Abstract
Although the effect of elevated CO2 (eCO2) on soybean yield has been well documented, few studies have addressed seed quality, particularly at the fresh edible (R6) and mature stages (R8). Under the current global scenario of increasing CO2 levels, this potentially threatens the nutritional content and quality of food crops. Using four soybean cultivars, we assessed the effects of eCO2 on the concentrations of crude protein, crude oil, and isoflavones and analyzed the changes in free amino acids, fatty acids, and mineral elements in seeds. At R6, eCO2 had no influence on soybean seed protein and oil concentrations. At R8, eCO2 significantly decreased seed protein concentration but increased seed oil concentration; it also significantly decreased total free amino acid concentration. However, at the same stage, the proportion of oleic acid (18:1) among fatty acids increased in response to eCO2 in the cultivars of Zhongke-maodou 2 (ZK-2) and Zhongke-maodou 3 (ZK-3), and a similar trend was found for linoleic acid (18:2) in Zhongke-maodou 1 (ZK-1) and Hei-maodou (HD). Total isoflavone concentrations increased significantly at both the R6 and R8 stages in response to eCO2. Compared with ambient CO2, the concentrations of K, Ca, Mg, P, and S increased significantly under eCO2 at R6, while the Fe concentration decreased significantly. The response of Zn and Mn concentrations to eCO2 varied among cultivars. At R8 and under eCO2, Mg, S, and Ca concentrations increased significantly, while Zn and Fe concentrations decreased significantly. These findings suggest that eCO2 is likely to benefit from the accumulation of seed fat and isoflavone but not from that of protein. In this study, the response of seed mineral nutrients to eCO2 varied between cultivars.
Keywords: soybean, climate change, mineral nutrients, protein, oil
Introduction
Atmospheric CO2 concentration has risen from 280 ppm to 390 ppm in the last 250 years and is predicted to increase to 550 ppm by 2050 (Stocker et al., 2013). As the primary substrate for photosynthesis, elevated CO2 (eCO2) concentration in the atmosphere significantly influences plant growth and productivity in many crop species, especially in C3 crops where the CO2 saturation point is much higher than the current atmospheric CO2 levels (Ainsworth et al., 2007; Wang et al., 2008; Leakey et al., 2009). The positive effects of eCO2 on C3 crop yields have been documented in rice, wheat, cowpea, and soybean (Krishnan et al., 2007; Yang et al., 2007; Zhu et al., 2008; Bishop et al., 2015; Dey et al., 2017); however, relatively little research has focused on soybean seed quality, which is as important as yield (Thomas et al., 2003, 2009; Ziska et al., 2007).
Soybean [Glycine max (L.) Merr.] is the world’s most important legume and a major source of protein and oil, which contain essential free amino acids and fatty acids. Results from a previous study suggest that eCO2 has no effect on soybean seed protein concentration (Taub et al., 2008). This is perhaps because soybean crops alleviate nitrogen (N) deficiency by increasing N2 fixation under eCO2 and, thus, maintain seed N concentration with increased seed yield (Allen and Boote, 2000; Li et al., 2017). However, there are few studies in regard to the influence of eCO2 on amino acid concentration. On the other hand, several investigations show that eCO2 has either no effect (Thomas et al., 2003; Taub et al., 2008) or a positive effect on soybean oil concentration (Heagle et al., 1998; Hao et al., 2014). Heagle et al. (1998) reported that eCO2 significantly increases oleic acid concentration, whereas Thomas et al. (2003) found that fatty acid level shows no response to eCO2. Amid this controversy, the relevant underlying mechanisms warrant specific investigation.
The concentrations of several elements in seeds, such as iron (Fe), zinc (Zn), calcium (Ca), and magnesium (Mg), are significantly influenced by eCO2. For instance, Fe and Zn concentration in wheat, barley, and rice decrease under eCO2 (Fangmeier et al., 1997; Lieffering et al., 2004; Erbs et al., 2010). A significant decrease of 9.3% for Zn and 5.1% for Fe in wheat seed was reported in a meta-analysis of 64 relevant studies (Myers et al., 2014). In another study, a decrease in seed nitrogen (N), phosphorus (P), potassium (K), and sulfur (S) concentrations was also observed in barley (free air CO2 enrichment, FACE), potato (open-top chamber, OTC), wheat (FACE), and sorghum (OTC) (Prior et al., 2008; Högy and Fangmeier, 2009; Erbs et al., 2010). In soybeans, Hao et al. (2016) found a significant increase of 31% and 26% in seed P and K concentrations, respectively, under eCO2. However, in northeast China, which produces 33% of the nation’s soybean crop (Liu and Herbert, 2002), the effects of eCO2 on the concentrations of mineral elements in soybean seed have not been studied to date.
Soybean seeds contain large quantities of isoflavones, including daidzein, genistein, and glycitein, which are considered beneficial to human health (Bennett et al., 2004; Morrison et al., 2008; Medic et al., 2014). These chemicals inhibit ovarian and colon cancer cell growth (MacDonald et al., 2005; Chang et al., 2007) and lower serum low-density lipoprotein cholesterol levels (Taku et al., 2007). Whether eCO2 favors the accumulation of isoflavones in soybean seed remains largely unknown. Theoretically, the synthesis of isoflavones in the seed is closely associated with the availability of photosynthetic carbon, which generally increases under eCO2.
Moreover, vegetable soybean (edamame), collected at the immature stage (R6) before pods turn yellow, is very popular in East Asian countries and is becoming more popular in the United States and western countries. However, no study has focused on the influence of eCO2 on seed nutritional status at the fresh edible stage.
Using four soybean cultivars, we assessed the effects of eCO2 on the nutritional quality of soybean seeds at the fresh edible and mature stages. Specifically, we examined the concentrations of crude protein, oil, and isoflavones and analyzed the changes in free amino acids, fatty acids, and mineral elements in seeds. The present results offer valuable information to drive improvement in human nutrition under the rising global atmospheric CO2 scenario.
Materials and Methods
Research Site and Experimental Design
A pot experiment was conducted in OTC at the Northeast Institute of Geography and Agroecology (45°73’N, 126°61’E), Chinese Academy of Sciences, Harbin, China. The experiment had a random block design comprising two values for atmospheric CO2 concentration and four vegetable soybean cultivars with six replicates. The four vegetable soybean cultivars were Zhongke-maodou 1 (ZK-1), Zhongke-maodou 2 (ZK-2), Zhongke-maodou 3 (ZK-3), and Hei-maodou (HD). Before sowing, uniform seeds were selected and germinated on moistened filter paper at 25°C. After 2 days of germination, six seeds were sown in each pot containing 9 L of soil and subsequently thinned to two plants 10 days after emergence. Therefore, there were 12 pots per cultivar grown in either ambient CO2 (aCO2) or eCO2. Soil water content was maintained at 80 ± 5% of field capacity by weighing and watering. Three replicates were harvested at the R6 and R8 stages (Fehr et al., 1971). Seeds were then dried at 70°C for 72 h.
There were six octagonal OTCs with three for eCO2 and the remainder for aCO2. The OTCs had a steel frame; the main body was 2.0 m high with a 0.5 m high canopy, which formed a 45° angle with the plane (Zhang et al., 2014). The OTCs were covered with polyethylene film (transparency ≥95%). A similar OTC design has been widely used in other studies of CO2 (Liu et al., 2016; Yu et al., 2016; Chaturvedi et al., 2017). A digital CO2-regulating system (Beijing VK2010, China) was installed to monitor the CO2 levels in OTCs and to automatically regulate the supply of CO2 gas (99.9%) to achieve concentrations of 550 ± 30 ppm for eCO2 and 390 ± 30 ppm for aCO2.
Chemical Analysis of Plant Samples
The Soxhlet extraction method was used to determine the total oil concentration in seeds. To achieve this, 0.5 g of dried sample was weighed and wrapped tightly using a weighted piece of filter paper and was placed into the Soxhlet apparatus in a water bath maintained at 60°C. Subsequently, 200 mL of ethyl ether was added to the Soxhlet apparatus to extract the oil. After a 48-h extraction period, the defatted sample was placed in an oven at 45°C for 12 h, and the weight was used to calculate the oil content (Li et al., 2014).
