Abstract
In this study, the effect of reduced graphene oxide (rGO) on interconnected Co3O4 nanosheets and the improved supercapacitive behaviors is reported. By optimizing the experimental parameters, we achieved a specific capacitance of ~1016.4 F g−1 for the Co3O4/rGO/NF (nickel foam) system at a current density of 1 A g−1. However, the Co3O4/NF structure without rGO only delivers a specific capacitance of ~520.0 F g−1 at the same current density. The stability test demonstrates that Co3O4/rGO/NF retains ~95.5% of the initial capacitance value even after 3000 charge–discharge cycles at a high current density of 7 A g−1. Further investigation reveals that capacitance improvement for the Co3O4/rGO/NF structure is mainly because of a higher specific surface area (~87.8 m2 g−1) and a more optimal mesoporous size (4–15 nm) compared to the corresponding values of 67.1 m2 g−1 and 6–25 nm, respectively, for the Co3O4/NF structure. rGO and the thinner Co3O4 nanosheets benefit from the strain relaxation during the charge and discharge processes, improving the cycling stability of Co3O4/rGO/NF.
Keywords: Supercapacitors, rGO, Co3O4 nanosheets, Strain relaxation
Highlights
Interconnected Co3O4 nanosheets anchored on rGO-coated nickel foam (NF) are facilely synthesized using a green, simple, and low-cost approach.
Because of the high specific surface area and optimal mesopore size distribution, high specific capacitances of ~1016.4 and 767.1 F g−1 are achieved for Co3O4/rGO/NF at current densities of 1 and 5 A g−1, respectively.
Excellent stability with ~95.5% capacity retention at a high current density of 7 A g−1 is achieved even after 3000 cycles.
Introduction
In recent years, extensive efforts have been dedicated to research related to supercapacitors owing to their higher power densities, longer cycling performance than Li-ion batteries, and larger energy densities than conventional dielectric capacitors [1–3]. Supercapacitors have a huge potential in applications requiring high-density power and long cycling lifetime such as electric vehicles and portable electronics [4].
Co3O4, an important supercapacitor material, has the advantages of high theoretical capacitance (~3560 F g−1) [5], low cost, environmental friendliness, and high chemical stability in alkaline electrolytes. It has, thus, attracted much attention recently. Geng et al. [6] prepared porous Co3O4 nanoplates with a specific capacitance of ~231 F g−1 at a current density of 1 A g−1 using a facile reflux method. Naveen et al. [7] synthesized Co3O4/graphene nanosheets by a chemical method and a high specific capacitance of ~650 F g−1 at a scan rate of 5 mV s−1. However, there still remain some challenges in the practical applications of Co3O4 as a high capacity electrode such as poor conductivity and cycling stability, and relatively lower experimental specific capacitances than the theoretical value.
As one kind of nanostructured carbon material, reduced graphene oxide (rGO) has been extensively investigated because of its superior mechanical and electronic properties, high specific surface area, and reasonable chemical stability [8]. These properties make graphene a preferred material for use in supercapacitors and Li-ion batteries as electrode materials and/or active material supporters [9–16]. Therefore, supercapacitors combining nanostructured Co3O4 and rGO can be expected to deliver high power and energy densities and long cycling lifetime [15, 17].
In this study, interconnected Co3O4 nanosheets anchored onto rGO-coated nickel foam (NF) are facilely synthesized using a green, simple, and low-cost approach. The effect of rGO on the microstructure and improved supercapacitive behaviors of Co3O4 nanosheets are investigated here. Because of the large specific area of ~87.8 m2 g−1 and a more optimal mesopore size distribution of ~4–15 nm, the Co3O4/rGO/NF architecture delivers higher specific capacitances of ~1016.4 and 767.1 F g−1 at current densities of 1 and 5 A g−1, respectively. In comparison, the Co3O4/NF structure has a relatively smaller specific area of ~67.1 m2 g−1 and a less optimal mesopore size distribution of ~6–25 nm, resulting in lower specific capacitances of ~520.0 and 485.8 F g−1 at current densities of 1 and 5 A g−1, respectively. Moreover, Co3O4/rGO/NF has excellent stability, with ~95.5% capacity retention at a high current density of 7 A g−1 even after 3000 cycles. This can be attributed to the thinned Co3O4 nanosheets and the presence of rGO, improving the electrical and mechanical properties of the Co3O4/rGO/NF system. For Co3O4/NF, the capacity retention after 3000 cycles at the corresponding current density is about 84.4%.
