Abstract
Background
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a rare neurological disorder of the peripheral nervous system. The economic burden of CIDP is not well understood.
Objectives
To assess the economic and clinical burden of CIDP and to compare the incremental burden relative to a matched control group without CIDP.
Methods
This retrospective case-control analysis was conducted using data from the IQVIA Real-World Data Adjudicated Claims. Adults newly diagnosed with CIDP between 7/1/2010 and 6/30/2014 were identified and direct matched to controls without CIDP. Baseline characteristics were assessed and compared over a 6-month pre-index period. Healthcare resource use, costs and clinical characteristics were assessed and compared over a 2-year follow-up. Total cost differences over the 2-year follow-up were compared between matched cohorts using a generalized estimating equation model.
Results
The final sample comprised a total of 790 cases matched to 790 controls. Over the 2-year follow-up, cases more frequently experienced neuropathic pain, back pain and osteoarthritis and more commonly utilized opioids, anti-convulsants and anti-depressants. Compared to controls, more cases had ≥1 hospitalization (26.2% vs. 9.0%), and cases had a higher mean number of outpatient prescription fills (62.8 vs. 32.0) and physician office visits (34.7 vs. 13.0) (all p<0.0001). Cases had 7.5x higher mean total costs ($116,330 vs. $15,586, p<0.0001). Important cost drivers were costs for outpatient ancillary, radiology and HCPCS drugs (mean $76,366 vs. $4,292) and costs for inpatient care (mean $16,357 vs. $2,862) (both p<0.0001). Among cases, CIDP therapy (inclusive of both outpatient pharmacy and medical claims) accounted for 51.2% of mean total costs. After further adjusting for baseline clinical characteristics, cases were associated with a 6.1x increase in total costs compared to controls (p<0.0001).
Conclusions
Our findings suggest a substantial clinical and economic burden among patients with CIDP relative to matched controls over a 2-year follow-up.
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a rare, chronic, acquired immune mediated disorder of the peripheral nervous system, characterized by progressive, symmetrical limb weakness, large fiber sensory loss and loss of reflexes [1–3]. Initial symptoms include sensory loss, usually beginning in the legs, and patients may report impaired ambulation (e.g., difficulty walking, climbing stairs) [3]. In the United States (US), the annual incidence of CIDP has been estimated at 1.6 per 100,000 people while the prevalence has been estimated at 8.9 per 100,000 people [4].
There has been increasing concern about misdiagnosis and over-diagnosis of CIDP in current clinical practice [5,6]. Not surprisingly, in view of the potential for misdiagnosis, stringent electro-diagnostic tests are required for the diagnosis of CIDP, along with the assessment of clinical and laboratory features [7]. Several US payers mandate positive electrophysiological findings on at least three of four tests (per guidelines from the American Academy of Neurology [AAN]) as part of medical necessity for CIDP treatment, particularly intravenous immunoglobulin (IVIg) [1,8,9]. Furthermore, a differential diagnosis is necessary to exclude disorders that may similarly present as CIDP, such as other peripheral nerve disorders [5]. Timely and appropriate initiation of therapy early on in the course of disease is critical to prevent permanent disability [10].
The primary goals of treatment for CIDP are to reduce symptoms, improve functional status (e.g., reduce disability and handicap) and maintain long-term remission as possible [3]. According to joint guidelines on the management of CIDP from the European Federation of Neurological Societies and the Peripheral Nerve Society, IVIg (level A recommendation) or corticosteroids (level C recommendation) should be considered for induction of treatment in patients with CIDP that present with disabling symptoms [7]. A report of the AAN has stated that IVIg is effective and should be offered in the long-term treatment of CIDP (level A); however, data are insufficient to address the comparative efficacy of other CIDP treatments (e.g., steroids) [11]. Other treatment options for CIDP include plasma exchange (PE) and the addition of immunosuppressant or immunomodulatory drugs (e.g., azathioprine, mycophenolate motefil, rituximab) [3].
The economic burden of CIDP is not well understood. Two published studies have identified substantial costs associated with CIDP [12,13]. No published studies have evaluated the incremental burden of CIDP. Given the limited real-world data on the economic burden of CIDP, the objectives of this analysis were to assess the economic burden of CIDP in the 2 years following diagnosis, and to quantify the incremental burden of CIDP relative to a control group without CIDP, from the payer perspective, using a large, nationally representative database in the US. Secondary objectives were to assess patient characteristics and the clinical burden of CIDP.
Methods
Study overview
This retrospective case-control analysis was conducted using IQVIA Real-World Data (RWD) Adjudicated Claims, using data from January 2010 through June 2016.
Data source
IQVIA RWD Adjudicated Claims is one of the largest US health plan claims databases and is comprised of adjudicated claims for more than 150 million unique enrollees across the US. The database is considered representative of the national, commercially insured population in terms of age and gender. Standard fields include inpatient and outpatient diagnoses and procedures, and retail and mail order prescription records and payments. All data are compliant with the Health Insurance Portability and Accountability Act (HIPAA) to protect patient’s privacy.
