Abstract
Abnormal initiation and control of voluntary movements are among the principal manifestations of Parkinson’s disease (PD). However, the processes underlying these abnormalities and their potential remediation by dopamine treatment remain poorly understood. Normally, movements depend on the integration of sensory information with the predicted consequences of action. This integration leads to a suppression in the intensity of predicted sensations, reflected in a ‘sensory attenuation’. We examined this integration process and its relation to dopamine in PD, by measuring sensory attenuation. Patients with idiopathic PD (n = 18) and population-derived controls (n = 175) matched a set of target forces applied to their left index finger by a torque motor. To match the force, participants either pressed with their right index finger (‘Direct’ condition) or moved a knob that controlled a motor through a linear potentiometer (‘Slider’ condition). We found that despite changes in sensitivity to different forces, overall sensory attenuation did not differ between medicated PD patients and controls. Importantly, the degree of attenuation was negatively related to PD motor severity but positively related to individual patient dopamine dose, as measured by levodopa dose equivalent. The results suggest that dopamine could regulate the integration of sensorimotor prediction with sensory information to facilitate the control of voluntary movements.
Introduction
A key manifestation of Parkinson’s disease (PD) is bradykinesia – that is, patients have slowness associated with marked difficulties in planning, initiating and executing voluntary movements1. This principal abnormality in motor control has been shown to correlate well with dopamine disruption in patients2, however, the exact mechanism remains poorly understood. In order to better understand the mechanisms underlying movement disorders, previous studies have used the framework of optimal control theory3–5.
According to this theory, normal motor control depends on the integration of peripheral sensory information with predictions arising from internal models of action. The integration is dependent on the relative uncertainty (i.e. the inverse of ‘precision’) of sensory information and predictions6, such that in a highly uncertain environment, for example, people’s movements rely more on prediction7. Dopamine has been suggested to play a central role in regulating the precision of sensory information relative to predictions8. Striatal dopamine deficit, which is a hallmark pathological feature in PD, is therefore expected to lead to reduced reliance on sensory information, which has indeed been demonstrated in decision-making tasks9,10. However, a common clinical observation in PD is that patients are more dependent on sensory cues for initiating movements11. For example, the withdrawal of visual feedback impairs patients more than healthy individuals in terms of both movement speed and accuracy12. These findings suggest that striatal dopamine deficit in PD could have a different impact on representing sensory uncertainty for movement. Here, we examine the integration between sensory signals and prediction for movement in PD, through sensorimotor attenuation.
Sensorimotor attenuation is the reduction in the perceived intensity of stimuli generated by one’s actions, compared to externally generated stimuli. It reflects the suppression of predicted sensory consequences from perception13. Intact precision of sensorimotor predictions are thought to be required for increasing the salience of external events, to facilitate the rapid initiation14 and correction of movements to unpredicted events6. In schizophrenia, for example, reduced sensory attenuation and ‘exaggerated’ increase in reliance on sensory information have been suggested to contribute to deficits in distinguishing between self-caused and external stimuli15. Deficits in the integration of prior prediction and sensory information, as reflected in sensory attenuation, can therefore shed light on the mechanism of neurological and psychiatric disorders16,17.
Sensory attenuation can be quantified by the force matching task. In the force matching task18, 98% of adults show attenuation19, applying a larger force when matching an external force directly with their hand (‘Direct’ condition). In contrast, people tend to be accurate when matching the force indirectly with a linear potentiometer that controls a motor18. The overcompensation of forces in the Direct condition is associated with the integrity of a fronto-striatal network19 that is strongly affected by dopamine deficits in PD (e.g. Lewis et al.20).
We tested patients with idiopathic PD on a force matching task to measure sensory attenuation. Patient measures were compared to normative data from a large epidemiological control cohort21. Patients were tested while ‘on’, after taking their regular dopaminergic medication, and we took advantage of the variability in disease severity and medication to examine between-subject differences in attenuation in the Direct condition in relation to motor severity of PD and dopamine dose. Sensory sensitivity and task-related bias were measured using the Slider condition. Our principal hypothesis was that with increasing motor severity, attenuation would be reduced, reflecting abnormal integration of sensory information with sensorimotor prediction22. We also hypothesised that dopaminergic medication would increase attenuation, expressing increased reliance on motor prediction.
Materials and Methods
Participants
Eighteen patients (12 men; aged 48–81 years, mean: 67; SD: 10) were recruited from the John van Geest Centre for Brain Repair, Parkinson’s disease research clinic. Patients met clinical diagnostic criteria of idiopathic PD, according to the UK PD brain bank criteria23, and were in the mild to moderate stages of disease [Hoehn and Yahr stages 1 to 3]24. Normative, population-derived controls were drawn from the Cambridge Centre for Ageing and Neuroscience (https://camcan-archive.mrc-cbu.cam.ac.uk/dataaccess/) using the same apparatus. Control subjects were selected from the data repository by age, such that all subjects within the patient age range were included in the study (n = 175, 89 men, mean age: 65, SD: 10). The research was carried out in accordance with guidelines and regulations approved by the Cambridgeshire 2 Research Ethics Committee (now ‘East of England – Cambridge Central’), who approved the experimental protocols. All participants gave full, informed, written consent before the experiment.
Assessment of motor and cognitive features in patients was performed at the beginning of the testing session. The severity of motor features was assessed with the Unified Parkinson’s Disease Rating Scale, motor subscale III25. Cognition was assessed with the Mini-Mental State Examination26 and Addenbrooke’s Cognitive Examination Revised27, excluding patients with ACE-R score below 84/10028. Patients were tested in the morning after taking their medication as normal. The time interval between levodopa self-administration and testing varied between one and three hours, such that all patients were in a relative ‘on’ state at the time they were assessed. Levodopa dose equivalent (LDE) was computed according to Tomlinson et al.29. Clinical data were collected and scored independently and blind to the behavioural results.