Experimental
Preparation of the Co3O4/rGO/NF Architecture
Figure 1 shows a schematic illustration of the preparation procedure of the Co3O4/rGO/NF architecture. First, the hydrothermal reduction process is used to prepare rGO-coated NF. After sequential ultrasonic cleaning in acetone, ethanol, and deionized (DI) water each for 10 min, the NFs are dried in air. GO aqueous solution (1.0 mg mL−1) is prepared by dispersing GO, which is prepared by the modified Hummers method [18], into DI water under ultrasonication for 30 min. Then, the GO aqueous solution (10 ml, 1.0 mg mL−1), ascorbic acid (L-AA, 0.02 g), and cleaned NFs of size 2 × 3 cm2 are placed into a beaker, which is then heated up to 95 °C and kept for 5.0 h for GO reduction and rGO coating onto the NFs. After the beaker is cooled down to room temperature, the products are taken out and dried at 50 °C for 3 h [19].
Fig. 1.
Schematic of the synthesis procedures of Co3O4/rGO/NF (top) and Co3O4/NF (bottom)
Then, Co(OH)2 nanosheets are electrochemically deposited at 70 °C in a three-electrode cell using rGO-coated NF as the working electrode, a platinum mesh (surface area: 2 × 2 cm2) as the counter electrode, and Ag/AgCl (sat. KCl) as the reference electrode. The aqueous electrolyte consists of 0.02 M Co(NO3)2·6H2O and 0.2 M NH4Cl. The electrodeposition potential is set at −3.0 V. After 500 s of electrodeposition, the resultant green foam is carefully washed using ethanol and DI water several times and finally dried in air. Then, the product is calcined at 250 °C for 2 h in a quartz tube to change Co(OH)2 into interconnected Co3O4 nanosheets [20]. A reference structure of interconnected Co3O4 nanosheets anchored onto NF is prepared following a similar process but without the rGO coating procedure, to determine the role of rGO.
Characterization
The resultant products are characterized by scanning electron microscopy (SEM, MIR A3, TESCAN), high-resolution transmission electron microscopy (HRTEM, FEI Tecnai G2 F30), micro-Raman spectroscopy (Jobin–Yvon Horiba HR800 with an excitation wavelength of 532 nm), X-ray powder diffraction (XRD, X’Pert Philips), and Brunauer–Emmett–Teller measurement (BET, ASAP 2020 Micromeritics).
Cyclic voltammetry (CV), galvanostatic charge/discharge (GCD), and electrochemical impedance spectroscopy (EIS) tests are conducted using an electrochemical workstation having a three-electrode configuration. Co3O4/rGO/NF or Co3O4/NF is employed as the working electrode using a 1 M KOH electrolyte. The applied potential window of CV measurements ranges from 0.0 to 0.6 V. GCD is performed at a constant current over a fixed potential range of 0.0–0.5 V. The capacitance retention test is performed from 0.0 to 0.5 V at a constant current density of 7 A g−1 for 3000 cycles. EIS measurement is performed under an AC voltage with a 5 mV amplitude over a frequency range of 0.01-100 kHz. The specific capacitance C s (F g−1) is calculated from the GCD data using C s = (I△t)/(m△V), where I is the charge–discharge current, Δt is the discharge time, m is the mass of Co3O4, and ΔV is the potential change.