Patient selection
US managed care enrollees were initially identified in IQVIA RWD Adjudicated Claims based on ≥1 medical claim with a diagnosis for CIDP (ICD-9-CM 357.81) between July 1, 2010 and June 30, 2014. Both confirmatory and non-confirmatory (i.e., ancillary) claims were considered. The date of the first claim was termed the “index date.” Patients were required to have confirmation of CIDP denoted by: 1) a subsequent confirmatory medical claim with a diagnosis for CIDP or 2) initiation of CIDP therapy within 1 year of the index date. CIDP therapy was identified based on a broad list of available therapies or procedures which have been proven or investigated in CIDP (Table 1) [3].
Table 1. CIDP therapies.
| IVIg | Etanercept |
| Corticosteroids | Interferon B1a |
| Alemtuzumab | Methotrexate |
| Azathioprine | Mycophenolate mofetil |
| Cyclophosphamide | Natalizumab |
| Cyclosporine A | Rituximab |
| Plasma exchange | Tacrolimus |
| Hematopoietic stem cell transplantation |
Patients were required to have ≥6 months of continuous enrollment (CE) in the health plan before the index date (i.e., the 6-month baseline or pre-index period) and to have ≥2-years CE following the index date (i.e., the 2-year follow-up or post-index period). Patients were required to be newly diagnosed with CIDP as of the index date and were excluded if they had any medical claims with a diagnosis for CIDP or if they had any claims for CIDP therapy in the 6-month pre-index period. Finally, only adults were included (≥18 years old at index) and patients were excluded if they had poor data quality (i.e., incomplete or invalid data).
Patients with CIDP (cases) were matched to patients without CIDP (controls). Cases were direct matched to controls based on: age, gender, geographic region, health plan type, payer type and Charlson Comorbidity Index (CCI) score. The index date for controls was set as the index date of the matched patient with CIDP. Controls had neither medical claims with a diagnosis for CIDP nor claims for CIDP therapy at any time during the study period. Controls were required to be ≥18 years old, to have ≥6-months pre- and ≥2-years post-index CE, and to have adequate data quality.
Study measures
Baseline demographic and clinical characteristics were assessed for cases and controls. Demographic characteristics included age, gender, health plan type, payer type and geographic region at index. Clinical characteristics were measured over the 6-month pre-index period and included the CCI, common and relevant comorbidities of interest, prior use of therapies of interest and total healthcare costs. Alternative pre-index diagnoses, i.e., occurring prior to confirmed CIDP diagnosis, which may be considered potential misdiagnoses [6] were also assessed.
Use of CIDP treatment was assessed over the 2-year follow-up among cases. Initiation of therapy was classified as either monotherapy or combination therapy based on the different therapies used within 30 days of therapy initiation (at the class level for corticosteroids or IVIg; at the generic product level for all other therapies). For instance, a patient with claims for two different IVIg products over the first 30 days of therapy initiation was classified as receiving IVIg monotherapy, while a patient with claims for a corticosteroid and an IVIg was classified as receiving combination therapy.
The clinical and economic burden of CIDP was assessed over the 2-year follow-up among cases and controls. Select clinical characteristics were also assessed over the 2-year follow-up, including new use of therapies of interest (without use in the pre-index). Healthcare resource use (HCRU) and costs were measured for cases and controls over the 2-year follow-up and compared to quantify the incremental burden of CIDP. Allowed healthcare costs, the amount paid by the health plan and patient combined, were evaluated on a per cohort member basis. Thus, the denominator included all patients in a cohort, regardless of whether they had utilization of a specific service. Costs were converted to 2016 US dollars (USD) using the medical component of the Consumer Price Index.
HCRU and costs were assessed for outpatient pharmacy and medical services. Medical services included the following mutually exclusive healthcare categories: hospitalizations and outpatient medical care (emergency room [ER] visits, physician office visits, outpatient surgical visits, laboratory and pathology, and outpatient ancillary, radiology and Healthcare Common Procedure Coding System [HCPCS] drugs). CIDP therapy costs (inclusive of both outpatient pharmacy and medical claims) were separately assessed.
Statistical analyses
Descriptive analyses were organized as follow. For categorical measures, reporting included the frequency (number of patients [N]) and percentage (%) for each cohort. For continuous variables, both the mean (standard deviation [SD]) and median were reported. Study outcomes were compared between the matched cohorts using paired t-test (mean) and the Wilcoxon signed-rank test (median) for continuous variables and McNemar’s or Bowker’s test for categorical variables.
A generalized estimating equation model (GEE) was developed, with a gamma distribution and log-link function, to evaluate differences in total healthcare costs over the 2-year follow-up between matched cohorts. The model included baseline clinical characteristics measured over the 6-month pre-index period that were significantly different between matched cases and controls, including back pain, use of anti-depressants, anti-anxiety medications, anti-convulsants, benzodiazepines, central muscle relaxants, NSAIDs, and opioids, number of outpatient physician office visits and total healthcare costs.
