Summary
Burns are considered mostly a serious illness with devastating consequences and prolonged length of hospital stay. Moreover, burns are among the traumatic lesions with the highest costs for care due to their hospitalization time, treatment required and the need for rehabilitation therapy. This study aims to evaluate the factors affecting length of hospital stay and mortality rates in acute burn patients. The study was conducted on 82 patients who presented with acute burn and were admitted to the Plastic and Reconstructive Surgery Department Burn Unit, Tanta University Hospital, in the period from June 2016 to June 2017. A prospective study was carried out using the data of acutely burned patients, and a statistical analysis conducted with the data collected on different factors affecting burn patient length of hospital stay and mortality. The mean age of our patients was 16.5 years, mean LOS was 24.23 days and mortality rate was 9.8% of the total admitted cases, with half of the cases with inhalation injury dying in hospital. The most influencing factors on prediction of length of hospital stay were: incidence of infection, wound depth, TBSA% and inhalation injury. The most influencing factors on patient mortality were: TBSA%, age of the patient, cause of burn and inhalation injury, therefore these factors should be carefully evaluated in every burn patient.
Keywords: burn, prognostic, hospital stay, mortality
Abstract
Les brûlures sont considérées comme une pathologie grave aux conséquences dévastatrices responsables d’hospitalisations prolongées. En outre, elles font partie des pathologies traumatiques responsables des coûts de prise en charge les plus élevés, en raison de la durée d’hospitalisation, des traitements nécessaires et de la nécessité de rééducation. Cette étude a pour but d’évaluer les facteurs influant sur la durée de séjour et la mortalité chez les brûlés. Elle a été réalisée chez 82 brûlés hospitalisés dans l’unité de brûlés du service de chirurgie plastique et reconstructrice du CHU Tanta entre juin 2016 et juin 2017. Les données des patients ont été recueillies de manière prospective et différents paramètres supposés prédictifs ont été analysés. L’âge moyen était de 16,5 ans; la durée de séjour de 24,23 jours et la mortalité de 9,8%. La moitié des brûlés avec lésion d’inhalation sont décédés. Les facteurs influant le plus clairement la durée de séjour sont la survenue d’une infection, la profondeur, la surface et l’inhalation de fumées. Ceux influant sur la mortalité sont la surface brûlée, l’âge, l’agent causal et l’inhalation. Ces facteurs doivent donc être précisément évalués chez tout brûlé.
Introduction
Burns are considered mostly a serious illness with devastating consequences and prolonged length of hospital stay. Moreover, burns are among the traumatic lesions with the highest costs for care, due to hospitalization time, treatment required and the need for rehabilitation therapy.1,2
Besides being a personal catastrophe for the patient, a severe burn is also a medical problem and an economic burden on the national health services. Burns are among the most expensive non-fatal injuries3 and account for a substantial direct economic loss.4,5 In the United States, burns are the fourth leading cause of death from accidental injury.6
Over 90% of deaths from burns caused by fire occur in middle- and low-income countries. Millions of people, mostly with a low socioeconomic status, suffer disability and disfigurement, with psychological, social and economic consequences for the survivors and their families.7
Careful evaluation of clinical risk factors has improved our understanding of morbidity and mortality after burn trauma. The size of the burn, the patient’s age, and the presence of inhalation injury have been shown to contribute to mortality.8
The aim of this work is to evaluate the factors affecting length of hospital stay (LOS) and mortality rates in acute burn patients.
