Abstract
The large and complex genome of Pseudomonas aeruginosa, which consists of significant portions (up to 20%) of transferable genetic elements contributes to the rapid development of antibiotic resistance. The whole genome sequences of 22 strains isolated from eye and cystic fibrosis patients in Australia and India between 1992 and 2007 were used to compare genomic divergence and phylogenetic relationships as well as genes for antibiotic resistance and virulence factors. Analysis of the pangenome indicated a large variation in the size of accessory genome amongst 22 stains and the size of the accessory genome correlated with number of genomic islands, insertion sequences and prophages. The strains were diverse in terms of sequence type and dissimilar to that of global epidemic P. aeruginosa clones. Of the eye isolates, 62% clustered together within a single lineage. Indian eye isolates possessed genes associated with resistance to aminoglycoside, beta-lactams, sulphonamide, quaternary ammonium compounds, tetracycline, trimethoprims and chloramphenicols. These genes were, however, absent in Australian isolates regardless of source. Overall, our results provide valuable information for understanding the genomic diversity of P. aeruginosa isolated from two different infection types and countries.
Introduction
The diverse and dynamic genetic composition of Pseudomonas aeruginosa enables this Gram-negative bacterium to colonise various environments, including humans where it can cause opportunistic infections1,2. P. aeruginosa is particularly associated with infections that are caused due to impaired anatomical structures or a weakened immune system. Such infections include microbial keratitis (MK), ventilator-associated pneumonia, wound infections, and respiratory infections in patients suffering from cystic fibrosis (CF)3–5. Several reports have shown that the prevalence of such infections by multidrug-resistant (MDR) strains is increasing rapidly worldwide6–9, which makes this bacterium difficult to treat and hence there is a high risk of mortality associated with infection by P. aeruginosa10. This pathogen has an exceptional capacity to develop resistance to antibiotics by the selection for genomic mutations and by exchange of transferable resistance determinants11. Knowledge of the genomic diversity of P. aeruginosa will help to understand differences in pathogenesis between strains and the mechanism of antibiotic resistance, which is important for controlling infections.
The genome size of P. aeruginosa varies greatly, ranging from 5.5 to 7 Mbp12,13. Such variation arises due to the presence of a large accessory genome. Accessory genomes are strain specific blocks of DNA and can occupy up to 20% of the whole genome14. They are composed of horizontally transferable elements which include prophages, transposons, insertion sequences (IS), genomic islands (GI) and plasmids15. Accessory genomes are important for carrying virulence and acquired antibiotic resistance genes. The lateral transfer of those genes between strains contributes to the development of MDR virulent strains16. Furthermore, mutational changes of chromosomal genes can also contribute to virulence and antibiotic resistance16,17. Therefore, unraveling the genetic content of P. aeruginosa helps to understand the gene modifications that are associated with more pathogenic and more resistant strains. Several studies have reported a comparison between genomes of P. aeruginosa in different infections at various points of time during infections16,18–21. However, most of those studies have centered around CF isolates and there is very limited comparative genomics of ocular isolates of P. aeruginosa.
This study aims to compare the genomic diversity between P. aeruginosa strains from MK and CF isolated in Australia and India. There are previous reports of genomic characterisation of Indian ocular isolates of P. aeruginosa22–24. A genotypic study of eye isolates of P. aeruginosa has shown that keratitis isolates from the UK are highly related25. However, information on genomic comparison amongst contemporary isolates of P. aeruginosa from eye infections in different geographical locations is still missing. This study focussed on 13 MK strains, which were isolated in India and Australia and nine strains from CF cases which were isolated in Australia. The whole genomes of all 22 strains were sequenced and a comparative genomic analysis was conducted to identify genomic divergence, evolutionary relationships, antibiotic resistance properties and virulence factors.
Results and Discussion
General features of genomes
A de novo assembly of the genomes of 22 P. aeruginosa strains generated a number of contigs from 56 in PA175 to 241 in PA37 (Median = 79). Like other published complete genomes of P. aeruginosa1,19,26,27, a mean C + G content of 66.4% and size of 6.1 to 7.1 Mbp was observed in the draft genomes. The genomic size varied widely between strains showing up to 900 kbp more DNA than PAO1, which was taken as the reference strain in this study. Similarly, the number of coding sequences (CDS), which were determined based on Prokka annotation pipeline, ranged from 5584 (in PA92) to 6645 (in PA37). Amongst 82 complete genomes of P. aeruginosa listed in the Pseudomonas genome database (PGDB)28 (accessed on 12/03/2018), PA92 has the lowest and PA37 has the second highest number of CDS. Wide variations in the tRNA copy numbers (65–73) per strain observed here is probably due to use of incomplete draft genome. In addition, different number of tRNAs in the same genome was observed when annotated using different pipelines. Table 1 shows the general features of the genomes.
Table 1.
General features of the genomes of P. aeruginosa strains.
| Strains | Sequence type# | No. of contigs | Length (bp) | GC (%) | CDS | tRNA | Accessory genes## | |
|---|---|---|---|---|---|---|---|---|
| Eye/India | PA31 | 308 | 137 | 7100578 | 66.02 | 6619 | 69 | 1709 |
| PA32 | 308 | 155 | 7101589 | 66.01 | 6611 | 69 | 1701 | |
| PA33 | 308 | 166 | 7092617 | 66.02 | 6609 | 69 | 1699 | |
| PA34 | 1284 | 130 | 6885314 | 65.95 | 6326 | 66 | 1416 | |
| PA35 | 308 | 156 | 7094960 | 66.02 | 6611 | 69 | 1701 | |
| PA37 | 308 | 241 | 7154765 | 65.94 | 6645 | 69 | 1735 | |
| PA82 | 1027 | 64 | 6387501 | 66.51 | 5810 | 65 | 900 | |
| Average number of accessory genes = 1552 | ||||||||
| Eye/Australia | PA17 | New | 60 | 6360710 | 66.45 | 5825 | 72 | 915 |
| PA40 | New | 109 | 6284606 | 66.44 | 5700 | 69 | 790 | |
| PA149 | New | 59 | 6314825 | 66.46 | 5745 | 68 | 835 | |
| PA157 | 386 | 56 | 6249622 | 66.53 | 5708 | 68 | 798 | |
| PA171 | 471 | 60 | 6339342 | 66.49 | 5812 | 69 | 902 | |
| PA175 | 309 | 62 | 6757641 | 66.2 | 6181 | 68 | 1271 | |
| Average number of accessory genes = 919 | ||||||||
| CF/Australia | PA55 | 549 | 77 | 6235554 | 66.57 | 5668 | 67 | 758 |
| PA57 | New | 73 | 6333117 | 66.48 | 5792 | 68 | 882 | |
| PA59 | New† | 78 | 6289887 | 66.55 | 5767 | 68 | 857 | |
| PA64 | 775 | 87 | 6264428 | 66.55 | 5713 | 65 | 803 | |
| PA66 | New† | 93 | 6337310 | 66.51 | 5828 | 68 | 918 | |
| PA86 | New† | 76 | 6170893 | 66.46 | 5685 | 68 | 775 | |
| PA92 | 775 | 81 | 6144573 | 66.59 | 5584 | 65 | 674 | |
| PA100 | 483 | 83 | 6310616 | 66.5 | 5732 | 66 | 822 | |
| PA102 | 1717 | 62 | 6245474 | 66.55 | 5710 | 69 | 800 | |
| Average number of accessory genes = 810 | ||||||||
| PAO1* | 549 | 1 | 6264404 | 66.6 | 5671 | 73 | 761 |
#Sequence types were determined by the multi locus sequence typing database. The sequence types not listed in the MLST database have been deemed as new.
##Accessory genes were determined by subtracting number of core genes (4910) from total number of CDS. †Same MLST allelic profile. *Reference strain.
A total of 9786 orthologs were detected in all 22 strains and the reference strain PAO1. As the pan-genome represents the cumulative genetic information within a set of bacterial genomes, its size increases with the number and diversity of strains used for evaluation. A study that included 17 P. aeruginosa reference strains from diverse sources has found 9344 orthologs in the pangenome29, which is comparable to the results observed here. The higher number of genes in the pan-genome in our study may be the result of the diverse nature of the studied strains. Out of the 9786 pan genes, 4910 genes were common in at least 99% of strains and this represents the core genome for the strains in the current study. Prior studies have reported core genomes of 531630, 523329, 502131, and 493414 in different P. aeruginosa strains. Although the other studies used smaller sets (5 to 17) of genomes, the results are broadly comparable. Many factors may be responsible for the smaller core genomes in the current study including a larger population of genomes used for alignment, use of incomplete draft genomes, the diverse nature of the study populations (ocular and lung; Australian and Indian) and a strict definition of the core genome (≥99% similarity in each strain). For example, pan-genome analysis of the same set of genomes of the current study but excluding PA57 and using ≥95% similarity resulted in 5287 core genes.