A p-value of <0.05 was considered statistically significant. All analyses used SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Patient sample
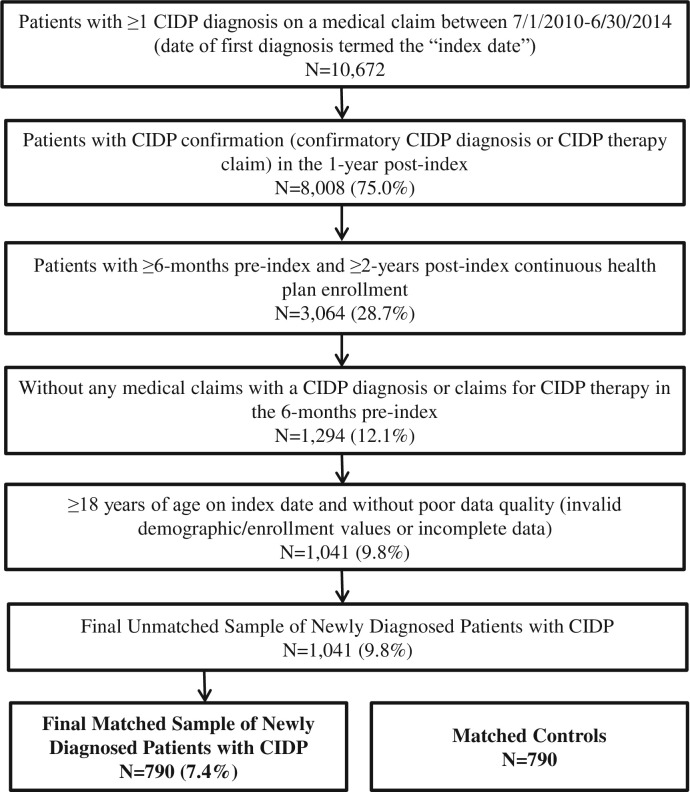
A total of 10,672 patients were identified with a medical claim with a diagnosis for CIDP between July 1, 2010 and June 30, 2014 and were evaluated for study inclusion (see Fig 1 for patient selection flow). Of the starting 10,672 patients, 8,008 (75.0%) had confirmation of CIDP within 1 year of the index date, while 3,064 (28.7%) met the CE requirements. The unmatched sample consisted of 1,041 (9.8%) patients with newly diagnosed CIDP. The final sample comprised 790 (7.4%) cases successfully matched to 790 controls without CIDP.
Fig 1. Study flow chart.
CIDP = chronic inflammatory demyelinating polyneuropathy.
Patient characteristics
Baseline demographic characteristics were direct matched between cases and controls (Table 2). Mean (SD) age at index for cases and controls was 49.7 (11.4). Half (53.7%) were male. Patients were most often located in the South (40.4%) and the majority was commercially-insured (63.9%).
Table 2. Baseline demographic characteristics and clinical characteristics in the 6-month pre-index period and 2-year follow-up.
| Baseline | 2-Year Follow-Up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases N = 790 |
Controls N = 790 |
P valueb | Cases N = 790 |
Controls N = 790 |
P valueb | |||||
| Characteristic | n | % | n | % | n | % | n | % | ||
| Age (years)a | ||||||||||
| Mean ± SD | 49.7 ± 11.4 | 49.7 ± 11.4 | ||||||||
| Median | 52 | 52 | ||||||||
| Male gendera | 424 | (53.7) | 424 | (53.7) | ||||||
| Regiona | ||||||||||
| Northeast | 219 | (27.7) | 219 | (27.7) | ||||||
| Midwest | 167 | (21.1) | 167 | (21.1) | ||||||
| South | 319 | (40.4) | 319 | (40.4) | ||||||
| West | 85 | (10.8) | 85 | (10.8) | ||||||
| Payer typea | ||||||||||
| Commercial | 505 | (63.9) | 505 | (63.9) | ||||||
| Medicaid | 19 | (2.4) | 19 | (2.4) | ||||||
| Medicare Risk | 9 | (1.1) | 9 | (1.1) | ||||||
| Self-insured | 257 | (32.5) | 257 | (32.5) | ||||||
| Health plan typea | ||||||||||
| Consumer-directed | 6 | (0.8) | 6 | (0.8) | ||||||
| HMO | 80 | (10.1) | 80 | (10.1) | ||||||
| Indemnity | 14 | (1.8) | 14 | (1.8) | ||||||
| POS | 16 | (2.0) | 16 | (2.0) | ||||||
| PPO | 674 | (85.3) | 674 | (85.3) | ||||||
| Index yeara | ||||||||||
| 2010 | 136 | (17.2) | 136 | (17.2) | ||||||
| 2011 | 226 | (28.6) | 226 | (28.6) | ||||||
| 2012 | 176 | (22.3) | 176 | (22.3) | ||||||
| 2013 | 175 | (22.2) | 175 | (22.2) | ||||||
| 2014 | 77 | (9.7) | 77 | (9.7) | ||||||
| CCIa | ||||||||||
| 0 | 580 | (73.4) | 580 | (73.4) | 334 | (42.3) | 495 | (62.7) | <.0001 | |
| 1 | 120 | (15.2) | 120 | (15.2) | 154 | (19.5) | 138 | (17.5) | 0.2893 | |
| 2 | 79 | (10.0) | 79 | (10.0) | 134 | (17.0) | 105 | (13.3) | 0.0388 | |
| 3+ | 11 | (1.4) | 11 | (1.4) | 168 | (21.3) | 52 | (6.6) | <.0001 | |
| Mean ± SD | 0.4 ± 0.8 | 0.4 ± 0.8 | 1.5 ± 1.9 | 0.7 ± 1.3 | <.0001 | |||||
| Median | 0 | 0 | 1 | 0 | <.0001 | |||||
| Common (≥5%) comorbidities of interest: | ||||||||||
| Asthma/COPD | 19 | (2.4) | 9 | (1.1) | 0.0588 | 65 | (8.2) | 17 | (2.2) | <.0001 |
| Back pain | 241 | (30.5) | 80 | (10.1) | <.0001 | 371 | (47.0) | 150 | (19.0) | <.0001 |