Materials and methods
This study was conducted on 82 patients who presented with acute burn at the Plastic and Reconstructive Surgery Department Burn Unit, Tanta University Hospital, in the period from June 2016 to June 2017. A prospective study of the acutely burned patients was carried out with statistical analysis of the collected data regarding the different factors affecting patient LOS and mortality. We included acutely burned patients with one or more of the following criteria for admission to our burn unit: partial-thickness burns of 10% total body surface area (TBSA) or more; isolated burns that involved the face, hands, feet, ears, genitalia, perineum or major joints; full thickness burn of 5% TBSA or more; electrical burns of any size; chemical burns; suspected inhalation injury; burns with associated co-morbidities (such as stroke, diabetes, hepatic disease, renal disease, cardiac disease, psychiatric/neurologic disease and neoplastic disorders); burns with associated trauma (traumatic brain injury and fractures) and burns associated with social problems (abuse, attempted suicide and lack of social conditions). We excluded the acutely burned patient who wouldn’t be admitted.
The collected data were organized, tabulated and statistically analyzed using SPSS software (Statistical Package for the Social Sciences, version 19, SPSS Inc. Chicago, IL, USA). For quantitative data, the range, mean and standard deviation were calculated. Boxplots were performed to illustrate median, upper and lower limits of the range, first and third quartiles and median of the quantitative data. For qualitative data, which describe a categorical set of data by frequency, percentage or proportion of each category, comparison between two groups and more was done using the Chi-square test ( 2). For comparison between means of two groups of parametric data of independent samples, the student t-test was used. To predict the presence or absence of an outcome based on a set of predictor variables, logistic regression was done, where the dependent variable was dichotomous. Logistic regression coefficients (B) were used to estimate Odds ratios (EXP (B)) for each of the independent variables. Significance was adopted at p > 0.05 for interpretation of results of tests of significance.
Results
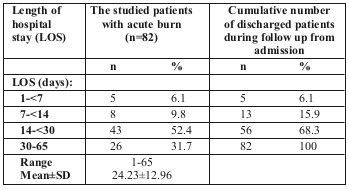
The total number of cases admitted to Tanta University Burn Unit from June 2016 to June 2017 was 82, with a mean hospitalization period of 24.23 days (Table I). In our study, the mean LOS was 24.23 days.
Table I. Length of hospital stay of the studied patients with acute burn (n=82).
We saw a higher incidence of children with burns (48 cases), representing 59.8% of the total number of cases, and only 34 adults, representing 40.2% of cases. The mean age of our cases was 16.5 years. More male patients than females were admitted, with a male/female ratio of 1:1.5. Relationship between LOS and age and sex of the studied patients is shown in Table II.
Table II. Relationship between LOS and age and sex of the studied patients with acute burn (n=82).
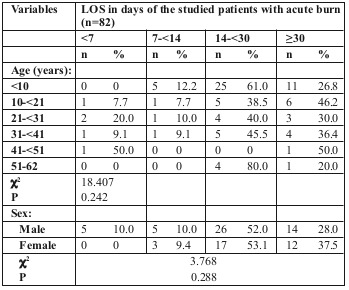
LOS was longer for flame burn cases, followed by scald burn then electric burn. The difference was statistically significant (Table III and Fig. 1).
Table III. Relationship between LOS and causes of burn injury among the studied patients with acute burn (n=82).
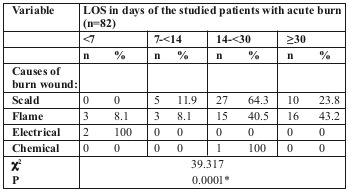
Fig. 1. Relationship between LOS and causes of burn injury among the studied patients with acute burn in box plot (n=82).
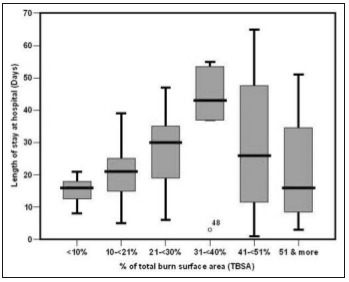
We noticed that patients with the longest LOS (>30 days) mainly had a TBSA burned of 31-41%, followed by those with 21-31% TBSA burned, as shown in Table IV and Fig. 2.
Table IV. Relationship between LOS and percentage of total burn wound surface area among the studied patients with acute burn (n=82).
