Abstract
Objective
To estimate preferences in relevant treatment characteristics evaluated by different groups involved in the management of patients with rheumatic diseases.
Subjects and methods
We surveyed patients with rheumatic diseases, and rheumatologists, nurses, and pharmacists with experience in treatment with/provision of biologic drugs for these patients. Through a discrete choice experiment, participants evaluated 16 possible scenarios in which pairs of similarly efficacious treatments were described with six characteristics: 1) frequency of administration; 2) mode and place of administration; 3) manner, helpfulness, efficiency, and courtesy of health personnel; 4) frequency of reactions at the site of drug administration; 5) severity of generalized undesired/allergic reactions; and 6) additional cost. The direction and strength of preferences toward each characteristic level and the relative importance of each characteristic were estimated through a random-effects conditional logistic regression model.
Results
In total, 513 patients, 110 rheumatologists, 51 nurses, and 46 pharmacists from 30 centers in Italy participated. Characteristics 3, 4, and 6 were the most important for every subgroup; 1 was least important for patients and rheumatologists, 2 was least important for pharmacists, and 2 and 5 were least important for nurses. For characteristic 2, pharmacists preferred subcutaneous self-injection with a syringe; nurses preferred assisted infusion at an infusion center close to the patient’s home; patients and rheumatologists preferred subcutaneous self-injection with a pen.
Conclusion
The different preferences for some characteristics shown by the different groups can play an important role, together with purely clinical aspects, in the choice and consequent benefit of treatments, contributing also to a more satisfactory use of resources.
Keywords: preferences, biologic drugs, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, decision making
Introduction
Rheumatoid arthritis (RA), ankylosing spondylitis (AS), and psoriatic arthritis (PsA) are among the most burdensome rheumatic diseases (RDs). They are chronic, progressive inflammatory conditions associated with severe morbidity and decline in functional status leading to a significant impairment of patients’ quality of life, limitations in activities, and restrictions in performance of social roles.1–4 At the societal level, the impact of these conditions includes high socioeconomic direct and indirect costs associated with increased use of health care resources, physical impairment, loss of working days, loss of efficiency at work, or loss of employment.5–8
Traditionally, drug management of RDs has included symptom-modifying therapies, mainly nonsteroidal anti-inflammatory drugs and corticosteroids, combined with disease-modifying antirheumatic drugs (DMARDs). Since the introduction of biologic agents, particularly anti-tumor necrosis factor α agents, outcomes for these diseases have changed: the addition of biologic agents to treatment strategies has improved the ability to control disease activity and slow the progression of joint damage, with significant improvements in symptoms, function, and quality of life.9,10
Despite the widespread availability of biologics, particularly the anti-tumor necrosis factor α agents, patient access to these agents differs significantly between countries. Potential barriers influencing availability may partly be explained by economic factors. Clinical practice also plays a key role in biologic access, and selecting the best treatment for each patient has become a challenging process for rheumatologists as well as payers.7,11 In agreement with the recently published recommendations of the European League Against Rheumatism for the management of RA with DMARDs,12 no evidence-based guidance is available for rheumatologists to preferentially prescribe a specific biologic drug, since direct comparisons between these agents at the start of treatment are lacking.
Recommendations for PsA13,14 have also been published recently to help clinicians in everyday practice manage patients treated with biologics. According to these recommendations, choosing a biologic over another drug should be guided by aspects such as safety, individual patient characteristics, patient preferences,15–22 and costs.23,24 However, management of patients with RDs involves a complex interaction between different parties, including patients, physicians, pharmacists, and nurses, each having a different but important role in the decision-making process. In the Italian health care system, for instance, physicians prescribe treatment after understanding each patient’s characteristics and needs, pharmacists interact with physicians to manage drugs provision according to requests and hospital budget, and nurses assist patients and interact with physicians to optimize treatment administration. Each group has their own set of preferences, influenced by the role they play in the health care system, their experiences, and their expectations.
Recent studies have shown that patients, physicians, budget holders, and other individuals involved do not necessarily agree on the importance of some aspects of possible treatment options.21,22,24–29
Reaching an optimal decision requires being informed and aware of the opinions and preferences of all interested parties. Understanding the similarities and discrepancies in treatment preferences of the different individuals involved can be useful for improving the communication between them to uncover alternative approaches to treatment strategy, with positive effects on patient satisfaction, compliance, and, consequently, the effectiveness and efficiency of the treatments. In RDs, comparisons of preferences between patients and physicians have been reported,26,28,29 but other parties involved in the decision-making process can further influence the choice of therapy.
The objective of the Conjoint Analysis in Rheumatic Diseases (CARA) study was to compare the preferences of patients, rheumatologists, nurses, and pharmacists in the choice of treatment with biologics used in RA, AS, and PsA.
Subjects and methods
A multicenter stated preference study was conducted, based on a discrete choice experiment (DCE), a type of conjoint analysis.30,31 DCEs are based on the idea that goods or services (called “items”) can be described by their characteristics (or attributes) and individual preferences for these items are dependent on the levels of their characteristics. Within a DCE, hypothetical scenarios are created with combinations of previously selected characteristic levels; these are presented to participants who choose between alternative options. DCEs make it possible to estimate whether a characteristic level is important, to compare the relative importance (RI) of one characteristic with others, and to determine how individuals are willing to trade between different characteristic levels, by estimating the marginal rate of substitution. If a cost characteristic is included in the choice sets, the marginal rate of substitution can be expressed in monetary terms, that is, in terms of willingness to pay (WTP) for a characteristic level.
The present DCE study, designed according to current guidelines32 and experience gained from previous research in the Italian health care system,25,26,33,34 was conducted in four main phases: 1) design of the DCE, consisting of choosing characteristics, assigning levels to each characteristic, and constructing the scenarios to be evaluated; 2) development of the survey instrument; 3) data collection; and 4) data analysis and interpretation of results. Details of the design process are reported in the Supplementary material. Table 1 lists the final characteristics and the levels selected. Particular attention should be paid to the cost characteristic, which we introduced as the possibility of increasing health care taxes for any citizen. In the Italian health care system, especially for expensive treatments such as biologics, this approach is more realistic than focusing on the price of the product or the cost of treatment, which is entirely paid by regional health services, and indirectly by citizens through taxes.27 The levels for the cost characteristic were decided by considering that the actual costs paid as health care taxes by Italian citizens correspond to a per capita monthly cost of €130 over a per capita gross income of €1,700 (corresponding to 8% of the gross income).27 Accordingly, we introduced the following three levels: 1) no increase in health care taxes to be paid (reference level); 2) health care taxes are twice those currently paid; and 3) health care taxes are three times those currently paid (details in Table 1).
Table 1.
