Abstract
Purpose
To investigate the impact of poor eating habits on glycemic and metabolic control, we analyzed the associations between eating behaviors and HbA1c and body mass index (BMI) in Japanese workers with type 2 diabetes mellitus (T2DM).
Subjects and methods
The Japan Medical Data Center database of workers’ medical health insurance claims was used to identify individuals with T2DM who were receiving antidiabetic medication between April 2012 and March 2015 (the primary analysis population). The database included routine medical check-up results and responses to questions on lifestyle and eating habits. Using these, we retrospectively analyzed the associations between the individuals’ eating habits and their HbA1c levels and BMIs.
Results
In total, 31,722 individuals were included in the primary analysis. The mean values of HbA1c and BMI were 7.27% and 26.29 kg/m2, respectively; these tended to be higher among the younger population. Approximately 36% of the individuals regularly ate supper within 2 hours of bedtime, 14.5% regularly consumed late-night snacks, and 13.4% regularly skipped breakfast. Each of these eating habits correlated significantly with higher HbA1c and BMI. In addition, the population with two or all three of these poor dietary habits showed the highest association with HbA1c ≥7.0% and BMI ≥25 kg/m2. Approximately 38% of workers ate fast. Fast eating was significantly associated with BMI ≥25 kg/m2 but not with HbA1c ≥7.0%.
Conclusion
Poor eating habits were significantly associated with poor glycemic and body weight control in Japanese workers with T2DM. Improved eating habits may help with glycemic and body weight management.
Keywords: type 2 diabetes, workers, eating habits, glycemic control, obesity
Introduction
People with type 2 diabetes mellitus (T2DM) are at high risk of developing diabetic complications and life-threatening complications such as cardiovascular diseases,1 which can reduce their quality of life and labor productivity.2,3 The Japan Epidemiology Collaboration on Occupational Health (J-ECOH) study, an epidemiological study of the workforces of several companies in Japan, reported that 8.0% of men and 3.3% of women had diabetes, whereas 14.1% of men and 9.2% of women had prediabetes, and that the prevalence of both T2DM and prediabetes increased with age, especially among middle-aged workers.4 Although T2DM is largely a self-managed disease, it may be difficult for Japanese workers to maintain daily metabolic control because many place a higher priority on their work than on their health.5 In addition, the dietary self-management of Japanese workers with T2DM is often affected by work-related factors, such as night shifts, workplace conformity, working overtime, and participation in workplace events.6
Eating habits show strong relationships with age and cultural, social, economic, and psychological determinants and are integrated into the individual’s daily routine over a long period. Meal frequency and times of eating can have a significant effect on various cardiac and metabolic parameters.7 Several epidemiological studies of healthy adults and patients with T2DM have shown that poor eating habits, such as eating supper or snacking late at night and skipping breakfast, are associated with hyperglycemia and obesity,8–11 as well as predisposing individuals to developing T2DM.12,13 The timing of meals plays a critical role in regulating human internal circadian rhythms.14 However, there have been only few investigations of the association between metabolic parameters and eating habits in workers with T2DM.
This retrospective, cross-sectional study using a medical claim database analyzed the relationship between eating habits and metabolic parameters in Japanese workers with T2DM in real world.
Subjects and methods
Database
Under the Industrial Safety and Health Act, workers in Japan are legally obliged to undergo a health examination at least annually, and medical records are held in databases of the health insurance societies.15 We obtained the available prescription and health examination records for the period from April 2009 to March 2015 held in the medical database of the Japan Medical Data Center (JMDC). This database stores a wealth of data on Japanese workers, uploaded from the databases of the health insurance societies. It holds not only medical claims but also records from medical check-ups, including clinical laboratory measurements and self-reports of lifestyle. It is possible to track individual workers over multiple periods of medical treatment. This study was not reviewed by an ethical committee and informed consent was not required, as this was a retrospective, observational study using a commercial database with anonymized data.
Study subjects
In this study, workers were defined as insured members of health insurance associations, but not their nonworking dependents. To exclude the workers with suspected T2DM, patients with a diagnosis of diabetes and a documented prescription for antihyperglycemic agents at least once during the period April–March of each year of the study period were included in this study. T2DM was identified by a disease classification code of either E11 (non-insulin-dependent diabetes mellitus) or E14 (unspecified diabetes mellitus) in the ICD (2003 version). Antihyperglycemic agents include those with the following Anatomical Therapeutic Chemical classification system subcodes: A10C, human insulins/insulin analogs; A10H, sulfonylureas; A10J, biguanides; A10K, thiazolidinediones; A10L, α-glucosidase inhibitors; A10M, glinides; A10N, dipeptidyl peptidase-4 (DPP-4) inhibitors; A10P, sodium-glucose co-transporter-2 inhibitors; and A10S, glucagon-like peptide-1 receptor agonists. The database included responses to questions asked in health check-ups, including about eating, exercise, and smoking habits, alcohol consumption, and sleep status.
The primary analysis set comprised workers who had measurements of HbA1c and body mass index (BMI) in a medical check-up conducted between April 2012 and March 2015 and who had provided answers to the following four questions on eating habits:
Do you eat supper (ie, the evening meal) within 2 hours of bedtime at least three times per week? (Yes/No)
Do you skip breakfast at least three times per week? (Yes/No)
Do you eat snacks after supper at least three times per week? (Yes/No)
How does your eating speed compare with that of other people? (Fast/Normal/Slow)
Data from April 2009 to March 2012 were excluded from the primary analysis set to avoid possible effects on HbA1c and BMI of the rapid increase in prescriptions of DPP-4 inhibitors (Figure S1A). The mean HbA1c and BMI levels for the population were constant between April 2012 and March 2015 (Figure S1B and C).
When two or more medical check-up records were available for one worker, the most recent data were used.
Outcome and data analysis
The primary end point was the associations of eating habits with HbA1c and BMI. For the primary analysis set, least squares (LS) means and standard errors were calculated for HbA1c and BMI, and covariance analysis was performed in which sex, age, exercise habits, sleep, alcohol consumption, and diabetes treatment history (prescription record of antihyperglycemic agents within 3 months before medical check-up) were considered as covariates in subgroups according to eating habits. For each eating habit, LS means and 95% CIs were calculated to analyze differences between groups (Yes vs No).