Crude protein concentration was determined using the combustion N analysis method by Elementar-Vario (Elementar Analysensysteme GmbH E-III, Germany). Total N was converted to crude protein content using a conversion factor of 6.25 (Saldivar et al., 2011). Free amino acid concentrations were determined by reverse-phase high-performance liquid chromatography (RP-HPLC), as described by Qin et al. (2014). The concentrations of 16 amino acids were measured and analyzed, these included the following: aspartate (Asp), threonine (Thr), serine (Ser), glutamate (Glu), proline (Pro), glycine (Gly), alanine (Ala), valine (Val), methionine (Met), isoleucine (Ile), leucine (Leu), tyrosine (Tyr), phenylalanine (Phe), histidine (His), lysine (Lys), and arginine (Arg).
The isoflavone concentration was determined by HPLC using the method described by Sun et al. (2011) with slight modifications. Dried seed samples (0.5 g) were placed in 10 mL of 70% methanol solution; after shaking for 8 h at 240 rpm, the mix was centrifuged at 4000 rpm for 10 min and then filtered through a 0.45 μm filter. A detailed method for the determination of isoflavone concentration is described by Wu et al. (2016).
Fatty acid concentration was determined by gas chromatography (GC) with a flame ionization detector. A total of 0.33 g of dried seed sample was placed in n-hexane solution for 5 h after a 0.5 min vortex. The supernatant was used for methyl esterification; later, the concentrations of the five fatty acids were determined according to the method described by Qin et al. (2014).
For seed digestion, 0.5 g of dried seed was placed in 10 mL of HNO3 and 2.5 mL of HClO4 acid (v/v 4:1) for 24 h at room temperature. Later, the seed samples were digested in the digestion instrument until clear liquid was obtained; subsequently, the liquid was diluted to 25 mL. The concentrations of Fe, Cu, Mg, Mn, and Zn were analyzed by ?ame atomic absorption spectrometry, and the concentrations of K and Ca were determined using a flame photometer. Each measurement was repeated five times.
Data Analysis
The mean data were compared according to Duncan’s multiple range test at 5% significance. Two-way analysis of variance (ANOVA) on variables such as the chemical element concentration, protein concentration, oil concentration, fatty acid concentration, and isoflavone concentration was performed to assess the interaction between CO2 and cultivar at levels of significance of P = 0.05, P = 0.01, and P = 0.001, using Genstat 13 (VSN International, Hemel Hempstead, United Kingdom).
Results
Protein and Free Amino Acids
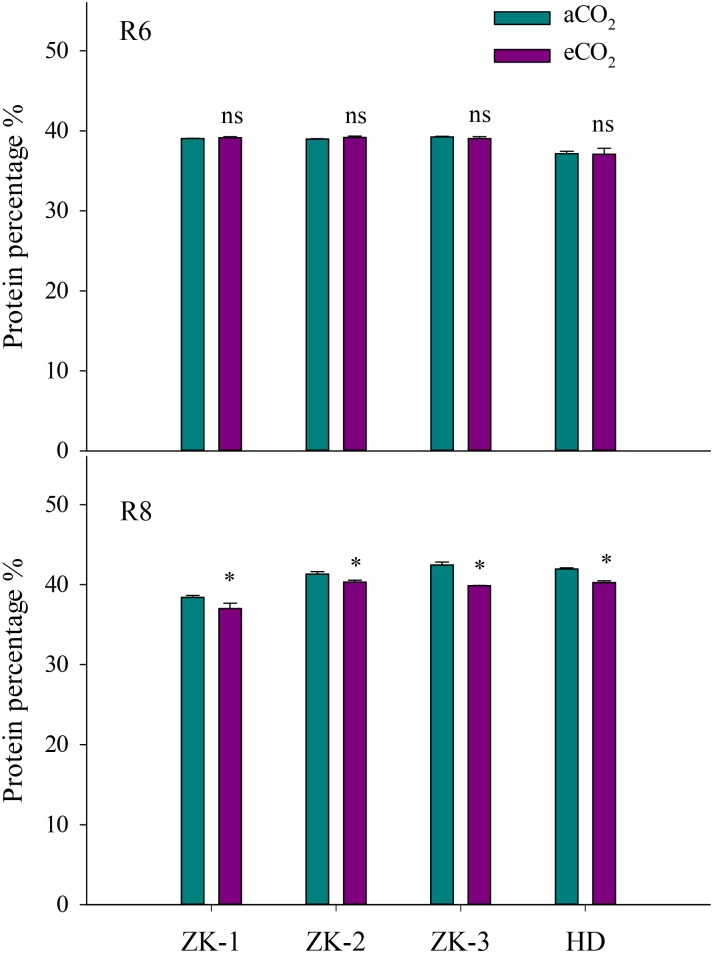
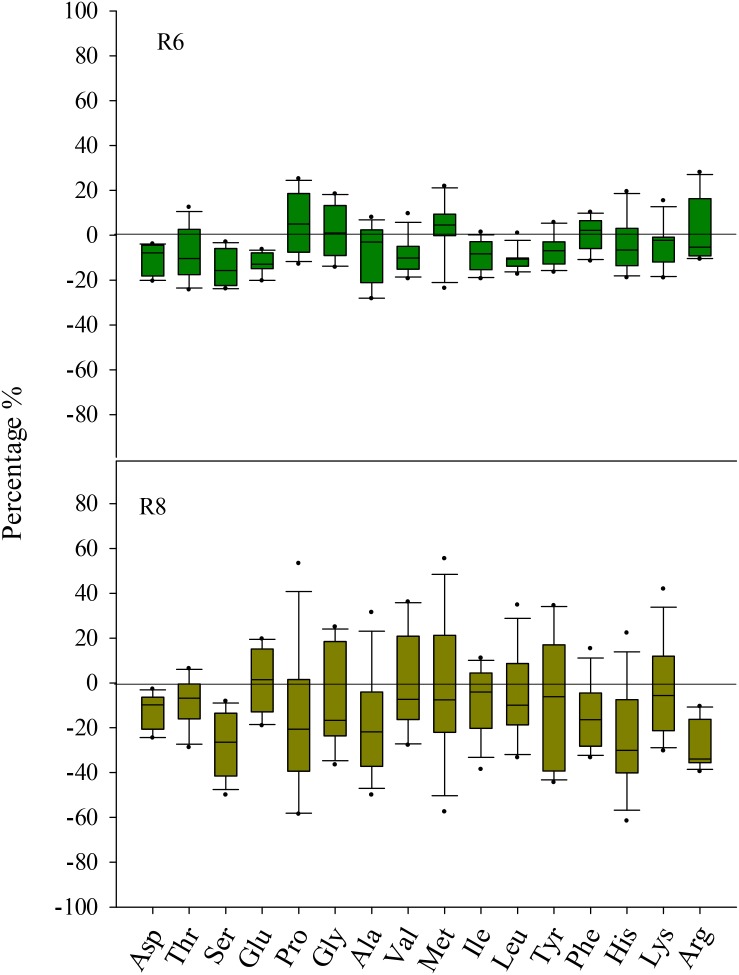
At R6, eCO2 had no influence on the protein concentration when compared with aCO2 (P > 0.05; Figure 1). However, eCO2 significantly (P < 0.05) decreased the free amino acid concentration at R6 (Supplementary Table S1). Except for Met, the concentrations of all other free amino acid decreased under eCO2 (P < 0.05). The extent of change in free amino acid concentrations in the seed in response to eCO2 varied between cultivars (P < 0.05; Figure 2), implying a significant CO2 × cultivar interaction (P < 0.05; Table 1).
FIGURE 1.
Influence of elevated CO2 on soybean seed protein concentrations at fresh edible (R6) and mature stages (R8) in the four soybean cultivars: Zhongke-maodou 1(ZK-1), Zhongke-maodou 2 (ZK-2), Zhongke-maodou 3(ZK-3), and Hei-maodou (HD); error bars represent the standard error (n = 3). Significant differences between CO2 treatments for each cultivar are noted with an asterisk at P < 0.05.
FIGURE 2.
Boxplot shows the mean response ratio of the seed free amino acid concentrations of four soybean cultivars under elevated CO2 at fresh edible (R6) and mature stages (R8).
Table 1.
