Abstract
The ternary transitional metal oxide NiCo2O4 is a promising anode material for sodium ion batteries due to its high theoretical capacity and superior electrical conductivity. However, its sodium storage capability is severely limited by the sluggish sodiation/desodiation reaction kinetics. Herein, NiCo2O4 double-shelled hollow spheres were synthesized via a microwave-assisted, fast solvothermal synthetic procedure in a mixture of isopropanol and glycerol, followed by annealing. Isopropanol played a vital role in the precipitation of nickel and cobalt, and the shrinkage of the glycerol quasi-emulsion under heat treatment was responsible for the formation of the double-shelled nanostructure. The as-synthesized product was tested as an anode material in a sodium ion battery, was found to exhibit a high reversible specific capacity of 511 mAh g−1 at 100 mA g−1, and deliver high capacity retention after 100 cycles.
Electronic supplementary material
The online version of this article (doi:10.1007/s40820-017-0164-2) contains supplementary material, which is available to authorized users.
Keywords: NiCo2O4, Double-shelled hollow sphere, Microwave, Sodium ion battery
Highlights
NiCo2O4 double-shelled hollow spheres were successfully synthesized via a rapid microwave-assisted solvothermal method in isopropanol with the aid of glycerol.
The roles of isopropanol, nitrate, glycerol, and the heating rate in the formation of the double shelled hollow spheres were systematically studied.
The as-synthesized NiCo2O4 double shelled hollow spheres showed good sodium storage performance with reversible specific capacity of 511 mAh g−1 at 100 mA g−1.
Introduction
Presently, due to increasing energy consumption, there is an increasing demand for energy storage materials. Lithium ion batteries (LIBs) offer high energy storage density, long cycling life, and excellent safety properties, thus dominating the market for portable electronic device power sources [1]. However, the depletion of lithium resources and the consequent high cost of lithium hinder the application of LIBs in several emerging areas, such as large-scale grid energy storage [2]. Sodium, another Group I element, is much more abundant and has a much lower cost. As such, sodium ion batteries (SIBs), which have a charging/discharging mechanism similar to that of LIBs, are promising energy storage devices for the future and have received great research attention in the past few years [3]. Nevertheless, the energy storage performance of SIBs is significantly limited by a lack of suitable electrode materials. For example, while graphite is used as the anode material in most commercial LIBs, it is nearly electrochemically inactive with sodium due to the large ionic radius of Na+ [4]. Although many other carbonaceous materials have been intensively investigated as anode materials for SIBs, their sodium storage capabilities are too low to meet the demands of practical applications.
Transitional metal oxides have been widely investigated as substitutes for carbonaceous anode materials in LIBs [5]. In particular, ternary transition metal oxides such as NiCo2O4 are extremely attractive, due to their high theoretical storage capacities (e.g., 890 mAh g−1 for NiCo2O4 compared to 372 mAh g−1 for graphite) and superior electrical conductivity (2 orders higher than that of single-component cobalt or nickel oxides) [6]. Theoretically, NiCo2O4 has equivalent storage capacities for both sodium and lithium. Recently, some work has been reported on the successful application of NiCo2O4 as an anode material for SIBs [7, 8]. However, due to the sluggish sodiation/desodiation reaction kinetics, as well as the large volume change during the charging/discharging process induced by the large ionic radius of Na+, the reported NiCo2O4 materials exhibit greatly inferior capacities for sodium storage. In order to increase the practical sodium storage capacity of this material, a new strategy to engineer robust nanostructured NiCo2O4 is urgently needed. One attractive avenue amongst the various approaches is the use of hollow multi-shelled spheres, due to their unique structural features [9–17].
Recently, microwave-assisted nanotechnology has attracted a great deal of research interest, due to the interest in green chemistry in both academia and industry. Microwaves heat the reactants directly via dielectric loss, rather than by heat convection as in the conventional heating method. This unique heating mechanism allows the use of microwaves to greatly enhance the fabrication rate of nanomaterials, thus saving both time and energy. Additionally, nanomaterials synthesized via microwave heating have been widely reported to exhibit excellent performance due to the formation of better dispersed nanoparticles with more uniform size distributions [18].
