Abstract
Background
Obesity and infertility are associated with poorer sexual function. We have previously shown that a lifestyle intervention in women with obesity and infertility reduced weight and improved cardiometabolic health and quality of life, which may positively affect sexual function. We now report on sexual function 5 years after randomization.
Methods and findings
In total 577 women, between 18–39 years of age, with infertility and a BMI ≥29 kg/m2 were randomized to a six-month lifestyle intervention targeting physical activity, diet and behavior modification or prompt infertility care as usual. Intercourse frequency and sexual function were assessed with the McCoy Female Sexuality Questionnaire (MFSQ), 5.4±0.8 years after randomization. 550 women could be approached for the follow-up study, of whom 84 women in the intervention and 93 in the control group completed the MFSQ. Results were adjusted for duration of infertility, polycystic ovary syndrome and whether women were attempting to conceive. The intervention group more often reported having had intercourse in the past 4 weeks compared to the control group (aOR: 2.3 95% CI 0.96 to 5.72). Among women reporting intercourse in the past 4 weeks, the intervention group (n = 75) had intercourse more frequently (6.6±5.8 vs. 4.9±4.0 times; 95% CI 0.10 to 3.40) and had higher scores for vaginal lubrication (16.5±3.0 vs. 15.4±3.5; 95% CI 0.15 to 2.32) and total ‘sexual function’ score (96.5±14.2 vs. 91.4±12.8; 95% CI 0.84 to 9.35) compared to the control group (n = 72). Sexual interest, satisfaction, orgasm and sex partner scores did not differ statistically between the groups. The intervention effect on sexual function was for 21% mediated by the change in moderate to vigorous physical activity.
Conclusion
A six-month lifestyle intervention in women with obesity and infertility led to more frequent intercourse, better vaginal lubrication and overall sexual function 5 years after the intervention. (Trial Registration: NTR1530).
Introduction
Sexual function plays an important role in the quality of life of adults and is determined by both biological and psychosocial factors [1, 2]. The multi-dimensional nature of sexual function and the diversity in assessment methods make it difficult to estimate the prevalence of female sexual dysfunction, but estimates are up to 40% worldwide [3–5].
Obesity and sexual function are associated through various mental and physical pathways [6, 7]. In women, obesity leads to decreased fecundability and increases the demand for assisted reproductive techniques [8–10]. Infertile couples undergoing infertility treatment report a poorer sexual function suggesting a causal relationship between infertility and sexual function, however a reciprocal or bidirectional association has also been suggested [11–14]. Apart from this effect on reproduction, obesity increases the risks of cardiometabolic diseases that are also associated with lower sexual function, like type two diabetes, dyslipidemia and hypertension [15, 16]. Endothelial function and genital blood flow seem to form an important link between cardiometabolic health status and sexual function, although evidence on the cause-effect relationship is scarce [17–21]. In addition, there is conflicting evidence on whether metabolic syndrome increases the risk of sexual dysfunction [22, 23]. Anxiety and depression are more prevalent in the obese population, and are directly and indirectly linked to sexual function [24, 25]. Obesity may thus be a common etiological factor for decreased sexual function, explaining the co-occurrence of multiple conditions, including mental, metabolic, reproductive and sexual problems [11, 26].
The prevalence of obesity has been increasing and affects around 48% of all women of childbearing age in the United States [27]. Lifestyle interventions are the first step in the treatment of obesity and have shown to improve several domains of physical health, mental health and increase quality of life [28–34]. Weight-loss is associated with an increase in female sexual function, although not all intervention studies found such an effect [35]. Studies in obese and overweight women with and without type 2 diabetes showed better sexual function after lifestyle interventions, especially in women who had been diagnosed with a sexual dysfunction prior to the intervention [36, 37].
We have previously reported on the LIFEstyle study, a Randomized Controlled Trial (RCT) in which women with obesity and infertility were allocated to a six month lifestyle intervention or infertility care as usual [38]. This intervention led to weight loss, improved cardiometabolic health by halving the odds of metabolic syndrome and improved physical quality of life [30, 39]. Due to these effects that are associated with sexual function, we hypothesized that a lifestyle intervention in women with obesity and infertility would improve sexual function. The current follow-up study of the LIFEstyle study aimed to investigate the effects of a lifestyle intervention in women with obesity and infertility on sexual function.
Methods
The current study is a follow-up study of the women who participated in the LIFEstyle study, a multicenter RCT. The study was conducted according to the principles of the Declaration of Helsinki and approved by the medical ethics committee of the University Medical Centre Groningen (UMCG) (METc code: 2008/284), as well as by the board of directors of the 22 other participating hospitals (Dutch trial register (NTR 1530)).
LIFEstyle study
The original LIFEstyle study was conducted in 23 medical centers in the Netherlands. The protocols of the original LIFEstyle study and the current follow-up study (WOMB project) have been published previously [38, 40]. From June 2009 until June 2012, 577 women between 18–39 years of age with infertility and a Body Mass Index (BMI) ≥29 kg/m2 were randomly allocated (1:1) to a lifestyle intervention or infertility care as usual. Infertility was defined as chronic anovulation [41] or unsuccessful conception for at least 12 months [42]. Women with severe endometriosis, premature ovarian insufficiency, endocrinopathy (e.g. diabetes type I, Cushing’s syndrome), and untreated pre-conception hypertension, or hypertension-related complications in a previous pregnancy were not eligible, as were women treated with donor sperm. Randomization was performed with an online program at the Academic Medical Centre in Amsterdam, stratified for trial center and ovulatory status.