Significance levels of main effects and interactions of CO2 and cultivars on soybean grain free amino acid concentrations at R6 and R8.
| R6 |
R8 |
|||||
|---|---|---|---|---|---|---|
| CO2 | Genotype | C × G | CO2 | Genotype | C × G | |
| Asp | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ |
| Thr | ∗∗∗ | ∗∗∗ | ∗∗∗ | n.s. | ∗∗∗ | n.s. |
| Ser | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ |
| Glu | ∗∗∗ | ∗∗∗ | ∗∗∗ | n.s. | ∗∗∗ | ∗∗∗ |
| Pro | ∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ |
| Gly | n.s. | ∗∗∗ | ∗∗ | n.s. | n.s. | n.s. |
| Ala | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | n.s. |
| Val | ∗∗∗ | ∗∗∗ | ∗ | n.s. | ∗∗∗ | n.s. |
| Met | n.s. | ∗∗∗ | n.s. | n.s. | n.s. | n.s. |
| Ile | ∗∗∗ | ∗∗∗ | ∗∗∗ | n.s. | ∗∗ | n.s. |
| Leu | ∗∗∗ | ∗∗∗ | n.s. | n.s. | ∗∗∗ | n.s. |
| Tyr | ∗∗∗ | ∗∗∗ | n.s. | n.s. | n.s. | n.s. |
| Phe | n.s. | ∗∗∗ | ∗∗∗ | ∗∗ | n.s. | n.s. |
| His | n.s. | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | n.s. |
| Lys | ∗ | ∗∗∗ | ∗∗ | n.s. | ∗∗∗ | n.s. |
| Arg | n.s. | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ |
| Total | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ |
∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; n.s., not significant (two-way ANOVA).
At R8, eCO2 significantly decreased seed protein concentration by 3.6%, 2.4%, 4.1%, and 6.1% in ZK-1, ZK-2, ZK-3, and HD, respectively. A significant effect of CO2 × cultivar interaction on protein concentration was observed (P < 0.001; Figure 1). Correspondingly, the free amino acid concentration decreased significantly (P < 0.05) under eCO2. The concentrations of Glu and Pro increased in response to eCO2 in ZK-1, whereas the concentration of Lys increased in response to eCO2 in ZK-2 and ZK-3. There was no influence of eCO2 on the concentrations of Thr, Gly, Val, Met, Ile, Leu, and Tyr (P > 0.05) in any of the cultivars (Table 1 and Supplementary Table S2).
Oil and Fatty Acids
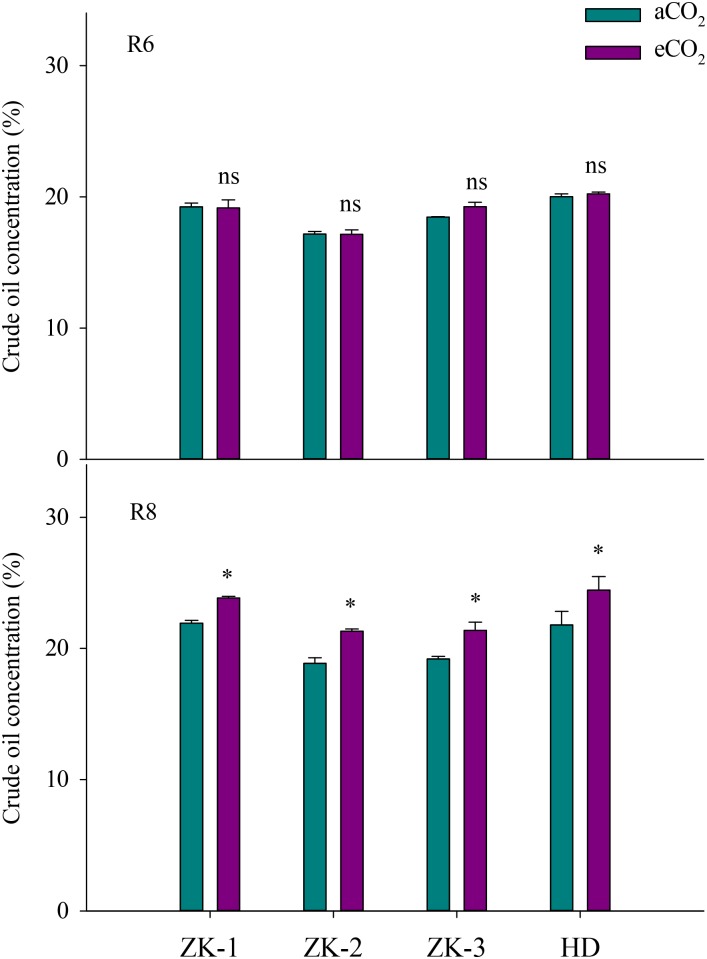
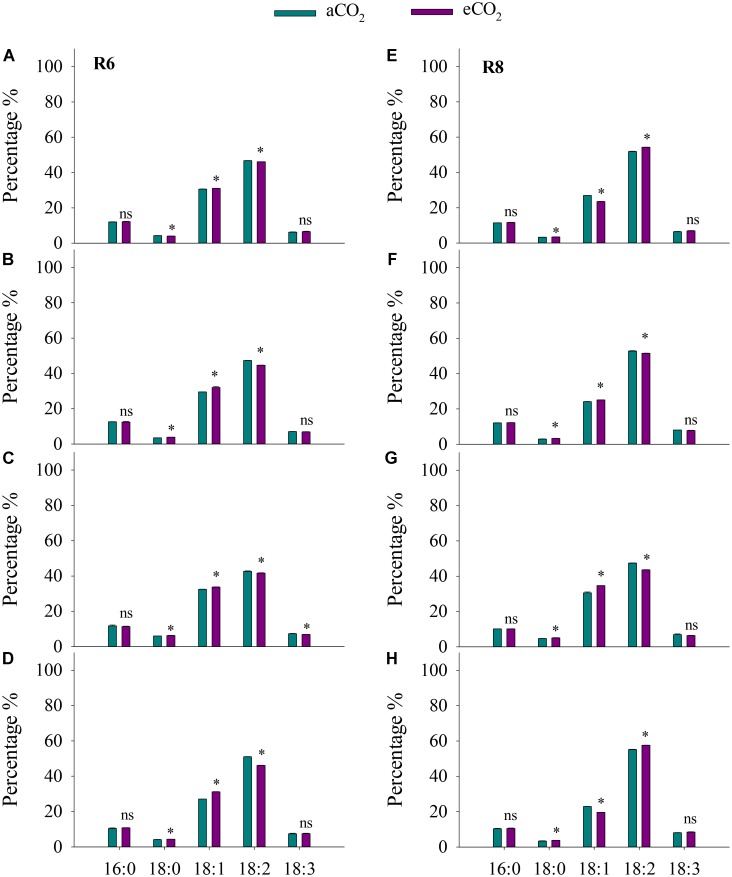
Elevated CO2 had no influence on soybean oil concentration in all cultivars at R6 (Figure 3). However, eCO2 significantly (P < 0.05) increased oleic acid (18:1) concentration by 1.4%, 8.5%, 3.8%, and 15% in ZK-1, ZK-2, ZK-3, and HD, respectively, and significantly decreased linoleic acid (18:2) concentration by 1.4%, 5.5%, 2.1%, and 9.6% (P < 0.05). Together, oleic acid and linoleic acid accounted for more than 75% of the total oil content. Furthermore, eCO2 significantly (P < 0.05) increased stearic acid (18:0) levels by 10%, 6.1%, and 4.8%, respectively, in ZK-2, ZK-3, and HD; however, this was significantly decreased by 6.3% in ZK-1 (P < 0.05). Elevated CO2 had no effect on either palmitic acid (16:0) or linoleic acid (18:3) concentration at R6 (P > 0.05; Figure 4).
FIGURE 3.
Influence of elevated CO2 on soybean seed oil concentrations at fresh edible (R6) and mature stages (R8) of four soybean cultivars: Zhongke-maodou 1 (ZK-1), Zhongke-maodou 2 (ZK-2), Zhongke-maodou 3 (ZK-3), and Hei-maodou (HD); error bars represent the standard error (n = 3). Significant differences between CO2 treatments for each cultivar are noted with an asterisk at P < 0.05.
FIGURE 4.
Influence of elevated CO2 on the percentage of palmitic (16:0), stearic acid (18:0), oleic acid (18:1), linoleic acid (18:2), and linoleic (18:3) acid of ZK-1 (A), ZK-2 (B), ZK-3 (C), and HD (D) at the fresh edible stage (R6) and ZK-1 (E), ZK-2 (F), ZK-3 (G), and HD (H) at the mature stage (R8); error bars represent the standard error (n = 3). Significant differences between CO2 treatments for each cultivar are noted with an asterisk at P < 0.05.
