Abstract
The structural transformation of high-nuclearity silver clusters from one to another induced by specific stimuli is of scientific significance in terms of both cluster synthesis and reactivity. Herein, we report two silver-thiolate clusters, [Mo6O22@Ag44] and [Mo8O28@Ag50], which are templated by isopolymolybdates inside and covered by iPrS− and PhCOO− ligands on the surfaces. Amazingly, the [Mo8O28@Ag50] can be transformed from [Mo6O22@Ag44] by adding PhCOOH which increases the degree of condensation of molybdates template from Mo6O228- to Mo8O288-, then enlarging the outer silver shell from Ag44 to Ag50. The evolution of solution species revealed by time-dependent electrospray ionization mass spectrometry (ESI-MS) suggests a breakage-growth-reassembly (BGR) transformation mechanism. These results not only provide a combined assembly strategy (anion-template + induced transformation) for the synthesis of silver-thiolate clusters but also help us to better understand the complex transformation process underpinning the assembly system.
Understanding how one metal nanocluster transforms into another is of synthetic and fundamental importance. Here, the authors use mass spectrometry to reveal an acid-induced structural transformation between two Ag clusters that proceeds via a breakage-growth-reassembly mechanism.
Introduction
With regard to their ubiquitous argentophilicity and variable coordination fashions1–3, Ag(I) coordination complexes, especially for silver clusters, are gorgeous in structural diversity and physicochemical properties4–6. However, the synthesis of high-nuclearity silver clusters is always tedious and frankly a trial-and-error process. Overwhelming these synthesis barriers has promoted the appearance of exquisite assembly strategies including anion templation and geometric polyhedral principle7,8, which have pushed the assembly of silver clusters to a higher level of sophistication9,10. From the known largest silver(I) solid cluster (Ag490)11 to the largest silver(I) cage (Ag180)12, we have witnessed the fruitful advances in this field. However, there is still vast room for improvement to realize the manipulation over such clusters at the molecular level, not just synthesizing them randomly. Inspired by the LEIST (ligand-exchange-induced size/structure transformation) methodology widely used in Aun(SR)m nanoclusters6, we would like to study whether the similar stories can be observed in their silver cousins. In 2012, Mak et. al., reported the reaction of a famous Cl@Ag14 cluster with AgClO4, which gave a larger Cl6Ag6@Ag30 cluster, thus realizing the cluster enlargement13. Following this, a polyoxovanadate-templated Ag30 cluster was subjected to acid/base stimulations, which only resulted in the reversible conversion of configurations of [VV10VIV2O34]10- from D3d to C2h, however, the silver shell was kept invariable14. These sporadic reports indicated the structural transformation of silver clusters inside and out by specific stimuli is still a challenging task.
In order to achieve structural transformation of silver clusters, two prerequisites are needed: (i) flexible silver shells and (ii) variable anion templates. Installing monocarboxylate ligands on the surface of silver clusters will endow the silver clusters some flexibility because the carboxylate belongs to hard base with respect to thiolate, thus forming relatively weak bonding with soft acid Ag(I) atoms15. As such, when the stimuli-induced post-reaction proceeds in solution, the carboxylates can partially disassociate then induce the rearrangement of surface silver atoms. For variable anion templates, polyoxometalates (POMs) are the best candidates due to their mutable forms depending on pH values16.
With all above considerations in mind, herein we use thiol and benzoic acid as mixed ligands to construct a silver cluster, [Mo6O22@Ag44(iPrS)20(PhCOO)16(CH3CN)2]·2CH3CN (SD/Ag44; SD = SunDi), which can be transformed to another larger silver cluster, [Mo8O28@Ag50(iPrS)24(PhCOO)18(CH3CN)2)]·4CH3CN (SD/Ag50) by the reaction with additional PhCOOH. To the best of our knowledge, this is the first report of the PhCOOH-induced structural transformation simultaneously involving enlargements of inner anion template (Mo6O228− → Mo8O288−) and outer silver shell (Ag44 → Ag50). Such transformation is also proved by the time-dependent ESI-MS of SD/Ag44 after adding PhCOOH and a breakage-growth-reassembly (BGR) mechanism is also proposed.
Results
Structures of SD/Ag44 and SD/Ag50
The SD/Ag44 was synthesized by solvothermal reaction of polymeric (iPrSAg)n precursor, PhCOOAg and (nBu4N)4(α-Mo8O26) in CH3CN at 65 °C (Fig. 1). After the reaction, the yellow tufted crystals can be collected as the bulk sample of SD/Ag44. If adding another portion of PhCOOH (0.32 mmol) into above reaction mother liquor without removing crystals of SD/Ag44, then continuing to react again under the same condition will produce yellow block crystals of SD/Ag50.
Fig. 1.
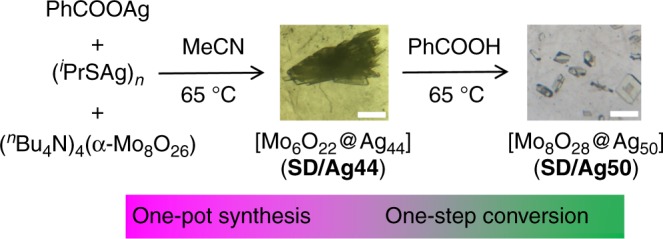
Synthesis and transformation routes for SD/Ag44 and SD/Ag50. The scale bar is 1 mm
The molecular structures of SD/Ag44 and SD/Ag50 were determined by single-crystal X-ray diffraction (SCXRD) analysis (Supplementary Table 1). Both of them crystallize in triclinic P-1 space group with a complete cluster in the asymmetric unit. As shown in Fig. 2a, the overall structure of SD/Ag44 is a slightly squashed spheroid composed of 44 silver atoms and covered by 20 iPrS−, 16 PhCOO− ligands and two CH3CN molecules. Interiorly, an unusual Mo6O228− anion in situ generated from (nBu4N)4(α-Mo8O26) supports the outer Ag44 shell. The diameter of SD/Ag44 is roughly 1.2 nm, if removing the organic shell. Based on their coordination modes, 20 iPrS− ligands are divided into two types comprised of 2 μ3 and 18 μ4 (Fig. 2b), which capped on the irregular silver trigons, tetragons or pentagons with the Ag-S bond lengths spanning from 2.3641(17) to 2.7589(18) Å. The PhCOO− ligands with μ2-κ1:κ1, μ3-κ1:κ2, μ4-κ2:κ2, and μ4-κ1:κ3 modes cap on the edges or faces of silver polygons (Supplementary Fig. 2). The Ag-Obenzoate bond lengths locate in the range of 2.114(5)−2.787(7) Å. Two additional CH3CN molecules finally finish the coverage of Ag44 shell (Ag-N: 2.338(7) and 2.395(6) Å), which is further consolidated by the Ag···Ag interactions (2.8272(7)−3.4289(8) Å)17,18.