In this work, we developed a synthesis method for NiCo2O4 double-shelled hollow spheres, using a fast microwave-assisted solvothermal treatment followed by annealing. Double-shelled hollow nanostructures are beneficial in facilitating a high specific surface area to expose more active materials for reaction, as well as in buffering the volume change during the charging/discharging process. The macropores in the hollow structure can act as a Na+ transport system, shortening pathways for the diffusion of Na+, thus leading to faster reaction kinetics in hollow nanostructures. The as-synthesized product was further tested as an anode material in SIBs and showed excellent sodium storage capability.
Experimental
Materials
All chemical materials were purchased from Aladdin Chemical Corporation and were of analytical grade. The materials were used without further purification.
Synthesis of NiCo2O4 Double-Shelled Hollow Spheres
In a typical procedure, 1 mmol of Co(NO3)2 and 0.5 mmol of Ni(NO3)2 were dissolved in 80 mL of isopropanol, to which 16 mL of glycerol was added. The mixture was stirred vigorously for 30 min. Subsequently, 60 mL of the mixture was pipetted into a 100-mL vessel and exposed to microwave solvothermal treatment in a microwave hydrothermal reactor (Xianghu, Beijing) at 180 °C for 30 min. To avoid any abnormal increases in pressure due to hot spots during the microwave heating, a ramping procedure was used to raise the temperature from room temperature to 180 °C over 20 min. Finally, the precipitate was collected, washed with ethanol and DI water, dried, and annealed in air at 350 °C for 2 h with a temperature ramping rate of 1 °C min−1.
Characterization of Materials
The crystal structure of the as-prepared samples was examined using X-ray diffraction (XRD, DX-2700, Cu Kα radiation, λ = 1.542 Å). The morphologies and the structural characterization of the products were observed using field emission scanning electron microscopy (FESEM, JEOL, JSM-7500F) and transmission electron microscopy (TEM, Zeiss, Libra200). N2 adsorption–desorption measurements were carried out using a NOVA1000e analyzer at 77 K. The pore size distributions of the samples were analyzed using the Barrett Joyner Halenda (BJH) method.
Electrochemical Measurements
The electrochemical performance of the material was evaluated using CR2032-type coin cells, which were assembled in a glove box filled with highly pure argon gas, with water and oxygen contents of less than 1 ppm. For the fabrication of the working electrode, the NiCo2O4 material, acetylene black, and the binder polyvinylidene fluoride (PVDF) were mixed in N-methyl-2-pyrrolidinone to form a homogeneous slurry with a weight ratio of 8:1:1, which was then coated on copper foil and finally dried for 12 h in a vacuum oven. The copper foil was cut into rounds with a diameter of 14 mm. The average loading mass of the active material was about 1 mg cm−2. Pure sodium foil was used as the counter electrode, and Whatman glass fiber (GF/C) was used as the separator. The electrolyte was 1 M NaClO4 in a mixture of ethylene carbonate and propylene carbonate (1:1, w/w). Galvanostatic charge/discharge tests were conducted using a Neware battery measurement system in the voltage range of 0.01–3.0 V (vs. Na+/Na). Cyclic voltammetry (CV) measurements were performed using a CHI 660E electrochemical workstation.