At R8, eCO2 significantly increased (P < 0.05) seed oil concentration by 8.7%, 13%, 11%, and 12% in ZK-1, ZK-2, ZK-3, and HD, respectively (Figure 3). The content of oil significantly increased as well (Supplementary Table S3). Elevated CO2 increased stearic acid (18:0) concentration (P < 0.05) by 5.4%, 13%, 7.8%, and 12% in ZK-1, ZK-2, ZK-3, and HD, respectively. Differential responses of oleic acid (18:1) concentration to eCO2 among cultivars were found. Elevated CO2 significantly (P < 0.05) increased oleic acid (18:1) concentration by 4.2% and 13% in ZK-2 and ZK-3 but not in ZK-1 and HD. On the contrary, the concentration of linoleic acid (18:2) was (P < 0.05) decreased by 2.3% and 8.1% in ZK-2 and ZK-3, but it increased (P < 0.05) by 4.7% and 4.5% in ZK-1 and HD under eCO2 (Figure 4).
Seed Isoflavones
Compared with aCO2, at R6, eCO2 increased total isoflavone concentration by 38% across the four cultivars (Figure 5). The increase in isoflavone concentration in response to eCO2 varied among cultivars (P < 0.001), implying a significant CO2 × cultivar interaction (P < 0.001). The concentrations of daidzin, glycitin, genistin, glycitein, and genistein were significantly (P < 0.01) increased in all cultivars in response to eCO2. Elevated CO2 increased the concentration of daidzein by 29% in HD (P < 0.05) but not in ZK-1, ZK-2, and ZK-3.
FIGURE 5.
Influence of elevated CO2 on isoflavone concentrations of ZK-1 (A), ZK-2 (B), ZK-3 (C), and HD (D) at the fresh edible stage (R6) and ZK-1 (E), ZK-2 (F), ZK-3 (G), and HD (H) at the mature stage (R8); error bars represent the standard error (n = 3). Significant differences between CO2 treatments for each cultivar are noted with an asterisk at P < 0.05.
At R8, eCO2 significantly increased total isoflavone concentration by 28% across the four cultivars. Significant increases in the concentrations of daidzein, daidzin, glycitin, genistin, glycitein, and genistein were observed (P < 0.001) in all cultivars under eCO2 (Figure 5).
Mineral Element Concentrations
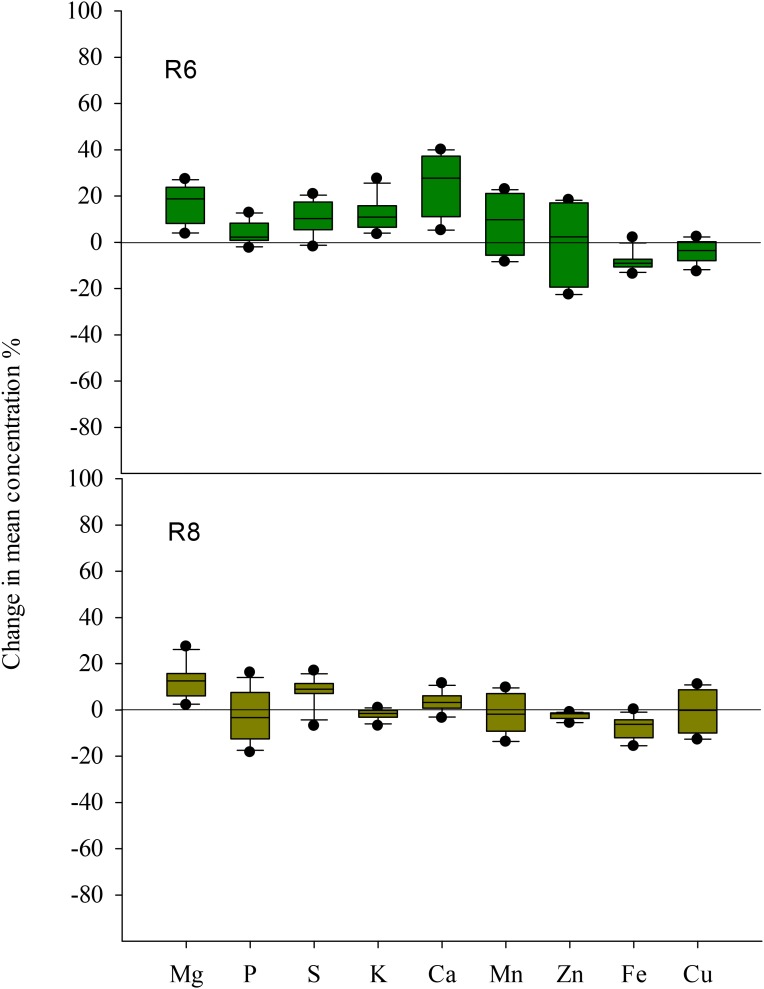
Elevated CO2 had no influence on Cu concentration in soybean seed at R6 (Table 2). However, the concentrations of other mineral elements responded differently to eCO2 among cultivars. Under eCO2, the Zn concentration was significantly decreased by 10% and 22% in ZK-1 and ZK-3, respectively, but significantly increased (P < 0.05) by 14% and 18% in ZK-2 and HD, respectively (Table 2 and Figure 6). The Fe concentration was decreased by 10%, 8.2%, 5.9%, and 13% (P < 0.05) in ZK-1, ZK-2, ZK-3, and HD, respectively (Table 2 and Figure 6). The Mn concentration was also decreased significantly by 8.3% in ZK-3 under eCO2 but increased in the other three cultivars. Elevated CO2 increased the P concentration by 12% in HD, whereas no changes were observed in other cultivars. The S concentration was increased by 10%, 13%, and 19% (P < 0.05) in ZK-1, ZK-2, and HD, respectively, under eCO2, whereas the concentration remained unchanged in ZK-3. Elevated CO2 increased Ca concentration (P < 0.05) by 23% and 34% in ZK-3 and HD, respectively, but it had no influence on Ca concentration in ZK-1 and ZK-2 (Table 3). Although Mg concentration changed only slightly in HD, a significant (P < 0.05) increase in ZK-1, ZK-2, and ZK-3 was found under eCO2. Consistent increases in K concentration among cultivars were found under eCO2, with 13%, 22%, 7.4%, and 6.1% increase in ZK-1, ZK-2, ZK-3, and HD, respectively (P < 0.05).
Table 2.
Effect of eCO2 on soybean seed element concentrations at R6 and R8.
| R6 |
R8 |
||||
|---|---|---|---|---|---|
| Cultivar | aCO2 | eCO2 | aCO2 | eCO2 | |
| Mg | ZK-1 | 4.96 | 5.93∗∗ | 6.39 | 7.31∗∗ |
| mg/g | ZK-2 | 5.66 | 6.96∗ | 5.65 | 6.09n.s. |
| ZK-3 | 4.96 | 6.03∗ | 5.96 | 6.53n.s. | |
| HD | 6.96 | 7.30∗ | 7.41 | 8.75∗ | |
| P | ZK-1 | 9.60 | 9.70n.s. | 14.65 | 12.72n.s. |
| mg/g | ZK-2 | 8.77 | 8.96n.s. | 11.40 | 12.17n.s. |
| ZK-3 | 7.79 | 7.87n.s. | 10.34 | 10.29n.s. | |
| HD | 8.45 | 9.44∗∗ | 13.15 | 12.48n.s. | |
| S | ZK-1 | 6.61 | 7.28∗ | 7.87 | 7.92n.s. |
| mg/g | ZK-2 | 5.25 | 5.94∗ | 8.02 | 8.98∗∗ |
| ZK-3 | 6.27 | 6.32n.s. | 7.79 | 8.63∗ | |
| HD | 6.21 | 7.42∗∗ | 8.27 | 9.00∗ | |
| K | ZK-1 | 25.0 | 28.2∗∗ | 34.3 | 34.1n.s. |
| mg/g | ZK-2 | 20.7 | 25.2∗ | 30.8 | 30.6n.s. |
| ZK-3 | 24.5 | 26.3n.s. | 32.3 | 32.1n.s. | |
| HD | 24.9 | 26.4∗∗ | 35.9 | 34.1n.s. | |
| Ca | ZK-1 | 3.77 | 4.64n.s. | 5.50 | 5.68n.s. |
| mg/g | ZK-2 | 3.29 | 3.60n.s. | 5.10 | 5.42∗∗ |
| ZK-3 | 4.07 | 5.45∗∗ | 3.82 | 3.85n.s. | |
| HD | 3.27 | 4.36∗∗ | 4.52 | 4.63n.s. | |
| Mn | ZK-1 | 20.4 | 23.9∗∗ | 16.9 | 18.4∗∗ |
| ug/g | ZK-2 | 15.5 | 15.9∗∗ | 14.6 | 14.8∗∗ |
| ZK-3 | 19.9 | 18.2∗∗ | 18.4 | 16.1∗∗ | |
| HD | 20.2 | 24.8∗∗ | 29.1 | 27.6∗∗ | |
| Zn | ZK-1 | 36.6 | 33.1∗∗ | 43.3 | 42.7∗ |
| ug/g | ZK-2 | 35.3 | 40.2∗∗ | 43.2 | 42.7n.s. |
| ZK-3 | 38.0 | 29.4∗∗ | 42.2 | 41.4∗∗ | |
| HD | 35.3 | 41.7∗∗ | 44.8 | 42.6∗∗ | |
| Fe | ZK-1 | 49.8 | 45.2∗∗ | 63.4 | 59.5∗ |
| ug/g | ZK-2 | 51.5 | 47.6∗∗ | 63.8 | 59.7∗ |
| ZK-3 | 45.2 | 42.7∗ | 55.1 | 53.2∗ | |
| HD | 45.1 | 39.9∗∗ | 54.1 | 46.2∗ | |
| Cu | ZK-1 | 22.9 | 20.5n.s. | 23.1 | 25.5∗∗ |
| ug/g | ZK-2 | 20.6 | 20.5n.s. | 18.6 | 19.2∗∗ |
| ZK-3 | 23.2 | 21.6n.s. | 24.4 | 23.5∗ | |
| HD | 20.2 | 20.5n.s. | 25.9 | 22.7∗ | |
∗, ∗∗, and n.s. indicate significance at 0.05, 0.01 level, and non-significant difference (t-test) between aCO2 and eCO2, respectively, for individual cultivars.
