Abstract
Mycobacterium simiae is one of the most common nontuberculous mycobacteria (NTM) microorganisms causing lung disease in many countries in the world. A reliable estimate of the extent of M. simiae pulmonary disease has not been well investigated in Iran. We systematically searched multiple databases to identify relative studies. Studies were excluded if they did not use the American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) diagnostic criteria for NTM diseases. Data were extracted independently and in duplicate. We assessed pooled estimate by using a random model effect, and sources of heterogeneity were assessed by using Cochran's Q and the I2 statistic. The potential for publication bias was explored by using Begg's and Egger's tests. All analyses were conducted with Stata 14.0 (StataCorp, College Station, TX, USA). Of 172 articles identified, seven met the inclusion criteria. Of 355 patients who were culture positive for NTM, 82 had M. simiae pulmonary disease according to the ATS/IDSA diagnostic criteria. The pooled frequency of M. simiae pulmonary disease among patients with NTM was 25.0% (95% confidence interval, 16.8–33.2). No evidence of publication bias was observed among the included studies (p >0.05 for Begg's and Egger's tests). Clinical isolates of M. simiae are increasingly being recognized as a cause of pulmonary disease in Iran and need further attention by health authorities.
Keywords: Iran, Mycobacterium simiae, systematic review
Introduction
Infections caused by nontuberculous mycobacteria (NTM) have been recently reported as an important public health problem in many parts of the world, especially in developing countries [1], [2], [3]. Iran is a tuberculosis (TB)-endemic country with an annual incidence of 22 cases per 100 000 population [4]. On the basis of the studies from this country, 5% to 10% of mycobacterial infections are caused by NTM [5]. Mycobacterium simiae is among the most prevalent NTM in Iran and has been recently recognized as an emerging pathogen [6], [7], [8], [9]. It causes pulmonary disease and disseminated infection in both immunocompromised and immunocompetent patients [6], [10].
M. simiae are commonly isolated from environmental sources such as soil, tap water and the water supply, and therefore its isolation does not necessarily imply disease because positive cultures may only represent colonization [6]. On the basis of the American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines, clinical, radiographic and microbiologic criteria are needed for the diagnosis of NTM diseases [11]. IDSA recommended that treatment regimens differ according to the NTM species, and management is a complicated process [11]. Pulmonary disease caused by M. simiae may be easily confused with Mycobacterium tuberculosis [7], [12]. Most isolates of M. simiae are resistant to all first-line anti-TB drugs, and for patients with M. simiae pulmonary disease, initial therapy usually consists of a regimen containing clarithromycin or moxifloxacin [11], [13], [14].
Clinical M. simiae isolation has been reported from many places in the world [15], [16], [17], [18], [19]. For example in the United states, India and Oman, M. simiae was among the most prevalent isolates of NTM [1]. Previous studies in Iran did not use ATS/IDSA criteria to report the prevalence of M. simiae. Furthermore, no specific information regarding the M. simiae pulmonary disease is available in Iran. Thus, a reliable estimate of the M. simiae pulmonary disease is needed for the programmatic management of the disease within the context of national TB control programmes.
In this study, we aimed to investigate the frequency of pulmonary M. simiae among NTM disease using a systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [20].
Methods
Search strategy
To identify relevant studies, PubMed, Web of Science, Embase and Iranian databases were searched for articles published from January 2000 to December 2017. Search terms included ‘mycobacterium,’ ‘mycobacterium simiae’ and ‘Iran.’ Titles and abstracts of all identified articles were screened by two authors. Likewise, full text of potentially relevant articles were assessed for eligibility independently and in duplicate by two investigators. In all included studies, we attempted to contact the authors for confirmation whether they used ATS/IDSA criteria.
Inclusion and exclusion criteria
We included all cross-sectional studies that evaluated the prevalence or frequency of M. simiae infections in Iran. Studies were included if they used ATS/IDSA diagnostic criteria [11] to report NTM infections and used standard methods for NTM diagnosis.
Studies that did not use ATS/IDSA diagnostic criteria for NTM diseases, as well as standard methods for NTM diagnosis, were excluded. Studies were also excluded if they did not report the number of M. simiae cases or if they considered only environmental samples.