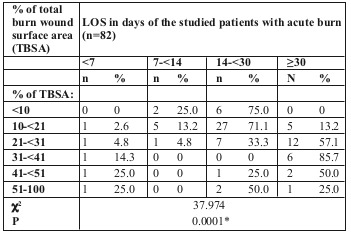
Fig. 2. Relationship between LOS and percentage of total burn wound surface area among the studied patients with acute burn in box plot (n=82).
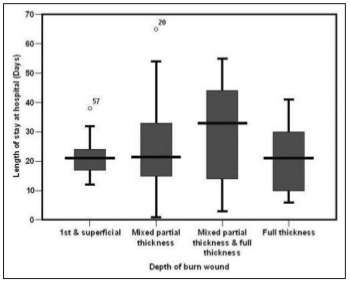
We found a statistically significant difference in LOS for the different degrees of burn depth, the majority of cases staying > 30 days having mixed partial thickness and full thickness followed by full thickness and mixed partial thickness burns (Table V and Fig. 3).
Table V. Relationship between LOS and depth of burn wound among the studied patients with acute burn (n=82).
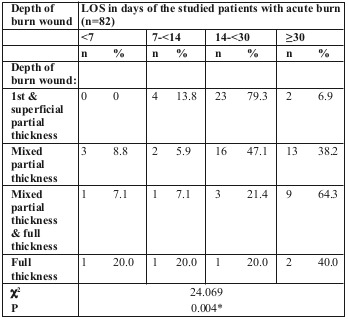
Fig. 3. Relationship between LOS and depth of burn wound among the studied patients with acute burn in box plot (n=82).
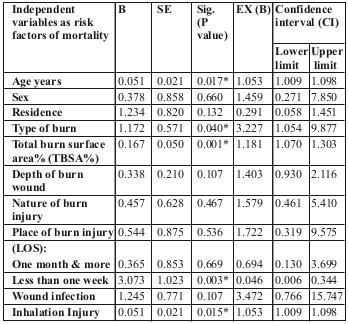
Binary regression analysis of the different variables among the studied patients, such as predictors for length of hospital stay (one month or more) and mortality, are shown in Tables VI and VII respectively.
Table VI. Binary regression analysis of the different variables as predictors for LOS (one month or more) among the studied patients with acute burn (n=82).
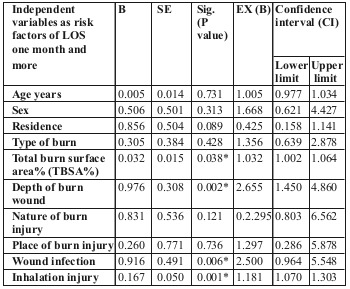
Table VII. Binary regression analysis of the different variables as predictors for mortality among the studied patients with acute burn (n=82).
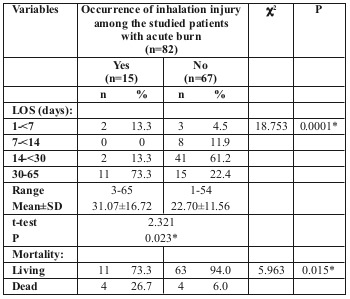
We found that the presence of inhalation injury greatly affected LOS (P>0.05), the mean hospital stay of patients with inhalation injury being about 31.07 days, while in the absence of inhalation injury the length of stay dropped to 22.70 days. Mortality also was greatly affected, as 50% of those who died were diagnosed with inhalation injury (Table VIII).
Table VIII. Relationship between inhalation injury and LOS and mortality among the studied patients with acute burn (n=82).