FIGURE 6.
Boxplot shows the mean response ratio of the grain chemical element concentrations of four soybean cultivars under elevated CO2 at the fresh edible (R6) and mature stages (R8).
Table 3.
Significance levels of main effects and interactions of CO2 and cultivars on soybean grain element nutrients concentrations at R6 and R8.
| R6 |
R8 |
|||||
|---|---|---|---|---|---|---|
| CO2 | Genotype | C × G | CO2 | Genotype | C × G | |
| Mg | ∗∗∗ | ∗∗∗ | n.s. | ∗∗∗ | ∗∗∗ | n.s. |
| P | ∗∗ | ∗∗∗ | ∗ | n.s. | ∗∗ | n.s. |
| S | ∗∗∗ | ∗∗∗ | ∗∗ | ∗∗ | ∗ | n.s. |
| K | ∗∗∗ | ∗∗∗ | ∗∗ | ∗ | ∗∗∗ | ∗∗ |
| Ca | ∗∗∗ | ∗∗∗ | n.s. | ∗ | ∗∗∗ | n.s. |
| Mn | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗ | ∗∗∗ | ∗∗∗ |
| Zn | ∗ | ∗∗∗ | ∗∗∗ | ∗∗ | ∗∗∗ | ∗∗∗ |
| Fe | ∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | ∗∗∗ | n.s. |
| Cu | n.s. | ∗∗ | n.s. | n.s. | ∗∗∗ | ∗∗∗ |
∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; n.s., not significant (two-way ANOVA).
At R8, eCO2 had no effect on P and K concentrations in seeds (Tables 2, 3). Elevated CO2 had a significantly positive effect on Mg, S, and Ca concentrations (Figure 6). On the contrary, Zn and Fe concentrations were significantly (P < 0.05) decreased under eCO2. The Mn concentration was decreased by 12% and 5.2% in ZK-3 and HD, respectively, but significantly increased by 9.3% and 1.7% in ZK-1 and ZK-2, respectively, under eCO2. Similarly, the Cu concentration was decreased by 3.8% and 14% in ZK-3 and HD, respectively, but significantly increased by 10% and 3.2% in ZK-1 and ZK-2, respectively, under eCO2 (Table 2). The protein and nutrient contents of the four cultivars increased under eCO2 compared with aCO2 (Supplementary Table S3).
Discussion
This study demonstrated that eCO2 had no influence on protein concentration in soybean seed at R6, but eCO2 significantly (P < 0.05) decreased protein concentration at R8 (Figure 1). This finding suggests that, although soybean plants are able to symbiotically fix N2 to mitigate N deficiency, shortfalls still occur after R6 under eCO2 when grown in Mollisols. Several studies argue that the lower seed protein concentration under eCO2 is attributed to the dilution effect, as eCO2 increases the accumulation of carbohydrates (Gifford et al., 2000; Wu et al., 2004). The increased carbon (C) gain under eCO2 might be used for protein synthesis, which requires a large amount of energy for the maintenance of the synthetic process (Fangmeier et al., 2002; Pérez-López et al., 2014). In this context, the CO2-induced reduction in protein concentration cannot be fully attributed to N limitation in soybeans under eCO2. Several other authors also reported that the decrease in protein concentration under eCO2 could not be diminished by additional N supply (Fangmeier et al., 1997; Weigel and Manderscheid, 2005). Therefore, soybean crops grown under eCO2 may have lower protein content, with nutritional implications for humans and animals that consume these crops as a food source. Specialized breeding strategies that intend to enhance seed quality are needed to address this issue (Bellaloui et al., 2015).
In the present study, eCO2 lowered total free amino acid concentrations both at the R6 and R8 stages. This indicated that the nutrient values of total free amino acids at both stages were reduced under eCO2 (Supplementary Table S1). Free amino acids are an important form of N storage in soybean seed during the processes of N assimilation and protein synthesis (Takahashi et al., 2003; Song et al., 2013). In particular, glutamine is the only product into which inorganic N is transformed (Pratelli and Pilot, 2014). Arginine is important in protein synthesis, and it is predominantly derived from glutamine in the urea cycle (Takahashi et al., 2003). Therefore, the decreased levels of free amino acids under eCO2 may be channeled toward the synthesis of functional proteins rather than those stored in the soybean seed.
The present study demonstrated that eCO2 significantly increased the oil concentration of soybeans at R8 (Figure 3); this result is supported by previous findings of an increase in oil concentration in soybean or other crops under eCO2 (Heagle et al., 1998; Högy et al., 2010; Hao et al., 2014). This phenomenon is reasonable, as oil synthesis and storage in plants, which are enhanced under eCO2, are involved in carbon and energy supply (Rawsthorne, 2002; Bates et al., 2013). Singh et al. (2016) also reported that the increased seed oil concentration under eCO2 is attributed to the direct stimulatory effect on photosynthesis. However, no studies have investigated oil composition in soybean under eCO2. To our knowledge, the present study is the first to report that eCO2 consistently increased oleic acid (18:1) concentrations but decreased linoleic acid (18:2) concentrations at R6 and R8. This finding indicates that eCO2 improves soybean oil quality, with potential benefits for human health. High levels of oleic acid (18:1) enhance the oxidative stability of soybean oil, giving it a longer shelf life (Clemente and Cahoon, 2009), whereas the increased levels of trans-fatty acids by partial hydrogenation of linoleic acid (18:2) are associated with heart disease (Demorest et al., 2016). Nevertheless, the mechanism underlying the increase in fatty acids under eCO2 is complex; major gaps remain in our understanding of the regulation of fatty acid synthesis, especially in tissues that store large amounts of oil (Rawsthorne, 2002; Bates et al., 2013).
The present study found that eCO2 significantly increased total and specific isoflavone concentrations at R6 and R8 (Figure 5). These results were in agreement with a previous study, which demonstrated that variation of isoflavones in soybeans was positively correlated with CO2 level (Kim et al., 2005). Theoretically, the synthesis of isoflavones via the phenylpropanoid pathway requires high levels of carbon (Ralston et al., 2005; Tsai et al., 2006). Weisshaar and Jenkins (1998) reported that approximately 20% of the carbon from photosynthesis is used to synthesize the phenolic compounds found in nature, including flavonoids and isoflavonoids. Larger amounts of carbon could be obtained to generate isoflavones from plants that were grown under eCO2 (Ainsworth et al., 2002; Kretzschmar et al., 2009; O’Neill et al., 2010). Therefore, environmental conditions, including CO2 level, strongly influence isoflavone concentration. Dhaubhadel et al. (2003) stated that the expression of two hydroxy isoflavanone synthase genes, IFS1 and IFS2, in different tissues, is influenced by environmental conditions. Owing to the health-promoting effects of isoflavones on human vasomotor symptoms, the cardiovascular system, the breast, uterus, bone, and cognition, foods with high levels of isoflavone have been recommended by the U.S. Food and Drug Administration (FDA) (Morrison et al., 2008; Clarkson et al., 2011). In the present study, the increase in isoflavone concentration of soybean observed in response to eCO2 suggests improved nutritional value of soybean under the scenario of rising CO2 levels.