Lifestyle intervention
Women allocated to the intervention group received a six-month structured lifestyle intervention, prior to receiving infertility treatment. The lifestyle intervention consisted of six face-to-face consultations of approximately 30 minutes at the outpatient clinics and four consultations by telephone and/or e-mail with trained research nurses. The goal of the lifestyle intervention was a 5–10% weight reduction, or a reduction in BMI below 29 kg/m2 within the intervention period. Women who reached this goal could stop with the lifestyle intervention program and proceed with infertility treatment. The intervention was discontinued if pregnancy occurred, but women could re-enter the intervention in case of a miscarriage.
The intervention was based on recommendations of the National Institute of Health [43], and consisted of a dietary, physical activity and a behavioral modification component. Women were advised to reduce their daily caloric intake by 600 kcal, but not below 1200 kcal/day. An online food diary was used to provide feedback on their diet. Women were recommended to be more physical active and to increase their daily step count to a minimum of 10,000 steps per day, supported by a pedometer that was worn daily. In addition, women had to be moderately physically active two to three times a week for a minimum of 30 minutes. Individual motivational counselling was used to set individual goals and create awareness of lifestyle factors predisposing to obesity [44]. Women were advised to adhere to their healthier lifestyle, also after finishing the intervention.
Control strategy
Women in the control group were treated according to the Dutch infertility guidelines, irrespective of their BMI, and were given information about the negative effects of obesity on fertility, as part of the usual care in the Netherlands [42].
Study procedures
All women who participated in the LIFEstyle study were eligible for the current follow-up study, five years after randomization. All women for whom valid contact information was available were sent an invitation letter in which they received information about the follow-up study. Women were contacted by telephone if they did not respond to the letter. All participants provided written informed consent. Participants could fill out a paper or online version of the questionnaires at home, without the presence of a researcher.
Outcome measures
Women filled out questionnaires concerning demographics, current lifestyle, reproductive health, quality of life (36-Item Short Form Survey), and anthropometrics [45]. The amount of moderate to vigorous physical activity (MVPA) in minutes per week was assessed with the validated Short QUestionnaire to ASsess Health-enhancing physical activity (SQUASH), which collects information about commuting activities, leisure time activities, household activities, activities at work and school, using three main questions: days per week, average time per day/week, and intensity [46].
The main outcomes of this paper were assessed using the Dutch version of the validated McCoy Female Sexuality Questionnaire (MFSQ) [47]. The MFSQ has a good test-retest reliability (r = 0.71–0.95) and discriminating capacity between women with and without sexual dysfunction. [48] In this 19-item questionnaire, 18 items are scored on a 7-point Likert scale. The remaining item asks about frequency of intercourse in the past 4 weeks. The questionnaire investigates five dimensions of sexual health: sexual interest; satisfaction; vaginal lubrication; orgasm and sex partner. Intercourse frequency, as part of the sexual satisfaction domain, is converted into a 7-point scale on a percentage-wise basis, in which the lowest frequency is converted to 1 point and the highest to 7 points. To calculate domain scores, ‘non applicable’ answers were replaced by the mean of at least 2 of the other items of the corresponding domain. The total score is calculated in complete cases by the sum score of all individual items. Only women who reported intercourse could complete all of the 19 items [47].
Statistical analyses
Comparison of baseline and follow-up characteristics was performed based on treatment group. We assessed potential selection bias by comparing the baseline characteristics between participants and non-participants. We analysed continuous variables using an independent sample t-test, binary and categorical outcomes with a Pearson Chi-Square, Fisher’s exact test or Fisher-Freeman-Halton exact test; p-values <0.05 were considered statistically significant. Sexual activity was assessed in the complete sample of women who filled out the questionnaire. In line with the MFSQ user manual, the frequency of intercourse, the five domains concerning sexual function and total MFSQ score were analysed for all women who reported having had intercourse at least once during the past four consecutive weeks [47]. We analysed the differences in outcomes between the intervention and control group by logistic and linear regression analyses. Duration of infertility at baseline, polycystic ovary syndrome (PCOS) and attempting to conceive (yes or no) were added as covariates to the adjusted model. PCOS was diagnosed by the Rotterdam 2003 criteria [49]. Results are presented as odds ratios for sexual activity or mean difference in intercourse frequency, domain scores or total MFSQ score between the intervention and control group. Confidence intervals (CI) for continuous outcomes are reported as bias corrected and accelerated (BCa) 95% CI, based on 5000 bootstrap samples [50]. Confidence intervals not including zero were considered statistically significant. Post-hoc mediation analyses were performed for the total score of the MFSQ, including delta values between baseline and follow-up of factors attributable to the intervention: weight, waist- and hip circumference, mental and physical quality of life and MVPA. The mediating effects were analysed for all potential mediators separately and combined. The mediation analyses were performed using model 4, with 5000 bootstrapped samples for the estimation of bias corrected 95% CI, of the PROCESS macro (V.2.16.3) for SPSS [51]. All statistical analyses were performed using IBM SPSS version 24.0 (Armonk, NY, USA). At the start of the original trial, no power calculation was performed for sexual function as a long-term outcome [38].