Fig. 2.
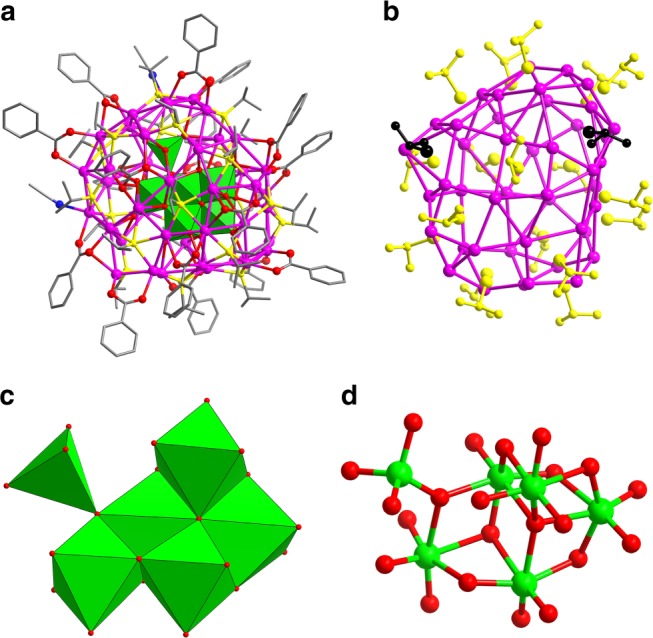
Single-crystal X-ray structure of SD/Ag44. a The molecular structure of SD/Ag44. The inner POM anion template is shown in polyhedral mode (Color legend: Ag: purple; S: yellow; Mo: green; O: red; C: gray; N: blue). b The distributions of iPrS− ligands on the Ag44 shell sorted by different coordination modes individually colored. Black: μ3 and yellow: μ4. The polyhedral (c) and ball-and-stick (d) modes showing the inner Mo6O228− anion
Notably, a Mo6O228− anion was trapped into the Ag44 cluster and should be in situ transformed from α-Mo8O264-. Bond-valence sum (BVS) calculations for six Mo atoms were performed and confirmed all of them are + 6 oxidation state (Mo1-Mo6: 5.744, 5.455, 5.792, 5.531, 5.766, and 6.173)19. This indicates that no redox reaction occurs under the reaction condition. The Mo6O228− in SD/Ag44 is constructed from five edge-shared MoO6 octahedra adding one MoO4 tetrahedron by sharing one vertex (Fig. 2c). Alternatively, it can be seen as three Mo3O4 opened cubanes fused together by sharing two Mo2O2 rhombus faces with another vertex-shared MoO4 outside (Fig. 2d). This Mo6O228− has 14 terminal O atoms, 5 μ2 bridging O atoms and 3 μ3 bridging O atoms. The Mo-O bond distances are in the range of 1.730(5)−2.328(4) Å, suggesting unusual elongated characteristic of Mo-O bonds compared to other Mo6O228− anions in classic POM chemistry20. Using the μ1 and μ2 bridging O atoms, Mo6O228− binds total 30 Ag atoms around it with the Ag-OPOM bond lengths ranging from 2.255(4) to 2.793(5) Å (Supplementary Fig. 3). To date, only three isomeric Mo6O228− anion templates have been reported in silver clusters such as face-shared Mo4O4 double cubanes21,22, two Mo3O4 opened cubanes sandwiching a Mo4O4 cubane by face-sharing23, and three face-shared Mo3O4 opened cubanes24, as summarized in Supplementary Table 2. By comparisons, the Mo6O228− in SD/Ag44 is the fourth isomer trapped by silver cluster. Moreover, such Mo6O228− is also different from those observed in POM-based inorganic–organic hybrids (Supplementary Table 3)25–27, suggesting a new Mo6O228− structure.
When adding another portion of PhCOOH to the mixture after the synthesis of SD/Ag44 for second step reaction, we can isolate a larger silver cluster, SD/Ag50. SCXRD analysis revealed that it is a larger cluster than SD/Ag44. The overall structure of SD/Ag50 comprised of 50 Ag atoms, forming a nearly ball-like shape with a diameter of ca. 1.4 nm, which is co-protected by 24 iPrS−, 18 PhCOO− ligands, and two CH3CN molecules (Fig. 3a). The ratio of iPrS/PhCOO in SD/Ag50 (4:3) is slightly larger than that in SD/Ag44 (5:4), indicating the re-organization of two kinds of ligands on the surface after structural transformation. As shown in Fig. 3b, 24 iPrS− ligands show μ4 and μ5 binding modes, whereas PhCOO− ligands show μ2-κ1:κ1, μ3-κ1:κ2, μ4-κ1:κ3, or μ4-κ2:κ2 modes. The Ag-S and Ag-Obenzoate bond lengths fall in the ranges of 2.248(7)−2.917(4) and 2.238(13)−2.771(12) Å, respectively. Due to the lack of regular silver polygons on the surface, such Ag50 cluster doesn’t exhibit any geometrical polyhedron feature. The Ag50 shell was further consolidated by rich Ag···Ag interactions falling in the range of 2.851(2)−3.431(2) Å.
Fig. 3.