The biogeochemical cycles of nutrients are affected by eCO2, and the resulting changes in seed nutrient concentration pose a potential challenge to human health. In this study, eCO2 greatly lowered the nutritional value of seed in terms of Zn and Fe content (Table 2); similar results have been found in rice, wheat, and barley (Myers et al., 2014; Zhu et al., 2018). This may increase the risk of micronutrient malnutrition and other related diseases. Several studies attribute this phenomenon to the dilution effect, which is caused by the increased growth of plants under eCO2. The eCO2-induced increase in grain nutrient content (Supplementary Table S3) also indicates that the demand for nutrients increases under such an environment, which may, to some extent, lead to dilution effect. As a result of the decrease in stomatal conductance under eCO2, plants tend to undergo reduced transpiration, leading to decreased mass flow (Rogers et al., 1999; Bunce, 2001) and absorption of mobile elements such as N (McDonald et al., 2002). However, P and K concentrations were not influenced by eCO2 in the present study (Table 2). This is likely because the increase in soil moisture due to reduced transpiration under eCO2 is beneficial for the diffusion of specific elements in soil to the roots, thus, increasing their availability. Nevertheless, the two primary mechanisms fail to explain the responses of all elements in seeds to eCO2, as significant increases in Mg, S, and Ca concentrations, or no change in Cu concentration, were found in this study (Figure 6). Pérez-López et al. (2014) reported that the increase in growth under eCO2 could be attributed to the stimulation of metabolic activity in plants, and, accordingly, to the requirement of nutrients that serve as enzyme cofactors in metabolic reactions (Ca, Mg, and Mn) and redox reactions (Fe, Zn, and Cu). Therefore, eCO2 has both positive and negative effects on the nutritional quality of soybean seeds. However, further study of the mechanism by which eCO2 influences seed nutrients is necessary, not only because the different elements show varying responses to eCO2 at the same growth stage but also because the same element shows differential responses at R6 and R8.
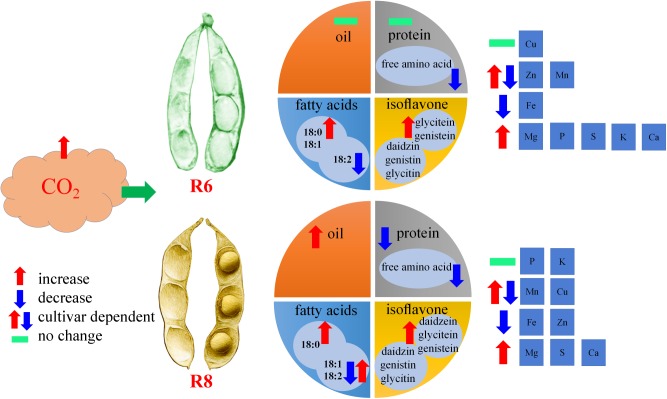
In summary, protein concentration in soybean seeds was significantly decreased under eCO2; however, oil concentration showed the opposite trend at R8. The free amino acid concentration was significantly decreased under eCO2, irrespective of the growth stage. Elevated CO2 resulted in an increase in oleic acid concentration (18:1) of all cultivars at R6. Total isoflavone concentrations were significantly increased at R6 and R8. The concentrations of Fe were significantly decreased at R6 and R8 under eCO2, while the changes in Zn and Mn concentrations varied among cultivars (Figure 7). These results suggest that eCO2 may promote fat content by enhancing oleic acid levels (18:1) but decrease the content of proteins and relevant amino acids.
FIGURE 7.
Diagram illustrating the impact of elevated CO2 on seed quality of soybean at the fresh edible (R6) and mature stages (R8).
Author Contributions
JJ and YL designed the experiments and managed the projects. YL, ZY, CL, QZ, JW, and CW performed the experiments. YL, JJ, GW, and XL performed the data analysis. YL, JJ, and XL wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer PL and handling editor declared their shared affiliation at time of review.
Acknowledgments
The authors thank Drs. Lu Xinchun, Hu Yanfeng, Liu Judong, and Xie Zhihuang for assisting with the measurement of mineral element levels.
Footnotes
Funding. The project was funded by the National Natural Science Foundation of China (31501259), Heilongjiang Provincial Funds for Distinguished Young Scientists (JC201413), Hundred Talents Program of Chinese Academy of Sciences, the Natural Science Foundation of Heilongjiang Province (QC2015045), the Foundation of Key Laboratory for Agricultural Environment, Ministry of Agriculture of China, and the Scientific Research Project of Beijing Municipal Education Commission (KM201810020006).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.01413/full#supplementary-material
References
- Ainsworth E. A., Davey P. A., Bernacchi C. J., Dermody O. C., Heaton E. A., Moore D. J., et al. (2002). A meta-analysis of elevated CO2 effects on soybean (Glycine max) physiology, growth and yield. Global Change Biol. 8 695–709. 10.1046/j.1365-2486.2002.00498.x [DOI] [Google Scholar]
- Ainsworth E. A., Rogers A., Leakey A. D., Heady L. E., Gibon Y., Stitt M., et al. (2007). Does elevated atmospheric [CO2] alter diurnal C uptake and the balance of C and N metabolites in growing and fully expanded soybean leaves? J. Exp. Bot. 58 579–591. 10.1093/jxb/erl233 [DOI] [PubMed] [Google Scholar]
- Allen L. H., Boote K. J. (2000). “Crop ecosystem responses to climatic change: soybean,” in Climate Change and Global Crop Productivity, eds Reddy K. R., Hodges H. F. (Oxon: CABI Publishing; ), 133–160. [Google Scholar]
- Bates P. D., Stymne S., Ohlrogge J. (2013). Biochemical pathways in seed oil synthesis. Curr. Opin. Plant Biol. 16 358–364. 10.1016/j.pbi.2013.02.015 [DOI] [PubMed] [Google Scholar]
- Bellaloui N., Bruns H. A., Abbas H. K., Mengistu A., Fisher D. K., Reddy K. N. (2015). Agricultural practices altered soybean seed protein, oil, fatty acids, sugars, and minerals in the Midsouth USA. Front. Plant Sci. 6:31. 10.3389/fpls.2015.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. O., Yu O., Heatherly L. G., Krishnan H. B. (2004). Accumulation of genistein and daidzein, soybean isoflavones implicated in promoting human health, is significantly elevated by irrigation. J. Agric. Food Chem. 52 7574–7579. 10.1021/jf049133k [DOI] [PubMed] [Google Scholar]
- Bishop K. A., Betzelberger A. M., Long S. P., Ainsworth E. A. (2015). Is there potential to adapt soybean (Glycine max Merr.) to future CO2? An analysis of the yield response of 18 genotypes in free-air CO2 enrichment. Plant Cell Environ. 38 1765–1774. 10.1111/pce.12443 [DOI] [PubMed] [Google Scholar]
- Bunce J. A. (2001). Direct and acclimatory responses of stomatal conductance to elevated carbon dioxide in four herbaceous crop species in the field. Global Change Biol. 7 323–331. 10.1046/j.1365-2486.2001.00406.x [DOI] [Google Scholar]
- Chang E. T., Lee V. S., Canchola A. J., Clarke C. A., Purdie D. M., Reynolds P., et al. (2007). Diet and risk of ovarian cancer in the California teachers study cohort. Am. J. Epidemiol. 165 802–813. 10.1093/aje/kwk065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi A. K., Bahuguna R. N., Pal M., Shah D., Maurya S., Jagadish K. S. V. (2017). Elevated CO2 and heat stress interactions affect grain yield, quality and mineral nutrient composition in rice under field conditions. Field Crop Res. 206 149–157. 10.1016/j.fcr.2017.02.018 [DOI] [Google Scholar]
- Clarkson T. B., Utian W. H., Barnes S., Gold E. B., Basaria S. S., Aso T., et al. (2011). The role of soy isoflavones in menopausal health: report of The North American Menopause Society/Wulf H. Utian Translational Science Symposium in Chicago, IL (October 2010). Menopause J. North Am. Menopause Soc. 18 732–753. 10.1097/gme.0b013e31821fc8e0 [DOI] [PubMed] [Google Scholar]
