.7% of the total variability in the metabolomics data (Supporting Information Fig. S2). BMI explained 2.2% of the total variability. The two main contributors to the variation were batch and country, explaining 14.9% and 13.6% of the total variability, respectively.
Analysis of individual metabolite concentrations
EPIC-Oxford sub-cohort
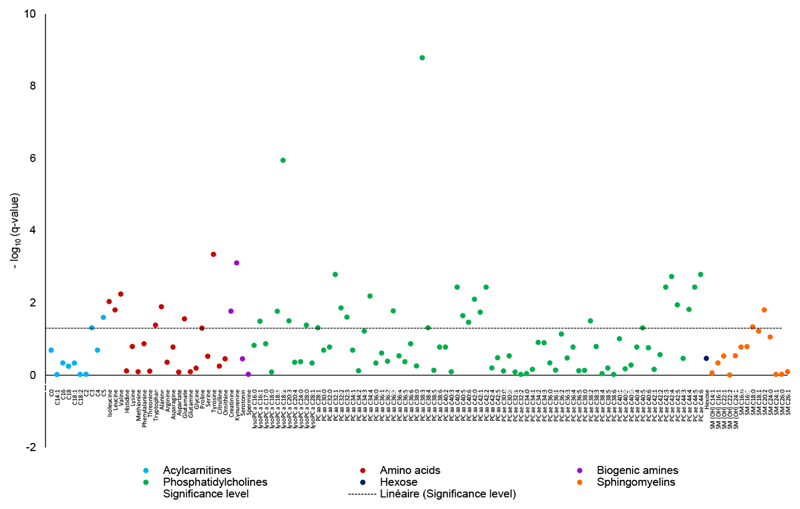
Overall, concentrations of 40 out of 128 metabolites (31.3%) were significantly associated with BMI in the EPIC-Oxford sub-cohort (Fig. 1; Supporting Information Table S2) after adjusting for confounders and multiple testing. Significant associations of BMI were found with 50% of biogenic amines, 45.4% of lysoPCs, 33.3% of amino acids, 32.8% of PCs, 20% of acylcarnitines, and 14.3% of sphingomyelins.
Figure 1.
Associations between body mass index (continuous) and metabolite concentrations plotted as –log10 (q-values) in the EPIC-Oxford cohort. The dashed line shows the corrected significance level (False Discovery Rate). The q-values were derived from linear regression adjusted for age (years, continuous), smoking status (never; former; current), the Cambridge index of physical activity (inactive; moderately inactive, moderately active; active), alcohol intake (<1; 1-7; 8-15; ≥16 g/d), diet group (meat eater; fish eater; vegetarian; vegan), batch (categorical), time since last food or drink at blood collection (min, continuous), time between blood collection and processing (fourths of the distribution corresponding to <25; 25-41; 41-72; ≥72h; unknown). The beta coefficient and 95% confidence intervals of metabolite concentrations are shown in Table S2.
Among the 10 acylcarnitines, C3 and C5 were significantly positively associated with BMI. Seven of the 21 amino acids were significantly related to BMI. Concentrations of leucine, isoleucine, valine, tryptophan, alanine, glutamate, and tyrosine all increased with increasing BMI. The biogenic amines kynurenine and creatinine were also positively associated with BMI. Twenty-two of the 67 PCs were significantly related to BMI, positively for the diacyl-PCs C28:1, C32:1 to :3, C34:4, C36:3, C38:3 to :4, C40:4 to :6, and inversely for all the significant acyl-alkyl PCs, i.e., C38:2, C40:5, C42:3 to :5, and C44:4 to :6, as well as for diacyl-PCs aaC42:0 to :2. In addition, 5 lysoPCs were associated with BMI, inversely for lysoPCs C18:1, C18:2, and C28:0, and positively for lysoPCs C16:1 and C20:3. Finally, concentrations of 2 out of 14 sphingolipids, namely SM C18:0 and SM C20:2, were significantly positively related to BMI. No association was observed between hexose and BMI. The sum of branched chain amino acids (BCAA) was significantly and positively related to BMI. Ratios of metabolites that were tested (i.e., monounsaturated fatty acids (MUFA) to saturated fatty acids (SFA); polyunsaturated fatty acids (PUFA) to SFA; kynurenine to tryptophan; and lysoPC to PC ratios did not exhibit significant associations with BMI (Supporting Information Table S2).