In addition to the large core genome, P. aeruginosa has accessory genomes that are not common in all strains15. The accessory genome can comprise of up to 20% of the total genome, and the majority of genes in this accessory genome are acquired horizontally. These genes include phages, transposons, IS and GI14. In the current study, the accessory genes were identified by subtracting the core genes (4910) from the total number of CDS. The frequency of accessory genes was 12% to 26%, which is more than the previously reported size of accessory genome29,32. However, the use of draft genomes may overestimate the number of accessory genes because of the presence of genomic repeats or transposable elements that may interrupt assembly and give an apparently larger genome than this actually present33. Accessory genomes may carry genes that help strains to persist in environments that may be unsuitable for others30. Like many other bacteria, the accessory genomes of P. aeruginosa encompasses genes related to virulence and antibiotic resistance34,35. The presence of a higher number of accessory genes in the set of ocular isolates indicates that eye strains may have acquired many genes to make this opportunistic species suitable to grow in the ocular environment. Furthermore, we examined the number of unique genes amongst accessory genes and found that the functions of the majority of the unique genes are unknown (Fig. 1a).
Figure 1.
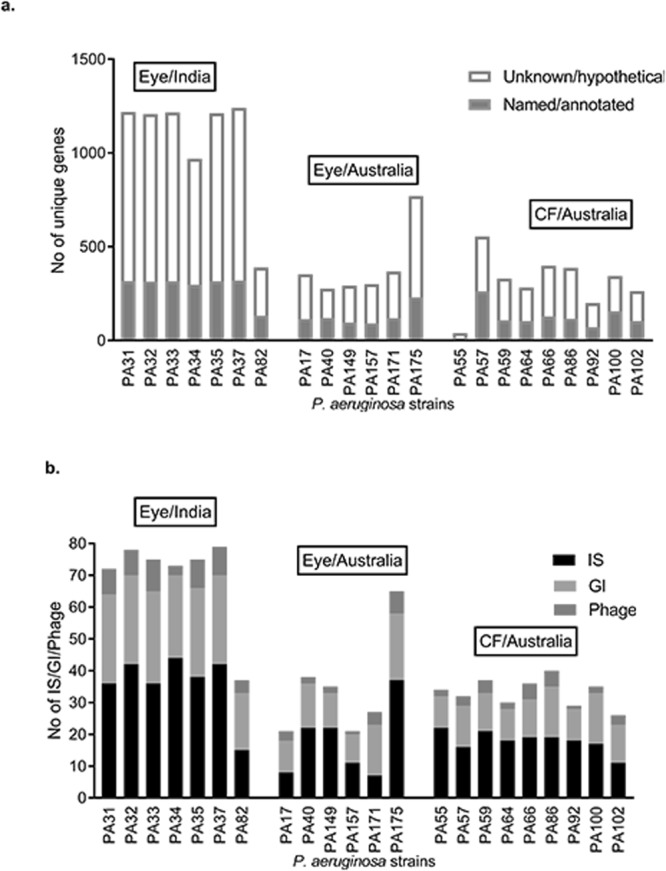
Composition of accessory genomes. (a) Distribution of unique genes. (b) Distribution of predicted no of insertion sequences (IS), genomic islands (GI) and phages.
The genomes were examined for the presence of insertion sequences (IS), genomic islands (GI) and prophages, which are the main elements of an accessory genome15. Contigs of draft genomes were reordered with reference to PAO1 and the ordered contigs were joined together and made into a single FASTA file before examining databases. The results show that the average predicated number of GI was 26 (range 29–18) in Indian eye isolates, which was greater than that of Australian isolates (average 13) irrespective of source. Similarly, the average predicted number of IS and phages were higher in Indian eye isolates (Fig. 1b). Twenty (PA157) to 75 (PA33) total accessory elements were observed in all draft genomes. In contrast, a study has noted 38 to 53 accessory elements that are integrated into 89 potential genomic loci (region of genomic plasticity) in the complete genome of several P. aeruginosa strains14. Complete genomes are required to ascertain the actual number of genes in accessory genome. Nevertheless, the predicted number of IS, GI and phages was well correlated with the size of the accessory genome indicating that they contribute to the genomic diversity as highlighted in other studies15,35,36.
From the pangenome and MLST analysis (below), five Indian strains, isolated from different patients with different histories, were found to be clonal and showed at least 99.98% sequence similarity with each other in MUMmer337 whole genome alignment. To avoid the overestimation of the accessory genome due to the dominance of a single clone, we obtained the nucleotide sequence of five additional Indian eye isolates from public databases22–24 and reran the pan genome analysis. The relative size of the accessory genome to PAO1 was examined (Supplementary Fig. S1). The results tend to show that the eye isolates have larger accessory genome than CF isolates. However, due to limited number of clonally diverse strains of Indian origin, further research on larger datasets is required to confirm this.
Based upon MLST analysis, 16 distinct sequence types (STs) were found, with seven of these constituting new types. The ST was assigned to each strain according to the matched number in the MLST database38. Any strain that did not match with the existing database was deemed to have a new ST. Five Indian ocular isolates (out of seven) belonged to ST 308, two Australian CF isolates corresponded to ST 775 and three Australian CF strains had identical allelic profiles but did not match with any existing ST in the MLST database (all MLST profiles are shown in Supplementary Table S4). The remaining 13 STs were unique, with only a single representative (Table 1). Our results show that these strains belong to a diverse range of STs and are not similar to previously described clinical epidemic isolates39,40. Five strains with ST 308, collected from keratitis patients from the same centre in India, indicate the strains were potentially acquired from the same source where these strains may persist. The most common genotype observed in this study, ST 308, was also reported in MDR hospital strains in France39. Although the MLST database does not contain all P. aeruginosa strains, our observations show the diverse nature of the strains, which were not related to so-called world epidemic STs (ST 235, ST 111, ST 175, ST 395)39. This result also contradicts the previous finding that some keratitis isolates were clonally related with ST 235 CF strains41.
Phylogenetics
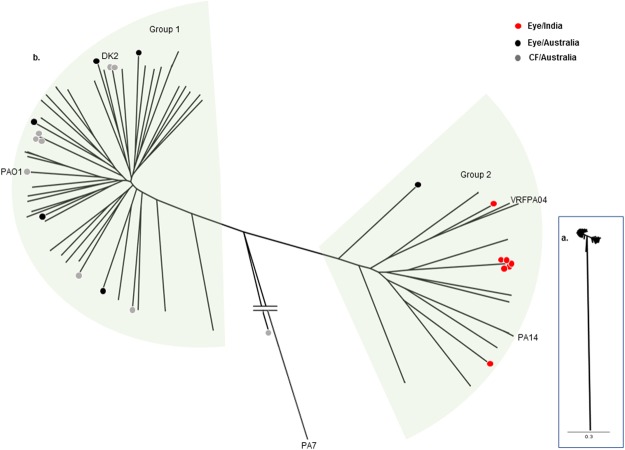
A total of 82 complete genomes of P. aeruginosa including PAO1 were downloaded from the NCBI database and used to compare the phylogenetic diversity of 22 strains from the current study. These 82 strains were listed in PGDB28 as a complete genome and could represent a global P. aeruginosa collection. Core genome alignment was generated using Parsnp of the Harvest Suite with PAO1 as the reference. The alignment was then used to construct a tree following previously described methods, with P. aeruginosa PA7, a taxonomic outliner27, as an outgroup. A multi-sample variant call file was generated from the core genome alignment and SNPs present in all strains were examined (Supplementary Tables S1 and S2). In total 284,252 SNP sites were identified amongst 104 isolates.
All strains, except PA57, were clustered into two groups (Fig. 2). This is in agreement with several studies which have also shown that P. aeruginosa strains from various sources tend to cluster into two major groups42–44, with group 1 being larger, and which contains the most widely studied stains PAO11 and some notable CF strains such as DK2 and LESB5845,46. Group 2 tends to be smaller and includes the well known virulent strain PA1413 and an Indian ocular isolate VRFPA04, a virulent MDR strain24. All seven Indian and one Australian eye isolates were clustered into three sub-lineages within the group 2. A typing-based population structure analysis has also unveiled that keratitis P. aeruginosa strains are closely related25. Furthermore, this supports the finding of the previous study that human P. aeruginosa are less diverse than isolates from the environment47. Similarly, all the CF strains and five Australian eye strains were of group 1 (See Supplementary Table S1 for phylogeny group classification of each strains and associated core genome SNPs). Amongst the CF isolates, continuous mutations have been shown to be an evolutionary process that may make a strain more pathogenic so that they rapidly transfer between humans16,21. However, previous studies have not focussed on ocular isolates. Our analysis showed that more than 60% of eye isolates clustered together in a single group, which is in aggrement with previous findings that 71% of MK isolates of P. aeruginosa from the UK clustered together in the same group48. Further studies should focus on the evolutionary changes in ocular isolates of P. aeruginosa over a prescribed period of time. A CF strain PA57 was in a separate cluster and did not show similarity with other strains. This strain could be another taxonomic outlier of the P. aeruginosa (group 3)44.
