Abstract
Agrilus mali (Coleoptera: Buprestidae) is an invasive wood borer pest that has caused considerable damage to the Xinjiang wild fruit forest. In this study, we investigated the bacterial and fungal intestinal microbial communities of A. mali during different developmental stages, including larvae, pupae and newly eclosed adults or fed different diets (leaves of Malus halliana and Malus pumila) using Illumina MiSeq high-throughput sequencing technology. The results showed that microbial alpha diversity first increased and then decreased during the developmental stages, with the most dominant bacteria and fungi exhibiting the dynamic patterns “Decrease”, “Increase” and “Fluctuation”. With respect to the different diets, the bacterial communities were similar between the newly eclosed adults and adults fed M. pumila leaves, while the structure of the fungal communities showed great differences between newly eclosed adults and adults fed different diets. Through a co-correlation network analysis, we observed complex microbial interactions among bacterial and fungal taxa that were associated with potential diverse functions and intricate biological processes in the intestinal microbiota of A. mali. Overall, the results of this study demonstrated that the invasive insect A. mali harbours diverse, dynamic, and presumably multifunctional microbial communities, an understanding of which could improve our ability to develop more effective management approaches to control A. mali.
Subject terms: Bacterial development, Microbiome
Introduction
Agrilus mali (Coleoptera: Buprestidae) is an invasive wood borer that is listed as a quarantine pest in China1, where it primarily attacks Malus pumila Mill, Crataegi cuneatae, Malus spectabilis, Prunus persica, Prunus armeniaca and other economically important fruit trees2. A. mali was first imported from Shandong Province to the Ili Kazakh autonomous prefecture in 1993, resulting in great financial losses from damage to economically important apple trees3. In particular, A. mali has caused extensive damage to the Xinjiang wild fruit forest tree Malus siversii, which is an endangered key and priority protected species in China4. During its long larval stage, A. mali larvae form crooked galleries in the phloem and cambium of trees by tunnelling under the bark. Two larvae can lead to the death of a 4-cm diameter tree branch, while twenty larvae can cause the death a tree with a 15-cm diameter1. In contrast, although A. mali adults feed on the leaves of host plants, they have a small appetite and often do not cause very severe damage. A. mali females prefer to lay eggs on tree trunks, in bark crevices and on buds on the sunny side of trees, with one female being able to oviposit 60–70 eggs over its lifespan. In recent years, the area damaged by A. mali in the wild fruit forest of Tianshan Mountain has rapidly increased from the original 33 hm2 to 4,866 hm2, with the affected area accounting for more than half of the total wild fruit forest4.
Insect guts harbour diverse microorganisms that have integral roles in organismal functions, including regulating the metabolism of hosts; promoting efficient digestion to allow the maximum amount of energy to be extracted from ingested foods; aiding in the detoxification of harmful compounds; developing and maintaining the immune system of insects; and protecting hosts from potentially harmful microbes5,6. Associations between microorganisms and insect hosts are widespread in nature and result from the co-evolution between the microbes and hosts to generate obligate symbioses7,8. Studies on caterpillars of the cabbage white butterfly revealed that their gut microbial community is dominated by common environmental taxa9. Additionally, the gut bacterial diversity of diverse termites and cockroaches showed that host phylogeny is an important factor that determines gut microbial composition10. Hosting bacteria can promote specific nutritional complementation for organisms living on a markedly imbalanced diet11,12. Insect gut microorganisms are recognized to originate from the environment and diet5. For example, the acquisition of nutrition-providing bacteria in mosquito larvae is likely dependent on the presence of a particular species present in the larval habitat or the ingestion of specific bacterial species by larvae throughout their development13. In addition, host ecological niches and feeding habits can influence the host microbial community and have important roles in shaping the gut microbial community5,6,14,15.
The relationships between phytophagous insects and their parasitic/mutualistic microbes have long been investigated to gain an understanding their evolutionary diversification. In one instance, indigenous facultative or obligate mutualistic microbes are associated with several species of phytophagous insect families, including the plant-feeding insect families Chrysomelidae (Coleoptera), Curculionidae (Coleoptera), plant-galling Cecidomyiidae (Diptera) and all plant-feeding hemipteran families16. Symbiotic microorganisms in termites provide essential ecosystem functions by digesting cellulosic materials (wood, litter, and humus) to promote soil formation and nutrient cycling10. Bacterial endosymbionts are thought to be beneficial to insects by providing essential amino acids and vitamins, recycling carbon sources and defending against enemies17. Although fungal mutualisms are not as prevalent in phytophagous insects compared with bacterial mutualisms18,19, they play important roles in insect development and fitness by providing nitrogen compounds, degrading high molecular weight molecules and producing pheromones for mating and communication20.
Recently, the use of high-throughput next-generation sequencing technologies has provided a better and more comprehensive understanding of insect intestinal microbiotas. This technique can detect significantly higher diversity in microbial populations than traditional culture-based and conventional molecular methods6. Using high-throughput sequencing technologies, specific studies on bacterial and fungal communities have been performed for many insects, including termites, ants, fire bugs, fruit flies, beetles and bees, but rarely for Buprestidae insects21–27. One of the few studies to do so characterized gut the microbial communities of Agrilus planipennis via a 16S rRNA gene-based clone library profiling analysis, which suggested that the invasive insect harbours a diverse, dynamic, and presumably multifunctional microbial communities that should be viewed as multispecies complexes28. Another study on tissue-specific gene expression in A. planipennis demonstrated that a high number of the midgut sequences encoded chitin-binding peritrophin and trypsin domains, while sequences obtained from fat bodies encoded a high number of cytochrome P450 and protein kinase domains29.
In this study, investigated the bacterial and fungal communities in A. mali in detail via Illumina MiSeq sequencing. Specifically, we addressed the following objectives: 1) revealing the dynamic changes in bacterial and fungal communities over the course of the developmental stages of A. mali from natural habitats; 2) characterizing the bacterial and fungal community diversity in adult A. mali fed two different diets; and 3) exploring the distribution and assembly of the core A. mali gut microbiota.
Results
Distribution of taxa and phylotypes
After the quality filtering and the removal of chimeric sequences, the entire A. mali gut microbiota sequencing dataset included 416,877 high-quality bacterial sequences (bacterial V3-V4 rRNA gene region) and 117,145 high-quality fungal sequences (fungal ITS2 region) for five groups, including wild larvae (Lar), pupae (Pup) and newly eclosed adults (EcA), and the lab-reared adults fed either the leaves of M. halliana (MhaA) or M. pumila (MpuA) (Table 1). The total number of bacterial and fungal OTUs identified was 20,704 and 1,969, respectively, defined at a 97% sequence similarity (Table 1). Rarefaction curves suggested that the majority of the bacterial and fungal taxa were recovered (see Fig. S1 in supplementary material). Among the bacterial OTUs, 99.02% (20,502 OTUs) were assigned to 39 phyla, 96 classes, 144 orders, 202 families, 334 genera and 216 species. Among the fungal OTUs, 89.54% (1,763 OTUs) were assigned to 5 phyla, 21 classes, 53 orders, 125 families, 255 genera and 423 species. In general, the number of bacterial OTUs was larger than that of fungi. Four bacterial phyla, including Proteobacteria, Actinobacteria, Firmicutes and Bacteroidetes were predominant (relative abundance >1%), and accounted for 77.42% of the total sequences. The phylum Proteobacteria, with a relative abundance of 70.68%, was represented by the classes Gammaproteobacteria (46.69%), Alphaproteobacteria (16.30%) and Betaproteobacteria (6.20%). In particular, Pup contained large number of specific phyla, including Caldithrix, Chlamydiae, Elusimicrobia, Gemmatimonadetes, Planctomycetes, Nitrospirae and Verrucomicrobia, which were not detected or showed low relative abundances in the other samples. At the fungal phylum level, Ascomycota and Basidiomycota were the predominant phyla, with relative abundances of 73.11% and 16.63%, respectively.