Data extraction
Data were extracted using an extraction form independently and in duplicate by two investigators. Information included the first author name, publication year, enrollment time, study name, location, design and population (i.e. sample size). Differences in data extraction between investigators were resolved by consensus.
Quality assessment of studies
We assessed study quality using checklist provided by the Joanna Briggs Institute [21].
Statistical analysis
Analyses were performed by using random-effects weights. The between-study heterogeneity was assessed by Cochran's Q and the I2 statistic. I2 values of 25%, 50% and 75% were considered to represent low, moderate and high heterogeneity, respectively [22]. Publication bias was assessed statistically by using Egger's and Begg's tests as well as the funnel plot (p < 0.05 was considered indicative of statistically significant publication bias; funnel plot asymmetry also suggests bias in meta-analysis) [23]. Analyses were conducted by STATA 14.0 (StataCorp, College Station, TX, USA).
Results
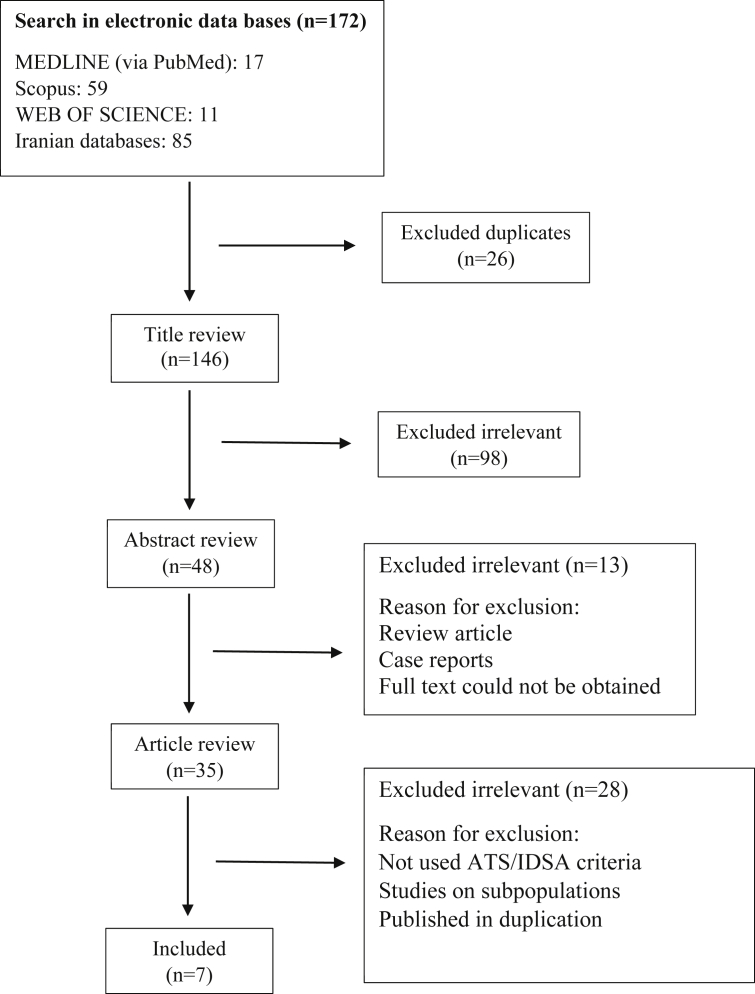
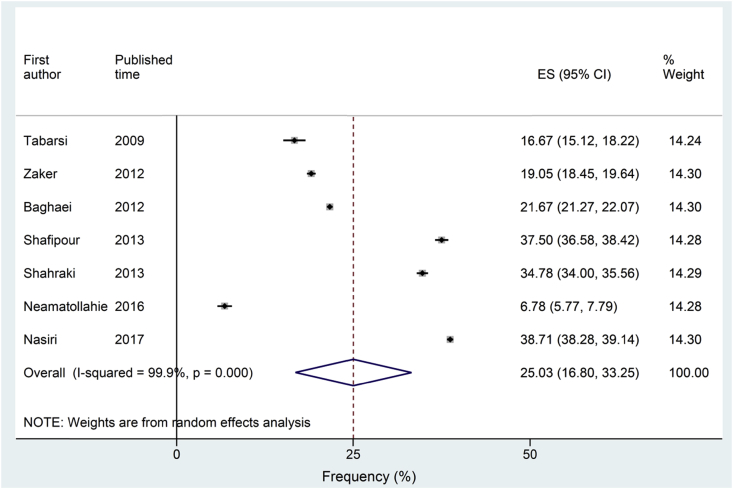
Of 172 articles identified, seven studies met the inclusion and exclusion criteria (Fig. 1). Because in Iran data on the prevalence or frequency of TB and NTM are from the TB suspected cases and not the general population, in all included studies TB suspected cases were investigated. From 355 patients who were culture positive for NTM, 82 had M. simiae pulmonary disease according to the ATS/IDSA diagnostic criteria (Table 1). Pooling all studies, the frequency of M. simiae pulmonary disease among patients with NTM was 25.0% (95% confidence interval, 16.8–33.2) (Fig. 2).