Results and Discussion
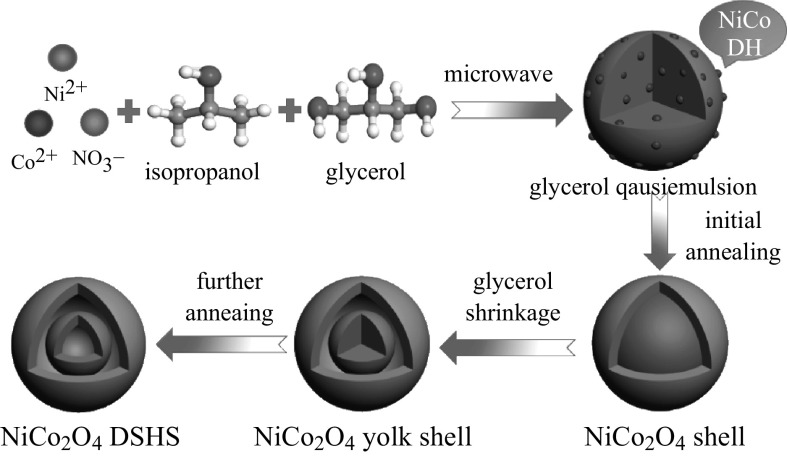
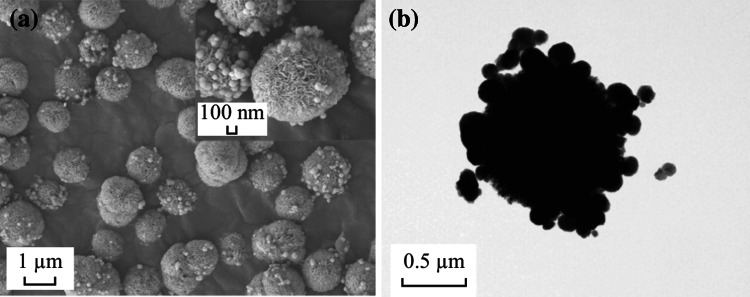
The synthesis route of the double-shelled hollow spherical NiCo2O4 is shown in Fig. 1. At 180 °C, isopropanol and NO3 − underwent a redox reaction, which is described by the formula: 4(CH3)2CHOH + NO3 − = 4CH3COCH3 + NH3 + OH− +2H2O, thus releasing hydroxyl ions that precipitated Ni2+ and Co2+ as Ni–Co double hydroxide (NiCo DH) [19]. Meanwhile, glycerol molecules self-assembled into quasi-emulsions in isopropanol via strong inter-molecular hydrogen bonding under solvothermal conditions, serving as a soft template for the growth of NiCo DH, resulting in NiCo DH-glycerol composites. A control experiment was also conducted to confirm the role that isopropanol and NO3 − played during the synthesis. When isopropanol was replaced with an equal amount of DI H2O, or when NO3 − was replaced by an equal amount of Cl−, no precipitation occurred, indicating that the reduction of NO3 − to NH3·H2O by isopropanol and the formation of NiCo DH was the main reason for precipitation, rather than the formation of Ni–Co glycerate as reported by Shen et al. [20]. The XRD spectra (Fig. 2a) of the as-obtained precursor precipitate showed no significant peaks, indicating that the NiCo DH was amorphous. This was probably due to the short reaction time (30 min), which was much shorter than the 6 h required in the conventional heating method. Compared to conventional solvothermal methods for the preparation of metal-glycerate spheres, the microwave-assisted solvothermal method dramatically enhanced the reaction kinetics, thus saving energy and time. The FESEM and TEM images of NiCo DH are shown in Fig. 2b, c. As can be seen, well-dispersed solid spheres of the NiCo DH-glycerol composite with a diameter of about 500 nm were obtained.
Fig. 1.
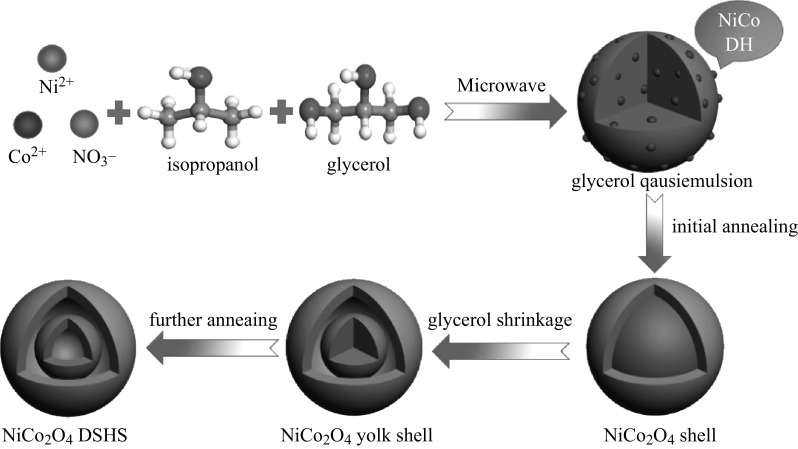
Schematic illustration of the formation process of NiCo2O4 double-shelled hollow spheres
Fig. 2.