The proportions of workers with HbA1c ≥7.0% and <7.0%, and with BMI ≥25 kg/m2 and <25 kg/m2, were calculated. A HbA1c level of 7.0% is the glycemic goal recommended by the Japanese Diabetes Society to avoid complications,16 and BMI ≥25 kg/m2 is the cut-off point for obesity defined by the Japan Society for the Study of Obesity.17 Subgroups were defined based on the responses to the eating habit questions (Yes vs No; Fast vs [Normal + Slow]; Fast vs Normal). Differences between these subgroups in the ratios of workers with HbA1c ≥7.0% vs <7.0% and BMI ≥25 kg/m2 vs <25 kg/m2 were evaluated using Fisher’s exact test. In addition, ORs and 95% CIs were calculated using a multivariate logistic regression model to analyze the differences between the eating habit subgroups with sex, age, exercise habits, sleep, alcohol consumption, and diabetes treatment history as covariates.
In this study, eating supper within 2 hours before bedtime, skipping breakfast, and eating snacks after supper were considered to be poor eating habits. We investigated the effects of single or multiple poor eating habits on HbA1c and BMI in an ad hoc analysis. Workers were considered to be without poor eating habits if they answered “No” to all three eating habit questions; this subgroup served as a reference. The remaining workers were grouped according to whether they answered “Yes” to one or to two or more of the three questions (referred to as “single” and “multiple” poor eating habits, respectively). The proportions of workers in these subgroups with HbA1c ≥7.0% and <7.0%, and with BMI ≥25 kg/m2 and <25 kg/m2, were compared with those in the control population using Fisher’s exact tests. Multivariate logistic regression models were used to examine the effect of multiple poor eating habits on glycemic control and obesity in the same way as was described above.
The statistical analyses were performed by JMDC using SAS ver.9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Characteristics of the primary analysis set
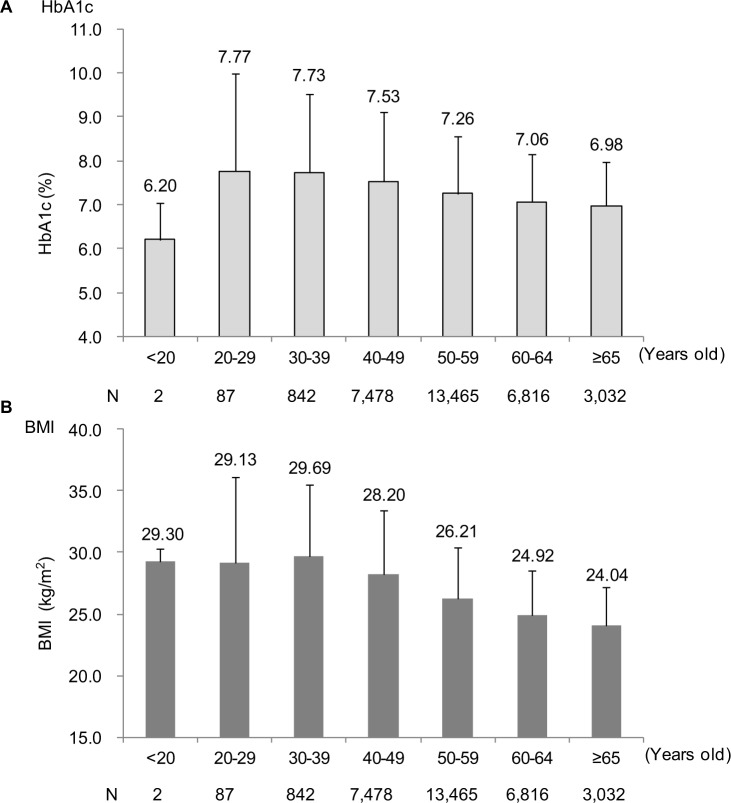
In total, 3,621,610 records were provided by JMDC, of which 1,836,555 were for insured members; 66,890 of these were identified as records of workers with T2DM receiving treatment with antihyperglycemic agents (Figure S2). Of these, 33,397 records met the inclusion criteria as those of workers with T2DM and medical records, and 31,722 workers with medical check-up records between April 2012 and March 2015 were included in the primary analysis set. Their characteristics are shown in Table 1. More than 90% of the workers were male, and the mean age was 54.7 years. The mean HbA1c and BMI were 7.27% and 26.29 kg/m2, respectively. There was no significant correlation between HbA1c levels and BMI (data not shown). Interestingly, HbA1c and BMI tended to be higher in the young age groups; the mean HbA1c and BMI values were the highest for the age group 20–40 years (Figure 1).
Table 1.