Results and Discussion
Figure 2a shows the XRD pattern of the Co(OH)2/rGO/NF structure. The peaks presented in the spectrum match well with those in the standard crystallographic spectrum of layered α-Co(OH)2 (JCPDS 46-0605) [21], except for the peaks at 44.5° and 51.8° attributed to the NF substrate (JCPDS 87-0712). Figure 2b exhibits the XRD spectra of the products of Co(OH)2/rGO/NF and Co(OH)2/NF after calcination at 250 °C for 2 h, and the XRD pattern of the rGO/NF structure serves as a reference. It is evident that except for the diffraction peaks from NF, all the other peaks are consistent with the (220), (311), (511), and (440) planes in the standard Co3O4 pattern (JCPDS 42-1467) [22]. It is worth noting that there are no obvious XRD signals of rGO, possibly because of the low mass loading or destruction of regular stacks of rGO [23]. Figure 2c shows the micro-Raman spectra of rGO/NF, Co3O4/NF, and Co3O4/rGO/NF composites. Two bands located around 1348.6 and 1609.5 cm−1 are assigned to the D and G band of rGO, respectively, indicating that GO has been successfully reduced [24]. For Co3O4-containing samples, five peaks of the crystalline Co3O4 phase corresponding to the A1g (691.5 cm−1), F2g (617.7 cm−1), F2g (522.8 cm−1), Eg (482.7 cm−1), and F2g (193.6 cm−1) modes are evident [25], and no obvious characteristic peaks related to Co(OH)2 can be observed. Accordingly, both XRD and Raman spectra indicate that Co(OH)2 is completely changed to Co3O4 after the aforementioned thermal treatment.
Fig. 2.
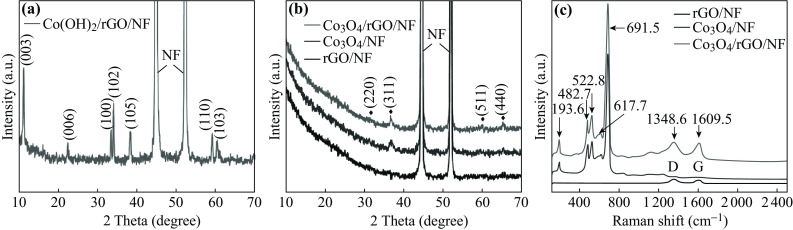
XRD patterns of a Co(OH)2/rGO/NF and b rGO/NF, Co3O4/NF, and Co3O4/rGO/NF. c Micro-Raman spectra of rGO/NF, Co3O4/NF, and Co3O4/rGO/NF composites
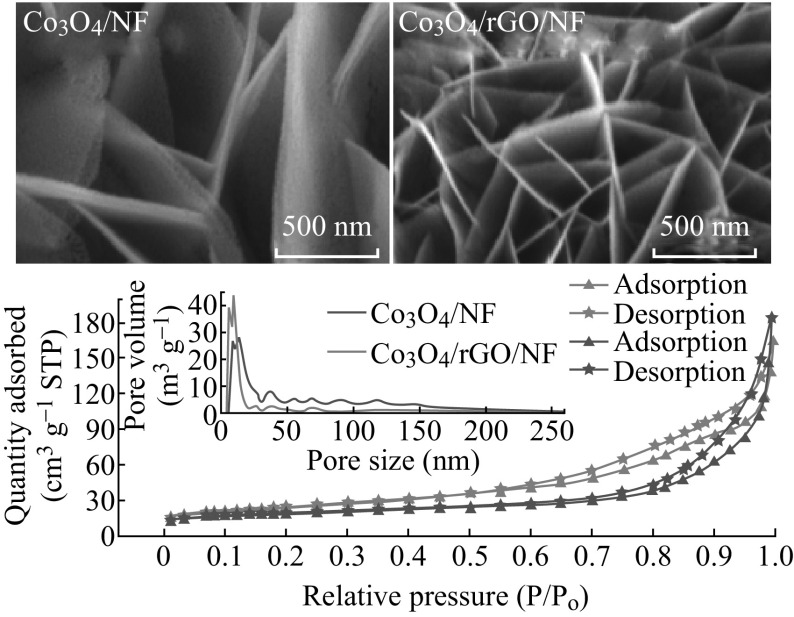
SEM images of rGO/NF, Co3O4/NF, and Co3O4/rGO/NF are shown in Fig. 3. It is obvious that the rGO coating onto NF is relatively uniform during the hydrothermal reducing process (see Fig. 3a). Figure 3b, c exhibits the top-view SEM images of Co3O4/NF and Co3O4/rGO/NF, respectively. Interconnected Co3O4 nanosheets can be synthesized on both NF and rGO-coated NF. This indicates that the growth of Co3O4 nanosheets is dependent more on the growth condition [26] rather than on the substrates. However, compared to the Co3O4 nanosheets on NF, the nanosheets on rGO-coated NF have a higher density and lower dimension because of the roughened NF surface due to rGO. Moreover, the corresponding high-magnification SEM images shown in the inset in Fig. 3b, c clearly show that the Co3O4 nanosheets on rGO-coated NF are thinner than the ones on NF. Accordingly, compared to Co3O4/NF, a higher specific surface area can be expected for Co3O4/rGO/NF. BET measurement is conducted to examine this hypothesis. Figure 3d shows the N2 adsorption–desorption isotherms of Co3O4/rGO/NF and Co3O4/NF. Two distinct hysteresis loops can be observed with a type IV sorption behavior, indicating the presence of a typical mesoporous microstructure for Co3O4/rGO/NF and Co3O4/NF. The pore size distribution of Co3O4 nanosheets on rGO (see the inset of Fig. 3d) indicates that the size of most pores is ~4–15 nm. In contrast, the size of most pores of the Co3O4 nanosheets on NF is 6–25 nm. The BET specific areas for Co3O4 nanosheets on rGO and on NF are ~87.8 and 67.1 m2 g−1, respectively. Accordingly, a higher specific capacitance can be expected for the Co3O4/rGO/NF structure.
Fig. 3.