EPIC Hepatobiliary controls sub-cohort
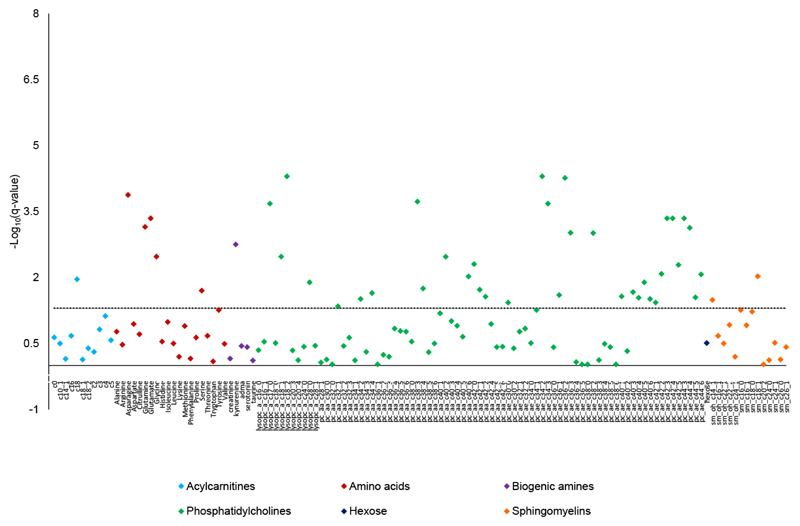
Overall, concentrations of 45 out of 130 metabolites (34.6%) were significantly associated with BMI in the EPIC-Hepatobiliary controls sub-cohort (Fig. 2; Supporting Information Table S2), after adjusting for confounders and multiple testing. Statistically significant associations of BMI were found with 44.1% of PCs, 36.4% of lysoPCs, 25% of amino acids, 21.4% of sphingomyelins, 20% of biogenic amines, and 9.1% of acylcarnitines.
Figure 2.
Associations between body mass index (continuous) and metabolite concentrations plotted as –log10 (q-values) in the EPIC-Hepatobiliary controls cohort. The dashed line shows the corrected significance level (False Discovery Rate). The q-values were derived from linear regression adjusted for sex (female; male), age (years, continuous), the Cambridge index of physical activity (inactive; moderately inactive, moderately active; active), alcohol intake (<1; 1-7; 8-15; ≥16 g/d), smoking status (never; former; current; missing), meat intake (quartiles in g/d: <68.5; 68.5-99.6; 99.6-144.3; ≥144.3); fish intake (quartiles in g/d: <16.1; 16.1-28.3; 28.3-46.3; ≥46.3); batch (categorical), time since last food or drink at blood collection (<3; 3 to 6; ≥6 hours; missing), and country (United Kingdom; Denmark; Greece; Germany; Spain; The Netherlands; Sweden; Italy). The beta coefficient and 95% confidence intervals of metabolite concentrations are shown in Table S2.
Among the 11 acylcarnitines, C18 was significantly and inversely associated with BMI. Five out of 20 amino acids were significantly related to BMI. Concentrations of asparagine, glutamine, glycine and serine decreased, whereas concentrations of glutamate increased with BMI. The biogenic amine kynurenine was positively associated with BMI. Thirty-one of the 68 PCs were significantly related to BMI, positively for the diacyl-PCs C32:1, C34:2, C34:4, C38:3, C38:4, and C40:6, and inversely for all the significant acyl-alkyl-PCs, i.e., C30:0, C34:2, C34:3, C36:1 to :3, C38:2, C40:1, C40:3 to :6, C42:1 to :5 and C44:3 to :6, as well as for diacyl PCs C40:2 and C42:0 to :2. In addition, 4 of the 11 lysoPCs, i.e., C17:0, C18:1, C18:2, and C28:0, were all inversely associated with BMI. Finally, concentrations of 3 out of 14 sphingolipids varied significantly with BMI, inversely for SM(OH) C14:1, and positively for SM C18:0 and SM C18:1. No association was seen between hexose and BMI.
The ratio of kynurenine to tryptophan was positively and significantly related to BMI. BMI was significantly negatively associated with the ratio of total lysoPCs to total PCs. No significant association of BMI was found with MUFA to SFA or PUFA to SFA ratios (Supporting Information Table S2).
Common associations of metabolites with BMI in both sub-cohorts
Twenty-two metabolites were statistically significantly associated with BMI in both the EPIC-Oxford and the EPIC-Hepatobiliary controls sub-cohorts (Fig. 3). The directions of 21 out of these 22 associations were consistent in both sub-cohorts; an opposing direction was seen for the association of lysoPCaC28:0 with BMI (positive in the EPIC-Oxford and inverse in the EPIC-Hepatobiliary controls sub-cohort). All those 22 compounds were correlated with BMI with a partial Pearson correlation coefficient (r) higher than |0.1| in both sub-cohorts (Table 2).