| Cardiac dysrhythmia | 34 | (4.3) | 28 | (3.5) | 0.4386 | 100 | (12.7) | 50 | (6.3) | <.0001 |
| Cerebrovascular disease | 25 | (3.2) | 12 | (1.5) | 0.0280 | 81 | (10.3) | 25 | (3.2) | <.0001 |
| CAD | 22 | (2.8) | 23 | (2.9) | 0.8788 | 62 | (7.8) | 52 | (6.6) | 0.3124 |
| Diabetes | 87 | (11.0) | 112 | (14.2) | 0.0176 | 172 | (21.8) | 147 | (18.6) | 0.0867 |
| Dyslipidemia | 215 | (27.2) | 237 | (30.0) | 0.1806 | 361 | (45.7) | 363 | (45.9) | 0.9113 |
| Hypertension | 233 | (29.5) | 223 | (28.2) | 0.5543 | 364 | (46.1) | 314 | (39.7) | 0.0064 |
| Hypothyroidism | 80 | (10.1) | 53 | (6.7) | 0.0126 | 128 | (16.2) | 86 | (10.9) | 0.0016 |
| IBD | 16 | (2.0) | 14 | (1.8) | 0.7150 | 46 | (5.8) | 25 | (3.2) | 0.0103 |
| Leukemia/lymphoma | 26 | (3.3) | 6 | (0.8) | 0.0004 | 84 | (10.6) | 34 | (4.3) | <.0001 |
| Neuropathic pain | 314 | (39.7) | 23 | (2.9) | <.0001 | 455 | (57.6) | 54 | (6.8) | <.0001 |
| Osteoarthritis | 65 | (8.2) | 26 | (3.3) | <.0001 | 188 | (23.8) | 62 | (7.8) | <.0001 |
| PVD | 17 | (2.2) | 3 | (0.4) | 0.0017 | 50 | (6.3) | 13 | (1.6) | <.0001 |
| Sleep apnea | 14 | (1.8) | 5 | (0.6) | 0.0290 | 59 | (7.5) | 23 | (2.9) | <.0001 |
| Common (≥5%) therapies of interest: | ||||||||||
| Anti-anxiety medications | 14 | (1.8) | 5 | (0.6) | 0.0389 | 50 | (6.3) | 17 | (2.2) | <.0001 |
| Anti-convulsants | 242 | (30.6) | 40 | (5.1) | <.0001 | 361 | (45.7) | 57 | (7.2) | <.0001 |
| Anti-depressants | 216 | (27.3) | 108 | (13.7) | <.0001 | 349 | (44.2) | 144 | (18.2) | <.0001 |
| Benzodiazepines | 121 | (15.3) | 43 | (5.4) | <.0001 | 207 | (26.2) | 75 | (9.5) | <.0001 |
| Central muscle relaxants | 93 | (11.8) | 42 | (5.3) | <.0001 | 206 | (26.1) | 71 | (9.0) | <.0001 |
| Lidocaine | 18 | (2.3) | 0 | (0.0) | - | 52 | (6.6) | 10 | (1.3) | <.0001 |
| NSAIDs | 142 | (18.0) | 71 | (9.0) | <.0001 | 253 | (32.0) | 137 | (17.3) | <.0001 |
| Opioids | 264 | (33.4) | 128 | (16.2) | <.0001 | 479 | (60.6) | 212 | (26.8) | <.0001 |
aCases (N = 790) and Controls (N = 790) were direct matched (exact matched) on demographic variables and CCI.
bMcNemar’s or Bowker’s test for categorical variables, and paired t-test (mean) and the Wilcoxon signed-rank test (median) for continuous variables.
CAD = Coronary artery disease; CCI = Charlson Comorbidity Index; COPD = chronic obstructive pulmonary disease; HMO = health maintenance organization; IBD = Inflammatory bowel disease; NSAIDs = nonsteroidal anti-inflammatory drugs; PAD = Peripheral vascular disease; POS = point of service; PPO = preferred provider organization; SD = standard deviation.
Baseline clinical characteristics can be found in Table 2. Patients were also direct matched on CCI score and the majority (73.4%) had a CCI score of 0 over the 6-month pre-index period. Among cases, alternative, pre-index diagnoses that may be considered as misdiagnosis of CIDP included multifocal motor neuropathy (31.1%), fibromyalgia (9.9%), Guillain-Barré syndrome (5.8%), multiple sclerosis (5.6%) and idiopathic small fiber neuropathy (4.7%). Overall, 47.7% of cases had a prior alternative diagnosis compared to 3.3% of controls (p<0.0001). A few comorbidities in the 6-month pre-index period were significantly higher among cases compared to controls including neuropathic pain (39.7% vs. 2.9%), back pain (30.5% vs. 10.1%) and osteoarthritis (8.2% vs. 3.3%) (all p<0.0001).