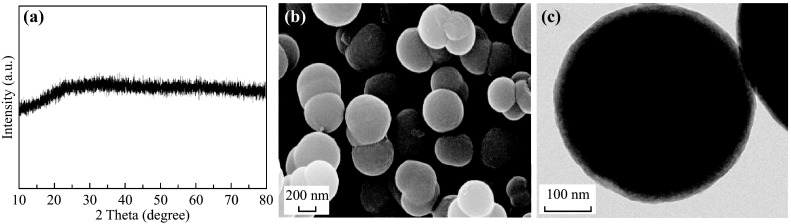
a XRD pattern, b FESEM image, c TEM image for NiCo DH-glycerol composite after microwave solvothermal treatment
The solid precursor spheres were then annealed at 350 °C in air with a temperature ramping rate of 1 °C min−1. During the initial annealing process, the large temperature gradient along the radial direction led to the rapid formation of a NiCo2O4 thin shell on the surface of the NiCo DH-glycerol composite spheres. Thereafter, the glycerol shrank toward the center due to the weight loss caused by oxidation, carrying the embedded NiCo DH with it. After further annealing, double-shelled hollow spherical NiCo2O4 was formed. The XRD pattern of the as-annealed sample in Fig. 3a showed peaks characteristic of the cubic spinel NiCo2O4 phase (JCPDS card No. 20-0781). No other phases were detected, indicating that pure crystalline NiCo2O4 was obtained. Figure 3b, c is the FESEM images of the as-obtained NiCo2O4, showing spheres with diameters of about 300–400 nm. The NiCo2O4 spheres were slightly smaller than those of the precursor precipitate, demonstrating the shrinkage of the spheres during annealing. The microstructure of the interior of the sample was further examined using TEM, as shown in Fig. 3d, e. Double-shelled hollow nanostructures are clearly observed, with a ~20 nm outer shell and a ~70 nm inner shell. The selected-area electron diffraction (SAED) pattern can be ascribed to polycrystallinity, and all rings can be indexed to the spinel NiCo2O4 phase (Fig. 3f). In order to confirm the role of glycerol in facilitating the formation of the hollow structure, a control experiment was carried out in which glycerol was replaced by an equal amount of isopropanol. After annealing at a heating rate of 1 °C min−1 (as shown in Fig. 4), solid microspheres with diameters of approximately 1 µm were obtained, demonstrating that glycerol is vital for fabricating the double-shelled hollow nanostructure, as well as to prevent the aggregation of NiCo DH into larger microspheres via a templating effect. Subsequently, to study the effect of the heating rate on the interior structure of the obtained NiCo2O4 spheres, the NiCo DH-glycerol composites obtained after solvothermal treatment were annealed at heating rates of 5 or 10 °C min−1 (TEM images are shown in Fig. S1). The outer shell was found to become thicker as the heating rate increased, and solid spheres were obtained at a heating rate of 10 °C min−1. The increase in shell thickness probably occurred because at a higher heating rate, the larger temperature gradient along the radial direction led to more rapid formation of a NiCo2O4 shell on the surface of the NiCo DH-glycerol composite spheres during the initial annealing process. Such an effect has been described in previous reports [20, 21].
Fig. 3.