Figure 3.
Statistically significant associations of metabolites with BMI and commonly associated metabolites in both EPIC-Oxford and EPIC-Hepatobiliary controls cohorts. Arrows indicate the direction of the associations: positive association (↗), negative association (↘) and inconsistent direction (?).
Table 2. Significant metabolites associated with body mass index in both the EPIC-Oxford and in the EPIC-Hepatobiliary control cohorts.
Betas, Partial R2 and Pearson correlation coefficients (r) are estimated from multivariable models adjusted for multiple testing (Q-value computed with False Discovery Rate, Simes method) and for potential confounders. In the EPIC-Oxford cohort, adjustment variables were age (years, continuous), Cambridge physical activity index (inactive; moderately inactive, moderately active; active), smoking status (never; former; current), alcohol intake (<1; 1-7; 8-15; ≥16 g/d), diet group (meat eater; fish eater; vegetarian; vegan), batch (categorical), time since last food or drink at blood collection (min, continuous), time between blood collection and processing (fourths of the distribution corresponding to <25; 25-41; 41-72; ≥72h; unknown). In the EPIC-Hepatobiliary control cohort, corresponding adjustment variables were age (years, continuous), sex (male; female), Cambridge physical activity index (inactive; moderately inactive, moderately active; active), smoking status (never; former; current; missing), alcohol intake (<1; 1-7; 8-15; ≥16 g/d), meat intake (quartiles in g/d: <68.5; 68.5-99.6; 99.6-144.3; ≥144.3); fish intake (quartiles in g/d: <16.1; 16.1-28.3; 28.3-46.3; ≥46.3); batch (categorical), time since last food or drink at blood collection (<3; 3 to 6; ≥6 hours; missing), and country (Denmark; Germany; Greece; Spain; Sweden; The Netherlands; Italy; United Kingdom).
| EPIC-Oxford (N = 392) | EPIC-Hepatobiliary controls (N = 327) | |||||||
|---|---|---|---|---|---|---|---|---|
| Metabolites | Beta | Q-value | Partial R2 | r* (Pearson) | Beta | Q-value | Partial R2 | r* (Pearson) |
| lysoPC a C18:1 | -0.0464 | 0.017 | 0.0247 | -0.157 | -0.0467 | 0.003 | 0.0411 | -0.203 |
| lysoPC a C18:2 | -0.0901 | <0.0001 | 0.0877 | -0.296 | -0.0659 | <0.0001 | 0.0814 | -0.285 |
| lysoPC a C28:0 | 0.0353 | 0.041 | 0.0185 | 0.136 | -0.0319 | 0.013 | 0.0349 | -0.187 |
| PC aa C32:1 | 0.0538 | 0.002 | 0.0443 | 0.210 | 0.0301 | 0.045 | 0.0201 | 0.142 |
| PC aa C34:4 | 0.0496 | 0.007 | 0.0327 | 0.181 | 0.0359 | 0.023 | 0.026 | 0.161 |
| PC aa C38:3 | 0.104 | <0.0001 | 0.1241 | 0.352 | 0.0568 | <0.0001 | 0.0676 | 0.260 |
| PC aa C38:4 | 0.039 | 0.049 | 0.0172 | 0.131 | 0.0374 | 0.018 | 0.0281 | 0.168 |
| PC aa C40:6 | 0.0307 | 0.035 | 0.0196 | 0.140 | 0.0404 | 0.010 | 0.0329 | 0.181 |
| PC aa C42:0 | -0.0497 | 0.008 | 0.0313 | -0.177 | -0.0433 | 0.005 | 0.0381 | -0.195 |
| PC aa C42:1 | -0.0426 | 0.018 | 0.0242 | -0.156 | -0.0377 | 0.019 | 0.0276 | -0.166 |
| PC aa C42:2 | -0.0561 | 0.004 | 0.037 | -0.192 | -0.0353 | 0.027 | 0.0243 | -0.156 |
| PC ae C38:2 | -0.0396 | 0.032 | 0.0205 | -0.143 | -0.0493 | 0.001 | 0.0506 | -0.225 |
| PC ae C40:5 | -0.0383 | 0.050 | 0.0168 | -0.130 | -0.0413 | 0.013 | 0.0303 | -0.174 |
| PC ae C42:3 | -0.0513 | 0.004 | 0.0364 | -0.191 | -0.0567 | <0.0001 | 0.0577 | -0.240 |
| PC ae C42:4 | -0.0635 | 0.002 | 0.0428 | -0.207 | -0.0575 | <0.0001 | 0.0587 | -0.242 |
| PC ae C42:5 | -0.0519 | 0.011 | 0.0288 | -0.170 | -0.048 | 0.005 | 0.0376 | -0.194 |
| PC ae C44:4 | -0.0488 | 0.015 | 0.0265 | -0.163 | -0.0553 | 0.001 | 0.0532 | -0.231 |
| PC ae C44:5 | -0.0596 | 0.004 | 0.0365 | -0.191 | -0.0373 | 0.028 | 0.0239 | -0.155 |
| PC ae C44:6 | -0.0654 | 0.002 | 0.0446 | -0.211 | -0.0439 | 0.009 | 0.034 | -0.184 |
| SM C18:0 | 0.0326 | 0.047 | 0.0176 | 0.133 | 0.0296 | 0.045 | 0.018 | 0.134 |
| Kynurenine | 0.0667 | 0.001 | 0.0502 | 0.224 | 0.0494 | 0.002 | 0.0465 | 0.216 |
| Glutamate | 0.0372 | 0.028 | 0.0213 | 0.146 | 0.0449 | <0.0001 | 0.0573 | 0.239 |
Discussion