- Clemente T. E., Cahoon E. B. (2009). Soybean oil: genetic approaches for modification of functionality and total content. Plant Physiol. 151 1030–1040. 10.1104/pp.109.146282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demorest Z. L., Coffman A., Baltes N. J., Stoddard T. J., Clasen B. M., Luo S., et al. (2016). Direct stacking of sequence-specific nuclease-induced mutations to produce high oleic and low linolenic soybean oil. BMC Plant Biol. 16:225. 10.1186/s12870-016-0906-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S. K., Chakrabarti B., Prasanna R., Pratap D., Singh S. D., Purakayastha T. J., et al. (2017). Elevated carbon dioxide level along with phosphorus application and cyanobacterial inoculation enhances nitrogen fixation and uptake in cowpea crop. Arch. Agron. Soil Sci. 63 1927–1937. 10.1080/03650340.2017.1315105 [DOI] [Google Scholar]
- Dhaubhadel S., McGarvey B. D., Williams R., Gijzen M. (2003). Isoflavonoid biosynthesis and accumulation in developing soybean seeds. Plant Mol. Biol. 53 733–743. [DOI] [PubMed] [Google Scholar]
- Erbs M., Manderscheid R., Jansen G., Seddig S., Pacholski A., Weigel H.-J. (2010). Effects of free-air CO2 enrichment and nitrogen supply on grain quality parameters and elemental composition of wheat and barley grown in a crop rotation. Agric. Ecosyst. Environ. 136 59–68. 10.1016/j.agee.2009.11.009 [DOI] [Google Scholar]
- Fangmeier A., De Temmerman L., Black C., Persson K., Vorne V. (2002). Effects of elevated CO2 and/or ozone on nutrient concentrations and nutrient uptake of potatoes. Eur. J. Agron. 17 353–368. 10.1016/s1161-0301(02)00071-0 [DOI] [Google Scholar]
- Fangmeier A., Gruters U., Högy P., Vermehren B., Jager H. J. (1997). Effects of elevated CO2 nitrogen supply and tropospheric ozone on spring wheat.2. Nutrients (N, P, K, S, Ca, Mg, Fe, Mn, Zn). Environ. Pollut. 96 43–59. 10.1016/s0269-7491(97)00013-4 [DOI] [PubMed] [Google Scholar]
- Fehr W. R., Caviness C. E., Burmood D. T., Pennington J. S. (1971). Stage of development descriptions for soybeans, Glycine max (L.) merrill. Crop Sci. 11 929–931. 10.2135/cropsci1971.0011183X001100060051x [DOI] [Google Scholar]
- Gifford R. M., Barrett D. J., Lutze J. L. (2000). The effects of elevated CO2 on the C : N and C : P mass ratios of plant tissues. Plant Soil 224 1–14. [Google Scholar]
- Hao X. Y., Gao J., Han X., Ma Z. Y., Merchant A., Ju H., et al. (2014). Effects of open-air elevated atmospheric CO2 concentration on yield quality of soybean (Glycine max (L.) Merr). Agric. Ecosyst. Environ. 192 80–84. 10.1016/j.agee.2014.04.002 [DOI] [Google Scholar]
- Hao X. Y., Li P., Han X., Norton R. M., Lam S. K., Zong Y. Z., et al. (2016). Effects of free-air CO2 enrichment (FACE) on N, P and K uptake of soybean in northern China. Agric. Forest. Meteorol. 21 261–266. 10.1016/j.agrformet.2015.12.061 [DOI] [Google Scholar]
- Heagle A. S., Miller J. E., Pursley W. A. (1998). Influence of ozone stress on soybean response to carbon dioxide enrichment: III. Yield and seed quality. Crop Sci. 38 128–134. 10.2135/cropsci1998.0011183X003800010022x [DOI] [Google Scholar]
- Högy P., Fangmeier A. (2009). Atmospheric CO2 enrichment affects potatoes: 2. Tuber quality traits. Eur. J. Agron. 30 85–94. 10.1016/j.eja.2008.07.006 [DOI] [Google Scholar]
- Högy P., Franzaring J., Schwadorf K., Breuer J., Schuetze W., Fangmeier A. (2010). Effects of free-air CO2 enrichment on energy traits and seed quality of oilseed rape. Agric. Ecosyst. Environ. 139 239–244. 10.1016/j.agee.2010.08.009 [DOI] [Google Scholar]
- Kim S. H., Jung W. S., Ahn J. K., Kim J. A., Chung I. M. (2005). Quantitative analysis of the isoflavone content and biological growth of soybean (Glycine max L.) at elevated temperature, CO2 level and N application. J. Sci. Food Agric. 85 2557–2566. 10.1002/jsfa.2294 [DOI] [Google Scholar]
- Kretzschmar F. D. S., Marinho Aidar M. P., Salgado I., Braga M. R. (2009). Elevated CO2 atmosphere enhances production of defense-related flavonoids in soybean elicited by NO and a fungal elicitor. Environ. Exp. Bot. 65 319–329. 10.1016/j.envexpbot.2008.10.001 [DOI] [Google Scholar]
- Krishnan P., Swain D. K., Bhaskar B. C., Nayak S. K., Dash R. N. (2007). Impact of elevated CO2 and temperature on rice yield and methods of adaptation as evaluated by crop simulation studies. Agric. Ecosyst. Environ. 122 233–242. 10.1016/j.agee.2007.01.019 [DOI] [Google Scholar]
- Leakey A. D., Ainsworth E. A., Bernacchi C. J., Rogers A., Long S. P., Ort D. R. (2009). Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J. Exp. Bot. 60 2859–2876. 10.1093/jxb/erp096 [DOI] [PubMed] [Google Scholar]
- Li Y. S., Du M., Zhang Q. Y., Wang G. H., Jin J., Herbert S., et al. (2014). Planting date influences fresh pod yield and seed chemical compositions of vegetable soybean. Hortscience 49 1376–1380. [Google Scholar]
- Li Y. S., Yu Z. H., Liu X. B., Mathesius U., Wang G. H., Tang C. X., et al. (2017). Elevated CO2 increases nitrogen fixation at the reproductive phase contributing to various yield responses of soybean cultivars. Front. Plant Sci. 8:1546. 10.3389/fpls.2017.01546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieffering M., Kim H. Y., Kobayashi K., Okada M. (2004). The impact of elevated CO2 on the elemental concentrations of field-grown rice grains. Field Crop Res. 88 279–286. 10.1016/j.fcr.2004.01.004 [DOI] [Google Scholar]
- Liu N. N., Tian Q. Y., Zhang W. H. (2016). Artemisia frigida and Stipa krylovii, two dominant species in Inner Mongolia steppe, differed in their responses to elevated atmospheric CO2 concentration. Plant Soil 409 117–129. 10.1007/s11104-016-2952-8 [DOI] [Google Scholar]
- Liu X. B., Herbert S. J. (2002). Fifteen years of research examining cultivation of continuous soybean in northeast China: a review. Field Crop Res. 79 1–7. 10.1016/s0378-4290(02)00042-4 [DOI] [Google Scholar]
- MacDonald R. S., Guo J. Y., Copeland J., Browning J. D., Sleper D., Rottinghaus G. E., et al. (2005). Environmental influences on isoflavones and saponins in soybeans and their role in colon cancer. J. Nutr. 135 1239–1242. 10.1093/jn/135.5.1239 [DOI] [PubMed] [Google Scholar]
- McDonald E. P., Erickson J. E., Kruger E. L. (2002). Can decreased transpiration limit plant nitrogen acquisition in elevated CO2? Funct. Plant Biol. 29 1115–1120. 10.1071/fp02007 [DOI] [PubMed] [Google Scholar]
- Medic J., Atkinson C., Hurburgh C. R., Jr. (2014). Current knowledge in soybean composition. J. Am. Oil Chem. Soc. 91 363–384. 10.1007/s11746-013-2407-9 [DOI] [Google Scholar]
- Morrison M. J., Cober E. K., Saleem M. F., McLaughlin N. B., Fregeau-Reid J., Ma B. L., et al. (2008). Changes in isoflavone concentration with 58 years of genetic improvement of short-season soybean cultivars in Canada. Crop Sci. 48 2201–2208. 10.2135/cropsci2008.01.0023 [DOI] [Google Scholar]
- Myers S. S., Zanobetti A., Kloog I., Huybers P., Leakey A. D., Bloom A. J., et al. (2014). Increasing CO2 threatens human nutrition. Nature 510 139–142. 10.1038/nature13179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill B. F., Zangerl A. R., Dermody O., Bilgin D. D., Casteel C. L., Zavala J. A., et al. (2010). Impact of elevated levels of atmospheric CO2 and herbivory on flavonoids of soybean (Glycine max Linnaeus). J. Chem. Ecol. 36 35–45. 10.1007/s10886-009-9727-0 [DOI] [PubMed] [Google Scholar]
