Abstract
We report a rare case of a patient with Moyamoya syndrome who presented with intracerebral hemorrhage resulting from rupture of a middle meningeal artery pseudoaneurysm. This 38-year-old woman was unconscious and hemiplegic when she was admitted to our hospital. The patient had mental retardation as a result of tuberculous meningitis infection at the age of one year. On radiologic examination, she had intracerebral hemorrhage in the right temporo-parietal lobe and an aneurysm in the middle meningeal artery with right internal carotid artery occlusion. The patient underwent surgical treatment for the hemorrhage and aneurysm. The radiologic data, intraoperative findings, and pathology were consistent with a diagnosis of pseudoaneurysm. In the current report, we describe a rare case of a patient with a history of tuberculous meningitis who developed Moyamoya syndrome and pseudoaneurysm, which resulted in a ruptured middle meningeal artery pseudoaneurysm and brain hemorrhage.
Keywords: Middle meningeal artery, Pseudoaneurysm, Moyamoya syndrome, Tuberculous meningitis
INTRODUCTION
Patients with Moyamoya syndrome (MMS) show distal internal carotid artery (ICA) occlusion, similar to patients with Moyamoya disease (MMD); however, MMS is associated with well-recognized underlying diseases, which distinguishes it from MMD.12) Various causes of MMS are recognized, including neurofibromatosis type 1, Down's syndrome, thyroid disease, and cranial irradiation.9) MMS with tuberculous meningitis (TBM) is rare.5),14) Intracranial aneurysms associated with MMD occasionally occur (3–14% of MMD patients),1) and aneurysm rupture has been frequently reported.2),4) However, the middle meningeal artery (MMA) is a rare location for aneurysms in MMD, and few cases of MMA aneurysmal rupture and hemorrhage have been reported.8) Moreover, MMA aneurysm rupture in patients with MMS is more rare than in MMD and has not been reported, especially related to TBM. Here, we present a delayed manifestation of MMS after TBM, presenting with an intracerebral hemorrhage as a result of MMA aneurysmal rupture.
CASE REPORT
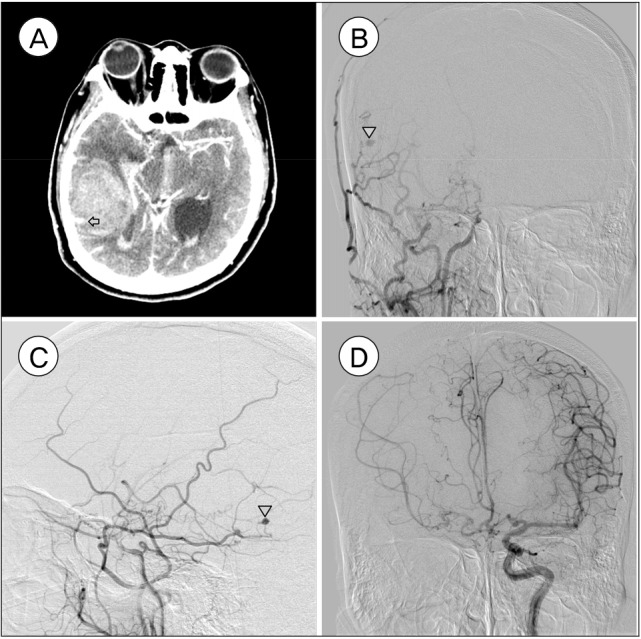
A 38-year-old unconscious female with left hemiplegia was admitted to out hospital. The neurologic evaluation on admission revealed that her consciousness level was drowsy (Glasgow coma scale 14, eye opening 3, verbal response 5, motor response 6), and motor grade was I on the left side. The patient had a history of TBM at the age of one, and she suffered from mental retardation as a consequence of the TBM. Except for mental retardation, no neurologic deficits were noted prior to the hemorrhage, and cerebral angiography had never been performed. Brain computed tomography (CT) scan disclosed a 35 mL hematoma in the right temporo-parietal lobe and an intraventricular hemorrhage (IVH) from the right lateral ventricle to the 4th ventricle (Fig. 1A). CT angiography revealed a saccular aneurysm, presumed to be the origin of the hemorrhage, at the right temporal area and hypotrophy of the branches of the right middle cerebral artery with M1 occlusion. For further evaluation, digital subtraction angiography was performed. The right distal ICA was occluded proximal to the ophthalmic artery with basal collaterals on the right common carotid artery (Fig. 1B, C). A small aneurysm was seen at the petrous branch of the right MMA. Angiography of the left common carotid artery revealed the left distal ICA and middle cerebral artery vessels to be patent, and the right middle cerebral artery and the anterior cerebral artery received blood supply through the anterior communicating artery channel (Fig. 1D).
Fig. 1. (A) Computed tomography scan showed an intracerebral hemorrhage and small vascular enhancement within the hematoma in the right temporo-parietal lobe (marked with arrow). (B, C) Digital subtraction angiography confirmed occlusion in the right distal internal carotid artery with fewer confluent basal collaterals and pseudoaneurysm in the right middle meningeal artery, petrous branch (marked with arrowhead). (D) Left common carotid artery angiography demonstrated blood flow in the left distal internal carotid artery and middle cerebral artery patency. The right middle cerebral artery and anterior cerebral artery received blood supply through the anterior communicating artery channel.