Figure 2.
Phylogenetic analysis of Pseudomonas aeruginosa isolates. Maximum likelihood phylogenetic tree built with core genome SNPs based on mapping to the PAO1 excluding SNPs identified in regions that had arisen by recombination. (a) The original tree where the scale bars represent the number of substitutions per site. (b) Magnified tree showing branches and groups. Strains used in this study are indicated by distinct colour circles. Relative positions of few reference strains are shown, which are P. aeruginosa VRFPA04, P. aeruginosa UCBPP-PA14, P. aeruginosa PAO1, and P. aeruginosa DK2.
Antibiotic resistance gene profiles
Horizontally acquired resistance genes were examined using the assembled contigs in the ResFinder database. Altogether, 13 distinct types of acquired resistance genes were detected in this study (Fig. 3). In common with other P. aeruginosa strains24, two beta-lactams (blaOXA-50, blaPAO) and one each for aminoglycoside (aph(3′)-IIb) fosfomycin (fosA) and chloramphenicol (catB7) resistance genes were present in all studied strains. Furthermore, six out of 22 strains had acquired additional resistance genes. Interestingly, all six strains were Indian eye isolates and possessed two aminoglycoside resistance genes (aph(3″)-Ib and aph(6)-Id), one sulphonamide resistance gene (sul1) and one quaternary ammonium compound resistance gene (qacEdelta1). The tetracycline efflux protein transporter gene tet(G) was detected in five Indian eye isolates, all of them are ST 308. An Indian eye strain PA34 possessed four unique resistance genes; blaNPS-1, aac(3)-IIb, dfrA15 and cmlA1 that can confer resistance to beta-lactams, aminoglycosides, trimethoprims and chloramphenicols, respectively. As horizontally acquired resistance genes may be associated with integrons, we analysed all of the draft sequences for the presence of integrons using Integron Finder version 1.5.149. Although sul1 and qacEdelta1 are indicative of class I integrons, only strain PA34 possessed a class 1 integron, in agreement with a recent publication50. The acquired resistance genes detected were comparable to previous observations for an Indian eye isolate of P. aeruginosa24. As all Indian isolates of the current study were from the same centre in India, it is possible that there was antibiotic selection pressure that led to the selection for strains that had acquired such resistance genes from the environment. The absence of such genes in Australian isolates indicates that the antibiotic selection pressure may be different between Australia and India or that the genes associated with resistance are not readily accessible to P. aeruginosa in their local Australian environment. Furthermore, isolates from India were more likely to carry more resistance genes than Australian isolates, potentially reflecting the relatively unregulated use of antibiotics in India compared to Australia51. Antibiotic susceptivity tests also shows that Indian eye isolates were resistance to gentamicin and at least one fluoroquinolone. Resistance to aminoglycoside and fluoroquinolone is however, low in Australian isolates (Table 2).
Figure 3.
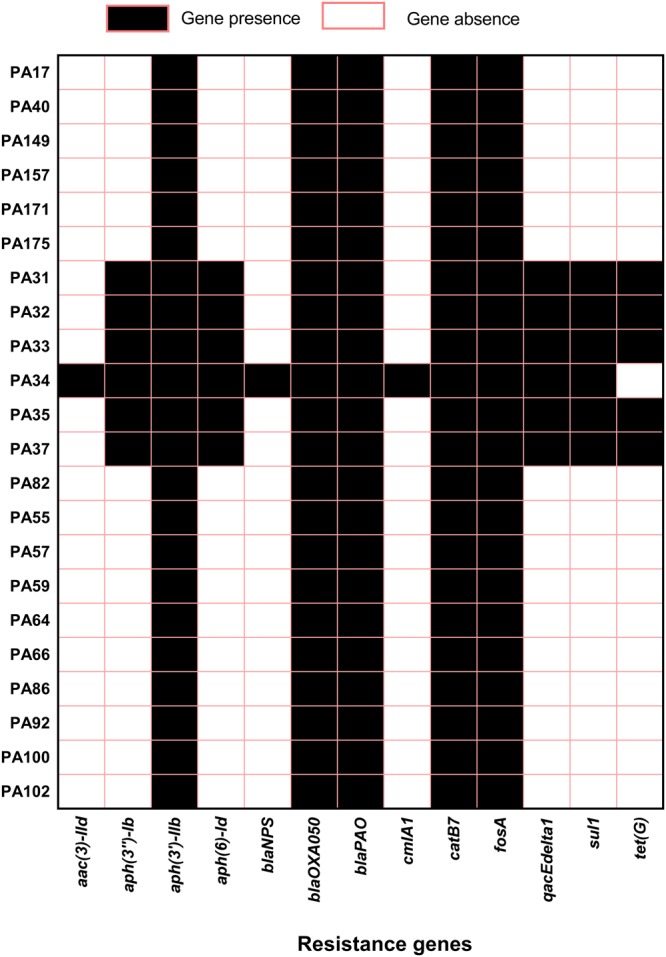
The presence and absence of resistance genes as detected by Resfinder database. Associated resistance: Beta lactams: - blaOXA-50, blaPAO,, blaNPS-1 Aminoglycosides: - aph(3′)-IIb, aph(6)-Id, aph(3″)-Ib, aac(3)-IId Fosfomycin: fosA Sulphonamide: sul1 Chroramphenicol: cmlA1, catB7 Tetracycline: tet(G). Quaternary ammonium compounds: qacEdelta1.
Table 2.
Antibiotic susceptibility profile of P. aeruginosa strains.
| Strains | Antibiotics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gentamicin | Ciprofloxacin | Levofloxacin | Moxifloxacin | Ceftazimidin | Cefepime | Imipenem | Ticarcillin | Aztronam | Polymyxin B | ||
| Eye/India | PA31 | R | R | R | R | I | I | I | I | I | I |
| PA32 | R | R | R | R | I | S | I | I | S | I | |
| PA33 | R | R | R | R | I | I | I | I | S | S | |
| PA34 | R | I | S | R | S | I | R | I | I | S | |
| PA35 | R | R | R | R | I | I | I | I | S | S | |
| PA37 | R | R | R | R | I | S | I | I | S | S | |
| PA82 | I | R | I | S | R | R | S | I | S | R | |
| Eye/Australia | PA17 | S | I | S | R | S | S | S | I | I | I |
| PA40 | S | R | S | S | S | S | I | I | S | S | |
| PA149 | S | S | S | S | I | S | S | S | S | S | |
| PA157 | S | S | S | S | I | S | S | I | S | S | |
| PA171 | S | S | S | S | I | S | I | I | S | S | |
| PA175 | S | I | S | S | I | S | I | I | S | S | |
| CF/Australia | PA55 | R | S | S | S | S | S | I | I | S | S |
| PA57 | R | S | S | S | S | S | S | I | S | S | |
| PA59 | R | S | S | S | S | S | S | I | S | S | |
| PA64 | I | R | R | R | S | R | S | I | S | S | |
| PA66 | R | I | I | R | S | R | S | I | S | S | |
| PA86 | S | S | S | S | I | S | S | I | S | S | |
| PA92 | I | I | I | R | I | R | S | S | I | S | |
| PA100 | I | I | S | R | I | S | S | S | S | S | |
| PA102 | R | I | I | R | I | S | S | S | S | S | |
On the basis of searches in the literature and online databases (Comprehensive Antibiotic Resistance Database (CARD), https://card.mcmaster.ca/home and the Pseudomonas genome database, http://www.pseudomonas.com), a set of 73 genes, which were related to antibiotic and disinfectant resistance in P. aeruginosa, were selected to examine variations in these genes between strains. Only high-quality, non-synonymous SNPs and indels were used for interpretation (Table 3). No insertions or deletions were detected in any of the strains. In terms of the number of SNPs and strains types, all Indian eye isolates and one Australian eye isolate (PA175) had relatively more variations (total SNPs >125) in the set of resistance genes than other strains. However, the CF strain PA57 had an exceptionally high number of SNPs in its resistome. Another CF strain, PA55, did not show any variations in its resistome. In terms of the total SNPs in resistance genes, the least number of variations (≤5 SNPs) were found in five efflux pump-related genes (oprM (5) cycB(1) mexF (4) nalD(5) and nfxB (2)), three target alternation genes (gyrB (5) tufA(2) tufB (0)) and one inactivation gene fosA (3); these are highly conserved genes in P. aeruginosa.
Table 3.