Force matching task procedures and analyses
On each trial of the Force Matching Task18, a lever attached to a torque motor applied the target force for 2.5 s to the left index finger (Fig. 1). The target force was pseudo-randomly selected from the set: 1.0, 1.5, 2.0 and 2.5 Newton (N): each target force was presented once within a cycle of four trials. At the end of the presentation period, the force was removed, and participants used their right index finger to match the force they had just sensed on their left finger (matching period 4.5 sec long). Premature (response during the presentation period) or late (>1 s) responses led to a warning “too fast” or “too slow” on the computer screen, and the trial was repeated. Because PD patients have altered force output [e.g. increased force irregularities and time to peak30], the matched force was calculated as the mean force measured within a 500 ms time window that was selected on a trial-by-trial basis. A sliding window was used to identify the 500 ms interval that had the minimum force variability. This procedure was implemented in both patient and control data.
Figure 1.
Force matching task illustration. Illustration of the force matching task. In each trial, a torque motor pseudorandomly applied one of four force levels (target force) through a lever to the participants’ left index finger. Participants were asked to match the force they had just sensed (matched force) either by pressing the lever with their right index finger (‘Direct’ condition); or by sliding a linear potentiometer which controlled the torque motor (‘Slider’ condition).
There were two conditions. In the Direct condition, participants matched the target force by pressing with their right index finger directly on top of the lever, mechanically transmitting the force to the left finger. In the Slider condition, participants matched the force with their right index finger by moving a slider (a linear potentiometer) which controlled the torque motor. A force sensor at the end of the lever measured both the target and matched forces applied to the left finger. All participants performed both the Direct and Slider conditions, in a counterbalanced order. For each condition, an initial familiarisation phase of eight trials (two cycles of the four target forces) was performed. The main experiment for each condition consisted of 32 trials.
For each condition, mean force overcompensation was calculated as the average difference between the matched and the target force on each trial across the four target force levels. Positive mean overcompensation in the Direct condition reflected an overall attenuation of self-generated sensations. In the Slider condition, mean overcompensation was used to measure general task-related bias. To examine force matching as a function of force levels, the intercept and slope from a linear regression of matched versus target force was calculated for each participant and condition. The Slider slope was used as a measure of sensory sensitivity for distinguishing between the different forces, reflecting sensory precision19. All statistical tests of behavioural data were performed with two-tailed tests, implemented with ‘R’ software31. Given the unequal sample size32, non-parametric Mann-Whitney test was used for the comparison between groups. After assessing the normality of errors using Kolmogorov-Smirnoff test, within PD group tests were performed with parametric tests. To examine the relationship between sensory attenuation and clinical variables in the PD group, multiple regression analyses were conducted with Direct force overcompensation as the dependent variables. This variable was used as this measure most closely reflects sensory attenuation18; it does not assume a linear relationship between matched and target force; it has been compared in various population groups15; and it has been associated with fronto-striatal circuits impaired in PD19. The independent variables were disease severity and patient LDE. Covariates of no interest were included to account for differences in sensory inflow and motor outflow, namely: Slider slope as a measure of sensory sensitivity19, mean within-trial force variability and unexplained variance of each patient linear regression of matched versus target force. All variables were Z-scaled before entering the model.
Results
Participant demographics
Patient clinical information is summarised in Table 1. Patients (n = 18) and controls (n = 175) were not different in terms of age (Mann-Whitney test; Z = 0.92, p = 0.36) and gender (χ2 = 1.64, p = 0.2).
Table 1.
Summary of patient clinical information.
| No. | Gender | Age | Disease duration (years) | Side* | Disease stage** | UPDRS motor subscale | ACE-R (MMSE) | LDE *** |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 63 | 11 | R | 1.5 | 16 | 94 (29) | 1315 |
| 2 | M | 74 | 16 | L | 2 | 19 | 98 (30) | 620 |
| 3 | M | 81 | 15 | L | 2.5 | 20 | 94 (29) | 410 |
| 4 | M | 54 | 15 | L | 1 | 26 | 89 (29) | 1815 |
| 5 | M | 56 | 11 | R | 3 | 14 | 86 (29) | 1210 |
| 6 | F | 73 | 13 | B | 2.5 | 13 | 95 (28) | 565 |
| 7 | F | 76 | 9 | R | 3 | 13 | 91 (30) | 855 |
| 8 | F | 81 | 13 | L | 2 | 16 | 97 (29) | 1355 |
| 9 | M | 77 | 13 | L | 2 | 37 | 97 (30) | 1720 |
| 10 | M | 64 | 6 | L | 1.5 | 15 | 94 (29) | 1175 |
| 11 | M | 48 | 6 | R | 2.5 | 24 | 88 (29) | 535 |
| 12 | M | 72 | 26 | L | 1 | 21 | 85 (28) | 460 |
| 13 | M | 57 | 14 | B | 1 | 21 | 94 (28) | 1180 |
| 14 | F | 66 | 17 | L | 2 | 21 | 95 (27) | 1740 |
| 15 | M | 76 | 12 | L | 1 | 31 | 87 (27) | 1500 |
| 16 | F | 64 | 11 | B | 3 | 19 | 96 (28) | 1000 |
| 17 | M | 77 | 9 | L | 3 | 18 | 88 (27) | 700 |
| 18 | F | 55 | 8 | L | 2 | 16 | 98 (29) | 200 |
| Average | 67 | 13 | 2 | 20 | 93 (29) | 1020 | ||
UPDRS = Unified Parkinson’s Disease Rating Scale; MMSE = Mini-Mental State Examination; ACE-R = Addenbrooke’s Cognitive Examination Revised; LDE = Levodopa dose equivalent; *Dominant side of motor symptoms: L = left, R = right, B = bilateral. **According to Hoehn and Yahr, 1967; ***Calculated according to Tomlinson et al.29.