In this population-based study involving two sub-cohorts from EPIC and using a targeted metabolomics approach, 21 specific metabolic signatures of BMI were identified as being consistent across the two sub-cohorts; those associations may be particularly robust as these two samples presented heterogeneous characteristics regarding blood matrix, and participants’ gender, BMI and dietary patterns. BMI increase was associated with increased blood concentrations of kynurenine; glutamate; SM C18:0; and diacyl-PCs C32:2, C34:4, C38:3, C38:4 and C40:6; and decreased blood concentrations of lysoPCs C18:1, and C18:2; diacyl-PCs C42:0, C42:1, and C42:2; and acyl-alkyl-PCs C38:2, C40:5, C42:3, C42:4, C42:5, C44:4, C44:5 and C44:6.
Our results are consistent with previous findings on metabolomics and body size. One cross-sectional study measured 127 metabolites in serum in 2,270 participants from the EPIC-Potsdam cohort with the same assay used in the current study.15,41 This study found 23 serum metabolites related to BMI with coefficients of Spearman’s partial rank correlation higher than |0.20|. Among the 21 metabolites that were consistently associated with BMI in our two datasets, 13 compounds were also correlated with BMI (with multivariable-adjusted Spearman’s rho>|0.20|) in the EPIC-Potsdam study41 in the same directions as in our study, including two lysoPCs (C18:1 and, C18:2), four diacyl-PCs (C38:3, C38:4, C40:6; and C42:0) and seven acyl-alkyl-PCs (C38:2, C42:3, C42:4, C42:5, C44:4, C44:5, and C44:6). For seven of these compounds (lysoPCs C18:1 and C18:2; PCaaC38:3; and acyl-alkyl-PCs C38:2, C42:3, C42:4 and C44:6), correlations exceeded |0.20| in at least one of our two sub-cohorts. LysoPCaC18:2, PCaaC38:3 and PCaeC42:4 showed correlations higher than |0.20| in our two sub-cohorts as well as in the EPIC-Potsdam, suggesting that the association of BMI with these three compounds was highly reproducible with a large magnitude of effect across different European EPIC population subsets. Biogenic amines, and especially kynurenine that correlated higher than |0.20| with BMI in our two sub-cohorts, were not measured in the EPIC-Potsdam.15,41
Additional studies have reported associations of PCs that were found to be associated with BMI in both our datasets.15,17,18,22,25 Present and former findings show that diacyl-PCs from C30 to C40 are increased in obese as compared to lean subjects, whereas diacyl-PCs from C42 to C44 and acyl-alkyl-PCs are systematically decreased with increasing BMI.15,18,22,25,42 LysoPCs compounds may have different biological and physical properties according to their degree of saturation and length of fatty acid chain.18,43 In our study, lysoPCs C18:1 and C18:2 were inversely associated with BMI in both datasets, which is in accordance with findings regarding obesity or diabetes in other studies.15,18,44
Kynurenine, a metabolite of tryptophan, was consistently positively related to BMI in both our datasets, and the ratio of kynurenine to tryptophan was positively associated with BMI in the EPIC-Hepatobiliary controls. Positive relations of kynurenine or the kynurenine to tryptophan ratio to BMI or obesity have been reported in other studies,19,20 and have also been related to the metabolic syndrome.19 High levels of kynurenine and its ratio to tryptophan are thought to reflect immune activation and low-grade systemic inflammation.19
Most amino acids were not consistently associated with BMI in our two sub-cohorts. In accordance with previous findings, results from the EPIC-Oxford sub-cohort suggest a BCAA-related metabolite signature of BMI with leucine, isoleucine and valine significantly associated with BMI. Tyrosine (aromatic amino acid) that was highlighted as a BMI signature in men from the EPIC-Potsdam (r=0.29),41 also showed a good correlation with BMI in our EPIC-Oxford sub-cohort (r=0.23). This is consistent with previous findings showing that aromatic amino acids and BCAA were positively associated with excess body weight,14–16,18,20,21,24 with a relatively high magnitude of effect observed for isoleucine, valine and tyrosine in the EPIC-Potsdam.41