Fig. 1.
Flowchart of study selection for inclusion.
Table 1.
Identified studies reporting frequency of Mycobacterium simiae pulmonary disease
| Study | Study time | City | No. of suspected TB cases | No. of culture-positive cases | No. of patients with NTM diseases | No. of patients with M. simiae pulmonary disease |
|---|---|---|---|---|---|---|
| Tabarsi (2009) [24] | 2002–2006 | Tehran | NR | NR | 12 | 2 |
| Zaker (2012) [25] | 2010–2011 | Tehran | 2385 | 270 | 63 | 12 |
| Baghaei (2012) [26] | 2002–2009 | Tehran | NR | NR | 120 | 26 |
| Shafipour (2013) [27] | 2010–2011 | Gorgan | 3336 | 319 | 16 | 6 |
| Hashemi-Shahraki (2013) [6] | 2009–2012 | Ahvaz | 190 | 117 | 23 | 8 |
| Nour-Neamatollahie (2016) [28] | 2011–2013 | Tehran | 10 377 | 380 | 59 | 4 |
| Nasiri (2017) [29] | 2014–2016 | Tehran | 7200 | 410 | 62 | 24 |
NR, not reported; NTM, nontuberculous mycobacteria; TB, tuberculosis.
Fig. 2.
Forest plots of studies investigating the frequency of Mycobacterium simiae pulmonary disease.
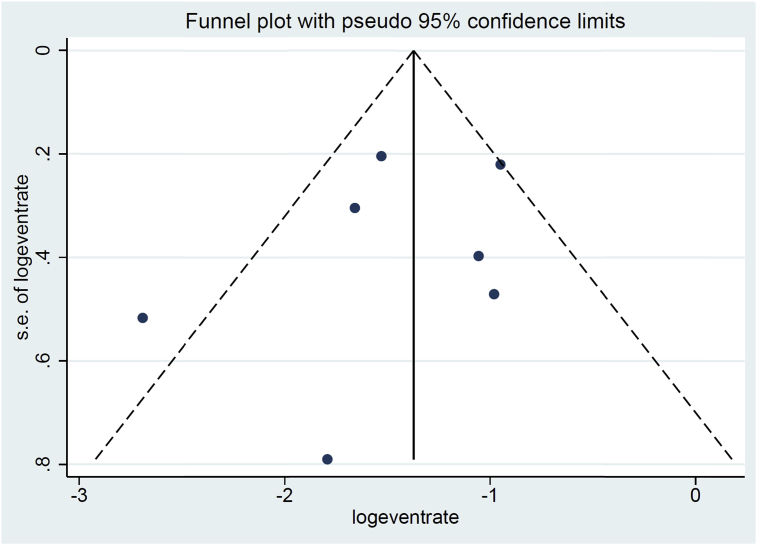
Using Cochran's Q and the I2 statistic analysis, heterogeneity was evident (I2 = 99, p < 0.001). The Begg's and Egger's tests provided no evidence for publication bias (p > 0.05). On visual inspection, the funnel plot did not indicate any publication bias (Fig. 3).
Fig. 3.
Funnel plot of studies to investigate publication bias (no evidence for publication bias was observed).
Discussion
This study indicated a relatively high frequency of M. simiae pulmonary disease among patients with NTM in Iran. These findings may have important diagnostic and therapeutic implications.