Characteristics of workers with T2DM (primary analysis set)
| No. of workers with T2DM | 31,722 | |||||
|---|---|---|---|---|---|---|
| Sex, male | n (%) | 29,147 (91.9) | ||||
| Age (years) | Mean ± SD | 54.7±8.2 | ||||
| HbA1c (%) | Mean ± SD | 7.27±1.34 | ||||
| Categories | <6.0, n (%) | 2,778 (8.8) | ||||
| 6.0–6.9, n (%) | 13,045 (41.1) | |||||
| 7.0–7.9, n (%) | 9,157 (28.9) | |||||
| 8.0–8.9, n (%) | 3,552 (11.2) | |||||
| ≥9.0, n (%) | 3,190 (10.1) | |||||
| BMI (kg/m2) | Mean ± SD | 26.29±4.58 | ||||
| Categories | <18.5, n (%) | 444 (1.4) | ||||
| 18.5–21.9, n (%) | 4,248 (13.4) | |||||
| 22.0–24.9, n (%) | 8,963 (28.3) | |||||
| 25.0–29.9, n (%) | 12,278 (38.7) | |||||
| ≥30.0, n (%) | 5,789 (18.2) | |||||
| Eating habits | Eating supper within 2 hours of bedtime | Yes | No | |||
| n (%) | 11,464 (36.1) | 20,258 (63.9) | ||||
| Skipping breakfast | Yes | No | ||||
| n (%) | 4,249 (13.4) | 27,473 (86.6) | ||||
| Eating snacks after supper | Yes | No | ||||
| n (%) | 4,594 (14.5) | 27,128 (85.5) | ||||
| Eating speed | Fast | Normal | Slow | |||
| n (%) | 11,947 (37.7) | 17,250 (54.4) | 2,525 (8.0) | |||
| Exercise habita | No | Yes | Unidentified/unlisted | |||
| n (%) | 23,767 (74.9) | 7,928 (25.0) | 27 (0.1) | |||
| Smoking habit | No | Yes | Unidentified/unlisted | |||
| n (%) | 20,131 (63.5) | 11,577 (36.5) | 14 (0.0) | |||
| Alcohol consumption | Daily | Sometimes | Rarely | Unidentified/unlisted | ||
| n (%) | 8,876 (28.0) | 9,908 (31.2) | 12,723 (40.1) | 215 (0.7) | ||
| Adequate sleep | No | Yes | Unidentified/unlisted | |||
| n (%) | 12,669 (39.9) | 18,553 (58.5) | 500 (1.6) | |||
Note:
Exercising regularly to a light sweat for >30 minutes at a time, twice weekly, for >1 year.
Abbreviations: BMI, body mass index; T2DM type 2 diabetes mellitus; HbA1c, glycated hemoglobin.
Figure 1.
Mean (A) HbA1c and (B) BMI by age (primary analysis set).
Note: Error bars show SDs of the mean.
Abbreviations: BMI, body mass index; HbA1c, glycated hemoglobin.
More than one-third of the workers regularly ate supper within 2 hours of bedtime, and 14.5% regularly ate snacks after supper (Table 1). The majority of workers (86.6%) ate breakfast five or more times a week, but 13.4% did not. Approximately 38% of workers ate fast compared with other people. Approximately 75% of the workers did not exercise regularly, 36.5% were smokers, and almost 60% consumed alcohol daily/sometimes.
Effect of eating habits on glycemic control and obesity
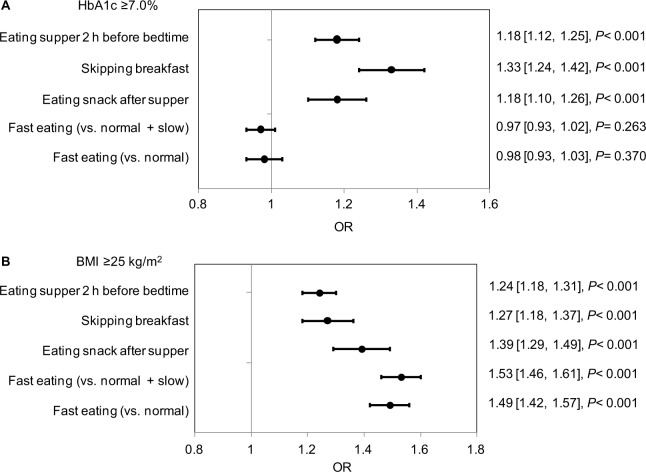
Workers who ate supper within 2 hours of bedtime, skipped breakfast, and ate snacks after supper had significantly higher HbA1c than those who did not (P<0.001; Table 2). The proportion of workers with HbA1c ≥7.0% was greater in the subgroups with these eating habits (P<0.001; Table 3). Multivariate logistic regression analysis confirmed that skipping breakfast was significantly associated with HbA1c ≥7.0% (OR 1.33, 95% CI 1.24–1.42; P<0.001) (Figure 2A). Eating supper within 2 hours of bedtime or eating snacks after supper was also significantly associated with HbA1c ≥7.0% (OR 1.18, 95% CI 1.12–1.25, and OR 1.18, 95% CI 1.10–1.26, respectively; both P<0.001). However, a fast eating speed was not associated with a change in HbA1c (Figure 2A).
Table 2.
The association of eating habits with HbA1c and BMI (primary analysis set)
| Question | Answer | N | HbA1c (%)
|
BMI (kg/m2)
|
||||
|---|---|---|---|---|---|---|---|---|
| LS mean ± SE | Difference in LS mean [95% CI] | P-value | LS mean ± SE | Difference in LS mean [95% CI] | P-value | |||
| Eating supper within | No | 20,258 | 7.65±0.04 | 27.79±0.14 | ||||
| 2 hours of bedtime | Yes | 11,464 | 7.76±0.04 | 0.10 [0.07, 0.13] | <0.001 | 28.21±0.14 | 0.42 [0.32, 0.52] | <0.001 |
| Skipping breakfast | No | 27,473 | 7.67±0.04 | 27.91±0.14 | ||||
| Yes | 4,249 | 7.89±0.05 | 0.22 [0.18, 0.26] | <0.001 | 28.37±0.15 | 0.46 [0.32, 0.60] | <0.001 | |
| Eating snacks after supper | No | 27,128 | 7.67±0.04 | 27.85±0.14 | ||||
| Yes | 4,594 | 7.80±0.05 | 0.13 [0.09, 0.17] | <0.001 | 28.41±0.15 | 0.56 [0.42, 0.69] | <0.001 | |
| Fast eatinga | No | 19,775 | 7.70±0.04 | 27.60±0.14 | ||||
| Yes | 11,947 | 7.68±0.04 | –0.02 [–0.05, 0.01] | 0.139 | 28.56±0.14 | 0.95 [0.86, 1.05] | <0.001 | |
| Fast eatingb | No | 17,250 | 7.68±0.04 | 27.58±0.14 | ||||
| Yes | 11,947 | 7.67±0.05 | –0.02 [–0.05, 0.01] | 0.266 | 28.50±0.15 | 0.91 [0.81, 1.01] | <0.001 | |
Notes:
No = Normal + Slow; Yes = Fast;
No = Normal; Yes = Fast. LS mean was calculated by analysis of covariance (factor: answer [No or Yes]; covariate: sex, age, exercise habits, sleep, alcohol consumption, and diabetes treatment history).