Characteristics and levels for the discrete choice experiment scenarios
| Characteristic | Levels |
|---|---|
| Frequency of administration | 1. Once every 7–15 days (FREQ1) |
| 2. Once every 1–2 months (FREQ2) | |
| 3. Once every 6 months (FREQ3) | |
| Mode and place of administration | 1. Subcutaneous self-injection with pen at home (ADM1) |
| 2. Subcutaneous self-injection with a syringe at home (ADM2) | |
| 3. Infusion, assisted by a nurse or doctor, at an infusion center close to home (ADM3) | |
| 4. Infusion assisted by a nurse or doctor, at the rheumatology center (ADM4) | |
| Manner, helpfulness, efficiency, and courtesy of health personnel (doctors, nurses) who assist the patient during treatment and related aspects (eg, side effects) and during follow-up visits | 1. Unsatisfactory for the patient (COMFORT1) |
| 2. Fairly satisfactory for the patient (COMFORT2) | |
| 3. Very satisfactory for the patient (COMFORT3) | |
| Frequency of reactions at the site of drug administration (eg, erythema, irritation, burning, pain at the injection site) | 1. Infrequently, that is, ≤3 times a year (LOCALR1) |
| 2. Frequent, ≥4 times per year (LOCALR2) | |
| Generalized undesired reactions or allergic reactions involving the whole body, due to the administration of the biologic drug | 1. Mild, such as redness, tightness of the throat, headache (GENERALR1) |
| 2. Serious, such as severe discomfort, shortness of breath, hypotensive shock (fainting) (GENERALR2) | |
| Additional cost to be paid | 1. None (taxes for health care remain the same already paid) (COST1) |
| 2. Health care taxes are doubled (€130 more per month on an assumed €1,700 of gross income) (COST2) | |
| 3. Health care taxes are tripled (€260 more per month on an assumed €1,700 of gross income) (COST3) |
Sixteen pairwise choice sets were generated, which demonstrated to be a reasonable number for evaluation by each respondent.27,33
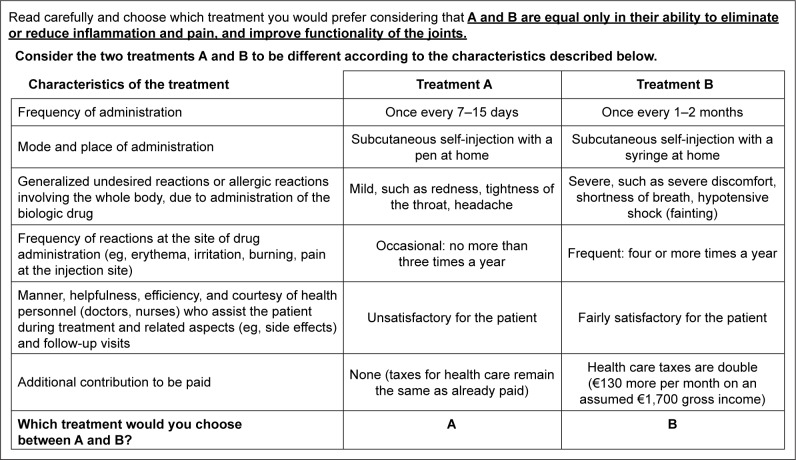
Each choice set (example in Figure 1) required choosing between two treatment options described by six characteristics with different levels. At the top of each scenario, a reminder that the two treatments must be assumed to be equivalent in clinical effectiveness was included, considering that clinical effects are similar for the different biologics. This was to guide the respondent to focus on characteristics other than clinical effectiveness, which was found to potentially dominate participant preferences, as confirmed by recent research.24
Figure 1.
An example of a choice set used in the discrete choice experiment.
Notes: Participants received 16 choice sets, in which only the combination of levels was changed. A reminder above each choice set specified that the two treatment options had to be considered as equal in terms of clinical effectiveness. Characteristics were listed on the left of each choice set and the two columns on the right presented combinations of levels to describe and compare two possible treatment options.
Survey instrument and data collection process
A paper questionnaire for self-completion was prepared and validated in a subgroup of participants. It included 16 choice sets and a number of other items identified as relevant to the study. In their questionnaires, physicians, nurses, and pharmacists gave some information on their sociodemographics and experience of biologic treatment. In patients’ questionnaires, physicians reported data on their clinical condition, and patients specified their sociodemographic characteristics and health-related quality of life using the EQ-5D-5L.35 These results are not reported in this paper, which focuses on preferences. In all the questionnaires, we included detailed descriptions of meanings of the characteristics and levels, and instructions on how to perform the exercise. To avoid a possible order effect on answers, we changed the order of the choice sets. All data were collected during routine practice. The questionnaire had to be completed independently at the hospital; however, a physician or nurse was on hand to address any uncertainties about the content.
Participants and setting
To obtain results that reflect the whole Italian health care system, we chose 30 key Italian university and hospital rheumatology centers from different regions of Northern, Central, and Southern Italy. Participants included patients, rheumatologists, nurses, and pharmacists involved in treatment with or management of biologics, who met the following inclusion criteria. Patients had to be ≥18 years old, recruited consecutively during a routine visit, and with a diagnosis of RA, AS, or PsA. Within each diagnosis, around half, classified as “naïve”, had no previous or current experience of treatment with biologics; however, at the time of recruitment, they had to be eligible for and were prescribed with a biologic. The other half of the patients were “experienced”, having received at the time of recruitment or during the previous 12 months and for ≥3 months treatment with a biologic for their RD. Patients were not recruited if they did not meet the inclusion criteria, were unable to understand or carry out the required tasks, were on a clinical trial of an investigational or marketed product, or had contraindications for use of any biologic. Rheumatologists and nurses had to operate in the Italian health care system, and have experience in treating the target patients with biologics (ie, managing at least ten patients per month treated with biologics for RA, AS, and/or PsA). Pharmacists were included if they were involved in managing and delivering biologics for RA, PsA, and/or AS in the center’s pharmacy.
The sample size of each subgroup was determined according to a formal calculation,36 with some practical considerations, clarified in the Supplementary material. Accordingly, we had to recruit ~90 patients in each subgroup of diagnosis (RA, AS, SpA) and treatment experience (naïve and experienced), for a total of up to 540 patients, ~100 rheumatologists, ~50 pharmacists, and ~50 nurses. The study was conducted in agreement with National Regulatory Requirements, International Conference on Harmonization Guidelines for Good Clinical Practice, and the 18th World Medical Assembly37 and all subsequent amendments. The ethics committees of the participating rheumatology centers accepted the study protocol. Participants had to sign an informed consent form, after receiving information on the aim of the study, type of data, and method of data collection.
Data analysis
Before conducting the statistical analyses, we assessed reliability of the data by checking if any respondent had answered fewer than half of the 16 choice scenarios, or if they had always chosen the same option (eg, always treatment A), as indicators of non-understanding or performing the task without due attention.
Responses from the DCE were analyzed according to the random utility theory,38 using a random-effects conditional logistic regression model, which allowed for multiple observations from individuals, in STATA program (Version 12).
The systematic utility V of each treatment option j was estimated as a linear and additive function of treatment characteristics and levels included in the choice sets analyzed (Equation 1):
| (1) |
Each regression coefficient β corresponds to the parameter estimates indicating the direction (ie, whether a characteristic level is more or less preferred) and the strength (ie, the dimension of the estimate) of preference for one level (eg, β2 for frequency of administration of once every 1–2 or every 6 months) compared with the reference level (eg, β3 for once every 7–15 days). The 95% CIs and the P-values for the characteristic levels are calculated to show the statistical significance of the parameter estimate of the corresponding level compared with the reference level. In particular, where CIs do not include the 0 value and when those of different levels of a particular characteristic do not overlap, it means that the mean estimates are different from 0 and from each other at the 5% level of statistical significance.
To understand which characteristic contributes more to the utility of treatment options under study, its RI was estimated by computing the ratio of the utility given to each characteristic (within the range of the levels assigned) to the sum of the utilities assigned to the level ranges of all characteristics included in the experiment.
We calculated the WTP in terms of increased health care taxes for the respondents, in order to improve, ceteris paribus, by one level the benefit of a specific treatment characteristic for all patients in the target health care system (eg, the amount of increased taxes that participants are willing to pay, so that all target patients receive a treatment administered once every 1–2 months instead of once every 7–15 days). WTP can be calculated as in Equation 2.
| (2) |
To calculate 95% CIs for WTP estimates, a nonparametric bootstrapping approach was used39 with 500 iterations for each WTP estimate. The results of the analyses were considered statistically significant if P<0.05, with two-tailed tests.
The analyses were conducted by splitting the sample into four subgroups to identify and investigate the different preferences between patients, rheumatologists, nurses, and pharmacists.