Non-synonymous SNPs detected in the 73 genes related to antibiotic resistance in the 22 isolates studied using PAO1 as the reference genome.
| Gene locus | Gene name | Mechanism | P. aeruginosa strains/number of SNPs | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PA31 | PA32 | PA33 | PA34 | PA35 | PA37 | PA82 | PA17 | PA40 | PA149 | PA157 | PA171 | PA175 | PA55 | PA57 | PA59 | PA64 | PA66 | PA86 | PA92 | PA100 | PA102 | Total SNPs | |||
| PA0156 | triA | Antibiotic efflux | 4 | 5 | 5 | 1 | 6 | 5 | 4 | 1 | 1 | 3 | 1 | 1 | 37 | ||||||||||
| PA0157 | triB | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 | ||||||||||
| PA0158 | triC | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 3 | 1 | 18 | ||||||||||||
| PA0424 | mexR | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 12 | ||||||||||||||
| PA0425 | mexA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | ||||||||||||||||
| PA0426 | mexB | 1 | 1 | 2 | 1 | 4 | 2 | 1 | 1 | 13 | |||||||||||||||
| PA0427 | oprM | 1 | 1 | 1 | 1 | 1 | 5 | ||||||||||||||||||
| PA1236 | farB | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11 | ||||||||||||
| PA1282 | lrfA | 6 | 6 | 9 | 4 | 6 | 8 | 6 | 8 | 5 | 5 | 6 | 4 | 4 | 7 | 3 | 4 | 3 | 3 | 4 | 3 | 4 | 108 | ||
| PA1316 | lrfA | 2 | 2 | 2 | 1 | 2 | 2 | 3 | 3 | 2 | 1 | 3 | 2 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 47 | ||
| PA1435 | mexM | 4 | 4 | 4 | 5 | 4 | 4 | 3 | 4 | 6 | 5 | 6 | 6 | 5 | 8 | 5 | 5 | 5 | 5 | 5 | 4 | 5 | 102 | ||
| PA1436 | mdtC | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 3 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 41 | ||||
| PA1754 | cysB | 1 | 1 | ||||||||||||||||||||||
| PA2018 | mexY | 5 | 5 | 5 | 5 | 5 | 5 | 3 | 1 | 1 | 1 | 4 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 55 | ||
| PA2019 | mexX | 4 | 4 | 4 | 4 | 4 | 4 | 5 | 3 | 4 | 5 | 3 | 3 | 3 | 6 | 3 | 4 | 3 | 3 | 4 | 3 | 4 | 80 | ||
| PA2389 | macA | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 18 | ||||||
| PA2390 | macB | 1 | 1 | 1 | 3 | 1 | 1 | 2 | 1 | 3 | 2 | 3 | 1 | 1 | 3 | 1 | 2 | 1 | 1 | 2 | 1 | 3 | 35 | ||
| PA2391 | opmQ | 6 | 5 | 6 | 4 | 6 | 6 | 4 | 1 | 3 | 2 | 1 | 2 | 4 | 5 | 4 | 5 | 4 | 4 | 5 | 3 | 1 | 81 | ||
| PA2491 | mexS | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 34 | ||
| PA2493 | mexE | 1 | 1 | 1 | 3 | 2 | 1 | 2 | 1 | 1 | 1 | 14 | |||||||||||||
| PA2494 | mexF | 1 | 1 | 2 | 4 | ||||||||||||||||||||
| PA2495 | oprN | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 15 | ||||||||||
| PA2525 | adeC | 3 | 5 | 1 | 1 | 2 | 2 | 2 | 3 | 1 | 20 | ||||||||||||||
| PA2526 | muxC | 1 | 1 | 1 | 1 | 1 | 1 | 6 | |||||||||||||||||
| PA2527 | muxB | 1 | 2 | 1 | 4 | ||||||||||||||||||||
| PA2837 | opmA | 3 | 3 | 3 | 4 | 3 | 3 | 5 | 1 | 1 | 3 | 4 | 6 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 50 | ||||
| PA3019 | taeA | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 4 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 32 | ||
| PA3137 | farB | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 24 | ||||
| PA3521 | opmE | 3 | 3 | 3 | 2 | 3 | 3 | 2 | 3 | 5 | 6 | 4 | 2 | 3 | 11 | 3 | 5 | 3 | 3 | 5 | 3 | 2 | 77 | ||
| PA3522 | mexQ | 4 | 4 | 4 | 4 | 4 | 4 | 6 | 4 | 5 | 2 | 2 | 3 | 3 | 3 | 4 | 6 | 4 | 4 | 5 | 2 | 3 | 80 | ||
| PA3523 | mexP | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 3 | 2 | 2 | 1 | 2 | 3 | 3 | 3 | 2 | 2 | 36 | ||||||
| PA3574 | nalD | 1 | 1 | 3 | 5 | ||||||||||||||||||||
| PA3676 | mexK | 1 | 1 | 1 | 2 | 1 | 1 | 5 | 1 | 2 | 2 | 2 | 3 | 6 | 4 | 2 | 4 | 4 | 2 | 2 | 2 | 48 | |||
| PA3677 | mexJ | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 36 | |||||||
| PA3678 | mexL | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 21 | ||||||
| PA3894 | adeC | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 16 | |||||||||||
| PA4205 | mexG | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 9 | ||||||||||||||||
| PA4206 | mexH | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 9 | |||||||||||||||
| PA4207 | mexI | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | |||||||
| PA4208 | opmD | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 5 | 1 | 1 | 4 | 6 | 1 | 38 | ||||||||||
| PA4374 | mexV | 2 | 2 | 2 | 4 | 2 | 2 | 3 | 2 | 1 | 1 | 2 | 2 | 4 | 1 | 2 | 1 | 1 | 2 | 1 | 3 | 40 | |||
| PA4375 | mexW | 2 | 2 | 2 | 1 | 2 | 2 | 3 | 3 | 1 | 2 | 3 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 32 | ||||
| PA4595 | yjjk | 1 | 1 | 2 | 1 | 2 | 1 | 3 | 1 | 2 | 1 | 1 | 1 | 17 | |||||||||||
| PA4597 | oprJ | 2 | 2 | 2 | 2 | 9 | 3 | 3 | 5 | 3 | 3 | 3 | 37 | ||||||||||||
| PA4598 | mexD | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 1 | 3 | 2 | 9 | 2 | 1 | 2 | 2 | 1 | 2 | 3 | 49 | ||
| PA4599 | mexC | 7 | 8 | 8 | 3 | 8 | 8 | 6 | 4 | 1 | 4 | 1 | 5 | 9 | 1 | 1 | 1 | 4 | 4 | 83 | |||||
| PA4600 | nfxB | 1 | 1 | 2 | |||||||||||||||||||||
| PA4974 | opmH | 2 | 1 | 1 | 1 | 5 | 5 | 1 | 2 | 1 | 5 | 1 | 5 | 1 | 1 | 3 | 4 | 39 | |||||||
| PA4990 | emrE | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | |||||||||||||||
| PA4997 | msbA | 2 | 3 | 3 | 2 | 2 | 3 | 4 | 4 | 3 | 1 | 1 | 1 | 29 | |||||||||||
| PA5158 | adeC | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 1 | 2 | 3 | 5 | 3 | 3 | 3 | 3 | 2 | 1 | 48 | |||||
| PA5160 | farB | 4 | 3 | 3 | 2 | 4 | 3 | 5 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 3 | 3 | 4 | 3 | 65 | |||
| PA5518 | rosB | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 29 | |||||||
| PA0706 | catB7 | Antibiotic inactivation | 4 | 4 | 4 | 5 | 4 | 4 | 3 | 3 | 2 | 2 | 2 | 3 | 4 | 1 | 2 | 4 | 2 | 2 | 4 | 3 | 3 | 65 | |
| PA1129 | fosA | 1 | 2 | 3 | |||||||||||||||||||||
| PA4109 | ampR | 2 | 2 | 2 | 3 | 2 | 2 | 1 | 2 | 2 | 3 | 21 | |||||||||||||
| PA4110 | ampC | 5 | 5 | 5 | 3 | 5 | 5 | 6 | 1 | 2 | 2 | 2 | 2 | 5 | 12 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 69 | ||
| PA4119 | Aph(3′)-IIb | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 4 | 3 | 1 | 1 | 1 | 2 | 31 | ||||||
| PA5514 | OXA-50 | 1 | 2 | 1 | 3 | 2 | 5 | 2 | 3 | 2 | 3 | 3 | 4 | 1 | 3 | 1 | 3 | 4 | 43 | ||||||
| PA0004 | gyrB | Antibiotic target alternation | 1 | 3 | 1 | 5 | |||||||||||||||||||
| PA0903 | alaS | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 13 | |||||||||||
| PA1972 | pmrC | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 1 | 1 | 4 | 1 | 1 | 2 | 3 | 2 | 3 | 2 | 2 | 3 | 4 | 1 | 49 | ||
| PA3002 | mfd | 1 | 2 | 2 | 3 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 27 | ||||||
| PA3168 | gyrA | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 11 | ||||||||||||||
| PA3946 | rosC | 6 | 8 | 8 | 3 | 7 | 6 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 1 | 1 | 2 | 2 | 1 | 56 | ||||
| PA4265 | tufA | 1 | 1 | 2 | |||||||||||||||||||||
| PA4277 | tufB | 0 | |||||||||||||||||||||||
| PA4560 | ileS | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 4 | 2 | 1 | 1 | 2 | 4 | 2 | 4 | 2 | 2 | 4 | 3 | 1 | 47 | ||
| PA4964 | parC | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 13 | |||||||||||||||
| PA4967 | parE | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 18 | |||||||
| PA3553 | pmrF | 3 | 1 | 1 | 1 | 1 | 7 | ||||||||||||||||||
| PA3554 | arnA | 2 | 4 | 4 | 5 | 4 | 4 | 3 | 2 | 3 | 3 | 4 | 6 | 2 | 2 | 3 | 4 | 55 | |||||||
| PA0920 | mprF | 6 | 6 | 6 | 4 | 6 | 6 | 9 | 2 | 1 | 1 | 2 | 7 | 8 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 76 | |||
| Total SNPs | 137 | 140 | 144 | 124 | 146 | 150 | 136 | 82 | 89 | 79 | 83 | 77 | 125 | 0 | 217 | 88 | 109 | 88 | 81 | 101 | 87 | 86 | |||
Virulence genes
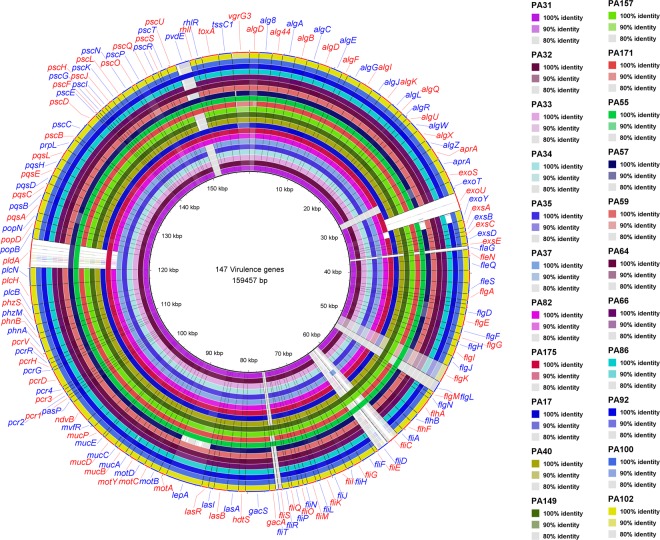