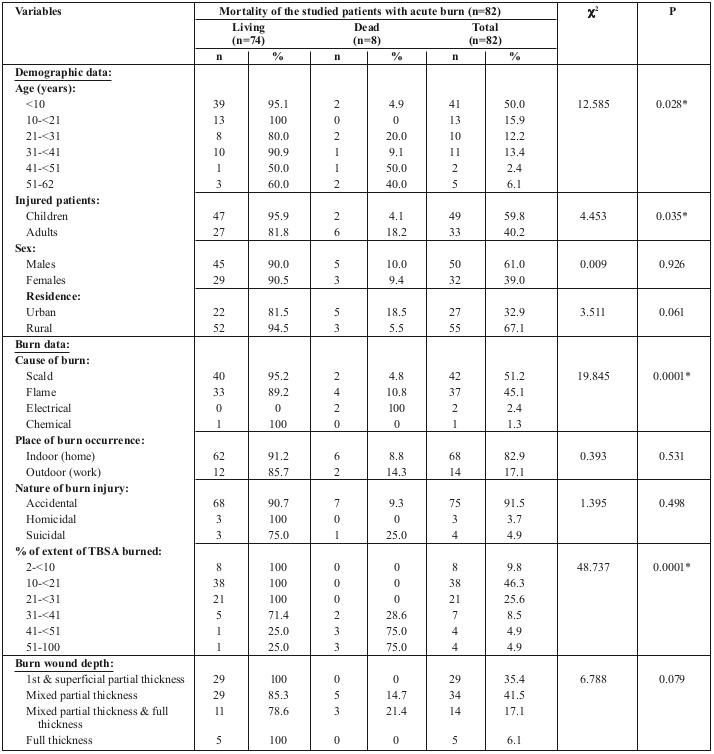
The mortality rate in our study was 9.8% of total admitted cases. Table IX shows the relationship between mortality of our studied patients and their other variables, with a statistically significant difference in mortality between the different age groups and the different causes of burn, as well as increasing TBSA% burned.
Table IV. Relationship between mortality of the studied patients with acute burn and their other variables (n=82).
Discussion
In our study, mean LOS was 24.23 days, while in a study at the Washington University Burn Centre it was 16.3 days.9
This could be explained by the higher TBSA burned in our study (22.88%) in comparison to the American study. Moreover, we had more cases with inhalation injury (18.3%) compared to their study (11.3%). Their mortality rate was 18.5% of total admitted cases compared to 9.8% in our study.
Hussain and Dunn conducted a systematic review of the literature on factors affecting LOS and found that the strongest predictors are the patient’s age and TBSA% burned, followed by full thickness burn%, female gender, inhalation injury, surgery including escharotomy and burn depth.10
We performed binary regression analysis of the different variables as predictors for LOS (one month or more) and mortality among our studied patients with acute burn. It showed that the most influencing factors for LOS were incidence of infection wound depth, TBSA% and inhalation injury. The most influencing factors for patient mortality were TBSA%, age of the patient, cause of burn and inhalation injury.
We found no significant relationship between LOS and patient age and sex. Rachel et al. also found that the total length of stay did not differ among different age groups.9 In contrast, other authors found that older patients stayed in hospital longer.8,11, 12,13,14
Mortality incidence was less than 5% in the under-10-yearolds, while it reached 50% in the 41-50 age group, and 40% in the 51-60 age group. There were no recorded deaths in the 10-21 age group, which means that mortality rates increase with age. This can be explained by the decreased healing power in the elderly burn patient population compared to children, and their decreased physiologic reserves.15
According to the University of Washington Burn Center, age alone (independent of comorbidities) was associated with risk of hospital mortality.9 Increased burn mortality with advancing age has also been reported by other authors.8,9,11,12,14,16,17,18,19,20,21,22,23,24 On the other hand, some studies found no significant relationship between age and mortality.24
Regarding patient gender, it was found to have no significant effect on LOS in our study and also in the study by Bartosch et al.,14 while Khaliq et al. found males to have a longer LOS.8 We did not find any significant effect of gender on burn mortality, and the same has been reported by some other authors. 14,22 On the contrary, many other authors found a significant relationship between gender and mortality, with all of them reporting increased burn mortality in female patients. 8,18,19,20,21,23,25 This increased mortality in female patients was explained by the suppressed cell-mediated immune response following thermal injury in females in an animal study.26
We found a strong statistical relationship between LOS and mortality and the causes of burn injury among the studied patients with acute burn: 88.1% of scald burn and 83.7% of flame burn cases stayed in hospital for more than two weeks before discharge. Khaliq et al. found a significantly higher LOS for thermal and electrical burns than other causes of burn,8 while Bartosch et al. found no relationship between the cause of burn and LOS in hospital.14 The effect of the cause of burn on mortality was a different situation, with the highest mortality in our cases occurring in electrical burns followed by thermal burns, while many other authors reported thermal burns to take 1st place,19,27 and others showed no effect of the cause of burn on mortality.21