Table 1.
Microbial community alpha-diversity characteristics in the guts of Agrilus mali at different developmental stages and fed different diets.
| Sample ID | Tag number | Alpha diversity indices | ||||
|---|---|---|---|---|---|---|
| OTU richness | Shannon-Wiener | Chao 1 | ACE | Coverage | ||
| Bacteria | ||||||
| Lar | 86,187 | 6,608 | 5.01 | 15,366.31 | 23,082.41 | 98.42% |
| Pup | 86,176 | 10,861 | 5.31 | 46,041.04 | 103,172.74 | 98.28% |
| EcA | 72,284 | 1,198 | 1.33 | 2,408.08 | 3,583.91 | 90.28% |
| MhaA | 83,728 | 2,140 | 2.14 | 4,987.25 | 8,717.10 | 95.46% |
| M.puA | 88,502 | 2,542 | 2.19 | 5,784.24 | 9,240.62 | 99.08% |
| Fungus | ||||||
| Lar | 6,862 | 433 | 3.86 | 793.00 | 1354.11 | 91.82% |
| Pup | 8,367 | 650 | 4.24 | 1,344.11 | 2071.72 | 95.32% |
| EcA | 91,179 | 541 | 1.74 | 976.76 | 1321.88 | 95.79% |
| MhaA | 3,216 | 447 | 4.36 | 985.33 | 1693.34 | 96.72% |
| MpuA | 7,521 | 642 | 4.29 | 1,320.86 | 1957.63 | 99.72% |
Microbial alpha and beta diversity in the guts of A. mali
The microbial alpha diversity indices, including the OTU richness (i.e., richness) and the Shannon-Wiener index (i.e., Shannon) were estimated in the guts of A. mali during three developmental stages and for insects fed two different diets (Fig. 1A–D). Across different developmental stages, the microbial alpha diversity showed a trend of first increasing and then decreasing. The Pup group showed highest OTU richness and Shannon-Wiener index for both bacterial and fungal communities. For the bacterial communities, the alpha diversity indices were lowest in the EcA group, while for the fungal communities, the OTU richness and Shannon-Wiener index was lowest in the Lar and EcA groups, respectively. Considering the effect of different diets, the MpuA group (adults fed M. pumila leaves) showed a higher bacterial Shannon-Wiener index than that of the MhaA group (adults fed M. halliana leaves), and the bacterial OTU richness differed little between the two gut microbiotas. In contrast, the fungal OTU richness was higher in the MpuA group than that observed in the MhaA group, whereas the Shannon-Wiener index differed little between the two gut microbiotas.
Figure 1.
Microbial diversity patterns for A. mali gut microbiotas. Alpha-diversity in bacterial (A,C) and fungal (B,D) gut communities of A. mali gut. Beta-diversity in bacterial (E) and fungal (F) gut communities estimated via principal coordinate analysis (PCoA) based on Bray-Curtis distance.
Principal coordinate analysis (PCoA) based on the Bray-Curtis distance (Fig. 1E,F) showed different results between the bacterial and fungal communities of the gut microbiota in A. mali. For the bacterial community, the microbiota varied in succession across the developmental stages, where the MpuA and EcA groups clustered together away from the MhaA group. For the fungal community during the different A. mali development stages, the Pup and Lar groups clustered together and were separated from the EcA group. In contrast, in the treatment of A. mali with different diets, the MpuA and MhaA groups clustered together and were separated from the EcA group.
The observed differences in beta-diversity resulted from the variations in community structure of the gut microbiotas (Supplementary Fig. S2). The relative abundance of Proteobacteria decreased along with the development stages and was much higher in the MhaA group (above 95%) than in the MpuA and EcA groups. The relative abundance of Ascomycota increased along with the development stages, reaching 98% in the EcA group. Greater differences were observed at the class level among the gut microbiotas (Supplementary Fig. S3). Gammaproteobacteria represented the predominant taxa in the phylum Proteobacteria, showing a consistent trend with that of Proteobacteria, while the fungal class Eurotiomycetes and phylum Ascomycota exhibited similar trends. Comparatively, the relative abundance of Alphaproteobacteria was highest in EcA group, and Betaproteobacteria were most abundant in the Pup group. The relative abundance of Actinobacteria was lowest in the MhaA group compared with that observed in the other microbiotas. Additionally, the relative abundances of Bacilli were 5.32% and 6.27% in the Lar and Pup groups, respectively but were very low in the EcA, MhaA and MpuA groups. In contrast, the fungal taxa Dothideomycetes and Sordariomycetes were the most abundant in the EcA group compared with those observed in the other samples In addition, the fungal taxa Wallemiomycetes, Tremellomycetes, Saccharomycetes and Agaricomycetes contributed a substantial fraction of reads in the Lar and Pup groups, but were rare in the EcA group. Moreover, the bacterial community structure in the EcA group was more similar to that of the MpuA group than the MhaA group, and the fungal community structure in the MhaA and MpuA groups were similar and different from that observed in the EcA group.
Gut microbial dynamic patterns during the development of A. mali
To explore the dynamic patterns of gut microbial communities during the development of A. mali, a cluster analysis was performed for the datasets obtained from the three developmental stages. As we focused on the abundant taxa, the 40 genera with the highest relative abundances were selected for this analysis. Clustering of these genera into three groups indicated that bacterial and fungal taxa showed similar dynamic patterns, including “Decrease”, “Increase” and “Fluctuation” patterns (Fig. 2). “Decrease” indicates microbes that were high in abundance in larvae; “Increase” indicates microbes that increased in abundance from larvae and pupae to eclosing adults; and “Fluctuate” represents microbes that were high in abundance in pupae. During the development of A. mali, the abundances of the bacterial taxa Erwinia, Pseudomonas, Acinetobacter, Klebsiella, Leuconostoc and Agrobacterium decreased continuously; Stenotrophomonas, Methylotenera, Serratia, Anoxybacillus, Sphingomonas and Staphylococcus first increased and then decreased; and Corynebacterium, Propionibacterium, Caulobacter, Rhodoplanes, Treponema, and Kocuria increased continuously. For the fungal taxa, Wallemia, Candida, Asterotremella, Cladosporium and Verticillium decreased continuously; Penicillium, Exophiala, Cryptococcus, Alternaria, Mortierella and Fusarium first increased and then decreased; and Aspergillus, Phoma, Trichothecium, Acremonium, Rhodotorula and Westerdykella increased continuously. In general, our results may indicate the presence of microbial community succession in the gut microbiota during the development of A. mali.