Virulence factors associated with keratitis and cystic fibrosis were selected based on the literature and published sequences in the Virulence Factor Data Base (VFDB)52 to examine the presence or absence of genes related to pathogenicity in the strains. A dataset of 147 virulence genes of PAO1 associated with adherence, protease production, the type IV secretion system, quorum sensing, alginate production/regulation and toxins were curated from VFDB and used in BLAST searches (Fig. 4). For the exoU gene, PA14 was taken as the reference because it is not present in PAO1. All instances where there was an absence of a gene were manually examined with orthologs from the most widely studied strains recommended by the PGDB28. Out of 147 genes, variation in virulence genes were found for 20 genes. This was most evident for a set of effector proteins (toxins) related to the type III secretion system (exoS, exoT, exoU and exoY)53,54. As in previous studies, exoS was predominantly found in CF strains (present in eight out of nine strains) and exoU was primarily found in eye strains (present in eight out of 13 eye isolates)25,55–58. Furthermore, as determined by previous studies, exoU and exoS were mutually exclusive59. However, neither exoU nor exoS was detected in the CF strain PA57. As the exoU gene is carried by genomic islands53,60, exoU possessing strains showed larger accessory genomes and cluster together in the same phylogenetic group. The exoT (100%) and exoY (86%) genes were the most prevalent secretory toxins in the strains and this result is in agreement with previous findings61. In a recent study, exoY (55%) and exoT (5%) were less prevalent than in the current study although the reason for these differences in distribution remains unclear62. One possible reason for this difference is that the study examined genes on the basis of PCR products, which may not be able to capture all different orthologs of genes.
Figure 4.
A circular representation of the genomes of studied isolates. The draft genomes of 22 strains were aligned against the 147 virulence genes curated from VFDB. Each genome is represented by a ring with different colours, which are shown in figure. Image was generated using BRIG (http://brig.sourceforge.net).
Flagellar genes help in the establishment of infections as they can be involved in adherence to surfaces and were also widely variable between strains63. Seven flagellar genes (flgK, flgL, fliC, flaG, fliD, fliS, and fliT) clustered between PA1086 and PA1096 in PAO1 were not matched with those of 19 strains that included both eye and CF isolates. However, these genes from 19 strains showed 90% to 99% similarity with genes between PA7_4275 and PA7_4291 of PA7, orthologs of the above seven flagellar genes. There was low sequence similarity (<50%) for the above flagellar genes between PAO1 and PA7. Studies involving CF isolates have shown that the activity of the fliC gene (that encodes flagellin) had been either downregulated64 or was absent in some strains63. As flagella are immunogenic, the loss of flagella may be an important antiphagocytic mechanism in chronic infection isolates65. Although it has been shown that non-flagellated strains are defective in acute infections65, 85% of eye isolates in this study had altered flagellar genes that may affect flagellar function. Previous work has shown that although fliC contributes to invasion of P. aeruginosa in eye infections, a lack of fliC did not cause complete loss of invasion66. Further studies will need to clarify the functionality of those flagellar genes on studied strains and their role in ocular P. aeruginosa infections.
A phospholipase D gene (pldA), a part of the type VI secretion system of P. aeruginosa is believed to promote chronic infections67,68. However, pldA was absent from 13 isolates, seven of which were CF isolates and yet over 50% eye isolates had pldA. Reports on the role of pldA in eye infections have not been published and this should be an area of future study. Another notable variation was observed in pvdE, a precursor for pyoverdin synthesis, which is essential for virulence of P. aeruginosa69,70. Eight strains, irrespective of their source of isolation, had a PAO1 homolog of pvdE. Similarly, DK2 and LES homologs of pvdE were equally distributed in 14 strains (Table 4) suggesting that these orthologs are evenly distributed in P. aeruginosa populations. PvdE can increase invasion of P. aeruginosa by inducing expression of the exoS71. Further studies will help understand role of pvdE variants in pathogenesis.
Table 4.
Distribution of pvdE orthologs among strains.
| Strains | pvdE orthologs (locus tag) |
|---|---|
| PA31, PA32, PA33, PA34, PA35, PA37 and PA175 | P. aeruginosa DK2 (DK2_13280) |
| PA82, PA17, PA171, PA175, PA55, PA57, PA64, and PA92 | P. aeruginosa PAO1 (PA2397) |
| PA40, PA149, PA59, PA66, PA86, PA100 and PA102 | P. aeruginosa LESB58 (PALES_28991) |
Conclusions
This study compared the genomic variations between Australian and Indian P. aeruginosa isolates from ocular infections. P. aeruginosa isolates from various sources showed diversity in the size of accessory genome, antibiotic resistance genes and virulence factors. We found a slightly smaller core genome than has been reported previously. Although all 22 strains were distributed throughout the global phylogeny of P. aeruginosa, certain clusters were observed in the eye isolates where five Indian eye isolates were clustered into a single clonal lineage in the group which also contains a well-studied and virulent strain PA14. Larger accessory genomes were associated with eye isolates of this group. Furthermore, the strains of this group had more SNPs in their set of 73 resistome suggesting possible positive antibiotic selection pressure. Variation in virulence factors, except for exoU, was not correlated with phylogeny. This study relied on draft genomes and may not be able to predict actual genomic diversity because the analysis could not ascertain the presence of the plasmids in any of the isolates. Further studies will focus on improvement of the assembly of these genomes. Overall, these findings have extended our understanding of the genomic diversity of P. aeruginosa in two different infections and information can be used to elucidate various mechanism that would help fight against virulent and drug resistant strains.
Methods
Bacterial strains and antibiotic susceptibility tests
Twenty two clinical isolates of P. aeruginosa from corneas of microbial keratitis and from the lungs of CF patients were selected for this study. Seven ocular isolates were obtained from a tertiary eye care centre in India (L.V. Prasad Eye Institute, Hyderabad, India), six ocular and nine CF isolates were acquired from various sources in Australia. All strains were collected from institutional repositories between 1992 and 2007 without identifiable patient data and all experiments followed the institutional guidelines, which were in place at the time (Table 5). Genome sequence data of an additional 82 P. aeruginosa strains, based on availability of complete genome sequence in Pseudomonas genome database (PGDB) version 17.228 including P. aeruginosa PAO1 (reference strain) were collected from public databases and used in this study to compare results and to build phylogenetic trees (all the reference strains used in this study are listed in Supplementary Table S1). The minimum inhibitory concentrations (MICs) of ceftazidime, cefepime, aztreonam, ticarcillin, imipenem, gentamicin, levofloxacin, ciprofloxacin, moxifloxacin and polymyxin were determined by broth microdilution according to CLSI guidelines and published standard breakpoints72–74.