In recent years, clinical isolation of M. simiae has been widely reported from different regions of the world such as Europe, the United States and the Middle East [7], [30], [31], [32], [33], [34], [35]. M. simiae was also reported to be the most common NTM species in India (22%), France (15.1%), Oman (14.3%), United States (3.0%) and Saudi Arabia (1.4%) [1], [36], [37]. Uses of newest laboratory diagnostic methods were assumed to be one of the reasons for increased reports of this organism. Furthermore, increasing the number of patients with underlying diseases such as prior pulmonary TB, silicosis, chronic obstructive pulmonary disease, non–cystic fibrosis bronchiectasis and other comorbidities, such as diabetes mellitus, cardiovascular diseases and malignancies, could predispose people to M. simiae infection [38], [39].
M. simiae is endemic to Iran, accounting for more than 30% of all NTM pathogens isolated in the country in 2014–2016 [29]. Previous studies emphasized that preexisting lung disease, particularly TB, is an important risk factor for pulmonary NTM infection [24], [39]. In our included studies, M. simiae were mostly isolated from patients who had been previously diagnosed as new TB cases or who were infected with multidrug-resistant TB. Treatment of patients with definite M. simiae disease is an important challenge because there are no evidence-based treatment regimens [14]. M. simiae is poorly susceptible to first-line anti-TB drugs [14]. A treatment regimen containing a macrolide, moxifloxacin and one or two additional drugs based on drug susceptibility testing results may be advisable to treat disease caused by M. simiae [14].
Unfortunately, there is not enough infection-control impact on hospitalized patients for M. simiae, and its isolation from respiratory specimens may indicate colonization rather than disease in most cases. According to the reports, M. simiae isolates recovered from humans are estimated to be clinically relevant in 9% to 21% of specimens [30]. Therefore, the distinction of M. simiae respiratory infection from pulmonary TB has significant practical importance. Furthermore, when the infection is considered to be clinically significant, selection of optimal treatment regimens should be taken into account by physicians.
Strengths and limitations
To our knowledge, this is the first study of status of M. simiae pulmonary disease in Iran. Our findings could help the programmatic management of the disease within the context of national TB control programmes. This meta-analysis had also some limitations which should be considered. First, there was a considerable heterogeneity between studies, which should be considered when interpreting results. To explore the heterogeneity of studies, we conducted subgroup and sensitivity analyses. Subgroup analyses found that variables such as number of included patients contributed to the heterogeneity. Second, because the frequency of M. simiae pulmonary disease are not yet studied in many regions of Iran, it cannot fully show the frequency of pulmonary M. simiae disease in the country.
Conclusions
In Iran, clinical isolates of M. simiae are increasingly being recognized as a cause of pulmonary disease; this finding merits further attention by health authorities. Further studies will provide more insights into the understanding of the epidemiology of this infection.
Conflict of interest
None declared.
Funding/support
This study was supported by Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- 1.Prevots D.R., Marras T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. 2015;36:13–34. doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haeili M., Darban-Sarokhalil D., Fooladi A.A.I., Javadpour S., Hashemi A., Siavoshi F. Spoligotyping and drug resistance patterns of Mycobacterium tuberculosis isolates from five provinces of Iran. Microbio Open. 2013;2:988–996. doi: 10.1002/mbo3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Supply P., Allix C., Lesjean S., Cardoso-Oelemann M., Rüsch-Gerdes S., Willery E. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol. 2006;44:4498–4510. doi: 10.1128/JCM.01392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . World Health Organization; Geneva: 2015. Global tuberculosis report, 2015. [Google Scholar]