A number of therapies of interest were more frequently used in the 6-month pre-index period among cases compared to controls including anti-convulsants (30.6% vs. 5.1%), opioids (33.4% vs. 16.2%) and anti-depressants (27.3% vs. 13.7%) (all p<0.0001). More cases had a hospitalization compared to controls (6.7% vs. 3.8%, p = 0.0056). Cases also had a higher mean number of outpatient physician office visits (8.0 vs. 4.1) and higher mean total costs ($8,316 vs. $3,748) (both p<0.0001). In the 6-month pre-index period, 39.4% of cases had ≥1 physician office visit to a neurologist and 10.4% had ≥1 physician office visit to an orthopedic surgeon, compared to 2.2% and 3.5% of controls, respectively.
Treatment utilization over the 2-year follow-up
Over the 2-year follow-up, 657 patients (83.2%) initiated CIDP therapy in a median of 52 days. Among patients that initiated CIDP therapy, the majority (57.4%) initiated monotherapy with corticosteroids, while 27.5% initiated monotherapy with IVIg and 8.2% initiated combination therapy with corticosteroids and IVIg. Among patients that initiated CIDP therapy, 83.1% had any use of corticosteroids and 41.2% had any use of IVIg over the entire 2-year follow-up; utilization of other CIDP treatments was infrequent (<5%) and included mycophenolate mofetil (4.4%), azathioprine (4.1%) and PE (4.0%).
Clinical burden over the 2-year follow-up
Over the 2-year follow-up, CCI score was higher for cases than controls (median 1 vs. 0, p<0.0001) (Table 2). A number of comorbidities of interest were significantly higher among cases compared to controls including neuropathic pain (57.6% vs. 6.8%), back pain (47.0% vs. 19.0%) and osteoarthritis (23.8% vs. 7.8%) (all p<0.0001). Several therapies of interest were more frequently used among cases compared to controls including opioids (60.6% vs. 26.8%), anti-convulsants (45.7% vs. 7.2%) and anti-depressants (44.2% vs. 18.2%) (all p<0.0001). More cases were new to therapies of interest (which were not observed in the pre-index) than controls, including opioids (31.4% vs. 19.5%, p<0.0001), NSAIDs (20.8% vs. 13.5%, p = 0.0002), anti-depressants (19.9% vs. 6.1%, p<0.0001) and anti-convulsants (19.2% vs. 2.8%, p<0.0001).
Economic burden over the 2-year follow-up
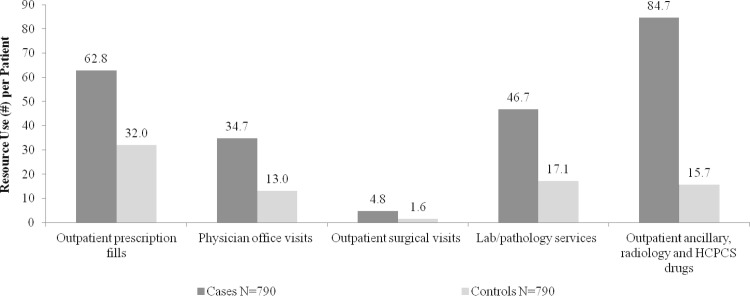
Over the 2-year follow-up, HCRU was significantly higher among cases. Proportion with utilization of a service was significantly higher among cases compared to controls including proportion with ≥1 hospitalization (26.2% vs. 9.0%), ER visit (42.2% vs. 21.9%) or outpatient surgical visit (77.1% vs. 45.9%) (all p<0.0001). More cases had ≥1 neurologist office visit (67.0% vs. 3.9%) or physical therapy visit (14.7% vs. 2.8%) (both p<0.0001). Number of healthcare services per patient was also significantly higher among cases including mean number of outpatient prescription fills (62.8 vs. 32.0) and physician office visits (34.7 vs. 13.0) (both p<0.0001) (Fig 2).
Fig 2. Mean resource use (#) per patient over the 2-year follow-up.
All p<0.0001. ER = emergency room; HCPCS = Healthcare Common Procedure Coding System.
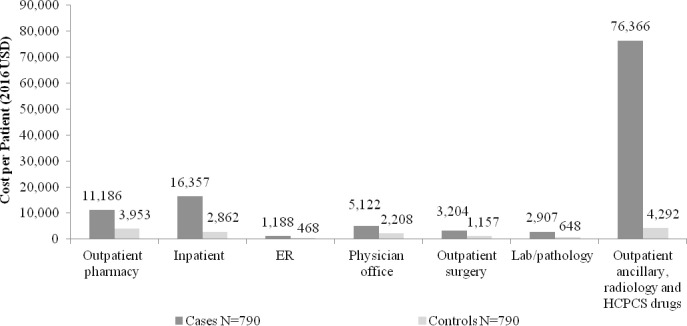
Over the 2-year follow-up, total costs were significantly higher for cases than controls (mean $116,330 vs. $15,586, p<0.0001) (See Table 3 and Fig 3). Compared to controls, cases had 7.5x higher mean total costs. Healthcare costs were significantly higher among cases for all resource categories, including mean per patient cost for outpatient ancillary, radiology and HCPCS drugs ($76,366 vs. $4,292), inpatient care ($16,357 vs. $2,862) and physician office visits ($5,122 vs. $2,208) (all p<0.0001). Compared to controls, cases had 17.8x higher costs for outpatient ancillary, radiology and HCPCS drugs, 9.0x higher medical costs and 5.7x higher inpatient costs. As a proportion of mean total costs for cases and controls, medical costs represented 90.4% and 74.6%, costs for outpatient ancillary, radiology and HCPCS drugs represented 65.6% and 27.5%, and inpatient costs represented 14.1% and 18.4%, respectively. Among cases, mean (SD) cost of CIDP therapy per patient (inclusive of outpatient pharmacy and medical claims) was $59,619 ($136,892), and CIDP therapy accounted for 51.2% of total costs. Note that 17% of cases used no CIDP therapy over the 2-year follow-up, contributing to the observed high standard deviation and wider distribution of costs.