- Pérez-López U., Miranda-Apodaca J., Mena-Petite A., Muñoz-Rueda A. (2014). Responses of nutrient dynamics in barley seedlings to the interaction of salinity and carbon dioxide enrichment. Environ. Exp. Bot. 99 86–99. 10.1016/j.envexpbot.2013.11.004 [DOI] [Google Scholar]
- Pratelli R., Pilot G. (2014). Regulation of amino acid metabolic enzymes and transporters in plants. J. Exp. Bot. 65 5535–5556. 10.1093/jxb/eru320 [DOI] [PubMed] [Google Scholar]
- Prior S. A., Runion G. B., Rogers H. H., Torbert H. A. (2008). Effects of atmospheric CO2 enrichment on crop nutrient dynamics under no-till conditions. J. Plant Nutr. 31 758–773. 10.1080/01904160801928364 [DOI] [Google Scholar]
- Qin P. Y., Song W. W., Yang X. S., Sun S., Zhou X. R., Yang R. P., et al. (2014). Regional distribution of protein and oil compositions of soybean cultivars in china. Crop Sci. 54 1139–1587. 10.2135/cropsci2013.05.0314 [DOI] [Google Scholar]
- Ralston L., Subramanian S., Matsuno M., Yu O. (2005). Partial reconstruction of flavonoid and isoflavonoid biosynthesis in yeast using soybean type I and type II chalcone isomerases. Plant Physiol. 137 1375–1388. 10.1104/pp.104.054502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawsthorne S. (2002). Carbon flux and fatty acid synthesis in plants. Prog. Lipid Res. 41 182–196. 10.1016/s0163-7827(01)00023-6 [DOI] [PubMed] [Google Scholar]
- Rogers H. H., Runion G. B., Prior S. A., Torbert H. A. (1999). Response of plants to elevated atmospheric CO2: root growth, mineral nutrition, and soil carbon. Carbon Dioxide Environ. Stress 75 215–244. 10.1371/journal.pone.0149301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldivar X., Wang Y. J., Chen P., Hou A. (2011). Changes in chemical composition during soybean seed development. Food Chem. 124 1369–1375. 10.1016/j.foodchem.2010.07.091 [DOI] [Google Scholar]
- Singh S. K., Barnaby J. Y., Reddy V. R., Sicher R. C. (2016). Varying response of the concentration and yield of soybean seed mineral elements, carbohydrates, organic acids, amino acids, protein, and oil to phosphorus starvation and CO2 enrichment. Front. Plant Sci. 7:1967. 10.3389/fpls.2016.01967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J. F., Liu C. Q., Li D. J., Gu Z. X. (2013). Evaluation of sugar, free amino acid, and organic acid compositions of different varieties of vegetable soybean (Glycine max L. Merr). Ind. Crop Prod. 50 743–749. 10.1016/j.indcrop.2013.08.064 [DOI] [Google Scholar]
- Stocker T. F., Qin D., Plattner G. K., Alexander L. V., Allen S. K., Bindoff N. L., et al. (2013). Technical summary. in: climate Change 2013: the physical science basis. contribution of working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Computat. Geom. 18 95–123. [Google Scholar]
- Sun J. M., Sun B. L., Han F. X., Yan S. R., Yang H. J., Kikuchi A. (2011). Rapid HPLC method for determination of 12 isoflavone components in soybean seeds. Agric. Sci. China 10 70–77. 10.1016/s1671-2927(11)60308-8 [DOI] [Google Scholar]
- Takahashi M., Uematsu Y., Kashiwaba K., Yagasaki K., Hajika M., Matsunaga R., et al. (2003). Accumulation of high levels of free amino acids in soybean seeds through integration of mutations conferring seed protein deficiency. Planta 217 577–586. 10.1007/s00425-003-1026-3 [DOI] [PubMed] [Google Scholar]
- Taku K., Umegaki K., Sato Y., Taki Y., Endoh K., Watanabe S. (2007). Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 85 1148–1156. 10.1093/ajcn/85.4.1148 [DOI] [PubMed] [Google Scholar]
- Taub D. R., Miller B., Allen H. (2008). Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Global Change Biol. 14 565–575. 10.1111/j.1365-2486.2007.01511.x [DOI] [Google Scholar]
- Thomas J. M. G., Boote K. J., Allen L. H., Gallo-Meagher M., Davis J. M. (2003). Elevated temperature and carbon dioxide effects on soybean seed composition and transcript abundance. Crop Sci. 43 1548–1557. 10.2135/cropsci2003.1548 [DOI] [Google Scholar]
- Thomas J. M. G., Prasad P. V. V., Boote K. J., Allen L. H., Jr. (2009). Seed composition, seedling emergence and early seedling vigour of red kidney bean seed produced at elevated temperature and carbon dioxide. J. Agron. Crop Sci. 195 148–156. 10.1111/j.1439-037X.2008.00348.x [DOI] [Google Scholar]
- Tsai C. J., Harding S. A., Tschaplinski T. J., Lindroth R. L., Yuan Y. (2006). Genome-wide analysis of the structural genes regulating defense phenylpropanoid metabolism in Populus. New Phytol. 172 47–62. 10.1111/j.1469-8137.2006.01798.x [DOI] [PubMed] [Google Scholar]
- Wang D., Heckathorn S. A., Barua D., Joshi P., Hamilton E. W., Lacroix J. J. (2008). Effects of elevated CO2 on the tolerance of photosynthesis to acute heat stress in C3. C4, and CAM species. Am. J. Bot. 95 165–176. 10.3732/ajb.95.2.165 [DOI] [PubMed] [Google Scholar]
- Weigel H. J., Manderscheid R. (2005). CO2 enrichment effects on forage and grain nitrogen content of pasture and cereal plants. J. Crop Imp. 13 73–89. 10.1300/J411v13n01_05 [DOI] [Google Scholar]
- Weisshaar B., Jenkins G. I. (1998). Phenylpropanoid biosynthesis and its regulation. Curr. Opin. Plant Biol. 1 251–257. 10.1016/s1369-5266(98)80113-1 [DOI] [PubMed] [Google Scholar]
- Wu D. X., Wang G. X., Bai Y. F., Liao J. X. (2004). Effects of elevated CO2 concentration on growth, water use, yield and grain quality of wheat under two soil water levels. Agric. Ecosyst. Environ. 104 493–507. 10.1016/j.agee.2004.01.018 [DOI] [Google Scholar]
- Wu T. T., Yao Y., Sun S., Wang C. J., Jia H. C., Man W. Q., et al. (2016). Temporospatial characterization of nutritional and bioactive components of soybean cultivars in China. J. Am. Oil Chem. Soc. 93 1637–1654. 10.1007/s11746-016-2908-4 [DOI] [Google Scholar]
- Yang L. X., Wang Y. L., Dong G. C., Gu H., Huang J. Y., Zhu J. G., et al. (2007). The impact of free-air CO2 enrichment (FACE) and nitrogen supply on grain quality of rice. Field Crop Res. 102 128–140. 10.1016/j.fcr.2007.03.006 [DOI] [Google Scholar]
- Yu Z. H., Li Y. S., Wang G. H., Liu J. J., Liu J. D., Liu X. B., et al. (2016). Effectiveness of elevated CO2 mediating bacterial communities in the soybean rhizosphere depends on genotypes. Agric. Ecosyst. Environ. 231 229–232. 10.1016/j.agee.2016.06.043 [DOI] [Google Scholar]
- Zhang W. W., Wang G. H., Liu X. B., Feng Z. Z. (2014). Effects of elevated O3 exposure on seed yield, N concentration and photosynthesis of nine soybean cultivars (Glycine max (L.) Merr.) in Northeast China. Plant Sci. 226 172–181. 10.1016/j.plantsci.2014.04.020 [DOI] [PubMed] [Google Scholar]
- Zhu C. W., Kobayashi K., Loladze I., Zhu J. G., Jiang Q., Xu X., et al. (2018). Carbon dioxide (CO2) levels this century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci. Adv. 4:eaaq1012. 10.1126/sciadv.aaq1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C. W., Zhu J. G., Liu G., Zeng Q., Xie Z. B., Pang J., et al. (2008). Elevated CO2 concentration enhances the role of the ear to the flag leaf in determining grain yield of wheat. Photosynthetica 46 318–320. 10.1007/s11099-008-0059-z [DOI] [Google Scholar]
- Ziska L. H., Palowsky R., Reed D. R. (2007). A quantitative and qualitative assessment of mung bean (Vigna mungo (L.) Wilczek) seed in response to elevated atmospheric carbon dioxide: potential changes in fatty acid composition. J. Sci. Food Agric. 87 920–923. 10.1002/jsfa.2818 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.