Abstract
Visible-light-responsive ternary metal tungstate (MWO4) photocatalysts are being increasingly investigated for energy conversion and environmental purification applications owing to their striking features, including low cost, eco-friendliness, and high stability under acidic and oxidative conditions. However, rapid recombination of photoinduced electron–hole pairs and a narrow light response range to the solar spectrum lead to low photocatalytic activity of MWO4-based materials, thus significantly hampering their wide usage in practice. To enable their widespread practical usage, significant efforts have been devoted, by developing new concepts and innovative strategies. In this review, we aim to provide an integrated overview of the fundamentals and recent progress of MWO4-based photocatalysts. Furthermore, different strategies, including morphological control, surface modification, heteroatom doping, and heterojunction fabrication, which are employed to promote the photocatalytic activities of MWO4-based materials, are systematically summarized and discussed. Finally, existing challenges and a future perspective are also provided to shed light on the development of highly efficient MWO4-based photocatalysts.
Keywords: Ternary metal tungstates, Micro- and nanostructures, Photocatalysis, Environmental purification, Water splitting
Highlights
A series of ternary tungstate-based photocatalysts and their applications in solar energy conversion and environmental purification are systematically introduced.
The relationship between intrinsic structures and unique properties of ternary tungstate-based photocatalysts is discussed and summarized in detail.
Various new concepts and innovative strategies are employed to enhance the photocatalytic performance of ternary tungstate-based photocatalysts
Introduction
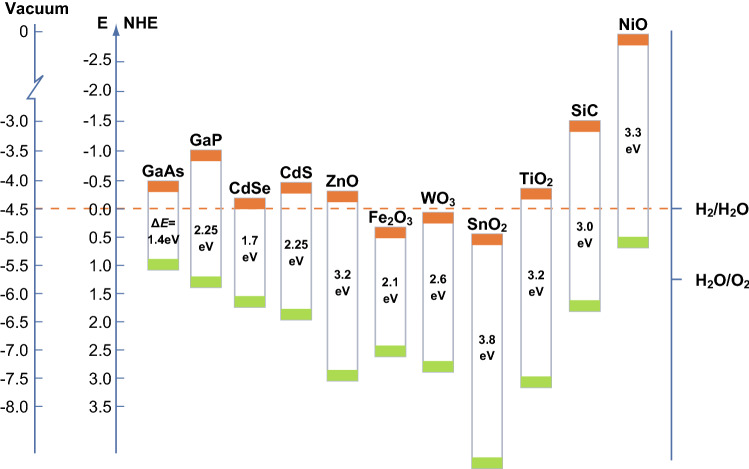
Since Fujishima and Honda in 1972 demonstrated that titanium dioxide (TiO2) can be used as photoanode to split water excited by ultraviolet light, photocatalysis technology has been viewed as among the most promising approaches to solve the global energy crisis and environmental problems [1–3]. In general, a complete semiconductor photocatalytic cycle involves light-harvesting, photogenerated charge carrier excitation, charge separation and transfer, and surface redox reactions [4–6] that allow for the formation of reactive oxygen species (ROSs), such as free electrons (e−), hydrogen peroxide (H2O2), hydroxyl (·OH), and superoxide radicals (·O2−) [7, 8]. The aforementioned ROSs play crucial roles in various important applications, including photocatalysis [9–12], photoelectrocatalysis [13–17], plasma photocatalysis [18, 19], and photothermocatalysis [20–22]. Until now, TiO2 has been among the most extensively studied semiconductor photocatalysts because of its strong oxidative ability, chemical stability, long durability, and nontoxicity [23–25]. However, the TiO2 photocatalyst possesses a wide band gap of ~ 3.2 eV that can only absorb ultraviolet (UV) light, which is a small fraction (~ 5%) of solar light, thereby hardly harvesting the remaining solar energy [26, 27]. To efficiently utilize the majority of the solar spectrum, Fe2O3- [28], WO3- [29, 30], Bi2WO6- [31], ZnO- [32], Bi2O3- [33], and NiO-based semiconductors [34] have been widely developed as photocatalysts for environmental treatment and solar water splitting (Fig. 1). Nevertheless, low sunlight utilization efficiency and quantum yield, rapid reverse reactions, and poor stability still hinder practical applications of these photocatalytic materials [35–39].
Fig. 1.
Schematic overview of the band positions of representative semiconductors
Desired photocatalysts should have a suitable band gap to achieve a high harvesting efficiency of sunlight, sufficient quantum yield, and an appropriate position of the band edge to trigger redox reactions [40–42]. For an ideal photocatalyst, the conduction band (CB) edge should be sufficiently negative to drive the photo-reduction reaction. In contrast, the valence band (VB) edge should be sufficiently positive to trigger the photo-oxidation reaction [43]. For example, in photocatalytic water splitting, when the VB edge position of the semiconductor photocatalyst is more positive than the potential of H2O/O2 (1.23 V vs. NHE) and the CB position is more negative than the potential of H2/H2O (0 V vs. NHE), the water splitting reaction can occur [44–47].
Recently, ternary tungstate-based complex oxides have been developed as potential candidates for efficient photocatalytic applications [48–50]. Tungstates are described by the general formula MWO4 (M denotes a bivalent cation) [51] and are widely used in the luminescence, microwave ceramics, and catalytic fields, given their self-activating fluorescence effect, microwave, and optical properties [52–55]. Owing to the variety of bivalent cations, the crystal structure of MWO4 is dependent on the size of cationic radii. According to the literature [56], MWO4 typically has a monoclinic wolframite structure for small M2+ cations (M = Fe, Co, Sn, and Ni) and a tetragonal scheelite structure for large M2+ cations (M = Ca, Ba, Pb, and Sr), as shown in Fig. 2 [57]. During the past few decades, MWO4 with large radii cations, including CaWO4 [58, 59], BaWO4 [60, 61], PbWO4 [62], and SrWO4 [63], has been prepared using different synthesis approaches. However, the band gaps of these MWO4 photocatalysts are much larger than that of TiO2 and are not suitable for practical photocatalytic applications. In contrast, the band gaps of MWO4 with small-radii cations are considerably smaller than that of TiO2 and could be a promising choice for the efficient utilization of solar energy [64–72]. The band-gap energies of representative MWO4 are summarized in Fig. 3 and Table 1. In addition, the band-gap energies of various MWO4 and the radii of the metal cations are plotted in Fig. 4. It can be seen that a flock of MWO4 is in the yellow area, in which each MWO4 has a smaller metal cation radius (< 0.73 Å) and narrower band-gap energy (< 3.2 eV) than those of the others. In contrast, MWO4 with a larger cation radius have larger band-gap energy. Notably, for specific MWO4, the band gaps presented in Table 1 are not the only ones, because the absorption light range of the semiconductors can be affected and controlled by various factors, including morphology, size, doping, and defects, thus resulting in one semiconductor material possessing several band-gap energies. By comparing the requirements of novel photocatalysts, MWO4 with small-radius cations is more advantageous and can be further developed as highly efficient photocatalysts. In addition, it is clearly observed that ternary MWO4 systems with a narrow band gap have transition metals as the cation component, which is earth-abundant, cost effective, and low-toxicity, benefiting wide usage in the future. However, for these visible-light-responsive pristine MWO4, photocatalytic activities remain inadequate for practical applications because of the rapid recombination of photogenerated holes and electrons.
Fig. 2.
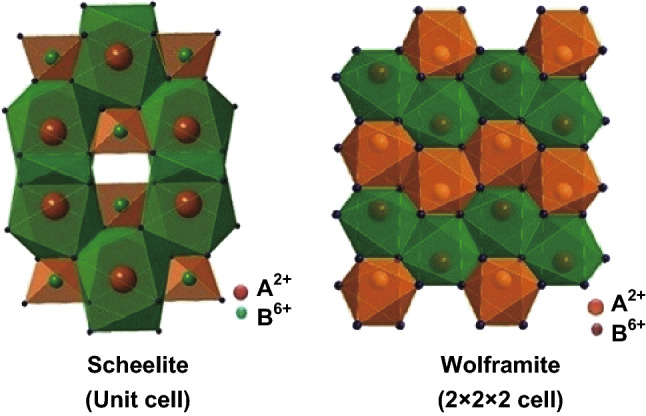
Illustration of unit cells of scheelite and wolframite. Reproduced with permission from Ref. [57]. Copyright 2014 Elsevier
Fig. 3.

Colors of different MWO4 materials. Reproduced with permission from Ref. [51]. Copyright 2014 American Chemical Society
Table 1.
Band gaps, crystal sizes, and effective ionic radii of different MWO4 materials
| Compounds | Ionic radius of cation M (Å) | Band gap Eg (eV) | Crystalline sizes (nm) via Scherrer equation | References |
|---|---|---|---|---|
| BaWO4 | 1.42 | 4.79 | 55 | [56] |
| PbWO4 | 1.29 | 3.2 | 11 | [73] |
| SrWO4 | 1.26 | 4.66 | 33 | [56] |
| CaWO4 | 1.12 | 4.27 | 32 | [56] |
| CdWO4 | 0.95 | 3.77 | 21 | [74] |
| ZnWO4 | 0.74 | 3.37 | 32 | [75] |
| CuWO4 | 0.73 | 3.2 | 36 | [76] |
| MgWO4 | 0.72 | 4.19 | 70 | [77] |
| SnWO4 | 0.69 | 2.27 | 36 | [78] |
| NiWO4 | 0.69 | 2.54 | 31 | [71] |
| MnWO4 | 0.66 | 2.97 | 29 | [79] |
| CoWO4 | 0.65 | 2.5 | 35 | [80] |
| FeWO4 | 0.61 | 2.16 | 50 | [81] |
Fig. 4.
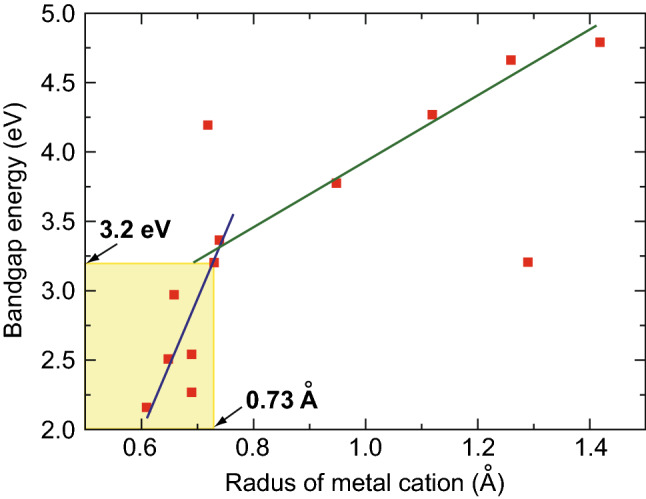
Relationship between band-gap energy of MWO4 and the radii of metal cations
Herein, we provide a comprehensive review of the evolution and current state of the development and application of ternary MWO4-based photocatalysts in environmental purification and solar water splitting. First, we discuss the fundamentals of ternary MWO4 systems, including the crystal composition, electronic structure, and relationship between the intrinsic structures and properties. Subsequently, versatile reported strategies to improve the photocatalytic activities of pristine MWO4 are systematically summarized. Finally, challenges and future developments of ternary MWO4-based photocatalysts are discussed. We believe that this review provides information on recent progress in ternary MWO4-based photocatalysts for environmental and energy applications and insight into future perspectives, which will aid the design of highly efficient semiconductor-based photocatalysts.
Ternary MWO4 Photocatalysts (M = bivalent metal cations)
The photocatalytic activity of semiconductor photocatalysts is known to be closely related to their crystal and electronic structures [82]. In this section, an overview of the crystal and electronic structures of ternary MWO4 is presented and the factors influencing their photocatalytic performance is explored.
Crystal Structure
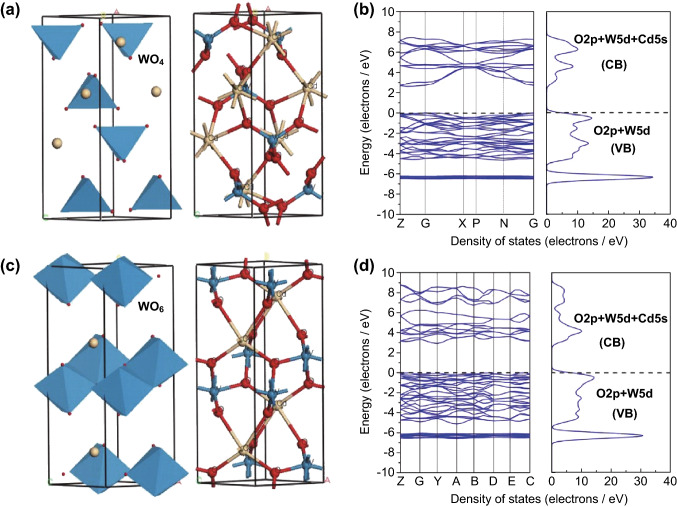
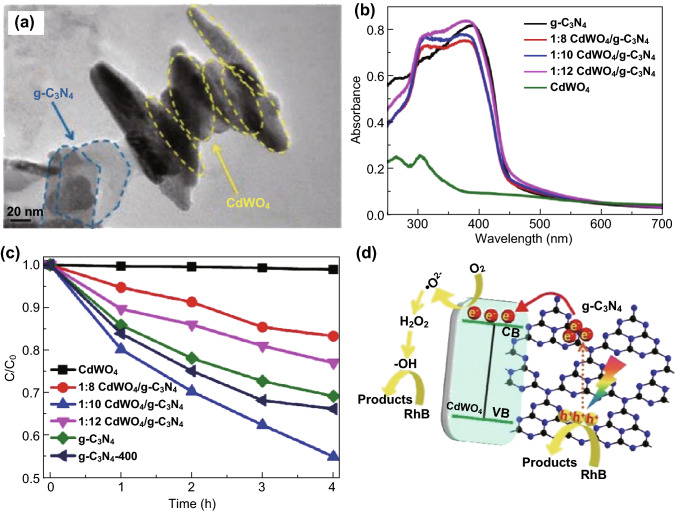
As ABO4-type compounds, ternary MWO4 complex materials possess a typical wolframite-type monoclinic crystal structure and scheelite-type tetragonal structure. In the scheelite crystal structure, one W atom coordinates with four O atoms to form the WO4 tetrahedral unit. In contrast, in the wolframite crystal structure, one W atom is encircled by six oxygen atoms to form the WO6 octahedral unit. For example, Yan et al. [83] reported a tetragonal structure in CdWO4 material, in which the W atom is situated in the center of the tetrahedra, forming four W–O bonds of the same bond length of 1.758 Å, with the coordination number of the Cd atom being eight. However, the monoclinic structure of CdWO4 is similar to that of previously reported MnWO4 [84]. Both W(VI) and Cd(II) have octahedral O coordination, in which each octahedron shares two corners with its neighbors. However, the configuration of the WO6 octahedron leads to severe distortion in which two W–O bonds are much shorter than the other four W–O bonds. The two crystal phases of CdWO4 are shown in Fig. 5. An investigation of methyl orange (MO) degradation showed that the photocatalytic performance of monoclinic CdWO4 was much higher than that of tetragonal CdWO4 and commercial TiO2 under UV light irradiation, which can be ascribed to the lower lattice symmetry of the monoclinic CdWO4. Furthermore, the electronic structures of the wolframite- and scheelite-type CdWO4 were investigated by theoretical computations and simulations based on density functional theory (DFT). As shown in Fig. 5b-d, both monoclinic and tetragonal CdWO4 are indirect-type semiconductors because the calculated bottom of the CB is not situated in the same line as the top of the VB. The calculated band gap of monoclinic CdWO4 was larger than that of tetragonal CdWO4, which was not consistent with the photocatalytic results. Actually, the monoclinic CdWO4 consisted of distorted WO6 octahedra, leading to the generation of dipole moments in the WO6 octahedral units, while the tetragonal CdWO4 comprised normal WO4 tetrahedra, forming a highly symmetric lattice in the absence of dipole moments. Based on its crystal and geometric structures, the monoclinic structure of CdWO4 is considered a highly efficient photocatalyst, and its catalytic performance has been extensively explored [74, 85, 86].
Fig. 5.