Sensory attenuation in PD
In order to calculate the matched force for each participant and trial, we calculated the mean force during a 500 ms interval that had the minimum force variability, found with a sliding window. There was no significant difference between initiation times of force matching in patients and controls (Mann-Whitney test; Z = −1.58, p = 0.11). Importantly the mean time at which patients and controls stabilised the force after initiation was not different (2.67 s versus 2.73 s in patients and controls; Mann-Whitney test; Z = −0.37, p = 0.71). In the Slider condition, initiation times of force matching were similar across groups (Z = 1.39, p = 0.16), and patients stabilised the forces slower than controls (2.61 s versus 2.36 s in patients and controls; Mann-Whitney test; Z = 2.78, p < 0.01). The distributions of calculated mean overcompensation (mean difference between matched and target force) for both conditions in patients were not different from normal distribution (one-sample Kolmogorov-Smirnov test; Direct: D = 0.228, p = 0.26, Slider: D = 0.137, p = 0.845).
All patients showed overall sensory attenuation, as indicated by a positive mean overcompensation in the Direct condition (Fig. 2A). Mean Direct force overcompensation was greater than zero (t17 = 6.94, p < SD = 0.92 N). Patients were more accurate when matching the force in the Slider condition (Fig. 2A), with smaller force overcompensation than in the Direct condition (t17 = −5.93, p < 0.001). Mean Slider force overcompensation was −0.01 N (SD = 0.36 N), and was not significantly different from zero (t17 = −0.12, p = 0.91). These results confirm that sensory attenuation is as robust in PD patients as in the general population19. Direct and Slider force overcompensation were not correlated in patients (r(16) = 0.29, p = 0.24), supporting the notion of a distinct underlying mechanism for sensory attenuation in the Direct condition.
Figure 2.
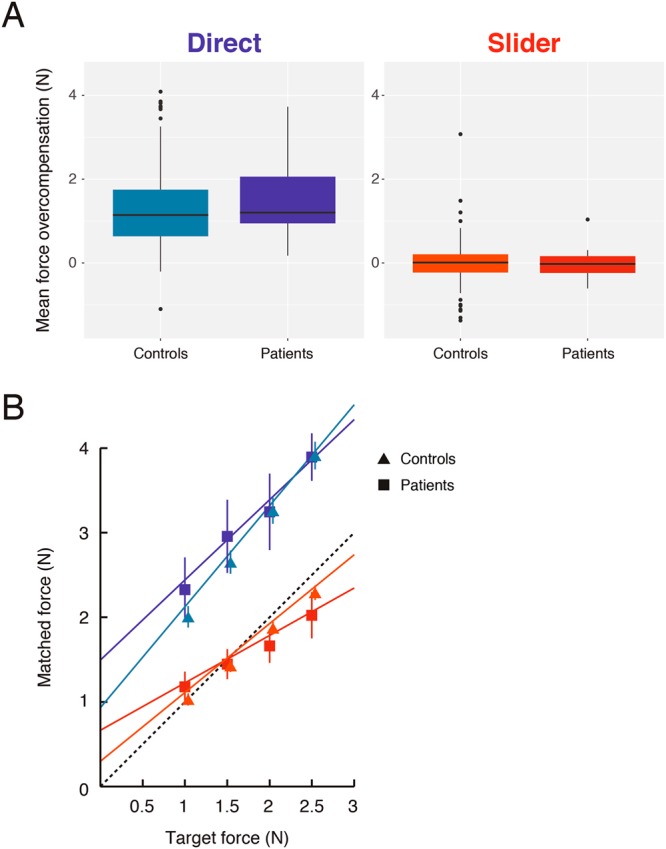
Differences in sensorimotor attenuation between PD patients and controls. (A) Standard boxplots showing the distribution of mean force overcompensation values across all patients and controls in the Direct (shades of blue) and Slider (shades of red) conditions. Positive value indicates sensory attenuation. (B) Mean regression plots of matched versus target force in the Direct and Slider conditions for both groups. Colour scheme is the same as in (A). Dashed line indicates the line of equality. Error bars indicate ± 2 standard error of group mean. Control data points and error bars are offset by 2 pixels for illustration.
To examine changes in attenuation in PD, we compared overall attenuation and attenuation as a function of force levels in patients with our age-matched, normative control data. There were no significant differences in mean Direct force overcompensation (Mann-Whitney test; Z = 1.00, p = 0.32), suggesting no group difference in overall attenuation. Similarly, no group differences emerged in mean Slider overcompensation (Z = −0.45, p = 0.65).
To compare force matching as a function of force level, we examined the linear regression fits of target force versus matched force (Fig. 2B). These linear regression fits may well change for a different range of forces, but provide a local approximation for the force stimuli used in the experiment. As there were no group differences in mean overcompensation in the Direct and Slider conditions, these measures provide more details on possible differences in the force matching procedure, namely: (i) differences in sensitivity to the target forces, reflected in varying levels of overcompensation across force levels and changes in the slope; and (ii) differences in response bias across all forces, reflected in a different intercept with normal slope.