Potential limitations of the current study include the relatively small sample size. Pre-analytic factors such as time since last food/drink at blood collection (and time from blood collection to processing in the EPIC-Oxford sub-cohort) contributed to less than 4% to the total variability in the metabolite data. To limit their potential impact on the concentrations of metabolites, pre-analytical factors were adjusted for in the statistical analyses. It is worth noting that in previous methodological work using the same assay a limited impact of fasting status on metabolites’ reliability or study results was reported in EPIC33,35,45 and the majority of metabolites (72% and 93%) were stable after non-centrifuged blood samples had been left at room temperature for 24 hours and after two thaw/freeze cycles, respectively.46 The main analytical discrepancy between the two sub-cohorts is that metabolites were quantified based on different matrices in blood samples: citrated plasma in the EPIC-Oxford and serum in the EPIC-Hepatobiliary controls. Yu et al47 assessed differences between human plasma and serum metabolite profiles with the same assay as the one we used in the current study. Although significantly higher concentrations were found in serum than in EDTA plasma for 64% of the metabolites, the overall correlation between the two matrices was high (mean r=0.81; range: 0.47-0.97), indicating a proportional change in plasma and serum concentrations.47 Moreover, both datasets were analyzed separately after log-transformation and standardization of metabolites concentrations. Therefore, the influence of blood matrices on our results should be very limited.
The main strength of our study was the ability to replicate associations in two populations with different general characteristics. The EPIC-Oxford involved only men with a relatively low BMI (only 3% obese), subjects had a median age of 41 years, and half the sub-cohort followed a vegan or vegetarian diet, whereas the EPIC-Hepatobiliary controls included men and women coming from 8 different European countries with a higher BMI (21% obese) and median age (60 years old) and all participants eating meat and/or fish. Still, 21 metabolites were similarly related to BMI in those two different populations. It is worth mentioning that those metabolites were measured using standard methods for laboratory analyses in both sub-cohorts and were identified after correction for multiple testing and potential confounding factors. Therefore, the findings which are common to the two studies are most likely to be robust and suggest consistent relationships of a metabolic phenotype reflecting body size in individuals with a wide range in BMI.
Conclusion
This study showed that variations in levels of BMI are paralleled by extensive and robust metabolic changes in PCs and kynurenine blood concentrations. Our results support the existence of a systemic metabolite profile reflecting body size and suggest that body size variation may be associated with a broadly modifiable metabolite phenotype. Our findings confirm PCs and kynurenine as candidate biomarkers that could mediate the relation of obesity to the risk of obesity-related diseases. Further research is warranted to investigate whether the identified metabolic signatures of BMI could mediate the relationship of adiposity with higher risk of certain non-communicable diseases (e.g., cancer and ischemic heart disease).
Acknowledgements
The work reported in this paper was undertaken during the tenure of a postdoctoral fellowship granted by the International Agency for Research on Cancer and the Fondation de France (project grant #2014-00050542) and a Senior Visiting Scientist Award granted by the International Agency for Research on Cancer. The data on the EPIC-Hepatobiliary dataset was generated through support from the French National Cancer Institute (L’Institut National du Cancer; INCA) (grant number 2009-139; PI: M. Jenab).