Models of band structures and calculated density of states for tetragonal (a, b) and monoclinic (c, d) CdWO4. Reproduced with permission from Ref. [83]. Copyright 2011 Elsevier
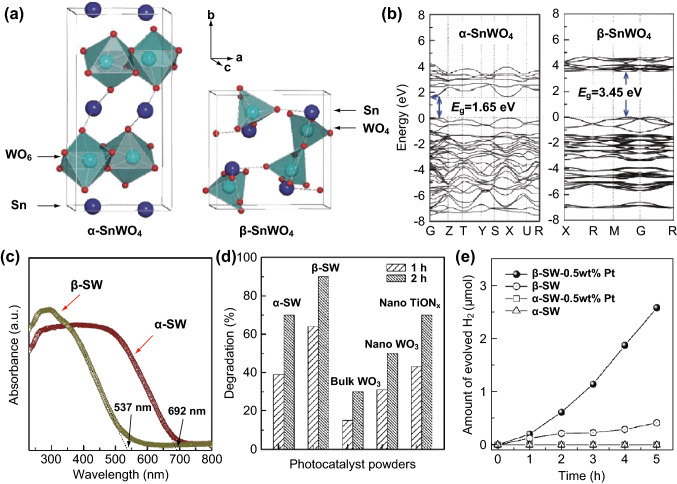
In addition, it has also been reported that SnWO4 has two crystal structures: α-SnWO4 and β-SnWO4 [87]. The orthorhombic α-SnWO4 is comparatively stable in the structure below 670 °C, whereas the cubic β-SnWO4 is a steady structure above 670 °C. In the crystal unit of orthorhombic α-SnWO4, a single W atom is bonded with six O atoms to constitute typical corner-shared WO6 octahedra, while the unshared WO4 tetrahedra are composed of a crystal unit of β-SnWO4. Owing to the lone-pair effects on the Sn(II) ion, distorted SnO6 octahedra are formed in both polymorphs. Cho et al. [88] prepared the aforementioned SnWO4 materials with different types of crystal structures and investigated the relationship of the crystal structure with the optical and catalytic properties of SnWO4 (Fig. 6). It was demonstrated that a difference in atom arrangement could result in an apparent difference in electronic distribution. The VB of SnWO4 is constituted through high hybridization between the Sn 5 s orbital and O 2p orbital, resulting from the strong interaction between the atomic orbitals with closer energy. Meanwhile, the Sn 5 s orbitals contribute to the lower and upper energy levels of the VB, while the O 2p orbitals are dedicated to the middle energy levels of the VB. Therefore, the VB and CB electronic structures of SnWO4 are totally different from those of the pristine WO3, in which the VB and CB comprise filled O 2p orbitals and empty W 5d orbitals, respectively. The band gap of α-SnWO4 was calculated to be 1.65 eV, which was lower than that of WO3 (1.77 eV), thereby accounting for the broadening effect of the VB, which can be attributed to the contribution of the Sn 5 s orbitals. In contrast, the calculated band gap of 3.45 eV for β-SnWO4 was apparently much larger than that of α-SnWO4 and WO3. Although the Sn 5 s orbitals also contribute to the VB of β-SnWO4 (Fig. 6b), the increase in the band gap stems from the decreased length of the W–O bonds and enhanced crystal field splits, thus resulting in the upshifting of the CB position. Furthermore, the experimental results showed that both α-SnWO4 and β-SnWO4 exhibited higher photocatalytic performance for the degradation of Rhodamine B (RhB), as compared to other visible-light-response photocatalysts, such as bulk- or nanoWO3 and nanoTiONx (Fig. 6d). Moreover, the β-SnWO4 showed a higher photocatalytic activity for H2 evolution in the presence of methanol, accompanying Pt as a co-catalyst under visible-light irradiation (Fig. 6e), which can be mainly ascribed to the higher CB edge of β-SnWO4 compared to that of α-SnWO4. Based on the aforementioned analysis, one can conclude that the MWO4 photocatalysts usually possess more than two types of crystal structure, thus leading to a difference in geometries and local lattice distortions. The non-bonded electrons in the metal ion should be considered an important factor in analyzing the crystal field, particularly in an MWO4-based asymmetric coordination environment. The distortion of the local crystal structure influences the electronic structure and band distribution, thus affecting the catalytic performances of MWO4-based photocatalysts.
Fig. 6.
a Crystal structures, b density of states, and c UV–Vis absorption spectra of α-SnWO4 and β-SnWO4. d Photocatalytic activity of α-SnWO4 and β-SnWO4 in RhB degradation. e H2 evolution. Reproduced with permission from Ref. [88]. Copyright 2009 American Chemical Society
Electronic Structure
Generally, tungstates are considered derivatives of H2WO4 and WO3 because of their similar elemental constitutions and crystal structures [89–91]. The DFT [92] and ab initio [93] calculations indicate that the CB of MWO4 consists of W 5d orbitals in tungstates, as in WO3, while the O 2p orbitals only comprise the upper part of the VB because the bivalent metal cation in tungstates contributes differently to the VB and CB positions given different outer electronic arrangements [55].
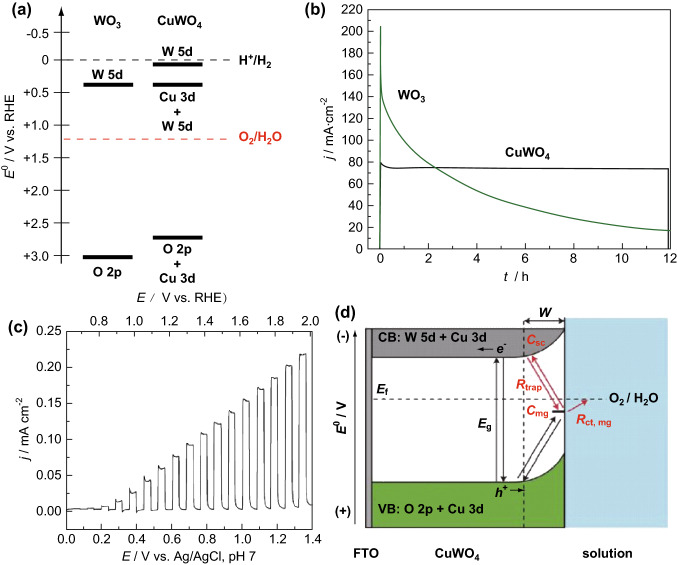
For instance, visible-light-driven CuWO4 is employed for photoelectrochemical (PEC) water splitting because of its suitable band gap of 2.3 eV [94–96]. For most binary metal oxides, the VB is mainly constituted of O 2p orbitals; thus, the VB energy is usually in the range of 2.5–3.0 eV, indicating that the top of the VB is not significantly shifted in the metal oxides. For ternary metal oxides, however, the mixing atomic orbitals between the O 2p orbitals and metal orbitals could result in an apparent shift in the top position of the VB [97]. Thus, for CuWO4, the VB shifts upward, in comparison to that of WO3, accounting for the hybridization between the O 2p orbitals and Cu 3d orbitals (Fig. 7a). The upward movement in the VB position indicates a decrease in the band gap, which results in an increased visible-light absorption range [98]. However, the composition of the CB is still a topic of hot debate. DFT calculations show that the VB of CuWO4 is composed of O 2p orbitals with a small portion of Cu 3d, whereas the CB of CuWO4 is composed of W 5d orbitals [99, 100]. Moreover, the Cu 3d orbitals may contribute to the CB of CuWO4 except for the top of the VB [101]. Experimental results demonstrate that the CB shift of CuWO4 relative to that of WO3 can be ascribed to the incorporation of Cu 3d orbitals into the energy level of the CB [102]. Yet, strong evidence of an accurate contribution proportion of Cu 3d orbitals to the CB of CuWO4 has not been obtained. Although the CB composition of CuWO4 is unclear, the CuWO4 photoanode presented a high photocatalytic activity with a photocurrent density of up to 0.07 mA cm−2 at 1.23 V and a high stability under AM 1.5G illumination (Fig. 7b, c), indicating that the CuWO4 photoanode has a thermodynamic potential for water oxidation. In this system, a physical model of the photogenerated charge carrier pathways in CuWO4 is proposed as shown in Fig. 7d. It shows that when the photogenerated electrons are transferred from a solution medium to a mid-gap state, the thermodynamic potential of the mid-gap state can be utilized to determine which elementary reaction is favored to occur and which is the rate-limiting reaction.
Fig. 7.
a Distribution of energy level and b chronoamperometry curves of CuWO4 and WO3. c Polarization curve of CuWO4 photoanode under AM1.5G irradiation. d Illustration of the physical model of charge carrier transfer in CuWO4. Reproduced with permission from Ref. [102]. Copyright 2016 American Chemical Society
Apart from CuWO4, the electronic structures of other MWO4 materials have also been previously studied. For example, Rajagopal and co-workers [103, 104] used X-ray emission spectroscopy and DFT computation to study the electronic structures and related properties of FeWO4 and CoWO4 photocatalysts. The theoretical calculation results demonstrated that O 2p orbitals mainly contributed to the VB of tungstates, while the unoccupied Fe 3d orbitals and Co 3d orbitals were dominantly dedicated to the CB of FeWO4 and CoWO4, respectively. In addition, the density of states showed that Co 3d and W 5d orbitals also contributed to the VB regions of CoWO4, similar to that of FeWO4. Hence, the VBs of FeWO4 and CoWO4 are composed of O 2p, W 5d, and Fe/Co 3d orbitals. However, X-ray emission spectroscopy results revealed that the W 5d orbitals and O 2p orbitals are dedicated to the entire VB of the tungstates, in which the O 2p orbitals are dedicated to the upper region of the VB and W 5d orbitals are dedicated to the lower region of the VB. The theoretical results agreed well with the experimental results for FeWO4 and CoWO4. In addition, the electronic structure of NiWO4 was obtained. For NiWO4, the CB predominantly consists of W 5d orbitals and Ni 3d orbitals, while the VB primarily consists of Ni 3d orbitals and O 2p orbitals [105]. It was found that the VB composition of NiWO4 is different from those of FeWO4 and CoWO4, which is related to the energy level distribution of the orbital electrons around the metal ions. Therefore, based on the aforementioned results, one can conclude that the electronic structures of ternary MWO4 systems mainly depend on the position of the M2+ cation in the periodic table, which affects the outer electronic arrangements and hybridized electrons of the atomic orbital to the M2+ cation.
Strategies for Enhanced Photocatalytic Activity
As described in Sect. 1, ternary MWO4 systems can act as highly promising photocatalysts for environmental purification and solar water splitting. However, among the major limiting factors in enhancing their photocatalytic activities is the rapid recombination of photogenerated electron and hole pairs. To overcome this problem and improve the overall catalytic performance of MWO4 photocatalysts, many research groups have endeavored to develop various techniques to enhance the separation and transfer efficiency of photoexcited charge carriers inside MWO4 or at the interface between different components. In this section, an overview of the developed strategies is provided to offer insights on their effects for the separation efficiency of photogenerated charge carriers and the corresponding catalytic performance of MWO4-based photocatalysts.
Morphological Control
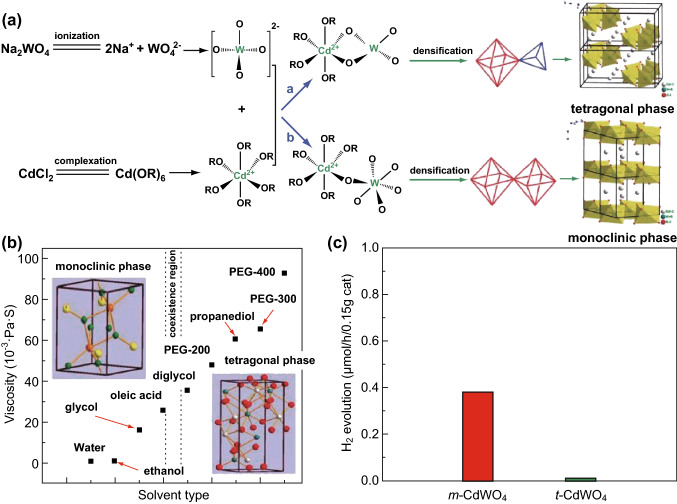
As is known, the morphology, facet exposure, and dimensions of photocatalysts have a significant influence on the photocatalytic performance. For example, nanostructured photocatalysts can exhibit excellent photocatalytic activities because the morphologies and particle sizes of the photocatalysts have a significant influence on the optical and electronic properties, which in turn affect the photocatalytic activities. For example, Yu et al. [106] prepared FeWO4 samples with different morphologies, including nanoparticles, flakes, nanorods (NRs), and a mixture of NRs with flakes and tiny granules, by varying pH values during the hydrothermal process, and systematically investigated their optical properties. The results indicated that the band-gap values of FeWO4 are correlated with specific morphologies. Hosseinpour-Mashkani and co-workers [75] synthesized ZnWO4 nanoparticles with different sizes through a precipitation route using different polymeric surfactants. The photocatalytic degradation experiments of MO demonstrated that the ZnWO4 with the smallest size of 27 nm exhibited the highest photoactivity, compared with other ZnWO4 samples under UV light illumination. It has been demonstrated that particle size can affect the band gap of semiconductors because of quantum size effects. The exposed surface area of nanoparticles increases with a decrease in nanoparticle size, which can provide more active sites for a surface catalytic reaction. In addition to morphologies and particle sizes, size-related crystallinity of MWO4 has an important influence on the photophysical and photocatalytic properties. For example, Tong et al. [74] reported nanostructured CdWO4 with controllable particle sizes via a hydrothermal process and studied the effects of the particle sizes on the lattice symmetry and crystallinity. It was found that the decreased size of the CdWO4 nanoparticles resulted in an expanded lattice, lowered crystallinity, and broadened band gap. Meanwhile, the decrease in particle sizes caused an apparent decrease in the photocatalytic activity of CdWO4. These results illustrate that size-related properties are closely correlated with the photocatalytic activity of MWO4. Apart from the effects of the aforementioned factors on photoactivity, the crystal phase of MWO4 is also regulated by various synthetic routes to obtain the desired physiochemical and optical properties, because different crystal phases can lead to distinct differences in the exposure of crystal facets and reactivities. For instant, different crystal phases of CdWO4 nanocrystals were prepared by adjusting the used solvents for photocatalytic H2 evolution (Fig. 8) [86]. The solvent significantly affected the chelation and growth of the CdWO4 material, thus leading to apparent differences in the crystal phase. m-CdWO4 nanocrystals with particle sizes ranging from 4.4 to 31 nm can be prepared using short-chain solvents, while t-CdWO4 nanocrystals can be easily prepared utilizing long-chain solvents. The obtained m-CdWO4 nanocrystals exhibited much higher H2 production than the t-CdWO4 nanocrystals.
Fig. 8.
a Formation mechanism of m-CdWO4 and t-CdWO4 and the b relationship between different crystal phases of CdWO4 and used solvents. c Photocatalytic H2 evolution of m-CdWO4 and t-CdWO4 under visible light irradiation. Reproduced with permission from Ref. [86]. Copyright 2012 Royal Society of Chemistry
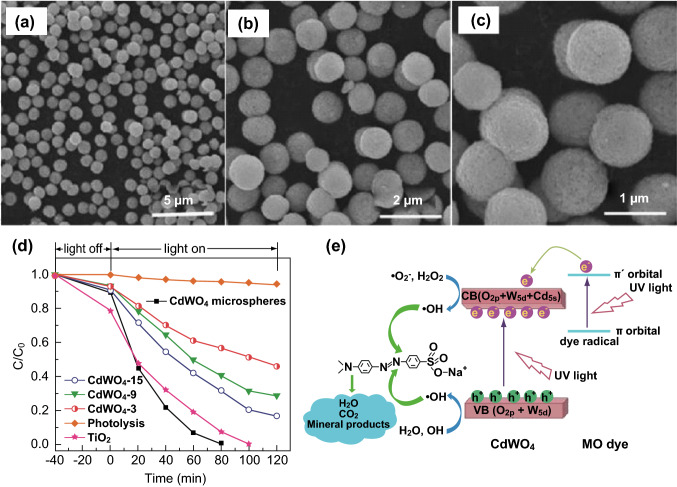
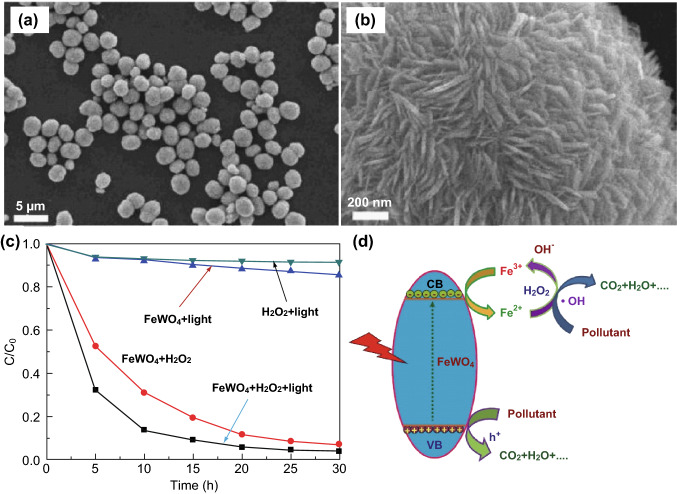
During recent years, the construction of hierarchical structures to tune the morphologies of MWO4 photocatalysts has attracted more attention, because hierarchical structures can offer more exposed surfaces and/or active sites. For example, highly efficient hydrophobic CdWO4 microspheres were synthesized by Hou et al. [107] through a microwave-assisted interfacial hydrothermal strategy. The CdWO4 microspheres showed enhanced photocatalytic activity for the degradation of MO under mercury lamp irradiation (Fig. 9). The advantages of a hierarchical structure for the enhanced photocatalytic activity of MWO4 are supported by several research groups [108–111]. Chen et al. [112] prepared FeWO4 microspheres by using an ethylene glycol-assisted solvothermal approach, in which ionic1-octyl-3-methylimidazolium tetrachloroferrate was used as one of the starting materials and played an important role as both a reactant and template. The microsphere-like FeWO4 material consisted of numerous nanosheets and exhibited a much better photo-Fenton activity in water purification (Fig. 10) because of the generation of hydroxyl radicals, which are produced from the chemical reaction between Fe2+ on the surface of FeWO4 and H2O2. The formed Fe3+ was further reduced by the photoinduced electrons to generate Fe2+, which is a virtuous cycle, to maintain high photocatalytic performance. Recently, Zhou et al. [113] synthesized an urchin-like MnWO4 hierarchical structure through a facile hydrothermal process with the assistance of cetyltrimethylammonium bromide (CTAB) as surfactant. The introduction of CTAB as the surfactant had significant effects on the morphology and magnetic properties of the MnWO4 nanocrystals. Subsequently, Xing et al. [114] provided a new route for the synthesis of a complex three-dimensional (3D) MnWO4 nanostructure. In this case, a 3D flower-like MnWO4 nanocomposite was synthesized using a microemulsion-based solvothermal approach. Moreover, Chen et al. [115] discussed the effect of the morphology of β-SnWO4 on its photocatalytic performance, in which the β-SnWO4 with a multi-armed architecture and hexahedral symmetry displayed much higher photocatalytic activities than those of both cubic β-SnWO4 and commercial WO3. Hence, more surface reaction sites induced by the hierarchically multi-armed architecture and the band structure reframing caused by incorporation of the Sn atom into WO3 contributed to the excellent photocatalytic activity.