The linear regression fit was better in controls, with patients showing smaller R2 compared to controls in both conditions (Direct: Z = 2.37, p < 0.05; Slider: Z = 3.30, p < 0.001). In the Direct condition, there was an increase in the intercept in patients compared to controls (Mann-Whitney test; Z = 2.25, p < 0.05, Bonferroni corrected), with no significant difference in the slope (Z = −1.8, p = 0.14, Bonferroni corrected). In the Slider condition, patients showed both reduced slope (Mann-Whitney test; Z = −2.82, p < 0.01, Bonferroni corrected) and a corresponding increase in intercept (Z = 2.75, p < 0.01, Bonferroni corrected). These results suggest reduced sensitivity to the different forces in patients in the Slider condition, but not in the Direct condition, as also found in relation to ageing19. We therefore accounted for these differences in the next analyses.
Before testing how sensory attenuation might be related to clinical features in patients, we conducted several control analyses to verify the patients’ abilities to match the forces in the Direct condition. Compared to controls, patients showed increased within-trial variability in the forces they applied (Mann-Whitney test; Z = 2.72, p < 0.01), consistent with previous findings in PD30. This is likely to arise from reduced force sensitivity due to increased sensory variability33, which, importantly, is not expected to introduce a systematic bias given the nature of the force matching task (see Discussion). However, increased within-trial force variability could also arise from the patients ‘overshooting’ and then slowly adjusting the force; an impaired ability to decide what force to apply; and/or from difficulties maintaining a steady force due to fatigue.
To explore the possibility of a systematic bias in the matching procedure, we performed additional analyses. First, for each trial, within the analysed time window, we fit a linear regression model of matched force against time. There was no linear trend in the matched force (regression slope not significantly different from zero; t17 = −0.96, p = 0.35), similar to controls (Mann-Whitney test; Z = 0.53, p = 0.53). Moreover, there was no consistent relationship between this linear trend and the magnitude of the force applied by each patient (mean Pearson correlation coefficient not different from zero in patients; t17 = −1.41, p = 0.18), which was again similar to controls (Mann-Whitney; Z = 0.15, p = 0.25). These results suggest it is unlikely that patients fatigued in the force they applied. Further, although not significantly different from controls, the slope in the Direct condition was overall smaller in patients relative to controls (see Fig. 2B). This raises the possibility that patients were more limited in their ability to match larger forces. However, a closer look at the Direct slope demonstrated it was not significantly different from a veridical slope of one (Wilcoxon signed-rank test; Z = −1.76, p = 0.08). To further explore patient performance in matching larger forces, we computed the Pearson coefficient of the correlation between the magnitude of matched forces and within-trial SD for each trial and for each subject. There was a consistently positive relationship between the matched force and within-trial SD in patients (mean Pearson correlation coefficient significantly greater than zero across patients; t17 = 8.09, p < 0.001), consistent with the well-known association between generated force variability and force level34. Importantly, however, this association did not differ from controls (Mann-Whitney test; Z = −1.21, p = 0.23). Taken together, these control analyses suggest that PD did not alter the matching procedure itself. Nevertheless, we accounted for group differences in matching procedure (within-trial variability and R2 of the matched against target force) in the next analysis.
Dopamine and sensory attenuation
To examine the relationship between sensory attenuation and patient dopamine dose, we next fit a linear regression model with Direct force overcompensation as the dependent variable. The independent variables were disease severity, as assessed using the Unified Parkinson’s disease Rating Scale motor subscale III, and levodopa dose equivalent (LDE; Tomlinson et al.29). To control for basic sensory and motor differences affecting the force matching procedure in patients, we included additional covariates of no interest. These were the Slider slope measuring sensory sensitivity19, patient force variability and unexplained variance of each patient linear fits (see Methods).
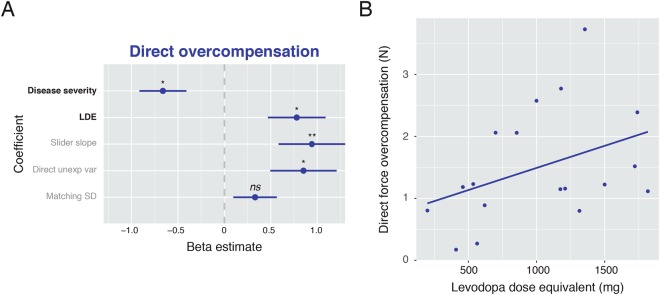
The regression model was statistically significant (F(5,12) = 3.24, p < 0.05; 40% of force overcompensation variance explained; Fig. 3A). Even though disease severity and LDE were marginally positively correlated (r(16) = 0.46, p = 0.056), they had opposite effects on Direct overcompensation in patients. Disease severity was a negative predictor (t12 = −2.62, p < 0.05), whereas LDE was a positive predictor (t12 = 2.51, p < 0.05). For illustration, the direct relationship between attenuation and dopamine dose is plotted in Fig. 3B. This pattern of results did not change when additionally co-varying for cognitive function in terms of Addenbrooke’s Cognitive Examination score; the laterality of dominantly affected side (p > 0.5 for the coefficients of both variables); or when entering Direct intercept (c.f. Wolpe et al.19) as the dependent variable. Moreover, when running a similar regression with mean Slider force overcompensation as the dependent variable, LDE did not predict overcompensation (indeed, unlike the Direct condition, the direction of effect was negative, t15 = −1.432, p = 0.173), whereas disease severity was a significant positive predictor (t15 = 2.573, p < 0.05). As this effect is in the opposite direction to the effect observed for Direct overcompensation, a generic effect of disease or drug on sensory, planning or execution systems cannot explain the impact of LDE and disease severity on sensory attenuation in the Direct condition. Together, these results suggest that dopamine treatment could restore parkinsonism-related reduction in sensory attenuation.