The coordination of EPIC is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), Federal Ministry of Education and Research (BMBF), Deutsche Krebshilfe, Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research (Germany); the Hellenic Health Foundation (Greece); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); Health Research Fund (FIS), PI13/00061 to Granada; PI13/01162 to EPIC-Murcia), Regional Governments of Andalucía, Asturias, Basque Country, Murcia (no. 6236) and Navarra, RTICC RD12/0036/0018, Instituto de Salud Carlos III, co-funded by FEDER funds (European Regional Development Fund, ERDF); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C570/A16491 and C8221/A19170 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk, MR/M012190/1 to EPIC-Oxford) (United Kingdom).
Footnotes
Associated Content Available
Appendix S1 - Material and methods.
Table S1 - Means and Standard Deviations (SD) of metabolites concentrations according to body mass index (BMI).
Table S2 - Relationship of metabolites with body mass index in the EPIC-Oxford and the EPIC-Hepatobiliary control cohorts.
Figure S1 - Proportion of variability explained by lifestyle, dietary and blood sample characteristics in the metabolomics data of the EPIC-Oxford cohort.
Figure S2 - Proportion of variability explained by lifestyle, dietary and blood sample characteristics in the metabolomics data of the EPIC-Hepatobiliary controls cohort.
Conflict of interest disclosure: The authors declare no competing financial interest.
References
- (1).Borrell LN, Samuel L. Body mass index categories and mortality risk in US adults: the effect of overweight and obesity on advancing death. Am J Public Health. 2014;104(3):512–9. doi: 10.2105/AJPH.2013.301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. Jama. 2007;298(17):2028–37. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- (3).Katzmarzyk PT, Reeder BA, Elliott S, Joffres MR, Pahwa P, Raine KD, Kirkland SA, Paradis G. Body mass index and risk of cardiovascular disease, cancer and all-cause mortality. Can J Public Health. 2012;103(2):147–51. doi: 10.1007/BF03404221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Alwan A. Global status report on noncommunicable diseases 2010. World Health Organization; 2011. [Google Scholar]
- (5).WCRF/AICR, Food, Nutrition, and Physical Activity: a Global Perspective. Washington DC: AICR; 2009. [Accessed August 21, 2015]. http:www.wcrf.org. [Google Scholar]
- (6).Hamilton MT, Hamilton DG, Zderic TW. Role of Low Energy Expenditure and Sitting in Obesity, Metabolic Syndrome, Type 2 Diabetes, and Cardiovascular Disease. Diabetes. 2007;56(11):2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- (7).Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–16. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- (8).Van Gaal LF, Mertens IL, Christophe E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- (9).Wishart DS. Metabolomics: applications to food science and nutrition research. Trends in Food Science & Technology. 2008;19(9):482–493. [Google Scholar]
- (10).Scalbert A, Brennan L, Fiehn O, Hankemeier T, Kristal BS, van Ommen B, Pujos-Guillot E, Verheij E, Wishart D, Wopereis S. Mass-spectrometry-based metabolomics: limitations and recommendations for future progress with particular focus on nutrition research. Metabolomics. 2009;5(4):435–458. doi: 10.1007/s11306-009-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Yang J, Xu G, Zheng Y, Kong H, Pang T, Lv S, Yang Q. Diagnosis of liver cancer using HPLC-based metabonomics avoiding false-positive result from hepatitis and hepatocirrhosis diseases. Journal of Chromatography B. 2004;813(1–2):59–65. doi: 10.1016/j.jchromb.2004.09.032. [DOI] [PubMed] [Google Scholar]
- (12).Brindle JT, Antti H, Holmes E, Tranter G, Nicholson JK, Bethell HWL, Clarke S, Schofield PM, McKilligin E, Mosedale DE, Grainger DJ. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat Med. 2002;8(12):1439–1445. doi: 10.1038/nm1202-802. [DOI] [PubMed] [Google Scholar]