- 5.Nasiri M.J., Dabiri H., Darban-Sarokhalil D., Shahraki A.H. Prevalence of non-tuberculosis mycobacterial infections among tuberculosis suspects in Iran: systematic review and meta-analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashemi-Shahraki A., Darban-Sarokhalil D., Heidarieh P., Feizabadi M.M., Deshmir-Salameh S., Khazaee S. Mycobacterium simiae: a possible emerging pathogen in Iran. Jpn J Infect Dis. 2013;66:475–479. doi: 10.7883/yoken.66.475. [DOI] [PubMed] [Google Scholar]
- 7.Maoz C., Shitrit D., Samra Z., Peled N., Kaufman L., Kramer M. Pulmonary Mycobacterium simiae infection: comparison with pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis. 2008;27:945. doi: 10.1007/s10096-008-0522-6. [DOI] [PubMed] [Google Scholar]
- 8.Heidarieh P., Mirsaeidi M., Hashemzadeh M., Feizabadi M.M., Bostanabad S.Z., Nobar M.G. In vitro antimicrobial susceptibility of nontuberculous mycobacteria in Iran. Microb Drug Resist. 2016;22:172–178. doi: 10.1089/mdr.2015.0134. [DOI] [PubMed] [Google Scholar]
- 9.Hashemi-Shahraki A., Bostanabad S.Z., Heidarieh P., Titov L.P., Khosravi A.D., Sheikhi N. Species spectrum of nontuberculous mycobacteria isolated from suspected tuberculosis patients, identification by multi locus sequence analysis. Infect Genet Evol. 2013;20:312–324. doi: 10.1016/j.meegid.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Shojaei H., Heidarieh P., Hashemi A., Feizabadi M.M., Naser A.D. Species identification of neglected nontuberculous mycobacteria in a developing country. Jpn J Infect Dis. 2011;64:265–271. [PubMed] [Google Scholar]
- 11.Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 12.Van Ingen J., Boeree M., Dekhuijzen P., Van Soolingen D. Clinical relevance of Mycobacterium simiae in pulmonary samples. Eur Respir J. 2008;31:106–109. doi: 10.1183/09031936.00076107. [DOI] [PubMed] [Google Scholar]
- 13.Philley J.V., Griffith D.E. Treatment of slowly growing mycobacteria. Clin Chest Med. 2015;36:79–90. doi: 10.1016/j.ccm.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 14.van Ingen J., Totten S.E., Heifets L.B., Boeree M.J., Daley C.L. Drug susceptibility testing and pharmacokinetics question current treatment regimens in Mycobacterium simiae complex disease. Int J Antimicrob Agents. 2012;39:173–176. doi: 10.1016/j.ijantimicag.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Sampaio J., Artiles N., Pereira R., Souza J., Leite J. Mycobacterium simiae infection in a patient with acquired immunodeficiency syndrome. Braz J Infect Dis. 2001;5:352–355. doi: 10.1590/s1413-86702001000600010. [DOI] [PubMed] [Google Scholar]
- 16.Legrand E., Devallois A., Horgen L., Rastogi N. A molecular epidemiological study of Mycobacterium simiae isolated from AIDS patients in Guadeloupe. J Clin Microbiol. 2000;38:3080–3084. doi: 10.1128/jcm.38.8.3080-3084.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Abdely H., Revankar S., Graybill J. Disseminated Mycobacterium simiae infection in patients with AIDS. J Infect. 2000;41:143–147. doi: 10.1053/jinf.2000.0700. [DOI] [PubMed] [Google Scholar]
- 18.Cruz A.T., Goytia V.K., Starke J.R. Mycobacterium simiae complex infection in an immunocompetent child. J Clin Microbiol. 2007;45:2745–2746. doi: 10.1128/JCM.00359-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun-Saro B., Esteban J., Jiménez S., Castrillo J.M., Fernández-Guerrero M.L. Mycobacterium simiae infection in an immunocompromised patient without acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34:e26–e27. doi: 10.1086/338874. [DOI] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 21.Joanna Briggs Institute . University of Adelaide, Faculty of Health and Medical Sciences, Joanna Briggs Institute; Adelaide, South Australia: 2011. Reviewers’ manual, 2011 edition. [Google Scholar]