Figure 3.
Association between dopamine and patient attenuation. (A) Illustration of the standardised beta estimates of all independent variable coefficients included in the multiple regression model (R2adj = 0.40), predicting Direct intercept. Clinical variables of interest were disease severity, which had a negative effect on attenuation, and levodopa doses, which had a positive effect on attenuation. Error bars indicate ± 1 standard error of group mean. LDE = Levodopa dose equivalent. Significance level indicated by *P < 0.05; **P < 0.01; ns = non-significant. (B) Illustration of the relationship between Direct force overcompensation and levodopa dose equivalent, before entered into the regression model.
As Slider slope differed between groups, we fit a regression model to the Slider slope in a final exploratory analysis. Disease severity and LDE were the only independent variables. In this model (F(2,15) = 6.974, p = 0.007; 41% of variance explained), disease severity was a positive predictor of Slider slope (b = 0.48, t15 = 2.29, p < 0.05) and LDE was a significant negative predictor (b = −0.77, t15 = −3.67, p = 0.002). The results did not change when including Direct force overcompensation as an additional covariate, suggesting these results cannot be readily explained by performance in the Direct condition.
Discussion
The principal result of our study is that sensorimotor attenuation is positively related to dopamine doses in Parkinson’s disease, whereas disease severity is related to reduced attenuation. These results can be interpreted in the context of optimal control theory, in which voluntary actions rely on the integration of sensory feedback with predictions of the consequences of one’s actions35. The integration is precision-dependent, such that low-precision signals are down-weighted relative to high-precision signals. For example, this means that when performing a task in a dark or foggy setting, the precision of sensory feedback is expected to be low, and the sensorimotor system therefore relies more strongly on prior predictions when performing an action7. Sensory attenuation is thought to reflect the precision of predictive signals36, relative to the precision of sensory feedback14,19. Our finding that PD motor severity is associated with reduced attenuation would therefore suggest that the precision of predictive signals may be compromised in PD22. This may underlie the changes of sensorimotor integration in PD37 and the dependence of patients on sensory cues, e.g. for the initiation and maintenance of their movement12.
Although the severity of parkinsonism and doses of dopamine replacement therapy were positively correlated, dopamine was associated with an opposite effect, namely increased attenuation. These results support the hypothesis that dopamine alleviates disorders of movement in PD by restoring the precision and hence the typical reliance on sensorimotor predictions22, at the expense of down-weighting the sensorium. This is further supported by the finding that an increase in dopamine dose was related to reduced force sensitivity in the Slider condition, reflecting reduced precision afforded to sensory signals. Finally, these results are further consistent with the exaggeration of age-related sensory deficits in medicated PD patients33, and the detrimental effects of dopaminergic therapy on sensory sensitivity in PD38,39. However, conflicting data on this issue exist40, and some studies revealed no adverse effect of dopamine on tactile perception, at least in terms of perceptual thresholds41.
The relationship between dopamine and the integration prediction with sensory information has been previously tested in PD. For example, dopamine increases the perceived temporal attraction or ‘binding’ between an action and its effect42 (but also see ref.43). As binding critically relies on sensorimotor prediction44,45, these results are consistent with the hypothesis that dopamine increases the reliance on sensorimotor prediction for action. Importantly, however, other studies reported the opposite association, in which dopaminergic treatment reduced the reliance on predictions in perceptual decision-making tasks, while increasing reliance on sensory information9,10. We propose that these are different types of predictions that are mediated by distinct brain mechanisms within a cortical hierarchy (c.f. Brown et al.14). “Low-level” sensorimotor predictions reflected in sensory attenuation depend on pre-SMA connectivity with dorsal striatum circuits19, and could play a key role in normal execution of movement14,22. On the other hand, high-level perceptual priors depend more on prefrontal connections with ventral striatum circuitry46,47. Since dopamine doses are tailored to alleviate patient motor symptoms, which mostly reflect dorsal striatal dopamine depletion, high dopamine doses can effectively “overdose” the ventral striatum48. This discrepancy might lead to the relative normalisation of low-level predictions for attenuation, but a weakening of high-level predictions for perceptual decision-making tasks10.
The positive association between attenuation and dopamine, and the combination of increased Direct intercept and reduced Slider slope in medicated PD patients mirror the impact of healthy ageing on sensory attenuation19. Increased attenuation found with normal ageing is associated with reduced connectivity in a fronto-striatal network19 that is strongly affected in PD. This network includes the caudate and putamen, dorsolateral prefrontal cortex and pre-SMA as the network hub. Interestingly, reduced fronto-striatal connectivity has also been associated with increased caudate dopamine synthesis as seen in healthy ageing49. Therefore, increasing dopamine synthesis – including by levodopa administration in PD – could increase sensory attenuation by altering connectivity of the pre-SMA within its fronto-striatal network.
Activity in the secondary somatosensory cortex, mediated via increased pre-SMA connectivity19,50, has also been suggested as the neural correlates of attenuation. Neurophysiological studies have indeed demonstrated that the perceived attenuation is closely related to late components of sensory evoked potentials, arising from the secondary somatosensory cortex51. In PD, however, early components of sensory evoked potentials, arising from fronto-striatal activity, are already altered37. The typical neurophysiological attenuation of these early components following a voluntary movement is absent in PD patients ‘off’ medication, and restored by dopamine treatment22. This reduced neurophysiological attenuation of the early components of sensory evoked potential has been attributed to a failure in sensory ‘gating’ in PD22, resulting from abnormal precision of sensory afferents14.