Figure 2.
Dynamic patterns of bacterial and fungal communities during the development of A. mali gut microbiotas, as determined using cluster analysis. The 40 genera with the highest relative abundances were selected and clustered into three groups on the basis of similar profiles (displayed as heatmaps). Each row in the heatmap has been standardized to have a mean of zero and a standard deviation of one. The intensity of the colour in the heatmap is proportional to the standardized relative abundances of the taxa.
The effect of different diets on the assembly of the A. mali gut microbiota
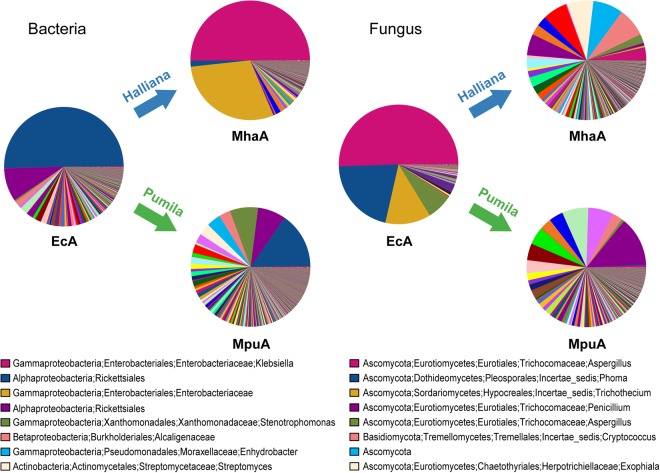
The effect of different diets on the assembly of the A. mali gut microbiota was assessed based on the observed OTUs (Fig. 3). In bacterial communities, the predominant OTUs in the EcA groups were also dominant in the MpuA group, which primarily belonged to the taxon Rickettsiales, whereas the dominant OTUs in the MhaA group primarily belonged to the taxa Klebsiella and Enterobacteriaceae, different from that observed for EcA. Some OTUs became abundant in the MpuA group compared with the EcA group, which were assigned to the genera Stenotrophomonas, Enhydrobacter and Streptomyces. For fungal communities, the predominant OTUs in the EcA group were not abundant in the MpuA and MhaA groups. In addition, the community compositions between the MpuA and MhaA groups were similar, but the relative abundances of the OTUs were differed greatly. For example, the taxa Cryptococcus podzolicus, Exophiala salmonis, Aspergillus cibarius, Stachybotrys microspora, Fusarium and Archaeorhizomyces were dominant in the MhaA group, while the taxa Penicillium citrinum, Penicillium menonorum, Candida, Russula, Penicillium commune and Trichosporon asahii were abundant in the MpuA group. Thus, these observations confirmed that the bacterial community structure in the EcA group was more similar with that of the MpuA group than the MhaA group, whereas the fungal community structures of the MhaA and MpuA groups were similar but differed from that of the EcA group.
Figure 3.
Pie charts of the bacterial and fungal OTU abundances in gut microbial communities of A. mali fed different diets of Halliana and Pumila leaves. The taxonomic information of the OTUs with the six highest relative abundances are displayed in the legends.
Co-correlation network among bacterial and fungal taxa
The correlation network was generated to unravel the microbial co-occurrence patterns among the bacterial and fungal taxa in the gut microbiota of A. mali (Fig. 4). In general, the network consisted of 36 nodes and 35 edges (with 21 positive edges and 14 negative edges). These nodes were distributed into four bacterial phyla, including Proteobacteria, Actinobacteria, Firmicutes and Bacteroidetes, and three fungal phyla, including Basidiomycota, Ascomycota and Chytridiomycota. We observed complex interactions among bacterial and fungal taxa. For example, Coniothyrium was negatively correlated with Erwinia, Pseudomonas and Anoxybacillus; Gibberella was positively correlated with Methyloversatilis and Pseudomonas and Anoxybacillus; Tomentella was linked by positive edges with Fusarium, Emericella, Stachybotrys, Nigrospora and Pyrenochaeta; and Leucoagaricus was linked by positive edges with Serratia, Paracoccus and Enhydrobacter.
Figure 4.
Co-correlation network of microbial bacteria and fungus genera in the A. mali gut microbiota. A connection indicates for Spearman’s correlation with a coefficient >0.6 (positive correlation, red edges) or <−0.6 (negative correlation, blue edges) and a significant (P < 0.01) correlation. The size of each node is proportional to the relative abundance. Diamonds indicate bacteria, and triangles indicate fungus. The nodes were coloured by phylum.
Core microbiota taxa of A. mali
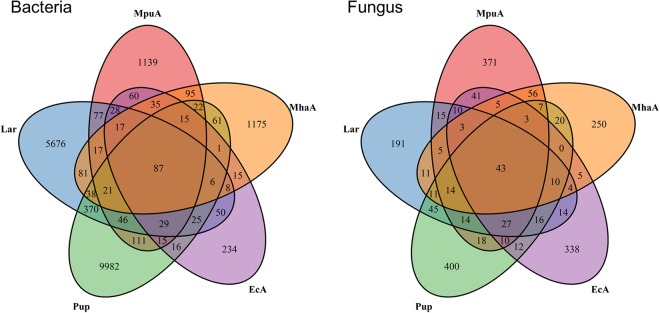
The persistent microbial taxa in the gut microbiota of A. mali were identified based on the OTUs present in all five samples (Fig. 5). A total of 87 and 43 OTUs were identified and defined as core taxa in the bacterial and fungal communities, respectively, accounting for low proportions of the total OTUs (means = 3.37% and 8.16% for bacteria and fungi, respectively). In contrast, these core taxa accounted for a substantial fraction of the reads (means = 44.43% and 59.56% for bacteria and fungi, respectively; Supplementary Fig. S4). The bacterial core taxa were primarily assigned to Klebsiella, Stenotrophomonas, Serratia, Enhydrobacter, Achromobacter, Corynebacterium, Micrococcus and Acinetobacter; and the fungal core taxa primarily belonged to Aspergillus, Wallemia, Phoma, Candida, Penicillium, Cryptococcus, Cladosporium and Fusarium (Supplementary Table S1 and S2).
Figure 5.
Venn diagram of the bacterial and fungal OTUs in different gut microbiotas of A. mali.
Discussion
Insects harbour diverse gut microorganisms that participate in many different activities, including the degradation of lignocellulose; the production of nutrients, vitamins and components of cohesion pheromones; nitrogen fixation and utilization of nitrogenous waste products; protection against parasites; the promotion of changes in body colouration; and sterol synthesis15,28–31. Although accumulating studies have described the microbial diversity in the insect gut, especially in Coleoptera insects15,28,30,32–37, to date, no report had described changes in A. mali gut microbial communities during successive life stages and in response to different diets. In the present study, we provide a novel description of the intestinal microbiota related to this apple buprestid via Illumina MiSeq high-throughput sequencing of the bacterial 16S rDNA gene and fungal ITS gene regions.