Fig. 9.
a–c FESEM images of CdWO4 microspheres. d Photocatalytic degradation efficiencies of MO in the presence of different photocatalysts. e Photocatalytic mechanism for CdWO4 microspheres. Reproduced with permission from Ref. [107]. Copyright 2014 Royal Society of Chemistry
Fig. 10.
a, b FESEM images of FeWO4 microspheres. c Photocatalytic performance of FeWO4 microspheres for the degradation of RhB. d Schematic illustration of photocatalysis mechanism for FeWO4 microspheres. Reproduced with permission from Ref. [112]. Copyright 2016 Elsevier
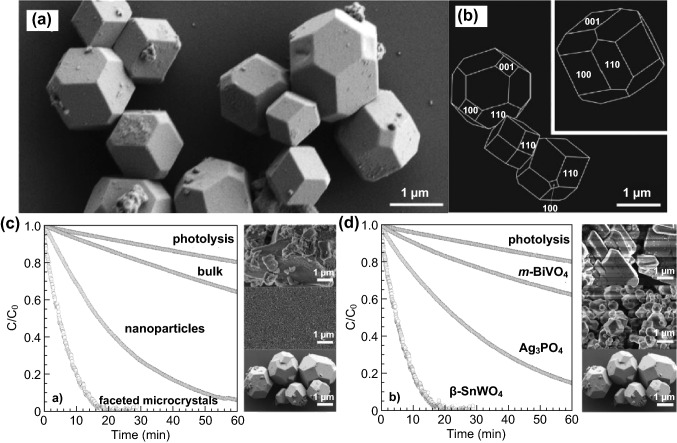
Previously, crystal facet exposure was viewed as an effective strategy to regulate the surface physicochemical and photophysical properties, thus optimizing the reactivity and selectivity of photocatalysts. In general, a crystal facet with a high percentage of unbonded atoms has superior reactivity in comparison to that with a low ratio of unpaired atoms. In addition, crystal facets possessing high surface energy are usually unstable during preparation. It is desirable to synthesize photocatalysts with a high exposure of high-energy crystal facets to enhance the photocatalytic reactivity and selectivity. Using DFT calculations, Qiu and co-workers [116] reported the atom distributions and electronic properties of MnWO4 and FeWO4 with specific facets. The calculated results showed that the {010} and {100} facets have the lowest surface energies in wolframite-type FeWO4 and MnWO4, respectively. Meanwhile, it was observed that the Fe and Mn atoms on the {010} and {001} planes as absorption sites can be used to absorb anions. These results indicated that the exposed {100} facet in MnWO4 and FeWO4 can provide a path for improving their photoactivities, while the other exposed facets can offer a certain selectivity to a specific reaction, such as a dichlorination reaction. Recently, Ungelenk et al. [117] synthesized phase-pure β-SnWO4 with truncated rhombic dodecahedrons using a microemulsion technique with CTAB as a co-surfactant with n-hexanol (Fig. 11). Benefitting from the rapid Na2WO4-induced nucleation and slow crystal growth controlled by the CTAB-mediated microemulsion reaction, the as-prepared β-SnWO4 with truncated rhombic dodecahedrons was encircled with highly exposed {100} and {110} facets. By comparing a series of β-SnWO4 to other photocatalysts of Ag3PO4 and m-BiVO4, the β-SnWO4 microcrystals with exposed {100} and {110} facets showed outstanding photocatalytic activity for the degradation of organic pollutants under daylight irradiation, which was far better than that of other photocatalysts, including bulk non-faceted β-SnWO4 and spherical-like β-SnWO4 nanoparticles. In addition, given the slight difference in specific surface area between the faceted β-SnWO4 and non-faceted β-SnWO4, it was concluded that optimized facet exposure was the predominant reason for the distinct photocatalytic activity. In addition, Tian et al. [118] reported a facile solvothermal method for the synthesis of hierarchical FeWO4 nanosheets with an exposed {100} facet, which exhibited excellent peroxidase-like catalytic activity for oxidizing the peroxidase substrate of 3,3′,5,5′-tetramethylbenzidine (TMB) because of the formation of hydroxyl radicals in the presence of H2O2. The surface Fe2+ in FeWO4 can activate the H2O2 molecule to produce active hydroxyl radicals for the oxidation of TMB. The results clearly indicated that the {100} facet of FeWO4 had a much higher ratio of Fe atoms than that of the {001} and {010} facets, which explained the enhanced catalytic activity of FeWO4 with the exposed {100} facet.
Fig. 11.
a, b FESEM images of faceted β-SnWO4 microcrystals. c, d FESEM images and photocatalytic activities for the degradation of MB over faceted β-SnWO4 microcrystals, bulk β-SnWO4, and β-SnWO4 nanoparticles under sunlight irradiation. Reproduced with permission from Ref. [117]. Copyright 2012 Royal Society of Chemistry
Owing to the high surface area and large absorption cross section it provides, a low-dimensional nanostructure can be constructed to manipulate and regulate optical, electrical, and magnetic properties [119, 120]. Low-dimensional nanostructures including those that are one-dimensional (1D) or two-dimensional (2D) cause the growth of a crystal along one or two directions, which can contribute to more exposure of specific surface facets. For example, 1D CdWO4 NRs were prepared using microwave or hydrothermal approaches and exhibited excellent photocatalytic activity for environmental treatments, as compared to nanoparticles [121–123]. Kovacs and co-workers [111] prepared a series of FeWO4 with different morphologies, including nanoparticles, NRs, and nanosheets, by varying the Fe precursors. The nanosheet-like FeWO4 with band-gap energy of ~ 2.2 eV exhibited excellent absorption ability throughout the UV and visible-light range, which was attributed to the formation of a cavity assembled by nanosheets that resulted in enhanced photon harvest. Therefore, the FeWO4 nanosheets showed higher photocatalytic activity than other control samples for the degradation of organic dyes under visible-light irradiation.
Surface Modification
Considering that the photocatalytic reaction proceeds on the surface of semiconductors, the surface physiochemical properties of semiconductor-based photocatalysts are important for improvement of photocatalytic activity. Several strategies, including chemical etching, surface coverage, and co-catalyst attachment, have been developed to tune the surface properties of semiconductor-based photocatalysts.
The purpose of etching techniques, such as chemical etching and laser or electron-beam irradiation, is to form non-stoichiometric or metal/oxygen vacancies on the surface of an inorganic semiconductor. The formation of metal or oxygen vacancies has proven to have an apparent influence on the electronic distribution because of the introduction of a new defect energy level, thus affecting the light absorption range and photocatalytic activity. For example, Aloysius-Sabu et al. [124] investigated the effects of intentional electron-beam irradiation on the crystal phase, size, and surface properties of CaWO4 that was prepared through chemical precipitation and heat treatment. The experimental results showed that the high-energy electron beam significantly affected the crystal size and surface structure, but not the crystal phase. When the CaWO4 photocatalyst was irradiated by an electron beam, the surface atomic layers of CaWO4 underwent stretching and compressive strain, which resulted in the formation of surface defects and a new energy level in the band gap. Therefore, an apparent absorption tail and narrowed band-gap energy were observed in the irradiated CaWO4 sample. In addition, Lin et al. [125] prepared a visible-light-driven Ag2WO4 photocatalyst through a laser irradiation route in liquid using commercial Ag2WO4 as a starting material for organics degradation and H2 evolution. Because of the laser irradiation, the crystal structure was recrystallized and a lattice defect was introduced in Ag2WO4, leading to the formation of intermediate energy levels with a decrease of 0.44 eV in the band gap. The synthesized Ag2WO4 exhibited a photocatalytic H2 evolution rate as high as 13.73 μmol (hg)−1 under visible-light illumination, while no H2 evolution was observed in the unirradiated commercial Ag2WO4 sample, which was ascribed to a large band gap of 3.22 eV for bulk Ag2WO4.
However, to enhance the solar conversion efficiency of tungstates, surface coverage has been adopted to increase the charge transfer efficiency by means of passivating the surface via deposition of a protective layer. For example, Karthiga and co-workers [71] reported the synthesis of NiWO4 nanoparticles modified by a plant extract, A. indica, as a capping agent through a precipitation route for enhanced photocatalytic activity. The introduction of A. indica, which possesses rich water-soluble heterocyclic groups, led to the formation of a passivation layer on the surface of the NiWO4 nanoparticles, which allowed the NiWO4 nanoparticles to separate well from each other. In comparison to the bare NiWO4 nanoparticles, the A. indica-coated NiWO4 exhibited a much higher photocatalytic activity for the degradation of organic contaminants under visible-light irradiation because of the formation of the passivation layer of the plant extract, which significantly suppressed the recombination of photoinduced electrons and holes. Meanwhile, modified NiWO4 presented higher antimicrobial activity as compared with pure NiWO4 nanoparticles.
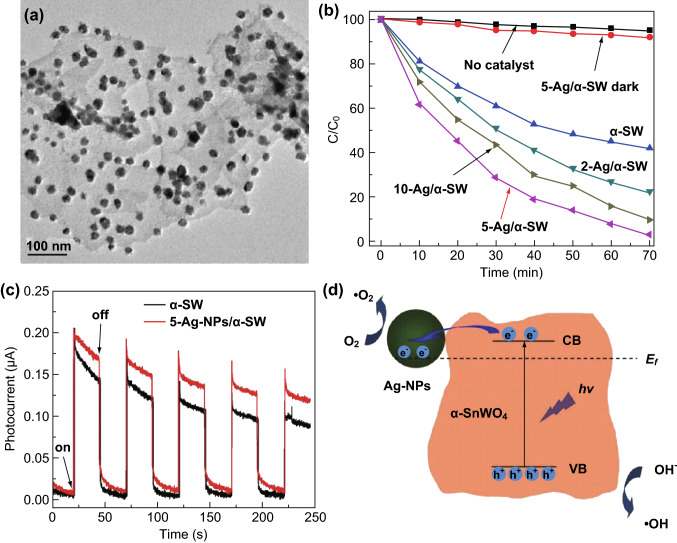
Apart from chemical etching and surface coverage strategies, attachment of noble metal co-catalysts (such as, Pt, Au, and Ag) is another effective means to tune the surface photophysical properties of photocatalysts because of the surface plasmon resonance (SPR) effect [126, 127]. The SPR effect not only significantly enhances visible-light absorption, but also produces a localized electromagnetic field, thus improving the separation efficiency of the photogenerated charge carriers at the interfaces between the metal and semiconductor [128, 129]. Furthermore, the metal–semiconductor heterostructure could efficiently suppress the recombination of photogenerated electrons and holes because of the formation of Schottky barriers at the contacted interface, thus enhancing photocatalytic performance [126]. Based on the aforementioned features, the introduction of a noble metal into MWO4 is a feasible approach to realize enhancement of its photocatalytic performance. Recently, Liu et al. [130] prepared Ag nanoparticles (NPs)/α-SnWO4 nanosheets through microwave-assisted anchoring of Ag NPs on SnWO4 nanosheets. The loading amount of the deposited Ag NPs was well-tuned by adjusting the initial concentration of the Ag+ precursor and CTAB surfactant (Fig. 12a). The obtained Ag NPs/α-SnWO4 hybrid showed enhanced light absorption ability and photocatalytic activity for the degradation of MO, compared to pure α-SnWO4, under visible-light irradiation. Moreover, the hybridized Ag NPs/α-SnWO4 system exhibited improved transient photocurrent density in comparison to that of pristine α-SnWO4 (Fig. 12c), indicating that more photoinduced charge carriers could be produced and further participated in the redox reaction. In addition, Yan et al. [131] synthesized Ag-loaded CdWO4 NRs using a photo-assisted co-precipitation method with the addition of the PEG-100 surfactant, which exhibited a higher photocatalytic activity than that of pure CdWO4 NRs because of the SPR effect. In another study, Au NPs were utilized to construct Schottky barriers in an MWO4-based system. For instance, Au NRs/MnWO4, with a diameter of 20-40 nm, was reported by Chakraborty et al. [132] to enhance the photocatalytic decomposition of 2-propanol and phenol. The Au NPs as co-catalysts in the Au/MnWO4 hybrid were beneficial to multi-electron O2 reduction and hole oxidation.
Fig. 12.
a TEM image and b photodegradation efficiency of Ag NPs/α-SnWO4 under visible-light irradiation. c Transient photocurrent response for different samples. d Illustration of photocatalytic mechanism of Ag NPs/α-SnWO4. Reproduced with permission from Ref. [130]. Copyright 2017 Elsevier
Heteroatom Doping
It has been demonstrated that the introduction of impurities via doping of heteroatoms into a semiconductor can influence the photocatalytic performance [133, 134]. However, heteroatom doping might either have positive or negative impacts for the photocatalytic performance of semiconductors, because there are two different doping levels—the shallow level near the surface and deep level inside the body [135]. Deep-level doping can usually provide a recombination center to intensify the meaningless dissipation of absorbed photons, thus undermining the photocatalytic activity. Therefore, appropriate heteroatom doping is vital to increasing the photocatalytic performance of heteroatom-doped photocatalysts. To overcome shortcomings, such as a narrow wavelength range and rapid recombination of photogenerated electron–hole pairs, a few recent reported types of heteroatom doping to enhance the photocatalytic performances of ternary MWO4 materials are reviewed and their roles discussed in detail.
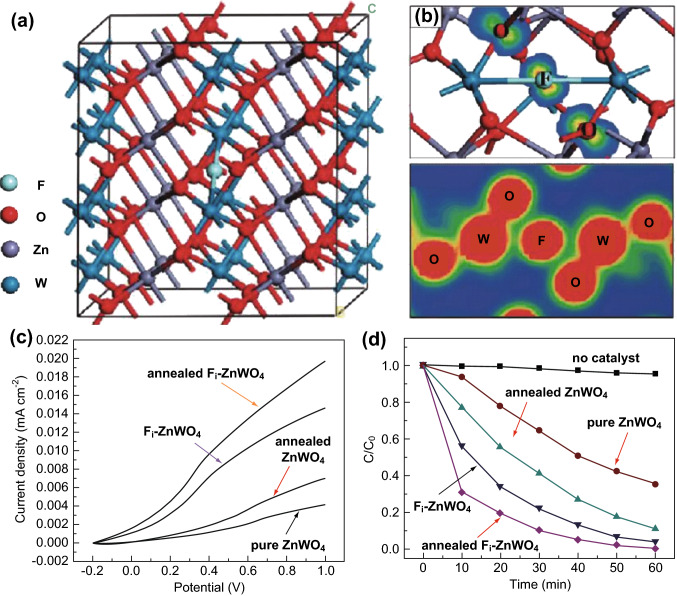
In heteroatom doping, the dopants are mainly non-metal elements such as B [136], Cl [137], and various transition metals (Zn, Ni, Fe, Co, etc.) [138, 139], which exhibit enhanced photocatalytic activity for mineralizing organic pollutants. For instance, Chen et al. [140] synthesized F-doped ZnWO4 nanocrystals (Fi-ZnWO4 nanocrystals) using a two-step hydrothermal process and investigated its chemical bonds via band structure calculations (Fig. 13). In comparison to undoped ZnWO4, the Fi-ZnWO4 nanocrystals exhibited significant red shifts and improved light absorption in the range of UV–visible light, which resulted in enhancement of photocatalytic activity for the degradation of RhB under mercury lamp irradiation. Additionally, the experimental results showed that the crystallinity and morphology of the prepared Fi-ZnWO4 was strongly related to the photocatalytic activity. Fi-ZnWO4 exhibited higher photocatalytic performance for the degradation of organic dyes than Fi-ZnWO4 NPs. Based on the theoretical calculation results, the enhancement of the photocatalytic activity of Fi-ZnWO4 might be ascribed to the following reasons. First, F-doping increased the absorptivity of Fi-ZnWO4, such that it could absorb more reactants to enhance the photocatalytic activity. Second, a new half-filled state was introduced into the original band gap of ZnWO4 accompanying the F-doping, which could provide more holes to enhance the photocurrent density of Fi-ZnWO4. Thus, heteroatom doping could efficiently improve the photocatalytic activities of MWO4 by introducing a new energy level to regulate the original electronic structure.