Results
Characteristics of participants
Overall, 720 individuals evenly distributed within different areas of Italy (71.3% were patients, 15.3% rheumatologists, 7.1% nurses, and 6.4% pharmacists) participated in the study. Data were collected from the rheumatology centers in 17 of 20 regions of Italy between July 2014 and December 2015. Compared with the Italian general population aged ≥18 years (men, 47.9%; mean age, 50.7 years),40 each subgroup had fewer men (42.5% of patients, 40.0% of rheumatologists, 13.7% of nurses, and 23.9% of pharmacists), whereas the mean age was equal for patients (50.0±13.6 years) and lower for other participants (43.7±10.5, 46.0±7.8, and 39.8±8.6 years for rheumatologists, nurses, and pharmacists, respectively). Table 2 shows the education level, working status, and clinical characteristics of patients. As planned, patients were balanced by diagnosis of RA, PsA, and AS and their experience with biologic treatment. Patients reported an average of 10.8 years since the appearance of first symptoms and 8 years since the first diagnosis of their disease. Overall, ten biologic drugs were used, more frequently for effectiveness not reached with previous treatments. Before the biologic treatment currently taken or prescribed, the patients had been treated with ≥1 drug treatment among methotrexate, DMARDs, nonsteroidal anti-inflammatory drugs, corticosteroids, or a different biologic drug.
Table 2.
Patients characteristics
| Variables | Values |
|---|---|
| Education, no (%) | |
| None (<5 years of primary school) | 7 (1.4) |
| Primary school (5 years) | 58 (11.3) |
| Lower secondary school (3 years) | 137 (26.7) |
| Upper secondary school (4–5 years) | 210 (40.9) |
| Graduate (4–6 years) | 84 (16.4) |
| Post-graduate (≥3 years’ specialization/doctorate) | 14 (2.7) |
| Working status, no (%)a | |
| Paid work | 272 (54.4) |
| Retired | 100 (19.8) |
| Housewife | 72 (14.3) |
| Unemployed | 47 (9.3) |
| Student | 10 (2.0) |
| Unpaid work (eg, volunteer) | 3 (0.6) |
| Diagnosis, no (%) | |
| Rheumatoid arthritis | 174 (33.9) |
| Psoriatic arthritis | 179 (34.9) |
| Ankylosing spondylitis | 160 (31.2) |
| Years from the appearance of first symptoms | |
| Mean (SD) | 10.8 (9.4) |
| Median (min–max) | 8.0 (0.2–50.0) |
| Years from diagnosis | |
| Mean (SD) | 8.0 (8.2) |
| Median (minb–max) | 5.0 (0–43.0) |
| Experience with biologic treatment, no (%) | |
| With experience and currently treated | 262 (51.1) |
| With experience, but currently not treated | 8 (1.5) |
| Naïve (ie, never treated before and with a new prescription) | 243 (47.4) |
| Biologic drugs used or prescribed, no (%) | |
| Abatacept | 38 (7.4) |
| Adalimumab | 105 (20.5) |
| Certolizumab | 31 (6.1) |
| Etanercept | 88 (17.2) |
| Golimumab | 90 (17.6) |
| Infliximab | 99 (19.3) |
| Rituximab | 12 (2.3) |
| Tocilizumab | 42 (8.2) |
| Ustekinumab | 6 (1.2) |
| Other (not specified) | 1 (0.2) |
| Reason for starting treatment with biologic drugs, no (%)c | |
| No effectiveness of previous treatment | 478 (93.2) |
| No tolerance to previous treatment | 60 (11.7) |
| Side effects from previous treatment | 26 (5.1) |
| Other reasons | 9 (1.8) |
| Treatment before biologic drugs, no (%)c | |
| Methotrexate | 316 (61.6) |
| ≥1 DMARD (not MTX) | 203 (39.6) |
| NSAIDs | 268 (52.2) |
| Corticosteroids | 231 (45.0) |
| Biologic drug | 102 (19.9) |
Notes:
The main work activity was considered, each patient was included in only one category.
Two patients were diagnosed <1 month before enrollment.
More than one reason could be reported.
Abbreviations: DMARD, disease-modifying antirheumatic drug; MTX, methotrexate; NSAIDs, nonsteroidal anti-inflammatory drugs.
Analysis of preferences
From our data quality check, responses of preferences for five participants (patients) were excluded from the analysis, as they were not considered reliable. Therefore, results from 715 participants (508 patients) are included.
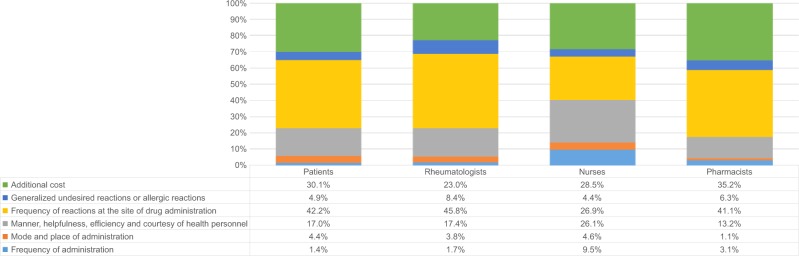
Generally, for RI assigned to each characteristic (Figure 2), the most important characteristics were “frequency of reactions at the site of drug administration” (26.9%–45.8% of importance relative to other characteristics); “additional cost” as health care taxes (23.0%–35.2%); and “manner, helpfulness, efficiency, and courtesy of health personnel” (13.2%–26.1%). “Frequency of administration” was the least important for patients and rheumatologists (<2%); “mode and place of administration” was least important for pharmacists (1.1%); and “mode and place of administration” and “generalized undesired reactions or allergic reactions involving the whole body” were the least important for nurses (<5% for each). “Frequency of administration” was considered relatively more important by nurses (9.5%) than other participants.
Figure 2.
Relative importance of each characteristic to patients, rheumatologists, nurses, and pharmacists.
Notes: Each column shows the RI estimated from the preferences elicited by each subgroup. Within each column, the height of the differently colored areas shows the amount of RI of each characteristic compared with the others.
Abbreviation: RI, relative importance.
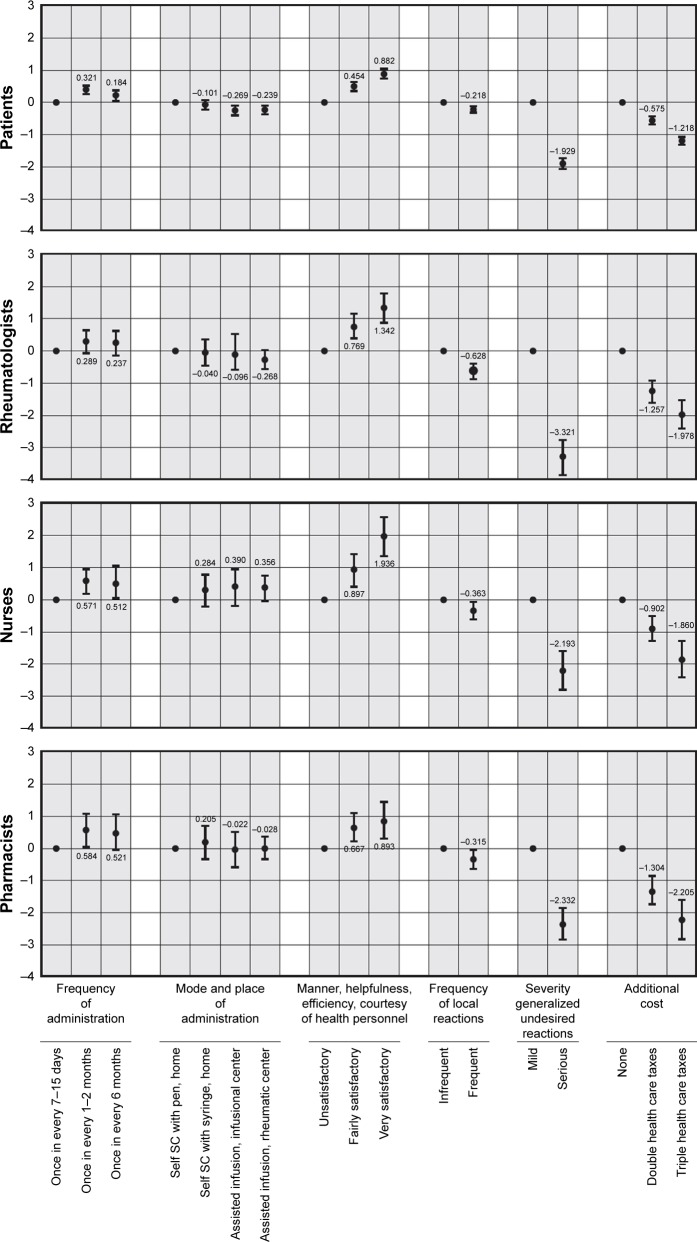
All the following results on direction and strength (using the statistical significance if CIs do not overlap with estimates of the other levels in the same characteristics) of preferences and on WTP within each characteristic must be considered ceteris paribus. All subgroups preferred a lower frequency of administration (Figure 3); however, a frequency of once every 1–2 months was preferred to once every 6 months (statistically significant for patients, pharmacists, and nurses), which was significantly preferred to once every 7–15 days by patients and nurses. Preferences for the mode and place of administration were different between subgroups, but statistically significant only for patients, who preferred subcutaneous self-injection with pen at home to any assisted mode of administration. Every subgroup was considered significantly important: a fairly or very satisfactory level of support from health personnel who assist patients with their treatment; mild, generalized, undesired, or allergic reactions to the whole body; and not paying twice or three times the health care taxes to make the described treatment available to patients.