Abbreviations: BMI, body mass index; LS, least squares; SE, standard error; HbA1c, glycated hemoglobin.
Table 3.
The association of eating habits with glycemic control and BMI (primary analysis set)
| Question | Answer | HbA1c
|
BMI
|
||||
|---|---|---|---|---|---|---|---|
| n % [95% CI] |
n % [95% CI] |
||||||
| <7.0% | ≥7.0% | P-value | <25 kg/m2 | ≥25 kg/m2 | P-value | ||
| Eating supper within 2 hours of bedtime Skipping breakfast | No | 10,541 | 9,717 | 9,406 | 10,852 | ||
| 52.0 [51.3, 52.7] | 48.0 [47.3, 48.7] | 46.4 [45.7, 47.1] | 53.6 [52.9, 54.3] | ||||
| Yes | 5,282 | 6,182 | 4,249 | 7,215 | |||
| 46.1 [45.2, 47.0] | 53.9 [53.0, 54.8] | <0.001 | 37.1 [36.2, 38.0] | 62.9 [62.0, 63.8] | <0.001 | ||
| No | 13,995 | 13,478 | 12,246 | 15,227 | |||
| 50.9 [50.3, 51.5] | 49.1 [48.5, 49.7] | 44.6 [44.0, 45.2] | 55.4 [54.8, 56.0] | ||||
| Yes | 1,828 | 2,421 | 1,409 | 2,840 | |||
| 43.0 [41.5, 44.5] | 57.0 [55.5, 58.5] | <0.001 | 33.2 [31.7, 34.6] | 66.8 [65.4, 68.3] | <0.001 | ||
| Eating snacks after supper | No | 13,732 | 13,396 | 12,087 | 15,041 | ||
| 50.6 [50.0, 51.2] | 49.4 [48.8, 50.0] | 44.6 [44.0, 45.1] | 55.4 [54.9, 56.0] | ||||
| Yes | 2,091 | 2,503 | 1,568 | 3,026 | |||
| 45.5 [44.1, 47.0] | 54.5 [53.0, 55.9] | <0.001 | 34.1 [32.8, 35.5] | 65.9 [64.5, 67.2] | <0.001 | ||
| Fast eatinga | No | 9,875 | 9,900 | 9,424 | 10,351 | ||
| 49.9 [49.2, 50.6] | 50.1 [49.4, 50.8] | 47.7 [47.0, 48.4] | 52.3 [51.6, 53.0] | ||||
| Yes | 5,948 | 5,999 | 4,231 | 7,716 | |||
| 49.8 [48.9, 50.7] | 50.2 [49.3, 51.1] | 0.799 | 35.4 [34.6, 36.3] | 64.6 [63.7, 65.4] | <0.001 | ||
| Fast eatingb | No | 8,636 | 8,614 | 8,120 | 9,130 | ||
| 50.1 [49.3, 50.8] | 49.9 [49.2, 50.7] | 47.1 [46.3, 47.8] | 52.9 [52.2, 53.7] | ||||
| Yes | 5,948 | 5,999 | 4,231 | 7,716 | |||
| 49.8 [48.9, 50.7] | 50.2 [49.3, 51.1] | 0.643 | 35.4 [34.6, 36.3] | 64.6 [63.7, 65.4] | <0.001 | ||
Notes:
No = Normal + Slow; Yes = Fast;
No = Normal; Yes = Fast. P-values for the difference in proportion of workers with or without HbA1c ≥7.0% or BMI ≥25 kg/m2 were determined by Fisher’s exact test.
Abbreviation: BMI, body mass index; HbA1c, glycated hemoglobin.
Figure 2.
ORs (95% CIs) for (A) HbA1c ≥7.0% and (B) BMI ≥25 kg/m2 by eating habit (primary analysis set).
Notes: The ORs, 95% CIs, and P-values were evaluated by multivariate logistic regression using sex, age, exercise habit, sleep, alcohol consumption, and diabetes treatment history as covariates. The reference subgroup was those workers who answered “No” to the questions about eating habits or “Normal” or “Normal” + “Slow” to the question about eating speed.
Abbreviation: BMI, body mass index.
The workers with the habit of eating supper within 2 hours of bedtime, skipping breakfast, eating snacks after supper, and eating faster had significantly higher BMIs (P<0.001) (Table 2). A significantly greater proportion of workers with these eating habits had BMI ≥25 kg/m2 (P<0.001) (Table 3). Multivariate logistic regression analysis showed that eating supper within 2 hours of bedtime, skipping breakfast, and eating snacks after supper were significantly associated with BMI ≥25 kg/m2 (OR 1.24, 95% CI 1.18–1.31; OR 1.27, 95% CI 1.18–1.37; and OR 1.39, 95% CI 1.29–1.49, respectively; all P<0.001). Eating fast was also significantly associated with BMI ≥25 kg/m2 (OR 1.53, 95% CI 1.46–1.61 [vs Normal + Slow]; OR 1.49, 95% CI 1.42–1.57 [vs Normal]; both P<0.001) (Figure 2B).
Effects of single and multiple poor eating habits
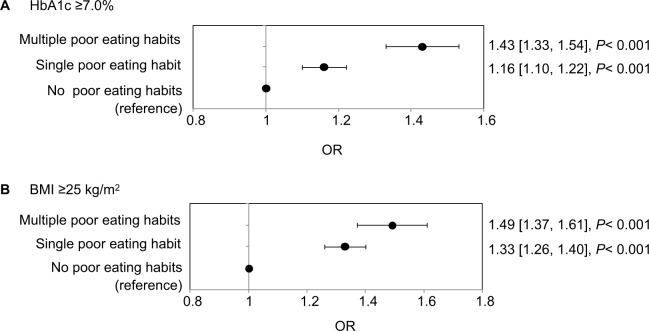
Because the three poor eating habits were associated with worse levels of HbA1c and BMI, we examined the association of glycemic and body weight control with single and multiple poor eating habits in an ad hoc analysis. The numbers of workers with single and multiple poor eating habits were 11,093 and 4,304, respectively (Table 4); these workers tended to be younger, to be smokers, to exercise less, and to have inadequate sleep. The prevalence of HbA1c ≥7.0% and BMI ≥25.0 kg/m2 was significantly greater among the workers with single and multiple poor eating habits than among those without (Table 4). Multivariate logistic regression analysis demonstrated that single and multiple poor eating habits were significantly associated with HbA1c ≥7.0% (OR 1.16, 95% CI 1.10–1.22, and OR 1.43, 95% CI 1.33–1.54, respectively; both P<0.001) (Figure 3A) and BMI ≥25.0 kg/m2 (OR 1.33, 95% CI 1.26–1.40, and OR 1.49, 95% CI 1.37–1.61, respectively; both P<0.001) (Figure 3B). The ORs for both HbA1c ≥7.0% and BMI ≥25.0 kg/m2 were higher for the workers with multiple poor eating habits than for those with a single poor eating habit.