Top-view SEM images of a rGO/NF, b Co3O4/NF, and c Co3O4/rGO/NF. d N2 adsorption–desorption isotherms of Co3O4/rGO/NF and Co3O4/NF. The inset in d shows the pore size distribution of both samples
To reveal further details of the microstructure of the Co3O4 nanosheets in Co3O4/rGO/NF, HRTEM characterization is performed. As demonstrated in Fig. 4a, the Co3O4 nanosheets are composed of small Co3O4 nanoparticles of size ~5–15 nm. The lattice fringes as exhibited in Fig. 4b show an interplanar spacing of 0.243 and 0.204 nm, attributed to the (311) and (400) planes of cubic Co3O4 [27, 28]. The polycrystalline structure of the obtained Co3O4 nanosheets is further confirmed by the selected area electron diffraction (SAED) pattern (see Fig. 4c). The homocentric diffraction rings (from the inside to the outside) can be assigned to the (220), (311), (400), (511), and (440) planes of Co3O4 [29]. The elemental distribution in the Co3O4 nanosheets is also characterized by scanning transmission electron microscopy (STEM) (see Fig. 4d) and X-ray elemental mapping images (see Fig. 4e–g). The elemental mapping further indicates that Co3O4 nanosheets were synthesized successfully.
Fig. 4.
a Low- and b high-magnification TEM images, c SAED patterns of a Co3O4 nanosheet taken from Co3O4/rGO/NF. d STEM image, and elemental mapping images of e and f Co and g O
Figure 5a presents the CV data for NF, rGO/NF, Co3O4/NF, and Co3O4/rGO/NF under a scanning rate of 3 mV s−1 and a potential range of 0.0–0.6 V. It is remarkable that the CV area of Co3O4/rGO/NF is evidently larger than that of Co3O4/NF owing to the high specific area of Co3O4/rGO/NF as mentioned above. The redox peaks for the Co3O4-containing samples originate from the conversion of different cobalt oxidation states. Here, it is worth noting that two redox peaks also emerge for NF and rGO/NF, which are attributed to the oxidized surface of NF during heat treatment of Co(OH)2. However, the contribution of the surface nickel oxide and rGO is not taken into consideration in the following study because of their negligible capacities compared to the Co3O4-containing samples. This is verified by the calculated areal specific capacitances of 84.0, 120.7, 627.3, and 1107.5 mF cm−2 for NF, rGO/NF, Co3O4/NF, and Co3O4/rGO/NF, respectively, as exhibited in Fig. 5b. The GCD results of Co3O4/NF and Co3O4/rGO/NF are exhibited in Fig. 5c, d with current densities of 1, 3, 5, 7, 9, and 10 A g−1 and a potential window of 0.0–0.5 V. For each charge/discharge current density, Co3O4/rGO/NF exhibits a larger specific capacitance than Co3O4/NF. The specific capacitance of Co3O4/rGO/NF is ~1016.4, 872.3, 767.1, 707.2, 657.3, and 633.0 F g−1 at the corresponding current density of 1–10 A g−1. As a distinct comparison, the Co3O4/NF electrode exhibits specific capacitances of 520.0, 513.7, 485.8, 456.8, 444.5, and 435.5 F g−1 at the corresponding current densities. Moreover, compared to other previously reported Co3O4/rGO composites with different Co3O4 microstructures [7, 16, 30], the Co3O4/rGO/NF electrode reported here also delivers superior specific capacitances especially at higher current densities. Here, it is worth noting that the specific capacitance decreases as the charge/discharge current density increases for both Co3O4/rGO/NF and Co3O4/NF. This is because of the insufficient supply of active material [31] and severe polarization [32] at higher current densities. Hence, how to improve the supercapacitive behaviors from these two aspects will be the main consideration in a future study.
