Abstract
Pain, especially chronic pain, can lead to cognitive deficits. Mismatch negativity (MMN) is a change-specific component of the auditory event-related brain potential (ERP) that is thought to provide a unique window into sensory memory processes. The present study was designed to determine how chronic and acute pain affects auditory sensory memory. In experiment 1, MMNs elicited by standard and deviant auditory stimuli at short and long inter-stimulus intervals (ISIs) were compared between trigeminal neuralgia (TN) patients and demographically matched healthy controls (HCs). The TN patients were found to have stronger attenuation of the MMN at longer ISIs than HCs. Correlation analysis revealed a significant positive correlation between the sensory subscale of McGill Pain Questionnaire and MMN amplitude reduction across ISI conditions. In experiment 2, MMNs recorded before, during, and after the cold pressor test were compared in healthy subjects. MMN amplitude was significantly reduced during pain exposure and recovered immediately thereafter. These results suggest that both chronic pain and acute pain can interfere with automatic change detection processes in the brain. This study provides the first evidence that chronic pain patients have a faster auditory memory trace decay than HCs.
Introduction
Pain is a subjective experience that involves interactions among sensory, affective, and cognitive factors1,2. When pain is chronic, it can produce sequela beyond the sensation of pain itself3–5. Indeed, substantial evidence indicates that chronic pain is associated with a general decline in cognitive functions, including attention, memory, and executive function6,7.
The division of resources theory holds that pain-related cognitive impairment may be a consequence of limited resources being consumed by pain processing and management3–5. According to this theory, competition between pain and cognition for processing resources may impair cognitive abilities in chronic pain patients. In a functional magnetic resonance imaging (fMRI) study of attention-related cerebral responses, Martinsen et al. found that, relative to healthy controls (HCs), chronic pain patients had reduced activation in brain regions implicated in cognitive processing8. In another fMRI study, Aizawa et al. found that patients with irritable bowel syndrome had cognitive flexibility impairments that were associated with altered activity in the dorsolateral prefrontal cortex and hippocampus9.
Electroencephalography (EEG) studies of pain effects on event-related potentials (ERPs) have also provided evidence of pain-associated cognitive impairment. The mismatch negativity (MMN) has been demonstrated to be affected by ongoing pain10–13. Experimentally, the MMN is elicited reliably by a detectable auditory change or regularity violation in a sequence of frequent standardized stimuli14. It is thought to arise from an automatic comparison between ongoing sensory input and the memory trace of the previous stimulus, and thus to reflect some aspect of sensory memory processing15. Two major generator sources of MMN in the brain have been proposed, one bilaterally generated by the auditory cortices (temporal component) and the other by the frontal cortex (frontal component)14,16. The temporal and frontal component has been suggested to reflect pre-perceptual change detection and involuntary attention switch to auditory change respectively17,18.
Dick et al. provided the first evidence that the MMN was affected by pain when they demonstrated that MMN amplitude was increased in pain patients following analgesic treatment12. Conversely, the same group did not find evidence of the MMN being affected by experimentally induced ischemic pain11. In a recent study, Yao et al. reported unchanged MMN amplitudes but delayed MMN latency in low back pain and neck pain patients13. Most recently, Choi et al. showed that the amplitude of MMN’s magnetoencephalographic equivalent was smaller in patients with fibromyalgia than in healthy subjects10.
To date, the effect of chronic pain on sensory memory, as reflected by the MNN response to ISI lengthening, has not been examined and the effect of experimentally induced pain on MMN has not been reexamined since Dick et al.’s 2006 study. The aim of the present study was to examine how both chronic and acute pain affect auditory sensory memory. MMNs elicited by auditory stimuli at short and long ISIs were compared between chronic pain patients and HCs. We hypothesized that MMN attenuation in long ISI trials would be stronger in chronic pain patients than in HCs. In addition, MMNs recorded before, during, and after acute pain in the cold pressor test (CPT) were compared in healthy subjects. We predicted that the MMN amplitude would be reduced by acute cold pain and would recover after the pain subsided.
Methods
Participants and design
A total of 71 individuals participated in this study, which consisted of experiments 1 and 2. Experiment 1 was designed to investigate the effects of chronic pain on the maintenance of the sensory memory trace, indexed by the MMN. It had a between-subject design with a group of 26 chronic pain patients suffering from trigeminal neuralgia (TN) and a group of 25 age and gender matched HCs. Experiment 2 was designed to examine the modulation of MMN by experimentally induced acute pain. It employed a within-subject design with a group of 20 healthy, undergraduate and graduate students who underwent the cold pressor test (CPT).
The TN patients were recruited from the Department of Neurosurgery, Chinese PLA General Hospital of Jinan Military Command, which receives TN patients from all over the country for microvascular decompression surgery. TN diagnosis was confirmed by a neurosurgeon (author F. Y.) based on inquiry, physical examination, and magnetic resonance imaging. Inclusion criteria for the TN patients were: (a) idiopathic TN, as defined by the International Headache Society19; (b) persistent chronic pain for at least 6 months; and (c) normal hearing and normal or corrected to normal vision. Patients were excluded if they had: (a) other types of acute or chronic pain; (b) a history of any other medical, neurological, or psychiatric disorder; or (c) current alcohol or substance abuse problems. All TN patients suffered from unilateral (13 left and 13 right) facial pain. The mean duration of pain history was 5.48 ± 5.59 years. The experiments were performed on the day before neurosurgical interventions (i.e., microvascular decompression). During the experiment, pain was controlled with carbamazepine. Based on the present pain intensity scale of the McGill Pain Questionnaire (MPQ), all patients rated their current pain less than 3 (mean 1.59 ± 1.37; range, 0–5).
The HCs in Experiment 1 were recruited through local advertisement posted in the local community. The HC group were demographically matched to the TN group. The exclusion criteria applied to the TN group were also applied to the HC group. Two patients who refused to undergo EEG were excluded from the study. Another 4 TN patients and 3 HC subjects were excluded because they could not understand the instructions. Additionally, 3 TN patients and 4 HCs were excluded from the analyses due to excessive EEG artifacts. The demographic information of the Experiment 1 participants is summarized in Table 1.
Table 1.
Demographic information and neuropsychological measurement scores in experiment 1.
| Variable | TN (n = 17) | HC (n = 18) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 48.94 | 13.63 | 46.06 | 5.43 |
| Education (levels)a | 2.29 | 1.21 | 2.11 | 0.83 |
| Fear of Pain Questionnaire (FPQ) (30–150) | 63.71 | 19.78 | 64.17 | 22.18 |
| Pain Anxiety Symptoms Scale (PASS-20) | ||||
| Total (0–100) | 41.53** | 16.99 | 19.61 | 20.19 |
| Cognitive anxiety (0–25) | 13.29** | 5.27 | 5.39 | 5.39 |
| Physiological anxiety (0–25) | 5.53 | 5.55 | 4.83 | 6.08 |
| Fear (0–25) | 5.76 | 4.97 | 3.11 | 4.17 |
| Avoidance (0–25) | 16.94** | 6.29 | 6.28 | 6.83 |
| Depression Anxiety and Stress Scale (DASS-21) | ||||
| Total (0–63) | 14.12 | 10.73 | 8.83 | 7.29 |
| Depression (0–21) | 4.59 | 3.59 | 2.06 | 2.26 |
| Anxiety (0–21) | 2.88 | 3.28 | 2.72 | 2.59 |
| Stress (0–21) | 6.65 | 5.79 | 4.06 | 3.28 |
| McGill Pain Questionnaire (SF-MPQ) | ||||
| Total (0–45) | 16.76 | 6.18 | ||
| Sensory index (0–33) | 12.75 | 5.32 | ||
| Affective index (0–12) | 4.01 | 2.11 | ||
| n | % | n | % | |
| Gender | ||||
| Male | 11 | 64.71 | 13 | 72.22 |
| Female | 6 | 35.29 | 5 | 27.78 |
aThe level of education is measured by the grading method, 1 represents the lowest degree (primary school), 7 represents the highest degree (doctor degree).