Table 3. Healthcare cost per patient over the 2-year follow-up.
| Cases | Controls | P value* | P value* | |||||
|---|---|---|---|---|---|---|---|---|
| N = 790 | N = 790 | |||||||
| Cost (2016 USD) per patient | Mean | SD | Median | Mean | SD | Median | Mean | Median |
| Outpatient Pharmacy | 11,186 | 24,947 | 3,014 | 3,953 | 10,574 | 848 | <.0001 | <.0001 |
| Medical | 105,144 | 177,800 | 33,206 | 11,633 | 55,090 | 3,144 | <.0001 | <.0001 |
| Inpatient | 16,357 | 66,522 | 0 | 2,862 | 17,257 | 0 | <.0001 | <.0001 |
| ER | 1,188 | 5,168 | 0 | 468 | 2,215 | 0 | <.0001 | 0.0003 |
| Physician office | 5,122 | 10,297 | 3,015 | 2,208 | 20,115 | 943 | <.0001 | 0.0003 |
| Outpatient surgery | 3,204 | 8,172 | 809 | 1,157 | 4,372 | 0 | <.0001 | <.0001 |
| Lab/pathology | 2,907 | 11,012 | 1,120 | 648 | 1,903 | 278 | <.0001 | <.0001 |
| Outpatient ancillary, radiology and HCPCS drugs | 76,366 | 155,863 | 12,355 | 4,292 | 23,125 | 800 | <.0001 | <.0001 |
| TOTAL COST | 116,330 | 179,116 | 47,827 | 15,586 | 56,692 | 5,823 | <.0001 | <.0001 |
| Total CIDP Therapy | 59,619 | 136,892 | 59 | |||||
*Paired t-test (mean) and the Wilcoxon signed-rank test (median).
CIDP = chronic inflammatory demyelinating polyneuropathy; ER = emergency room; HCPCS = Healthcare Common Procedure Coding System; USD = US dollar.
Fig 3. Mean healthcare cost per patient over the 2-year follow-up.
All p<0.0001 except for ER (p = 0.0003). ER = emergency room; HCPCS = Healthcare Common Procedure Coding System; USD = US dollar.
In the GEE of total healthcare costs over the 2-year follow-up, cases were associated with a 6.1x increase in total costs compared to controls (p<0.0001).
Discussion
This study represents to our knowledge the first real-world database study to quantify the incremental burden of CIDP using a matched case-control analysis. Our findings suggest a substantial clinical and economic burden among patients with CIDP compared to matched controls. Over the 2-year follow-up, common symptoms of CIDP included neuropathic pain, back pain and osteoarthritis. Medications such as anti-convulsants, anti-depressants and even opioids were frequently utilized. A third of cases newly used opioids following initial diagnosis. We observed that a number of clinical characteristics appeared to increase for cases in the 2-year follow-up compared to the 6-month pre-index including CCI score, and presence of neuropathic pain, back pain and osteoarthritis. Over the 2-year follow-up, cases had significantly higher HCRU compared to controls, including more patients with ≥1 hospitalization and a higher number of physician office visits. Cases were associated with 7.5x higher mean total healthcare costs compared to controls. Outpatient ancillary, radiology and HCPCS drugs comprised the majority of total costs, while inpatient care was also an important cost driver. Half of total costs for cases over the 2-year follow-up were attributable to CIDP therapy (outpatient prescriptions, HCPCS drugs and therapeutic procedures). Following further adjustment in the GEE, cases were associated with a 6-fold increase in total healthcare costs compared to controls.
The frequent use of opioids observed among CIDP cases is concerning. We have no way of identifying whether opioids were prescribed for the CIDP or, as is likely, for observed comorbid neuropathic or chronic pain due to the limitations of the study database. While severe pain is unusual in CIDP, it appears that comorbid pain-related conditions were relatively common among our cases. We found that almost half of cases experienced back pain and almost a quarter experienced osteoarthritis over the follow-up. Yet, the use of opioids, even if for neuropathic pain, remains controversial and opioids do not provide improvements in physical functioning among those with neuropathic pain [14,15]. Further, opioids should not be used as first-line or routine therapy for chronic pain, and their use must be carefully considered given the potential for misuse, abuse and overdose [16].