- 22.Higgins J., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 23.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 24.Tabarsi P., Baghaei P., Farnia P., Mansouri N., Chitsaz E., Sheikholeslam F. Nontuberculous mycobacteria among patients who are suspected for multidrug-resistant tuberculosis—need for earlier identification of nontuberculosis mycobacteria. Am J Med Sci. 2009;337:182–184. doi: 10.1097/maj.0b013e318185d32f. [DOI] [PubMed] [Google Scholar]
- 25.Zaker S., Heidarieh P., Sheikhi N., Ghalami M., Shahraki A.H. Identification of nontuberculous mycobacteria isolated from clinical samples. Mol Cell Biotechnol. 2012;2:49–65. [Google Scholar]
- 26.Baghaei P. Pulmonary disease caused by Mycobacterium simiae in Iran’s national referral center for tuberculosis. J Infect Dev Ctries. 2012;6:23–28. doi: 10.3855/jidc.1297. [DOI] [PubMed] [Google Scholar]
- 27.Shafipour M., Ghane M., Alang S.R., Livani S., Javid N., Shakeri F. Non-tuberculosis mycobacteria isolated from tuberculosis patients in Golestan province, North of Iran. Ann Biol Res. 2013;4:133–137. [Google Scholar]
- 28.Nour-Neamatollahie A., Ebrahimzadeh N., Siadat S.D., Vaziri F., Eslami M., Akhavan Sepahi A. Distribution of non-tuberculosis mycobacteria strains from suspected tuberculosis patients by heat shock protein 65 PCR-RFLP. Saudi J Biol Sci. 2017;24:1380–1386. doi: 10.1016/j.sjbs.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasiri M.J., Dabiri H., Fooladi A.A.I., Amini S., Hamzehloo G., Feizabadi M.M. High rates of nontuberculous mycobacteria isolation from patients with presumptive tuberculosis in Iran. New Microbe. New Infect. 2018;21:12–17. doi: 10.1016/j.nmni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Sahly H.M., Septimus E., Soini H., Septimus J., Wallace R.J., Pan X. Mycobacterium simiae pseudo-outbreak resulting from a contaminated hospital water supply in Houston, Texas. Clin Infect Dis. 2002;35:802–807. doi: 10.1086/342331. [DOI] [PubMed] [Google Scholar]
- 31.Hamblion E.L., Le Menach A., Anderson L.F., Lalor M.K., Brown T., Abubakar I. Recent TB transmission, clustering and predictors of large clusters in London, 2010–2012: results from first 3 years of universal MIRU-VNTR strain typing. Thorax. 2016;71:749–756. doi: 10.1136/thoraxjnl-2014-206608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caulfield A.J., Wengenack N.L. Diagnosis of active tuberculosis disease: from microscopy to molecular techniques. J Clin Tuberc Other Mycobacterial Dis. 2016;4:33–43. doi: 10.1016/j.jctube.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black A.T., Hamblion E.L., Buttivant H., Anderson S.R., Stone M., Casali N. Tracking and responding to an outbreak of tuberculosis using MIRU-VNTR genotyping and whole genome sequencing as epidemiological tools. J Public Health (Oxf) 2018;40:e66–e73. doi: 10.1093/pubmed/fdx075. [DOI] [PubMed] [Google Scholar]
- 34.Globan M., Lavender C., Leslie D., Brown L., Denholm J., Raios K. Molecular epidemiology of tuberculosis in Victoria, Australia, reveals low level of transmission. Int J Tuberc Lung Dis. 2016;20:652–658. doi: 10.5588/ijtld.15.0437. [DOI] [PubMed] [Google Scholar]
- 35.Bicmen C., Coskun M., Gunduz A.T., Senol G., Cirak A.K., Tibet G. Nontuberculous mycobacteria isolated from pulmonary specimens between 2004 and 2009: causative agent or not? New Microbiologica. 2010;33:399–403. [PubMed] [Google Scholar]
- 36.Varghese B., Memish Z., Abuljadayel N., Al-Hakeem R., Alrabiah F., Al-Hajoj S.A. Emergence of clinically relevant non-tuberculous mycobacterial infections in Saudi Arabia. PLoS Negl Trop Dis. 2013;7:e2234. doi: 10.1371/journal.pntd.0002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coolen-Allou N., Touron T., Belmonte O., Gazaille V., Andre M., Allyn J. Clinical, radiological, and microbiological characteristics of Mycobacterium simiae infection in 97 patients. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.00395-18. e00395–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasser M. All about Mycobacterium simiae in brief. J Med Microb Diagn. 2014;4(175):2. [Google Scholar]
- 39.Shitrit D., Peled N., Bishara J., Priess R., Pitlik S., Samra Z. Clinical and radiological features of Mycobacterium kansasii infection and Mycobacterium simiae infection. Respir Med. 2008;102:1598–1603. doi: 10.1016/j.rmed.2008.05.004. [DOI] [PubMed] [Google Scholar]