Operation and postoperative course
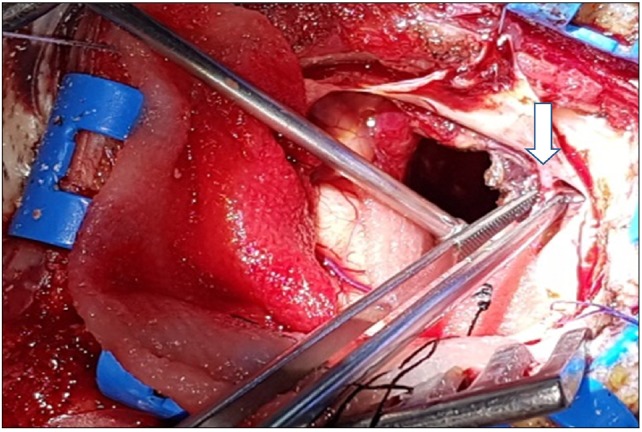
Under general anesthesia, the patient underwent an emergency operation to evacuate the massive hematoma and to control the acute hydrocephalus with IVH. As the dura was incised, an aneurysm-shaped vessel was identified penetrating into the dura from the MMA (Fig. 2). After coagulating the vessels of the dura and removing the hematoma, the temporal horn of the lateral ventricle was exposed. The wall was reconstructed with Tachosil (Takeda Inc, Osaka, Japan), and cranioplasty was performed with a mesh plate to facilitate ingrowth of the branches of the arteries. An external ventricular drainage tube was inserted, and the operation was concluded.
Fig. 2. An aneurysm-shaped vessel was identified penetrating into the dura from the middle meningeal artery (marked with arrow).
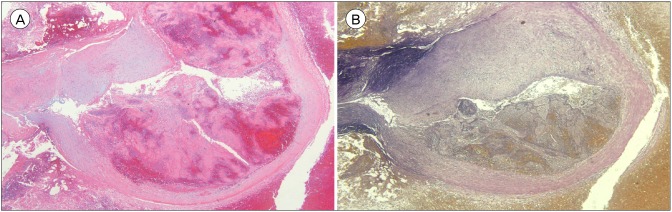
A 6 × 6 × 4-mm-sized aneurysm was biopsied. Hematoxylin and eosin staining revealed fibromyxoid degeneration of the vascular wall with partial rupture, and pseudoaneurysmal rupture was confirmed. The vessel wall did not stain on elastic stain, a typical finding of pseudoaneurysm (Fig. 3).
Fig. 3. (A) Hematoxylin and eosin stain revealed fibromyxoid degeneration of the vascular wall with partial rupture. (B) On elastic stain, the vessel wall was not stained. Original magnification, ×40.
Postoperatively, the patient's consciousness was drowsy, although conversation was possible despite grade II weakness on her left side. Delayed communicating hydrocephalus developed, a ventricular peritoneal shunt was inserted, and the patient was transferred to the department of rehabilitation medicine.
DISCUSSION
MMS has a variety of risk factors, including infectious meningitis and especially TBM.14) TBM is caused by Mycobacterium tuberculosis and frequently affects children under 4 years of age.13) In vessel studies of TBM patients, ICA stenosis is characteristically observed, which is assumed to be the result of chronic and repetitive inflammation of the brain parenchyme and great arteries. Subsequent to the ICA stenosis, demand for perfusion flow induces development of collateral vessels in ischemic areas, which might be the cause of hemorrhage with significant neurologic deficit.5),7),10) Pinardi et al.10) reviewed several cases of post-infectious Moyamoya disease, including cases of MMD caused by Mycobacterium tuberculosis infection. Nakayama et al.7) reported a case of intracerebral hemorrhage with Moyamoya phenomenon caused by tuberculous arteritis.
MMA aneurysms associated with MMD have been reported in previous studies. Borota et al.1) reported a case with a saccular aneurysm at the MMA posterior branch with subarachnoid hemorrhage and subdural hemorrhage, and the patient died 4 days after surgery. Park et al.8) presented experiences of repeated MMA aneurysm rupture in an MMD patient treated with craniotomy and aneurysmal neck clipping.
However, MMA aneurysms are less common in patients with MMS than in patients with MMD, and only one case of pure intracerebral hemorrhage following a ruptured MMA aneurysm in an MMS patient has been reported.3) In the study of Koebbe and Horowitz,3) an MMS patient with Down's syndrome had an aneurysm located in a collateral branch between the MMA and occipital arteries, and the patient was treated with an endovascular approach by occlusion of the parent branch.