The neurophysiological gating of sensory afferent signals before and during movement has been proposed to be required for the initiation processes of voluntary movements14,52. This theoretical account suggests that a relative reduction in the precision of sensory signals, leading to sensory attenuation, enables high-level predictions to drive normal movement through hierarchical networks in the central nervous system14. In PD, deficient precision of predictions would be overwhelmed by sensory evidence for a lack of movement, resulting in bradykinesia14. Although the neurophysiological attenuation of early components of sensory evoked potentials, shown to be impaired in PD and remediated with dopamine, may not directly underlie the behavioural phenomenon of attenuation51, our behavioural results are consistent with these previous studies. Our findings that PD motor severity is associated with reduced attenuation while dopamine dose is related to increased attenuation, support the hypothesis that bradykinesia in Parkinson’s disease could be considered in terms of pathological imprecision of sensorimotor prediction, which are alleviated by dopamine treatment.
Our results have two main interpretative limitations that should be considered. Firstly, we opted for the force matching task, as it is a simple, highly intuitive and robust task19, however, it requires the application of (albeit small) forces for matching the perceived pressure intensities. Preliminary testing indeed showed that the task is not easily performed by PD patients ‘off’ medication, mainly because of tremor and akinesia in light of the timed, dexterous movements required for matching the forces. Explicit measures of attenuation such as intensity ratings (e.g.53) may go around patient motor difficulties, but introduce additional confounders due to cognitive and metacognitive difficulties in patients. We therefore included only an ‘on’ state, in which patients were well able to generate forces (even larger forces on average, see Fig. 2). Further, we accounted for differences in motor outflow by: (1) using a moving average method that captures the time window with the lowest variability in matched force; time windows that were revealed to be similar across groups. (2) performing several control analyses revealing that matching procedure was not significantly altered in patients. (3) accounting for differences in motor abilities in the main analysis linking attenuation and levodopa and disease severity.
Secondly, PD patients can show intrinsic sensory deficits41, and differences in sensory inflow must therefore be ruled out as a potential confound. In a matching task any absolute bias is factored out and therefore sensory deficits, which are common in PD33,41, can increase performance variability, as observed in our study, but are unlikely to introduce a systematic bias (e.g. with a force of ‘X’ N sensed as X/2N, patients would still have to match X N to experience the same X/2N intensity). Performance in the control Slider condition indeed showed a minimal bias, similar to controls, but it also suggested reduced sensory sensitivity. Similarly, unexplained variance of matched versus target force in the Direct condition was increased in patients, suggesting reduced sensory precision19. Importantly, however, these sensory differences in patients (measured as Slider slope and Direct unexplained variance) were accounted for in the main regression analyses, suggesting that sensory deficits were unlikely to provide a sufficient explanation for the significant associations between attenuation, disease severity and dopamine dose.
In conclusion, our study suggests that dopamine is related to an increase in sensory attenuation in Parkinson’s disease, and that dopamine increases the precision of sensorimotor prediction for movement. We propose that dopamine has distinct effects for representing sensory uncertainty in perceptual decision-making and motor control. The results support the hypothesis that bradykinesia in movement disorders like Parkinson’s disease can, in part, be considered in terms of pathological (im)precision of sensorimotor predictions14, which can be modulated by dopamine22. This may provide a common framework for understanding the role of dopamine in perceptual, cognitive and motor function.
Acknowledgements
We are grateful to the patients for their participation in the study. We are also indebted to the Cam-CAN respondents and their primary care teams in Cambridge for their participation. We thank Professor Roger Barker for his helpful comments on the manuscript and the support of the John van Geest Centre for Brain Repair, Parkinson’s disease research clinic. The study was funded by grants to JBR from the Wellcome Trust (103838), Medical Research Council (SUAG/004 RG91365) and James S. McDonnell Foundation 21st Century Science Initiative: Scholar Award in Understanding Human Cognition. NW was funded by Gates Cambridge. DMW received grants from the Wellcome Trust (097803), Human Frontier Science Program and the Royal Society Noreen Murray Professorship in Neurobiology. Cam-CAN was supported by the Biotechnology and Biological Sciences Research Council (BB/H008217/1). JZ was funded by the European Research Council Starting Grant (716321).
Author Contributions
N.W. conceived the study; N.W., C.N. and Cam-CAN piloted the study and collected the data; N.W., J.Z. and J.N.I. analysed the data; N.W., J.B.R., J.N.I. and D.M.W. wrote the manuscript; N.W. prepared the figures.
Data Availability
Patient data and analysis code are available from the corresponding author upon request. Cam-CAN raw data and analysis code are available upon signing a data sharing request form (http://www.mrc-cbu.cam.ac.uk/datasets/camcan/).
Competing Interests
The authors declare no competing interests.
Footnotes
A comprehensive list of consortium members appears at the end of the paper
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/22/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
Contributor Information
Noham Wolpe, Email: nw305@medschl.cam.ac.uk.