Results
Participation
Flow of participants
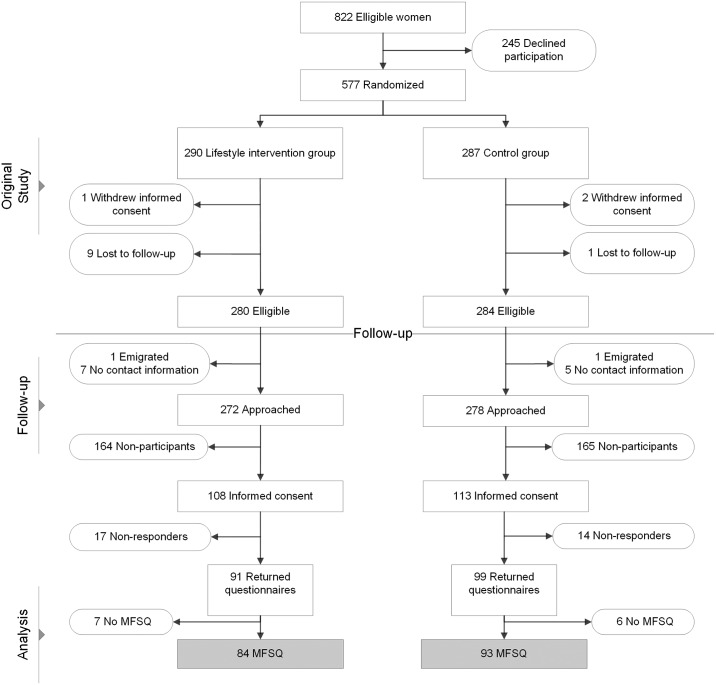
During the original trial, 577 women were randomized, of which 3 women withdrew their informed consent and 10 women were lost to follow-up. Of the 564 women who completed the original trial, 14 women could not be contacted for the current follow-up study, because of missing contact information or immigration out of the Netherlands. All remaining 550 women (98%) were approached for the follow-up study, of whom 272 women in the intervention group and 278 women in the control group. A total of 106 of the approached women (39%) in the intervention and 113 women (41%) in the control group gave written informed consent. 31 of the women who gave informed consent did not respond to the complete set of questionnaires, and 13 women did not fill out the MFSQ specifically, because of personal reasons (not specified). In total, 84 of the approached women (31%) in the intervention group and 93 women (33%) in the control group filled out the MFSQ and were included in the analyses (Fig 1). Of the women in the intervention group, 13 women (15,5%) did not complete the intervention program.
Fig 1. Flowchart of participants.
Abbreviations: MFSQ, McCoy Female Sexuality Questionnaire.
Participants and non-participants
The comparison of women who filled out the MFSQ (N = 177) and women who did not (N = 397) is shown in S2 Table. Women who filled out the MFSQ were more often Caucasian, were more often diagnosed with PCOS, but had shorter duration of infertility and a higher score for mental quality of life at baseline than women who did not fill out the MFSQ.
Characteristics of treatment groups
The characteristics of the women who participated in the follow-up are reported separately for the intervention and control group in Tables 1 and 2 respectively. Women in the intervention group had a longer duration of infertility at baseline (Table 1).
Table 1. Baseline characteristics of the participants.
| Variables | n | Intervention group | n | Control group | P-value a |
|---|---|---|---|---|---|
| Age, years–mean (SD) | 84 | 30.2 (4.1) | 93 | 29.7 (4.3) | 0.40 |
| Weight, kg–mean (SD) | 84 | 104.8 (12.8) | 93 | 103.6 (11.9) | 0.52 |
| Waist circumference, cm mean–(SD) | 80 | 107.6 (9.6) | 93 | 108.7 (9.4) | 0.42 |
| Hip circumference, cm mean–(SD) | 82 | 124.7 (8.7) | 93 | 125.1 (8.4) | 0.76 |
| Caucasian–no. (%) | 84 | 79 (94.0) | 93 | 90 (96.8) | 0.48 |
| Education–no. (%) | 81 | 91 | 0.66 | ||
| Primary school, age 4–12 year | 3 (3.7) | 1 (1.1) | |||
| Secondary education | 15 (18.5) | 20 (22.0) | |||
| Intermediate vocational education | 44 (54.3) | 51 (56.0) | |||
| Advanced vocational education or university | 19 (23.5) | 19 (20.9) | |||
| Current smoker–no. (%) | 83 | 20 (24.1) | 92 | 17 (18.5) | 0.36 |
| Nulliparous–no. (%) | 84 | 65 (77.4) | 93 | 68 (73.1) | 0.51 |
| Duration of infertility–median (IQR) | 84 | 20.5 (14.0–37.0) | 93 | 17.0 (12.0–24.5) | 0.04 |
| Polycystic Ovary Syndrome b—no. (%) | 84 | 31 (36.9) | 93 | 42 (45.2) | 0.27 |
| Physical Quality of Life–median (IQR) | 68 | 53.0 (47.6–55.4) | 83 | 51.3 (45.6–54.4) | 0.12 |
| Mental Quality of Life–median (IQR) | 68 | 53.8 (50.1–57.1) | 83 | 53.8 (48.7–56.2) | 0.42 |
| Weekly intercourse frequency, median (IQR) | 65 | 3.0 (2.0–3.0) | 81 | 2.0 (2.0–3.0) | 0.85 |
a P-values of continuous outcomes based on student t-test or Mann-Whitney-U test. P-values of dichotomous and categorical outcomes are based on the Pearson Chi-Square test, the Fisher’s exact test or Fisher-Freeman-Halton exact test.
b Diagnosed by Rotterdam 2003 criteria [49].