**P < 0.01 for TN vs. HC.
TN, Trigeminal neuralgia patients; HC, healthy controls.
The participants in Experiment 2 (10 males; mean age 23.50 ± 3.83 years) were recruited through advertisements posted on local college campuses. The exclusion criteria were: (a) a history of cardiovascular disease, (b) a history of fainting or seizures, (c) a history of Reynaud’s syndrome, or (d) a recent injury or open cut affecting the hand20. Two participants could not endure the cold pressor pain and quit the study, and another 2 were excluded due to excessive EEG artifacts. Thus, the final sample size in Experiment 2 was 16 (9 males; mean age, 23.44 ± 3.33 years).
All study participants gave their written informed consent after they had received a detailed explanation of the experimental protocol. They were informed that they could terminate the experiment at any time. The study was performed in accordance with relevant guidelines and regulations. Ethical approval was granted by the Institutional Review Board of the Institute of Psychology, Chinese Academy of Sciences.
Experiment 1
Questionnaires
Questionnaires were used to collect information on demographics, medical history, and emotional status. All participants were asked to fill out Chinese versions of the Fear of Pain Questionnaire (FPQ), the 20-item Pain Anxiety Symptoms Scale (PASS-20), and the 21-item Depression Anxiety and Stress Scale (DASS-21). TN patients also completed the short form (SF)-MPQ, to provide a quantitative evaluation of the sensory and affective aspects of their pain. If the participant could not read, the experimenter read the scales to them, and the participant reported their answers verbally.
The FPQ is a 30-item survey that has been widely used as a self-reported measure of pain-related fear in (chronic) pain syndromes as well as in non-clinical samples. For each painful situation described on the FPQ, respondents rate how fearful they are on a scale from 1 (not at all) to 5 (extreme)21. This scale has a good reported internal consistency, with Cronbach’s α of 0.93. The Chinese FPQ (edition III) used in the experiment was translated and validated by a research group at Southwest University, Chongqing, China22.
The PASS-20 consists of four 5-item subscales (cognitive, escape/avoidance, fear, and physical anxiety) and is intended to measure pain-related anxiety and fear responses23. All items are rated on a frequency scale from 0 (never) to 5 (always). The PASS-20, a short form version of the original 40-item PASS, has been demonstrated to have good internal consistency (mean α = 0.81) and to correlate strongly (mean r = 0.95) with the original scales23. The four subscales and full scale of the Chinese PASS-20, used in this study, have been shown to have good internal consistency with a Cronbach’s α of 0.72–0.9224.
The DASS-21 is a self-reported questionnaire that is widely used to assess depression, anxiety, and stress dimensions in clinical and nonclinical groups25. Each of its 21 items are rated on a 4-point (0–3) scale of frequency or severity of respondent experiences over the last week. Respondents rated how much each item applied to them (0 = does not apply to me at all, 3 = applies to me very much). This scale has high reliability, with Henry reporting a Cronbach’s α of 0.9326. The Chinese version of DASS-21 has internal consistency indices (Cronbach’sα values) of 0.83, 0.80, and 0.82 for its Depression, Anxiety, and Stress subscales, respectively, and 0.92 for the total scale24.
Pain intensity was assessed with the SF-MPQ, which consists of 15 pain descriptors (11 sensory and 4 affective) rated on a 4-point scale (0 = none, 1 = mild, 2 = moderate, 3 = severe)27. The SF-MPQ provides information on the sensory, affective, and overall intensity of pain with a sensory index, affective index, and pain rating index, respectively. It includes the present pain intensity index of the standard MPQ and a visual analogue scale. The sensory and affective dimensions of the SF-MPQ were shown to have good internal consistency (Cronbach’sα valuesof 0.78 and 0.76, respectively), and were translated into a Chinese version and standardized28.
Stimuli and procedure
Auditory stimuli consisted of standard tone (1000 Hz) and occasional deviant tone (2000 Hz). Both were pure sine wave tones with an intensity of 80 dB SPL (sound pressure level) and a duration of 50 ms with 5-ms rise and fall times. Stimuli were presented in two blocks, each having a constant ISI of 500 ms (short) or 2500 ms (long). Each block contained 500 auditory stimuli and the order of blocks (short-ISI and long-ISI) was counterbalanced among participants. The standard and deviant tone occurrence probabilities were fixed at 90% and 10% for both ISI conditions, resulting in 450 standard and 50 deviant tones in each block. Within each block, stimuli were presented pseudo-randomly with the constraints of at least three standard tones being displayed at the beginning of the block and that deviant tones were separated by at least two standard tones29.
Participants were seated in a comfortable armchair in a dim, electrically shielded room. The stimuli were generated by a PC running E-prime software (Psychology Software Tools, Inc., Sharpsburg, PA) and were delivered binaurally through earphones. During EEG recordings, participants watched a silent documentary movie (Winged Migration) while listening passively to auditory stimuli via earphones. Subjects were instructed to pay attention to the video clips and to ignore the tones. They were told that they would be quizzed on content of the movie. During EEG recordings, participants were asked to refrain from frequent blinking and head movements. Between blocks, they were allowed to take a short break. On average, the task lasted for a total of about 30 min.
EEG recording and data preprocessing
EEG data were recorded with a 64-channel Biosemi Active Two system (BioSemi, Amsterdam, The Netherlands) with electrodes positioned according to the extended 10–20 system. Signals were referenced online to the common mode sense-driven right leg ground and digitized at 512 Hz with 0.01-Hz high-pass and 100-Hz low-pass filtering. The impedance of each electrode was <5 kΩ. Vertical eye movements were monitored with bipolar recordings above and below the left eye. Horizontal eye movements were monitored with electrodes placed at the outer canthus of each eye. Two additional EEG signals were recorded from the bilateral mastoids, M1 and M2, for computing temporal MMN, and another electrode was placed at the tip of the nose for off-line referencing.
MATLAB (version 8.3., The MathWorks Inc., Natick, MA) and EEGLAB toolbox30 were used for the offline analysis of EEG data. All data were first re-referenced to nose tip and then re-referenced to M1 and M2. They were all band-pass filtered in the range of 1–25 Hz. Trial epochs starting 100 ms before and ending 400 ms after the onset of auditory stimulus were extracted and then baseline corrected. Artifacts related to eye movements and cardiac responses were removed by independent component analysis31. Trials with EEG amplitudes larger than 100 μV were excluded from further analysis. At least 70% artifact-free standard and deviant trials were preserved for each ISI condition (short and long). The remaining trial epochs were averaged separately to compute standard and deviant ERPs under each experimental condition.
To obtain the MMN, a difference wave was computed by subtracting the individual grand average responses to standard stimuli from those to deviant stimuli for each ISI condition32. Based on visual inspection of the difference waveforms, as well as previous studies12,33,34, the MMN component was measured as the mean amplitude of the difference wave within the 150–200 ms post-stimulus time window. The MMN was recognized as a characteristic negative deflection over the frontal and central scalp sites and as positive wave over the posterior temporal regions35,36, and was thus analyzed within frontocentral (F3, Fz, F4, C3, Cz, C4) and temporal (M1, M2) regions of interest (ROIs). For mastoid re-referenced data, the MMN was analyzed only within the frontocentral region. To assess the responses elicited by standard and deviant tones per se, peak amplitude and peak latency of the N1 (50–175 ms) and P2 (120–280 ms) components were measured33.