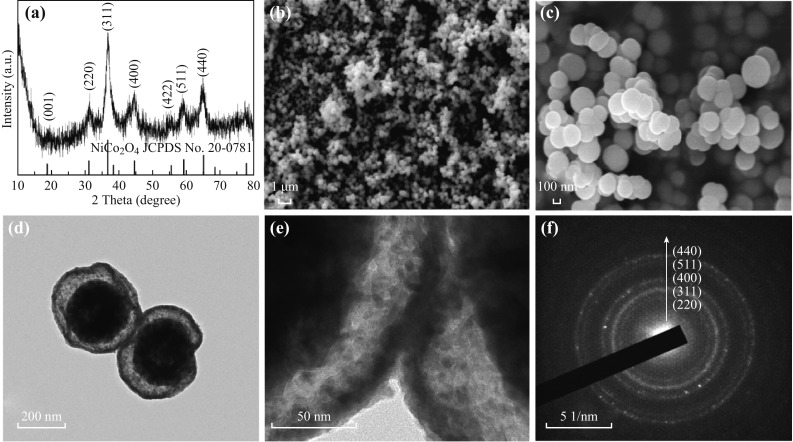
a XRD pattern, b, c FESEM images, d, e TEM images, f SAED pattern of as-synthesized NiCo2O4 double-shelled hollow spheres
Fig. 4.
a FESEM image, b TEM image of the sample prepared without glycerol
From the N2 adsorption/desorption isotherm curve (shown in Fig. S2), the surface area of the as-synthesized double-shelled hollow product was determined to be 30.7 m2 g−1, with the pore size distribution centered around 6.9 nm. This porous hollow structure is beneficial, both because the high surface area exposes more active materials for reaction during the charging/discharging process and because it could help to buffer the volume change during the sodiation/desodiation process. Thus, this morphology is vital to improve the storage capacity and cycling stability of the electrode.
Electrochemical Performance
The electrochemical performance of the as-synthesized NiCo2O4 double-shelled hollow spheres as an anode material for SIBs was investigated. Figure 5a shows the CV curve at a scan rate of 0.2 mV s−1. The peak at 1.2 V in the first cathodic cycle, which disappeared in subsequent cycles, was assigned to the formation of a solid electrolyte interphase layer [22]. Two additional weak peaks at 0.34 and 0.47 V corresponded to the reduction of NiCo2O4 into metallic Ni and Co, accompanied by the formation of Na2O. The peaks at 0.68, 0.85, and 1.17 V in the anodic scan corresponded to the re-oxidation of metallic Ni and Co to NiCo2O4. Subsequently, the oxidation/reduction peaks for the conversion reaction overlapped and stabilized, indicating the high reversibility of the sodiation/desodiation reaction after the first cycle. Figure 5b shows the typical galvanostatic charge–discharge profiles of the product at a current density of 100 mA g−1 during the 1st, 2nd, and 5th cycles. During the first discharge process, the electrode exhibited a long potential plateau at around 0.2 V that disappeared during subsequent cycles. This plateau was ascribed to the phase decomposition of the spinel structure. Similar results have been reported previously [23]. The initial discharge and charge capacities were 814 and 513 mAh g−1, respectively, corresponding to a coulombic efficiency of 63%. The irreversibility of the capacity was mainly caused by irreversible SEI film formation reactions as a result of electrolyte decomposition [8]. The discharge and charge curves for the second and the fifth cycles were basically coincident, demonstrating the good cycling stability. The rate capacity of the cells is shown in Fig. 5c. The cell with the NiCo2O4 electrode delivered high reversible specific capacities of 511, 412, 353, and 251 mAh g−1 at 100, 200, 500, and 1000 mA g−1, respectively. The capacity recovered to 451 mAh g−1 when the current density was switched to 100 mA g−1, which was close to the value obtained during the initial cycle, demonstrating the good electrochemical reversibility and structural stability during the charge/discharge process. The cycling performance of the NiCo2O4 double-shelled hollow spheres at a current density of 100 mA g−1 is shown in Fig. 5d. The coulombic efficiency of the cell increases significantly upon cycling, eventually reaching about 98%, illustrating that the charge/discharge process was highly reversible. After 100 continuous cycles, a high reversible discharge capacity of 341 mAh g−1 was retained, which corresponded to retention of 66% of the second cycle discharge capacity. The sodium storage properties achieved in the present study were superior to those of many reported NiCo2O4 materials [7, 24–26] (as shown in Table S1). Clearly, the unique double-shelled hollow sphere structure of NiCo2O4 played an important role in its excellent electrochemical performance. Specifically, the large surface area and the porous structure were beneficial for promoting Na-ion permeation and the electrochemical reaction between the electrode material and electrolyte, resulting in a high-specific capacity and outstanding rate performance. In addition, the hollow structure can serve as a buffer to resist volume change during the sodiation/desodiation process. As a result, this special structure can endow outstanding cycling stability.