Abbreviations: n, number; SD, Standard Deviation.
Table 2. Follow-up characteristics of the participants.
| Variables | n | Intervention group | n | Control group | P-value a |
|---|---|---|---|---|---|
| Age at follow-up, years–mean (SD) | 84 | 35.6 (4.3) | 93 | 35.2 (4.4) | 0.49 |
| Follow-up duration, years–mean (SD) | 84 | 5.4 (0.9) | 93 | 5.5 (0.7) | 0.55 |
| Weight, kg–mean (SD) | 84 | 99.6 (15.1) | 93 | 99.8 (16.5) | 0.95 |
| Waist circumference, cm mean–(SD) | 82 | 107.3 (13.5) | 92 | 108.3 (13.3) | 0.62 |
| Hip circumference, cm mean–(SD) | 82 | 120.0 (11.4) | 92 | 120.5 (14.4) | 0.79 |
| Long-term relationship b –no. (%) | 84 | 75 (89.3) | 93 | 87 (93.5) | 0.42 |
| Childlessness–no. (%) | 84 | 18 (21.4) | 93 | 14 (15.1) | 0.27 |
| History of miscarriage c—no. (%) | 84 | 28 (33.3) | 93 | 31 (33.3) | 1.00 |
| Attempting to conceive—no. (%) | 84 | 23 (27.4) | 93 | 15 (16.1) | 0.07 |
a P-values of continuous outcomes based on student t-test or Mann-Whitney-U test. P-values of dichotomous and categorical outcomes are based on the Pearson Chi-Square test or the Fisher’s exact test.
b Women who are in a relationship with the same partner as during the intervention.
c including three women with a history of extra uterine gravidity.
Abbreviations: n, number; SD, Standard Deviation.
Sexual intercourse occurrence
Of the 177 women who filled out the MFSQ, 75 of the 84 women (89.3%) in the intervention group compared to 72 of the 93 women (77.4%) in the control group reported having had intercourse in the past four weeks, resulting in an Odds Ratio (OR) of 2.4 (95% CI 1.04–5.66; p = 0.04). However, the OR was not statistically significant after adjusting for duration of infertility at baseline, PCOS and whether women were attempting to conceive (aOR: 2.3 95% CI 0.96–5.72; p = 0.06). (Table 2).
In the group of women who reported having had intercourse (irrespective of the treatment group), the prevalence of PCOS (44.2% versus 26.7%; p = 0.08) as well as the percentage of women attempting to conceive (24.5% versus 6.7% p = 0.03) was higher in comparison to women who reported not to have had intercourse. Furthermore, women who reported not to have had intercourse had a lower frequency of intercourse at baseline compared to women who had intercourse at follow-up (median 2.0 (IQR 1.0–3.0) versus 3.0 (2.0–3.0) per week; p = 0.03) (S3 Table).
Intercourse frequency and sexual function
Baseline- and follow-up characteristics of women reporting intercourse
The between group comparison of characteristics of women who reported having had intercourse in the last four weeks, at baseline and follow-up is reported in S4 Table. Women in the intervention group had a longer duration of infertility at baseline (20.0 (IQR 14.0–40.0) versus 17.0 (12.0–23.8) months; p = 0.04).
Intervention effect on intercourse frequency and sexual function
Among women reporting intercourse, the frequency of intercourse was higher in the intervention group than in the control group (Table 3). Women in the intervention group also had higher total MFSQ scores and higher scores on the sexual satisfaction and vaginal lubrication domains (Table 3). After adjusting for duration of infertility at randomization, PCOS and whether women were attempting to conceive at time of the outcome assessment, the difference in sexual satisfaction scores was not statistically significant. Sexual interest, orgasm and sex partner domain scores were higher in the intervention group, but were not statistically significant (Table 3).
Table 3. Comparison of intercourse frequency, MFSQ domains and total score in women reporting intercourse in the past four weeks.
| Outcomes | Unadjusted | Adjusted a | ||||
|---|---|---|---|---|---|---|
| Intervention group (n = 75) |
Control group (n = 72) |
Mean difference | 95% CI b | Mean difference | 95% CI b | |
| Intercourse frequency, number per 4 weeks–mean (SD) | 6.6 (5.8) | 4.9 (4.0) | 1.7 | 0.18–3.25 | 1.7 | 0.10–3.40 |
| Sexual interest, score–mean (SD) | 28.1 (6.2) | 26.3 (5.8) | 1.9 | -0.11–3.83 | 1.8 | -0.07–3.67 |
| Sexual satisfaction, score–mean (SD) | 11.8 (2.6) | 10.9 (2.6) | 0.9 | 0.07–1.75 | 0.9 | -0.03–1.73 |
| Vaginal lubrication, score–mean (SD) | 16.5 (3.0) | 15.4 (3.5) | 1.1 | 0.07–2.21 | 1.3 | 0.15–2.32 |
| Orgasm, score–mean (SD) | 20.8 (5.0) | 19.5 (5.2) | 1.2 | -0.41–2.85 | 1.3 | -0.27–2.81 |
| Sex partner, score–mean (SD) | 19.3 (1.9) | 19.0 (2.0) | 0.2 | -0.39–0.86 | 0.2 | -0.43–0.87 |
| Total MFSQ, score–mean (SD) | 96.5 (14.2) | 91.4 (12.8) | 5.1 | 0.87–9.48 | 5.1 | 0.84–9.35 |
a Adjusted for duration of infertility at randomization, PCOS and whether women were attempting to conceive at time of the outcome assessment.
b Bias corrected and accelerated 95% CIs based on 5000 bootstrap re-samples; CI not containing zero indicate statistical significance.