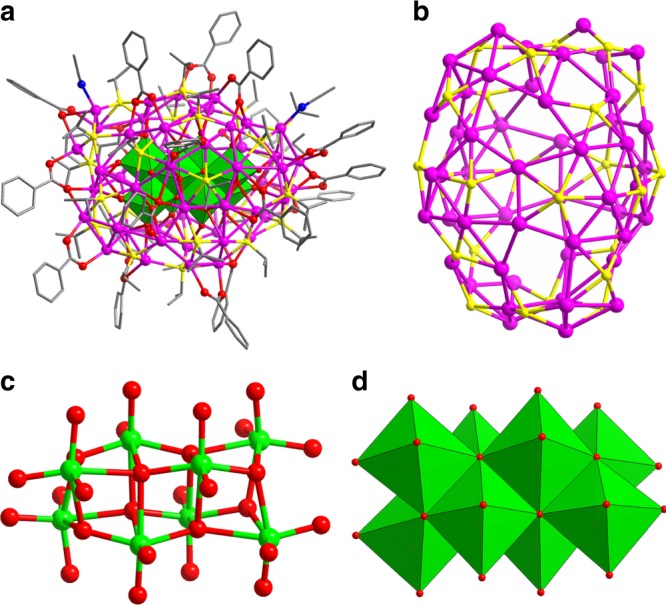
Single-crystal X-ray structure of SD/Ag50. a Molecular structure of SD/Ag50 trapping a Mo8O288− anion as template (Color legend: Ag: purple; S: yellow; Mo: green; O: red; C: gray; N: blue). b The ball-and-stick mode of Ag50S24 shell. The ball-and-stick (c) and polyhedral (d) modes showing the inner Mo8O288− anion
This 50-nucleus cluster is also templated by a novel rod-like POM, formulated as Mo8O288−, which can be seen as three Mo4O4 cubanes fused together by sharing two Mo2O2 rhombus faces (Fig. 3c), or described to eight edge-shared MoO6 octahedra (Fig. 3d). The BVS19 also verified + 6 oxidation state of eight Mo atoms (Mo1-Mo8: 5.865, 5.431, 5.648, 5.889, 5.868, 5.748, 6.092, and 5.738). The surface of Mo8O288− has 20 terminal, 4 μ2 bridging and 4 μ4 bridging O atoms. Among these O atoms, only 20 terminal O atoms participate to the coordination with Ag atoms (Supplementary Fig. 4). Compared to the starting material α-Mo8O264-, Mo8O288− has higher negative charges and more O atoms, which endow it as a better template in the assembly of silver clusters. The Mo8O288− in SD/Ag50, to the best of our knowledge, has never been reported in POM-templated silver clusters and classic POM chemistry.
Inspired by the above discussed structure features of SD/Ag44, both PhCOO− ligands and weakly coordinated CH3CN molecules are the potential reaction active sites, and the POM template can be also varied depending on the solution acidity. In fact, we found the transformation of SD/Ag44 to SD/Ag50 can facilely work by adding additional PhCOOH without changing any other reaction conditions. Based on the comparisons of their structures, such cluster-to-cluster structural transformation is a rare occurrence that simultaneously involved inner anion template (Mo6O228− → Mo8O288−) and outer silver shell (Ag44 → Ag50). In this transformation process, the degree of condensation of molybdate was increased, resulting in the growth of Mo6O228− to Mo8O288−. The larger POM template thus enlarges the outer silver shell from Ag44 to Ag50. We also tried to synthesize SD/Ag50 without the transformation step by directly adding more PhCOOH into the reaction system, however, the SD/Ag50 can be only isolated with a quite low yield ( < 5%), whereas using the transformation synthesis route, it can reach up to more than 50%. Thus, PhCOOH-induced structural transformation might provide a new method for the high-yield synthesis of some silver clusters that are otherwise difficult to access. Moreover, this transformation reaction should be a thermodynamics controlled reaction, so SD/Ag50 should be more stable than SD/Ag44.
ESI-MS of SD/Ag44 and SD/Ag50
In order to investigate solution behaviors of both clusters, the ESI-MS of SD/Ag44 and SD/Ag50 dissolved in dichloromethane were measured in the positive ion mode. As shown in Fig. 4a, there are a series of +2 species (1a-1k) centered at the m/z range of 2000–8000. The most dominant peak centered at 4392.48 (1e) was assigned to [Δ(iPrS)26(PhCOO)8(CH2Cl2)2(H2O)]2+ based on the superimposable observed and simulated isotope patterns (Cal. 4392.49; Δ = Mo6O22@Ag44 hereafter). Similar formula assignment for each labeled species in ESI-MS was also performed as listed in Supplementary Table 4. Based on the assigned formula, we found the core of SD/Ag44 is quite stable just with some surface ligand exchange between PhCOO− and iPrS− such as those observed in paired species of 1b and 1c, 1 g and 1 h, and 1j and 1k. The 11 identified species existed in the solution, suggesting a typical coordination-disassociation equilibrium state, which may be broken by introducing some exotic stimuli, then producing some new species.
Fig. 4.
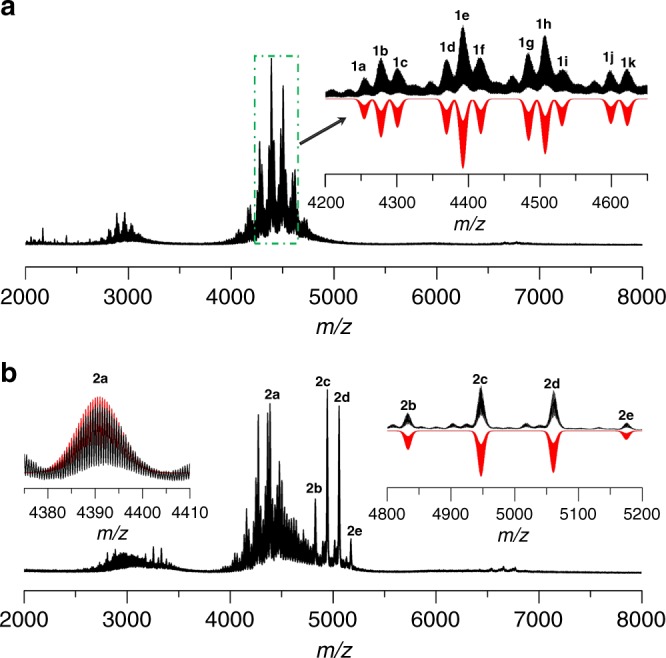
ESI-MS. a Positive ion mode ESI-MS of the crystals of SD/Ag44 dissolved in dichloromethane. Inset: The zoom-in mass spectrum of experimental (black line) and simulated (red line) isotope patterns for each labeled species. b Positive ion mode ESI-MS of the crystals of SD/Ag50 dissolved in dichloromethane. Insets: The zoom-in mass spectrum of experimental (black lines) and simulated (red lines) isotope patterns for each labeled species
More complicated ESI-MS was observed for SD/Ag50 in its dichloromethane solution (Fig. 4b). Carefully analyzing the isotope distribution of the observed peaks in the m/z range of 2000–8000, we found all these peaks were bivalent species with a parent ion peak of SD/Ag50 detected at the m/z 5175.19 (2e), corresponding to [Mo8O28@Ag50(iPrS)24(PhCOO)16]2+ (Cal. 5175.23), which can be formed by losing all the acetonitrile molecules and two PhCOO− ligands from SD/Ag50. The neighbor peak centered at m/z 5060.72 (2d) can be assigned to [Mo8O28@Ag49(iPrS)24(PhCOO)15]2+ (Cal. 5060.76), corresponding to loss one PhCOOAg from 2e. The most dominant peak (2c) was observed at m/z 4947.25, assigned to [Mo8O28@Ag47(iPrS)22(PhCOO)15(CH2Cl2)(H2O)3]2+ (Cal. 4947.32). The other identified species were shown in Supplementary Table 5. Of note, a series of peaks in the m/z range of 4200–4700 are quite similar to those in ESI-MS of SD/Ag44. For example, the peak (2a) centered at 4391.01 can be ascribed to [Δ(iPrS)25(PhCOO)9(CH2Cl2)(H2O)3]2+ (Cal. 4391.03). These results demonstrated (i) SD/Ag50 can maintain its integrity in dichloromethane, and (ii) SD/Ag50 can partially decompose to the closely related species to SD/Ag44.