Peripheral artery aneurysms in MMD have been sporadically reported, and most are confirmed on pathologic examination as false aneurysms, also known as pseudoaneurysms. The arterial walls are completely disrupted and are often contained only by a cavitated clot. Since the walls contain collagen and fibrin without elastic fibers, the aneurysms have a tendency to rapidly grow in the early stage.1),11) Because peripheral pseudoaneurysms have fragile walls and tend to repeatedly bleed and rapidly grow, immediate treatment is essential. The MMA and its dural branches play important roles in collateral blood supply in both MMD and MMS,6) and both neurosurgeons and neuro-interventionists should consider the increased MMA flow during treatment to prevent ischemic complications. Both surgical excision and endovascular embolization could be excellent treatment options for a ruptured aneurysm, and treatment options should be carefully selected under multidisciplinary considerations regarding the patient's neurologic status, coexisting brain lesions such as ICH and IVH, and collateral vessel involvement. In this study, we chose surgical treatment for aneurysmal occlusion because removing the ICH and draining the hydrocephalus were helpful in the patient's recovery. The patient's life was saved, but the motor weakness on her left side remained.
CONCLUSION
We reported our experience with a very rare case of ruptured MMA pseudoaneurysm associated with MMS in a patient with TBM. It is helpful to evaluate the cerebral vessels with an imaging modality for lowering the mortality and morbidity of patients with TBM. Early detection of vascular changes, including aneurysm formation, may prevent more significant neurologic complications.
Footnotes
Disclosure: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Borota L, Marinkovic S, Bajic R, Kovacevic M. Intracranial aneurysms associated with moyamoya disease. Neurol Med Chir (Tokyo) 996 Dec;36(12):860–864. doi: 10.2176/nmc.36.860. [DOI] [PubMed] [Google Scholar]
- 2.Hamada J, Hashimoto N, Tsukahara T. Moyamoya disease with repeated intraventricular hemorrhage due to aneurysm rupture. Report of two cases. J Neurosurg. 1994 Feb;80(2):328–331. doi: 10.3171/jns.1994.80.2.0328. [DOI] [PubMed] [Google Scholar]
- 3.Koebbe CJ, Horowitz MB. A rare case of a ruptured middle meningeal aneurysm causing intracerebral hematoma in a patient with moyamoya disease. AJNR Am J Neuroradiol. 2004 Apr;25(4):574–576. [PMC free article] [PubMed] [Google Scholar]
- 4.Kuroda S, Houkin K, Kamiyama H, Abe H. Effects of surgical revascularization on peripheral artery aneurysms in moyamoya disease: report of three cases. Neurosurgery. 2001 Aug;49(2):463–467. doi: 10.1097/00006123-200108000-00039. [DOI] [PubMed] [Google Scholar]
- 5.Mathew NT, Abraham J, Chandy J. Cerebral angiographic features in tuberculous meningitis. Neurology. 1970 Oct;20(10):1015–1023. doi: 10.1212/wnl.20.10.1015. [DOI] [PubMed] [Google Scholar]
- 6.Matsukawa H, Fujii M, Murakata A, Shinoda M, Takahashi O. Foramen spinosum and middle meningeal artery in moyamoya disease: preliminary results of a pilot study. Brain Inj. 2015 Jun;29(10):1246–1251. doi: 10.3109/02699052.2015.1035333. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama Y, Tanaka A, Nagasaka S, Ikui H. Intracerebral hemorrhage in a patient with moyamoya phenomenon caused by tuberculous arteritis: a case report. No Shinkei Geka. 1999 Aug;27(8):751–755. [PubMed] [Google Scholar]
- 8.Park YS, Suk JS, Kwon JT. Repeated rupture of a middle meningeal artery aneurysm in moyamoya disease: case report. J Neurosurg. 2010 Oct;113(4):749–752. doi: 10.3171/2009.11.JNS09895. [DOI] [PubMed] [Google Scholar]
- 9.Phi JH, Wang KC, Lee JY, Kim SK. Moyamoya syndrome: a window of moyamoya disease. J Korean Neurosurg Soc. 2015 Jun;57(6):408–414. doi: 10.3340/jkns.2015.57.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinardi F, Stracciari A, Spinardi L, Guarino M. Postpneumococcal Moyamoya syndrome case report and review of the postinfective cases. BMJ Case Rep. 2013 Feb;2013:bcr2012006726. doi: 10.1136/bcr-2012-006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadatoh A, Yonekawa Y, Morooka Y, Imakita T. A case of moyamoya disease with repeated intraventricular hemorrhage due to a ruptured pseudoaneurysm. No Shinkei Geka. 1989 Aug;17(8):755–758. [PubMed] [Google Scholar]
- 12.Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009 Mar;360(12):1226–1237. doi: 10.1056/NEJMra0804622. [DOI] [PubMed] [Google Scholar]
- 13.Torok ME. Tuberculous meningitis: advances in diagnosis and treatment. Br Med Bull. 2015 Mar;113(1):117–131. doi: 10.1093/bmb/ldv003. [DOI] [PubMed] [Google Scholar]
- 14.Zipfel GJ, Fox DJ, Jr, Rivet DJ. Moyamoya disease in adults: the role of cerebral revascularization. Skull Base. 2005 Feb;15(1):27–41. doi: 10.1055/s-2005-868161. [DOI] [PMC free article] [PubMed] [Google Scholar]