Abbreviations: n, number; SD, Standard Deviation; CI, Confidence Interval; MFSQ, McCoy Female Sexuality Questionnaire.
Mediation of the lifestyle intervention effect on sexual function
The mediation analyses showed that 21% of the total intervention effect on the total MFSQ score at time of follow-up was mediated by MVPA. No statistically significant mediating effects of change in weight, change in waste- and hip circumference, or change in mental or physical quality of life were observed on the intervention effect on the total MFSQ score. The combination of the mediators into one model explained 37% of the total intervention effect on MFSQ total score (Table 4).
Table 4. Mediation of change in measures of anthropometrics, physical activity and quality of life on MFSQ total score.
| Mediator | n | Indirect effect, (95% CI) a | Mediation effect, % (95% CI) b |
|---|---|---|---|
| Δ Weight, kg | 146 | 0.10 (-0.26–1.11) | 1.9% (-5.1–21.6) |
| Δ Waist circumference, cm | 140 | -0.00 (-0.46–0.48) | 0.0% (-9.7–10.2) |
| Δ Hip circumference, cm | 142 | 0.17 (-0.21–1.33) | 3.8% (-4.6–28.8) |
| Δ Mental quality of life score | 118 | -0.02 (-0.77–0.34) | -0.4% (-13.8–6.0) |
| Δ Physical quality of life score | 118 | 0.17 (-0.58–1.92) | 3.1% (-10.3–34.1) |
| Δ MVPA, minutes/week c | 129 | 1.05 (0.13–2.90) | 20.7% (2.6–56.9) |
| Combined model d | 113 | 1.91 (0.14–4.47) | 36.9% (2.8–86.3) |
a Bias corrected 95% CIs based on 5000 bootstrap re-samples. CI not containing zero indicate statistical significance.
b Mediation effect is indirect effect / total effect x 100%. CI not containing zero indicate statistical significance.
c MVPA = Moderate to vigorous physical activity in minutes per week based on SQUASH questionnaire.
d Model includes Δ weight, Δ waist circumference, Δ hip circumference, Δ mental quality of life score, Δ physical quality of life score, Δ MVPA between baseline and follow-up as mediator variables.
Abbreviations: n, Number; BC, Bias Corrected; CI, Confidence Interval.
Discussion
This five-years follow-up study of an RCT shows that a lifestyle intervention in women with obesity and infertility improves sexual function. Five years after the intervention, women in the intervention group reported a higher intercourse frequency, more vaginal lubrication and a better overall sexual function than women in the control group. The intervention group also scored higher on the sexual interest, satisfaction, orgasm and sex partner domains, but these effects were not statistically significant. Our finding that the lifestyle intervention did not only reduced weight, improved cardiovascular health and physical quality of life in the short term, but also had lasting beneficial effects on sexual function shows that lifestyle interventions have beneficial effects for health in a broad range of areas of health and wellbeing [39].
Randomized studies of lifestyle interventions in women with other comorbidities like type two diabetes, that were not specifically performed in women with obesity and infertility reported positive as well as absent effects on sexual function during or directly after a lifestyle intervention [35, 37, 52]. A non-randomized study with a follow-up period of 2 years reported improved sexual quality of life after a lifestyle intervention that successfully reduced weight among obese women. Regaining body weight did not eliminate the increase in sexual quality of life 2 years later [53]. We found similar results showing better sexual function in the intervention group, despite the absence of a difference in weight at follow-up. This suggest that a lifestyle intervention can have beneficial long-term effects on sexual health, even in the absence of a long-term effect on weight.
The intervention effect on sexual function was partly mediated by change in physical activity. In our study, a decrease in physical activity was associated with a lower sexual function (R = 0.23; p <0.01). Although throughout the study, physical activity decreased over time, the intervention group reduced their physical activity less than the control group (mean delta: -111.4 vs. -492 min/week; p = 0.03), which seems to have contributed to a better sexual function in the intervention group compared to the control group.
This study is the first to report experimental evidence of better long-term sexual function in women with obesity and infertility after a lifestyle intervention. The validated MFSQ was part of a more extensive survey that was filled out in private, without the presence of a researcher, which probably has reduced the risks of performance bias and social desirability bias, increasing the reliability of our results [54].
We were unable to assess the change in sexual function over time, because sexual function was not assessed at the start of the study. Whether the difference between the intervention and control group was based on an improved sexual function in the intervention group or decreased sexual function in the control group could not be determined. Adjustments for duration of infertility at randomization, PCOS, and attempting to conceive had little effect on our results, therefore it seems unlikely that our finding of a better sexual function in the intervention group was caused by these potential confounders. However, we found indications for selective attrition by comparing the 177 participants (31.4%) with the non-participants. Women who participated in the follow-up were more often Caucasian, were more often diagnosed with PCOS, but had a shorter duration of infertility and higher mental quality of life at start of the intervention. PCOS has been associated with a poorer sexual health, whereas shorter infertility duration and higher mental quality of life have been associated with a better sexual function [12, 34, 55]. Due to these opposed associations with sexual function and small absolute differences it seems unlikely that the selection in participants (S2 Table) had a substantial impact on our findings. However, the high percentage (>95%) of Caucasian women in our sample does limit the representativeness of our findings to the Caucasian population. Whether similar effects can be found in non-Caucasian women needs to be further investigated.