Statistical analysis
Demographics and pain-related neuropsychological assessment results were compared between patient and control groups with Student’s t-tests and chi-squared analyses where appropriate. The N1 and P2 components elicited by auditory stimuli at Cz were analyzed with three-way group (TN vs. HC) × stimulus type (standard vs. deviant) × ISI (short vs. long) repeated measures (rm) analyses of variance (ANOVA) of peak amplitude and peak latency. For the nose-referenced MMN, three-way group × ISI × hemisphere rmANOVAs were conducted separately at frontocentral sites and temporal sites. To better elucidate the ISI effects, separate two-way group × ISI ANOVAs, split by hemisphere, were performed. Post-hoc pairwise comparisons with Bonferroni corrections were carried out as follow-up analyses of ANOVA revealed significant interactions. The Greenhouse-Geisser correction was applied to control for non-sphericity where appropriate. Corrected probability values are reported. The same analyses were conducted on mastoid-referenced MMN components recorded at frontocentral sites. To examine whether the electrophysiological activities were associated with pain perception, Pearson correlation analyses were performed between MMN amplitude (at each ROI for each ISI condition) and MPQ scores (including subscale scores). All statistical analyses were conducted in SPSS version 19.0 (SPSS Inc., Chicago, IL). The significance level was set at P < 0.05.
Experiment 2
Stimuli and procedure
The CPT was conducted in a custom-made plastic tank (25 × 25 × 16 cm3) holding ice and water. A thermostat-controlled electric pump was used to propel water flow, while maintaining temperature 4 ± 1 °C. Ice was packed into a mesh pocket submersed in the water to prevent direct contact between the ice and the participant’s skin. A similar tank of warm water (32–34 °C) was used for hand immersion before the CPT task, to reduce inter-subject differences in hand temperature.
The physical parameters of the auditory stimuli were the same as in Experiment 1. Standard and deviant auditory stimuli were presented pseudo-randomly across three blocks (i.e., CPT baseline, immersion, recovery; details below) with a 400 ms ISI. Each block contained 500 auditory stimuli (400 standard and 100 deviant), and lasted approximately 4 min.
The CPT commenced with each participant placing his or her nondominant hand in the warm water for 4 min (baseline phase), during which auditory stimuli were delivered binaurally through earphones and EEG signals were recorded. Then, the participant transferred the immersed hand from the warm water immediately to the cold water, where it remained immersed for 4 min (maximum tolerance time) or until the participant felt too uncomfortable to continue (immersion phase). Subsequently, the participant’s hand was exposed to the room air (26 °C) for 4 min (recovery phase).
During the immersion and recovery phases, participants reported their perceived pain intensity and unpleasantness on 0–9 scales at 15 s, 30 s, 60 s, 120 s, 180 s, 240 s, 300 s, 360 s, 420 s, and 480 s after the beginning of the immersion. Auditory stimuli were delivered and EEG signals were recorded throughout the experiment.
EEG recording and data analysis
EEG recording and data processing were performed as in Experiment 1 except that the ERPs were analyzed with nose-referenced data only. The self-reported pain intensity and unpleasantness ratings were analyzed with one-way rmANOVAs followed by Dunnett’s tests.
Peak amplitudes and peak latencies of auditory stimulus elicited N1 and P2 components recorded at Cz were analyzed with two-way condition (baseline, immersion, recovery) × stimulus type (standard vs. deviant) rmANOVAs. The MMN datasets at frontocentral (F3, Fz, F4, C3, Cz, C4) and at temporal (M1, M2) sites were each subjected to two-way condition × hemisphere rmANOVAs. Condition effects were detected with one-way ANOVAs followed by Dunnett’s tests. Analyses of the correlations between MMN amplitude and self-reported ratings were also conducted. All statistical analyses were conducted in SPSS version 19.0 (SPSS Inc., Chicago, IL). The significance level was set at P < 0.05.
Results
Chronic clinical pain
Demographics and neuropsychological measures
The groups’ demographic characteristics and neuropsychological findings are presented in Table 1. The TN patient and HC groups were well matched for age (t = 0.83, P = 0.411), gender (χ2 = 0.23, P = 0.725), and education level (t = 0.52, P = 0.609). The TN patients exhibited a higher level of pain-related anxiety than HCs, manifested as more anxiety thoughts (PASS cognitive subscale, 13.29 ± 5.27 vs. 5.39 ± 5.39, t = 4.39, P < 0.001) and increased avoidance behaviors (PASS avoidance subscale, 16.94 ± 6.29 vs. 6.28 ± 6.83, t = 4.81, P < 0.001) when experiencing suffering. No other differences were observed between the two groups.
ERPs elicited by standard and deviant tones
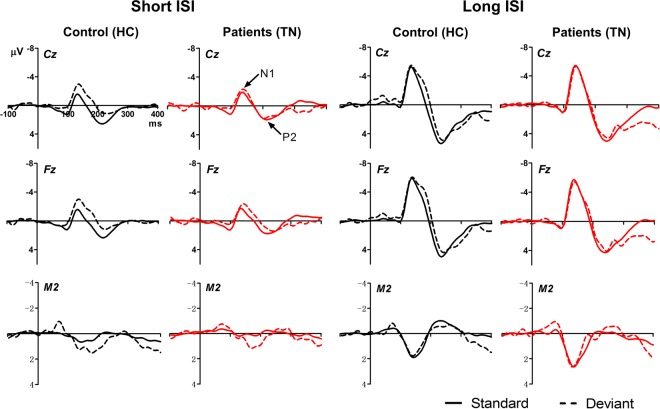
Grand average ERPs elicited by auditory stimuli with respect to stimulus type (standard vs. deviant), experimental group (TN vs. HC), ISI condition (short vs. long), and electrode site (Cz, Fz, and right mastoid) are shown in Fig. 1. The peak amplitudes and latencies of the N1 and P2 components at Cz are presented in Table 2. We observed main effects of ISI on N1 (F1,33 = 33.16, P < 0.001, ηp2 = 0.50) and P2 (F1,33 = 60.69, P < 0.001, ηp2 = 0.65), with larger amplitudes being recorded in the long-ISI condition than in the short-ISI condition. There was also a main effect of stimulus type on N1 amplitude (F1,33 = 21.27, P < 0.001, ηp2 = 0.39), with N1 being larger with deviant stimuli than with standard stimuli.
Figure 1.
ERPs produced in response to standard and deviant tones in Experiment 1. Grand average waveforms for TN patients (red) and HCs (black) are shown for Cz, Fz, and M2 electrodes under short- (left panel) and long-ISI (right panel) conditions.
Table 2.
Peak amplitude and latency of N1 and P2 to standard and deviant tones under different ISI conditions at Cz electrode in experiment 1.
| Amplitude (μV) | Latency (ms) | |||
|---|---|---|---|---|
| TN | HC | TN | HC | |
| Standard N1 | ||||
| 500-ms ISI | −2.05 (1.72) | −1.81 (1.56) | 136.90 (6.17) | 125.26 (28.42) |
| 2500-ms ISI | −5.77 (3.19) | −5.63 (2.11) | 139.66 (9.66) | 132.42 (19.20) |
| Standard P2 | ||||
| 500-ms ISI | 2.34 (1.48) | 2.96 (1.41) | 210.43 (31.59) | 212.07 (30.76) |
| 2500-ms ISI | 5.60 (2.23) | 6.08 (1.69) | 241.45 (17.20) | 238.98 (21.25) |
| Deviant N1 | ||||
| 500-ms ISI | −3.00 (2.43) | −3.70 (1.71) | 134.38 (24.89) | 129.38 (21.64) |
| 2500-ms ISI | −6.15 (3.71) | −6.62 (2.19) | 137.13 (25.42) | 148.48 (19.00) |
| Deviant P2 | ||||
| 500-ms ISI | 3.36 (2.04) | 2.77 (2.31) | 229.27 (31.91) | 242.45 (26.80) |
| 2500-ms ISI | 5.91 (3.18) | 5.83 (3.34) | 243.75 (20.16) | 242.23 (18.91) |
TN, Trigeminal neuralgia patients; HC, healthy controls.