Figure 3.
Preferences for each characteristic level by group.
Notes: The position of each dot indicates the amount of estimated preference of each characteristic level moving from the reference level of that characteristic: the greater the difference, the higher (for positive estimates) or the lower (for negative estimates) the importance of that level is, compared with the reference level within that characteristic. The vertical segments represent the 95% CIs of each preference estimate. If the CIs do not overlap between the different levels of a particular characteristic, the mean estimates are significantly different from each other at the 5% level of significance. Otherwise, when the CIs overlap between each other or with the dot, the preferences for the involved levels are not statistically significant.
Abbreviation: SC, subcutaneous.
The estimated mean WTP was similar among the four subgroups; however, it was significant only for patients and some of the pharmacist responses (Table 3). In particular, the highest significant mean WTP was estimated for mild vs severe generalized undesired or allergic reactions among patients, corresponding to €391 per month. The second highest mean WTP was estimated for having a very satisfactory level of support from the health personnel, which reached up to €258 per month among nurses, but was statistically significant only among patients and pharmacists with about €130–170 per month. However, a significant WTP among patients and pharmacists was estimated also for a fairly satisfactory level of support, corresponding to about €83 and €95 per month, respectively, and reaching almost €123 per month among nurses, although not significant from a statistical perspective. The third highest mean WTP was estimated for a treatment administered every 1–2 months instead of every 7–15 days, reaching up to €79 per month among nurses, but being significant only for patients and pharmacists with €65 and €75 per month, respectively. Then, patients would pay on average an additional €45–50 per month to have a treatment subcutaneously self-administered with a pen at home (since this was included as a reference, negative WTP estimates were produced in the other levels), instead of a treatment requiring assisted infusion at the infusion center or the rheumatology center. A mean WTP, albeit not statistically significant, of about €42–68 per month was estimated among nurses. A mean WTP corresponding to up to €82 per month was estimated among the four groups for infrequent local reactions, but it was significant only for patients with almost €45 per month.
Table 3.
Willingness to pay
| Characteristics | Patients
|
Rheumatologists
|
Nurses
|
Pharmacists
|
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| Frequency of administration | ||||
| Once every 7–15 days (reference) | – | – | – | – |
| Once every 1–2 months | 65.68 (36.11, 95.25) | 45.96 (−70.12, 162.04) | 79.30 (−238.70, 397.30) | 75.31 (3.36, 147.26) |
| Once every 6 months | 35.31 (−3.77, 74.39) | 30.43 (−39.38, 100.24) | 69.72 (−216.44, 355.88) | 66.52 (−19.13, 152.17) |
| Mode and place of administration | ||||
| Self SC with pen, at home (reference) | – | – | – | – |
| Self SC with syringe, at home | −17.96 (−45.87, 9.96) | 8.90 (−95.64, 113.45) | 46.76 (−79.82, 173.33) | 28.03 (−63.60, 119.66) |
| Assisted infusion, at infusion center | −51.40 (−86.13, −16.67) | −14.98 (−139.60, 109.65) | 68.70 (−283.42, 420.82) | −16.31 (−122.38, 89.75) |
| Assisted infusion, at rheumatology center | −45.88 (−78.38, −13.39) | −35.52 (−106.91, 35.86) | 42.11 (−222.90, 307.12) | 0.42 (−74.51, 75.35) |
| Manner, helpfulness, and so on of personnel | ||||
| Unsatisfactory (reference) | – | – | – | – |
| Fairly satisfactory | 83.16 (52.98, 113.33) | 96.45 (−85.94, 278.84) | 122.78 (−358.10, 603.67) | 94.71 (9.26, 180.16) |
| Very satisfactory | 169.59 (119.18, 220.01) | 175.93 (−114.83, 466.70) | 257.96 (−629.26, 1,145.19) | 132.62 (21.03, 244.21) |
| Frequency of local reactions | ||||
| Infrequent (reference) | – | – | – | – |
| Frequent | −44.63 (−61.66, −27.61) | −81.66 (−221.13, 57.80) | −50.91 (−217.53, 115.71) | −45.53 (−111.09, 20.02) |
| Severity of generalized reactions | ||||
| Mild (reference) | – | – | – | – |
| Serious | −390.87 (−494.76, −286.99) | 410.76 (−990.21, 168.68) | −268.21 (−1,290.75, 754.34) | 297.83 (−447.89, 147.77) |
Note: The WTP estimates statistically significant at P, 0.05, that is, those with CIs not including the 0 value and not overlapping with each other within the same characteristic, are reported in bold.
Abbreviations: SC, subcutaneous; WTP, willingness to pay.
Discussion
Although the literature reports studies in which patients’ and physicians’ opinions were compared, to the best of our knowledge, this is the first study showing how four different groups of individuals involved in different ways in treating patients with RDs or managing biologics can disagree on the RI of some characteristics of treatment.
In particular, when looking at the RI of the six characteristics included in the DCE, pharmacists, patients, and physicians considered “frequency of reactions at the site of drug administration” and “additional cost” to be the most important characteristics. For nurses, “manner, helpfulness, and efficiency of health personnel” was equally as important as the two characteristics above. In contrast, this characteristic was less important for the other subgroups, since according to their responses, we ranked it third in importance. This disagreement between nurses and the other participants can be attributed to a better knowledge and awareness of this process characteristic, compared with physicians and pharmacists, because of nurses’ experience in assisting patients at the centers during treatment administration and routine visits. A higher agreement may be expected between patients and nurses, since they are in close relationship; instead, we found that patients were more concerned than nurses about the severity of possible generalized undesired reactions.