Our study shows that even in the absence of a sustained effect on weight, a lifestyle intervention in women with obesity and infertility leads to more frequent intercourse, better vaginal lubrication and overall sexual function 5 years later. Thus besides short-term improvements in cardiometabolic health and quality of life, lifestyle interventions can contribute to a better long-term sexual function in women who are at greater risk of sexual problems.
Supporting information
(PDF)
(SAV)
(PDF)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank all the women who participated in this study. We thank all participating hospitals and their staff for their contribution to this study, and the lifestyle coaches, research nurses, research midwives and office members of the Dutch Consortium (www.studies-obsgyn.nl) for their hard work and dedication. Furthermore, we thank all members of the WOMB-project who contributed to the follow-up study; with special thanks to our colleague PhD students, post-docs, research assistants and students.
Data Availability
All relevant data are within the paper and its Supporting Information files (S2 File: Minimal dataset).
Funding Statement
A.H. has received a grant to conduct the LIFEstyle study from the Netherlands Organization for Health Research and Development [50-50110-96-518] (https://www.zonmw.nl/en/). T.R. has received a grant to conduct the follow-up study from the Dutch Heart Foundation [2013T085] (https://www.hartstichting.nl/). B.W.M. is supported by a National Health and Medical Research Council Practitioner Fellowship grant [GNT1082548] (https://www.nhmrc.gov.au/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Berman JR. Physiology of female sexual function and dysfunction. International Journal Of Impotence Research. 2005;17:S44 10.1038/sj.ijir.3901428 [DOI] [PubMed] [Google Scholar]
- 2.Flynn KE, Lin L, Bruner DW, Cyranowski JM, Hahn EA, Jeffery DD, et al. Sexual Satisfaction and the Importance of Sexual Health to Quality of Life Throughout the Life Course of US Adults. The journal of sexual medicine. 2016;13(11):1642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual Problems and Distress in United States Women: Prevalence and Correlates. Obstetrics & Gynecology. 2008;112(5):970–8. 10.1097/AOG.0b013e3181898cdb [DOI] [PubMed] [Google Scholar]
- 4.Fugl-Meyer KS, Arrhult H, Pharmanson H, Bäckman AC, Fugl-Meyer AM, Fugl-Meyer AR. ORIGINAL RESEARCH—EPIDEMIOLOGY: A Swedish Telephone Help-line for Sexual Problems: A 5-year Survey. The Journal of Sexual Medicine. 1(3):278–83. [DOI] [PubMed] [Google Scholar]
- 5.Laumann EO, Nicolosi A, Glasser DB, Paik A, Gingell C, Moreira E, et al. Sexual problems among women and men aged 40–80 y: prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. International Journal Of Impotence Research. 2004;17:39 10.1038/sj.ijir.3901250 [DOI] [PubMed] [Google Scholar]
- 6.Larsen SH, Wagner G, Heitmann BL. Sexual function and obesity. International Journal Of Obesity. 2007;31:1189 10.1038/sj.ijo.0803604 [DOI] [PubMed] [Google Scholar]
- 7.Hruby A, Manson JE, Qi L, Malik VS, Rimm EB, Sun Q, et al. Determinants and Consequences of Obesity. American Journal of Public Health. 2016;106(9):1656–62. 10.2105/AJPH.2016.303326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Human Reproduction. 2007;22(2):414–20. 10.1093/humrep/del400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Steeg JW, Steures P, Eijkemans MJC, Habbema JDF, Hompes PGA, Burggraaff JM, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Human Reproduction. 2008;23(2):324–8. 10.1093/humrep/dem371 [DOI] [PubMed] [Google Scholar]
- 10.Koning AMH, Mutsaerts MAQ, Kuchenbecher WKH, Broekmans FJ, Land JA, Mol BW, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women†. Human Reproduction. 2012;27(2):457–67. 10.1093/humrep/der416 [DOI] [PubMed] [Google Scholar]
- 11.Millheiser LS, Helmer AE, Quintero RB, Westphal LM, Milki AA, Lathi RB. Is infertility a risk factor for female sexual dysfunction? A case-control study. Fertility and Sterility. 2010;94(6):2022–5. 10.1016/j.fertnstert.2010.01.037 [DOI] [PubMed] [Google Scholar]
- 12.Winkelman WD, Katz PP, Smith JF, Rowen TS. The Sexual Impact of Infertility Among Women Seeking Fertility Care. Sexual Medicine. 2016;4(3):e190–e7. 10.1016/j.esxm.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao P, Coates R, Maycock B. The impact of infertility on sexuality: A literature review. The Australasian Medical Journal. 2011;4(11):620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith NK, Madeira J, Millard HR. Sexual Function and Fertility Quality of Life in Women Using In Vitro Fertilization. The Journal of Sexual Medicine. 2015;12(4):985–93. 10.1111/jsm.12824 [DOI] [PubMed] [Google Scholar]