The incidence of inhalation injury in our study was 18.29% and this caused a statistically significant increase in LOS in hospital with a mean of 31.07 days and 22.70 days in the presence and absence of inhalation injury, respectively. The same was reported by Taylor et al.11 and Khaliq et al.,8 while Bartosch et al. found inhalation injury to have no significant effect on LOS.14 There was a statistically significant difference in mortality rate between cases with (26.7%) and without (6%) inhalation injury, with 50% of those who died having been diagnosed with inhalation injury before their death. This relationship has been reported to be significant in many studies8,9,11,14,16,17,23,24,28 while Yang et al. found it insignificant, and they correlated mortality with age >60 years, female sex, TBSA>50% and plasma and urine levels of neutrophil gelatinase-associated lipocalin.18 Our study verified that with the increase in TBSA%, LOS is also increased up to 40% TBSA. After that, LOS relatively decreased by 15% due to an increased mortality rate above 40% TBSA. This finding is in accordance with reports by previous authors.8,11,13,24 LOS was found to increase by 1.406 days for each 1% of TBSA burned.14 Total burn size (TBSA%) was independently related to the overall LOS. Baseline comorbidities, independent of hospital complications, were not associated with increased LOS.9 The significant impact of increased TBSA burned on mortality that was observed in our study confirms what has been previously reported, without controversy, in the literature,8,9,11,14,16,17,18,19,20,22,23,24 and it has been reported to be the strongest predictor of mortality after adjusting for age and mechanism of burn.29
We found that LOS has a statistically significant proportional relationship to the depth of burn, which has been reported also in other studies.12,13 Increased burn wound thickness was also reported to increase mortality,12,21 but in our study we found this to be insignificant.
Gomez et al. performed autopsies on burn cases who died and found that the causes of death were: infection (61%); disorders of the pulmonary (55%), cardiac (36%), renal (27%), gastrointestinal (27%) and central nervous (11%) systems; and multi-organ dysfunction (15%).29 Nitzschke et al. found a significant difference in mortality between patients with healed and non-healed wounds at day 20 following burn.30
The mortality rate at the Rotterdam Burn Centre was 6.9%; the most frequent cause of death appeared to be multisystem organ failure in 64.9% of cases (93% of these had systemic inflammatory response syndrome at the time of death), and in 45.9%, infection was deemed responsible for the fatal clinical deterioration (in 21.3% sepsis was proven and in 24.6% it was highly suspected).31
There are several potential limitations to this study. We did not examine the impact of management decisions such as time to operate, use of skin substitutes, and limb amputation on outcome. We didn’t perform a long-term follow up of patients to detect post-discharge mortality and quality of life. We couldn’t account for retrospective data from previous cases due to lack of information. The predisposing factors, demographic and epidemiological features of burn injuries differ in each country. It is therefore important that Burn Units in every nation carry out their own epidemiological studies on burned patients.
Conclusion
The most influencing factors on prediction of length of hospital stay in acute burn patients were: incidence of infection, wound depth, TBSA% and inhalation injury. The most influencing factors on patient mortality were: TBSA%, age of the patient, cause of burn and inhalation injury. As regards wound infection, the most influencing factors on infection rate were: TBSA%, wound depth and length of stay.