Fig. 13.
a Geometric structures for the Fi-ZnWO4. Spin density (top) and total charge density (bottom) for Fi-ZnWO4. c Photocurrent density of the synthesized samples under UV-light irradiation. Photocatalytic degradation of RhB over different samples under UV light irradiation. Reprinted with permission from Ref. [140]. Copyright 2010 American Chemical Society
Apart from the introduction of non-metal elements, transition metal elements can also be a potential dopant. For example, Su et al. [141] prepared Zn2+-doped SnWO4 nanocrystals, and reported that the morphological alteration of SnWO4 nanocrystals from nanosheets to nanowires can be controlled by Zn2+ doping. Consequently, the Zn2+-doped SnWO4 exhibited a greater Brunauer–Emmett–Teller surface area (54 and 100 m2 g−1 for pure SnWO4 and Zn2+-doped SnWO4, respectively), narrowed band gap (2.68 and 2.64 eV for pure SnWO4 and Zn2+-doped SnWO4, respectively), and highly enhanced photocatalytic performance for the degradation of MO (95% and 30% for pure SnWO4 and Zn2+-doped SnWO4, respectively) compared to that of pure SnWO4. In addition, Song et al. [142] reported the synthesis of Zn-doped CdWO4 NRs using a hydrothermal process to enhance the photocatalytic conversion efficiency of organic pollutants into low-toxicity small molecules under simulated sunlight irradiation. The influence of the Zn-doping amounts on the crystal phase, morphology, and optoelectronic properties of CdWO4 NRs was also systematically investigated. Compared to the undoped sample, the Zn-doped CdWO4 NRs exhibited much higher photocatalytic activity, which was assigned to the narrowed band gap due to Zn-doping. Heteroatom doping could be an effective means to tune the distribution of the energy level and further enhance the photocatalytic performance of MWO4 without consuming excess foreign substances.
Recently, rare earth element-doped photocatalysts have attracted more attention because of their special 4f electron configuration, which could be beneficial for introducing a suitable energy level into the original band gap of MWO4 [143]. Phuruangrat et al. [144] synthesized Ce-doped ZnWO4 using a hydrothermal process and investigated the influence of Ce doping on the crystal phase, morphology, electronic structure, and photocatalytic activity of ZnWO4. After the introduction of Ce atoms, the photocatalytic activity of ZnWO4 improved with the increase in Ce content, owing to the following two reasons. First, the introduction of the Ce3+ dopant led to the generation of defects on the surface of Ce-doped ZnWO4. Second, the Ce4+ ions on the surface of ZnWO4 could efficiently trap electrons at the CB by the reduction of Ce4+ into Ce3+ ions, thus efficiently suppressing the recombination of photoinduced electrons and holes in the Ce-doped ZnWO4. Therefore, rare earth elements with variable valence, such as Ce, La, and Eu, are promising dopants to improve the photocatalytic activity by trapping photogenerated electrons, consequently limiting the recombination of photogenerated charge carriers.
Heterojunction Fabrication
Hybridization with Semiconductors
Among the aforementioned approaches, constructing a semiconductor heterostructure is an effective means to obtain highly efficient photocatalysts [145–148]. When heterojunctions are composed of different semiconductors that have matching band potentials to form type-I or type-II heterojunctions through realignment of the energy level, the excited photogenerated holes and electrons can be moved from one semiconductor to another in opposite directions driven by the formed built-in electric fields [149, 150], thus strengthening the separation efficiency of the photoinduced electrons and holes and further enhancing photocatalytic performance.
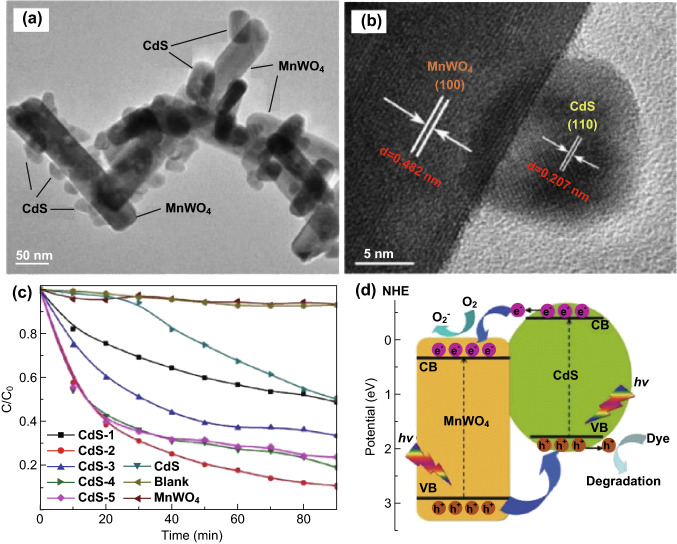
For constructing MWO4-based heterojunctions, semiconductors with a narrow band gap were chosen as a counterpart component to form the heterojunction system, which exhibited remarkable advantages for enhancing the photocatalytic performance. Among most semiconductors with a narrow band gap, cadmium sulfide (CdS) has attracted increasing attention for heterojunction fabrication because of its narrow band gap and high CB position, which could be beneficial for photocatalytic H2 production [151, 152]. Therefore, various CdS/MWO4 heterojunction photocatalysts have been developed, which exhibit enhanced photocatalytic activities in water purification and energy conversion [153, 154]. For instance, Yan et al. [155] prepared a CdS/MnWO4 heterojunction using a facile hydrothermal method to mineralize MB and methyl violet (MV) under visible-light irradiation. Owing to the overlapping of energy bands and tightly contacted interfaces between CdS and MnWO4, the holes at the VB of MnWO4 could transfer to the VB of CdS, and the excited electrons at the CB of CdS could in turn move into the CB of MnWO4. This model could efficiently limit the recombination rate of the photogenerated electrons and holes, thus intensifying the separation of the photogenerated charge carriers in the hybrid system (Fig. 14). Nevertheless, it is apparent that the introduced CdS amount was not synchronous with the photocatalytic activities of the heterojunction, while there was an optimal amount of CdS in the hybrid. Excessive CdS amounts caused severe agglomeration of MnWO4, damaging the heterojunction and worsening the separation efficiency of the photogenerated electrons and holes. This phenomenon was nearly discovered in the heterojunctions by combining two or more components into one system. In addition, Xu et al. [156] prepared a CdS/ZnWO4 heterojunction consisting of ZnWO4 NRs and CdS NPs using a hydrothermal method for the photodegradation of ciprofloxacin antibiotics. Compared to the ZnWO4 NRs and CdS NPs, the CdS/ZnWO4 hybrids showed higher photocatalytic activity than bare ZnWO4 NRs and CdS NPs, which was ascribed to the highly efficient separation of the photogenerated electrons and holes in the hybrid structure.
Fig. 14.
a, b TEM and HRTEM images of a CdS/MnWO4 heterojunction. c Photocatalytic degradation of MB over CdS/MnWO4 with different CdS contents. d Schematic photocatalytic mechanism of CdS/MnWO4 heterojunction. Reproduced with permission from Ref. [155]. Copyright 2017 Elsevier
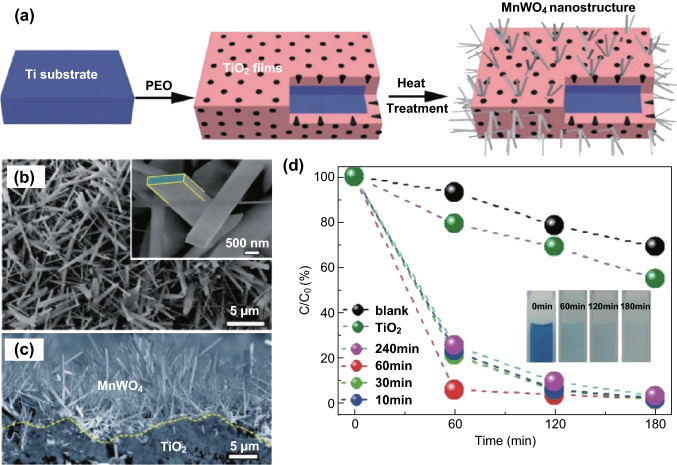
The CdS/CdWO4 heterojunction also exhibited enhanced efficiency in the photocatalytic H2 production. For instance, Jia [157] and Wang et al. [158] deposited CdS on the surface of CdWO4 via an ion-exchange and in situ growth route, and the fabricated Z-scheme CdS/CdWO4 hybrid exhibited significantly enhanced photocatalytic performance for H2 evolution compared to that of the pure CdWO4 and CdS. As discusses in the previous section, the MWO4 with a small M2+ cation has a narrow band-gap energy, which can be considered an efficient solar energy harvester to connect with wide band-gap semiconductors for constructing MWO4-based heterojunctions [159–161]. Thus, Jiang et al. [162] fabricated an MnWO4/TiO2 heterojunction with excellent mechanical adhesion by the in situ growth of MnWO4 on a porous TiO2 film; it presented extremely high photocatalytic performance for degrading MB because of its high crystallinity, large surface area, and strong mechanical properties (Fig. 15). Similarly, Buvaneswari et al. [163] prepared an FeWO4/ZnO heterojunction via a simple co-precipitation route; the band gap of the prepared FeWO4/ZnO heterojunction was estimated to be 2.12 eV, which was apparently smaller than that of ZnO (3.01 eV). In comparison to pristine ZnO, the photocatalytic activity of the FeWO4/ZnO hybrid was substantially enhanced because of the formation of a FeWO4/ZnO heterojunction structure. Additionally, the CuWO4/ZnO hybrid consisting of ZnO NRs and CuWO4 NPs fabricated by Mavric et al. [164] showed enhanced photocatalytic activity compared to that of pure CuWO4 NPs and ZnO NRs.
Fig. 15.
a Schematic diagram describing the formation of MnWO4 nanoplates. b–c FESEM images of the MnWO4 nanoplates and MnWO4 nanoplates on TiO2 film. d Photodegradation efficiency of different samples heated at 850 °C for different times. Reproduced with permission from Ref. [162]. Copyright 2016 Royal Society of Chemistry
Furthermore, a MOx/MWO4 hybrid as a smart-built heterojunction was fabricated using a facile one-pot synthetic strategy to enhance the interaction between MOx and MWO4, in which M is generally reported to be Fe, Ni, Co, or Cu [165–168]. Cao et al. [169] fabricated a novel p-n heterojunction consisting of Fe3O4 NPs and FeWO4 nanowires. The calculated band gap of the FeWO4/Fe3O4 heterojunction was 2.50 eV, lower than that of pristine FeWO4 nanowires. The FeWO4/Fe3O4 heterojunction exhibited enhanced photo-Fenton activity compared to that of the bare FeWO4 nanowires under UV–visible-light irradiation with the addition of H2O2. In addition, a α-SnWO4/SnO2 heterostructure was synthesized with CTAB as the surfactant [170] and displayed enhanced photocatalytic performance compared to that of pure α-SnWO4. Considering that WO3 can be obtained by dehydration from tungstate acid, WO3 is considered to be simultaneously produced during the synthesis of MWO4 and is likely to form a MWO4/WO3 heterojunction, such as CoWO4/WO3 [171, 172], NiWO4/WO3 [173, 174], or CuWO4/WO3 [175, 176]. Aslam et al. [177] prepared a CdWO4/WO3 heterojunction using a hydrothermal and chemisorption method, and reported enhanced photocatalytic activities toward the degradation of organic pollutants, compared with pure CdWO4 and WO3. This section clearly demonstrates in detail that the construction of MWO4-based heterojunction systems is an effective and controllable method for enhancing the photocatalytic activities of MWO4-based semiconductors.
Hybridization with Carbon-Rich Materials
Carbon-rich materials, including carbon nanotubes (CNTs), graphene, and graphitic carbon nitride (g-C3N4), possess unique physical and chemical properties such as a large surface area, high absorption co-efficiency, and chemical stability, ensuring excellent and long-lasting applications in the fields of photochemical and PEC water treatment, photovoltaic devices, and water splitting [178–183]. Carbon-rich materials have a conjugative π structure and unique sp2/sp3 hybrid carbon network, which are suitable substrates for constructing hybrid photocatalysts to intensify the separation and transportation of photoinduced charge carriers inside carbon-rich networks, thus improving the photocatalytic performance [184–187]. Based on this strategy, Gaillard et al. [188] synthesized a novel photoelectrode consisting of CuWO4 and a multi-wall CNT (MWCNT) to tune the photogenerated charge transfer in the nanocomposite film for enhancing the performance of solar-driven PEC water splitting. Compared to the bare CuWO4 photoelectrode, the resistance and photocurrent density of the CuWO4/MWCNT composite photoelectrode decreased and increased by 30% and 26%, respectively. This was mainly attributed to the complete dispersion of the MWCNT as an electron collector in the entire CuWO4 layer. Compared to CNTs, graphene nanosheets produced via the chemical oxidation treatment of graphite have more sp3 hybridized edge structure because of the destroyed perfect sp2 structure. It is well-known that a perfect sp2 carbon structure (CNT) is beneficial for rapid charge mobility, and that an sp3-hybridized carbon structure (graphene) can lead to a small band gap in the semiconductor [189, 190]. Meanwhile, layer-structure carbon materials have a richer porosity substructure assembly from graphene stacking and surface defects, which could provide more reactive sites, in comparison to tube-like carbon materials. Recently, Bai et al. [191] designed a ZnWO4/graphene hybrid, demonstrating that graphene could act as a photo-sensitizer in the hybrid and improve the production of ·OH and ·O2− radicals. The excited photogenerated electrons at the CB of ZnWO4 could be easily injected into the lowest unoccupied molecular orbital (LUMO) of graphene, resulting in a beneficial spatial separation between the holes and electrons inside the ZnWO4/graphene hybrid, in which more holes and electrons can participate in the production of active species, in comparison to bare ZnWO4. Xu et al. [192] reported a CdWO4/graphene hybrid using a hydrothermal process, in which the formed heterojunction showed significantly enhanced photocatalytic activity compared to that of the bare ZnWO4. However, it was found that excessive graphene could have a negative effect on the photocatalytic performance because of the reduced light absorption efficiency of CdWO4 with the addition of the superfluous graphene.
Apart from graphene, other carbon-rich materials, such as g-C3N4 and mesoporous carbon materials, have been considered as promising candidates to build MWO4-based heterojunctions. g-C3N4, which is regarded as an allotrope of C3N4, possesses excellent chemical stability, a suitable band gap of 2.7 eV, and a high specific surface area, which are beneficial for anchoring various other semiconductor materials [193–195]. For instance, Sun et al. [196] synthesized g-C3N4/ZnWO4 NRs via a thermal treatment route, and investigated the microcosmic mechanisms of the enhanced photocatalytic activity of C3N4/ZnWO4 in comparison to pristine ZnWO4 NRs and C3N4. Together with theoretical calculation results, it was found that the well-matched energy level alignment of the C3N4 and ZnWO4 NRs was the primary reason for the enhancement of the photocatalytic performance, in which the electrons at the VB top edge of the C3N4, when excited by the incident light, quickly jumped to the CB bottom edge of C3N4 and then transferred to the CB of ZnWO4 because of the staged alignment, thus enhancing the charge transfer and separation efficiency. In addition, Tian et al. [197] synthesized a CdWO4/C3N4 hybrid using a hydrothermal process, followed by a mixed-calcination treatment. Compared to pure CdWO4, the CdWO4/C3N4 heterojunction exhibited higher photocatalytic activity (Fig. 16c), the proposed mechanism for which is shown in Fig. 16d. The matched band structures between the two components contributed to the separation and transfer process, in which the excited electrons at the CB of C3N4 moved to the CB of CdWO4 following a downward staged band, such that the photoinduced electrons and holes were efficiently separated, thus enhancing the overall photocatalytic activity. Considering its appropriate band energy level, g-C3N4 is usually used to construct a Z-scheme hybrid system to enhance photocatalytic activities. Instead of a type-II heterojunction, the direct Z-scheme configuration not only forms a built-in electronic field to promote the separation and transfer efficiency of photogenerated electrons and holes, but it also maintains the reductive and oxidative ability of electrons and holes [198, 199]. Recently, Zhu and co-workers [200] reported a direct Z-scheme heterojunction by combining g-C3N4 with Ag2WO4 through a facile precipitation route. Because of the use of g-C3N4 as a support in the precursor solution, Ag2WO4 was able to nucleate and grow on the surface of the layered C3N4, thus resulting in the Ag2WO4 evenly distributing on the surface of the layered g-C3N4-nanosheets. In comparison to the bare Ag2WO4 and g-C3N4, the Z-scheme of the g-C3N4/Ag2WO4 hybrid system exhibited a much higher photoactivity for the degradation of MO, owing to the efficient separation between the photoinduced electrons and holes in the direct Z-scheme configuration. Given the aforementioned examples and explanations, it is obvious that carbon-rich materials possess a high charge carrier mobility and large specific surface area, which could efficiently promote the separation efficiency of photoexcited electrons and holes in the MWO4, ultimately leading to improvement of the photocatalytic activity.