When looking at the strength of preferences, as expected, every subgroup assigned a statistically significant higher preference to “fewer local undesired reactions”, “mild generalized undesired reactions”, and “no additional cost to pay”. Furthermore, every subgroup assigned a significantly higher importance to a satisfactory level of helpfulness from health personnel. However, only patients assigned a statistically significant lower preference for triple compared with double additional costs, and a higher desirability for a very satisfactory compared with a fairly satisfactory service. For the frequency of administration, every subgroup considered having one administration every 1–2 months preferable, although this higher preference was only statistically significant in patients and physicians. For the mode and place of administration, only patients assigned a significantly higher preference to having an assisted infusion compared with a subcutaneous self-injection. WTP was statistically significant for patients in all the characteristics (although not all the levels) and for pharmacists in support of health personnel and generalized undesired or allergic reactions, although several WTP amounts estimated for these subjects were similar to those of the other groups of respondents. This apparent inconsistency between amount and statistical significance of WTP estimates in the different groups of participants can be attributed to the fact that estimates from the patients are more precise, as shown with the smaller CIs, since they are derived from many more observations (>8,000 choices from 16 choice sets answered by 513 patients) than those from the other subgroups (1,700 from the rheumatologists, about 800 and 700 from the nurses and pharmacists, respectively). Hence, we could consider the nonsignificant results with relatively high WTP estimates also potentially relevant. The highest WTP was estimated for mild generalized undesired or allergic reactions, followed by a very satisfactory support by the health personnel. Interestingly, among the patients only (as shown by not overlapping CIs of these estimates), we also found a significantly higher WTP for very satisfactory vs fairly satisfactory support by the health personnel, showing the high importance assigned by patients to this characteristic, which was specifically requested to be included in the choice tasks incidentally during the preliminary study.
In other countries, several studies have been conducted in recent years on preferences of patients with RDs,15–19 while others have also involved the perspective of rheumatologists toward different aspects of drug treatments, with or without biologics.24,26,41–46 These studies used different methods than ours, so results cannot be directly compared. For instance, in the recent study by Nolla et al,26 only patients and physicians were involved and only four characteristics were used to describe possible treatment options, of which only administration method and risk of adverse events were included in our study. In addition, a full-profile ranking exercise was used, that is, treatment options were ordered from most to least important, whereas our exercise consisted of a series of pairwise choices between two options each. Although we cannot directly compare results, we can make some general comparisons. First, both studies indicate that patients and rheumatologists have similar preferences: in our study, both the RI of each characteristic and the strength of preferences assigned to the single characteristic levels were more similar between patients and rheumatologists than pharmacists or nurses. Second, in the Spanish study, both patients and rheumatologists significantly preferred subcutaneous self-injection at home to intravenous administration by a health care professional at the hospital; our study showed a similar result among patients, but physicians as well as pharmacists were not concerned about this characteristic. Nurses were shown to be in favor of assisted infusion, although the strength of these preferences was not statistically significant.
Other studies available in the literature underline the importance of preference for route and place of administration as potentially influencing the acceptance of and adherence to treatment.42,44–46 In a study conducted in Italy some years ago,45 it was reported that intravenous administration was preferred for safety, rapidity, and reassurance, whereas the subcutaneous route was preferred for convenience. The authors of a study conducted in France42 found that almost half of the patients among those using intravenous administrations preferred to keep this route. The authors interpreted these results as a patient’s need for reassuring medical assistance and hospital administration, in apparent contrast to available evidence demonstrating a similar safety profile of the two modes of administration, suggesting that patients are probably given insufficient information about this.
Results from a systematic review conducted by the Italian experts show how the benefit of treatment with biologic drugs in patients with RA, PsA, and AS can significantly depend on patient satisfaction and compliance with treatment, and knowledge on reason of discontinuation together with appropriate awareness of patients’ characteristics may contribute to achieve good results.20 In their systematic review focusing on patient-reported outcomes in subjects with AS and PsA, Torre-Alonso et al underlined that the clinical benefits of biologic treatments demonstrated in randomized clinical trials may actually be reduced by poor compliance and early discontinuation in clinical practice, with the consequent need for more aggressive treatments and increase of costs.22
Therefore, considering patients’ preferences and involving them in the treatment decision making through a satisfactory communication process with clinicians can have positive implications on patients’ satisfaction, compliance, and benefits from treatment, and can reduce unnecessary costs. With the CARA study, we show that other subjects involved in the process can have their own preferences that differ from those of patients and clinicians, such as nurses who assist patients during treatment administration and even pharmacists who interact with clinicians for the management of the hospital budgets allocated for drugs delivery, showing how their interactions can contribute further in the final decisions.
The importance attributed to the cost of treatment has been investigated in a DCE study in which 559 rheumatologists from 12 European countries, including Italy, participated to assess the value assigned to some characteristics of treatment used in patients with RDs.43 Participants showed willingness to trade between treatment efficacy, safety, and patient preference, as they would not choose a treatment not preferred by patients, and costs. In our study, all participants valued the cost characteristic included in the scenarios, proving they can take this aspect into account when evaluating different treatment options. It is important to note that preferences for treatment cost could also depend on the type of health care system, especially on the entity, that is, national health service, health insurance, or patient pays for the treatment, with consequent different impacts on individual perceptions and opinions. To present the scenarios realistic for our context, we used as a cost characteristic the possible increase of health care taxes for any citizen, that is, potentially including all the participants in the study, to ensure the availability of the described treatment to all the target patients, which could not be applicable in other contexts. However, although the CARA study may not strictly apply in all other health care systems, it shows how different and not necessarily as expected can be the preferences of the different parties involved in the prescription, use, and payment for biologic treatments and can guide the optimization of benefits and a more efficient use of resources attributable to them.
Conclusion
While all respondents agreed on the preferences assigned to some characteristics, they did not agree on others. The results of the present study are useful to understand which treatment characteristics could influence the behavior of different persons, according to their role and perspective, and related consequences on treatment satisfaction, compliance and final benefits for patients, together with the overall costs for the whole health care system.
For many years, decisions on health technologies have been mainly physician centered; instead, observations derived from patients and the different health care professionals may be helpful in modifying the perspectives of the decision-making process. Scientific studies estimating and comparing preferences between different parties constitute an important opportunity to provide useful information for guiding interactions and decisions at different levels: physician–budget holder, nurse–physician, nurse–patient, and patient–physician. Taking into account the results of the CARA study could guide the conduct of good choices aimed at optimizing benefits and efficiently allocating resources.
Supplementary materials
Details of the discrete choice experiment and study design
Overview of the discrete choice experiment technique
The discrete choice experiment (DCE) is a technique for eliciting preferences and a useful tool widely employed for estimating values of non-market goods and services. As the DCE elicitation process consists of a trade-off between characteristics during the decision-making process, it has the potential to meet the economic criteria for measuring benefit. In the past 20 years, DCEs have been increasingly used to elicit preferences for health care interventions and to allow inclusion of more than just health outcomes.1 A number of publications are available on DCEs applied in rheumatology.2–5
To conduct such a study, a series of steps included in two main phases were carried out. Importantly, to obtain an efficient set of choice sets, both the number and type of characteristics and levels had to be selected carefully, since the dimension and, hence, the complexity of the experimental design exponentially increase with the number of these elements, with possible negative effects on the capability of the tasks to capture all the information of interest.
The first phase consisted of designing the DCE and is described below; the second phase consisted of recruiting the participants, collecting and analyzing the data, and interpreting the results.
Design of the DCE
The design of the present DCE consisted of three steps: 1) identifying and selecting the characteristics and assigning levels to each characteristic; 2) constructing the scenarios to be evaluated; and 3) developing the survey instrument.
1. Identification and selection of characteristics and levels
We conducted a review of the literature to identify the attributes of treatments potentially relevant for the target population. Then, within a focus group with expert rheumatologists (n=3) and patients with all three target conditions (n=9), we identified the final set of attributes and related levels.
2. Construction of the scenarios to be evaluated
A fractional factorial experimental design was used to define the scenarios of treatments to be evaluated. The factorial approach consists of combining every attribute level to create possible treatment options. However, a full factorial design would generate too many scenarios to be valued, implying a too high cognitive burden to the participants. In particular, in the present study, we obtained the combination 33*41*22, which would produce 432 possible treatment options, corresponding to 186,192 possible scenarios of two options each. Hence, a fraction of all the possible scenarios had to be selected in a way that also guaranteed statistical efficiency of the design to obtain reliable estimates of preferences. In this study, with an orthogonal statistically efficient design,6 we selected 16 treatment descriptions suitable for main effects estimations. The 16 profiles were then paired to others by applying a fold-over approach to ensure a minimum overlap between levels within ≥1 characteristics, so that the respondents would make choices by evaluating every characteristic. Sixteen pairwise choice sets were finally produced.