- 15.Lyall DM, Celis-Morales C, Ward J, et al. Association of body mass index with cardiometabolic disease in the uk biobank: A mendelian randomization study. JAMA Cardiology. 2017;2(8):882–9. 10.1001/jamacardio.2016.5804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowland DL, McNabney SM, Mann AR. Sexual Function, Obesity, and Weight Loss in Men and Women. Sexual Medicine Reviews. 2017;5(3):323–38. 10.1016/j.sxmr.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 17.Giraldi A, Kristensen E. Sexual Dysfunction in Women with Diabetes Mellitus. The Journal of Sex Research. 2010;47(2–3):199–211. 10.1080/00224491003632834 [DOI] [PubMed] [Google Scholar]
- 18.Miner M, Esposito K, Guay A, Montorsi P, Goldstein I. Cardiometabolic Risk and Female Sexual Health: The Princeton III Summary (CME). The Journal of Sexual Medicine. 2012;9(3):641–51. 10.1111/j.1743-6109.2012.02649.x [DOI] [PubMed] [Google Scholar]
- 19.Allahdadi KJ, Hannan JL, Ergul A, Tostes RC, Webb RC. Internal Pudendal Artery from Type 2 Diabetic Female Rats Demonstrate Elevated Endothelin-1-Mediated Constriction. The journal of sexual medicine. 2011;8(9):2472–83. 10.1111/j.1743-6109.2011.02375.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nappi R, Salonia A, Traish AM, Van Lunsen RHW, Vardi Y, Kodiglu A, et al. ORIGINAL RESEARCH—PATHOPHYSIOLOGY: Clinical Biologic Pathophysiologies of Women’s Sexual Dysfunction. The Journal of Sexual Medicine. 2005;2(1):4–25. [DOI] [PubMed] [Google Scholar]
- 21.Battaglia C, Battaglia B, Mancini F, Persico N, Nappi RE, Paradisi R, et al. Cigarette Smoking Decreases the Genital Vascularization in Young Healthy, Eumenorrheic Women. The Journal of Sexual Medicine. 2011;8(6):1717–25. 10.1111/j.1743-6109.2011.02257.x [DOI] [PubMed] [Google Scholar]
- 22.Kim YH, Kim SM, Kim JJ, Cho IS, Jeon MJ. Does Metabolic Syndrome Impair Sexual Function in Middle to Old Aged Women? The Journal of Sexual Medicine. 2011;8(4):1123–30. [DOI] [PubMed] [Google Scholar]
- 23.Otunctemur A, Dursun M, Ozbek E, Sahin S, Besiroglu H, Koklu I, et al. Effect of metabolic syndrome on sexual function in pre-And postmenopausal women. Journal of Sex and Marital Therapy. 2015;41(4):440–9. 10.1080/0092623X.2014.918068 [DOI] [PubMed] [Google Scholar]
- 24.Mather AA, Cox BJ, Enns MW, Sareen J. Associations of obesity with psychiatric disorders and suicidal behaviors in a nationally representative sample. Journal of Psychosomatic Research. 66(4):277–85. 10.1016/j.jpsychores.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 25.Laurent SM, Simons AD. Sexual dysfunction in depression and anxiety: Conceptualizing sexual dysfunction as part of an internalizing dimension. Clinical Psychology Review. 2009;29(7):573–85. 10.1016/j.cpr.2009.06.007 [DOI] [PubMed] [Google Scholar]
- 26.Yaylali GF, Tekekoglu S, Akin F. Sexual dysfunction in obese and overweight women. Int J Impot Res. 2010;22(4):220–6. 10.1038/ijir.2010.7 [DOI] [PubMed] [Google Scholar]
- 27.Dudenhausen JW, Grünebaum A, Kirschner W. Prepregnancy body weight and gestational weight gain—recommendations and reality in the USA and in Germany. American Journal of Obstetrics & Gynecology. 213(4):591–2. [DOI] [PubMed] [Google Scholar]
- 28.Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, et al. Pharmacological Management of Obesity: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism. 2015;100(2):342–62. 10.1210/jc.2014-3415 [DOI] [PubMed] [Google Scholar]
- 29.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction. Circulation. 2010;121(4):586 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 30.van Dammen L, Wekker V, van Oers AM, Mutsaerts MAQ, Painter RC, Zwinderman AH, et al. Effect of a lifestyle intervention in obese infertile women on cardiometabolic health and quality of life: A randomized controlled trial. PLOS ONE. 2018;13(1):e0190662 10.1371/journal.pone.0190662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carroll S, Borkoles E, Polman R. Short-term effects of a non-dieting lifestyle intervention program on weight management, fitness, metabolic risk, and psychological well-being in obese premenopausal females with the metabolic syndrome. Applied Physiology, Nutrition, and Metabolism. 2007;32(1):125–42. 10.1139/h06-093 [DOI] [PubMed] [Google Scholar]
- 32.Borkoles E, Carroll S, Clough P, Polman RCJ. Effect of a non-dieting lifestyle randomised control trial on psychological well-being and weight management in morbidly obese pre-menopausal women. Maturitas. 2016;83:51–8. 10.1016/j.maturitas.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 33.Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K, et al. Impact of a Weight Management Program on Health-related Quality of Life In Overweight Adults with Type 2 Diabetes. Archives of internal medicine. 2009;169(2):163–71. 10.1001/archinternmed.2008.544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nappi PRE, Cucinella L, Martella S, Rossi M, Tiranini L, Martini E. Female sexual dysfunction (FSD): Prevalence and impact on quality of life (QoL). Maturitas. 94:87–91. 10.1016/j.maturitas.2016.09.013 [DOI] [PubMed] [Google Scholar]