Additionally, we found main effects of ISI on N1 (F1,33 = 6.98, P = 0.013, ηp2 = 0.18) and P2 (F1,33 = 19.97, P < 0.001, ηp2 = 0.38) latencies, where the peak latencies in the long-ISI condition were delayed compared to those in the short-ISI condition. There was also a main effect of stimulus type on P2 latency (F1,33 = 10.45, P = 0.003, ηp2 = 0.24) and a stimulus type × ISI interaction (F1,33 6.98, P = 0.012, ηp2 = 0.18), with the peak P2 latency in response to standard stimuli being faster than that in response to deviant ones in the short-ISI condition, and no stimulus difference in the long-ISI condition (Table 2). There were no other significant group effects or interactions.
MMN
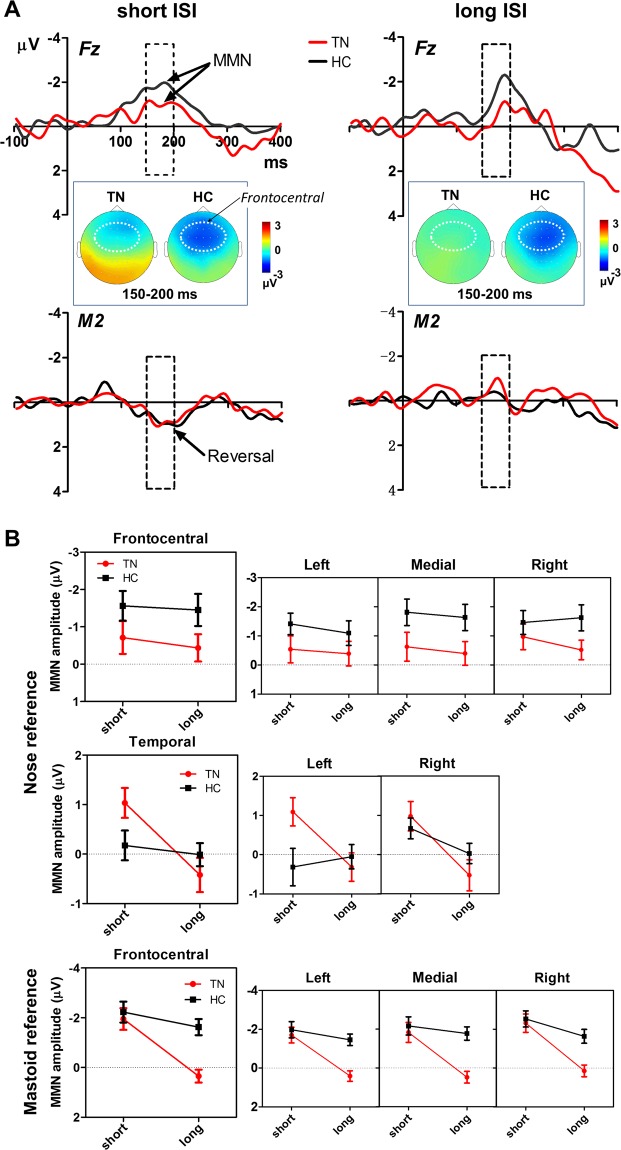
The difference waveforms (deviant minus standard) that constitute the MMN in short- and long-ISI conditions are presented in Fig. 2. The MMN was evident for both groups. In the short-ISI condition, a typical negative deflection at Fz and a polarity reversal (positive deflection) at the mastoid were seen for both groups. In the long-ISI condition, there was a marked negativity at Fz for both groups, but not a strong polarity reversal at the mastoid.
Figure 2.
Comparison of MMN amplitudes between TN patients and HCs in different ISI conditions. (A) MMN grand average waveforms at frontal and temporal regions shown with scalp topographical maps. The dashed boxes superimposed on waveforms mark the MMN time window (150–200 ms). Scalp topographies of MMN amplitude are shown in this time window, with a white dotted oval indicating the frontocentral ROI. Note the typical negative deflection at Fz and a polarity reversal (positive deflection) at the mastoid. (B) Nose-referenced and mastoid re-referenced MMN data at frontocentral and temporal regions. Reduction of MMN amplitude with ISI prolongation was more pronounced in the TN group than in the HC group. Error bars indicate standard errors.
Frontocentral MMN
There was a main effect of group (nose reference) on mean MMN amplitude (Fig. 2B) at frontocentral sites, with TN patients having smaller amplitudes than the control group (F1,33 = 5.43, P = 0.026, ηp2 = 0.14), as well as a main effect of hemisphere, with higher MMN amplitudes being recorded from the right hemisphere (F4, C4) than from the left hemisphere (F3, C3) (F2,66 = 3.56, P = 0.034, ηp2 = 0.10). No ISI effects on frontocentral MMNs were found. Group × ISI two-way ANOVAs for each hemisphere revealed main effects of group on the MMN at medial sites (Fz, Cz) (F1,33 = 7.13, P = 0.012, ηp2 = 0.18) and at right hemisphere sites (F1,33 = 4.21, P = 0.048, ηp2 = 0.11), but not at left hemisphere sites.
Similarly, three-way ANOVAs of the mastoid-referenced data (Fig. 2B) revealed main effects of group (F1,33 = 8.59, P = 0.006, ηp2 = 0.21) and hemisphere (F2,66 = 7.27, P = 0.005, ηp2 = 0.18) on MMN amplitude. Unlike the nose-referenced data, there was a significant effect of ISI on MMN amplitude (F1,33 = 16.94, P < 0.001, ηp2 = 0.34) and a significant group × ISI interaction (F1,33 = 5.78, P = 0.022, ηp2 = 0.15). Subsequent post-hoc analysis indicated that the TN patients had a smaller MMN than the HC group in the long-ISI (P < 0.001), but not the short-ISI (P = 0.643), condition. Within-group contrasts showed a marked reduction in MMN amplitude for TN patients from the short to the long ISI (P < 0.001), whereas the MMN amplitude remained relatively stable across ISIs in the HC group (P = 0.258).
Temporal MMN
A three-way group × ISI × hemisphere ANOVA at temporal sites (left and right mastoids) revealed a main effect of ISI (F1,33 = 8.80, P = 0.006, ηp2 = 0.21), indicating that temporal MMN amplitude decreased with ISI prolongation. This decrease was more pronounced in the TN patient group than in the HC group, yielding an ISI × group interaction (F1,33 = 5.27, P = 0.028, ηp2 = 0.14) (Fig. 2B). A Bonferroni post-hoc analysis confirmed that the TN patients had a significant reduction in MMN amplitude from the short to the long ISI (P = 0.001), whereas MMN amplitude did not differ significantly between the two ISI conditions in the HC group (P = 0.634). In addition, the post-hoc comparisons showed a marginally significant difference between TN vs. HC for the short ISI (P = 0.053). No other significant differences were found.