Only one identified study has previously evaluated total healthcare costs among CIDP patients in the US [12]. A total of 73 patients with CIDP were identified from 9 small commercial health plans. The mean health plan paid cost per patient in 2011 was $56,953. A quarter (26%) of patients had claims for IVIg, while 16% had claims for prednisone. This study highlighted the high costs of IVIg: 90% of pharmacy costs were related to IVIg, which was administered to only a minority (26%). Our study, conducted using a large, nationally representative database and with a much larger sample size, found a mean 2-year total cost of $116,330, consistent with being approximately double the annual cost found by Guptill et al. An older study quantified the cost-of-illness of CIDP in 2008 in southeast England [13]. The total annual cost-of-illness per patient was £22,085 for CIDP. The use of IVIg was the most important determinant of cost.
We did not evaluate costs of specific CIDP therapies in this analysis. In an exploratory analysis, we observed that patients initiating monotherapy with IVIg had higher CIDP therapy costs than patients initiating monotherapy with corticosteroids. From treatment initiation to the end of the 24-month follow-up, corticosteroid patients had mean (SD) CIDP therapy costs of about $7,900 ($35,000) while IVIg patients had mean CIDP therapy costs of $165,000 ($170,000). IVIg patients had higher costs, in part, related to longer persistence on IVIg compared to corticosteroids. The long-term clinical and economic impact of greater discontinuation of steroids compared to IVIg is unknown. Further, the claims data do not provide any insights into clinical effectiveness. Yet, our observation of high CIDP therapy costs, and high costs of IVIg specifically, highlights the importance of optimally managing the treatment of CIDP while considering both clinical benefit and costs. Setting of care has also been identified as an important cost driver for IVIg, with IVIg administration in the outpatient hospital setting associated with higher costs compared to IVIg administration in the home setting [17,18]. While we did not specifically evaluate IVIg costs, setting of care is not comprehensively recorded in the database for IVIg administration, limiting our ability to investigate this cost driver.
This study has limitations inherent to retrospective database studies, as well as to the data source and study design. Results from retrospective studies should be interpreted with understanding of their inherent limitations, and in context of results from other similar studies. Administrative databases do not provide as much clinical detail, for example, treatment outcomes or disease severity, as medical records. We relied on diagnosis and therapy codes on administrative billing claims to identify patients with CIDP; however, CIDP is often misdiagnosed [6,7] Although we required an additional CIDP diagnosis claim and/or initiation of CIDP-specific treatment to confirm the CIDP diagnosis, in the absence of clinical data (electronic medical records, chart review, etc.) to confirm CIDP, some diagnostic uncertainty still remains. There is limited visibility into healthcare resource use or prescriptions obtained outside of the plan benefit. However, 70+ plans contribute to the database and any unobserved out-of-network claims are likely to be very limited. Our study only captures the direct cost of CIDP as measured by administrative claims data. The data provide no insight into indirect costs of CIDP such as loss of productivity or unemployment, or insight into the quality of life impacts of CIDP, such as functional limitations, fatigue, pain, anxiety and depression [19]. Thus, the comprehensive societal impact of CIDP remains unknown. Continuous health plan enrollment was required for inclusion in the study to eliminate the impact of insurance coverage interruptions and to have full visibility into healthcare resource utilization and associated costs obtained through the plan benefit. This requirement may bias the analysis towards a healthier sample (by excluding patients who disenrolled due to death, changes in employment, or for other reasons during this period). And because CIDP is a chronic condition, it is possible that establishment of a treatment schedule could take several years for some patients. Thus, the economic burden of CIDP quantified in this study may represent a conservative estimate. Finally, since the study sample employed was largely commercially- or self-insured, these findings are not readily generalizable to uninsured, Medicare or Medicaid populations. Despite these limitations, our study is the first to report estimates of the healthcare resource utilization and costs associated with CIDP at a national level in the US, as well as the incremental burden of CIDP.
Our study constitutes the largest retrospective real-world database study of the economic burden of CIDP in the US and is the only study to quantify the incremental burden of CIDP relative to matched controls. Our findings suggest a substantial clinical and economic burden associated with CIDP. Over the 2-year follow-up, cases had higher healthcare resource utilization which was associated with 7.5x higher mean total healthcare costs compared to controls. This study provides important insights into the economic burden of CIDP; however, future studies are necessary to understand the economic burden among different patient populations as well as from indirect and societal perspectives. Future research is also needed to investigate optimal therapeutic strategies in CIDP to balance the costs and outcomes of therapy.
Acknowledgments
The authors take full responsibility for the content of the paper.
Data Availability
Original de-identified data used in this analysis were obtained from and are the property of IQVIA. IQVIA has restrictions prohibiting the authors from making the minimal data set publicly available. Interested researchers may contact IQVIA to apply to gain access to the study’s data in the same way the authors obtained the data (https://www.iqvia.com/contact/sf). The inclusion criteria specified in the Methods section would allow other researchers to identify the same cohort of patients we used for these analyses. The authors confirm that they did not have any special privileges to access the data that other researchers would not have.