Fig. 5.

a CV curves of NF, rGO/NF, Co3O4/NF, and Co3O4/rGO/NF composites at 3 mV s−1. b Charge–discharge curves of NF, rGO/NF, Co3O4/NF, and Co3O4/rGO/NF composites at 5 mA cm−2. Charge–discharge curves of c Co3O4/NF and d Co3O4/rGO/NF at different current densities. e Cycling performance of Co3O4/rGO/NF at a current density of 7 A g−1 (the inset is a top-view SEM image of Co3O4/rGO/NF after the 3000 cycles shown in e. f Nyquist plots of Co3O4/rGO/NF and Co3O4/NF
To evaluate the cycling performance, the electrochemical stability of Co3O4/rGO/NF and Co3O4/rGO is tested using the GCD technique. The cycling performance of Co3O4/rGO/NF at a current density of 7 A g−1 is recorded over the potential range of 0.0–0.5 V (see Fig. 5e). For the Co3O4/rGO/NF structure, ~95.5% of the initial specific capacitance can be retained after 3000 cycles even at such a high current density compared to other related reports [7, 33]. However, ~84.4% of the initial specific capacitance is retained after the same cycling period for Co3O4/rGO. To understand this excellent cycling stability, SEM characterization is performed on the Co3O4/rGO/NF sample after 3000 cycles and is shown in the inset of Fig. 5e. It is obvious that even after 3000 cycles, the microstructure is maintained very well, showing no evident change compared to the microstructures before cycling (see Fig. 3c). It is noted that during the first cycle, there is a significant increase in the specific capacitance. Previous reports mentioned this phenomenon as an activation process [34]. However, such a strong ‘activation’ has been rarely reported and needs to be further investigated to reveal the underlying mechanism.
EIS is used to further understand the electrical properties of the related material/structural system. Figure 5f shows the Nyquist plots of Co3O4/rGO/NF and Co3O4/NF. The linear portion of the Nyquist plots in the low-frequency region corresponds to the Warburg impedance, which is related to electrolyte diffusion into the pores in the electrodes. If the impedance plot increases sharply and tends to become a vertical line, it indicates a pure capacitive behavior. In the high-frequency region, the Z’-intercept represents the equivalent series resistance (ESR) including the ionic resistance of the electrolyte, intrinsic resistance of the substrate, and contact resistance at the interface of the active material and current collector [35]. The ESR of Co3O4/rGO/NF and Co3O4/NF is ~1.18 and 1.22 Ω, respectively (see the inset in Fig. 5f), indicating a lower solution resistance and Faradaic resistance for the Co3O4/rGO/NF architecture. In this study, the improved performance of Co3O4/rGO/NF is mainly attributed to the introduction of rGO, which not only provides effective electrolyte accessible channels, thus shortening the ion diffusion distance, but also improves the specific surface area of Co3O4 and optimizes the mesopore size distribution for facilitating an enhancement in the capacitance performance.