Correlations
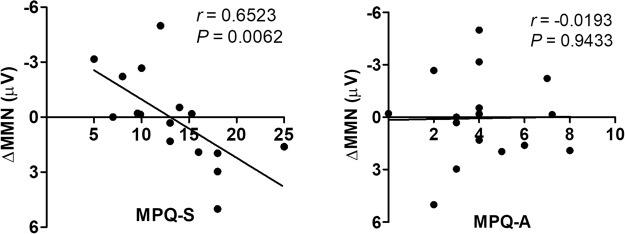
There was a significant correlation between the sensory, but not the affective, MPQ subscale and MMN amplitude reduction across ISI conditions (long minus short) (r = 0.6523, P = 0.0062) (Fig. 3), indicating that a more intense pain experience results in a more rapid MMN amplitude attenuation with increasing ISI.
Figure 3.
Correlation between MPQ subscale scores and MMN amplitude reduction across ISI conditions in TN patients. The sensory (left panel), but not the affective (right panel), subscale of the MPQ correlated with MMN amplitude reduction.
Acute experimental pain
CPT
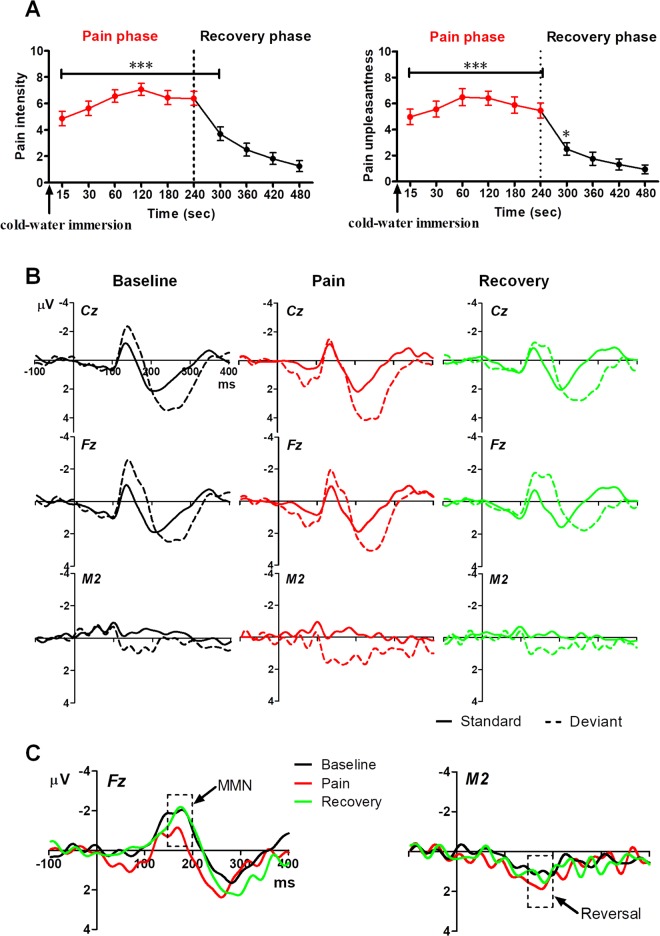
Changes in perceived pain intensity and unpleasantness ratings over time during (15 s, 30 s, 60 s, 120 s, 180 s, 240 s) and after ice-water immersion (300 s, 360 s, 420 s, 480 s) are illustrated in Fig. 4A. One-way rmANOVAs revealed revealed significant main effects of time on pain intensity (F9,135 = 36.11, P < 0.001, ηp2 = 0.71) and pain unpleasantness (F9,135 = 35.93, P < 0.001, ηp2 = 0.71). Post-hoc analysis indicated that the perceived pain intensity and unpleasantness ratings at time points in the range of 15–300 s were significantly higher than those at 480 s (i.e., the end of recovery phase) (all Ps < 0.05).
Figure 4.
ERPs produced in response to standard and deviant tones and MMN grand average waveforms in Experiment 2. (A) Ratings of pain intensity and unpleasantness in cold-induced pain (red) and recovery (black) phases. *P < 0.05, ***P < 0.001, compared to ratings at 480 s. (B) Grand average waveforms generated in response to standard and deviant tones in the baseline (black), cold pain (red), and recovery (green) periods. (C) MMN grand average waveforms in different experimental phases. The dashed boxes superimposed on the waveforms mark the time window of the MMN (150–200 ms).
ERPs elicited by standard and deviant tones
ERPs generated in response to standard and deviant auditory stimuli were compared during the baseline, immersion, and recovery phases. Grand average waveforms recorded at Cz, Fz, and M2 are shown in Fig. 4B. N1 and P2 peak amplitudes and latencies at Cz are presented in Table 3. We observed a main effect of stimulus type on both N1 amplitude (F1,15 = 15.27, P = 0.001, ηp2 = 0.51) and P2 amplitude (F1,15 = 15.48, P = 0.001, ηp2 = 0.51), with both being larger in deviant trials than in standard trials. There was a main effect of phase (baseline, pain, recovery) (F2,30 = 6.70, P = 0.007, ηp2 = 0.31) on N1 peak latency and a main effect of stimulus type on P2 peak latency (F1,15 = 21.93, P < 0.001, ηp2 = 0.59). No other effects were found.
Table 3.
Peak amplitude and latency of N1 and P2 to standard and deviant tones in cold pressor test at Cz electrode in experiment 2.
| Amplitude (μV) | Latency (ms) | |||||
|---|---|---|---|---|---|---|
| Baseline | Pain | Recovery | Baseline | Pain | Recovery | |
| Standard | ||||||
| N1 | −1.33 (1.32) | −1.42 (1.42) | −1.07 (1.09) | 128.52 (18.87) | 125.10 (24.16) | 135.84 (10.27) |
| P2 | 2.60 (1.03) | 2.53 (1.40) | 2.37 (1.01) | 213.97 (23.48) | 213.23 (22.38) | 204.20 (12.42) |
| Deviant | ||||||
| N1 | −2.94 (2.05) | −1.88 (1.87) | −2.23 (1.86) | 130.47 (24.37) | 133.15 (14.39) | 151.47 (18.43) |
| P2 | 4.41 (3.18) | 5.03 (3.08) | 3.91 (2.26) | 231.30 (46.77) | 234.47 (20.71) | 236.67 (25.40) |
MMN
MMN waveforms corresponding to recordings made before (baseline), during (immersion), and after (recovery) the CPT at Fz and M2 are shown in Fig. 4C electrodes. Note that the MMN had a negative deflection at Fz and a positive deflection at M2.
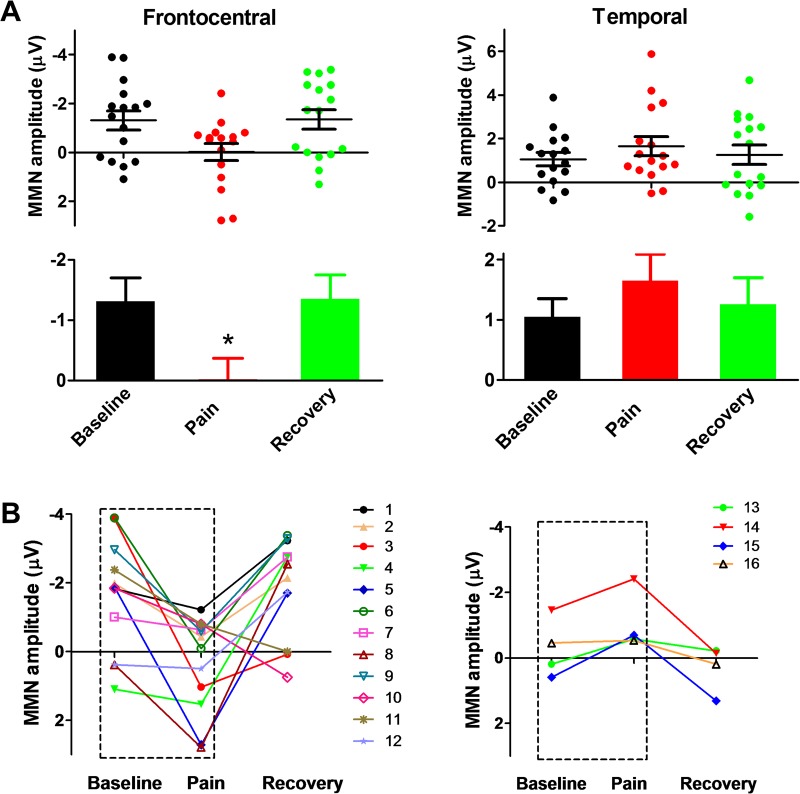
Because a phase (baseline, immersion, recovery) × hemisphere two-way ANOVA did not detect a significant interaction, data were collapsed across the hemispheres. One-way ANOVAs across the three phases at frontocentral areas and at temporal areas (Fig. 5A) revealed a main effect of phase for the frontocentral MMN (F2,30 = 4.05, P = 0.031, ηp2 = 0.21). Post-hoc tests revealed that the MMN amplitude was reduced during ice-water immersion compared to that during the baseline (P = 0.038) and recovery phases (P = 0.032). No effect was found for the temporal MMN.