Fig. 16.
a TEM image, b UV–vis diffused reflectance spectra, and c photocatalytic degradation curves of CdWO4/C3N4. d Mechanism of photocatalytic reaction on the CdWO4/C3N4. Reproduced with permission from Ref. [197]. Copyright 2015 Elsevier
Summary and Outlook
This review summarized the development of novel strategies to enhance the photocatalytic performance of MWO4-based materials with a special emphasis on their applications in environmental purification and solar water splitting. Although significant improvements have been achieved in the construction of highly efficient ternary MWO4-based oxides, challenges remain that need to be addressed. First, the recombination rate of photogenerated charge carriers for MWO4-based photocatalysts is still considerably high, accounting for poor reduction ability in the photoexcited electrons at a low potential of the CB edge, which are easily quenched by defects and holes. Second, although morphological engineering could improve the photocatalytic activity of MWO4-based systems by regulating the crystal structure, particle size, and surface area, the current synthetic methods are inadequate for large-scale preparation, particularly for nanosized materials, which would significantly improve the separation efficiency of the photogenerated charge carriers. Third, the surface effect, particularly the crystal surface effect on the photocatalytic performance of MWO4-based systems, has not been synergistically and comprehensively investigated. It is thought that the atom configurations and surface defects should be paid more attention, to provide important information for designing highly efficient photocatalysts. Fourth, based on this review, it is clear that the MWO4 materials consisting of a single valence metal ion, such as Cd, Zn, or Sn, have been well-developed in the past, while those composed of a versatile valence metal, for instance, Co, Fe, or Ni, have been insufficiently explored in surface engineering and theoretical computation.
To overcome these challenges, future research needs to focus on the exploration of novel photocatalytic materials. Although the sunlight-harvesting ability and separation efficiency of photogenerated charge carriers could be strengthened by heteroatom doping or heterojunction fabrication as reported by previous literature, traditional material screening, high-throughput screening, and computational materials design can guide the construction of photocatalysts with a proper band edge position and suitable band gap, thereby shortening the experimental period, reducing the workload, and saving experimental cost. In other fundamental studies, combining experiment and theory would enable understanding the photocatalytic principles and screen alternative high-performance photocatalysts. Future work also needs to focus on facile synthetic approaches for constructing stable MWO4 materials with high active crystal surface and/or quantum size, and developing advanced techniques for large-scale production. It is expected that further progress in ternary MWO4-based photocatalysts for applications in environmental purification and solar water splitting will be made in future studies.
Acknowledgements
Y. Hou thanks the support of NSFC 51702284, Fundamental Research Funds for the Central Universities (112109*172210171) and the Startup Foundation for Hundred-Talent Program of Zhejiang University (112100-193820101/001/022). J. Ke thanks the support of the NSFC 21501138, the Science Research Foundation of Wuhan Institute of Technology (K201513).
Contributor Information
Jie Liu, Email: liujiedut@hotmail.com.
Yang Hou, Email: yhou@zju.edu.cn.
References
- 1.Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238:37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- 2.Chen XP, Zhang ZX, Chi L, Nair AK, Shangguan WF, Jiang Z. Recent advances in visible-light-driven photoelectrochemical water splitting: catalyst nanostructures and reaction systems. Nano-Micro Lett. 2016;8(1):1–12. doi: 10.1007/s40820-015-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou Y, Li X, Zhao Q, Chen G, Raston CL. Role of hydroxyl radicals and mechanism of Escherichia coli inactivation on Ag/AgBr/TiO2 nanotube array electrode under visible light irradiation. Environ. Sci. Technol. 2012;46(7):4042–4050. doi: 10.1021/es204079d. [DOI] [PubMed] [Google Scholar]
- 4.Cheng L, Xiang QJ, Liao YL, Zhang HW. CdS-Based photocatalysts. Energ. Environ. Sci. 2018;11(6):1362–1391. doi: 10.1039/c7ee03640j. [DOI] [Google Scholar]
- 5.Wang F, Li Q, Xu D. Recent progress in semiconductor-based nanocomposite photocatalysts for solar to chemical energy conversion. Adv. Energy Mater. 2017;7(23):1700529. doi: 10.1002/aenm.201700529. [DOI] [Google Scholar]
- 6.Hou Y, Zhuang X, Feng X. Recent advances in earth-abundant heterogeneous electrocatalysts for photoelectrochemical water splitting. Small Methods. 2017;1(6):1700090. doi: 10.1002/smtd.201700090. [DOI] [Google Scholar]
- 7.Hou Y, Li X, Zhao Q, Chen G. ZnFe2O4 multi-porous microbricks/graphene hybrid photocatalyst: facile synthesis, improved activity and photocatalytic mechanism. Appl. Catal. B Environ. 2013;142–143:80–88. doi: 10.1016/j.apcatb.2013.04.062. [DOI] [Google Scholar]
- 8.Hou Y, Li X, Zhao Q, Quan X, Chen G. Electrochemically assisted photocatalytic degradation of 4-chlorophenol by ZnFe2O4-modified TiO2 nanotube array electrode under visible light irradiation. Environ. Sci. Technol. 2010;44(13):5098–5103. doi: 10.1021/es100004u. [DOI] [PubMed] [Google Scholar]
- 9.Teoh WY, Scott JA, Amal R. Progress in heterogeneous photocatalysis: from classical radical chemistry to engineering nanomaterials and solar reactors. J. Phys. Chem. Lett. 2012;3(5):629–639. doi: 10.1021/jz3000646. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee S, Pillai SC, Falaras P, O’Shea KE, Byrne JA, Dionysiou DD. New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 2014;5(15):2543–2554. doi: 10.1021/jz501030x. [DOI] [PubMed] [Google Scholar]
- 11.Schneider J, Matsuoka M, Takeuchi M, Zhang J, Horiuchi Y, Anpo M, Bahnemann DW. Understanding TiO2 photocatalysis: mechanisms and materials. Chem. Rev. 2014;114(19):9919–9986. doi: 10.1021/cr5001892. [DOI] [PubMed] [Google Scholar]
- 12.Kale MJ, Avanesian T, Christopher P. Direct photocatalysis by plasmonic nanostructures. ACS Catal. 2013;4(1):116–128. doi: 10.1021/cs400993w. [DOI] [Google Scholar]
- 13.Augugliaro V, Camera-Roda G, Loddo V, Palmisano G, Palmisano L, Soria J, Yurdakal S. Heterogeneous photocatalysis and photoelectrocatalysis: from unselective abatement of noxious species to selective production of high-value chemicals. J. Phys. Chem. Lett. 2015;6(10):1968–1981. doi: 10.1021/acs.jpclett.5b00294. [DOI] [PubMed] [Google Scholar]
- 14.Xie S, Zhang Q, Liu G, Wang Y. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures. Chem. Commun. 2016;52(1):35–59. doi: 10.1039/c5cc07613g. [DOI] [PubMed] [Google Scholar]
- 15.García A, Fernandez-Blanco C, Herance JR, Albero J, García H. Graphenes as additives in photoelectrocatalysis. J. Mater. Chem. A. 2017;5(32):16522–16536. doi: 10.1039/c7ta04045h. [DOI] [Google Scholar]
- 16.Laursen AB, Kegnæs S, Dahl S, Chorkendorff I. Molybdenum sulfides-efficient and viable materials for electro- and photoelectrocatalytic hydrogen evolution. Energy Environ. Sci. 2012;5(2):5577–5591. doi: 10.1039/c2ee02618j. [DOI] [Google Scholar]
- 17.Yang Y, Ajmal S, Zheng X, Zhang L. Efficient nanomaterials for harvesting clean fuels from electrochemical and photoelectrochemical CO2 reduction. Sustain. Energ. Fuels. 2018;2(3):510–537. doi: 10.1039/c7se00371d. [DOI] [Google Scholar]
- 18.Zhou R, Zhou R, Zhang X, Li J, Wang X, et al. Synergistic effect of atmospheric-pressure plasma and TiO2 photocatalysis on inactivation of Escherichia coli cells in aqueous media. Sci. Rep. 2016;6:39552. doi: 10.1038/srep39552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou JJ, Liu CJ, Zhang YP. Control of the metal-support interface of NiO-loaded photocatalysts via cold plasma treatment. Langmuir. 2006;22(5):2334–2339. doi: 10.1021/la052135u. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Kong G, Meng Y, Tian J, Zhang L, Wan S, Lin J, Wang Y. Direct coupling of thermo- and photocatalysis for conversion of CO2-H2O into fuels. Chemsuschem. 2017;10(23):4709–4714. doi: 10.1002/cssc.201701472. [DOI] [PubMed] [Google Scholar]
- 21.Kho ET, Tan TH, Lovell E, Wong RJ, Scott J, Amal R. A review on photo-thermal catalytic conversion of carbon dioxide. Green Energy Environ. 2017;2(3):204–217. doi: 10.1016/j.gee.2017.06.003. [DOI] [Google Scholar]
- 22.Jia J, Wang H, Lu Z, O’Brien PG, Ghoussoub M, et al. Photothermal catalyst engineering: hydrogenation of gaseous CO2 with high activity and tailored selectivity. Adv. Sci. 2017;4(10):1700252. doi: 10.1002/advs.201700252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Nanayakkara CE, Grassian VH. Titanium dioxide photocatalysis in atmospheric chemistry. Chem. Rev. 2012;112(11):5919–5948. doi: 10.1021/cr3002092. [DOI] [PubMed] [Google Scholar]
- 24.Wan L, Long M, Zhou D, Zhang L, Cai W. Preparation and characterization of freestanding hierarchical porous TiO2 monolith modified with graphene oxide. Nano-Micro Lett. 2012;4(2):90–97. doi: 10.1007/bf03353698. [DOI] [Google Scholar]
- 25.Boehme M, Ensinger W. Mixed phase anatase/rutile titanium dioxide nanotubes for enhanced photocatalytic degradation of methylene blue. Nano-Micro Lett. 2011;3(4):236–241. doi: 10.1007/bf03353678. [DOI] [Google Scholar]
- 26.Li X, Li J, Bai J, Dong Y, Li L, Zhou B. The inhibition effect of tert-butyl alcohol on the TiO2 nano assays photoelectrocatalytic degradation of different organics and its mechanism. Nano-Micro Lett. 2016;8(3):221–231. doi: 10.1007/s40820-015-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Jiang Y, Cheng W, Li Y, Xu X, Lin K. Mesoporous TiO2/carbon beads: one-pot preparation and their application in visible-light-induced photodegradation. Nano-Micro Lett. 2015;7(3):243–254. doi: 10.1007/s40820-015-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou Y, Zuo F, Dagg A, Feng P. Visible light-driven α-Fe2O3 nanorod/graphene/BiV1–xMoxO4 core/shell heterojunction array for efficient photoelectrochemical water splitting. Nano Lett. 2012;12(12):6464–6473. doi: 10.1021/nl303961c. [DOI] [PubMed] [Google Scholar]
- 29.Ke J, Zhou H, Liu J, Duan X, Zhang H, Liu S, Wang S. Crystal transformation of 2D tungstic acid H2WO4 to WO3 for enhanced photocatalytic water oxidation. J. Colloid Interface Sci. 2018;514:576–583. doi: 10.1016/j.jcis.2017.12.066. [DOI] [PubMed] [Google Scholar]
- 30.Hou Y, Zuo F, Dagg AP, Liu J, Feng P. Branched WO3 nanosheet array with layered C3N4 heterojunctions and CoOx nanoparticles as a flexible photoanode for efficient photoelectrochemical water oxidation. Adv. Mater. 2014;26(29):5043–5049. doi: 10.1002/adma.201401032. [DOI] [PubMed] [Google Scholar]
- 31.Luo S, Ke J, Yuan M, Zhang Q, Xie P, Deng L, Wang S. CuInS2 quantum dots embedded in Bi2WO6 nanoflowers for enhanced visible light photocatalytic removal of contaminants. Appl. Catal. B: Environ. 2018;221:215–222. doi: 10.1016/j.apcatb.2017.09.028. [DOI] [Google Scholar]
- 32.Kumar SG, Rao KSRK. Zinc oxide based photocatalysis: tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015;5(5):3306–3351. doi: 10.1039/c4ra13299h. [DOI] [Google Scholar]
- 33.Ke J, Liu J, Sun H, Zhang H, Duan X, et al. Facile assembly of Bi2O3/Bi2S3/MoS2 n-p heterojunction with layered n-Bi2O3 and p-MoS2 for enhanced photocatalytic water oxidation and pollutant degradation. Appl. Catal. B: Environ. 2017;200:47–55. doi: 10.1016/j.apcatb.2016.06.071. [DOI] [Google Scholar]
- 34.Liu J, Li Y, Ke J, Wang S, Wang L, Xiao H. Black NiO-TiO2 nanorods for solar photocatalysis: recognition of electronic structure and reaction mechanism. Appl. Catal. B: Environ. 2018;224:705–714. doi: 10.1016/j.apcatb.2017.11.028. [DOI] [Google Scholar]
- 35.Kumar SG, Devi LG. Review on modified TiO2 photocatalysis under UV/visible light: selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A. 2011;115(46):13211–13241. doi: 10.1021/jp204364a. [DOI] [PubMed] [Google Scholar]
- 36.Ni M, Leung MKH, Leung DYC, Sumathy K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energ. Rev. 2007;11(3):401–425. doi: 10.1016/j.rser.2005.01.009. [DOI] [Google Scholar]
- 37.Ke J, Li X, Zhao Q, Liu B, Liu S, Wang S. Upconversion carbon quantum dots as visible light responsive component for efficient enhancement of photocatalytic performance. J. Colloid Interface Sci. 2017;496:425–433. doi: 10.1016/j.jcis.2017.01.121. [DOI] [PubMed] [Google Scholar]
- 38.Fujishima A, Zhang X, Tryk D. Heterogeneous photocatalysis: from water photolysis to applications in environmental cleanup. Int. J. Hydrog. Energy. 2007;32(14):2664–2672. doi: 10.1016/j.ijhydene.2006.09.009. [DOI] [Google Scholar]
- 39.Zou X, Dong Y, Li S, Ke J, Cui Y. Facile anion exchange to construct uniform AgX (X = Cl, Br, I)/Ag2CrO4 NR hybrids for efficient visible light driven photocatalytic activity. Sol. Energy. 2018;169:392–400. doi: 10.1016/j.solener.2018.05.017. [DOI] [Google Scholar]
- 40.Hoffmann MR, Martin ST, Choi WY, Bahnemann DW. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995;95(28):69–96. doi: 10.1021/cr00033a004. [DOI] [Google Scholar]
- 41.Chen D, Zhang XG, Lee AF. Synthetic strategies to nanostructured photocatalysts for CO2 reduction to solar fuels and chemicals. J. Mater. Chem. A. 2015;3(28):14487–14516. doi: 10.1039/c5ta01592h. [DOI] [Google Scholar]