Microbiota richness and diversity of A. mali Gut
In general, the annotation results revealed that A. mali contained a larger number of bacterial than fungal taxa. Similar results have been reported in members of the families Passalidae and Elateridae, while members of the family Cerambycidae have shown a high diversity of fungi and a moderate diversity of bacteria38. A previous study demonstrated that the coverage of bacterial gut communities is often sufficient between individuals, while fungal diversity is generally underestimated30. Additionally, we observed that the dominant gut microbial communities in A. mali only occupied a relatively low numbers, consistent with previous findings in pea aphids and red palm weevils39,40. Across the different A. mali development stages, pupae showed the highest microbial diversity in gut microbial communities, a result that was also observed for the guts of the emerald ash borer28. Insect gut microorganisms are recognized to originate from the environment and diet of a given species5. The high gut microbial diversity observed in A. mali pupae may have resulted from the vast microbial influx occurring via a specific nutritional complementation from a markedly imbalanced diet11–13. Another explanation is that the sequencing effort might cause a greater number of transient, non-specific bacteria to be present in pupae, since the pupae lose much of their core gut bacteria during the process of pupation.
Compared with the newly eclosed adults, A. mali adults fed different diets showed increased diversity in gut microbiota. We hypothesized that feeding might stimulate the growth of microbiota and provide new taxa from the diet to restore the community richness in the insect gut41. However, our results showed that there were substantially more unique OTUs that shared OTUs between any two life stages, which seemed odd given the life history of this insect and might indicate contamination of our samples. Thus, more stringent procedures for controlling contamination will be conducted in our future research.
The comprehensive community analysis across the developmental stages of A. mali and in insects fed different diets revealed that four bacterial phyla, including Proteobacteria, Actinobacteria, Firmicutes and Bacteroidetes, and two fungal phyla, including Ascomycota and Basidiomycota, were predominant. This result is supported by a previous study is similar to that of a previous study showing that Proteobacteria and Firmicutes were the predominant bacterial phyla in 81 insect gut samples6. Essentially, species belonging to all major bacterial taxa have been isolated from bark beetles, including Alphaproteobacteria, Bacteriodetes, Firmicutes, Betaproteobacteria, Gammaproteobacteria and Actinobacteria42. The dominant fungal phyla observed in A. mali is consistent with previous observations of a high diversity of Basidiomycota and Ascomycota isolates from Cerambycidae, Passalidae, Elateridae and the Hemiptera insect genus Dactylopius20,38. The assembly of these communities in insect alimentary tract microbiota could be determined by the physiological and biochemical conditions in this environment, such as gut morphology, pH and oxygen availability5,43.
Gut microbiota variation during different developmental stages
According to the PCoA analysis, the gut microbiota varied in succession across the A. mali developmental stages, with pupae being clearly separated from larvae and newly eclosed adults. Compared with larvae and adults, the pupal gut undergoes a decrease in metabolic activity and undergoes morphological changes during the insect metamorphosis that may influence the associated microbial communities36.
A complex microbial consortium is considered fundamental for normal survival and complete development of insects from larvae to adults13. During the development of A. mali, the dominant gut bacterial and fungal communities primarily exhibited three dynamic patterns, including “Decrease”, “Increase” and “Fluctuation”. For the bacterial community, cellulolytic bacteria, including members of the genera Erwinia, Pseudomonas, Acinetobacter, Klebsiella, Leuconostoc and Agrobacterium were more abundant in xylophagous A. mali larvae, which feed on phloem and cambium28. Interestingly, Klebsiella has been used in cellulose degradation and fermentation processes44. In addition, Klebsiella sp. are recognized as recurrent diazotrophs that contribute to beetle nitrogen requirements and sometimes are closely related to pathogenic bacteria obtained from the environment32,33. Pseudomonas has been isolated in most Coleoptera insects due to its encoded cellulolytic enzyme capacity, which may be involved in terpene transformation of plant resin compounds36,45,46. For the fungal community, a continuous decrease in the fungal taxa Wallemia, Candida, Asterotremella, Cladosporium and Verticillium were also observed to be abundant in larvae. Ascomycetous yeast of the genus Candida and basidiomycetous yeast of the genus Asterotremella were shown to produce xylanase and carboxymethyl cellulose in insects and their natural habit47. Wallemia and Cladosporium are very common environmental fungal genera that associate with many plants as commensals and pathogens48. These microbial groups are widespread, ecologically diverse, and functionally redundant in organic matter decomposition and lignocellulose digestion and may be transiently acquired by insects from feeding materials and the environment10.
Some other bacteria and fungi showed “Increase” patterns during developmental stages that were relatively abundant in newly eclosed adults. The abundances of the bacterial genera Propionibacterium and Kocuria were extremely high in newly eclosed adults, which has been observed in the guts of other insect, e.g., the bark beetle Dendroctonus rhizophagus, A. planipennis and the hornworm Manduca sexta32,36. Interestingly, Propionibacterium, which was identified in A. mali adults, was reported to cause skin acne in humans15. For the fungal community, genera in the classes Dothideomycetes, Sordariomycetes, Eurotiomycetes were primarily detected in newly eclosed adults. These groups are involved in organic matter decomposition and are saprobes or endophytes that associate with plants, arthropods, and mammals10. The observed “Fluctuation” pattern of microbiota included the bacterial genera Stenotrophomonas, Serratia, Sphingomonas and Staphylococcus and the fungal genera Penicillium, Cryptococcus and Fusarium. Stenotrophomonas has been described to protect hosts against an entomopathogenic fungus in the fly Stomoxys calcitrans. Sphingomonas is known as an endosymbiont of ticks14,15. Serratia is often isolated from the guts of insects in the wood-feeding Coleoptera order, possessing potential characteristics related to the degradation of lignocellulose and xylan and for fermentative metabolism34,38. Staphylococcus is commonly present in the environment and is a symbiont in the guts of many insect, including Drosophila melanogaster, Culex quinquefasciatus, Analeptes trifasciata and Asobara tabida46,49. Cryptococcus and Penicillium, yeast-like symbionts, were reported to be involved in uric acid (UA) catabolism via turning UA into amino acids for insects20. In addition, Fusarium was observe to be associated with amino acid salvage and the recycling of nitrogenous waste products35. The evolutionary innovation of holometaboly also created distinct niches for colonization by distinct microbial symbionts. Over the holometabolous life cycle of the host, variation in diet and internal physicochemical conditions could support communities functionally specialized for a particular life stage41.
Gut microbiota variation with different diets
Gut microbial communities can be influenced by host diet, as they adapt to dietary changes through the induction of enzyme production and changes in community structure30. The bacterial communities were similar between the newly eclosed adults and adults fed M. pumila leaves, whereas great differences in fungal community structures were observed between newly eclosed adults and adults fed either diet. This result indicates that bacterial taxa inhabiting the gut of A. mali are more adapted to M. pumila leaves and that the fungal community is more easily affected by different diets.