Funding Statement
This study was financially supported by CSL Behring, King of Prussia, PA. Rajiv Mallick and Girishanthy Krishnarajah are employees and shareholders of CSL Behring. CSL Behring provided support in the form of salaries for authors RM and GK, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section. Victoria Divino and Mitch DeKoven are employees of IQVIA. IQVIA provided support in the form of salaries for authors VD and MD, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.AAN Task Force. Research criteria for diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). Neurology. 1991;41: 617–18. [PubMed] [Google Scholar]
- 2.Bouchard C, Lacroix C, Planté V, Adams D, Chedru F, Guglielmi JM, et al. Clinicopathologic findings and prognosis of chronic inflammatory demyelinating polyneuropathy. Neurology. 1999;52: 498–503. [DOI] [PubMed] [Google Scholar]
- 3.Gorson KC. An update on the management of chronic inflammatory demyelinating polyneuropathy. Ther Adv Neurol Disord. 2012;5: 359–73. 10.1177/1756285612457215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughlin RS, Dyck PJ, Melton LJ 3rd, Leibson C, Ransom J, Dyck PJ. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology. 2009;73: 39–45. 10.1212/WNL.0b013e3181aaea47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorson KC, Gooch CL. The (mis)diagnosis of CIDP: The high price of missing the mark. Neurology. 2015;85: 488–9. 10.1212/WNL.0000000000001838 [DOI] [PubMed] [Google Scholar]
- 6.Allen J, Lewis R. CIDP diagnostic pitfalls and perceptions of patient benefit. Neurology 2015;85: 498–504. 10.1212/WNL.0000000000001833 [DOI] [PubMed] [Google Scholar]
- 7.Van den Bergh PY, Hadden RD, Bouche P, Cornblath DR, Hahn A, Illa I, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. Eur J Neurol. 2010;17: 356–63. 10.1111/j.1468-1331.2009.02930.x [DOI] [PubMed] [Google Scholar]
- 8.United Healthcare. Immune Globulin (IVIG and SCIG). 1 Feb 2017. Available from: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Drug%20Policies/IVIG_policy.pdf. Cited 31 Jan 2018.
- 9.Cigna. Cigna Drug and Biologic Coverage Policy. 15 Oct 2017. Available from: https://cignaforhcp.cigna.com/public/content/pdf/coveragePolicies/pharmacy/ph_5026_coveragepositioncriteria_Immune_Globulin_Intravenous_IGIV.pdf. Cited 31 Jan 2018.
- 10.Köller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. N Engl J Med. 2005;352: 1343–56. 10.1056/NEJMra041347 [DOI] [PubMed] [Google Scholar]
- 11.Patwa HS, Chaudhry V, Katzberg H, Rae-Grant AD, So YT. Evidence-based guideline: intravenous immunoglobulin in the treatment of neuromuscular disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2012;78: 1009–15. 10.1212/WNL.0b013e31824de293 [DOI] [PubMed] [Google Scholar]
- 12.Guptill JT, Bromberg MB, Zhu L, Sharma BK, Thompson AR, Krueger A, et al. Patient demographics and health plan paid costs in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2014;50: 47–51. 10.1002/mus.24109 [DOI] [PubMed] [Google Scholar]
- 13.Mahdi-Rogers M, McCrone P, Hughes RA. Economic costs and quality of life in chronic inflammatory neuropathies in southeast England. Eur J Neurol. 2014;21: 34–9. 10.1111/ene.12245 [DOI] [PubMed] [Google Scholar]
- 14.McNicol ED, Midbari A, Eisenberg E. Opioids for neuropathic pain. Cochrane Database Syst Rev. 2013;CD006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bostick GP, Toth C, Carr EC, Stitt LW, Morley-Forster P, Clark AJ. Physical Functioning and Opioid use in Patients with Neuropathic Pain. Pain Med. 2015;16: 1361–8. 10.1111/pme.12702 [DOI] [PubMed] [Google Scholar]
- 16.Centers For Disease Control And Prevention. Guideline for Prescribing Opioids for Chronic Pain: Recommendations. Available from: https://www.cdc.gov/drugoverdose/pdf/Guidelines_Factsheet-a.pdf. Cited 31 Jan 2018.
- 17.Le Masson G, Solé G, Desnuelle C, Delmont E, Gauthier-Darnis M, Puget S. Home versus hospital immunoglobulin treatment for autoimmune neuropathies: A cost minimization analysis. Brain Behav. 2018;8: e00923 10.1002/brb3.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luthra R, Quimbo R, Iyer R, Luo M. An Analysis of Intravenous Immunoglobin Site of Care: Home Versus Outpatient Hospital. Am J Pharm Benefits. 2014;6: e41–e49. [Google Scholar]
- 19.Merkies IS, Kieseier BC. Fatigue, Pain, Anxiety and Depression in Guillain-Barré Syndrome and Chronic Inflammatory Demyelinating Polyradiculoneuropathy. Eur Neurol. 2016;75: 199–206. 10.1159/000445347 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original de-identified data used in this analysis were obtained from and are the property of IQVIA. IQVIA has restrictions prohibiting the authors from making the minimal data set publicly available. Interested researchers may contact IQVIA to apply to gain access to the study’s data in the same way the authors obtained the data (https://www.iqvia.com/contact/sf). The inclusion criteria specified in the Methods section would allow other researchers to identify the same cohort of patients we used for these analyses. The authors confirm that they did not have any special privileges to access the data that other researchers would not have.