Figure 5.
Comparisons of MMN amplitude between Experiment 2 phases and among individuals. (A) Reduced MMN amplitude in the ice-water immersion (pain) phase compared to that in the baseline and recovery phases. *P < 0.05. (B) Different-colored numbers indicate different participants. Note the different response patterns observed.
Changes in MMN amplitudes in frontocentral areas before, during, and after cold-water immersion for individual subjects are shown in Fig. 5B. MMN attenuation (towards positive deflection) due to cold-induced pain was observed in 12 of 16 subjects (75%). In the remaining 4 subjects, the reverse pattern occurred, with the MMN amplitude increasing (more negative deflection) in response to cold pain. No correlations were found between MMN amplitude and self-reported pain ratings.
Discussion
The present study represents a first attempt to determine how pain affects auditory sensory memory in chronic and acute pain paradigms. Experiment 1 showed that TN patients had a smaller frontocentral MMN than the HCs with a right hemisphere dominance. This effect was observed in both nose- and mastoid re-referenced data analysis. When the ISI was increased, temporal MMN amplitudes decreased more rapidly in TN patients than in HCs, consistent with our hypothesis predicting that chronic pain patients would exhibit greater MMN attenuation in response to extending the ISI than HCs. Similar results were obtained in the mastoid-re-referenced data, corroborating the notion that chronic pain patients have impaired auditory sensory memory. Experiment 2 showed that MMN amplitude was reduced by acute cold pain and recovered after the pain was relieved, as we had hypothesized.
We observed a right hemisphere dominance of the MMN in both TN patients and HCs, consistent with previous studies showing a predominant right hemisphere scalp distribution of the MMN37–39. Likewise, a recent auditory evoked magnetic field study conducted in fibromyalgia patients showed that the MMN magnetoencephalographic equivalent has a right-hemisphere dominance lateralization pattern in response to tone stimuli10. This consistent laterality suggests that some MMN generation process is stronger in the right hemisphere than in the left hemisphere. Moreover, there appears to be a predominant right hemisphere frontal process associated with involuntary attention switching in response to an auditory change11.
It is noteworthy that our TN patients had a smaller frontocentral MMN than the HCs. This effect was observed in both nose- and mastoid re-referenced data analyzed across ISI conditions. These results are in agreement with Dick et al.’s findings of an increased MMN amplitude following analgesic treatment in chronic pain patients12. This pattern of findings may indicate a pain-related deficit in the initial encoding of sound stimulus properties. According to Eccleston et al.’s model, chronic pain, which is behaviorally distracting and disruptive, may in fact disrupt cognitive neurophysiological processes at very early processing stages associated with the MMN40. Empirically demonstrated attentional deficits in chronic pain patients, particularly in attention switching and attention interference tasks41,42, may be due to pain competing with other attention-demanding stimuli for limited cognitive resources40,43. Thus, chronic pain-induced reduction of the MMN may reflect disruption of pre-attentive processing.
The present data also provided evidence that the lifetime of a memory trace was reduced faster in chronic pain patients than in HCs. Both mastoid re-referenced frontocentral data and nose-re-referenced temporal data indicated that MMN amplitude decreased as a function of ISI in TN patients but not HCs. Generally, the MMN is thought to reflect an automatic change detection mechanism that is supported primarily by the perception of an incoming stimulus and the maintenance of its trace in memory44. According to Bartha-Doering et al., the duration and decay of auditory sensory memory can be examined by increasing ISIs, such that a prolongation of the ISI decreases MMN amplitude45–47. Consistent with this view, the significant main effect of ISI in the present study can be interpreted as a fading of the memory trace and the mastoid data are indicative of a hastened trace decay in chronic pain patients. This decreased MMN with a longer ISI may reflect a specific deficit in auditory sensory information maintenance and an impairment of echoic memory early in the progression of TN. Indeed, pain can slow stimulus information processing and impede working memory function48. Reports of poor memory and concentration among chronic pain patients are well known5,49.
In the present study, neither chronic pain nor acute induced pain affected temporal MMN, although there was a near-significant increase in the chronic pain condition for the short ISI. This finding may seem inconsistent with the main finding. We speculated that the increased temporal MMN in TN patients might be partially explained by a heightened vigilance (i.e., increased attention) to external stimulation in those suffering from chronic pain. The hypervigilance may result in a general enhancement in processing of sensory perception, including auditory discrimination, reflected as a somewhat larger temporal MMN.
Our finding of a significant correlation between MPQ sensory subscale score and MMN amplitude reduction across ISI conditions (long minus short) further confirmed that the greater the perceived intensity of pain, the faster auditory sensory memories decay. It was the sensory, but not the affective, component of pain that was associated with the sensory memory decay in chronic pain patients. It has been suggested that this MMN reduction results mainly from pain-related emotional disturbances, such as anxiety, distress, or depression50,51. Following Melzack et al.’s model of sensory, motivational, and cognitive determinants of pain52, the affective-motivational dimension of pain has been brought clearly into focus by clinical pain studies. Psychological factors have been shown to have a selective influence on the affective, versus the sensory, dimension of pain53,54. Nonetheless, the affective component of pain often covaries with pain intensity, a key feature of the sensory component of pain55,56. Pain intensity and unpleasantness ratings have often been reported to correlate strongly with each other57,58; the anterior cingulate cortex and medial thalamic nuclei, implicated in the encoding of the affective dimension of pain59–61, have also been found to exhibit pain intensity-related activation55,62.
The present study was the first to demonstrate that experimentally induced acute pain can disrupt the MMN. In contrast to Dick et al., who found that experimentally induced pain did not disrupt generation of the MMN11, we found that MMN amplitude was reduced by cold pressor pain and recovered after the pain was relieved. This inconsistency may be due to the different models used in the two studies, ischemic pain versus cold pressor pain. The former is thought to be caused by accumulation of algesic metabolites, occlusion of blood vessels below the inflated cuff, and mechanical pressure of the cuff, which activates mechano-sensitive nociceptors directly63. This physiological mechanism differs from that of cold-induced pain wherein direct activation of high-threshold thermo-sensitive nociceptors produces pain64. Another possible explanation for the discrepancy between findings is that the present study used an easy-to-detect tone differential (1000 Hz vs. 2000 Hz) while Dick et al.11 used both a difficult-to-detect (1000 Hz vs. 1020 Hz) and an easy-to-detect (1000 Hz vs. 1500 Hz) distinction. According to Sams et al., a greater standard-deviant distinction causes a larger MMN65. Thus, when the MMN is used as an index of cognitive impairment, the stimulus parameters used should be considered to ensure better sensitivity.