- (13).Assfalg M, Bertini I, Colangiuli D, Luchinat C, Schäfer H, Schütz B, Spraul M. Evidence of different metabolic phenotypes in humans. Proceedings of the National Academy of Sciences. 2008;105(5):1420–1424. doi: 10.1073/pnas.0705685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, Palma MJ, Roberts LD, Dejam A, Souza AL, Deik AA, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125(18):2222–31. doi: 10.1161/CIRCULATIONAHA.111.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Floegel A, Wientzek A, Bachlechner U, Jacobs S, Drogan D, Prehn C, Adamski J, Krumsiek J, Schulze MB, Pischon T, Boeing H. Linking diet, physical activity, cardiorespiratory fitness and obesity to serum metabolite networks: findings from a population-based study. Int J Obes (Lond) 2014;38(11):1388–96. doi: 10.1038/ijo.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Gaudet MM, Falk RT, Stevens RD, Gunter MJ, Bain JR, Pfeiffer RM, Potischman N, Lissowska J, Peplonska B, Brinton LA, Garcia-Closas M, et al. Analysis of serum metabolic profiles in women with endometrial cancer and controls in a population-based case-control study. J Clin Endocrinol Metab. 2012;97(9):3216–23. doi: 10.1210/jc.2012-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hanamatsu H, Ohnishi S, Sakai S, Yuyama K, Mitsutake S, Takeda H, Hashino S, Igarashi Y. Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutrition & Diabetes. 2014;4:e141. doi: 10.1038/nutd.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Kim JY, Park JY, Kim OY, Ham BM, Kim HJ, Kwon DY, Jang Y, Lee JH. Metabolic profiling of plasma in overweight/obese and lean men using ultra performance liquid chromatography and Q-TOF mass spectrometry (UPLC-Q-TOF MS) J Proteome Res. 2010;9(9):4368–75. doi: 10.1021/pr100101p. [DOI] [PubMed] [Google Scholar]
- (19).Mangge H, Summers KL, Meinitzer A, Zelzer S, Almer G, Prassl R, Schnedl WJ, Reininghaus E, Paulmichl K, Weghuber D, Fuchs D. Obesity-related dysregulation of the tryptophan-kynurenine metabolism: role of age and parameters of the metabolic syndrome. Obesity (Silver Spring) 2014;22(1):195–201. doi: 10.1002/oby.20491. [DOI] [PubMed] [Google Scholar]
- (20).Moore S, Matthews C, Sampson J, Stolzenberg-Solomon R, Zheng W, Cai Q, Tan Y, Chow W-H, Ji B-T, Liu D, Xiao Q, et al. Human metabolic correlates of body mass index. Metabolomics. 2014;10(2):259–269. doi: 10.1007/s11306-013-0574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–26. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Oberbach A, Bluher M, Wirth H, Till H, Kovacs P, Kullnick Y, Schlichting N, Tomm JM, Rolle-Kampczyk U, Murugaiyan J, Binder H, et al. Combined proteomic and metabolomic profiling of serum reveals association of the complement system with obesity and identifies novel markers of body fat mass changes. J Proteome Res. 2011;10(10):4769–88. doi: 10.1021/pr2005555. [DOI] [PubMed] [Google Scholar]
- (23).Pietiläinen KH, Sysi-Aho M, Rissanen A, Seppänen-Laakso T, Yki-Järvinen H, Kaprio J, Orešič M. Acquired Obesity Is Associated with Changes in the Serum Lipidomic Profile Independent of Genetic Effects – A Monozygotic Twin Study. PLoS ONE. 2007;2(2):e218. doi: 10.1371/journal.pone.0000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Wurtz P, Wang Q, Kangas AJ, Richmond RC, Skarp J, Tiainen M, Tynkkynen T, Soininen P, Havulinna AS, Kaakinen M, Viikari JS, et al. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med. 2014;11(12):e1001765. doi: 10.1371/journal.pmed.1001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Zhang A, Sun H, Wang X. Power of metabolomics in biomarker discovery and mining mechanisms of obesity. Obes Rev. 2013;14(4):344–9. doi: 10.1111/obr.12011. [DOI] [PubMed] [Google Scholar]
- (26).Wurtz P, Makinen VP, Soininen P, Kangas AJ, Tukiainen T, Kettunen J, Savolainen MJ, Tammelin T, Viikari JS, Ronnemaa T, Kahonen M, et al. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes. 2012;61(6):1372–80. doi: 10.2337/db11-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Riboli E, Kaaks R. The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26(Suppl 1):S6–14. doi: 10.1093/ije/26.suppl_1.s6. [DOI] [PubMed] [Google Scholar]
- (28).Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, Overvad K, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6b):1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- (29).Davey GK, Spencer EA, Appleby PN, Allen NE, Knox KH, Key TJ. EPIC-Oxford: lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003;6(3):259–69. doi: 10.1079/PHN2002430. [DOI] [PubMed] [Google Scholar]