3. Development of the survey instrument
Together with rheumatologists, we developed the questionnaire including the choice sets, information, and instructions on how to interpret the choice set and how to complete the task, and the other questions considered relevant to be collected during the study (details in the main paper). Subsequently, we tested the questionnaire within a pilot study involving nine patients, two pharmacists, and three nurses. These participants autonomously completed the questionnaire and then underwent a telephone interview to complete a cognitive debriefing. We assessed the feasibility, acceptability, and validity of the questionnaire. The results of the pilot study were discussed with the physicians, small changes were made, and the questionnaire was finalized for the data collection process.
Sample size calculation
The sample size for each subgroup of participants was decided according to a formal calculation and some practical considerations. Basically, the sample size was determined by the desired level of accuracy of the estimated probabilities of choosing one of two treatment options. In particular, we applied the following formula:7,8
where:
n is the minimum sample size of respondents;
p is the true choice proportion of the relevant population for an alternative. We used the results of the pilot study previously conducted, corresponding to a mean P=0.54 (0.33–0.69);
q is defined as 1–p, hence q=0.46;
[Φ−1 (1-α/2)] is the inverse cumulative distribution function of a standard normal distribution (ie,Ñ(0,1)) taken at (1-α/2);
a is the level of allowable deviation as a percentage between the estimated p and the true choice proportion p. To apply it in this study, we considered two levels of a using our results from the pilot study. Since we had data from the pilot study for nine patients, two nurses, and three pharmacists, we considered the estimate of choice probability for health professionals weaker than that for patients. Accordingly, for patients, we used a=5% and for health professionals, we used a=6%; and
S is the number of choice tasks per respondent, which we decided to be 16.
Accordingly, the formula gave the following n (minimum sample sizes) in each subgroup:
Patients with each diagnosis in each treatment situation (experienced vs naïve)=82. We planned to receive data from 90 patients accounting for possible missing data.
Nurses=48. We planned to receive data from 50 nurses accounting for possible missing data.
Pharmacists=48. We planned to receive data from 50 pharmacists accounting for possible missing data.
Physicians=48. However, we planned to receive data from 100 physicians, for some practical considerations. Although all physicians are specialists in rheumatology in the key centers identified for this study, each physician generally focuses on one category (disease condition) of patients. Consequently, we decided to involve ≥3 clinicians from each center to recruit all the target patients. Therefore, they were also involved in the completion of the questionnaire, since this allowed us to obtain, for a relatively small additional effort, preferences data from a sample of physicians that overall was balanced in terms of specific experience. We did not apply this to nurses or pharmacists, who were more homogeneous in terms of experience. As they did not care for one specific category of patients, it was not necessary to increase the sample size of these professionals.
List of ethical committees
Ethical Committee of Policlinico Universitario, Campus Bio-Medico, Roma
Ethical Committee of Ospedale Santa Chiara, Azienda Ospedaliero Universitaria Pisana, Pisa
Ethical Committee of Ospedale La Colletta, Arenzano, Genova
Ethical Committee of Ospedali Riuniti di Foggia, Foggia
Ethical Committee of Policlinico Università degli Studi di Udine
Ethical Committee of Ospedale Niguarda Ca’ Granda, Milano
Ethical Committee of Complesso Integrato Columbus, Università Cattolica del Sacro Cuore, Roma
Ethical Committee of Policlinico Vittorio Emanuele, Catania
Ethical Committee of Ospedale Policlinico di Borgo Roma, Verona
Ethical Committee of Policlinico S. Maria alle Scotte, Azienda Ospedaliero Universitaria Senese, Siena
Ethical Committee of Ospedale Civile dello Spirito Santo, Pescara
Ethical Committee of spedale S. Maria della Misericordia, Perugia
Ethical Committee of Ospedale S. Salvatore, Azienda Sanitaria Locale L’Aquila
Ethical Committee of Ospedale “C. Urbani”, Azienda Sanitaria Unica Regionale Marche, Area vasta 2, Iesi, Ancona
Ethical Committee of Policlinico Universitario di Germaneto, Catanzaro
Ethical Committee of Università di Bari
Ethical Committee of Azienda Ospedaliera Papa Giovanni XXIII, Bergamo
Ethical Committee of Azienda Ospedaliero Universitaria Careggi, Firenze
Ethical Committee of Ospedale S. Pietro Fatebenefratelli, Roma
Ethical Committee of Azienda Ospedaliera “S. Camillo Forlanini”, Roma
Ethical Committee of Fondazione Istituto di Ricerca e Cura a Carattere Scientifico Policlinico San Matteo, Pavia
Ethical Committee of Ospedale Galateo, San Cesario (Lecce)
Ethical Committee of Ospedale San Carlo di Potenza and Ospedale Madonna delle Grazie di Matera
Ethical Committee of Azienda Ospedaliero Universitaria di Sassari
Ethical Committee of Ospedale Cardarelli, Napoli
Ethical Committee of Azienda Ospedaliero Universitaria Federico II, Napoli
Ethical Committee of Azienda Ospedaliero Universitaria Policlinico Palermo
Ethical Committee of Azienda Ospedaliero Universitaria della Seconda Università degli Studi di Napoli
Ethical Committee of Ospedale Universitario L. Sacco, Milano
Ethical Committee of Ospedale G. Pini, Milano
References
- 1.Ryan M, Gerard K, Amaya-Amaya M. Using Discrete Choice Experiments to Value Health and Health Care. Dordrecht: Springer; 2008. [Google Scholar]
- 2.Hifinger M, Hiligsmann M, Ramiro S, et al. Patients’ preferences and economic considerations play an important role in treatment decisions: a discrete choice experiment among rheumatologists. Rheumatology (Oxford) 2017;56(1):68–76. doi: 10.1093/rheumatology/kew328. [DOI] [PubMed] [Google Scholar]
- 3.Nolla JM, Rodríguez M, Martin-Mola E, et al. Patients’ and rheumatologists’ preferences for the attributes of biological agents used in the treatment of rheumatic diseases in Spain. Patient Prefer Adherence. 2016;10:1101–1113. doi: 10.2147/PPA.S106311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen H, Schumacher HR, Li X, et al. Comparison of expectations of physicians and patients with rheumatoid arthritis for rheumatology clinic visits: a pilot, multicenter, international study. Int J Rheum Dis. 2012;15(4):380–389. doi: 10.1111/j.1756-185X.2012.01752.x. [DOI] [PubMed] [Google Scholar]
- 5.Willeke P, Becker H, Wassenberg S, Pavenstädt H, Jacobi AM. Patient/rheumatologist evaluation of infusion treatment for rheumatoid arthritis. Z Rheumatol. 2011;70(3):232–234. 236–238. doi: 10.1007/s00393-011-0752-3. [DOI] [PubMed] [Google Scholar]
- 6.Hahn GJ, Shapiro SS. Report 66-C-165. Schenectady, NY: General Electric Research and Development Centre; 1966. A Catalog and Computer Program for the Design and Analysis of Orthogonal Symmetric and Asymmetric Fractional Factorial Experiments. [Google Scholar]
- 7.Hensher DA, Rose JM, Greene WH. A Primer. Cambridge, UK: Cambridge University Press; 2005. Applied Choice Analysis. [Google Scholar]
- 8.Louviere JJ, Hensher DA, Swait JD. Stated Choice Methods Analysis and Application. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