Transformation mechanism study
How the transformation process happened and proceeded are intriguing issues in this system. To gain further insights into the transformation mechanism, we carried out a series of specific experiments including pH values measurements, comparable synthesis, and solution species tracking by ESI-MS during the transformation.
After synthesis of SD/Ag44, the pH of mother liquor is 10.45, then decreases to 8.20 upon adding PhCOOH, which is favorable to the further condensation reaction of molybdates28. To rule out the Mo8O288− being transformed from the residual molybdate species in mother liquor, we also used fresh crystals of SD/Ag44 and clean CH3CN as solvent to do the transformation reaction and SD/Ag50 can also be formed, which suggested the Mo8O288− must be transformed from the interior Mo6O228− in SD/Ag44. Meanwhile, this result also unambiguously indicated that the smaller-to-larger silver nanocluster conversion from SD/Ag44 to SD/Ag50 genuinely started from SD/Ag44 instead of other silver precursors.
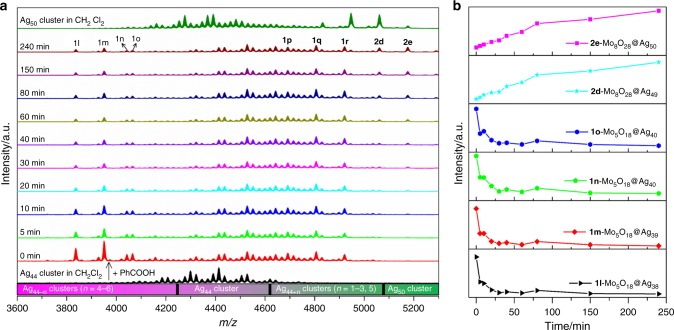
As we know, ESI-MS is a promising analytical tool that provides considerable speciation information in solution29–32. Cronin group have contributed largely to this field by using ESI-MS to detect new POM species and track transformation of POMs33–35. This technique was also profoundly used by Xie group to study growth mechanism of gold nanoclusters or reactivity36–40. However, using ESI-MS to study transformation or reactivity of silver clusters has remained a black box to date. To shed light on the details of transformation process from Ag44 to Ag50, we tracked the solution species evolution over the course of 240 min upon addition of PhCOOH (0.32 mmol, 39.1 mg) in the CH2Cl2 solution of Ag44 cluster by time-dependent ESI-MS. In order to guarantee comparable intensity of signals for each species at different times, a generalized operation for the sampling and analysis was established and uniform instrument parameters were maintained. At scheduled time intervals, 500 μL aliquots of the reaction solution were extracted, and immediately infused (240 μL/h) to the mass spectroscopy without dilution for subsequent measurement. To set the baseline and finishing line for this monitoring process, we also incorporated the ESI-MS spectra of Ag44 (black line) and Ag50 (dark green line) in CH2Cl2 into Fig. 5, respectively, although in-source fragmentation of them under our chosen ESI source parameters have been studied in above section. All formulae of labeled species in the ESI-MS in this section are listed in Supplementary Table 6 and hereafter only inner core compositions are shown below for clarity. Zoom-in ESI-MS including the simulated isotope distributions of all these species are shown in Supplementary Fig. 5. As depicted in Fig. 5a (0 min), once adding PhCOOH, four doubly charged species 1l-1o appeared in the lower m/z range of 3600–4200 which are absent in original ESI-MS of Ag44 solution. The assigned formulae for 1l-1o are [Mo5O18@Ag38] (1l), [Mo5O18@Ag39] (1m), and [Mo5O18@Ag40] (1n and 1o). In the higher m/z range of 4600–5300, the other five larger divalent species (1p, 1q, 1r, 2d, and 2e) are observed and can be assigned to [Mo8O28@Ag45] (1p), [Mo8O28@Ag46] (1q), [Mo8O28@Ag47] (1r), [Mo8O28@Ag49] (2d), and [Mo8O28@Ag50] (2e). Of note, the 2e species is the parent ion of SD/Ag50. All these intermediates are smaller or larger than original Ag44 cluster and their formations are intensely dependent on the addition of PhCOOH.
Fig. 5.
Time-course ESI-MS. a Time-course ESI-MS of transformation from Ag44 to Ag50 cluster induced by adding PhCOOH. The detailed formulae of labeled species are listed in Supplementary Table 6. b Plots of ESI-MS peak intensity of selected intermediate species vs time during the transformation process
The evolutions of species were further represented by the plots of signal intensity vs. time as shown in Fig. 5b. Upon adding PhCOOH, we observed four species (1l-1o) smaller than Ag44, indicating the breakage of Ag44 shell was initiated by PhCOOH. As time going on (5–240 mins), the signal intensities of [Mo5O18@Ag38] (1l), [Mo5O18@Ag39] (1m), and [Mo5O18@Ag40] (1n and 1o) gradually decrease, whereas those of [Mo8O28@Ag49] (2d) and [Mo8O28@Ag50] (2e) become more intense, which suggested that the formation of Ag50 cluster is at the expense of smaller intermediate species such as 1l-1o at this stage. Moreover, we also noted the POM template encapsulated in silver shell undergo a condensation reaction from Mo6O228− to Mo8O288− and the excess oxygen coordination sites permit for the linkage of more silver atoms, thus forming the cluster larger than Ag44.
Based on above observations, we speculated that the outer silver shell was attacked and partially broken by PhCOOH during the initial stage, which resulted in the breakage of Ag44 shell in some extent, followed by the growth of inner Mo6O228− anion to Mo8O288−. Due to the addition of PhCOOH, the solution becomes more acidity, thus larger Mo8O288− was formed via condensation reaction. The decomposed fragments further reassemble around the new template to form final large Ag50 cluster. Finally, the overall breakage-growth-reassembly (BGR) mechanism was established for this transformation process (Fig. 6).
Fig. 6.