- 42.Cheng HF, Fuku K, Kuwahara Y, Mori K, Yamashita H. Harnessing single-active plasmonic nanostructures for enhanced photocatalysis under visible light. J. Mater. Chem. A. 2015;3(10):5244–5258. doi: 10.1039/c4ta06484d. [DOI] [Google Scholar]
- 43.Wenderich K, Mul G. Methods, mechanism, and applications of photodeposition in photocatalysis: a review. Chem. Rev. 2016;116(23):14587–14619. doi: 10.1021/acs.chemrev.6b00327. [DOI] [PubMed] [Google Scholar]
- 44.Hisatomi T, Kubota J, Domen K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014;43(22):7520–7535. doi: 10.1039/c3cs60378d. [DOI] [PubMed] [Google Scholar]
- 45.Zhang G, Liu G, Wang L, Irvine JTS. Inorganic perovskite photocatalysts for solar energy utilization. Chem. Soc. Rev. 2016;45(21):5951–5984. doi: 10.1039/c5cs00769k. [DOI] [PubMed] [Google Scholar]
- 46.Fabian DM, Hu S, Singh N, Houle FA, Hisatomi T, Domen K, Osterloh FE, Ardo S. Particle suspension reactors and materials for solar-driven water splitting. Energy Environ. Sci. 2015;8(10):2825–2850. doi: 10.1039/c5ee01434d. [DOI] [Google Scholar]
- 47.Takata T, Domen K. Development of non-oxide semiconductors as light harvesting materials in photocatalytic and photoelectrochemical water splitting. Dalton Trans. 2017;46(32):10529–10544. doi: 10.1039/c7dt00867h. [DOI] [PubMed] [Google Scholar]
- 48.Mal P, Bera G, Rambabu P, Turpu GR, Chakraborty B, et al. Electronic, magnetic and spectroscopic properties of doped Mn(1-x) AxWO4 (A = Co, Cu, Ni and Fe) multiferroic: an experimental and DFT study. J. Phys. Condens. Matter. 2017;29(7):075901. doi: 10.1088/1361-648X/aa4e64. [DOI] [PubMed] [Google Scholar]
- 49.Zawawi SMM, Yahya R, Hassan Z, Ekramul Mahmud EHNM, Daud NM. Structural and optical characterization of metal tungstates (MWO4; M = Ni, Ba, Bi) synthesized by a sucrose-templated method. Chem. Cent. J. 2013;7:80. doi: 10.1186/1752-153X-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.López XA, Fuentes AF, Zaragoza MM, Díaz Guillén JA, Gutiérrez JS, Ortiz AL, Collins-Martínez V. Synthesis, characterization and photocatalytic evaluation of MWO4 (M = Ni Co, Cu and Mn) tungstates. Int. J. Hydrog. Energy. 2016;41(48):23312–23317. doi: 10.1016/j.ijhydene.2016.10.117. [DOI] [Google Scholar]
- 51.Dey S, Ricciardo RA, Cuthbert HL, Woodward PM. Metal-to-metal charge transfer in AWO4 (A = Mg, Mn Co, Ni, Cu, or Zn) compounds with the wolframite structure. Inorg. Chem. 2014;53(9):4299–4394. doi: 10.1021/ic4031798. [DOI] [PubMed] [Google Scholar]
- 52.Nikl M, Bohacek P, Mihokova E, Kobayashi M, Ishii M, et al. Excitonic emission of scheelite tungstates AWO4 (A = Pb, Ca, Ba, Sr) J. Lumin. 2000;87–89:1136–1139. doi: 10.1016/S0022-2313(99)00569-4. [DOI] [Google Scholar]
- 53.Pullar RC, Farrah S, Alford NM. MgWO4, ZnWO4, NiWO4 and CoWO4 microwave dielectric ceramics. J. Eur. Ceram. Soc. 2007;27(2–3):1059–1063. doi: 10.1016/j.jeurceramsoc.2006.05.085. [DOI] [Google Scholar]
- 54.Zhang G, Jia R, Wu Q. Preparation, structural and optical properties of AWO4 (A = Ca, Ba, Sr) nanofilms. Mater. Sci. Eng. B. 2006;128(1–3):254–259. doi: 10.1016/j.mseb.2005.11.040. [DOI] [Google Scholar]
- 55.Lacomba-Perales R, Ruiz-Fuertes J, Errandonea D, Martínez-García D, Segura A. Optical absorption of divalent metal tungstates: correlation between the band-gap energy and the cation ionic radius. EPL. 2008;83(3):37002. doi: 10.1209/0295-5075/83/37002. [DOI] [Google Scholar]
- 56.Shivakumara C, Saraf R, Behera S, Dhananjaya N, Nagabhushana H. Scheelite-type MWO4 (M = Ca, Sr, and Ba) nanophosphors: facile synthesis, structural characterization, photoluminescence, and photocatalytic properties. Mater. Res. Bull. 2015;61:422–432. doi: 10.1016/j.materresbull.2014.09.096. [DOI] [Google Scholar]
- 57.Ramarao SD, Roopas Kiran S, Murthy VRK. Structural, lattice vibrational, optical and microwave dielectric studies on Ca1−xSrxMoO4 ceramics with scheelite structure. Mater. Res. Bull. 2014;56:71–79. doi: 10.1016/j.materresbull.2014.04.064. [DOI] [Google Scholar]
- 58.Su YG, Li GS, Xue YF, Li LP. Tunable physical properties of CaWO4 nanocrystals via particle size control. J. Phys. Chem. C. 2007;111(18):6684–6689. doi: 10.1021/jp068480p. [DOI] [Google Scholar]
- 59.Ghoreishi SM. Facile synthesis and characterization of CaWO4 nanoparticles using a new Schiff base as capping agent: enhanced photocatalytic degradation of methyl orange. J. Mater. Sci. Mater. Electron. 2017;28(19):14833–14838. doi: 10.1007/s10854-017-7354-z. [DOI] [Google Scholar]
- 60.Mohamed Jaffer Sadiq M, Samson Nesaraj A. Soft chemical synthesis and characterization of BaWO4 nanoparticles for photocatalytic removal of Rhodamine B present in water sample. J. Nanostruct. Chem. 2014;5(1):45–54. doi: 10.1007/s40097-014-0133-y. [DOI] [Google Scholar]
- 61.Oliveira MC, Gracia L, Nogueira IC, Carmo Gurgel MFD, Mercury JMR, Longo E, Andrés J. Synthesis and morphological transformation of BaWO4 crystals: experimental and theoretical insights. Ceram. Int. 2016;42(9):10913–10921. doi: 10.1016/j.ceramint.2016.03.225. [DOI] [Google Scholar]
- 62.Yu C, Cao F, Li X, Li G, Xie Y, Yu JC, Shu Q, Fan Q, Chen J. Hydrothermal synthesis and characterization of novel PbWO4 microspheres with hierarchical nanostructures and enhanced photocatalytic performance in dye degradation. Chem. Eng. J. 2013;219:86–95. doi: 10.1016/j.cej.2012.12.064. [DOI] [Google Scholar]
- 63.Chen D, Liu Z, Ouyang SX, Ye JH. Simple room-temperature mineralization method to SrWO4 micro/nanostructures and their photocatalytic properties. J. Phys. Chem. C. 2011;115(32):15778–15784. doi: 10.1021/jp202406n. [DOI] [Google Scholar]
- 64.Ye D, Li DZ, Zhang WJ, Sun M, Hu Y, Zhang YF, Fu XZ. A new photocatalyst CdWO4 prepared with a hydrothermal method. J. Phys. Chem. C. 2008;112(44):17351–17356. doi: 10.1021/jp8059213. [DOI] [Google Scholar]
- 65.Priya AM, Selvan RK, Senthilkumar B, Satheeshkumar MK, Sanjeeviraja C. Synthesis and characterization of CdWO4 nanocrystals. Ceram. Int. 2011;37(7):2485–2488. doi: 10.1016/j.ceramint.2011.03.040. [DOI] [Google Scholar]
- 66.Zhang C, Zhang H, Zhang K, Li X, Leng Q, Hu C. Photocatalytic activity of ZnWO4: band structure, morphology and surface modification. ACS Appl. Mater. Interfaces. 2014;6(16):14423–14432. doi: 10.1021/am503696b. [DOI] [PubMed] [Google Scholar]
- 67.Guo J, Zhou X, Lu Y, Zhang X, Kuang S, Hou W. Monodisperse spindle-like FeWO4 nanoparticles: controlled hydrothermal synthesis and enhanced optical properties. J. Solid State Chem. 2012;196:550–556. doi: 10.1016/j.jssc.2012.07.026. [DOI] [Google Scholar]
- 68.Sivakumar M, Madhu R, Chen SM, Veeramani V, Manikandan A, Hung WH, Miyamoto N, Chueh YL. Low-temperature chemical synthesis of CoWO4 nanospheres for sensitive nonenzymatic glucose sensor. J. Phys. Chem. C. 2016;120(30):17024–17028. doi: 10.1021/acs.jpcc.6b04116. [DOI] [Google Scholar]
- 69.Ahmadi F, Rahimi-Nasrabadi M, Eghbali-Arani M. The synthesize of CuWO4 nanoparticles by a new morphological control method, characterization of its photocatalytic activity. J. Mater. Sci. Mater. Electron. 2016;28(7):5244–5249. doi: 10.1007/s10854-016-6181-y. [DOI] [Google Scholar]
- 70.Vosoughifar M. Preparation, characterization, and morphological control of MnWO4 nanoparticles through novel method and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2016;28(2):2135–2140. doi: 10.1007/s10854-016-5777-6. [DOI] [Google Scholar]
- 71.Karthiga R, Kavitha B, Rajarajan M, Suganthi A. Photocatalytic and antimicrobial activity of NiWO4 nanoparticles stabilized by the plant extract. Mater. Sci. Semicond. Process. 2015;40:123–129. doi: 10.1016/j.mssp.2015.05.037. [DOI] [Google Scholar]
- 72.Zhu G, Que W, Zhang J, Zhong P. Photocatalytic activity of SnWO4 and SnW3O9 nanostructures prepared by a surfactant-assisted hydrothermal process. Mater. Sci. Eng. B. 2011;176(18):1448–1455. doi: 10.1016/j.mseb.2011.08.003. [DOI] [Google Scholar]
- 73.Pourmasoud S, Eghbali-Arani M, Ahmadi F, Rahimi-Nasrabadi M. Synthesis, characterization, and morphological control of PbWO4 nanostructures through precipitation method and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2017;28(22):17089–17097. doi: 10.1007/s10854-017-7635-6. [DOI] [Google Scholar]
- 74.Tong WM, Li LP, Hu WB, Yan TJ, Li GS. Systematic control of monoclinic CdWO4 nanophase for optimum photocatalytic activity. J. Phys. Chem. C. 2010;114(3):1512–1519. doi: 10.1021/jp910284u. [DOI] [Google Scholar]
- 75.Hosseinpour-Mashkani SM, Maddahfar M, Sobhani-Nasab A. Precipitation synthesis, characterization, morphological control, and photocatalyst application of ZnWO4 nanoparticles. J. Electron. Mater. 2016;45(7):3612–3620. doi: 10.1007/s11664-016-4532-3. [DOI] [Google Scholar]
- 76.Hosseinpour-mashkani SM, Sobhani-Nasab A. Simple synthesis and characterization of copper tungstate nanoparticles: investigation of surfactant effect and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2016;27(7):7548–7553. doi: 10.1007/s10854-016-4735-7. [DOI] [Google Scholar]
- 77.Wannapop S, Thongtem T, Thongtem S. Photoemission and energy gap of MgWO4 particles connecting as nanofibers synthesized by electrospinning-calcination combinations. Appl. Surf. Sci. 2012;258(11):4971–4976. doi: 10.1016/j.apsusc.2012.01.133. [DOI] [Google Scholar]
- 78.Raj AT, Thangavel S, Rose A, Jipsa CV, Jose M, Nallamuthu G, Kim SJ, Venugopal G. Influence of morphology and common oxidants on the photocatalytic property of β-SnWO4 nanoparticles. J. Nanosci. Nanotechnol. 2016;16(3):2541–2547. doi: 10.1166/jnn.2016.10961. [DOI] [PubMed] [Google Scholar]
- 79.Tong WM, Li LP, Hu WB, Yan TJ, Guan XF, Li GS. Kinetic control of MnWO4 nanoparticles for tailored structural properties. J. Phys. Chem. C. 2010;114(36):15298–15305. doi: 10.1021/jp103879c. [DOI] [Google Scholar]
- 80.Ahmed MI, Adam A, Khan A, Rehman AU, Qamaruddin M, Siddiqui MN, Qamar M. Improved photoelectrochemical water oxidation under visible light with mesoporous CoWO4. Mater. Lett. 2016;183:281–284. doi: 10.1016/j.matlet.2016.07.137. [DOI] [Google Scholar]
- 81.Gao Q, Liu Z. FeWO4 nanorods with excellent UV-Visible light photocatalysis. Prog. Nat. Sci. Mater. 2017;27(5):556–560. doi: 10.1016/j.pnsc.2017.08.016. [DOI] [Google Scholar]
- 82.Di J, Xiong J, Li H, Liu Z. Ultrathin 2D photocatalysts: electronic-structure tailoring, hybridization, and applications. Adv. Mater. 2018;30(1):1704548. doi: 10.1002/adma.201704548. [DOI] [PubMed] [Google Scholar]
- 83.Yan T, Li L, Tong W, Zheng J, Wang Y, Li G. CdWO4 polymorphs: selective preparation, electronic structures, and photocatalytic activities. J. Solid State Chem. 2011;184(2):357–364. doi: 10.1016/j.jssc.2010.12.013. [DOI] [Google Scholar]
- 84.Cai LG, Liu FM, Zhang D, Zhong WW. Dependence of optical properties of monoclinic MnWO4 on the electric field of incident light. Phys. B. 2012;407(17):3654–3659. doi: 10.1016/j.physb.2012.05.044. [DOI] [Google Scholar]
- 85.Hosseinpour-Mashkani SM, Sobhani-Nasab A. A simple sonochemical synthesis and characterization of CdWO4 nanoparticles and its photocatalytic application. J. Mater. Sci. Mater. Electron. 2015;27(4):3240–3244. doi: 10.1007/s10854-015-4150-5. [DOI] [Google Scholar]
- 86.Wang Y, Guan X, Li L, Lin H, Wang X, Li G. Solvent-driven polymorphic control of CdWO4 nanocrystals for photocatalytic performances. New J. Chem. 2012;36(9):1852–1858. doi: 10.1039/c2nj40504k. [DOI] [Google Scholar]
- 87.Dan M, Cheng M, Gao H, Zheng H, Feng C. Synthesis and electrochemical properties of SnWO4. J. Nanosci. Nanotechnol. 2014;14(3):2395–2399. doi: 10.1166/jnn.2014.8497. [DOI] [PubMed] [Google Scholar]
- 88.Cho IS, Kwak CH, Kim DW, Lee SW, Hong KS. Photophysical, photoelectrochemical, and photocatalytic properties of novel SnWO4 oxide semiconductors with narrow band gaps. J. Phys. Chem. C. 2009;113(24):10647–10653. doi: 10.1021/jp901557z. [DOI] [Google Scholar]
- 89.Chen D, Gao L, Yasumori A, Kuroda K, Sugahara Y. Size- and shape-controlled conversion of tungstate-based inorganic-organic hybrid belts to WO3 nanoplates with high specific surface areas. Small. 2008;4(10):1813–1822. doi: 10.1002/smll.200800205. [DOI] [PubMed] [Google Scholar]
- 90.Liang L, Zhang J, Zhou Y, Xie J, Zhang X, Guan M, Pan B, Xie Y. High-performance flexible electrochromic device based on facile semiconductor-to-metal transition realized by WO3▪2H2O ultrathin nanosheets. Sci. Rep. 2013;3:1936. doi: 10.1038/srep01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang ZF, Song J, Pan L, Zhang X, Wang L, Zou JJ. Tungsten oxides for photocatalysis, electrochemistry, and phototherapy. Adv. Mater. 2015;27(36):5309–5327. doi: 10.1002/adma.201501217. [DOI] [PubMed] [Google Scholar]
- 92.Abraham Y, Holzwarth NAW, Williams RT. Electronic structure and optical properties of CdMoO4 and CdWO4. Phys. Rev. B. 2000;62(3):1733–1741. doi: 10.1103/PhysRevB.62.1733. [DOI] [Google Scholar]
- 93.Shepard S, Smeu M. Ab initio study of structural and electronic properties of copper and nickel tungstate. Comput. Mater. Sci. 2018;143:301–307. doi: 10.1016/j.commatsci.2017.11.021. [DOI] [Google Scholar]
- 94.Lima AEB, Costa MJS, Santos RS, Batista NC, Cavalcante LS, Longo E, Luz GE. Facile preparation of CuWO4 porous films and their photoelectrochemical properties. Electrochim. Acta. 2017;256:139–145. doi: 10.1016/j.electacta.2017.10.010. [DOI] [Google Scholar]