Fig. 5.
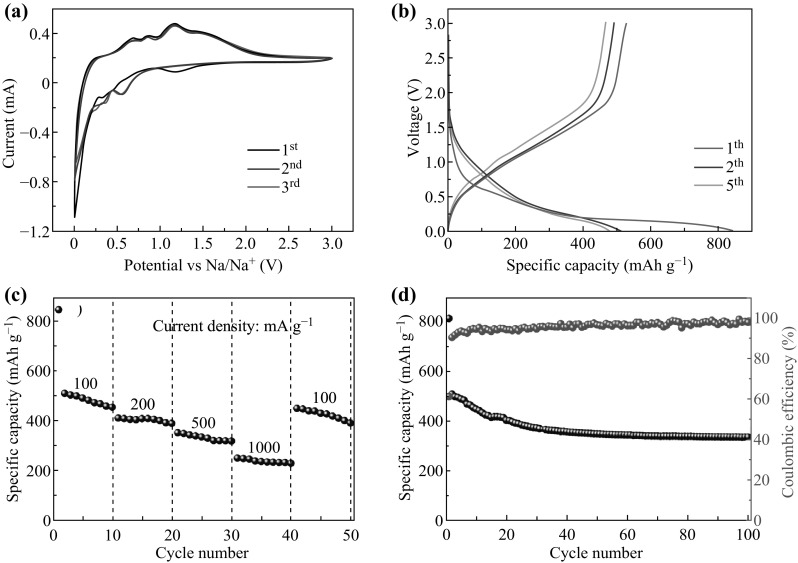
a CV curve of the as-synthesized product in the scan rate of 0.2 mV s−1. b Discharge–charge voltage profiles of as-synthesized product at a current density of 100 mA g−1. c Rate capacity of as-synthesized product at different current density between 0.01 and 3 V. d Cycling stability of as-synthesized product at a current density of 100 mA g−1
Conclusions
In summary, a microwave-assisted fast solvothermal synthetic procedure for NiCo2O4 double-shelled hollow spheres in the presence of isopropanol and glycerol was developed. Both isopropanol and glycerol played a vital role in the synthetic procedure. The as-synthesized product exhibited excellent sodium storage performance when tested as an anode material in SIBs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The work was financially supported by the Science Foundation of Sichuan Province (Grant No. 2016FZ0070) and the Natural Science Foundation of China (NSFC, 201476145). The authors also appreciate the technical support for Materials Characterization from The Analytical and Testing Center of Sichuan University.
Contributor Information
Yanping Zhou, Email: ypzhou11@scu.edu.cn.
Wei Chu, Email: chuwei1965@scu.edu.cn.