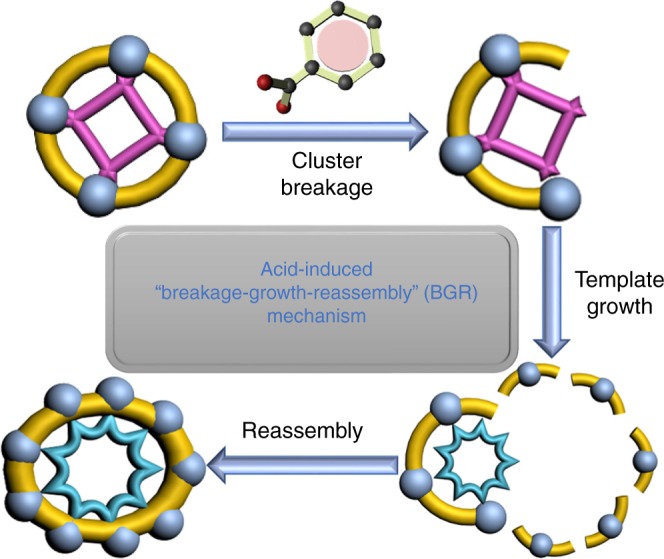
The proposed breakage-growth-reassembly mechanism for the transformation process from SD/Ag44 to SD/Ag50
Universality of acid-induced transformation
We also introduced different substituted benzoic acids such as 4-methylbenzoic acid (4-MePhCOOH) and 3-methylbenzoic acid (3-MePhCOOH) into above transformation process (Supplementary Fig. 1) to check the universality of transformation. Of note, the similar Ag50 clusters can be also isolated after acid induction and characterized by SCXRD analysis, which showed their structures are quite similar with inner Mo8O288− and outer Ag50 shell but just with different substituted degree of benzoates on the surface. The details of molecular structures and crystallography tables were shown in Supplementary Figs. 6–8 and Supplementary Table 1, respectively. Their formulae were determined as [Mo8O28@Ag50(iPrS)24(4-MePhCOO)14(PhCOO)4(CH3CN)4] (SD/Ag50a), [Mo8O28@Ag50(iPrS)24(4-MePhCOO)16.5(PhCOO)1.5] (SD/Ag50b), and [Mo8O28@Ag50(iPrS)24(3-MePhCOO)18(3-MePhCOOH)(CH3CN)2] (SD/Ag50c). The degree of substitution reaction between substituted benzoates and PhCOO− is intensely depended on the dose of substituted benzoates. For example, when increasing amount of 4-MePhCOOH from 0.3 to 0.6 mmol, the SD/Ag50b can be formed instead of SD/Ag50a. These results not only justified a universal acid-induced transformation fashion to synthesize larger sized silver clusters but also may bring new functionalities for silver clusters by ligand exchanges.
UV-Vis absorption spectra and luminescence properties
The solid state UV/Vis absorption spectra of SD/Ag44, SD/Ag50, and (iPrSAg)n precursor were measured at room temperature. As shown in Supplementary Fig. 19, SD/Ag44 and SD/Ag50 exhibit one intense absorption centered at 344 and 356 nm in the UV region. The UV absorption peaks can be attributed to the n → π* transition of iPrS−, as similar observed in the spectrum of the precursor (iPrSAg)n. The HOMO–LUMO gaps were determined as 2.35, 2.33, and 2.52 eV for SD/Ag44, SD/Ag50, and (iPrSAg)n precursor by using the transformed Kubelka–Munk plots, respectively (Supplementary Fig. 20), which are consistent with their yellow appearances.
We also checked the emission behaviors of SD/Ag44 and SD/Ag50 at both 298 and 77 K using hand-held UV light (λex = 365 nm). Preliminarily, both SD/Ag44 and SD/Ag50 emit red luminescence at 77 K but become emission-silent at 298 K. The on-off phenomena of them are reversible between 298 and 77 K and can be directly observed by naked eyes (See the insets in Fig. 7). For detailed studies of such observations, varied-temperature luminescent spectra of SD/Ag44 and SD/Ag50 were measured from 93 to 293 K in the solid state. As shown in Fig. 7a, SD/Ag44 emits near-infrared (NIR) light (λem = 719 nm) at 93 K and is almost non-emissive from 213–293 K. The maximum emission peak gradually red-shifts to 725 nm upon warming to 193 K along with obvious decrease of emission intensity. For SD/Ag50, its maximum emission peak red-shifts from 674 nm at 93 K to 686 nm at 233 K with gradually decayed emission intensity, after which no obvious emissions can be detected above 253 K. These NIR emissions at cryogenic temperature can be similarly attributed to the ligand-to-metal charge transfer (LMCT, charge transfer from S 3p to Ag 5 s) perturbed by Ag···Ag interaction41,42. The temperature-dependent emissions should be in the connection with the variations of molecule rigidity and argentophilicity under different temperatures43,44. The emission lifetimes of both SD/Ag44 and SD/Ag50 at 93 K fall in the microsecond scale (Supplementary Fig. 21), suggesting the phosphorescent triplet excitation state45. It is worth to noting that the emission intensities have good linearity correlation with respect to temperature in the low temperature regions (Supplementary Fig. 22). The linearity equations can be described as Imax = −3977T + 876273 (correlation coefficient = 0.985) and Imax = −1461T + 294270 (correlation coefficient = 0.979) for SD/Ag44 (93–213 K) and SD/Ag50 (93–193 K), respectively.
Fig. 7.