Cam-CAN:
Lorraine K. Tyler, Carol Brayne, Edward T. Bullmore, Andrew C. Calder, Rhodri Cusack, Tim Dalgleish, John Duncan, Fiona E. Matthews, William D. Marslen-Wilson, Meredith A. Shafto, Teresa Cheung, Linda Geerligs, Anna McCarrey, Abdur Mustafa, Darren Price, David Samu, Matthias Treder, Kamen A. Tsvetanov, Janna van Belle, Nitin Williams, Lauren Bates, Andrew Gadie, Sofia Gerbase, Stanimira Georgieva, Claire Hanley, Beth Parkin, David Troy, Tibor Auer, Marta Correia, Lu Gao, Emma Green, Rafael Henriques, Jodie Allen, Gillian Amery, Liana Amunts, Anne Barcroft, Amanda Castle, Cheryl Dias, Jonathan Dowrick, Melissa Fair, Hayley Fisher, Anna Goulding, Adarsh Grewal, Geoff Hale, Andrew Hilton, Frances Johnson, Patricia Johnston, Thea Kavanagh-Williamson, Magdalena Kwasniewska, Alison McMinn, Kim Norman, Jessica Penrose, Fiona Roby, Diane Rowland, John Sargeant, Maggie Squire, Beth Stevens, Aldabra Stoddart, Cheryl Stone, Tracy Thompson, Ozlem Yazlik, Dan Barnes, Marie Dixon, Jaya Hillman, Joanne Mitchell, and Laura Villis
References
- 1.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 2.Vingerhoets FJ, Schulzer M, Calne DB, Snow BJ. Which clinical sign of Parkinson’s disease best reflects the nigrostriatal lesion? Ann. Neurol. 1997;41:58–64. doi: 10.1002/ana.410410111. [DOI] [PubMed] [Google Scholar]
- 3.Garbarini F, et al. Moving’ a paralysed hand: bimanual coupling effect in patients with anosognosia for hemiplegia. Brain. 2012;135:1486–97. doi: 10.1093/brain/aws015. [DOI] [PubMed] [Google Scholar]
- 4.Garbarini F, Piedimonte A, Dotta M, Pia L, Berti A. Dissociations and similarities in motor intention and motor awareness: the case of anosognosia for hemiplegia and motor neglect. J. Neurol. Neurosurg. Psychiatry. 2013;84:416–419. doi: 10.1136/jnnp-2012-302838. [DOI] [PubMed] [Google Scholar]
- 5.Wolpe Noham, Moore James W., Rae Charlotte L., Rittman Timothy, Altena Ellemarije, Haggard Patrick, Rowe James B. The medial frontal-prefrontal network for altered awareness and control of action in corticobasal syndrome. Brain. 2013;137(1):208–220. doi: 10.1093/brain/awt302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franklin DW, Wolpert DM. Computational mechanisms of sensorimotor control. Neuron. 2011;72:425–42. doi: 10.1016/j.neuron.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Körding KP, Wolpert DM. Bayesian integration in sensorimotor learning. Nature. 2004;427:244–247. doi: 10.1038/nature02169. [DOI] [PubMed] [Google Scholar]
- 8.Friston KJ, et al. Dopamine, affordance and active inference. PLoS Comput. Biol. 2012;8:e1002327. doi: 10.1371/journal.pcbi.1002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vilares I, Kording KP. Dopaminergic medication increases reliance on current information in Parkinson’s disease. Nat. Hum. Behav. 2017;1:0129. doi: 10.1038/s41562-017-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolpe, N., Nombela, C. & Rowe, J. B. Dopaminergic modulation of positive expectations for goal-directed action: evidence from Parkinson’s disease. Front. Psychol. 6 (2015). [DOI] [PMC free article] [PubMed]
- 11.Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease: normalization strategies and underlying mechanisms. Brain. 1996;119:551–568. doi: 10.1093/brain/119.2.551. [DOI] [PubMed] [Google Scholar]
- 12.Klockgether T, Dichgans J. Visual control of arm movement in Parkinson’s disease. Mov. Disord. 1994;9:48–56. doi: 10.1002/mds.870090108. [DOI] [PubMed] [Google Scholar]
- 13.Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat. Neurosci. 1998;1:635–640. doi: 10.1038/2870. [DOI] [PubMed] [Google Scholar]
- 14.Brown H, Adams RA, Parees I, Edwards M, Friston K. Active inference, sensory attenuation and illusions. Cogn. Process. 2013;14:411–427. doi: 10.1007/s10339-013-0571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. Am. J. Psychiatry. 2005;162:2384–6. doi: 10.1176/appi.ajp.162.12.2384. [DOI] [PubMed] [Google Scholar]
- 16.Pareés I, et al. Loss of sensory attenuation in patients with functional (psychogenic) movement disorders. Brain. 2014;137:2916–21. doi: 10.1093/brain/awu237. [DOI] [PubMed] [Google Scholar]
- 17.Wolpe N, Rowe JB. Beyond the ‘urge to move’: objective measures for the study of agency in the post-Libet era. Front. Hum. Neurosci. 2014;8:450. doi: 10.3389/fnhum.2014.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shergill SS, Bays PM, Frith CD, Wolpert DM. Two eyes for an eye: the neuroscience of force escalation. Science (80-.). 2003;301:187. doi: 10.1126/science.1085327. [DOI] [PubMed] [Google Scholar]
- 19.Wolpe N, et al. Ageing increases reliance on sensorimotor prediction through structural and functional differences in frontostriatal circuits. Nat. Commun. 2016;7:13034. doi: 10.1038/ncomms13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J. Neurosci. 2003;23:6351–6. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shafto MA, et al. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: a cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 2014;14:204. doi: 10.1186/s12883-014-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macerollo A, et al. Dopaminergic treatment modulates sensory attenuation at the onset of the movement in Parkinson’s disease: A test of a new framework for bradykinesia. Mov. Disord. 2016;31:143–146. doi: 10.1002/mds.26493. [DOI] [PubMed] [Google Scholar]
- 23.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/WNL.17.5.427. [DOI] [PubMed] [Google Scholar]
- 25.Fahn, S. & Elton, R. In Recent developments in Parkinsons disease (eds Fahn, S., Marsden, C., Goldstein, M. & Calne, D.) 153–163 (Macmillan Healthcare Information, 1987).