References
- 1.Bruce PG, Scrosati B, Tarascon JM. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008;47(16):2930–2946. doi: 10.1002/anie.200702505. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Miao X, Chu W, Wu P, Tong DG. Hollow amorphous NaFePO4 nanospheres as a high-capacity and high-rate cathode for sodium-ion batteries. J. Mater. Chem. A. 2015;3(16):8265–8271. doi: 10.1039/C5TA01191D. [DOI] [Google Scholar]
- 3.Zhu Y, Nie P, Shen L, Dong S, Sheng Q, Li H, Luo H, Zhang X. High rate capability and superior cycle stability of a flower-like Sb2S3 anode for high-capacity sodium ion batteries. Nanoscale. 2015;7(7):3309–3315. doi: 10.1039/C4NR05242K. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Wang Z, Li L, Peng S, Zhang L, Srinivasan M, Ramakrishna S. Preparation of nitrogen and phosphorous co-doped carbon microspheres and their superior performance as anode in sodium-ion batteries. Carbon. 2016;99:556–563. doi: 10.1016/j.carbon.2015.12.066. [DOI] [Google Scholar]
- 5.Ban C, Wu Z, Gillaspie DT, Chen L, Yan Y, Blackburn JL, Dillon AC. Nanostructured Fe3O4/SWCNT electrode: binder-free and high-rate Li-ion anode. Adv. Mater. 2010;22(20):145–149. doi: 10.1002/adma.200904285. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Cao C. A simple synthesis of two-dimensional ultrathin nickel cobaltite nanosheets for electrochemical lithium storage. Electrochim. Acta. 2015;176(10):141–148. doi: 10.1016/j.electacta.2015.06.130. [DOI] [Google Scholar]
- 7.Chen J, Ru Q, Mo Y, Hu S, Hou X. Design and synthesis of hollow NiCo2O4 nanoboxes as anodes for lithium-ion and sodium-ion batteries. Phys. Chem. Chem. Phys. 2016;18(28):18949–18957. doi: 10.1039/C6CP02871C. [DOI] [PubMed] [Google Scholar]
- 8.Mo Y, Ru Q, Chen J, Song X, Guo L, Hu S, Peng S. Three-dimensional NiCo2O4 nanowire arrays: preparation and storage behavior for flexible lithium-ion and sodium-ion batteries with improved electrochemical performance. J. Mater. Chem. A. 2015;3(39):19765–19773. doi: 10.1039/C5TA05931C. [DOI] [Google Scholar]
- 9.Wu G, Wu H, Wang K, Zheng C, Wang Y, Feng A. Facile synthesis and application of multi-shelled SnO2 hollow spheres in lithium ion battery. RSC Adv. 2016;6(63):58069–58076. doi: 10.1039/C6RA11771F. [DOI] [Google Scholar]
- 10.Wang Y, Yu L, Lou XW. Formation of triple-shelled molybdenum-polydopamine hollow spheres and their conversion into MoO2/carbon composite hollow spheres for lithium-ion batteries. Angew. Chem. Int. Ed. 2016;55(47):14668–14672. doi: 10.1002/anie.201608410. [DOI] [PubMed] [Google Scholar]
- 11.Shen L, Yu L, Wu HB, Yu XY, Zhang X, Lou XW. Formation of nickel cobalt sulfide ball-in-ball hollow spheres with enhanced electrochemical pseudocapacitive properties. Nat. Commun. 2015;6:6694. doi: 10.1038/ncomms7694. [DOI] [PubMed] [Google Scholar]
- 12.Wu H, Wu G, Ren Y, Li X, Wang L. Multishelled metal oxide hollow spheres: easy synthesis and formation mechanism. Chem. Eur. J. 2016;22(26):8864–8871. doi: 10.1002/chem.201504358. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Qu S, Cheng Y, Zheng C, Chen S, Wu H. Facile synthesis of Co3O4 spheres and their unexpected high specific discharge capacity for lithium-ion batteries. Appl. Surf. Sci. 2017;416:338–343. doi: 10.1016/j.apsusc.2017.04.194. [DOI] [Google Scholar]