Conclusions
This work reports the effect of rGO on the interconnected Co3O4 nanosheets and the improved supercapacitive behaviors. It is found that rGO can help to optimize the microstructures of the interconnected Co3O4 nanosheets, including an increased specific surface area and a more optimal mesopore size distribution, which result in the specific capacitance of the Co3O4/rGO/NF architecture being higher that that of the Co3O4/NF structure. Further, the Co3O4/rGO/NF structure possesses excellent cycling stability owing to the improved mechanical and electrical properties associated with the thinned Co3O4 nanosheets and incorporation of rGO.
Acknowledgements
This work is financially supported from the National Natural Science Foundation of China (Grant Nos.: 61376068, 11304132, 11304133, and 11405144), the Specialized Research Fund of the Doctoral Program of Higher Education (Grant Nos.: 20120211120003 and 20130211120009), and the Fundamental Research Funds for the Central Universities (Grant Nos.: lzujbky-2013-36 and lzujbky-2014-30).
Contributor Information
Junshuai Li, Email: jshli@lzu.edu.cn.
Deyan He, Email: hedy@lzu.edu.cn.
References
- 1.Simon P, Gogotsi Y. Materials for electrochemical capacitors. Nat. Mater. 2008;7(11):845–854. doi: 10.1038/nmat2297. [DOI] [PubMed] [Google Scholar]
- 2.Wang G, Zhang L, Zhang J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012;41(2):797–828. doi: 10.1039/C1CS15060J. [DOI] [PubMed] [Google Scholar]
- 3.Li D, Gong Y, Wang M, Pan C. Preparation of sandwich-like NiCo2O4/rGO/NiO heterostructure on nickel foam for high performance supercapacitor electrodes. Nano-Micro Lett. 2017;9(2):16. doi: 10.1007/s40820-016-0117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaempgen M, Chan CK, Ma J, Cui Y, Gruner G. Printable thin film supercapacitors using single-walled carbon nanotubes. Nano Lett. 2009;9(5):1872–1876. doi: 10.1021/nl8038579. [DOI] [PubMed] [Google Scholar]
- 5.Deng J, Kang L, Bai G, Li Y, Li P, Liu X, Yang Y, Gao F, Liang W. Solution combustion synthesis of cobalt oxides (Co3O4 and Co3O4/CoO) nanoparticles as supercapacitor electrode materials. Electrochim. Acta. 2014;132:127–135. doi: 10.1016/j.electacta.2014.03.158. [DOI] [Google Scholar]
- 6.Geng T, Zhang L, Wang H, Zhang K, Zhou X. Facile synthesis of porous Co3O4 nanoplates for supercapacitor applications. Bull. Mater. Sci. 2015;38(5):1171–1175. doi: 10.1007/s12034-015-0997-6. [DOI] [Google Scholar]
- 7.Naveen AN, Manimaran P, Selladurai S. Cobalt oxide (Co3O4)/graphene nanosheets (GNS) composite prepared by novel route for supercapacitor application. J. Mater. Sci. Mater. Electron. 2015;26(11):8988–9000. doi: 10.1007/s10854-015-3582-2. [DOI] [Google Scholar]
- 8.Yang Z, Gao R, Hu N, Chai J, Cheng Y, Zhang L, Wei H, Kong ES-W, Zhang Y. The prospective two-dimensional graphene nanosheets: preparation, functionalization, and applications. Nano-Micro Lett. 2012;4(1):1–9. doi: 10.1007/BF03353684. [DOI] [Google Scholar]
- 9.Chen Y, Zhang X, Yu D, Ma Y. High performance supercapacitors based on reduced graphene oxide in aqueous and ionic liquid electrolytes. Carbon. 2011;49(2):573–580. doi: 10.1016/j.carbon.2010.09.060. [DOI] [Google Scholar]