- 26.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke’s Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int. J. Geriatr. Psychiatry. 2006;21:1078–1085. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- 28.Reyes MA, et al. Addenbrooke’s Cognitive Examination validation in Parkinson’s disease. Eur. J. Neurol. 2009;16:142–147. doi: 10.1111/j.1468-1331.2008.02384.x. [DOI] [PubMed] [Google Scholar]
- 29.Tomlinson CL, et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010;25:2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 30.Stelmach, G. E., Teasdale, N., Phillips, J. & Worringham, C. J. Force production characteristics in Parkinson’s disease. Exp. Brain Res. 76 (1989). [DOI] [PubMed]
- 31.R Core Team, R: A language and environment for statistical computing. (2016).
- 32.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947;18:50–60. doi: 10.1214/aoms/1177730491. [DOI] [Google Scholar]
- 33.Konczak J, et al. Parkinson’s disease accelerates age-related decline in haptic perception by altering somatosensory integration. Brain. 2012;135:3371–9. doi: 10.1093/brain/aws265. [DOI] [PubMed] [Google Scholar]
- 34.Jones KE, Hamilton AF, Wolpert DM. Sources of signal-dependent noise during isometric force production. J. Neurophysiol. 2002;88:1533–44. doi: 10.1152/jn.2002.88.3.1533. [DOI] [PubMed] [Google Scholar]
- 35.Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat. Rev. Neurosci. 2011;12:739–51. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- 36.Bays PM, Flanagan JR, Wolpert DM. Attenuation of self-generated tactile sensations is predictive, not postdictive. PLoS Biol. 2006;4:e28. doi: 10.1371/journal.pbio.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov. Disord. 2003;18:231–240. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- 38.O’Suilleabhain P, Bullard J, Dewey RB. Proprioception in Parkinson’s disease is acutely depressed by dopaminergic medications. J. Neurol. Neurosurg. Psychiatry. 2001;71:607–10. doi: 10.1136/jnnp.71.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konczak J, et al. Proprioception and motor control in Parkinson’s disease. J. Mot. Behav. 2009;41:543–52. doi: 10.3200/35-09-002. [DOI] [PubMed] [Google Scholar]
- 40.Li K, Pickett K, Nestrasil I, Tuite P, Konczak J. The effect of dopamine replacement therapy on haptic sensitivity in Parkinson’s disease. J. Neurol. 2010;257:1992–8. doi: 10.1007/s00415-010-5646-9. [DOI] [PubMed] [Google Scholar]
- 41.Conte A, Khan N, Defazio G, Rothwell JC, Berardelli A. Pathophysiology of somatosensory abnormalities in Parkinson disease. Nat. Rev. Neurol. 2013;9:687–697. doi: 10.1038/nrneurol.2013.224. [DOI] [PubMed] [Google Scholar]
- 42.Moore JW, et al. Dopaminergic medication boosts action–effect binding in Parkinson’s disease. Neuropsychologia. 2010;48:1125–1132. doi: 10.1016/j.neuropsychologia.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 43.Saito N, et al. Altered awareness of action in Parkinson’s disease: Evaluations by explicit and implicit measures. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore J, Haggard P. Awareness of action: Inference and prediction. Conscious. Cogn. 2008;17:136–144. doi: 10.1016/j.concog.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Wolpe N, Haggard P, Siebner HR, Rowe JB. Cue integration and the perception of action in intentional binding. Exp. Brain Res. 2013;229:467–74. doi: 10.1007/s00221-013-3419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolpe, N., Wolpert, D. M. & Rowe, J. B. Seeing what you want to see: priors for one’s own actions represent exaggerated expectations of success. Front. Behav. Neurosci. 8 (2014). [DOI] [PMC free article] [PubMed]
- 47.Vilares I, Howard JD, Fernandes HL, Gottfried JA, Kording KP. Differential representations of prior and likelihood uncertainty in the human brain. Curr. Biol. 2012;22:1641–1648. doi: 10.1016/j.cub.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci. Biobehav. Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 49.Klostermann EC, Braskie MN, Landau SM, O’Neil JP, Jagust WJ. Dopamine and frontostriatal networks in cognitive aging. Neurobiol. Aging. 2012;33:623.e15–623.e24. doi: 10.1016/j.neurobiolaging.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shergill SS, et al. Modulation of somatosensory processing by action. Neuroimage. 2013;70:356–62. doi: 10.1016/j.neuroimage.2012.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmer CE, Davare M, Kilner JM. Physiological and perceptual sensory attenuation have different underlying neurophysiological correlates. J. Neurosci. 2016;36:10803–10812. doi: 10.1523/JNEUROSCI.1694-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barker R. How does the brain control its own activity? A new function for the basal ganglia. J. Theor. Biol. 1988;131:497–507. doi: 10.1016/S0022-5193(88)80044-4. [DOI] [PubMed] [Google Scholar]
- 53.Blakemore SJ, Frith CD, Wolpert DM. Spatio-temporal prediction modulates the perception of self-produced stimuli. J. Cogn. Neurosci. 1999;11:551–9. doi: 10.1162/089892999563607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Patient data and analysis code are available from the corresponding author upon request. Cam-CAN raw data and analysis code are available upon signing a data sharing request form (http://www.mrc-cbu.cam.ac.uk/datasets/camcan/).