References
- 1.Patel DD, Rosenberg L, Rosenberg M, Leal J. The epidemiology of burns in young children from Mexico treated at a U.S hospital. Burns, 2016;42:1825–1830. doi: 10.1016/j.burns.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Brusselaers N, Monstrey S, Vogelaers D, Hoste E. Severe burn injury in Europe: a systematic review of the incidence, etiology, morbidity and mortality. Crit Care. 2010;14(5):R188. doi: 10.1186/cc9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chien WC, Pai L, Lin CC, Chen HC. Epidemiology of hospitalized burns patients in Taiwan. Burns. 2003;29:582–2003. doi: 10.1016/s0305-4179(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 4.Olaitan PB, Jiburum BC. Analysis of burn mortality in a burns centre. Ann Burns Fire Disasters. 2006;19(2):59–62. [PMC free article] [PubMed] [Google Scholar]
- 5.Han TH, Kim JH, Yang MS, Han KW. A retrospective analysis of 19,157 burn patients: 18-year experience from Hallym Burn Center in Seoul, Korea. Burns. 2005;31:465–470. doi: 10.1016/j.burns.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Liao CC, Rossignol AM. Landmarks in burn prevention. Burns. 2000;26:422–434. doi: 10.1016/s0305-4179(00)00026-7. [DOI] [PubMed] [Google Scholar]
- 7.Sierra Zúñiga MF, Castro Delgado OE, Merchán-Galvis AM, Caicedo JCC. Factors associated with length of hospital stay in minor and moderate burns at Popayan, Colombia, analysis of a cohort study. Burns. 2016;42:190–195. doi: 10.1016/j.burns.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Khaliq MF, Noorani MM, Siddiqui UA, Ebran EA. Factors associated with duration of hospitalization and outcome in burns patients: a cross sectional study from Government Tertiary Care Hospital in Karachi, Pakistan. Burns. 2013;39X:150–154. doi: 10.1016/j.burns.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Lundgren RS, Kramer CB, Rivara FB, Wang J. Influence of comorbidities and age on outcome following burn injury in older adults. J Burn Care Res. 2009;30(2):307–314. doi: 10.1097/BCR.0b013e318198a416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.J Burn Care Res A, Dunn KW. Predicting length of stay in thermal burns: a systematic review of prognostic factors. Burns. 2013;39:1331–1340. doi: 10.1016/j.burns.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 11.Taylor SL, Sen S, Greenhalgh DG, Lawless L. A competing risk analysis for hospital length of stay in patients with burns. JAMA Surg. 2015;150(5):450–456. doi: 10.1001/jamasurg.2014.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton KR, Sharma VK, Harrop R, Lindsay R. A population-based study of the epidemiology of acute adult burn injuries in the Calgary Health Region and factors associated with mortality and hospital length of stay from 1995 to 2004. Burns. 2009;35:572–579. doi: 10.1016/j.burns.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Zuniga MFS, Delgado OES, Merchan-Galvis AM, Caicedo JCC. Factors associated with length of hospital stay in minor and moderate burns at Popayan, Colombia. Analysis of a cohort study. Burns. 2016;42:190–195. doi: 10.1016/j.burns.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Bartosch I, Bartosch C, Egipto P, Silva A. Factors associated with mortality and length of stay in the Oporto burn unit (2006–2009). Burns. 2013;39:477–482. doi: 10.1016/j.burns.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Demling RH. The incidence and impact of pre-existing protein energy malnutrition on outcome in the elderly burn patient population. J Burn Care Rehabil. 2005;26:94–100. doi: 10.1097/01.bcr.0000150302.71007.80. [DOI] [PubMed] [Google Scholar]