Table 4.
The association between single/multiple poor eating habits and HbA1c and BMI
| Poor eating habits
|
||||
|---|---|---|---|---|
| 0a | Singleb | Multiplec | ||
| N | 16,325 | 11,093 | 4,304 | |
| Age (years) | Mean ± SD | 56.3±8.2 | 53.6±7.8 | 51.5±7.6 |
| <65 years, % | 86.5 | 93.8 | 96.6 | |
| Exercise habits, n (%) | No | 11,567 (70.9) | 8,645 (77.9) | 3,555 (82.6) |
| Yes | 4,743 (29.1) | 2,438 (22.0) | 747 (17.4) | |
| Unidentified/unlisted | 15 (0.1) | 10 (0.1) | 2 (0.0) | |
| Smoking habit, n (%) | No | 11,084 (67.9) | 6,750 (60.8) | 2,297 (53.4) |
| Yes | 5,235 (32.1) | 4,337 (39.1) | 2,005 (46.6) | |
| Unidentified/unlisted | 6 (0.0) | 6 (0.1) | 2 (0.0) | |
| Alcohol consumption, n (%) | Daily | 4,215 (25.8) | 3,507 (31.6) | 1,154 (26.8) |
| Sometimes | 5,254 (32.2) | 3,327 (30.0) | 1,327 (30.8) | |
| Rarely | 6,748 (41.3) | 4,169 (37.6) | 1,806 (42.0) | |
| Unidentified/unlisted | 108 (0.7) | 90 (0.8) | 17 (0.4) | |
| Adequate sleep, n (%) | No | 5,479 (33.6) | 4,941 (44.5) | 2,249 (52.3) |
| Yes | 10,668 (65.3) | 5,959 (53.7) | 1,926 (44.7) | |
| Unidentified/unlisted | 178 (1.1) | 193 (1.7) | 129 (3.0) | |
| HbA1c (%) | Mean ± SD | 7.17±1.26 | 7.31±1.35 | 7.55±1.53 |
| Percentage of workers, % [95% CI] | <7.0% | 53.1 [52.3, 53.8] | 48.2 [47.3, 49.2] | 42.1 [40.6, 43.5] |
| ≥7.0% | 46.9 [46.2, 47.7] | 51.8 [50.8, 52.7] | 57.9 [56.5, 59.4] | |
| P-value | Fisher’s exact test | – | <0.001 | <0.001 |
| BMI (kg/m2) | Mean ± SD | 25.71±4.44 | 26.70±4.59 | 27.46±4.75 |
| Percentage of workers, % [95% CI] | <25 kg/m2 | 48.9 [48.2, 49.7] | 38.6 [37.7, 39.5] | 32.2 [30.8, 33.6] |
| ≥25 kg/m2 | 51.1 [50.3, 51.8] | 61.4 [60.5, 62.3] | 67.8 [66.4, 69.2] | |
| P-value | Fisher’s exact test | – | <0.001 | <0.001 |
Note:
Answered “No” to all questions;
Answered “Yes” to one of the three questions;
Answered “Yes” to two or three questions.
Abbreviation: BMI, body mass index; HbA1c, glycated hemoglobin.
Figure 3.
ORs (95% CIs) for (A) HbA1c ≥7.0% and (B) BMI ≥25 kg/m2 by poor eating habit.
Notes: The ORs, 95% CIs, and P-values were evaluated by multivariate logistic regression using sex, age, exercise habit, sleep, alcohol consumption, and diabetes treatment history as covariates. The reference subgroup was those workers who answered “No” to all three poor eating habit questions.
Abbreviations: BMI, body mass index; HbA1c, glycated hemoglobin.
Discussion
This study analyzed data from the JMDC database for medical insurance associations, which is one of the largest medical databases with medical check-up data in Japan. Because the selected data sets were for insured members with a diagnosis of T2DM and prescribed antihyperglycemic medication, the subjects of this study were considered to be workers with T2DM. The results of the study showed clear associations between some eating habits and poor glycemic control and obesity, even under treatment with antidiabetic medication.
In the primary analysis set, mean HbA1c and BMI values were similar to those previously reported for workers with T2DM.4 Approximately half of the workers had not achieved the therapeutic goal of HbA1c <7.0%, more than half were obese with BMI ≥25.0 kg/m2, and approximately one-fifth were severely obese with BMI ≥30 kg/m2. Taken together, obesity and poor glycemic control remain issues for workers with T2DM, despite taking antihyperglycemic medication.
The HbA1c and BMI levels tended to be higher in the younger workers. In the general population, young people often show less healthy eating habits compared with older generations.18–20 Of the 929 workers aged 20–40 years in the primary analysis set, 428 (46.1%) regularly ate supper within 2 hours of bedtime, 225 (24.2%) skipped breakfast more than three times a week, and 159 (17.1%) regularly ate snacks after supper; these percentages were higher than for the workers aged ≥40 years (data not shown). Consistent with our data, poor glycemic control and obesity were more frequently reported in younger patients with T2DM.21–23 Because early stage of T2DM is often asymptomatic, younger patients being busy with their job may be less motivated to their diabetic condition, have less time to foster a healthy lifestyle, and tend to be less adherent to medication.22,23 The National Health and Nutrition Survey in Japan and a study of Japan Clinicians Diabetes Association also reported the higher treatment discontinuation rate for males in the 40’s or those diagnosed with T2DM in their 40’s.24,25 Therefore, a higher rate of poor glycemic control and obesity in young patients is possibly due to poor compliance to proper dietary habits and to treatment. The results suggested that more effective intervention or changes in the lifestyle may be necessary for younger diabetic workers.