In the bacterial community, the predominance of the order Rickettsiales in the EcA group was also observed in the MpuA group but not the MhaA group, in which Klebsiella and Enterobacteriaceae were dominant. In the present study, larvae were fed cambium and phloem, while adults were fed foliage, possibly indicating that the bacterial community was conserved in larvae and adults (MpuA) regardless the shift in diet after metamorphosis. Previous studies have demonstrated that dietary influences on adult gut microbiota were obscured by colonization history33,50. Because adult A. mali ingest large amounts of foliage, which contains potentially useful and harmful microbes associated, it makes sense for the host to efficiently control its gut microbiota and quickly eliminate invading microbes51. Rickettsiales species are widespread insect symbionts that have mutualistic fitness effects in their hosts and commonly cause pathogenicity or reproductive alterations15,32. Members of the family Enterobacteriaceae are often dominant taxa in insect guts due to their various abilities, including hydrolysing and fermenting carbohydrates, catalysing nitrogen fixation, and producing vitamins and pheromones33. In addition, shifts in diet may be beneficial to the colonization of Enterobacteriaceae taxa, which often showed strong adaptability.
For the fungal communities, the predominant OTUs in the EcA group were not abundant in the MpuA and MhaA groups, and the community compositions between the MpuA and MhaA groups were similar differed greatly in abundance. The feeding behaviour of A. mali adults may explain the similar fungal composition between the MpuA and MhaA groups10. Both groups were fed leaves of different plant species collected from the same location, and many insects derive their gut microbiota from the surrounding environment, including the phylloplane of food plants43.
Complex interactions among gut microbiota in A. mali
Microorganisms form complex interaction webs within specific ecological niches, and understanding the interactions among microorganisms is important to explore the complexity of functional processes52. In the present study, we observed complex interactions among bacterial and fungal microbes. The bacterial genera Corynebacterium and Kocuria were observed to be positively correlated in the A. mali gut, two genera that were previously observed to co-occur in the bacterial gut microbiota of the bark beetle53. With respect to fungi in the A. mali gut, Tomentella was positively correlated with Fusarium, Emericella, Stachybotrys, Nigrospora and Pyrenochaeta, suggesting that they potentially share similar ecological niches in the intestinal microenvironment. More intricate linkages were observed between bacterial and fungal microbes. In general, there were 12 positive and 10 negative edges between taxa of these two groups. Interactions among microorganisms may be associated with specific functions possessed by gut microbiota. In the co-correlation network, Coniothyrium, reported as an antimicrobial fungus54, was negatively correlated with the bacterial genera Erwinia, Anoxybacillus and Pseudomonas. Kocuria, which was negatively correlated with the fungal genera Archaeorhizomyces and Trichoderma, has been observed to have strong antifungal activity55. Overall, the complexity of microbial interactions was associated with multiple functions of gut microbiota, indicating that intricate biological processes exist in intestinal microenvironment of A. mali.
Core taxa associated with potential gut microbiota functions in A. mali
In the A. mali gut microbiota, we identified only a few core taxa, which accounted for a substantial fraction of the total reads. Their persistence in the gut microbiota during different development stages and with different diets suggest that they may be important for maintaining microbial diversity and intestinal health in A. mali56. Some of specific enzymatic degrading activities of these core bacteria are thought to have important roles in the intestinal microenvironment of A. mali, including the detoxification of plant compounds, the production of metabolites against pathogens, and plant-insect interactions. For example, Stenotrophomonas and some other Proteobacterial bacteria were reported to have cellulose and/or aromatics degradation capabilities57,58. Members of the fungal genus Fusarium are abundant members in the A. mali gut, similar to the A. glabripennis larval midgut and are capable of secreting numerous plant cell wall degrading enzymes, detoxification enzymes, and laccases with potential involvement in lignin degradation35,59. Insects tolerant microbial intruders both inside and outside their bodies, and symbiotic microbiota simultaneously supply enzymes that generate metabolites for host utilization. The presence of core microbiota in the gut indicated that there may well be core microbial functions in healthy individuals56. In a previous study, insects were observed to produce a large number of transcripts predicted to break down plant cell-wall carbohydrates, whereas the gut microbial community expressed numerous transcripts predicted to be involved in the assimilation and fermentation of wood sugars35. Moreover, the gut microbiota in Anoplophora glabripennis can also contribute to nitrogen availability by recycling nitrogenous waste products that are reincorporated into both essential and non-essential amino acids60.
Conclusion
In this study, we comprehensively investigated the gut microbiota of A. mali during development and in adults fed different diets obtained from the same locality. During development, most dominant bacteria and fungi showed the dynamic patterns of “Decrease”, “Increase” and “Fluctuation”. The bacterial communities were similar between the newly eclosed adults and adults fed M. pumila leaves, whereas the structure of fungal communities from these two groups exhibited large differences between newly eclosed adults and adults fed with different diets. These results indicated that bacterial taxa in the A. mali gut were more adapted for M. pumila leaves, whereas the fungal community was more easily affected by different diets. In addition, we observed that complex microbial interactions may be linked to diverse functions of the gut microbiota, reflecting intricate biological processes in the intestinal microenvironment of A. mali. The results of this study could improve our ability to develop more effective management approaches in controlling A. mali in China, especially in regions that commonly suffer from outbreaks, such as Xinjiang Province. However, additional studies must still be performed, such as characterizing the specific members and roles of gut microbiota in A. mali. Thus, future research should determine the mechanisms of microbial passage and loss among the different life stages of A. mali and determine processes by which gut flora affect host plant utilization and cause host illness.
Materials and Methods
Sample Collection
Damaged branches of Malus siversii were collected from the wild fruit forest located in Yining city, Ili Kazakh autonomous prefecture, Xinjiang Province, China, on June 26, 2015. The larvae, pupae and some newly eclosed adults of A. mali were collected from the damaged branches and stored in centrifuge tubes, after which the guts were immediately dissected. The remaining newly eclosed adults, which were hatched from larvae collected from the damaged branches of Malus siversii, were reared with fresh leaves from different apple tree species, including Malus halliana Koehne and Malus pumila Miller, in an artificial climate chamber at 25 ± 1 °C with a 70 ± 5% relative humidity and a photoperiod cycle of 16/8 (L/D). The experimental feeding period was 20 days (from July 4 to July 24, 2015) to ensure the formation of intestinal microflora. Twenty individuals from each sample were manually selected and gut dissection was performed within 4 h.
Gut Dissection and DNA Extraction
Twenty individuals each of the five sample groups were used for gut dissection, including the wild larvae (Lar), pupae (Pup) and newly eclosed adults (EcA), and the lab-reared adults fed either the leaves of M. halliana (MhaA) or M. pumila (MpuA). Each sample was superficially disinfected with 75% ethanol for 1–3 min and then rinsed repeatedly with sterile water. Next, the insects were transported to a horizontal clean bench, and the guts were extracted with sterile fine tip forceps under a stereoscope and placed into 1.5-ml microcentrifuge tubes. Gut dissections were performed under sterile conditions and included the removal of the hepatopancreatic gland. The guts of twenty individuals from each sample group were pooled in one tube and crushed gently with a pestle in liquid nitrogen. Then, the gut tissue was transferred into 100 μl of sterile PBS. The tissue samples were macerated with sterile polypropylene micro pestles inside 1.5 ml tubes, after which the tubes were centrifuged at low speed to pellet the macerated gut tissue. Total DNA was extracted from each sample using a General AllGen Kit (ComWin Biotech Co., Ltd., Beijing, China) according to the manufacturer’s instructions. The extracted DNA was stored at −80 °C and reserved for further use.