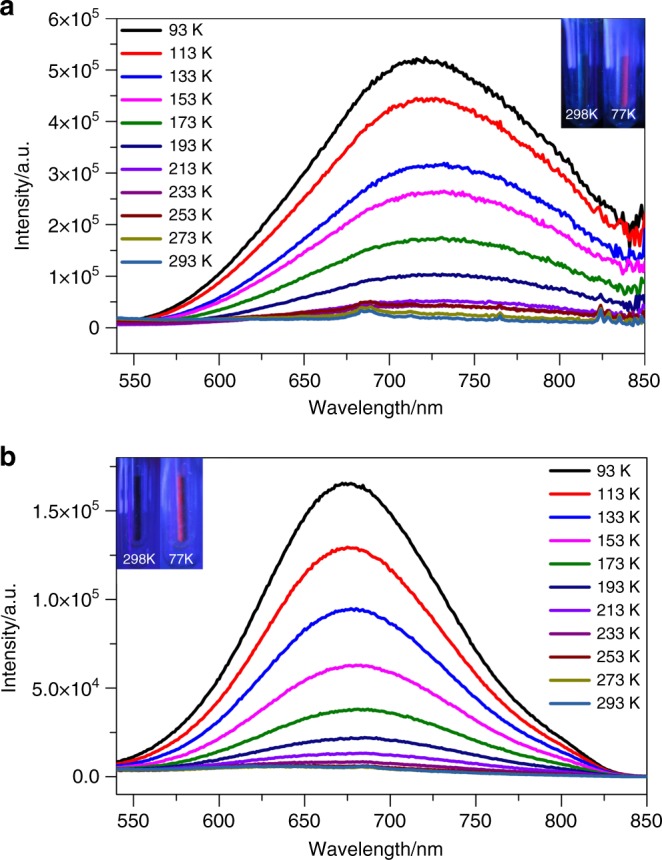
Luminescence properties of SD/Ag44 and SD/Ag50. Varied-temperature emission spectra of SD/Ag44 (a) and SD/Ag50 (b) in solid state under the excitation of 463 nm, Insets: the photographs of SD/Ag44 and SD/Ag50 irradiated by hand-held UV light (λex = 365 nm) at 298 and 77 K
Discussion
In conclusion, we synthesized and characterized two thiolate/benzoate co-protected Ag44 and Ag50 clusters. The latter can be facilely synthesized by PhCOOH-induced transformation reaction, resulting in the enlargement of both inner anion template (Mo6O228− → Mo8O288−) and outer silver shell (Ag44 → Ag50). The solution behaviors of Ag44 and Ag50 clusters were studied in details using ESI-MS technique. The overall breakage-growth-reassembly (BGR) transformation mechanism was also established based on the time-dependent ESI-MS, which revealed several small fragmented species ([Mo5O18@Ag38]~[Mo5O18@Ag40]) and large intermediates ([Mo8O28@Ag45]~[Mo8O28@Ag49]), as well as their abundance evolutions along with reaction going on. To our knowledge, this is the first example of the transformation of a smaller silver cluster into a larger one via anion template growth. This work not only revealed the important reactivity of silver clusters with acids but also provide a novel method for synthesizing larger silver clusters that are otherwise difficult to obtain.
Methods
Synthesis of (iPrSAg)n
(iPrSAg)n was prepared by the following reported procedure43. The solution of AgNO3 (30 mmol, 5.1 g) in 75 mL acetonitrile was mixed with 100 mL ethanol containing iPrSH (30 mmol, 2.8 mL) and 5 mL Et3N under stirring for 3 h in the dark at room temperature, then the yellow powder of (iPrSAg)n was isolated by filtration and washed with 10 mL ethanol and 20 mL ether, then dried in the ambient environment (yield: 97 %, based on AgNO3).
Synthesis of SD/Ag44
Typically, the mixture of PhCOOAg (0.1 mmol, 22.9 mg), (iPrSAg)n (0.05 mmol, 9.2 mg), and (nBu4N)4(α-Mo8O26) (0.0002 mmol, 4.2 mg) were dissolved in 5 mL acetonitrile then sealed into 25 mL Teflon-lined autoclave and heated at 65 °C under autogenous pressure for 2000 min. After cooling to room temperature, yellow crystals were isolated with a yield of 45 % (based on (iPrSAg)n). Elemental analyses calc. (found) for SD/Ag44 (C180H232Ag44Mo6N4O54S20): C, 23.30 (23.18); H, 2.52 (2.47); N 0.60 (0.55) %. Selected IR peaks (cm−1): 3682 (w), 2961 (m), 1589 (w), 1523 (m), 1454 (w), 1371 (s), 1241 (w), 1149 (w), 1032 (s), 1014 (m), 811 (w), 715 (m), 675 (m), 588 (w).
Synthesis of SD/Ag50
Another portion of PhCOOH (0.32 mmol, 39.1 mg) was added into the mother liquor after the synthesis of SD/Ag44 but without removing crystals of SD/Ag44, then this mixture was again sealed into 25 mL Teflon-lined autoclave and heated at 65 °C under autogenous pressure for 2000 min. After cooling to room temperature, yellow crystals were isolated with a yield of 50% (based on (iPrSAg)n). Elemental analyses calc. (found) for SD/Ag50 (C210H276Ag50Mo8N6O64S24): C, 23.09 (23.18); H, 2.57 (2.48); N 0.78 (0.70) %. Selected IR peaks (cm−1): 2955 (w), 2907 (w), 1589 (w), 1528 (s), 1454 (w), 1371 (s), 1241 (m), 1144 (w), 1046 (m), 910 (w), 880 (m), 830 (m), 800 (m), 714 (s), 671 (m), 635 (m), 610 (m).
Synthesis of SD/Ag50a
The synthesis conditions were similar to those described for SD/Ag50 except using 4-MePhCOOH (0.33 mmol, 44.9 mg) instead, yellow crystals were isolated with a yield of 36 % (based on (iPrSAg)n). Elemental analyses calc. (found) for SD/Ag50a (C220H298Ag50Mo8N4O64S24): C, 24.12 (23.97); H, 2.74 (2.58); N 0.51 (0.46) %. Selected IR peaks (cm−1): 3670 (w), 2949 (w), 2912 (w), 1584 (w), 1520 (m), 1453 (w), 1368 (s), 1242 (m), 1169 (m), 1148 (m), 1052 (m), 916 (m), 878 (m), 831 (m), 796 (s), 759 (s), 712 (m), 632 (s), 606 (s).
Synthesis of SD/Ag50b
The synthesis conditions were similar to those described for SD/Ag50 except using 4-MePhCOOH (0.57 mmol, 77.6 mg) instead, yellow crystals were isolated with a yield of 30 % (based on (iPrSAg)n). Elemental analyses calc. (found) for SD/Ag50b (C214.5H291Ag50Mo8O64S24): C, 23.80 (23.59); H, 2.71 (2.58) %. Selected IR peaks (cm−1): 3674 (w), 2971 (w), 2905 (w), 1584 (w), 1524 (m), 1453 (w), 1368 (s), 1237 (m), 1173 (w), 1149 (w), 1047 (s), 911 (m), 877 (s), 834 (m), 798 (m), 763 (s), 712 (m), 632 (s), 605 (s).
Synthesis of SD/Ag50c
The synthesis conditions were similar to those described for SD/Ag50 except using 3-MePhCOOH (0.57 mmol, 77.6 mg), yellow crystals were isolated with a yield of 33 % (based on (iPrSAg)n). Elemental analyses calc. (found) for SD/Ag50c (C234H317Ag50Mo8N5O66S24): C, 25.12 (24.98); H, 2.86 (2.74); N 0.63 (0.57) %. Selected IR peaks (cm−1): 3673 (w), 2955 (w), 2912 (w), 1592 (w), 1528 (m), 1450 (w), 1368 (s), 1236 (m), 1149 (m), 1078 (m), 1049 (m), 914 (m), 875 (m), 780 (s), 714 (m), 671 (s), 631 (s), 601 (s).