Although regular exercise improves insulin sensitivity and promotes better glycemic control in patients with T2DM,26 it is often difficult for patients to maintain regular exercise habits. In our study, approximately 75% of workers reported no regular exercise habits, whereas 72.2% of Japanese male workers without T2DM reported that they did not exercise regularly.27 These results suggest that lack of regular, habitual exercise may be a common issue for workers in Japan.
The primary analysis revealed that the three poor eating habits were closely associated with both poor glycemic control and obesity. Previous reports have shown that night eating, including late-night snack, promoted weight gain because of decreased metabolic rate during sleep28 and fat oxidation.29 This pattern of eating also enhanced postprandial glucose excursion and elevated 24-hour average blood glucose levels.30 In addition, there was an inverse relationship between the frequency of breakfast consumption and the risk of obesity and T2DM.31–33 The mechanism of weight gain after skipping breakfast may include possible consumption of large meals as lunch and supper, and reduced thermogenesis, as the thermic effect of food in the morning is higher than it is during midday and night time.34 Skipping breakfast is associated with a significantly higher HbA1c and a higher hyperglycemic response to lunch and supper in people with T2DM.35,36 It has been shown that when breakfast was skipped, insulin, C-peptide, and intact glucagon-like peptide-1 levels were low, whereas glucagon and fatty acid levels were increased after lunch and supper.36 This resulted in higher postprandial blood glucose levels. Subgroups with single and multiple poor eating habits were associated with significantly higher HbA1c and BMI, and subgroups with multiple poor eating habits had higher ORs for hyperglycemia and obesity compared with the subgroup without any of these poor eating habits. These results suggest that each poor eating habit contributes to poor glycemic control and the development of obesity in workers with T2DM.
The subgroup of workers who described their eating speed as Fast tended to have higher BMIs than the subgroups whose eating speeds were Normal or Normal + Slow. A fast eating habit has been reported to be associated with high BMI in nondiabetic obese subjects37–39 and in people with T2DM.40 In contrast, HbA1c level was not associated with fast eating in the present study. A previous study showed no difference in post-meal changes in blood glucose and the secretion of insulin in people with T2DM between those given liquid nutrition over 20 minutes and over 5 minutes.41 Eating slowly while masticating may increase diet-induced thermogenesis after a meal, contributing to reduced body weight in obese subjects.42,43 Thus, the association between fast eating and high BMI but not HbA1c in the workers with T2DM in this study is consistent with that for people with T2DM in general population.
A limitation of this study was the lack of information about meal sizes, total energy consumption, constituents of meal or snack, and intake of nutraceutical/functional food, which plays a role in preventing metabolic syndrome,44 by the individual workers over the course of the day. In addition, the self-reported lifestyle assessment was qualitative and may have included reporting bias. Although these questionnaires are widely used for medical check-ups in Japan, cautious interpretation of results is necessary because the definition of each response criterion in questionnaires differs depending on studies. The insured members of health insurance associations were not representative of all types of work, and 92% of the workers in this study population were male. We could not define the workers diagnosed with T2DM who were treated by lifestyle intervention alone from medical claim, as a large number of workers with suspected T2DM was included in this database. Finally, this was a retrospective, cross-sectional, epidemiological study, which cannot prove causal relationships, with incomplete database records, in some cases.
Despite these limitations, this database study demonstrated associations between poor eating habits and poor glycemic control and obesity in Japanese workers with T2DM treated with antidiabetic agents. Because a divided supper ameliorates postprandial hyperglycemia in patients with T2DM,45 the results of this study suggest that improving eating habits would lead to improved glycemic and body weight control in workers with T2DM, as recommended in the American Heart Association’s scientific statement.7 As the JMDC database includes medical check-up data for a large proportion of insured Japanese workers, a systematic analysis of this database may provide the opportunity to examine relationships between lifestyle and diseases.
Conclusion
In summary, the results of our database study using medical claim and medical check-up data showed that eating time, skipping breakfast, and eating speed were significantly associated with glycemic control and body weight in T2DM workers treated with antidiabetic agents. Improving eating habits may aid weight control and help in the glycemic management of T2DM patients.
Supplementary material
Time course changes in (A) Prescription of anti-diabetic agents, (B) HbA1c, and (C) BMI in workers with T2DM.
Note: Data in graphs (B) and (C) are means ± standard deviation.
Abbreviations: BMI, body mass index; DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; HbA1c, glycated hemoglobin; SGLT2. sodium-glucose co-transporter-2.
Sampling design.
Note: (1) Workers were defined as insured members of health insurance associations between 2009 and 2015; (2) T2DM was identified by a disease classification code of E11 or E14 in the International Classification of Diseases and antihyperglycemic agents include those with the following Anatomical Therapeutic Chemical classification system sub-code (A10C, A10H, A10J, A10K, A10L, A10M, A10N, A10P, A10S); (3) The data set of T2DM workers include medical check-up data including both HbA1c and BMI values; (4) Primary analysis set included workers with T2DM with a latest medical check-up record between 2012 and 2015.
Abbreviation: T2DM, type 2 diabetes mellitus.
Acknowledgments
The authors would like to express gratitude to the following co-workers: Hiroaki Matsuda and Takumi Tajima from Mitsubishi Tanabe Pharma Corporation, Chie Ito, Satoshi Kusakabe, and Rie Nishikino from the JMDC for valuable advice of the study and conducting the data analysis, and Akira Saito, PhD (International Medical Translation Service, Inc.), for providing medical writing support funded by Mitsubishi Tanabe Pharma Corporation.