In conclusion, the current study represents a first attempt to determine how pain affects auditory sensory memory in chronic and acute pain conditions. We found that the auditory memory trace decayed faster in chronic pain patients than in HCs, and that experimentally-induced cold pain impaired pre-attentive auditory processing. Thus, we provided evidence that both trait pain and state pain can interfere with automatic change detection processes. Our study indicates that the MMN has the potential to be used as an objective index of clinical pain and therapeutic response. In future studies, it will be important to set up specific MMN recording parameters for different pain disorder etiologies.
Acknowledgements
This work was funded by an NNSF (National Natural Science Foundation of China) grant (31271092) to J.-Y.W., an NNSF grant (31671140) to N.W., NNSF grants (31171067, 31471061) to F. L., and grants from CAS Key Laboratory of Mental Health, Institute of Psychology (KLMH 2014G01, KLMH2016K02).
Author Contributions
J.-Y.W. conceived and designed the study. L.F., Y.-B.S., Z.-K.S. and F.Y. performed the experiment. L.F. and J.-Y.W. analyzed the data. All authors contributed to the manuscript.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Feng Yu, Email: yufeng.yf@163.com.
Jin-Yan Wang, Email: wangjy@psych.ac.cn.
References
- 1.McCracken LM, Vowles KE. Psychological flexibility and traditional pain management strategies in relation to patient functioning with chronic pain: An examination of a revised instrument. J Pain. 2007;8:700–707. doi: 10.1016/j.jpain.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Tamburin S, Magrinelli F, Zanette G. Diagnostic and Therapeutic Pitfalls in Considering Chronic Pain as a Disease. Pain Med. 2014;15:1640–1642. doi: 10.1111/pme.12495. [DOI] [PubMed] [Google Scholar]
- 3.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moriarty O, Finn DP. Cognition and pain. Curr Opin Support Pa. 2014;8:130–136. doi: 10.1097/SPC.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 5.Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog Neurobiol. 2011;93:385–404. doi: 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Oosterman JM, de Vries K, Dijkerman HC, de Haan EH, Scherder EJ. Exploring the relationship between cognition and self-reported pain in residents of homes for the elderly. Int Psychogeriatr. 2009;21:157–163. doi: 10.1017/S1041610208007941. [DOI] [PubMed] [Google Scholar]
- 7.Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The Relationship Between Pain, Neuropsychological Performance, and Physical Function in Community‐Dwelling Older Adults with Chronic Low Back Pain. Pain Med. 2006;7:60–70. doi: 10.1111/j.1526-4637.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 8.Martinsen S, et al. Fibromyalgia Patients Had Normal Distraction Related Pain Inhibition but Cognitive Impairment Reflected in Caudate Nucleus and Hippocampus during the Stroop Color Word Test. Plos One. 2014;9:9. doi: 10.1371/journal.pone.0108637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aizawa E, et al. Altered Cognitive Function of Prefrontal Cortex During Error Feedback in Patients With Irritable Bowel Syndrome, Based on fMRI and Dynamic Causal Modeling. Gastroenterology. 2012;143:1188–1198. doi: 10.1053/j.gastro.2012.07.104. [DOI] [PubMed] [Google Scholar]
- 10.Choi W, Lim M, Kim JS, Kim DJ, Chung CK. Impaired pre-attentive auditory processing in fibromyalgia: A mismatch negativity (MMN) study. Clin Neurophysiol. 2015;126:1310–1318. doi: 10.1016/j.clinph.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Dick BD, et al. Effects of experimentally induced pain on Mismatch Negativity. J Psychophysiol. 2006;20:21–31. doi: 10.1027/0269-8803.20.1.21. [DOI] [Google Scholar]
- 12.Dick BD, et al. The disruptive effect of chronic pain on mismatch negativity. Clin Neurophysiol. 2003;114:1497–1506. doi: 10.1016/S1388-2457(03)00133-0. [DOI] [PubMed] [Google Scholar]
- 13.Yao S, Liu X, Yang W, Wang X. Preattentive processing abnormalities in chronic pain: neurophysiological evidence from mismatch negativity. Pain Med. 2011;12:773–781. doi: 10.1111/j.1526-4637.2011.01097.x. [DOI] [PubMed] [Google Scholar]
- 14.Näätänen R, Gaillard AW, Mäntysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta psychol. 1978;42:313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- 15.Tiitinen H, May P, Reinikainen K, Naatanen R. Attentive Novelty Detection In Humans Is Governed by Pre-Attentive Sensory Memory. Nature. 1994;372:90–92. doi: 10.1038/372090a0. [DOI] [PubMed] [Google Scholar]
- 16.Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clin Neurophysiol. 2007;118:2544–2590. doi: 10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Giard MH, Perrin F, Pernier J, Bouchet P. Brain Generators Implicated in the Processing of Auditory Stimulus Deviance: A Topographic Event‐Related Potential Study. Psychophysiology. 1990;27:627–640. doi: 10.1111/j.1469-8986.1990.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 18.Rinne T, Alho K, Ilmoniemi RJ, Virtanen J, Näätänen R. Separate time behaviors of the temporal and frontal mismatch negativity sources. Neuroimage. 2000;12:14–19. doi: 10.1006/nimg.2000.0591. [DOI] [PubMed] [Google Scholar]
- 19.Manzoni GC, et al. Classification Of Chronic Daily Headache by International-Headache-Society Criteria - Limits And New Proposals. Cephalalgia. 1995;15:37–43. doi: 10.1046/j.1468-2982.1995.1501037.x. [DOI] [PubMed] [Google Scholar]
- 20.von Baeyer CL, Piira T, Chambers CT, Trapanotto M, Zeltzer LK. Guidelines for the cold pressor task as an experimental pain stimulus for use with children. J Pain. 2005;6:218–227. doi: 10.1016/j.jpain.2005.01.349. [DOI] [PubMed] [Google Scholar]
- 21.McNeil DW, Berryman ML. Components of dental fear in adults? Behav Res Ther. 1989;27:233–236. doi: 10.1016/0005-7967(89)90041-7. [DOI] [PubMed] [Google Scholar]
- 22.Yang Z, et al. The Reliability and Validity of the Fear of Pain Questionnaire-III. Chin J Clin Psychol. 2013;21:768–763. [Google Scholar]
- 23.McCracken LM, Dhingra L. A short version of the Pain Anxiety Symptoms Scale (PASS-20): preliminary development and validity. Pain Res Manag. 2002;7:45–50. doi: 10.1155/2002/517163. [DOI] [PubMed] [Google Scholar]
- 24.Wong WS, McCracken LM, Fielding R. Factor structure and psychometric properties of the Chinese version of the 20-item Pain Anxiety Symptoms Scale (ChPASS-20) J Pain Symptom Manag. 2012;43:1131–1140. doi: 10.1016/j.jpainsymman.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Antony MM, Bieling PJ, Cox BJ, Enns MW, Swinson RP. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol Assess. 1998;10:176. doi: 10.1037/1040-3590.10.2.176. [DOI] [Google Scholar]
- 26.Henry JD, Crawford JR. The short‐form version of the Depression Anxiety Stress Scales (DASS‐21): Construct validity and normative data in a large non‐clinical sample. Br J Clin psychol. 2005;44:227–239. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- 27.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 28.Luo, Y. Assessment of Clinical Application of Short-Form McGill Pain Questionnaire. Chin J Rehabilitation (1992).