Acknowledgments
The authors thank all the patients, pharmacists, nurses, and rheumatologists who participated in the study and completed the questionnaire with their preferences. A special thanks to Dr Paolo Cozzolino for his technical support during the analyses of data. The study was supported by MSD Italia S.r.l. CARA Study Group members: Afeltra Antonella, U.O.C. Medicina Clinica e Reumatologia, Policlinico Universitario, Campus Bio-Medico, Roma; Bazzichi Laura, U.O. Reumatologia, Ospedale Santa Chiara, AOU Pisana, Pisa; Bianchi Gerolamo, U.O di Reumatologia, Ospedale La Colletta, Arenzano (Genova); Bucci Romano, Struttura Semplice Dipartimentale Reumatologia Ospedaliera, Ospedali Riuniti di Foggia, Foggia; De Vita Salvatore, Clinica di Reumatologia, Policlinico Università degli Studi di Udine; Epis Oscar, Divisione di Reumatologia, Ospedale Niguarda Ca’ Granda, Milano; Ferraccioli Gianfranco, U.O.C. di Reumatologia, Complesso Integrato Columbus, Università Cattolica del Sacro Cuore, Roma; Foti Rosario, U.O. di Reumatologia, A.O.U. Policlinico Vittorio Emanuele, Catania; Fracassi Elena, Unità Operativa di Reumatologia, Policlinico G. B. Rossi, Ospedale Policlinico di Borgo Roma, Verona; Frediani Bruno, U.O.S. Reumatologia, Policlinico S. Maria alle Scotte, AOU Senese, Siena; Gabini Marco, U.O.C. di Reumatologia, Ospedale Civile dello Spirito Santo, Pescara; Gerli Roberto, Dip. Medicina Clinica Sperimentale Cattedra di Reumatologia; U.O. Diagnosi e Cura delle Malattie Reumatiche, Ospedale S. Maria della Misericordia, Perugia; Giacomelli Roberto, Dipartimento di Medicina, UOC di Immunoreumatologia, Ospedale S. Salvatore, ASL L’Aquila; Grassi Walter, Clinica Reumatologica, Ospedale “C. Urbani”, ASUR Marche, Area vasta 2, Iesi (Ancona); Grembiale Rosa Daniela, Rheumatology Research Unit, Dipartimento di Scienze Mediche e Chirurgiche, Policlinico Universitario di Germaneto, Catanzaro; Lapadula Giovanni, Unità di Reumatologia, Università di Bari; Limonta Massimiliano, U.O. Reumatologia, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo; Matucci Cerinic Marco, Dipartimento di Medicina Sperimentale e Clinica, S.O.D. Reumatologia, A.O.U. Careggi, Firenze; Migliore Alberto, Divisione di Reumatologia, Ospedale S. Pietro Fatebenefratelli, Roma; Minisola Giovanni, UOC di Reumatologia, Azienda Ospedaliera “S. Camillo Forlanini”, Roma; Montecucco Carlomaurizio, Facoltà di Medicina e Chirurgia, Università di Pavia, Fondazione IRCCS Policlinico San Matteo, Pavia; Muratore Maurizio, U.O. Reumatologia, Ospedale Galateo (ASL Lecce), San Cesario (Lecce); Olivieri Ignazio, Dipartimento di Reumatologia della Lucania, Ospedale San Carlo di Potenza, e Ospedale Madonna delle Grazie di Matera; Passiu Giuseppe, UOC di Reumatologia, A.O.U. di Sassari; Russo Romualdo, UOS Reumatologia, UC Medicina Interna III Ospedale Cardarelli, Napoli; Sarzi-Puttini Piercarlo, Unità di Reumatologia, Ospedale Universitario L. Sacco, Milano; Scarpa Raffaele, Area Funzionale di Reumatologia e Riabilitazione Reumatologica, Dipartimento Assistenziale Integrato di Medicina Clinica e Sperimentale, AOU Federico II, Napoli; Sinigaglia Luigi, Unità di Reumatologia, Ospedale G. Pini, Milano; Triolo Giovanni, U.O.C. di Reumatologia, Azienda Ospedaliero Universitaria Policlinico Palermo; Valentini Gabriele, U.O. di Reumatologia, Dipartimento di Internistica Clinica e Sperimentale, A.O.U. della Seconda Università degli Studi di Napoli.
We remember with fondness Prof Ignazio Olivieri for his professionalism and compassion.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
PAC received speaking fees from Pfizer; AMG and MM are employees of MSD Italia, Rome, Italy; GL received consultancies, speaking fees, and honoraria from MSD, Janssen, Sanofi, Mundifarma, and BMS; CM received consultancies, speaking fees, and honoraria from MSD; LSi received consultancies, speaking fees, and honoraria from Abbvie, UCB, Eli Lilly, and Roche. The authors report no other conflicts of interest in this work.
References
- 1.Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol Suppl. 2006;78:4–11. [PubMed] [Google Scholar]
- 2.Geuskens GA, Burdorf A, Hazes JM. Consequences of rheumatoid arthritis for performance of social roles – a literature review. J Rheumatol. 2007;34(6):1248–1260. [PubMed] [Google Scholar]
- 3.Gladman DD, Stafford-Brady F, Chang CH, Lewandowski K, Russell ML. Longitudinal study of clinical and radiological progression in psoriatic arthritis. J Rheumatol. 1990;17(6):809–812. [PubMed] [Google Scholar]
- 4.Hunsche E, Chancellor JV, Bruce N. The burden of arthritis and non-steroidal anti-inflammatory treatment. A European literature review. Pharmacoeconomics. 2001;19(1):1–15. doi: 10.2165/00019053-200119001-00001. [DOI] [PubMed] [Google Scholar]
- 5.Cortesi PA, Scalone L, D’Angiolella L, et al. Systematic literature review on economic implications and pharmacoeconomic issues of psoriatic arthritis. Clin Exp Rheumatol. 2012;30(4 Suppl 73):S126–S131. [PubMed] [Google Scholar]
- 6.Furneri G, Mantovani LG, Belisari A, et al. Systematic literature review on economic implications and pharmacoeconomic issues of rheumatoid arthritis. Clin Exp Rheumatol. 2012;30(4 Suppl 73):S72–S84. [PubMed] [Google Scholar]
- 7.Olivieri I, Cortesi PA, de Portu S, et al. Long-term costs and outcomes in psoriatic arthritis patients not responding to conventional therapy treated with tumour necrosis factor inhibitors: the extension of the Psoriatic Arthritis Cost Evaluation (PACE) study. Clin Exp Rheumatol. 2016;34(1):68–75. [PubMed] [Google Scholar]
- 8.Palla I, Trieste L, Tani C, et al. A systematic literature review of the economic impact of ankylosing spondylitis. Clin Exp Rheumatol. 2012;30(4 Suppl 73):S136–S141. [PubMed] [Google Scholar]
- 9.Hjardem E, Hetland ML, Ostergaard M, Krogh NS, Kvien TK, Danish Database for Biological Therapies in Rheumatology Study G Prescription practice of biological drugs in rheumatoid arthritis during the first 3 years of post-marketing use in Denmark and Norway: criteria are becoming less stringent. Ann Rheum Dis. 2005;64(8):1220–1223. doi: 10.1136/ard.2004.031252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kavanaugh A, Cohen S, Cush JJ. The evolving use of tumor necrosis factor inhibitors in rheumatoid arthritis. J Rheumatol. 2004;31(10):1881–1884. [PubMed] [Google Scholar]
- 11.Kobelt G, Andlin-Sobocki P, Brophy S, Jönsson L, Calin A, Braun J. The burden of ankylosing spondylitis and the cost-effectiveness of treatment with infliximab (Remicade) Rheumatology. 2004;43(9):1158–1166. doi: 10.1093/rheumatology/keh271. [DOI] [PubMed] [Google Scholar]
- 12.Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 13.Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 14.Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510. doi: 10.1136/annrheumdis-2015-208337. [DOI] [PubMed] [Google Scholar]
- 15.Barton JL. Patient preferences and satisfaction in the treatment of rheumatoid arthritis with biologic therapy. Patient Prefer Adherence. 2009;3:335–344. doi: 10.2147/ppa.s5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Augustovski F, Beratarrechea A, Irazola V, et al. Patient preferences for biologic agents in rheumatoid arthritis: a discrete-choice experiment. Value Health. 2013;16(2):385–393. doi: 10.1016/j.jval.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Kromer C, Schaarschmidt ML, Schmieder A, Herr R, Goerdt S, Peitsch WK. Patient preferences for treatment of psoriasis with biologicals: a discrete choice experiment. PLoS One. 2015;10(6):e0129120. doi: 10.1371/journal.pone.0129120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazlewood GS, Bombardier C, Tomlinson G, et al. Treatment preferences of patients with early rheumatoid arthritis: a discrete-choice experiment. Rheumatology. 2016;55(11):1959–1968. doi: 10.1093/rheumatology/kew280. [DOI] [PubMed] [Google Scholar]