- 10.Liu WW, Yan XB, Lang JW, Peng C, Xue QJ. Flexible and conductive nanocomposite electrode based on graphene sheets and cotton cloth for supercapacitor. J. Mater. Chem. 2012;22(33):17245–17253. doi: 10.1039/c2jm32659k. [DOI] [Google Scholar]
- 11.Dong L, Xu C, Yang Q, Fang J, Li Y, Kang F. High-performance compressible supercapacitors based on functionally synergic multiscale carbon composite textiles. J. Mater. Chem. A. 2015;3(8):4729–4737. doi: 10.1039/C4TA06494A. [DOI] [Google Scholar]
- 12.Guo X, Qin S, Bai S, Yue H, Li Y, Cheng Q, Li J, He D. Vertical graphene nanosheets synthesized by thermal chemical vapor deposition and the field emission properties. J. Phys. D-Appl. Phys. 2016;49(38):385301. doi: 10.1088/0022-3727/49/38/385301. [DOI] [Google Scholar]
- 13.Jost K, Perez CR, McDonough JK, Presser V, Heon M, Dion G, Gogotsi Y. Carbon coated textiles for flexible energy storage. Energy Environ. Sci. 2011;4(12):5060–5067. doi: 10.1039/c1ee02421c. [DOI] [Google Scholar]
- 14.Chen P, Su Y, Liu H, Wang Y. Interconnected tin disulfide nanosheets grown on graphene for Li-ion storage and photocatalytic applications. ACS Appl. Mater. Interfaces. 2013;5(22):12073–12082. doi: 10.1021/am403905x. [DOI] [PubMed] [Google Scholar]
- 15.Xiang C, Liu M, Zhi M, Manivannan A, Wu N. A reduced graphene oxide/Co3O4 composite for supercapacitor electrode. J. Power Sources. 2013;226:65–70. doi: 10.1016/j.jpowsour.2012.10.064. [DOI] [Google Scholar]
- 16.Yuan C, Long Y, Hou L, Li J, Sun Y, Zhang X, Lu X, Xiong S, Lou XWD. Flexible hybrid paper made of monolayer Co3O4 microsphere arrays on rGO/CNTs and their application in electrochemical capacitors. Adv. Funct. Mater. 2012;22(12):2560–2566. doi: 10.1002/adfm.201102860. [DOI] [Google Scholar]
- 17.Nguyen TT, Nguyen VH, Deivasigamani RK, Kharismadewi D, Iwai Y, Shim J-J. Facile synthesis of cobalt oxide/reduced graphene oxide composites for electrochemical capacitor and sensor applications. Solid State Sci. 2016;53:71–77. doi: 10.1016/j.solidstatesciences.2016.01.006. [DOI] [Google Scholar]
- 18.Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM. Improved synthesis of graphene oxide. ACS Nano. 2010;4(8):4806–4814. doi: 10.1021/nn1006368. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Zhang W, Li D, Gao L, Hou C, Zhang Y, Liu Y. Facile synthesis of MnO2/rGO/Ni composite foam with excellent pseudocapacitive behavior for supercapacitors. J. Alloys Compd. 2015;649:579–584. doi: 10.1016/j.jallcom.2015.07.212. [DOI] [Google Scholar]
- 20.Zou Y, Kinloch IA, Dryfe RAW. Mesoporous vertical Co3O4 nanosheet arrays on nitrogen-doped graphene foam with enhanced charge-storage performance. ACS Appl. Mater. Interfaces. 2015;7(41):22831–22838. doi: 10.1021/acsami.5b05095. [DOI] [PubMed] [Google Scholar]
- 21.Zhong J-H, Wang A-L, Li G-R, Wang J-W, Ou Y-N, Tong Y-X. Co3O4/Ni(OH)2 composite mesoporous nanosheet networks as a promising electrode for supercapacitor applications. J. Mater. Chem. 2012;22(12):5656–5665. doi: 10.1039/c2jm15863a. [DOI] [Google Scholar]
- 22.Dong X-C, Xu H, Wang X-W, Huang Y-X, Chan-Park MB, Zhang H, Wang L-H, Huang W, Chen P. 3D Graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano. 2012;6:3206–3213. doi: 10.1021/nn300097q. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Wang X, Xiao X, Xu F, Sun Y, Li Z. Reduced graphene oxide/nickel cobaltite nanoflake composites for high specific capacitance supercapacitors. Electrochim. Acta. 2013;111:937–945. doi: 10.1016/j.electacta.2013.08.094. [DOI] [Google Scholar]