- (30).Schmidt JA, Rinaldi S, Ferrari P, Carayol M, Achaintre D, Scalbert A, Cross AJ, Gunter MJ, Fensom GK, Appleby PN, Key TJ, et al. Metabolic profiles of male meat eaters, fish eaters, vegetarians, and vegans from the EPIC-Oxford cohort. Am J Clin Nutr. 2015;102(6):1518–26. doi: 10.3945/ajcn.115.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Schmidt JA, Rinaldi S, Scalbert A, Ferrari P, Achaintre D, Gunter MJ, Appleby PN, Key TJ, Travis RC. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: a cross-sectional analysis in the EPIC-Oxford cohort. Eur J Clin Nutr. 2016;70(3):306–12. doi: 10.1038/ejcn.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Fedirko V, Duarte-Salles T, Bamia C, Trichopoulou A, Aleksandrova K, Trichopoulos D, Trepo E, Tjonneland A, Olsen A, Overvad K, Boutron-Ruault MC, et al. Prediagnostic circulating vitamin D levels and risk of hepatocellular carcinoma in European populations: a nested case-control study. Hepatology. 2014;60(4):1222–30. doi: 10.1002/hep.27079. [DOI] [PubMed] [Google Scholar]
- (33).Floegel A, Drogan D, Wang-Sattler R, Prehn C, Illig T, Adamski J, Joost HG, Boeing H, Pischon T. Reliability of serum metabolite concentrations over a 4-month period using a targeted metabolomic approach. PLoS One. 2011;6(6):e21103. doi: 10.1371/journal.pone.0021103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5(4):561–5. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- (35).Fages A, Ferrari P, Monni S, Dossus L, Floegel A, Mode N, Johansson M, Travis R, Bamia C, Sánchez-Pérez M-J, Chiodini P, et al. Investigating sources of variability in metabolomic data in the EPIC study: the Principal Component Partial R-square (PC-PR2) method. Metabolomics. 2014;10(6):1074–1083. [Google Scholar]
- (36).R, A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. http://www.R-project.org/ [Google Scholar]
- (37).Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73(3):751–754. [Google Scholar]
- (38).Broadhurst D, Kell D. Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics. 2006;2(4):171–196. [Google Scholar]
- (39).Storey JD. A direct approach to false discovery rates. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2002;64(3):479–498. [Google Scholar]
- (40).Storey JD. The positive false discovery rate: a Bayesian interpretation and the q-value. Annals of statistics. 2003:2013–2035. [Google Scholar]
- (41).Bachlechner U, Floegel A, Steffen A, Prehn C, Adamski J, Pischon T, Boeing H. Associations of anthropometric markers with serum metabolites using a targeted metabolomics approach: results of the EPIC-potsdam study. Nutr Diabetes. 2016;6:e215. doi: 10.1038/nutd.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Szymanska E, Bouwman J, Strassburg K, Vervoort J, Kangas AJ, Soininen P, Ala-Korpela M, Westerhuis J, van Duynhoven JP, Mela DJ, Macdonald IA, et al. Gender-dependent associations of metabolite profiles and body fat distribution in a healthy population with central obesity: towards metabolomics diagnostics. Omics. 2012;16(12):652–67. doi: 10.1089/omi.2012.0062. [DOI] [PubMed] [Google Scholar]
- (43).Kougias P, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Lysophosphatidylcholine and secretory phospholipase A2 in vascular disease: mediators of endothelial dysfunction and atherosclerosis. Med Sci Monit. 2006;12(1):Ra5–16. [PubMed] [Google Scholar]
- (44).Floegel A, Stefan N, Yu Z, Muhlenbruch K, Drogan D, Joost HG, Fritsche A, Haring HU, Hrabe de Angelis M, Peters A, Roden M, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62(2):639–48. doi: 10.2337/db12-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Carayol M, Licaj I, Achaintre D, Sacerdote C, Vineis P, Key TJ, Onland Moret NC, Scalbert A, Rinaldi S, Ferrari P. Reliability of Serum Metabolites over a Two-Year Period: A Targeted Metabolomic Approach in Fasting and Non-Fasting Samples from EPIC. PLoS ONE. 2015;10(8):e0135437. doi: 10.1371/journal.pone.0135437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Breier M, Wahl S, Prehn C, Fugmann M, Ferrari U, Weise M, Banning F, Seissler J, Grallert H, Adamski J, Lechner A. Targeted metabolomics identifies reliable and stable metabolites in human serum and plasma samples. PLoS One. 2014;9(2):e89728. doi: 10.1371/journal.pone.0089728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Yu Z, Kastenmuller G, He Y, Belcredi P, Moller G, Prehn C, Mendes J, Wahl S, Roemisch-Margl W, Ceglarek U, Polonikov A, et al. Differences between human plasma and serum metabolite profiles. PLoS One. 2011;6(7):e21230. doi: 10.1371/journal.pone.0021230. [DOI] [PMC free article] [PubMed] [Google Scholar]