- 95.Wang L, Ke F, Wang Q, Yan J, Liu C, et al. Effect of crystallization water on the structural and electrical properties of CuWO4 under high pressure. Appl. Phys. Lett. 2015;107(20):201603. doi: 10.1063/1.4935978. [DOI] [Google Scholar]
- 96.Wang D, Bassi PS, Qi H, Zhao X, Gurudayal, Wong LH, Xu R, Sritharan T, Chen Z. Improved charge separation in WO3/CuWO4 composite photoanodes for photoelectrochemical water oxidation. Materials. 2016;9(5):348. doi: 10.3390/ma9050348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun S, Wang W. Advanced chemical compositions and nanoarchitectures of bismuth based complex oxides for solar photocatalytic application. RSC Adv. 2014;4(88):47136–47152. doi: 10.1039/c4ra06419d. [DOI] [Google Scholar]
- 98.Atuchin VV, Troitskaia BI, Khyzhun OY, Bekenev VL, Solonin YM. Electronic properties of h-WO3 and CuWO4 nanocrystals as determined from X-ray spectroscopy and first principles band structure calculations. Int. J. Appl. Phys. Math. 2011;1(1):19–23. doi: 10.7763/ijapm.2011.V1.4. [DOI] [Google Scholar]
- 99.Khyzhun OY, Bekenev VL, Solonin YM. First-principles calculations and X-ray spectroscopy studies of the electronic structure of CuWO4. J. Alloy. Compd. 2009;480(2):184–189. doi: 10.1016/j.jallcom.2009.01.119. [DOI] [Google Scholar]
- 100.Khyzhun OY, Strunskus T, Cramm S, Solonin YM. Electronic structure of CuWO4: XPS, XES and NEXAFS studies. J. Alloy. Compd. 2005;389(1–2):14–20. doi: 10.1016/j.jallcom.2004.08.013. [DOI] [Google Scholar]
- 101.Lalić MV, Popović ZS, Vukajlović FR. Ab initio study of electronic, magnetic and optical properties of CuWO4 tungstate. Comp. Mater. Sci. 2011;50(3):1179–1186. doi: 10.1016/j.commatsci.2010.11.018. [DOI] [Google Scholar]
- 102.Lhermitte CR, Bartlett BM. Advancing the chemistry of CuWO4 for photoelectrochemical water oxidation. Acc. Chem. Res. 2016;49(6):1121–1129. doi: 10.1021/acs.accounts.6b00045. [DOI] [PubMed] [Google Scholar]
- 103.Rajagopal S, Nataraj D, Khyzhun OY, Djaoued Y, Robichaud J, Mangalaraj D. Hydrothermal synthesis and electronic properties of FeWO4 and CoWO4 nanostructures. J. Alloy. Compd. 2010;493(1–2):340–345. doi: 10.1016/j.jallcom.2009.12.099. [DOI] [Google Scholar]
- 104.Rajagopal S, Bekenev VL, Nataraj D, Mangalaraj D, Khyzhun OY. Electronic structure of FeWO4 and CoWO4 tungstates: first-principles FP-LAPW calculations and X-ray spectroscopy studies. J. Alloy. Compd. 2010;496(1–2):61–68. doi: 10.1016/j.jallcom.2010.02.107. [DOI] [Google Scholar]
- 105.Hoang K, Oh M, Choi Y. Electronic structure, polaron formation, and functional properties in transition-metal tungstates. RSC Adv. 2018;8(8):4191–4196. doi: 10.1039/c7ra13436c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu F, Cao L, Huang J, Wu J. Effects of pH on the microstructures and optical property of FeWO4 nanocrystallites prepared via hydrothermal method. Ceram. Int. 2013;39(4):4133–4138. doi: 10.1016/j.ceramint.2012.10.269. [DOI] [Google Scholar]
- 107.Hou L, Lian L, Zhang L, Wu T, Yuan C. Microwave-assisted interfacial hydrothermal fabrication of hydrophobic CdWO4 microspheres as a high-performance photocatalyst. RSC Adv. 2014;4(5):2374–2381. doi: 10.1039/c3ra45784b. [DOI] [Google Scholar]
- 108.Ling Y, Zhou L, Tan L, Wang Y, Yu C. Synthesis of urchin-like CdWO4 microspheres via a facile template free hydrothermal method. CrystEngComm. 2010;12(10):3019–3026. doi: 10.1039/b927420k. [DOI] [Google Scholar]
- 109.Kang S, Li Y, Wu M, Cai M, Shen PK. Synthesis of hierarchically flower-like FeWO4 as high performance anode materials for Li-ion batteries by a simple hydrothermal process. Int. J. Hydrog. Energy. 2014;39(28):16081–16087. doi: 10.1016/j.ijhydene.2014.02.101. [DOI] [Google Scholar]
- 110.Liu S, Tian S, Xing R. CaWO4 hierarchical nanostructures: hydrothermal synthesis, growth mechanism and photoluminescence properties. CrystEngComm. 2011;13(24):7258–7261. doi: 10.1039/c1ce05790a. [DOI] [Google Scholar]
- 111.Kovács TN, Pokol G, Gáber F, Nagy D, Igricz T, Lukács IE, Fogarassy Z, Balázsi K, Szilágyi IM. Preparation of iron tungstate (FeWO4) nanosheets by hydrothermal method. Mater. Res. Bull. 2017;95:563–569. doi: 10.1016/j.materresbull.2017.08.031. [DOI] [Google Scholar]
- 112.Chen ZG, Ma HJ, Xia JX, Zeng J, Di J, Yin S, Xu L, Li HM. Ionic liquid-induced strategy for FeWO4 microspheres with advanced visible light photocatalysis. Ceram. Int. 2016;42(7):8997–9003. doi: 10.1016/j.ceramint.2016.02.117. [DOI] [Google Scholar]
- 113.Zhou YX, Zhang Q, Gong JY, Yu SH. Surfactant-assisted hydrothermal synthesis and magnetic properties of urchin-like MnWO4 microspheres. J. Phys. Chem. C. 2008;112(35):13383–13389. doi: 10.1021/jp804211w. [DOI] [Google Scholar]
- 114.Xing Y, Song S, Feng J, Lei Y, Li M, Zhang H. Microemulsion-mediated solvothermal synthesis and photoluminescent property of 3D flowerlike MnWO4 micro/nanocomposite structure. Solid State Sci. 2008;10(10):1299–1304. doi: 10.1016/j.solidstatesciences.2008.01.019. [DOI] [Google Scholar]
- 115.Chen YC, Lin YG, Hsu LC, Tarasov A, Chen PT, Hayashi M, Ungelenk J, Hsu YK, Feldmann C. β-SnWO4 photocatalyst with controlled morphological transition of cubes to spikecubes. ACS Catal. 2016;6(4):2357–2367. doi: 10.1021/acscatal.5b02444. [DOI] [Google Scholar]
- 116.Qiu XY, Huang HW, Gao YD. Effects of surface properties of 010}, {001 and 100 of MnWO4 and FeWO4 on absorption of collector. Appl. Surf. Sci. 2016;367:354–361. doi: 10.1016/j.apsusc.2016.01.190. [DOI] [Google Scholar]
- 117.Ungelenk J, Feldmann C. Synthesis of faceted beta-SnWO4 microcrystals with enhanced visible-light photocatalytic properties. Chem. Commun. 2012;48(63):7838–7840. doi: 10.1039/c2cc33224h. [DOI] [PubMed] [Google Scholar]
- 118.Tian T, Ai L, Liu X, Li L, Li J, Jiang J. Synthesis of hierarchical FeWO4 architectures with {100}-faceted nanosheet assemblies as a robust biomimetic catalyst. Ind. Eng. Chem. Res. 2015;54(4):1171–1178. doi: 10.1021/ie504114v. [DOI] [Google Scholar]
- 119.Hou Y, Li X, Zou X, Quan X, Chen G. Photoeletrocatalytic activity of a Cu2O-loaded self-organized highly oriented TiO2 nanotube array electrode for 4-chlorophenol degradation. Environ. Sci. Technol. 2009;43(3):858–863. doi: 10.1021/es802420u. [DOI] [PubMed] [Google Scholar]
- 120.Hou Y, Li X, Zhao Q, Quan X, Chen G. Electrochemical method for synthesis of a ZnFe2O4/TiO2 composite nanotube array modified electrode with enhanced photoelectrochemical activity. Adv. Funct. Mater. 2010;20(13):2165–2174. doi: 10.1002/adfm.200902390. [DOI] [Google Scholar]
- 121.Yan J, Shen Y, Cao R, Li T. CdWO4 nanorods: ultrafast synthesis via a PEG-1000 polymer-assisted enhanced microwave synthesis route and their photoluminescence property. Ceram. Int. 2014;40(6):8081–8085. doi: 10.1016/j.ceramint.2014.01.003. [DOI] [Google Scholar]
- 122.Liao HW, Wang YF, Liu XM, Li YD, Qian YT. Hydrothermal preparation and characterization of luminescent CdWO4 nanorods. Chem. Mater. 2000;12(10):2819–2821. doi: 10.1021/cm000096w. [DOI] [Google Scholar]
- 123.Zhang C, Guo D, Xu W, Hu C, Chen Y. Radiative/nonradiative recombination affected by defects and electron-phone coupling in CdWO4 nanorods. J. Phys. Chem. C. 2016;120(22):12218–12225. doi: 10.1021/acs.jpcc.6b04145. [DOI] [Google Scholar]
- 124.Aloysius Sabu N, Priyanka KP, Ganesh S, Varghese T. Modifications in the structural and optical properties of nanocrystalline CaWO4 induced by 8 MeV electron beam irradiation. Radiat. Phys. Chem. 2016;123:1–5. doi: 10.1016/j.radphyschem.2016.02.006. [DOI] [Google Scholar]
- 125.Lin Z, Li J, Zheng Z, Yan J, Liu P, Wang C, Yang G. Electronic reconstruction of α-Ag2WO4 nanorods for visible-light photocatalysis. ACS Nano. 2015;9(7):7256–7265. doi: 10.1021/acsnano.5b02077. [DOI] [PubMed] [Google Scholar]
- 126.Hou W, Cronin SB. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 2013;23(13):1612–1619. doi: 10.1002/adfm.201202148. [DOI] [Google Scholar]
- 127.Hou Y, Zuo F, Ma Q, Wang C, Bartels L, Feng P. Ag3PO4 oxygen evolution photocatalyst employing synergistic action of Ag/AgBr nanoparticles and graphene sheets. J. Phys. Chem. C. 2012;116(38):20132–20139. doi: 10.1021/jp303219j. [DOI] [Google Scholar]
- 128.Wang F, Wong RJ, Ho JH, Jiang Y, Amal R. Sensitization of Pt/TiO2 using plasmonic Au nanoparticles for hydrogen evolution under visible-light irradiation. ACS Appl. Mater. Interfaces. 2017;9(36):30575–30582. doi: 10.1021/acsami.7b06265. [DOI] [PubMed] [Google Scholar]
- 129.Liu J, Li Y, Li Z, Ke J, Xiao H, Hou Y. In situ growing of Bi/Bi2O2CO3 on Bi2WO6 nanosheets for improved photocatalytic performance. Catal. Today. 2018;314:2–9. doi: 10.1016/j.cattod.2017.12.001. [DOI] [Google Scholar]
- 130.Liu X, Liang B, Zhang M, Long Y, Li W. Enhanced photocatalytic properties of alpha-SnWO4 nanosheets modified by Ag nanoparticles. J. Colloid Interface Sci. 2017;490:46–52. doi: 10.1016/j.jcis.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 131.Yan Y, Xing H, Han C, Yang A. Synthesis and photocatalytic activity of Ag-CdWO4 nanorods. Ceram. Int. 2017;43(4):3905–3909. doi: 10.1016/j.ceramint.2016.11.186. [DOI] [Google Scholar]
- 132.Chakraborty AK, Ganguli S, Kebede MA. Photocatalytic degradation of 2-propanol and phenol using Au loaded MnWO4 nanorod under visible light irradiation. J. Clust. Sci. 2012;23(2):437–448. doi: 10.1007/s10876-012-0450-6. [DOI] [Google Scholar]
- 133.Zong X, Sun C, Yu H, Chen ZG, Xing Z, Ye D, Lu GQ, Li X, Wang L. Activation of photocatalytic water oxidation on N-doped ZnO bundle-like nanoparticles under visible light. J. Phys. Chem. C. 2013;117(10):4937–4942. doi: 10.1021/jp311729b. [DOI] [Google Scholar]
- 134.Mei Z, Zhang B, Zheng J, Yuan S, Zhuo Z, et al. Tuning Cu dopant of Zn0.5Cd0.5S nanocrystals enables high-performance photocatalytic H2 evolution from water splitting under visible-light irradiation. Nano Energy. 2016;26:405–416. doi: 10.1016/j.nanoen.2016.05.051. [DOI] [Google Scholar]
- 135.Ambast AK, Sharma SK. Variation of trap depth by dopant/codopant and heating rate in CaWO4 phosphors. Opt. Quant. Electron. 2017;49(2):58. doi: 10.1007/s11082-017-0889-7. [DOI] [Google Scholar]
- 136.Liu Z, Tian J, Zeng D, Yu C, Zhu L, Huang W, Yang K, Li D. A facile microwave-hydrothermal method to fabricate B doped ZnWO4 nanorods with high crystalline and highly efficient photocatalytic activity. Mater. Res. Bull. 2017;94:298–306. doi: 10.1016/j.materresbull.2017.06.021. [DOI] [Google Scholar]
- 137.Huang G, Zhu Y. Synthesis and photoactivity enhancement of ZnWO4 photocatalysts doped with chlorine. CrystEngComm. 2012;14(23):8076–8082. doi: 10.1039/c2ce26005k. [DOI] [Google Scholar]
- 138.Tian X, Jiang G, Chen Y, Yin M. Eu3+, Li+ doped CaWO4 nanorods: synthesis, emission-decay curves and effective-refractive index. Curr. Appl. Phys. 2014;14(12):1612–1615. doi: 10.1016/j.cap.2014.09.010. [DOI] [Google Scholar]
- 139.Choo CK, Suzuki S, Tanaka K. Photoluminescence intensity enhancement of ion-doped CdWO4 thin films prepared with pulsed laser deposition. Thin Solid Films. 2004;458(1–2):179–185. doi: 10.1016/j.tsf.2003.12.062. [DOI] [Google Scholar]
- 140.Chen SH, Sun SX, Sun HG, Fan WL, Zhao X, Sun X. Experimental and theoretical studies on the enhanced photocatalytic activity of ZnWO4 nanorods by fluorine doping. J. Phys. Chem. C. 2010;114(17):7680–7699. doi: 10.1021/jp911952v. [DOI] [Google Scholar]
- 141.Su Y, Hou L, Du C, Peng L, Guan K, Wang X. Rapid synthesis of Zn2+ doped SnWO4 nanowires with the aim of exploring doping effects on highly enhanced visible photocatalytic activities. RSC Adv. 2012;2(15):6266–6273. doi: 10.1039/c2ra20401k. [DOI] [Google Scholar]
- 142.Song XC, Cui X, Huang WZ, Zhang Y, Yin HY, Zheng YF. Photoactivity enhancement of Zn-doped CdWO4 prepared with a hydrothermal method. Mater. Sci. Eng. B. 2015;197:31–35. doi: 10.1016/j.mseb.2015.03.007. [DOI] [Google Scholar]
- 143.Ye D, Li D, Chen W, Shao Y, Xiao G, Sun M, Fu X. Characterization and properties of Eu3+-doped CdWO4 prepared by a hydrothermal method. Res. Chem. Intermed. 2009;35(6–7):675–683. doi: 10.1007/s11164-009-0104-y. [DOI] [Google Scholar]
- 144.Phuruangrat A, Dumrongrojthanath P, Thongtem T, Thongtem S. Effect of Ce dopant on structure, morphology, photoabsorbance and photocatalysis of ZnWO4 nanostructure. J. Ceram. Soc. Jpn. 2017;125(2):62–64. doi: 10.2109/jcersj2.16176. [DOI] [Google Scholar]
- 145.Wei H, Wang L, Li Z, Ni S, Zhao Q. Synthesis and photocatalytic activity of one-dimensional CdS@TiO2 core-shell heterostructures. Nano-Micro Lett. 2011;3(1):6–11. doi: 10.1007/bf03353645. [DOI] [Google Scholar]
- 146.Zhang C, Hua H, Liu J, Han X, Liu Q, Wei Z, Shao C, Hu C. Enhanced photocatalytic activity of nanoparticle-aggregated Ag-AgX (X = Cl, Br)@TiO2 microspheres under visible light. Nano-Micro Lett. 2017;9(4):49. doi: 10.1007/s40820-017-0150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu C, Wang F, Zhang J, Wang K, Qiu Y, Liang Q, Chen Z. Efficient photoelectrochemical water splitting by g-C3N4/TiO2 nanotube array heterostructures. Nano-Micro Lett. 2018;10(2):37. doi: 10.1007/s40820-018-0192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu J, Li Y, Ke J, Wang Z, Xiao H. Synergically improving light harvesting and charge transportation of TiO2 nanobelts by deposition of MoS2 for enhanced photocatalytic removal of Cr(VI) Catalysts. 2017;7(12):30. doi: 10.3390/catal7010030. [DOI] [Google Scholar]