- 29.Sams M, Alho K, Näätänen R. Short‐Term Habituation and Dishabituation of the Mismatch Negativity of the ERP. Psychophysiology. 1984;21:434–441. doi: 10.1111/j.1469-8986.1984.tb00223.x. [DOI] [PubMed] [Google Scholar]
- 30.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Belouchrani, A., Abed-Meraim, K., Cardoso, J. & Moulines, E. In Proc. Int. Conf. Digital Signal Processing. 346–351 (Citeseer, 1993).
- 32.Näätänen R, Kujala T, Winkler I. Auditory processing that leads to conscious perception: a unique window to central auditory processing opened by the mismatch negativity and related responses. Psychophysiology. 2011;48:4–22. doi: 10.1111/j.1469-8986.2010.01114.x. [DOI] [PubMed] [Google Scholar]
- 33.Cooper RJ, Todd J, McGill K, Michie PT. Auditory sensory memory and the aging brain: A mismatch negativity study. Neurobiol Aging. 2006;27:752–762. doi: 10.1016/j.neurobiolaging.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Ruzzoli M, Pirulli C, Brignani D, Maioli C, Miniussi C. Sensory memory during physiological aging indexed by mismatch negativity (MMN) Neurobiol Aging. 2012;33:625. e621–625. e630. doi: 10.1016/j.neurobiolaging.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Giard M, et al. Separate representation of stimulus frequency, intensity, and duration in auditory sensory memory: an event-related potential and dipole-model analysis. J Cogn Neurosci. 1995;7:133–143. doi: 10.1162/jocn.1995.7.2.133. [DOI] [PubMed] [Google Scholar]
- 36.Sams M, Hari R, Rif J, Knuutila J. The human auditory sensory memory trace persists about 10 sec: neuromagnetic evidence. J Cogn Neurosci. 1993;5:363–370. doi: 10.1162/jocn.1993.5.3.363. [DOI] [PubMed] [Google Scholar]
- 37.Kasai K, et al. Brain lateralization for mismatch response to across- and within-category change of vowels. Neuroreport. 2001;12:2467–2471. doi: 10.1097/00001756-200108080-00036. [DOI] [PubMed] [Google Scholar]
- 38.Näätänen R, et al. The mismatch negativity: an index of cognitive decline in neuropsychiatric and neurological diseases and in ageing. Brain. 2011;134:3432–3450. doi: 10.1093/brain/awr064. [DOI] [PubMed] [Google Scholar]
- 39.Takegata R, Nakagawa S, Tonoike M, Naatanen R. Hemispheric processing of duration changes in speech and non-speech sounds. Neuroreport. 2004;15:1683–1686. doi: 10.1097/01.wnr.0000134929.04561.64. [DOI] [PubMed] [Google Scholar]
- 40.Eccleston C, Crombez G. Pain demands attention: A cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125:356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- 41.Bosma FK, Kessels RPC. Cognitive impairments, psychological dysfunction, and coping styles in patients with chronic whiplash syndrome. Neuropsy Neuropsy Be. 2002;15:56–65. [PubMed] [Google Scholar]
- 42.Grisart JM, Plaghki LH. Impaired selective attention in chronic pain patients. Eur J Pain-London. 1999;3:325–333. doi: 10.1016/S1090-3801(99)90014-9. [DOI] [PubMed] [Google Scholar]
- 43.Grisart JM, Van der Linden M. M. Conscious and automatic uses of memory in chronic pain patients. Pain. 2001;94:305–313. doi: 10.1016/S0304-3959(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 44.Cowan N. On short and long auditory stores. Psychol Bull. 1984;96:341. doi: 10.1037/0033-2909.96.2.341. [DOI] [PubMed] [Google Scholar]
- 45.Bartha‐Doering L, Deuster D, Giordano V, Am Zehnhoff‐Dinnesen A, Dobel C. A systematic review of the mismatch negativity as an index for auditory sensory memory: From basic research to clinical and developmental perspectives. Psychophysiology. 2015;52:1115–1130. doi: 10.1111/psyp.12459. [DOI] [PubMed] [Google Scholar]
- 46.Cowan N, Winkler I, Teder W, Naatanen R. Memory prerequisites of mismatch negativity in the auditory event-related potential (ERP) J Exp Psychol Learn Mem Cogn. 1993;19:909–921. doi: 10.1037/0278-7393.19.4.909. [DOI] [PubMed] [Google Scholar]
- 47.Tiitinen H, Sinkkonen J, May P, Naatanen R. The Auditory Transient 40-Hz Response Is Insensitive to Changes in Stimulus Features. Neuroreport. 1994;6:190–192. doi: 10.1097/00001756-199412300-00048. [DOI] [PubMed] [Google Scholar]
- 48.Berryman C, et al. Evidence for working memory deficits in chronic pain: a systematic review and metaanalysis. Pain. 2013;154:1181. doi: 10.1016/j.pain.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Oosterman JM, Dijkerman HC, Kessels RPC, Scherder EJA. A unique association between cognitive inhibition and pain sensitivity in healthy participants. Eur J Pain. 2010;14:1046–1050. doi: 10.1016/j.ejpain.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Price DD. Neuroscience - Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 51.Villemure C, Bushnell MC. Mood Influences Supraspinal Pain Processing Separately from Attention. J Neurosci. 2009;29:705–715. doi: 10.1523/JNEUROSCI.3822-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melzack, R. & Casey, K. L. Sensory, motivational and central control determinants of pain: a new conceptual model. The skin senses1 (1968).
- 53.Kupers RC, Konings H, Adriaensen H, Gybels JM. Morphine differentially affects the sensory and affective pain ratings in neurogenic and idiopathic forms of pain. Pain. 1991;47:5–12. doi: 10.1016/0304-3959(91)90004-H. [DOI] [PubMed] [Google Scholar]
- 54.Price DD, Harkins SW, Baker C. Sensory-affective relationships among different types of clinical and experimental pain. Pain. 1987;28:297–307. doi: 10.1016/0304-3959(87)90065-0. [DOI] [PubMed] [Google Scholar]
- 55.Buchel C, et al. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: A parametric single-trial laser functional magnetic resonance imaging study. J Neurosci. 2002;22:970–976. doi: 10.1523/JNEUROSCI.22-03-00970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmidt-Wilcke T, et al. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Flaten MA, Aslaksen PM, Lyby PS, Bjørkedal E. The relation of emotions to placebo responses. Philos Trans R Soc Lond B Biol Sci. 2011;366:1818–1827. doi: 10.1098/rstb.2010.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kunz M, Lautenbacher S, LeBlanc N, Rainville P. Are both the sensory and the affective dimensions of pain encoded in the face? Pain. 2012;153:350–358. doi: 10.1016/j.pain.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 59.Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. 1996;384:258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- 60.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 61.Sawamoto N, et al. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J Neurosci. 2000;20:7438–7445. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 63.Pertovaara A, Nurmikko T, Pöntinen PJ. Two separate components of pain produced by the submaximal effort tourniquet test. Pain. 1984;20:53–58. doi: 10.1016/0304-3959(84)90810-8. [DOI] [PubMed] [Google Scholar]
- 64.Johnson MI, Tabasam G. A single-blind placebo-controlled investigation into the analgesic effects of interferential currents on experimentally induced ischaemic pain in healthy subjects. Clin Physiol Funct I. 2002;22:187–196. doi: 10.1046/j.1475-097X.2002.00416.x. [DOI] [PubMed] [Google Scholar]
- 65.Sams M, Paavilainen P, Alho K, Naatanen R. Auditory Frequency Discrimination and Event-Related Potentials. Electroen Clin Neuro. 1985;62:437–448. doi: 10.1016/0168-5597(85)90054-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.