Table 5.
List of strains used in this study.
| Strains | Collection date# | Geographical location | Associated infections |
|---|---|---|---|
| PA31 | 02/10/1997 | LVPEI, Hyderabad, India | Microbial Keratitis |
| PA32 | 08/10/1997 | LVPEI, Hyderabad, India | Microbial Keratitis |
| PA33 | 29/08/1997 | LVPEI, Hyderabad, India | Microbial Keratitis |
| PA34 | 28/08/1997 | LVPEI, Hyderabad, India | Microbial Keratitis |
| PA35 | 09/08/1997 | LVPEI, Hyderabad, India | Microbial Keratitis |
| PA37 | 11/07/1997 | LVPEI, Hyderabad, India | Microbial Keratitis |
| PA82 | 11/05/2004 | LVPEI, Hyderabad, India | Microbial Keratitis |
| PA17 | 15/09/1992 | Flinders, Adelaide, Australia | Microbial Keratitis |
| PA40 | 02/02/1999 | SEH, Sydney, Australia | Microbial Keratitis |
| PA149 | 04/03/2004 | Flinders, Adelaide, Australia | Microbial Keratitis |
| PA157 | 29/04/2006 | PAH, Brisbane, Australia | Microbial Keratitis |
| PA171 | 16/03/2006 | PAH, Brisbane, Australia | Microbial Keratitis |
| PA175 | 07/10/2006 | PAH, Brisbane, Australia | Microbial Keratitis |
| PA55 | 2003 | RPAH, Sydney, Australia | Cystic Fibrosis |
| PA57 | 2003 | RPAH, Sydney, Australia | Cystic Fibrosis |
| PA59 | 2003 | RPAH, Sydney, Australia | Cystic Fibrosis |
| PA64 | 2003 | RPAH, Sydney, Australia | Cystic Fibrosis |
| PA66 | 2003 | RPAH, Sydney, Australia | Cystic Fibrosis |
| PA86 | 2004 | RPAH, Sydney, Australia | Cystic Fibrosis |
| PA92 | 2004 | RPAH, Sydney, Australia | Cystic Fibrosis |
| PA100 | 2004 | RPAH, Sydney, Australia | Cystic Fibrosis |
| PA102 | 2004 | RPAH, Sydney, Australia | Cystic Fibrosis |
LVPEI = LV Prasad Eye Institute; Flinders = Flinders University, SEH = Sydney Eye Hospital; PAH = Princes Alexandra Hospital; RPAH = Royal Prince Alfred Hospital CF Clinic, Sydney, Australia.
#All cystic fibrosis isolates were obtained from Royal Prince Alfred Hospital CF Clinic, Sydney, Australia, between 2003 and 2004. Information on exact date of collection is missing in our record.
Whole genome sequencing
Genomic DNA was extracted from overnight cultures using the DNeasy® Blood and Tissue Kit (QIAGEN®, Germany) following the manufacturer’s instructions. The paired-end library was prepared using Nextera XT DNA library preparation kit (Illumina®, San Diego, CA, USA). Libraries were then sequenced on Illumina® MiSeq bench top sequencer (Illumina), generating 300 bp paired-end reads. All of the libraries were multiplexed on one MiSeq run.
Genome assembly and sequence analysis
The MiSeq sequencing resulted an average of 760,773 reads (range 632,180 to 1,193,844) per isolate. FastQC version 0.11.7 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc) was used to assess the quality of raw reads, which were then quality trimmed to remove adaptor sequences using Trimmomatic version 0.36 and with the setting of minimum read length of 36 and minimum coverage of 1575. A de novo assembly was performed by SPAdes version 3.11.176. with the default setting. The annotations of the assembled genomes were performed using Prokka version 1.7. using GenBank® compliance flag77. The genome of P. aeruginosa PAO1 (RefSeq accession number NC_002516.2), which was used as the reference in this study, was re-annotated with Prokka to avoid annotation bias. Whenever necessary, the contigs of the draft genomes were reordered and/or aligned with the reference genome using MAUVE multiple-genome alignment software78,79. Artemis, a genome browser tool80, was used to concatenate the ordered contigs to get a single fragment of genomes which were used to examine insertion sequence using web tool ISsaga (http://issaga.biotoul.fr/ISsaga2/issaga_index.php), genomic islands using IslandViewer 481 and prophages using PHASTER82 Multi locus sequence type (MLST) was determined using pubMLST database38 to find sequence type (ST) of each strain.
Pan-genome and Phylogenetics
The pangenome analysis was performed using Roary version 3.12.083 which uses the GFF3 files produced by Prokka. The program was run using the default settings, which uses BLASTp for all-against-all comparison with a percentage sequence identity of 95%. Core-genes were taken as the genes which were common in at least 99% of strains. The accessory genome was obtained as the genes present in the genome of each strain minus core genes. The Roary “gene_presence_absence.csv” file was further examined for unique genes using “union” and “difference” command. Parsnp version 1.2 in the Harvest Suite84 was used to align the genomes of 104 P. aeruginosa strains (82 complete genomes from the PGDB and 22 draft genomes from this study), followed by the construction of a maximum likelihood tree based on core genome single nucleotide polymorphisms (SNPs), excluding SNPs identified in regions that had arisen by recombination.
Variant calling
The paired-end reads for each isolate were aligned against the genome of the P. aeruginosa PAO1 using Bowtie2 version 2.3.285 following “score-min” command to avoid alignments that score less than the default minimum score threshold and with “local” flag for better score. Genomic variants were compiled using “mpileup” in SAMtools, version 1.786. A minimum quality score of 50 was set to list the SNPs and Indels. The genomic variants were annotated using SnpEff version 4.387 with the default options to obtain the nucleotide changes and the predicted effects at the protein level.
Antibiotic resistance and virulence genes
Genomes were examined for the presence of acquired resistance genes using Resfinder 3.0 (Centre for Genomic Epidemiology, DTU, Denmark)88. Furthermore, a set of 73 genes related to antibiotic and disinfectant resistance in P. aeruginosa were selected from searches in the online databases Comprehensive Antibiotic Resistance Database (CARD) (https://card.mcmaster.ca/home)89 and Pseudomonas genome database (http://www.pseudomonas.com)28. These 73 genes were manually examined for the presence of non-synonymous SNPs to predict genotypic changes in the resistome (see Supplementary Table S3).
A dataset of 146 virulence genes of PAO1 and one virulence gene (exoU) of Pseudomonas aeruginosa UCBPP-PA14 (NC_008463.1) associated with adherence (flagella), protease production, type IV secretion system, quorum sensing, alginate production/regulation and toxins were curated from the Virulence Factor Data Base (VFDB)90 and used in BLAST searches to match them with the genomes of the strains studied here. BLAST Ring Image Generator (BRIG)91 was used to generate an image that shows presence or absence of virulence genes in multiple genomes. (List of virulence genes used is shown in Supplementary Table S4). The contigs were joined together before searching them in BRIG to avoid false matching due to fragmented genomes. The absence of a gene in this analysis was confirmed by manual BLASTn searching using orthologs from a widely-studied panel of P. aeruginosa suggested by PGDB. These include PA14, P. aeruginosa LESB58 (NC_011770.1), P. aeruginosa PA7 (NC_009656.1) and P. aeruginosa DK2 (CP003149.1).
Nucleotide accession
The nucleotide sequences are available in the GenBank under the Bioproject accession number PRJNA431326.
Electronic supplementary material
Acknowledgements
The authors would like to acknowledge the Singapore Centre for Environmental Life Sciences Engineering (SCELSE), whose research is supported by the National Research Foundation Singapore, Ministry of Education, Nanyang Technological University and National University of Singapore, under its Research Centre of Excellence Programme. Sequencing of DNA was carried out with the help of Daniela Moses and Stephan Schuster using the sequencing facilities at SCELSE. We are also thankful to UNSW high performance computing facility KATANA for providing us cluster time for data analysis.
Author Contributions
D.S. designed the study, performed the experiments, analysed the data and wrote the drafts of the article. A.K.V. supervised D.S. and edited the article. G.S.K. helped with computational analyses and design of experiments. S.A.R. contributed to the design and implementation of the research, to the analysis of the results and edited the article. M.W. devised the project, developed the theoretical framework, edited the article.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34020-7.