- 35.Kolotkin RL, Zunker C, Østbye T. Sexual Functioning and Obesity: A Review. Obesity. 2012;20(12):2325–33. 10.1038/oby.2012.104 [DOI] [PubMed] [Google Scholar]
- 36.Aversa A, Bruzziches R, Francomano D, Greco EA, Violi F, Lenzi A, et al. Weight Loss by Multidisciplinary Intervention Improves Endothelial and Sexual Function in Obese Fertile Women. The Journal of Sexual Medicine. 2013;10(4):1024–33. 10.1111/jsm.12069 [DOI] [PubMed] [Google Scholar]
- 37.Wing RR, Bond DS, Gendrano IN, Wadden T, Bahnson J, Lewis CE, et al. Effect of Intensive Lifestyle Intervention on Sexual Dysfunction in Women With Type 2 Diabetes: Results from an ancillary Look AHEAD study. Diabetes Care. 2013;36(10):2937–44. 10.2337/dc13-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mutsaerts MAQ, Groen H, ter Bogt NCW, Bolster JHT, Land JA, Bemelmans WJE, et al. The LIFESTYLE study: costs and effects of a structured lifestyle program in overweight and obese subfertile women to reduce the need for fertility treatment and improve reproductive outcome. A randomised controlled trial. BMC Women’s Health. 2010;10(1):22 10.1186/1472-6874-10-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mutsaerts MAQ, van Oers AM, Groen H, Burggraaff JM, Kuchenbecker WKH, Perquin DAM, et al. Randomized Trial of a Lifestyle Program in Obese Infertile Women. New England Journal of Medicine. 2016;374(20):1942–53. 10.1056/NEJMoa1505297 . [DOI] [PubMed] [Google Scholar]
- 40.van de Beek C, Hoek A, Painter RC, Gemke RJBJ, van Poppel MNM, Geelen A, et al. Women, their Offspring and iMproving lifestyle for Better cardiovascular health of both (WOMB project): a protocol of the follow-up of a multicentre randomised controlled trial. BMJ Open. 2018;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The ECWG. Anovulatory infertility*. Human Reproduction. 1995;10(6):1549–53. 10.1093/HUMREP/10.6.1549 [DOI] [PubMed] [Google Scholar]
- 42.(NVOG). DSoOaG. Data sheet (http://nvog-documenten.nl/index.php?pagina=/richtlijn/pagina.php&fSelectNTG_112=113&fSelectedSub=112).
- 43.Executive Summary. Obesity Research. 1998;6(S2):51S–179S. 10.1002/j.1550-8528.1998.tb00690.x9813653 [DOI] [Google Scholar]
- 44.Patrick K S J, Long B, Calfas KJ, Wooten W, Heath G, Pratt M. A new tool for encouraging activity. Project PACE. The physician and sportsmedicine. 1994:45–55. 10.1080/00913847.1994.11947706 [DOI] [PubMed] [Google Scholar]
- 45.Aaronson NK, Muller M, Cohen PDA, Essink-Bot M-L, Fekkes M, Sanderman R, et al. Translation, Validation, and Norming of the Dutch Language Version of the SF-36 Health Survey in Community and Chronic Disease Populations. Journal of Clinical Epidemiology. 1998;51(11):1055–68. 10.1016/S0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 46.Wendel-Vos GCW, Schuit AJ, Saris WHM, Kromhout D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. Journal of Clinical Epidemiology. 2003;56(12):1163–9. 10.1016/S0895-4356(03)00220-8. [DOI] [PubMed] [Google Scholar]
- 47.McCoy NL. The McCoy Female Sexuality Questionnaire. Quality of Life Research. 2000;9(1):739–45. 10.1023/A:1008925906947 [DOI] [Google Scholar]
- 48.Giraldi A, Rellini A, Pfaus JG, Bitzer J, Laan E, Jannini EA, et al. Questionnaires for Assessment of Female Sexual Dysfunction: A Review and Proposal for a Standardized Screener. The Journal of Sexual Medicine. 2011;8(10):2681–706. 10.1111/j.1743-6109.2011.02395.x [DOI] [PubMed] [Google Scholar]
- 49.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and Sterility. 2004;81(1):19–25. 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 50.DiCiccio TJ, Efron B. Bootstrap confidence intervals. Statist Sci. 1996;11(3):189–228. 10.1214/ss/1032280214 [DOI] [Google Scholar]
- 51.Hayes AF, Rockwood NJ. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behaviour Research and Therapy. 2017;98:39–57. 10.1016/j.brat.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 52.Jamali S, Zarei H, Rasekh Jahromi A. The relationship between body mass index and sexual function in infertile women: A cross-sectional survey. Iranian Journal of Reproductive Medicine. 2014;12(3):189–98. [PMC free article] [PubMed] [Google Scholar]
- 53.Kolotkin RL, Binks M, Crosby RD, Østbye T, Mitchell JE, Hartley G. Improvements in sexual quality of life after moderate weight loss. International Journal Of Impotence Research. 2008;20:487 10.1038/ijir.2008.32 [DOI] [PubMed] [Google Scholar]
- 54.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the united states: Prevalence and predictors. JAMA. 1999;281(6):537–44. 10.1001/jama.281.6.537 [DOI] [PubMed] [Google Scholar]
- 55.Lizneva D, Walker WJ, Gavrilova-Jordan L, Diamond MP, Azziz R, Suturina L, et al. Sexual function and polycystic ovary syndrome: a systematic review and meta-analysis. Fertility and Sterility. 2016;106(3):e261 10.1016/j.fertnstert.2016.07.752 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(SAV)
(PDF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files (S2 File: Minimal dataset).