Footnotes
Author contributions
All authors contributed toward the study design, data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
All authors are employees of Mitsubishi Tanabe Pharma Corporation, Japan. The authors report no other conflicts of interest in this work.
References
- 1.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavigne JE, Phelps CE, Mushlin A, Lednar WM. Reductions in individual work productivity associated with type 2 diabetes mellitus. Pharmacoeconomics. 2003;21(15):1123–1134. doi: 10.2165/00019053-200321150-00006. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–928. doi: 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uehara A, Kurotani K, Kochi T, et al. Prevalence of diabetes and pre-diabetes among workers: Japan Epidemiology Collaboration on Occupational Health Study. Diabetes Res Clin Pract. 2014;106(1):118–127. doi: 10.1016/j.diabres.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Gotay CC, Shimizu H, Muraoka M, Ishihara Y, Tsuboi K, Ogawa H. Health attitudes and behaviors: comparison of Japanese and Americans of Japanese and European Ancestry. Health Place. 2004;10(2):153–161. doi: 10.1016/S1353-8292(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 6.Sato M, Yamazaki Y. Work-related factors associated with self-care and psychological health among people with type 2 diabetes in Japan. Nurs Health Sci. 2012;14(4):520–527. doi: 10.1111/j.1442-2018.2012.00729.x. [DOI] [PubMed] [Google Scholar]
- 7.St-Onge MP, Ard J, Baskin ML, et al. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation. 2017;135(9):e96–e121. doi: 10.1161/CIR.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morse SA, Ciechanowski PS, Katon WJ, Hirsch IB. Isn’t this just bedtime snacking? The potential adverse effects of night-eating symptoms on treatment adherence and outcomes in patients with diabetes. Diabetes Care. 2006;29(8):1800–1804. doi: 10.2337/dc06-0315. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe Y, Saito I, Henmi I, et al. Skipping Breakfast is Correlated with Obesity. J Rural Med. 2014;9(2):51–58. doi: 10.2185/jrm.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakajima K, Suwa K. Association of hyperglycemia in a general Japanese population with late-night-dinner eating alone, but not breakfast skipping alone. J Diabetes Metab Disord. 2015;14:16. doi: 10.1186/s40200-015-0147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakurai M, Yoshita K, Nakamura K, et al. Skipping breakfast and 5-year changes in body mass index and waist circumference in Japanese men and women. Obes Sci Pract. 2017;3(2):162–170. doi: 10.1002/osp4.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bi H, Gan Y, Yang C, Chen Y, Tong X, Lu Z. Breakfast skipping and the risk of type 2 diabetes: a meta-analysis of observational studies. Public Health Nutr. 2015;18(16):3013–3019. doi: 10.1017/S1368980015000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uemura M, Yatsuya H, Hilawe EH, et al. Breakfast Skipping is Positively Associated With Incidence of Type 2 Diabetes Mellitus: Evidence From the Aichi Workers’ Cohort Study. J Epidemiol. 2015;25(5):351–358. doi: 10.2188/jea.JE20140109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison KC, Goel N. Timing of eating in adults across the weight spectrum: Metabolic factors and potential circadian mechanisms. Physiol Behav. 2018;192:158–166. doi: 10.1016/j.physbeh.2018.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuwahara K, Uehara A, Yamamoto M, et al. Current status of health among workers in Japan: Results from the Japan Epidemiology Collaboration on Occupational Health Study. Ind Health. 2016;54(6):505–514. doi: 10.2486/indhealth.2016-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haneda M, Noda M, Origasa H, et al. Japanese Clinical Practice Guideline for Diabetes 2016. J Diabetes Investig. 2018;9(3):657–697. doi: 10.1111/jdi.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito Y, Shirai K, Nakamura T, et al. Diagnostic criteria for obesity 2011. J Jpn Soc Study Obesity. 2011;17(s):1–78. [Google Scholar]
- 18.Nishiyama M, Muto T, Minakawa T, Shibata T. The combined unhealthy behaviors of breakfast skipping and smoking are associated with the prevalence of diabetes mellitus. Tohoku J Exp Med. 2009;218(4):259–264. doi: 10.1620/tjem.218.259. [DOI] [PubMed] [Google Scholar]
- 19.Sakata K, Matumura Y, Yoshimura N, et al. Relationship between skipping breakfast and cardiovascular disease risk factors in the national nutrition survey data. Nihon Koshu Eisei Zasshi. 2001;48(10):837–841. in Japanese. [PubMed] [Google Scholar]
- 20.Hattori T, Konno S, Munakata M. Gender Differences in Lifestyle Factors Associated with Metabolic Syndrome and Preliminary Metabolic Syndrome in the General Population: The Watari Study. Intern Med. 2017;56(17):2253–2259. doi: 10.2169/internalmedicine.8578-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36(8):2271–2279. doi: 10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quah JH, Liu YP, Luo N, How CH, Tay EG. Younger adult type 2 diabetic patients have poorer glycaemic control: a cross-sectional study in a primary care setting in Singapore. BMC Endocr Disord. 2013;13:18. doi: 10.1186/1472-6823-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu H, Hori A, Nishiura C, et al. Hba1c, Blood Pressure, and Lipid Control in People with Diabetes: Japan Epidemiology Collaboration on Occupational Health Study. PLoS One. 2016;11(7):e0159071. doi: 10.1371/journal.pone.0159071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ministry of Health, Labour and Welfare Overview of the National Health and Nutrition survey results. 2012. [Accessed July 23, 2018]. Available from: http://www.nibiohn.go.jp/eiken/english/research/pdf/nhns2012.pdf.