References
- 1.Stover CK, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 2.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton F, Carnt N. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye (Lond) 2012;26:185–193. doi: 10.1038/eye.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaez H, et al. Efflux pump regulatory genes mutations in multidrug resistance Pseudomonas aeruginosa isolated from wound infections in Isfahan hospitals. Adv Biomed Res. 2014;3:117. doi: 10.4103/2277-9175.133183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding C, et al. Prevalence of Pseudomonas aeruginosa and antimicrobial-resistant Pseudomonas aeruginosa in patients with pneumonia in mainland China: a systematic review and meta-analysis. International Journal of Infectious Diseases. 2016;49:119–128. doi: 10.1016/j.ijid.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Gad GF, El-Domany RA, Zaki S, Ashour HM. Characterization of Pseudomonas aeruginosa isolated from clinical and environmental samples in Minia, Egypt: prevalence, antibiogram and resistance mechanisms. J Antimicrob Chemother. 2007;60:1010–1017. doi: 10.1093/jac/dkm348. [DOI] [PubMed] [Google Scholar]
- 8.Kouda S, et al. Increased prevalence and clonal dissemination of multidrug-resistant Pseudomonas aeruginosa with the blaIMP-1 gene cassette in Hiroshima. J Antimicrob Chemother. 2009;64:46–51. doi: 10.1093/jac/dkp142. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud, A., Zahran, W., Hindawi, G., Labib, A. & Galal, R. Prevalence of multidrug-resistant Pseudomonas aeruginosa in patients with nosocomial infections at a University hospital in Egypt, with special reference to typing methods. Journal of Virology & Microbiology, 1–13, 10.5171/2013.290047 (2013).
- 10.Pena C, et al. Prospective multicenter study of the impact of carbapenem resistance on mortality in Pseudomonas aeruginosa bloodstream infections. Antimicrob Agents Chemother. 2012;56:1265–1272. doi: 10.1128/AAC.05991-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis. 2002;34:634–640. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt KD, Tummler B, Romling U. Comparative genome mapping of Pseudomonas aeruginosa PAO with P. aeruginosa C, which belongs to a major clone in cystic fibrosis patients and aquatic habitats. J Bacteriol. 1996;178:85–93. doi: 10.1128/jb.178.1.85-93.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DG, et al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 2006;7:R90. doi: 10.1186/gb-2006-7-10-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klockgether J, Cramer N, Wiehlmann L, Davenport CF, Tummler B. Pseudomonas aeruginosa Genomic Structure and Diversity. Front Microbiol. 2011;2:150. doi: 10.3389/fmicb.2011.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kung VL, Ozer EA, Hauser AR. The accessory genome of Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2010;74:621–641. doi: 10.1128/MMBR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeukens J, et al. Comparative genomics of isolates of a Pseudomonas aeruginosa epidemic strain associated with chronic lung infections of cystic fibrosis patients. PLoS One. 2014;9:e87611. doi: 10.1371/journal.pone.0087611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashish A, Shaw M, Winstanley C, Ledson MJ, Walshaw MJ. Increasing resistance of the Liverpool Epidemic Strain (LES) of Pseudomonas aeruginosa (Psa) to antibiotics in cystic fibrosis (CF)–a cause for concern? J Cyst Fibros. 2012;11:173–179. doi: 10.1016/j.jcf.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 18.De Soyza A, et al. Developing an international Pseudomonas aeruginosa reference panel. Microbiologyopen. 2013;2:1010–1023. doi: 10.1002/mbo3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeukens, J., Kukavica-Ibrulj, I., Emond-Rheault, J. G., Freschi, L. & Levesque, R. C. Comparative genomics of a drug-resistant Pseudomonas aeruginosa panel and the challenges of antimicrobial resistance prediction from genomes. FEMS Microbiol Lett364, 10.1093/femsle/fnx161 (2017). [DOI] [PubMed]
- 20.Klockgether J, et al. Intraclonal diversity of the Pseudomonas aeruginosa cystic fibrosis airway isolates TBCF10839 and TBCF121838: distinct signatures of transcriptome, proteome, metabolome, adherence and pathogenicity despite an almost identical genome sequence. Environ Microbiol. 2013;15:191–210. doi: 10.1111/j.1462-2920.2012.02842.x. [DOI] [PubMed] [Google Scholar]
- 21.Marvig RL, Johansen HK, Molin S, Jelsbak L. Genome analysis of a transmissible lineage of Pseudomonas aeruginosa reveals pathoadaptive mutations and distinct evolutionary paths of hypermutators. PLoS Genet. 2013;9:e1003741. doi: 10.1371/journal.pgen.1003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aggarwal, R. K., Dawar, C., Das, S. & Sharma, S. Draft genome sequences of two drug-resistant isolates of Pseudomonas aeruginosa obtained from keratitis patients in India. Genome Announc3, 10.1128/genomeA.01404-14 (2015). [DOI] [PMC free article] [PubMed]
- 23.Murugan N, Malathi J, Umashankar V, Madhavan HN. Resistome and pathogenomics of multidrug resistant (MDR) Pseudomonas aeruginosa VRFPA03, VRFPA05 recovered from alkaline chemical keratitis and post-operative endophthalmitis patient. Gene. 2016;578:105–111. doi: 10.1016/j.gene.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Murugan N, Malathi J, Umashankar V, Madhavan HN. Unraveling genomic and phenotypic nature of multidrug-resistant (MDR) Pseudomonas aeruginosa VRFPA04 isolated from keratitis patient. Microbiol Res. 2016;193:140–149. doi: 10.1016/j.micres.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Stewart RM, et al. Genetic characterization indicates that a specific subpopulation of Pseudomonas aeruginosa is associated with keratitis infections. J Clin Microbiol. 2011;49:993–1003. doi: 10.1128/JCM.02036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyoshi-Akiyama T, Kuwahara T, Tada T, Kitao T, Kirikae T. Complete genome sequence of highly multidrug-resistant Pseudomonas aeruginosa NCGM2.S1, a representative strain of a cluster endemic to Japan. J Bacteriol. 2011;193:7010. doi: 10.1128/JB.06312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy PH, et al. Complete genome sequence of the multiresistant taxonomic outlier Pseudomonas aeruginosa PA7. PLoS One. 2010;5:e8842. doi: 10.1371/journal.pone.0008842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winsor GL, et al. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 2016;44:D646–653. doi: 10.1093/nar/gkv1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valot B, et al. What It Takes to Be a Pseudomonas aeruginosa? The Core Genome of the Opportunistic Pathogen Updated. PLoS One. 2015;10:e0126468. doi: 10.1371/journal.pone.0126468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozer EA, Allen JP, Hauser AR. Characterization of the core and accessory genomes of Pseudomonas aeruginosa using bioinformatic tools Spine and AGEnt. BMC Genomics. 2014;15:737. doi: 10.1186/1471-2164-15-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathee K, et al. Dynamics of Pseudomonas aeruginosa genome evolution. Proc Natl Acad Sci USA. 2008;105:3100–3105. doi: 10.1073/pnas.0711982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pohl S, et al. The extensive set of accessory Pseudomonas aeruginosa genomic components. FEMS Microbiol Lett. 2014;356:235–241. doi: 10.1111/1574-6968.12445. [DOI] [PubMed] [Google Scholar]
- 33.Ricker N, Qian H, Fulthorpe RR. The limitations of draft assemblies for understanding prokaryotic adaptation and evolution. Genomics. 2012;100:167–175. doi: 10.1016/j.ygeno.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Ho Sui SJ, Fedynak A, Hsiao WWL, Langille MGI, Brinkman FSL. The Association of Virulence Factors with Genomic Islands. PLoS ONE. 2009;4:e8094. doi: 10.1371/journal.pone.0008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen K, et al. Extensive genomic plasticity in Pseudomonas aeruginosa revealed by identification and distribution studies of novel genes among clinical isolates. Infect Immun. 2006;74:5272–5283. doi: 10.1128/iai.00546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy Chowdhury Piklu, Scott Martin, Worden Paul, Huntington Peter, Hudson Bernard, Karagiannis Thomas, Charles Ian G., Djordjevic Steven P. Genomic islands 1 and 2 play key roles in the evolution of extensively drug-resistant ST235 isolates ofPseudomonas aeruginosa. Open Biology. 2016;6(3):150175. doi: 10.1098/rsob.150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurtz Stefan, Phillippy Adam, Delcher Arthur L, Smoot Michael, Shumway Martin, Antonescu Corina, Salzberg Steven L. Genome Biology. 2004;5(2):R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jolley KA, Maiden MC. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cholley P, et al. Most multidrug-resistant Pseudomonas aeruginosa isolates from hospitals in eastern France belong to a few clonal types. J Clin Microbiol. 2011;49:2578–2583. doi: 10.1128/JCM.00102-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen SH, Chen RY, Xu XL, Chen HT. Multilocus sequencing typing of Pseudomonas aeruginosa isolates and analysis of potential pathogenicity of typical genotype strains from occupational oxyhelium saturation divers. Undersea Hyperb Med. 2014;41:135–141. [PubMed] [Google Scholar]