- 19.Rothery C, Bojke L, Richardson G, et al. A discrete choice experiment to explore patients’ willingness to risk disease relapse from treatment withdrawal in psoriatic arthritis. Clin Rheumatol. 2016;35(12):2967–2974. doi: 10.1007/s10067-016-3452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantini F, Niccoli L, Nannini C, et al. Second-line biologic therapy optimization in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Semin Arthritis Rheum. 2017;47(2):183–192. doi: 10.1016/j.semarthrit.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Dures E, Hewlett S, Lord J, et al. Important treatment outcomes for patients with psoriatic arthritis: a multisite qualitative study. Patient. 2017;10(4):455–462. doi: 10.1007/s40271-017-0221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torre-Alonso JC, Queiro R, Comellas M, Lizán L, Blanch C. Patient-reported outcomes in European spondyloarthritis patients: a systematic review of the literature. Patient Prefer Adherence. 2018;12:733–747. doi: 10.2147/PPA.S162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Angelo S, Tramontano G, Gilio M, Leccese P, Olivieri I. Review of the treatment of psoriatic arthritis with biological agents: choice of drug for initial therapy and switch therapy for non-responders. Open Access Rheumatol. 2017;9:21–28. doi: 10.2147/OARRR.S56073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hifinger M, Hiligsmann M, Ramiro S, et al. Influence of disease activity on RA treatment choices in countries with restricted access to expensive, innovative drugs: a discrete choice experiment among rheumatologists. RMD Open. 2017;3(2):e000453. doi: 10.1136/rmdopen-2017-000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faggioli G, Scalone L, Mantovani LG, Borghetti F, Stella A, PREFER study group Preferences of patients, their family caregivers and vascular surgeons in the choice of abdominal aortic aneurysms treatment options: the PREFER study. Eur J Vasc Endovasc Surg. 2011;42(1):26–34. doi: 10.1016/j.ejvs.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Nolla JM, Rodríguez M, Martin-Mola E, et al. Patients’ and rheumatologists’ preferences for the attributes of biological agents used in the treatment of rheumatic diseases in Spain. Patient Prefer Adherence. 2016;10:1101–1113. doi: 10.2147/PPA.S106311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scalone L, Mantovani LG, Borghetti F, von Mackensen S, Gringeri A. Patients’, physicians’, and pharmacists’ preferences towards coagulation factor concentrates to treat haemophilia with inhibitors: results from the COHIBA Study. Haemophilia. 2009;15(2):473–486. doi: 10.1111/j.1365-2516.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- 28.Wen H, Ralph Schumacher H, Li X, et al. Comparison of expectations of physicians and patients with rheumatoid arthritis for rheumatology clinic visits: a pilot, multicenter, international study. Int J Rheum Dis. 2012;15(4):380–389. doi: 10.1111/j.1756-185X.2012.01752.x. [DOI] [PubMed] [Google Scholar]
- 29.Willeke P, Becker H, Wassenberg S, Pavenstädt H, Jacobi AM. Patient/rheumatologist evaluation of infusion treatment for rheumatoid arthritis. Z Rheumatol. 2011;70234(3):232236–232238. doi: 10.1007/s00393-011-0752-3. [DOI] [PubMed] [Google Scholar]
- 30.Hakim Z, Pathak DS. Modelling the EuroQol data: a comparison of discrete choice conjoint and conditional preference modelling. Health Econ. 1999;8(2):103–116. doi: 10.1002/(sici)1099-1050(199903)8:2<103::aid-hec393>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 31.Ryan M. A role for conjoint analysis in technology assessment in health care? Int J Technol Assess Health Care. 1999;15(3):443–457. [PubMed] [Google Scholar]
- 32.Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13. doi: 10.1016/j.jval.2012.08.2223. [DOI] [PubMed] [Google Scholar]
- 33.Moia M, Mantovani LG, Carpenedo M, et al. Patient preferences and willingness to pay for different options of anticoagulant therapy. Intern Emerg Med. 2013;8(3):237–243. doi: 10.1007/s11739-012-0844-3. [DOI] [PubMed] [Google Scholar]
- 34.Scalone L, Carminati M, Bonhoeffer P, et al. Patients’ and physicians’ needs, experiences and preferences in the treatment of right ventricular outflow tract dysfunction. Ital J Public Health. 2012;9(2):73–83. [Google Scholar]
- 35.Scalone L, Ciampichini R, Fagiuoli S, et al. Comparing the performance of the standard EQ-5D 3L with the new version EQ-5D 5L in patients with chronic hepatic diseases. Qual Life Res. 2013;22(7):1707–1716. doi: 10.1007/s11136-012-0318-0. [DOI] [PubMed] [Google Scholar]
- 36.Louviere JJ, Hensher DA, Swait JD. Stated Choice Methods Analysis and Application. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 37.World Medical Association WMA Declaration of Helsinki – ethical principles for medical research involving human subjects. 2013. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ [DOI] [PubMed]
- 38.Manski CF. The structure of random utility models. Theory Decis. 1977;8(3):229–254. [Google Scholar]
- 39.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton: CRC Press; 1994. [Google Scholar]
- 40.DEMO ISTAT. [Accessed October 5, 2017]. Available from: http://demo.istat.it/pop2014/index.html.
- 41.Bolge SC, Goren A, Brown D, Ginsberg S, Allen I. Openness to and preference for attributes of biologic therapy prior to initiation among patients with rheumatoid arthritis: patient and rheumatologist perspectives and implications for decision making. Patient Prefer Adherence. 2016;10:1079–1090. doi: 10.2147/PPA.S107790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desplats M, Pascart T, Jelin G, et al. Are abatacept and tocilizumab intravenous users willing to switch for the subcutaneous route of administration? A questionnaire-based study. Clin Rheumatol. 2017;36(6):1395–1400. doi: 10.1007/s10067-017-3587-8. [DOI] [PubMed] [Google Scholar]
- 43.Hifinger M, Hiligsmann M, Ramiro S, et al. Patients’ preferences and economic considerations play an important role in treatment decisions: a discrete choice experiment among rheumatologists. Rheumatology. 2017;56(1):68–76. doi: 10.1093/rheumatology/kew328. [DOI] [PubMed] [Google Scholar]
- 44.Huynh TK, Østergaard A, Egsmose C, Madsen OR. Preferences of patients and health professionals for route and frequency of administration of biologic agents in the treatment of rheumatoid arthritis. Patient Prefer Adherence. 2014;8:93–99. doi: 10.2147/PPA.S55156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scarpato S, Antivalle M, Favalli EG, et al. Patient preferences in the choice of anti-TNF therapies in rheumatoid arthritis. Results from a questionnaire survey (RIVIERA study) Rheumatology. 2010;49(2):289–294. doi: 10.1093/rheumatology/kep354. [DOI] [PubMed] [Google Scholar]
- 46.Sylwestrzak G, Liu J, Stephenson JJ, Ruggieri AP, Devries A. Considering patient preferences when selecting anti-tumor necrosis factor therapeutic options. Am Health Drug Benefits. 2014;7(2):71–81. [PMC free article] [PubMed] [Google Scholar]