- 25.Sugimoto H, Nakaishi S, Isogaya H, et al. A multiclinical study on the cessation of treatment for type 2 diabetic patients. J Japan Diab Soc. 2013;56(10):744–752. [Google Scholar]
- 26.Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84(7 Suppl 1):S15–S21. doi: 10.3949/ccjm.84.s1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakurai M, Nakamura K, Miura K, et al. Self-reported speed of eating and 7-year risk of type 2 diabetes mellitus in middle-aged Japanese men. Metabolism. 2012;61(11):1566–1571. doi: 10.1016/j.metabol.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Katayose Y, Tasaki M, Ogata H, Nakata Y, Tokuyama K, Satoh M. Metabolic rate and fuel utilization during sleep assessed by whole-body indirect calorimetry. Metabolism. 2009;58(7):920–926. doi: 10.1016/j.metabol.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Hibi M, Masumoto A, Naito Y, et al. Nighttime snacking reduces whole body fat oxidation and increases LDL cholesterol in healthy young women. Am J Physiol Regul Integr Comp Physiol. 2013;304(2):R94–R101. doi: 10.1152/ajpregu.00115.2012. [DOI] [PubMed] [Google Scholar]
- 30.Sato M, Nakamura K, Ogata H, et al. Acute effect of late evening meal on diurnal variation of blood glucose and energy metabolism. Obes Res Clin Pract. 2011;5(3):e169–e266. doi: 10.1016/j.orcp.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Pereira MA, Erickson E, Mckee P, et al. Breakfast frequency and quality may affect glycemia and appetite in adults and children. J Nutr. 2011;141(1):163–168. doi: 10.3945/jn.109.114405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timlin MT, Pereira MA. Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr Rev. 2007;65(6 Pt 1):268–281. doi: 10.1301/nr.2007.jun.268-281. [DOI] [PubMed] [Google Scholar]
- 33.Horikawa C, Kodama S, Yachi Y, et al. Skipping breakfast and prevalence of overweight and obesity in Asian and Pacific regions: a meta-analysis. Prev Med. 2011;53(4-5):260–267. doi: 10.1016/j.ypmed.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 34.Romon M, Edme JL, Boulenguez C, Lescroart JL, Frimat P. Circadian variation of diet-induced thermogenesis. Am J Clin Nutr. 1993;57(4):476–480. doi: 10.1093/ajcn/57.4.476. [DOI] [PubMed] [Google Scholar]
- 35.Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol Int. 2014;31(1):64–71. doi: 10.3109/07420528.2013.821614. [DOI] [PubMed] [Google Scholar]
- 36.Jakubowicz D, Wainstein J, Ahren B, Landau Z, Bar-Dayan Y, Froy O. Fasting until noon triggers increased postprandial hyperglycemia and impaired insulin response after lunch and dinner in individuals with type 2 diabetes: a randomized clinical trial. Diabetes Care. 2015;38(10):1820–1826. doi: 10.2337/dc15-0761. [DOI] [PubMed] [Google Scholar]
- 37.Otsuka R, Tamakoshi K, Yatsuya H, et al. Eating fast leads to obesity: findings based on self-administered questionnaires among middle-aged Japanese men and women. J Epidemiol. 2006;16(3):117–124. doi: 10.2188/jea.16.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohkuma T, Fujii H, Iwase M, et al. Impact of eating rate on obesity and cardiovascular risk factors according to glucose tolerance status: the Fukuoka Diabetes Registry and the Hisayama Study. Diabetologia. 2013;56(1):70–77. doi: 10.1007/s00125-012-2746-3. [DOI] [PubMed] [Google Scholar]
- 39.Ohkuma T, Hirakawa Y, Nakamura U, Kiyohara Y, Kitazono T, Ninomiya T. Association between eating rate and obesity: a systematic review and meta-analysis. Int J Obes (Lond) 2015;39(11):1589–1596. doi: 10.1038/ijo.2015.96. [DOI] [PubMed] [Google Scholar]
- 40.Saito A, Kawai K, Yanagisawa M, et al. Self-reported rate of eating is significantly associated with body mass index in Japanese patients with type 2 diabetes. Japan Diabetes Clinical Data Management Study Group (JDDM26. Appetite. 2012;59(2):252–255. doi: 10.1016/j.appet.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Kamiko K, Aoki K, Kamiyama H, Taguri M, Terauchi Y. Comparison of Plasma Glucose and Gut Hormone Levels Between Drinking Enteral Formula Over a Period of 5 and 20 Minutes in Japanese Patients With Type 2 Diabetes: A Pilot Study. J Clin Med Res. 2016;8(10):749–752. doi: 10.14740/jocmr2686w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamada Y, Kashima H, Hayashi N. The number of chews and meal duration affect diet-induced thermogenesis and splanchnic circulation. Obesity (Silver Spring) 2014;22(5):E62–E69. doi: 10.1002/oby.20715. [DOI] [PubMed] [Google Scholar]
- 43.Hamada Y, Miyaji A, Hayashi N. Effect of postprandial gum chewing on diet-induced thermogenesis. Obesity (Silver Spring) 2016;24(4):878–885. doi: 10.1002/oby.21421. [DOI] [PubMed] [Google Scholar]
- 44.Scicchitano P, Cameli M, Maiello M, et al. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J Funct Foods. 2014;6:11–32. [Google Scholar]
- 45.Imai S, Kajiyama S, Hashimoto Y, et al. Divided consumption of late-night-dinner improves glycemic excursions in patients with type 2 diabetes: A randomized cross-over clinical trial. Diabetes Res Clin Pract. 2017;129:206–212. doi: 10.1016/j.diabres.2017.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time course changes in (A) Prescription of anti-diabetic agents, (B) HbA1c, and (C) BMI in workers with T2DM.
Note: Data in graphs (B) and (C) are means ± standard deviation.
Abbreviations: BMI, body mass index; DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; HbA1c, glycated hemoglobin; SGLT2. sodium-glucose co-transporter-2.
Sampling design.
Note: (1) Workers were defined as insured members of health insurance associations between 2009 and 2015; (2) T2DM was identified by a disease classification code of E11 or E14 in the International Classification of Diseases and antihyperglycemic agents include those with the following Anatomical Therapeutic Chemical classification system sub-code (A10C, A10H, A10J, A10K, A10L, A10M, A10N, A10P, A10S); (3) The data set of T2DM workers include medical check-up data including both HbA1c and BMI values; (4) Primary analysis set included workers with T2DM with a latest medical check-up record between 2012 and 2015.
Abbreviation: T2DM, type 2 diabetes mellitus.