Electronic supplementary material
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 21822107, 21571115, 21827801, and 21701133), the Natural Science Foundation of Shandong Province (Nos. JQ201803 and ZR2017MB061), the Qilu Youth Scholar Funding of Shandong University and the Fundamental Research Funds of Shandong University (104.205.2.5). H.-F.S. thanks the President Research Funds from Xiamen University (20720170100).The authors also give special thanks to Dr. Zhao-Zhen Cao from Shandong University for her help with the varied-temperature luminescence spectra measurements.
Author contributions
Original idea was conceived by D.S., experiments and data analyses were performed by Z.W. and D.S., ESI-MS data were collected by H.-F.S., structure characterization was performed by Z.W. and D.S., manuscript was drafted by D.S., Z.W., C.-H.T. and L.-S.Z. All authors have given approval to the manuscript.
Data availability
The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC: 1837115–1837119 for SD/Ag44, SD/Ag50, and SD/Ag50a-SD/Ag50c. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zhi Wang, Hai-Feng Su.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-06755-4.
References
- 1.Young AG, Hanton LR. Square planar silver(I) complexes: a rare but increasingly observed stereochemistry for silver(I) Coord. Chem. Rev. 2008;252:1346–1386. doi: 10.1016/j.ccr.2007.07.017. [DOI] [Google Scholar]
- 2.Schmidbaur H, Schier A. Argentophilic interactions. Angew. Chem. Int. Ed. Engl. 2015;54:746–784. doi: 10.1002/anie.201405936. [DOI] [PubMed] [Google Scholar]
- 3.Liu JW, et al. Anisotropic assembly of Ag52 and Ag76 nanoclusters. J. Am. Chem. Soc. 2018;140:1600–1603. doi: 10.1021/jacs.7b12777. [DOI] [PubMed] [Google Scholar]
- 4.Corrigan JF, Fuhr O, Fenske D. Metal chalcogenide clusters on the border between molecules and materials. Adv. Mater. 2009;21:1867–1871. doi: 10.1002/adma.200802897. [DOI] [Google Scholar]
- 5.Xie YP, Jin JL, Duan GX, Lu X, Mak TCW. High-nuclearity silver(I) chalcogenide clusters: a novel class of supramolecular assembly. Coord. Chem. Rev. 2017;331:54–72. doi: 10.1016/j.ccr.2016.10.007. [DOI] [Google Scholar]
- 6.Jin RC, Zeng CJ, Zhou M, Chen YX. Atomically precise colloidal metal nanoclusters and nanoparticles: fundamentals and opportunities. Chem. Rev. 2016;116:10346–10413. doi: 10.1021/acs.chemrev.5b00703. [DOI] [PubMed] [Google Scholar]
- 7.Rais D, et al. Anion-templated syntheses of rhombohedral silver-alkynyl cage compounds. Angew. Chem. Int. Ed. Engl. 2001;40:3464–3467. doi: 10.1002/1521-3773(20010917)40:18<3464::AID-ANIE3464>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 8.Li XY, et al. A platonic solid templating Archimedean solid: an unprecedented nanometre-sized Ag37 cluster. Nanoscale. 2015;7:8284–8288. doi: 10.1039/C5NR01222H. [DOI] [PubMed] [Google Scholar]
- 9.Wang QM, Lin YM, Liu KG. Role of anions associated with the formation and properties of silver clusters. Acc. Chem. Res. 2015;48:1570–1579. doi: 10.1021/acs.accounts.5b00007. [DOI] [PubMed] [Google Scholar]
- 10.Li S, et al. Atom-precise modification of silver(I) thiolate cluster by shell ligand substitution: a new approach to generation of cluster functionality and chirality. J. Am. Chem. Soc. 2018;140:594–597. doi: 10.1021/jacs.7b12136. [DOI] [PubMed] [Google Scholar]
- 11.Anson CE, et al. Synthesis and crystal structures of the ligand-stabilized over chalcogenide clusters [Ag154Se77(dppxy)18], [Ag320(StBu)60S130(dppp)12], [Ag352S128(StC5H11)96], and [Ag490S188(StC5H11)114] Angew. Chem. Int. Ed. Engl. 2008;47:1326–1331. doi: 10.1002/anie.200704249. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, et al. Assembly of silver trigons into a buckyball-like Ag180 nanocage. Proc. Natl Acad. Sci. USA. 2017;114:12132–12137. doi: 10.1073/pnas.1711972114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hau SCK, Cheng PS, Mak TCW. Enlargement of globular silver alkynide cluster via core transformation. J. Am. Chem. Soc. 2012;134:2922–2925. doi: 10.1021/ja211438q. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, et al. Acid-base-triggered structural transformation of a polyoxometalate core inside a dodecahedrane-like silver thiolate shell. Angew. Chem. Int. Ed. Engl. 2016;55:3699–3703. doi: 10.1002/anie.201511765. [DOI] [PubMed] [Google Scholar]
- 15.Parr RG, Pearson RG. Absolute hardness-companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983;105:7512–7516. doi: 10.1021/ja00364a005. [DOI] [Google Scholar]
- 16.Chalmers RA, Sinclair AG. Organic molybdate complexes. J. Inorg. Nucl. Chem. 1967;29:2065–2080. doi: 10.1016/0022-1902(67)80467-6. [DOI] [Google Scholar]
- 17.Huang RW, et al. Hypersensitive dual-function luminescence switching of a silver-chalcogenolate cluster-based metal-organic framework. Nat. Chem. 2017;9:689–697. doi: 10.1038/nchem.2718. [DOI] [PubMed] [Google Scholar]
- 18.Dhayal RS, et al. [Ag21{S2P(OiPr)2}12]+: an eight-electron superatom. Angew. Chem. Int. Ed. Engl. 2015;54:3702–3706. doi: 10.1002/anie.201410332. [DOI] [PubMed] [Google Scholar]
- 19.Brown ID, Altermatt D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal-structure database. Acta Crystallogr. B. 1985;41:244–247. doi: 10.1107/S0108768185002063. [DOI] [Google Scholar]
- 20.Li YW, et al. Two unprecedented POM-based inorganic organic hybrids with concomitant heteropolytungstate and molybdate. Inorg. Chem. 2017;56:2481–2489. doi: 10.1021/acs.inorgchem.6b02601. [DOI] [PubMed] [Google Scholar]
- 21.Li XY, et al. Atom-precise polyoxometalate-Ag2S core-shell nanoparticles. Chem.-Asian J. 2015;10:1295–1298. doi: 10.1002/asia.201500201. [DOI] [PubMed] [Google Scholar]