- 14.Wang Y, Yu L, Lou XW. Synthesis of highly uniform molybdenum-glycerate spheres and their conversion into hierarchical MoS2 hollow nanospheres for lithium-ion batteries. Angew. Chem. Int. Ed. 2016;55(26):7423–7426. doi: 10.1002/anie.201601673. [DOI] [PubMed] [Google Scholar]
- 15.Wu HJ, Wu GL, Wang LD. Peculiar porous α-Fe2O3, γ-Fe2O3 and Fe3O4 nanospheres: facile synthesis and electromagnetic properties. Powder Technol. 2015;269:443–451. doi: 10.1016/j.powtec.2014.09.045. [DOI] [Google Scholar]
- 16.Wu H, Wu G, Ren Y, Yang L, Wang L, Li X. Co2+/Co3+ ratio dependence of electromagnetic wave absorption in hierarchical NiCo2O4–CoNiO2 hybrids. J. Mater. Chem. C. 2015;3(29):7677–7690. doi: 10.1039/C5TC01716E. [DOI] [Google Scholar]
- 17.Wu H, Wang Y, Zheng C, Zhu J, Wu G, Li X. Multi-shelled NiO hollow spheres: easy hydrothermal synthesis and lithium storage performances. J. Alloy. Compd. 2016;685:8–14. doi: 10.1016/j.jallcom.2016.05.264. [DOI] [Google Scholar]
- 18.Zhang ZJ, Wang YX, Chou SL, Li HJ, Liu HK, Wang JZ. Rapid synthesis of α-Fe2O3/rGO nanocomposites by microwave autoclave as superior anodes for sodium-ion batteries. J. Power Sources. 2015;280:107–113. doi: 10.1016/j.jpowsour.2015.01.092. [DOI] [Google Scholar]
- 19.Ren Y, Gao L. From three-dimensional flower-like α-Ni(OH)2 nanostructures to hierarchical porous NiO nanoflowers: microwave-assisted fabrication and supercapacitor properties. J. Am. Ceram. Soc. 2010;93(11):3560–3564. doi: 10.1111/j.1551-2916.2010.04090.x. [DOI] [Google Scholar]
- 20.Shen L, Yu L, Yu XY, Zhang X, Lou XW. Self-templated formation of uniform NiCo2O4 hollow spheres with complex interior structures for lithium-ion batteries and supercapacitors. Angew. Chem. Int. Ed. 2015;54(6):1868–1872. doi: 10.1002/anie.201409776. [DOI] [PubMed] [Google Scholar]
- 21.Liu BJ, Li XY, Zhao QD, Hou Y, Chen GH. Self-templated formation of ZnFe2O4 double shelled hollow microspheres for photocatalytic degradation of gaseous o-dichlorobenzene. J. Mater. Chem. A. 2017;5:8909–8915. doi: 10.1039/C7TA02048A. [DOI] [Google Scholar]
- 22.Zhang D, Sun W, Chen Z, Zhang Y, Luo W, Jiang Y, Dou SX. Two-dimensional cobalt-/nickel-based oxide nanosheets for high-performance sodium and lithium storage. Chem. Eur. J. 2016;22(50):18060–18065. doi: 10.1002/chem.201604115. [DOI] [PubMed] [Google Scholar]
- 23.Rahman MM, Glushenkov AM, Ramireddy T, Chen Y. Electrochemical investigation of sodium reactivity with nanostructured Co3O4 for sodium-ion batteries. Chem. Commun. 2014;50(39):5057–5060. doi: 10.1039/C4CC01033G. [DOI] [PubMed] [Google Scholar]
- 24.Alcantara R, Jaraba M, Lavela P, Tirado JL. NiCo2O4 spinel: first report on a transition metal oxide for the negative electrode of sodium-ion batteries. Chem. Mater. 2002;14:2847–2848. doi: 10.1021/cm025556v. [DOI] [Google Scholar]
- 25.Zhou K, Hong Z, Xie C, Dai H, Huang Z. Mesoporous NiCo2O4 nanosheets with enhance sodium ion storage properties. J. Alloy. Compd. 2015;651:24–28. doi: 10.1016/j.jallcom.2015.08.130. [DOI] [Google Scholar]
- 26.Fu F, Li J, Yao Y, Qin X, Dou Y, Wang H, Tsui J, Chan KY, Shao M. Hierarchical NiCo2O4 micro- and nanostructures with tunable morphologies as anode materials for lithium- and sodium-ion batteries. ACS Appl. Mater. Interfaces. 2017;9(19):16194–16201. doi: 10.1021/acsami.7b02175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.