PCR amplification and sequence data processing
The V3 and V4 hypervariable regions of the bacterial 16S rRNA gene were amplified using the specific barcoded primers 341 F (5′-CCTAYGGGRBGCASCAG-3′) and 806 R (5′-GGACTACNNGGGTATCTAAT-3′). The ITS2 region of the ITS rDNA was amplified using the specific barcoded primers F (5′-GCATCGATGAAGAACGCAGC-3′) and R (5′-ATATGTAGGATGAAGAACGYAGYRAA-3′) to assess fungal diversity. The PCR reaction mixtures (25 μl) contained 10 pmol of each primer, 5–10 ng DNA and 1× GeneAmp PCR Gold Buffer, 3.5 mM MgCl2, 0.2 mM GeneAmp dNTPs, and 0.025 U/μl AmpliTaq Gold DNA Polymerase, LD (Applied Biosystems, Foster City, CA). The PCR amplification procedure was as follows: 95 °C for 10 min, followed by 35 cycles of 95 °C for 30 s, an incubation at the appropriate annealing temperature (see below) for 30 s and 72 °C for 25 s, and a final extension step of 72 °C for 10 min. The annealing temperatures used were 52 °C and 57 °C for the bacterial V3-V4 and fungal ITS2 regions, respectively. All samples were amplified in triplicate, and no-template controls were included at all steps of the process. The PCR reaction products were detected by agarose gel electrophoresis and purified using a QIAquick Gel Extraction Kit (QIAGEN, cat#28706). The purified DNA was quantified using a QuantiFluor Fluorometer (Promega, SA3060) and equivalent amounts were subsequently mixed. Barcoded V3-V4 and ITS2 amplicons were ligated with adapters to construct sequencing libraries, which were sequenced using the paired-end method with an Illumina MiSeq (250-bp paired-end reads) with a 6 cycle index read at GENE DENOVO Co., Ltd. (Guangzhou, China).
Raw reads were removed that had contaminating adaptors, were of low quality and had polymeric sequences longer than 10 bp, had ambiguous bases, or had mismatched primers. For the V3-V4 and ITS2 paired-end reads, only clean sequences with overlaps of longer than 10 bp and with a mismatch rate of lower than 0.02 were assembled according to their overlapping sequences. The assembled reads (called tags) were used to remove redundant sequences with Mothur v.1.34.061. Sequences with ≥97% similarity was assigned to the same OTU (operational taxonomic unit). The low-abundance OTUs were eliminated from the OTU table if they did not have at least 2 counts across all the samples in the experiment. Representative sequences for each OTU were assigned to taxonomic groups using the Ribosomal Database Project naïve Bayesian rRNA classifier with the Greengenes database.
Data analysis
Alpha diversity indices, including the OTU richness, the Shannon-Wiener index, the bias-corrected Chao1 richness estimator, and the abundance-based coverage estimator (ACE) were determined using Mothur v1.34.0 and were used to analyse the microbial species diversity in each sample62. To identify linkages between the samples, the beta diversity among different samples was estimated based on the pairwise Bray-Curtis dissimilarity distances. PCoA was performed on the distance matrices to visualize the sample relationships. Dynamic patterns of bacterial and fungal communities during the development of the A. mali gut microbiota were determined using cluster analysis. The 40 genera with the highest relative abundances were selected and clustered into three groups-based similar profiles, which were displayed in a heatmap. A pie chart was constructed to analyse community variations in A. mali gut microbiotas resulting from different diets. Venn diagrams were generated to evaluate shared and unique bacterial and fungal OTUs to describe the similarities and differences among the different samples and treatments.
A network analysis was used to explore interactions between bacterial and fungal taxa. Bacterial and fungal genera with relative abundances above 0.05% were selected for this analysis. A Spearman’s correlation between two genera was considered significantly robust if the coefficient (ρ) was >0.6 or <-0.6, and the P-value was <0.01. All of the robust correlations identified from pairwise comparisons of the genera abundances formed a correlation network, where each node represents one genus and each edge stands for a strong and significant correlation between the nodes. The co-correlation network was visualized using CYTOSCAPE.
All statistical analyses were performed in the R environment (http://www.r-project.org) using the vegan63, igraph64 and gplots65 packages unless otherwise indicated.
Electronic supplementary material
Acknowledgements
We are grateful to Chang Yong and DINH, NGOC HIÊN for assistance with material collection. This study was financially supported by the National Natural Science Foundation of China (U1503102).
Author Contributions
Z.Z. and M.L. conceived and designed the experiments; Z.Z. performed the experiments, with the help of and X.L.; and Z.Z. and S.J. analysed the data and wrote the manuscript.
Data Availability
The sequences produced in this study are available in the NCBI Sequence Read Archive (Bioproject PRJNA358858). The accession numbers of the bacteria and fungi in the different biosamples are SAMN06186591-SAMN06186600.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/23/2019
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has not been fixed in the paper.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34127-x.
References
- 1.Wang Z, Zhang Y, Yang Z, Wang X. Determination of Larval Instars of Agrilus marl Matsumura (Coleoptera: Buprestidae) Forest Research. 2013;26:786–789. [Google Scholar]
- 2.Yi Z, Liu D, Cui X, Shang Z. Morphology and Ultrastructure of Antennal Sensilla in Male and Female Agrilus mali (Coleoptera: Buprestidae) Journal of Insect Science. 2016;16:87. doi: 10.1093/jisesa/iew073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji Y, Ji R, Huang R. Invasive species–Agrilus Mali Matsumura and damage in Xinjiang. Xin jiang Agricultural Sciences. 2004;41:31–33. [Google Scholar]
- 4.Cui X, Liu D, Liu A. Research progress in integrated management of Agrilus mali. Plant Protection. 2015;41:16–23. [Google Scholar]
- 5.Engel P, Moran NA. The gut microbiota of insects–diversity in structure and function. FEMS Microbiol Rev. 2013;37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- 6.Yun J-H, et al. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl Environ Microb. 2014;80:5254–5264. doi: 10.1128/AEM.01226-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumann P, Moran NA. Non-cultivable microorganisms from symbiotic associations of insects and other hosts. Antonie van Leeuwenhoek. 1997;72:39–48. doi: 10.1023/A:1000239108771. [DOI] [PubMed] [Google Scholar]
- 8.Buchner, P. Endosymbiosis of animals with plant microorganims. Interscience Publishers, Inc, New York. (1965).