- 41.Hall AJ, et al. Intraclonal genetic diversity amongst cystic fibrosis and keratitis isolates of Pseudomonas aeruginosa. J Med Microbiol. 2013;62:208–216. doi: 10.1099/jmm.0.048272-0. [DOI] [PubMed] [Google Scholar]
- 42.Stewart L, et al. Draft genomes of 12 host-adapted and environmental isolates of Pseudomonas aeruginosa and their positions in the core genome phylogeny. Pathog Dis. 2014;71:20–25. doi: 10.1111/2049-632X.12107. [DOI] [PubMed] [Google Scholar]
- 43.Kos VN, et al. The resistome of Pseudomonas aeruginosa in relationship to phenotypic susceptibility. Antimicrob Agents Chemother. 2015;59:427–436. doi: 10.1128/AAC.03954-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freschi L, et al. Clinical utilization of genomics data produced by the international Pseudomonas aeruginosa consortium. Front Microbiol. 2015;6:1036. doi: 10.3389/fmicb.2015.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiehlmann L, Cramer N, Tummler B. Habitat-associated skew of clone abundance in the Pseudomonas aeruginosa population. Environ Microbiol Rep. 2015;7:955–960. doi: 10.1111/1758-2229.12340. [DOI] [PubMed] [Google Scholar]
- 46.Rau MH, Marvig RL, Ehrlich GD, Molin S, Jelsbak L. Deletion and acquisition of genomic content during early stage adaptation of Pseudomonas aeruginosa to a human host environment. Environ Microbiol. 2012;14:2200–2211. doi: 10.1111/j.1462-2920.2012.02795.x. [DOI] [PubMed] [Google Scholar]
- 47.Winstanley C, et al. Newly introduced genomic prophage islands are critical determinants of in vivo competitiveness in the Liverpool Epidemic Strain of Pseudomonas aeruginosa. Genome Res. 2009;19:12–23. doi: 10.1101/gr.086082.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shankar J, et al. Genotypic analysis of UK keratitis-associated Pseudomonas aeruginosa suggests adaptation to environmental water as a key component in the development of eye infections. FEMS Microbiol Lett. 2012;334:79–86. doi: 10.1111/j.1574-6968.2012.02621.x. [DOI] [PubMed] [Google Scholar]
- 49.Cury J, Jove T, Touchon M, Neron B, Rocha EP. Identification and analysis of integrons and cassette arrays in bacterial genomes. Nucleic Acids Res. 2016;44:4539–4550. doi: 10.1093/nar/gkw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subedi Dinesh, Vijay Ajay Kumar, Kohli Gurjeet Singh, Rice Scott A, Willcox Mark. Nucleotide sequence analysis of NPS-1 β-lactamase and a novel integron (In1427)-carrying transposon in an MDR Pseudomonas aeruginosa keratitis strain. Journal of Antimicrobial Chemotherapy. 2018;73(6):1724–1726. doi: 10.1093/jac/dky073. [DOI] [PubMed] [Google Scholar]
- 51.Laxminarayan R, et al. Access to effective antimicrobials: a worldwide challenge. The Lancet. 2016;387:168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 52.Chen L, et al. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Research. 2005;33:D325–D328. doi: 10.1093/nar/gki008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato H, et al. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 2003;22:2959–2969. doi: 10.1093/emboj/cdg290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yahr TL, Goranson J, Frank DW. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol Microbiol. 1996;22:991–1003. doi: 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi S, et al. Genotypic analysis of Pseudomonas aeruginosa isolated from ocular infection. J Infect Chemother. 2014;20:407–411. doi: 10.1016/j.jiac.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 56.de Almeida Silva KCF, et al. Molecular characterization of multidrug-resistant (MDR) Pseudomonas aeruginosa isolated in a burn center. Burns. 2017;43:137–143. doi: 10.1016/j.burns.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Georgescu M, et al. Virulence and resistance features of Pseudomonas aeruginosa strains isolated from chronic leg ulcers. BMC Infect Dis. 2016;16(Suppl 1):92. doi: 10.1186/s12879-016-1396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tingpej P, et al. Phenotypic characterization of clonal and nonclonal Pseudomonas aeruginosa strains isolated from lungs of adults with cystic fibrosis. J Clin Microbiol. 2007;45:1697–1704. doi: 10.1128/JCM.02364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berthelot P, et al. Genotypic and phenotypic analysis of type III secretion system in a cohort of Pseudomonas aeruginosa bacteremia isolates: evidence for a possible association between O serotypes and exo genes. J Infect Dis. 2003;188:512–518. doi: 10.1086/377000. [DOI] [PubMed] [Google Scholar]
- 60.Kulasekara BR, et al. Acquisition and evolution of the exoU locus in Pseudomonas aeruginosa. J Bacteriol. 2006;188:4037–4050. doi: 10.1128/JB.02000-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feltman H, et al. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology. 2001;147:2659–2669. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- 62.Azimi S, et al. Presence of exoY, exoS, exoU and exoT genes, antibiotic resistance and biofilm production among Pseudomonas aeruginosa isolates in Northwest Iran. GMS Hyg Infect Control. 2016;11:Doc04. doi: 10.3205/dgkh000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feldman M, et al. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun. 1998;66:43–51. doi: 10.1128/iai.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolfgang MC, Jyot J, Goodman AL, Ramphal R, Lory S. Pseudomonas aeruginosa regulates flagellin expression as part of a global response to airway fluid from cystic fibrosis patients. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6664–6668. doi: 10.1073/pnas.0307553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67:159–173. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 66.Fleiszig SMJ, Arora SK, Van R, Ramphal R. FlhA, a Component of the Flagellum Assembly Apparatus of Pseudomonas aeruginosa, Plays a Role in Internalization by Corneal Epithelial Cells. Infect Immun. 2001;69:4931–4937. doi: 10.1128/iai.69.8.4931-4937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spencer C, Brown HA. Biochemical characterization of a Pseudomonas aeruginosa phospholipase D. Biochemistry. 2015;54:1208–1218. doi: 10.1021/bi501291t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilderman PJ, Vasil AI, Johnson Z, Vasil ML. Genetic and biochemical analyses of a eukaryotic-like phospholipase D of Pseudomonas aeruginosa suggest horizontal acquisition and a role for persistence in a chronic pulmonary infection model. Mol Microbiol. 2001;39:291–303. doi: 10.1046/j.1365-2958.2001.02282.x. [DOI] [PubMed] [Google Scholar]
- 69.McMorran BJ, Merriman ME, Rombel IT, Lamont IL. Characterisation of the pvdE gene which is required for pyoverdine synthesis in Pseudomonas aeruginosa. Gene. 1996;176:55–59. doi: 10.1016/0378-1119(96)00209-0. [DOI] [PubMed] [Google Scholar]
- 70.Meyer JM, Neely A, Stintzi A, Georges C, Holder IA. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okuda J, et al. Complementation of the exoS gene in the pvdE pyoverdine synthesis gene-deficient mutant of Pseudomonas aeruginosa results in recovery of the pvdE gene-mediated penetration through the intestinal epithelial cell barrier but not the pvdE-mediated virulence in silkworms. Journal of Infection and Chemotherapy. 2012;18:332–340. doi: 10.1007/s10156-011-0340-0. [DOI] [PubMed] [Google Scholar]
- 72.Clinical and Laboratory Standards Institute(CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition. CLSI32 (2012).
- 73.Clinical and Laboratory Standards Institute(CLSI). Performance standards for antimicrobial susceptibility testing; twenty-second information supplement. CLSI document M100-S2232 (2012).
- 74.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 6.0: The European Committee on Antimicrobial Susceptibility Testing, http://www.eucast.org/clinical_breakpoints/ (2016).
- 75.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bankevich A, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 78.Darling AE, Mau B, Perna N. T. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One. 2010;5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rissman AI, et al. Reordering contigs of draft genomes using the Mauve aligner. Bioinformatics. 2009;25:2071–2073. doi: 10.1093/bioinformatics/btp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carver T, et al. Artemis and ACT: viewing, annotating and comparing sequences stored in a relational database. Bioinformatics. 2008;24:2672–2676. doi: 10.1093/bioinformatics/btn529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bertelli C, et al. IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017;45:W30–W35. doi: 10.1093/nar/gkx343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arndt D, et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44:W16–21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Page AJ, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Treangen TJ, Ondov BD, Koren S, Phillippy AM. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014;15:524. doi: 10.1186/PREACCEPT-2573980311437212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cingolani P, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zankari E, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jia B, et al. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017;45:D566–d573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen L. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Research. 2004;33(Database issue):D325–D328. doi: 10.1093/nar/gki008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.