- 16.Stylianou N, Buchan I, Dunn KW. A model of British in-hospital mortality among burns patients. Burns. 2014;40(7):1316–1321. doi: 10.1016/j.burns.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Moore EC, Pilcher DV, Bailey MJ, Cleland H. simple tool for mortality prediction in burns patients: APACHE III score and FTSA. Burns. 2010;36:1086–1091. doi: 10.1016/j.burns.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Yang HT, Yim H, Cho YS, Kym D. Assessment of biochemical markers in the early post-burn period for predicting acute kidney injury and mortality in patients with major burn injury: comparison of serum creatinine, serum cystatin-C, plasma and urine neutrophil gelatinaseassociated lipocalin. Crit Care. 2014;18(4):R151. doi: 10.1186/cc13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qader AR. Burn mortality in Iraq. Burns. 2012;38:772–75. doi: 10.1016/j.burns.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Laloe V. Epidemiology and mortality of burns in a general hospital of Eastern Sri Lanka. Burns. 2002;28(8):778–781. doi: 10.1016/s0305-4179(02)00202-4. [DOI] [PubMed] [Google Scholar]
- 21.Moore EC, Pilcher DC, Bailey MJ, Stephens H. The Burns Evaluation and Mortality Study (BEAMS): predicting deaths in Australian and New Zealand burn patients admitted to intensive care with burns. J Trauma Acute Care Surg. 2013;75(2):298–303. doi: 10.1097/TA.0b013e318295409d. [DOI] [PubMed] [Google Scholar]
- 22.Steinvall I, Fredrikson M, Bak Z, Sjoberg F. Mortality after thermal injury: no sex-related difference. J Trauma. 2011;70(4):959–964. doi: 10.1097/TA.0b013e3181e59dbe. [DOI] [PubMed] [Google Scholar]
- 23.Othman N, Kendrick D. Burns in Sulaymaniyah Province, Iraq: epidemiology and risk factors for death in patients admitted to hospital. J Burn Care Res. 2011;32(4):e126–e134. doi: 10.1097/BCR.0b013e3182223ef5. [DOI] [PubMed] [Google Scholar]
- 24.Hwee J, Song C, Tan KC, Tan BK. The trends of burns epidemiology in a tropical regional burns centre. Burns. 2016;42:682–686. doi: 10.1016/j.burns.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Kerby JD, McGwin G, George RL, Cross JA. Sex differences in mortality after burn injury: results of analysis of the national burn repository of the American Burn Association. J Burn Care Res. 2006;27(4):452–456. doi: 10.1097/01.BCR.0000225957.01854.EE. [DOI] [PubMed] [Google Scholar]
- 26.Gregory MS, Faunce DE, Duffner LA, Kovacs EJ. Gender difference in cell-mediated immunity after thermal injury is mediated, in part, by elevated levels of interleukin-6. J Leukoc Biol. 2000;67(3):319–326. [PubMed] [Google Scholar]
- 27.Senel E, Yasti AC, Reis E, Doganay M. Effects on mortality of changing trends in the management of burned children in Turkey: Eight years’ experience. Burns. 2009;35:372–377. doi: 10.1016/j.burns.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 28.El-Helbawy RH, Ghareeb FM. Inhalation injury as a prognostic factor for mortality in burn patients. Ann Burns Fire Disasters. 2011;24(2):82–88. [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez R, Murray CK, Hospenthal DR, Cancio LC. Causes of mortality by autopsy findings of combat casualties and civilian patients admitted to a burn unit. J Am Coll Surg. 2009;208(3):348–354. doi: 10.1016/j.jamcollsurg.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Nitzschke SL, Aden JK, Serio-Melvin ML, Shingleton SK. Wound healing trajectories in burn patients and their impact on mortality. J Burn Care Res. 2014;35(6):474–479. doi: 10.1097/BCR.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 31.Dokter J, Felix M, Krijnen P, Vloemans JFPM. Mortality and causes of death of Dutch burn patients during the period 2006-2011. Burns. 2015;41:235–240. doi: 10.1016/j.burns.2014.10.009. [DOI] [PubMed] [Google Scholar]