- 9.Robinson CJ, Schloss P, Ramos Y, Raffa K, Handelsman J. Robustness of the bacterial community in the cabbage white butterfly larval midgut. Microbial Ecol. 2010;59:199–211. doi: 10.1007/s00248-009-9595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santana RH, et al. The gut microbiota of workers of the litter-feeding termite Syntermes wheeleri (Termitidae: Syntermitinae): archaeal, bacterial, and fungal communities. Microbial Ecol. 2015;70:545–556. doi: 10.1007/s00248-015-0581-z. [DOI] [PubMed] [Google Scholar]
- 11.Rouhbakhsh D, et al. The tryptophan biosynthetic pathway of aphid endosymbionts (Buchnera): genetics and evolution of plasmid-associated anthranilate synthase (trpEG) within the Aphididae. J Mol Evol. 1996;42:414–421. doi: 10.1007/BF02498635. [DOI] [PubMed] [Google Scholar]
- 12.Paoletti MG, et al. A unique midgut-associated bacterial community hosted by the cave beetle Cansiliella servadeii (Coleoptera: Leptodirini) reveals parallel phylogenetic divergences from universal gut-specific ancestors. BMC microbiology. 2013;13:129. doi: 10.1186/1471-2180-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duguma D, et al. Bacterial communities associated with Culex mosquito larvae and two emergent aquatic plants of bioremediation importance. PLoS One. 2013;8:e72522. doi: 10.1371/journal.pone.0072522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bili M, et al. Bacterial community diversity harboured by interacting species. PloS one. 2016;11:e0155392. doi: 10.1371/journal.pone.0155392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meriweather M, Matthews S, Rio R, Baucom RS. A 454 survey of the community composition and core microbiome of the common bed bug, Cimex lectularius, reveals significant microbial community structure across an urban landscape. arXiv preprint arXiv. 2012;1210:3707. doi: 10.1371/journal.pone.0061465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janson EM, Stireman JO, Singer MS, Abbot P. Phytophagous insect–microbe mutualisms and adaptive evolutionary diversification. Evolution. 2008;62:997–1012. doi: 10.1111/j.1558-5646.2008.00348.x. [DOI] [PubMed] [Google Scholar]
- 17.Minard G, Mavingui P, Moro CV. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasites & vectors. 2013;6:1. doi: 10.1186/1756-3305-6-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fermaud M, Menn RL. Association of Botrytis cinerea with grape berry moth larvae. Phytopathology. 1989;79:651–656. doi: 10.1094/Phyto-79-651. [DOI] [Google Scholar]
- 19.Six DL. Bark beetle-fungus symbioses. Insect symbiosis. 2003;1:97–114. doi: 10.1201/9780203009918.ch7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de León, A. V.-P., Sanchez-Flores, A., Rosenblueth, M. & Martínez-Romero, E. Fungal community associated with Dactylopius (Hemiptera: Coccoidea: Dactylopiidae) and its role in uric acid metabolism. Frontiers in Microbiology7 (2016). [DOI] [PMC free article] [PubMed]
- 21.Boucias DG, et al. The hindgut lumen prokaryotic microbiota of the termite Reticulitermes flavipes and its responses to dietary lignocellulose composition. Mol Ecol. 2013;22:1836–1853. doi: 10.1111/mec.12230. [DOI] [PubMed] [Google Scholar]
- 22.Engel P, Martinson VG, Moran NA. Functional diversity within the simple gut microbiota of the honey bee. Proc Natl Acad Sci USA. 2012;109:11002–11007. doi: 10.1073/pnas.1202970109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hulcr J, et al. Mycangia of ambrosia beetles host communities of bacteria. Microbial Ecol. 2012;64:784–793. doi: 10.1007/s00248-012-0055-5. [DOI] [PubMed] [Google Scholar]
- 24.Köhler T, Dietrich C, Scheffrahn RH, Brune A. High-resolution analysis of gut environment and bacterial microbiota reveals functional compartmentation of the gut in wood-feeding higher termites (Nasutitermes spp.) Appl Environ Microb. 2012;78:4691–4701. doi: 10.1128/AEM.00683-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poulsen M, Sapountzis P. Behind every great ant, there is a great gut. Mol Ecol. 2012;21:2054–2057. doi: 10.1111/j.1365-294X.2012.05510.x. [DOI] [PubMed] [Google Scholar]
- 26.Sudakaran S, Salem H, Kost C, Kaltenpoth M. Geographical and ecological stability of the symbiotic mid ‐ gut microbiota in European firebugs, Pyrrhocoris apterus (Hemiptera, Pyrrhocoridae) Mol Ecol. 2012;21:6134–6151. doi: 10.1111/mec.12027. [DOI] [PubMed] [Google Scholar]
- 27.Toju H, Fukatsu T. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: relevance of local climate and host plants. Mol Ecol. 2011;20:853–868. doi: 10.1111/j.1365-294X.2010.04980.x. [DOI] [PubMed] [Google Scholar]
- 28.Vasanthakumar A, Handelsman J, Schloss PD, Bauer LS, Raffa KF. Gut microbiota of an invasive subcortical beetle, Agrilus planipennis Fairmaire, across various life stages. Environ Entomol. 2008;37:1344–1353. doi: 10.1603/0046-225X(2008)37[1344:GMOAIS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Mittapalli O, et al. Tissue-specific transcriptomics of the exotic invasive insect pest emerald ash borer (Agrilus planipennis) PLoS One. 2010;5:e13708. doi: 10.1371/journal.pone.0013708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franzini PZ, et al. The gut microbiomes of two Pachysoma MacLeay desert Dung Beetle Species (Coleoptera: Scarabaeidae: Scarabaeinae) Feeding on Different Diets. PloS one. 2016;11:e0161118. doi: 10.1371/journal.pone.0161118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klepzig KD, Adams A, Handelsman J, Raffa K. Symbioses: a key driver of insect physiological processes, ecological interactions, evolutionary diversification, and impacts on humans. Environ Entomol. 2009;38:67–77. doi: 10.1603/022.038.0109. [DOI] [PubMed] [Google Scholar]
- 32.Weiss B, Kaltenpoth M. Bacteriome-localized intracellular symbionts in pollen-feeding beetles of the genus Dasytes (Coleoptera, Dasytidae) Frontiers in Microbiology. 2016;7:1–10. doi: 10.3389/fmicb.2016.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizzi A, et al. Characterization of the bacterial community associated with larvae and adults of Anoplophora chinensis collected in Italy by culture and culture-independent methods. BioMed research international. 2013;2013:1–12. doi: 10.1155/2013/420287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arias-Cordero E, et al. Comparative evaluation of the gut microbiota associated with the below-and above-ground life stages (larvae and beetles) of the forest cockchafer, Melolontha hippocastani. PLoS One. 2012;7:e51557. doi: 10.1371/journal.pone.0051557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scully ED, et al. Functional genomics and microbiome profiling of the Asian longhorned beetle (Anoplophora glabripennis) reveal insights into the digestive physiology and nutritional ecology of wood feeding beetles. BMC genomics. 2014;15:1–21. doi: 10.1186/1471-2164-15-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morales-Jiménez J, Zúñiga G, Ramírez-Saad HC, Hernández-Rodríguez C. Gut-associated bacteria throughout the life cycle of the bark beetle Dendroctonus rhizophagus Thomas and Bright (Curculionidae: Scolytinae) and their cellulolytic activities. Microbial Ecol. 2012;64:268–278. doi: 10.1007/s00248-011-9999-0. [DOI] [PubMed] [Google Scholar]