- 24.Athouël L, Moser F, Dugas R, Crosnier O, Bélanger D, Brousse T. Variation of the MnO2 birnessite structure upon charge/discharge in an electrochemical supercapacitor electrode in aqueous Na2SO4 electrolyte. J. Phys. Chem. C. 2008;112(18):7270–7277. doi: 10.1021/jp0773029. [DOI] [Google Scholar]
- 25.Varghese B, Teo CH, Zhu Y, Reddy MV, Chowdari BVR, Wee ATS, Tan VBC, Lim CT, Sow CH. Co3O4 nanostructures with different morphologies and their field-emission properties. Adv. Funct. Mater. 2007;17(12):1932–1939. doi: 10.1002/adfm.200700038. [DOI] [Google Scholar]
- 26.Jagadale AD, Kumbhar VS, Bulakhe RN, Lokhande CD. Influence of electrodeposition modes on the supercapacitive performance of Co3O4 electrodes. Energy. 2014;64:234–241. doi: 10.1016/j.energy.2013.10.016. [DOI] [Google Scholar]
- 27.Yuan C, Yang L, Hou L, Shen L, Zhang X, Lou XW. Growth of ultrathin mesoporous Co3O4 nanosheet arrays on Ni foam for high-performance electrochemical capacitors. Energy Environ. Sci. 2012;5(7):7883–7887. doi: 10.1039/c2ee21745g. [DOI] [Google Scholar]
- 28.Hong W, Wang J, Li Z, Yang S. Fabrication of Co3O4@Co-Ni sulfides core/shell nanowire arrays as binder-free electrode for electrochemical energy storage. Energy. 2015;93:435–441. doi: 10.1016/j.energy.2015.09.053. [DOI] [Google Scholar]
- 29.Min S, Zhao C, Chen G, Zhang Z, Qian X. One-pot hydrothermal synthesis of 3D flower like rGO/Co3O4/Ni(OH)2 composite film on nickel foam for high-performance supercapacitors. Electrochim. Acta. 2014;135:336–344. doi: 10.1016/j.electacta.2014.05.032. [DOI] [Google Scholar]
- 30.Yuan CZ, Zhang L, Hou L, Pang G, Oh W-C. One-step hydrothermal fabrication of strongly coupled Co3O4 nanosheets–reduced graphene oxide for electrochemical capacitors. RSC Adv. 2014;4(28):14408–14413. doi: 10.1039/C4RA00762J. [DOI] [Google Scholar]
- 31.Zhu YG, Wang Y, Shi Y, Huang ZX, Fu L, Yang HY. Phase transformation induced capacitance activation for 3D graphene-CoO nanorod pseudocapacitor. Adv. Energy Mater. 2014;4(9):1079–1098. doi: 10.1002/aenm.201301788. [DOI] [Google Scholar]
- 32.Wang B, He X, Li H, Liu Q, Wang J, Yu L, Yan H, Li Z, Wang P. Optimizing the charge transfer process by designing Co3O4@PPy@MnO2 ternary core-shell composite. J. Mater. Chem. A. 2014;2(32):12968–12973. doi: 10.1039/C4TA02380C. [DOI] [Google Scholar]
- 33.Jing M, Yang Y, Zhu Y, Hou H, Wu Z, Ji X. An asymmetric ultracapacitors utilizing α-Co(OH)2/Co3O4 flakes assisted by electrochemically alternating voltage. Electrochim. Acta. 2014;141:234–240. doi: 10.1016/j.electacta.2014.07.075. [DOI] [Google Scholar]
- 34.Xia X, Tu J, Zhang Y, Wang X, Gu C, Zhao X-B, Fan HJ. High-quality metal oxide core/shell nanowire arrays on conductive substrates for electrochemical energy storage. ACS Nano. 2012;6(6):5531–5538. doi: 10.1021/nn301454q. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Zhang F, Yang Z, Huang S. One-pot hydrothermal synthesis of reduced graphene oxide/carbon nanotube/α-Ni(OH)2 composites for high performance electrochemical supercapacitor. J. Power Sources. 2013;243:555–561. doi: 10.1016/j.jpowsour.2013.04.076. [DOI] [Google Scholar]