- 149.Liu J, Ke J, Li Y, Liu B, Wang L, Xiao H, Wang S. Co3O4 quantum dots/TiO2 nanobelt hybrids for highly efficient photocatalytic overall water splitting. Appl. Catal. B Environ. 2018;236:396–403. doi: 10.1016/j.apcatb.2018.05.042. [DOI] [Google Scholar]
- 150.Pan L, Wang S, Xie J, Wang L, Zhang X, Zou JJ. Constructing TiO2 p-n homojunction for photoelectrochemical and photocatalytic hydrogen generation. Nano Energy. 2016;28:296–303. doi: 10.1016/j.nanoen.2016.08.054. [DOI] [Google Scholar]
- 151.Xu Y, Huang Y, Zhang B. Rational design of semiconductor-based photocatalysts for advanced photocatalytic hydrogen production: the case of cadmium chalcogenides. Inorg. Chem. Front. 2016;3(5):591–615. doi: 10.1039/c5qi00217f. [DOI] [Google Scholar]
- 152.Kment S, Riboni F, Pausova S, Wang L, Wang L, et al. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting-superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017;46(12):3716–3769. doi: 10.1039/c6cs00015k. [DOI] [PubMed] [Google Scholar]
- 153.Huo P, Tang Y, Zhou M, Li J, Ye Z, Ma C, Yu L, Yan Y. Fabrication of ZnWO4-CdS heterostructure photocatalysts for visible light induced degradation of ciprofloxacin antibiotics. J. Ind. Eng. Chem. 2016;37:340–346. doi: 10.1016/j.jiec.2016.03.043. [DOI] [Google Scholar]
- 154.Vignesh K, Priyanka R, Hariharan R, Rajarajan M, Suganthi A. Fabrication of CdS and CuWO4 modified TiO2 nanoparticles and its photocatalytic activity under visible light irradiation. J. Ind. Eng. Chem. 2014;20(2):435–443. doi: 10.1016/j.jiec.2013.04.038. [DOI] [Google Scholar]
- 155.Yan X, Liu K, Shi W. Facile synthesis of CdS/MnWO4 heterojunction with enhanced visible-light-driven photocatalytic activity and mechanism investigation. Colloids Surf. A Physicochem. Eng. Asp. 2017;520:138–145. doi: 10.1016/j.colsurfa.2017.01.065. [DOI] [Google Scholar]
- 156.Xu M, Ye T, Dai F, Yang J, Shen J, et al. Rationally designed n-n heterojunction with highly efficient solar hydrogen evolution. Chemsuschem. 2015;8(7):1218–1225. doi: 10.1002/cssc.201403334. [DOI] [PubMed] [Google Scholar]
- 157.Jia X, Tahir M, Pan L, Huang ZF, Zhang X, Wang L, Zou JJ. Direct Z-scheme composite of CdS and oxygen-defected CdWO4: an efficient visible-light-driven photocatalyst for hydrogen evolution. Appl. Catal. B: Environ. 2016;198:154–161. doi: 10.1016/j.apcatb.2016.05.046. [DOI] [Google Scholar]
- 158.Wang L, Wang W. In situ synthesis of CdS modified CdWO4 nanorods and their application in photocatalytic H2 evolution. CrystEngComm. 2012;14(9):3315–3320. doi: 10.1039/c2ce06656d. [DOI] [Google Scholar]
- 159.Bera S, Rawal SB, Kim HJ, Lee WI. Novel coupled structures of FeWO4/TiO2 and FeWO4/TiO2/CdS designed for highly efficient visible-light photocatalysis. ACS Appl. Mater. Interfaces. 2014;6(12):9654–9663. doi: 10.1021/am502079x. [DOI] [PubMed] [Google Scholar]
- 160.Habibi-Yangjeh A, Shekofteh-Gohari M. Novel magnetic Fe3O4/ZnO/NiWO4 nanocomposites: enhanced visible-light photocatalytic performance through p-n heterojunctions. Sep. Purif. Technol. 2017;184:334–346. doi: 10.1016/j.seppur.2017.05.007. [DOI] [Google Scholar]
- 161.Hassan MS, Amna T, Al-Deyab SS, Kim HC, Khil MS. Monodispersed 3D MnWO4-TiO2 composite nanoflowers photocatalysts for environmental remediation. Curr. Appl. Phys. 2015;15(6):753–758. doi: 10.1016/j.cap.2015.03.022. [DOI] [Google Scholar]
- 162.Jiang YN, Liu BD, Yang WJ, Yang B, Liu XY, Zhang XL, Mohsin MA, Jiang X. New strategy for the in situ synthesis of single-crystalline MnWO4/TiO2 photocatalysts for efficient and cyclic photodegradation of organic pollutants. CrystEngComm. 2016;18(10):1832–1841. doi: 10.1039/c5ce02445e. [DOI] [Google Scholar]
- 163.Buvaneswari K, Karthiga R, Kavitha B, Rajarajan M, Suganthi A. Effect of FeWO4 doping on the photocatalytic activity of ZnO under visible light irradiation. Appl. Surf. Sci. 2015;356:333–340. doi: 10.1016/j.apsusc.2015.08.060. [DOI] [Google Scholar]
- 164.Mavric T, Valant M, Forster M, Cowan AJ, Lavrencic U, Emin S. Design of a highly photocatalytically active ZnO/CuWO4 nanocomposite. J. Colloid Interf. Sci. 2016;483:93–101. doi: 10.1016/j.jcis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 165.Wang H, Wang C, Cui X, Qin L, Ding R, Wang L, Liu Z, Zheng Z, Lv B. Design and facile one-step synthesis of FeWO4/Fe2O3 di-modified WO3 with super high photocatalytic activity toward degradation of quasi-phenothiazine dyes. Appl. Catal. B Environ. 2018;221:169–178. doi: 10.1016/j.apcatb.2017.09.011. [DOI] [Google Scholar]
- 166.Kim TH, Kwak CH, Lee JH. NiO/NiWO4 composite yolk-shell spheres with nanoscale NiO outer layer for ultrasensitive and selective detection of subppm-level p-xylene. ACS Appl. Mater. Interfaces. 2017;9(37):32034–32043. doi: 10.1021/acsami.7b10294. [DOI] [PubMed] [Google Scholar]
- 167.Ding K, Zhang X, Li J, Yang P, Cheng X. Formation of dandelion-like Co3O4/CoWO4 heterojunctions for enhanced supercapacitive performance. ChemElectroChem. 2017;4(11):3011–3017. doi: 10.1002/celc.201700704. [DOI] [Google Scholar]
- 168.Polyakov B, Kuzmin A, Vlassov S, Butanovs E, Zideluns J, Butikova J, Kalendarev R, Zubkins M. A comparative study of heterostructured CuO/CuWO4 nanowires and thin films. J. Cryst. Growth. 2017;480:78–84. doi: 10.1016/j.jcrysgro.2017.10.011. [DOI] [Google Scholar]
- 169.Cao X, Chen Y, Jiao S, Fang Z, Xu M, Liu X, Li L, Pang G, Feng S. Magnetic photocatalysts with a p-n junction: Fe3O4 nanoparticle and FeWO4 nanowire heterostructures. Nanoscale. 2014;6(21):12366–12370. doi: 10.1039/c4nr03729d. [DOI] [PubMed] [Google Scholar]
- 170.Yao S, Zhang M, Di J, Wang Z, Long Y, Li W. Preparation of α-SnWO4/SnO2 heterostructure with enhanced visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2015;357:1528–1535. doi: 10.1016/j.apsusc.2015.10.012. [DOI] [Google Scholar]
- 171.Shao GQ, Guo JK, Xie JR, Duan XL, Wu BL, Yuan RZ. The Preparation of CoWO4/WO3 nanocomposite powder. J. Wuhan Univ. Technol. 2004;19(2):1–3. doi: 10.1007/BF03000154. [DOI] [Google Scholar]
- 172.Zhang H, Tian W, Li Y, Sun H, Tadé MO, Wang S. Heterostructured WO3@CoWO4 bilayer nanosheets for enhanced visible-light photo, electro and photoelectro-chemical oxidation of water. J. Mater. Chem. A. 2018;6(15):6265–6272. doi: 10.1039/c8ta00555a. [DOI] [Google Scholar]
- 173.Do TH, Van Nguyen C, Tsai KA, Quynh LT, Chen JW, et al. Superior photoelectrochemical activity of self-assembled NiWO4-WO3 heteroepitaxy. Nano Energy. 2016;23:153–160. doi: 10.1016/j.nanoen.2016.03.021. [DOI] [Google Scholar]
- 174.Anis SF, Lalia BS, Palmisano G, Hashaikeh R. Photoelectrochemical activity of electrospun WO3/NiWO4 nanofibers under visible light irradiation. J. Mater. Sci. 2017;53(3):2208–2220. doi: 10.1007/s10853-017-1633-1. [DOI] [Google Scholar]
- 175.Yourey JE, Kurtz JB, Bartlett BM. Water oxidation on a CuWO4-WO3 composite electrode in the presence of [Fe(CN)6]3−: toward solar Z-scheme water splitting at zero bias. J. Phys. Chem. C. 2012;116(4):3200–3205. doi: 10.1021/jp211409x. [DOI] [Google Scholar]
- 176.Zhan F, Li J, Li W, Liu Y, Xie R, Yang Y, Li Y, Chen Q. In situ formation of CuWO4/WO3 heterojunction plates array films with enhanced photoelectrochemical properties. Int. J. Hydrog. Energy. 2015;40(20):6512–6520. doi: 10.1016/j.ijhydene.2015.03.131. [DOI] [Google Scholar]
- 177.Aslam I, Cao C, Tanveer M, Farooq MH, Khan WS, Tahir M, Idrees F, Khalid S. A novel Z-scheme WO3/CdWO4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of organic pollutants. RSC Adv. 2015;5(8):6019–6026. doi: 10.1039/c4ra15847d. [DOI] [Google Scholar]
- 178.Hou Y, Wen Z, Cui S, Ci S, Mao S, Chen J. An advanced nitrogen-doped graphene/cobalt-embedded porous carbon polyhedron hybrid for efficient catalysis of oxygen reduction and water splitting. Adv. Funct. Mater. 2014;25(6):872–882. doi: 10.1002/adfm.201403657. [DOI] [Google Scholar]
- 179.Qin Y, Long M, Tan B, Zhou B. RhB adsorption performance of magnetic adsorbent Fe3O4/rGO composite and its regeneration through a Fenton-like reaction. Nano-Micro Lett. 2014;6(2):125–135. doi: 10.1007/bf03353776. [DOI] [Google Scholar]
- 180.Russo P, Hu A, Compagnini G. Synthesis, properties and potential applications of porous graphene: a review. Nano-Micro Lett. 2013;5(4):260–273. doi: 10.1007/bf03353757. [DOI] [Google Scholar]
- 181.Wang A, Wang C, Fu L, Wong-Ng W, Lan Y. Recent advances of graphitic carbon nitride-based structures and applications in catalyst, sensing, imaging, and LEDs. Nano-Micro Lett. 2017;9(4):47. doi: 10.1007/s40820-017-0148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pan L, Muhammad T, Ma L, Huang ZF, Wang S, Wang L, Zou JJ, Zhang X. MOF-derived C-doped ZnO prepared via a two-step calcination for efficient photocatalysis. Appl. Catal. B Environ. 2016;189:181–191. doi: 10.1016/j.apcatb.2016.02.066. [DOI] [Google Scholar]
- 183.Liu J, Ke J, Li D, Sun H, Liang P, et al. Oxygen vacancies in shape controlled Cu2O/reduced graphene oxide/In2O3 hybrid for promoted photocatalytic water oxidation and degradation of environmental pollutants. ACS Appl. Mater. Interfaces. 2017;9(13):11678–11688. doi: 10.1021/acsami.7b01605. [DOI] [PubMed] [Google Scholar]
- 184.Singh AK, Mathew K, Zhuang HL, Hennig RG. Computational screening of 2D materials for photocatalysis. J Phys. Chem. Lett. 2015;6(6):1087–1098. doi: 10.1021/jz502646d. [DOI] [PubMed] [Google Scholar]
- 185.Zhang G, Lan ZA, Wang X. Surface engineering of graphitic carbon nitride polymers with cocatalysts for photocatalytic overall water splitting. Chem. Sci. 2017;8(8):5261–5274. doi: 10.1039/c7sc01747b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hou Y, Qiu M, Zhang T, Ma J, Liu S, Zhuang X, Yuan C, Feng X. Efficient electrochemical and photoelectrochemical water splitting by a 3D nanostructured carbon supported on flexible exfoliated graphene foil. Adv. Mater. 2017;29(3):1604480. doi: 10.1002/adma.201604480. [DOI] [PubMed] [Google Scholar]
- 187.Ke J, Duan X, Luo S, Zhang H, Sun H, Liu J, Tade M, Wang S. UV-assisted construction of 3D hierarchical rGO/Bi2MoO6 composites for enhanced photocatalytic water oxidation. Chem. Eng. J. 2017;313:1447–1453. doi: 10.1016/j.cej.2016.11.048. [DOI] [Google Scholar]
- 188.Gaillard N, Chang Y, DeAngelis A, Higgins S, Braun A. A nanocomposite photoelectrode made of 2.2 eV band gap copper tungstate (CuWO4) and multi-wall carbon nanotubes for solar-assisted water splitting. Int. J. Hydrog. Energy. 2013;38(8):3166–3176. doi: 10.1016/j.ijhydene.2012.12.104. [DOI] [Google Scholar]
- 189.Wadhwa P, Kumar S, Dhilip Kumar TJ, Shukla A, Kumar R. Effect of edge defects on band structure of zigzag graphene nanoribbons. J. Appl. Phys. 2018;123(16):161416. doi: 10.1063/1.5011310. [DOI] [Google Scholar]
- 190.Liu ZB, Zhao X, Zhang XL, Yan XQ, Wu YP, Chen YS, Tian JG. Ultrafast dynamics and nonlinear optical responses from sp2- and sp3-hybridized domains in graphene oxide. J. Phys. Chem. Lett. 2011;2(16):1972–1977. doi: 10.1021/jz2008374. [DOI] [Google Scholar]
- 191.Bai XJ, Wang L, Zhu YF. Visible photocatalytic activity enhancement of ZnWO4 by graphene hybridization. ACS Catal. 2012;2(12):2769–2778. doi: 10.1021/cs3005852. [DOI] [Google Scholar]
- 192.Xu J, Chen M, Wang Z. Preparation of CdWO4-deposited reduced graphene oxide and its enhanced photocatalytic properties. Dalton Trans. 2014;43(9):3537–3544. doi: 10.1039/c3dt52120f. [DOI] [PubMed] [Google Scholar]
- 193.Hao J, Zhang S, Ren F, Wang Z, Lei J, Wang X, Cheng T, Li L. Synthesis of TiO2@g-C3N4 core-shell nanorod arrays with Z-scheme enhanced photocatalytic activity under visible light. J. Colloid Interface Sci. 2017;508:419–425. doi: 10.1016/j.jcis.2017.08.065. [DOI] [PubMed] [Google Scholar]
- 194.Hou Y, Wen Z, Cui S, Guo X, Chen J. Constructing 2D porous graphitic C3N4 nanosheets/nitrogen-doped graphene/layered MoS2 ternary nanojunction with enhanced photoelectrochemical activity. Adv. Mater. 2013;25(43):6291–6297. doi: 10.1002/adma.201303116. [DOI] [PubMed] [Google Scholar]
- 195.Liu Y, Zhang H, Ke J, Zhang J, Tian W, et al. 0D (MoS2)/2D (g-C3N4) heterojunctions in Z-scheme for enhanced photocatalytic and electrochemical hydrogen evolution. Appl. Catal. B: Environ. 2018;228:64–74. doi: 10.1016/j.apcatb.2018.01.067. [DOI] [Google Scholar]
- 196.Sun L, Zhao X, Jia C-J, Zhou Y, Cheng X, Li P, Liu L, Fan W. Enhanced visible-light photocatalytic activity of g-C3N4–ZnWO4 by fabricating a heterojunction: investigation based on experimental and theoretical studies. J. Mater. Chem. A. 2012;22(44):23428–23438. doi: 10.1039/c2jm34965e. [DOI] [Google Scholar]
- 197.Tian N, Huang H, Zhang Y. Mixed-calcination synthesis of CdWO4/g-C3N4 heterojunction with enhanced visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2015;358:343–349. doi: 10.1016/j.apsusc.2015.07.154. [DOI] [Google Scholar]
- 198.Huang ZF, Song J, Wang X, Pan L, Li K, Zhang X, Wang L, Zou JJ. Switching charge transfer of C3N4/W18O49 from type-II to Z-scheme by interfacial band bending for highly efficient photocatalytic hydrogen evolution. Nano Energy. 2017;40:308–316. doi: 10.1016/j.nanoen.2017.08.032. [DOI] [Google Scholar]
- 199.Pan L, Zhang J, Jia X, Ma YH, Zhang X, Wang L, Zou JJ. Highly efficient Z-scheme WO3–x quantum dots/TiO2 for photocatalytic hydrogen generation. Chin. J. Catal. 2017;38(2):253–259. doi: 10.1016/s1872-2067(16)62576-7. [DOI] [Google Scholar]
- 200.Zhu B, Xia P, Li Y, Ho W, Yu J. Fabrication and photocatalytic activity enhanced mechanism of direct Z-scheme g-C3N4/Ag2WO4 photocatalyst. Appl. Surf. Sci. 2017;391:175–183. doi: 10.1016/j.apsusc.2016.07.104. [DOI] [Google Scholar]