- 22.Li XY, et al. Anion-templated nanosized silver clusters protected by mixed thiolate and diphosphine. Nanoscale. 2017;9:3601–3608. doi: 10.1039/C6NR09632H. [DOI] [PubMed] [Google Scholar]
- 23.Qiao J, Shi K, Wang QM. A giant silver alkynyl cage with sixty silver(I) ions clustered around polyoxometalate templates. Angew. Chem., Int. Ed. 2010;49:1765–1767. doi: 10.1002/anie.200906815. [DOI] [PubMed] [Google Scholar]
- 24.Gao GG, Cheng PS, Mak TCW. Acid-induced surface functionalization of polyoxometalate by enclosure in a polyhedral silver-alkynyl cage. J. Am. Chem. Soc. 2009;131:18257–18259. doi: 10.1021/ja907900b. [DOI] [PubMed] [Google Scholar]
- 25.Su ZH, Zhou BB, Zhao ZF, Zhang X. A novel 1D chain compound constructed from copper-complex fragments-substituted dilacunary beta-octamolybdate units and saturated beta-octamolybdate clusters. Inorg. Chem. Commun. 2008;11:334–337. doi: 10.1016/j.inoche.2008.01.002. [DOI] [Google Scholar]
- 26.Wang JP, Du XD, Niu JY. A novel 1D organic-inorganic hybrid based on alternating heteropolyanions [GeMo12O40]4- and isopolyanions [Mo6O22]8- J. Solid. State Chem. 2006;179:3260–3264. doi: 10.1016/j.jssc.2006.06.014. [DOI] [Google Scholar]
- 27.Dai L, Wang E, You W, Zhang Z. Synthesis and electrochemical properties of a new 1D organic-inorganic hybrid compound based on Keggin-type heteropolyanions and isopolyanions decorated by transition metal fragments. J. Clust. Sci. 2008;19:511–519. doi: 10.1007/s10876-008-0190-9. [DOI] [Google Scholar]
- 28.Cruywagen JJ. Protonation, oligomerization, and condensation reactions of vanadate(V), molybdate(VI), and tungstate(VI) Adv. Inorg. Chem. 2000;49:127–182. doi: 10.1016/S0898-8838(08)60270-6. [DOI] [Google Scholar]
- 29.Hu YQ, et al. Tracking the formation of a polynuclear Co16 complex and its elimination and substitution reactions by mass spectroscopy and crystallography. J. Am. Chem. Soc. 2013;135:7901–7908. doi: 10.1021/ja3123784. [DOI] [PubMed] [Google Scholar]
- 30.Newton GN, Cooper GJT, Koegerler P, Long DL, Cronin L. Trading templates: supramolecular transformations between {CoII13} and {CoII12} nanoclusters. J. Am. Chem. Soc. 2008;130:790–791. doi: 10.1021/ja0768183. [DOI] [PubMed] [Google Scholar]
- 31.Deng YK, et al. Hierarchical assembly of a {MnII15MnIII4} brucite disc: step-by-step formation and ferrimagnetism. J. Am. Chem. Soc. 2016;138:1328–1334. doi: 10.1021/jacs.5b11736. [DOI] [PubMed] [Google Scholar]
- 32.Guo LY, et al. Core-shell {Mn7⊂(Mn,Cd)12} assembled from core {Mn7} disc. J. Am. Chem. Soc. 2017;139:14033–14036. doi: 10.1021/jacs.7b08679. [DOI] [PubMed] [Google Scholar]
- 33.Surman AJ, et al. Sizing and discovery of nanosized polyoxometalate clusters by mass spectrometry. J. Am. Chem. Soc. 2016;138:3824–3830. doi: 10.1021/jacs.6b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cameron JM, et al. Investigating the transformations of polyoxoanions using mass spectrometry and molecular dynamics. J. Am. Chem. Soc. 2016;138:8765–8773. doi: 10.1021/jacs.6b02245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miras HN, et al. Oscillatory template exchange in polyoxometalate capsules: a ligand-triggered, redox-powered, chemically damped oscillation. J. Am. Chem. Soc. 2012;134:6980–6983. doi: 10.1021/ja302861z. [DOI] [PubMed] [Google Scholar]
- 36.Yuan X, et al. Balancing the rate of cluster growth and etching for gram-scale synthesis of thiolate-protected Au25 nanoclusters with atomic precision. Angew. Chem. Int. Ed. Engl. 2014;53:4623–4627. doi: 10.1002/anie.201311177. [DOI] [PubMed] [Google Scholar]
- 37.Luo Z, et al. Toward understanding the growth mechanism: tracing all stable intermediate species from reduction of Au(I)-thiolate complexes to evolution of Au25 nanoclusters. J. Am. Chem. Soc. 2014;136:10577–10580. doi: 10.1021/ja505429f. [DOI] [PubMed] [Google Scholar]
- 38.Yao Q, et al. Precise control of alloying sites of bimetallic nanoclusters via surface motif exchange reaction. Nat. Commun. 2017;8:1555. doi: 10.1038/s41467-017-01736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao Q, et al. Understanding seed-mediated growth of gold nanoclusters at molecular level. Nat. Commun. 2017;8:927. doi: 10.1038/s41467-017-00970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao Q, et al. Revealing isoelectronic size conversion dynamics of metal nanoclusters by a noncrystallization approach. Nat. Commun. 2018;9:1979. doi: 10.1038/s41467-018-04410-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yam VWW, Au VKM, Leung SYL. Light-emitting self-assembled materials based on d8 and d10 transition metal complexes. Chem. Rev. 2015;115:7589–7728. doi: 10.1021/acs.chemrev.5b00074. [DOI] [PubMed] [Google Scholar]
- 42.Barbieri, A., Accorsi, G. & Armaroli, N. Luminescent complexes beyond the platinum group: the d10 avenue. Chem Commun. 0, 2185–2193 (2008). [DOI] [PubMed]
- 43.Wang Z, et al. Johnson solids: anion-templated silver thiolate clusters capped by sulfonate. Chemistry. 2018;24:1640–1650. doi: 10.1002/chem.201704298. [DOI] [PubMed] [Google Scholar]
- 44.Huang RW, et al. Tandem silver cluster isomerism and mixed linkers to modulate the photoluminescence of cluster-assembled materials. Angew. Chem. Int. Ed. Engl. 2018;57:8560–8566. doi: 10.1002/anie.201804059. [DOI] [PubMed] [Google Scholar]
- 45.Wang ZY, et al. Atomically precise site-specific tailoring and directional assembly of superatomic silver nanoclusters. J. Am. Chem. Soc. 2018;140:1069–1076. doi: 10.1021/jacs.7b11338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC: 1837115–1837119 for SD/Ag44, SD/Ag50, and SD/Ag50a-SD/Ag50c. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.