- 37.Taerum SJ, et al. Large shift in symbiont assemblage in the invasive red turpentine beetle. PloS one. 2013;8:e78126. doi: 10.1371/journal.pone.0078126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rojas-Jiménez K, Hernández M. Isolation of fungi and bacteria associated with the guts of tropical wood-feeding Coleoptera and determination of their lignocellulolytic activities. International journal of microbiology. 2015;2015:1–11. doi: 10.1155/2015/285018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gauthier J-P, Outreman Y, Mieuzet L, Simon J-C. Bacterial communities associated with host-adapted populations of pea aphids revealed by deep sequencing of 16S ribosomal DNA. PloS one. 2015;10:e0120664. doi: 10.1371/journal.pone.0120664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montagna M, et al. Effects of the diet on the microbiota of the red palm weevil (Coleoptera: Dryophthoridae) PloS one. 2015;10:e0117439. doi: 10.1371/journal.pone.0117439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammer TJ, McMillan WO, Fierer N. Metamorphosis of a butterfly-associated bacterial community. PLoS One. 2014;9:e86995. doi: 10.1371/journal.pone.0086995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popa V, Deziel E, Lavallée R, Bauce E, Guertin C. The complex symbiotic relationships of bark beetles with microorganisms: a potential practical approach for biological control in forestry. Pest Manag Sci. 2012;68:963–975. doi: 10.1002/ps.3307. [DOI] [PubMed] [Google Scholar]
- 43.Dillon R, Dillon V. The gut bacteria of insects: nonpathogenic interactions. Annual Reviews in Entomology. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- 44.Okeke BC, Lu J. Characterization of a defined cellulolytic and xylanolytic bacterial consortium for bioprocessing of cellulose and hemicelluloses. Appl Biochem Biotech. 2011;163:869–881. doi: 10.1007/s12010-010-9091-0. [DOI] [PubMed] [Google Scholar]
- 45.Huang S, Sheng P, Zhang H. Isolation and identification of cellulolytic bacteria from the gut of Holotrichia parallela larvae (Coleoptera: Scarabaeidae) International journal of molecular sciences. 2012;13:2563–2577. doi: 10.3390/ijms13032563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oyedokun A, Adeniyi D. Microbial diversity in the gut of Cashew Stem Girdler, Analeptes trifasciata Fabricius (Coleoptera: Cerambycidae), in Ibadan, Nigeria. International journal of insect science. 2016;8:17–22. doi: 10.4137/IJIS.S31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thongekkaew J, Khumsap A, Chatsa-nga P. Yeasts in mixed deciduous forest areas of Phujong Nayoy National Park and their ability to produce xylanase and carboxymethyl cellulose. Songklanakarin J Sci Techonol. 2012;34:157–163. [Google Scholar]
- 48.Iasur-Kruh L, et al. Microbial associates of the vine mealybug Planococcus ficus (Hemiptera: Pseudococcidae) under different rearing conditions. Microbial Ecol. 2015;69:204–214. doi: 10.1007/s00248-014-0478-2. [DOI] [PubMed] [Google Scholar]
- 49.Zouache K, Voronin D, Tran-Van V, Mavingui P. Composition of bacterial communities associated with natural and laboratory populations of Asobara tabida infected with Wolbachia. Appl Environ Microb. 2009;75:3755–3764. doi: 10.1128/AEM.02964-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colman DR, Toolson EC, Takacs‐Vesbach C. Do diet and taxonomy influence insect gut bacterial communities? Mol Ecol. 2012;21:5124–5137. doi: 10.1111/j.1365-294X.2012.05752.x. [DOI] [PubMed] [Google Scholar]
- 51.Chen, B. et al. Biodiversity and activity of the gut microbiota across the life history of the insect herbivore Spodoptera littoralis. Scientific Reports6 (2016). [DOI] [PMC free article] [PubMed]
- 52.Faust K, Raes J. Microbial interactions: from networks to models. Nat Rev Microbiol. 2012;10:538–550. doi: 10.1038/nrmicro2832. [DOI] [PubMed] [Google Scholar]
- 53.Briones-Roblero CI, et al. Structure and dynamics of the gut bacterial microbiota of the bark beetle, Dendroctonus rhizophagus (Curculionidae: Scolytinae) across their life stages. PloS one. 2017;12:e0175470. doi: 10.1371/journal.pone.0175470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomprefa N, McQuilken M, Hill R, Whipps J. Antimicrobial activity of Coniothyrium minitans and its macrolide antibiotic macrosphelide A. J Appl Microbiol. 2009;106:2048–2056. doi: 10.1111/j.1365-2672.2009.04174.x. [DOI] [PubMed] [Google Scholar]
- 55.Chaiharn M, Chunhaleuchanon S, Lumyong S. Screening siderophore producing bacteria as potential biological control agent for fungal rice pathogens in Thailand. World J Microb Biot. 2009;25:1919–1928. doi: 10.1007/s11274-009-0090-7. [DOI] [Google Scholar]
- 56.Robinson CJ, Bohannan BJ, Young VB. From structure to function: the ecology of host-associated microbial communities. Microbiol Mol Biol R. 2010;74:453–476. doi: 10.1128/MMBR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bugg TD, Ahmad M, Hardiman EM, Singh R. The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotech. 2011;22:394–400. doi: 10.1016/j.copbio.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 58.Calderón-Cortés N, Quesada M, Watanabe H, Cano-Camacho H, Oyama K. Endogenous plant cell wall digestion: a key mechanism in insect evolution. Annual Review of Ecology, Evolution, and Systematics. 2012;43:45–71. doi: 10.1146/annurev-ecolsys-110411-160312. [DOI] [Google Scholar]
- 59.Scully ED, Hoover K, Carlson J, Tien M, Geib SM. Proteomic analysis of Fusarium solani isolated from the Asian longhorned beetle, Anoplophora glabripennis. PLoS One. 2012;7:e32990. doi: 10.1371/journal.pone.0032990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ayayee P, et al. Gut microbes contribute to nitrogen provisioning in a wood-feeding cerambycid. Environ Entomol. 2014;43:903–912. doi: 10.1603/EN14045. [DOI] [PubMed] [Google Scholar]
- 61.Schloss PD, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microb. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kemp PF, Aller JY. Bacterial diversity in aquatic and other environments: what 16S rDNA libraries can tell us? FEMS Microbiol Ecol. 2004;47:161–177. doi: 10.1016/S0168-6496(03)00257-5. [DOI] [PubMed] [Google Scholar]
- 63.Oksanen, J. et al. The vegan package. Community ecology package10 (2007).
- 64.Csardi G, Nepusz T. The igraph software package for complex network research. InterJournal, Complex Systems. 2006;1695:1–9. [Google Scholar]
- 65.Warnes, G. R. et al. gplots: Various R programming tools for plotting data. R package version2 (2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequences produced in this study are available in the NCBI Sequence Read Archive (Bioproject PRJNA358858). The accession numbers of the bacteria and fungi in the different biosamples are SAMN06186591-SAMN06186600.