Abstract
Vacuum therapy has been widely used for penile rehabilitation after radical prostatectomy (RP), but its efficacy and safety are unclear. The study was to evaluate the efficacy and safety of the early use of vacuum therapy for post-RP men. Randomized clinical trials were selected according to predefined inclusion and exclusion criteria. RevMan 5.3 software was used for meta-analyses. In total, six randomized controlled trials were included with a total of 273 post-RP patients. The meta-analysis revealed that the early use of vacuum therapy could significantly improve the five-item International Index of Erectile Function and penile shrinkage in post-RP patients. Few adverse events were reported across the included studies. This review suggests that the early use of vacuum therapy appears to have excellent therapeutic effect on post-RP patients and no serious side effects were identified. Due to overall limited quality of the included studies, the therapeutic benefit of vacuum therapy in penile rehabilitation needs be substantiated to a limited degree in the future. Better methodological, large controlled trials are expected to verify the therapeutic effect of vacuum therapy in penile rehabilitation.
Keywords: vacuum therapy, penile rehabilitation, radical prostatectomy, erectile dysfunction, sexuality, penile shrinkage
Prostate cancer is the most frequently diagnosed and the second leading cause of cancer death in men (Siegel, Miller, & Jemal, 2017). In 2014, there are an estimated 3,085,209 men living with prostate cancer in the United States, and the overall risk of an individual male dying from prostate cancer is 2.6% (Primeau, Paterson, & Nabi, 2017). Radical prostatectomy (RP) is one of the most commonly used curative procedures for the treatment of localized prostate cancer. Accumulating studies have documented that there are a higher frequency of adverse events in the patients underwent RP, such as erectile dysfunction (ED) and penile shrinkage (Lee et al., 2018; Wilt et al., 2017). Since 1997, the concept of penile rehabilitation was introduced, and many therapeutic approaches have been attempted with the aim of reducing the ED and penile shrinkage in patients after RP (Montorsi et al., 1997).
A previous study assessed the penile morphometrics of post-RP men, and indicated that denervation muscular atrophy is most apparent between the first 4–8 months after RP (Fraiman, Lepor, & McCullough, 1999). This study suggested that the post-RP men should follow the early penile rehabilitation protocol to maintain the vascular and cellular integrity of the penis (Fraiman et al., 1999). The main therapeutic approaches for penile rehabilitation include oral phosphodiesterase-5 inhibitor (PDE-5i), intraurethral or intracavernosal vasoactiveagents, vacuum therapy, or combination therapy (Liu, Lopez, Chen, & Wang, 2017; Qin et al., 2018). Oral PDE-5i (such as sildenafil and vardenafil) is the most popular initial choice for ED patients after RP. Unfortunately, it was not very effective in elderly patients, or patients with moderate to severe diabetes, hypertension, and coronary artery disease (Naccarato, Reis, Ferreira, & Denardi, 2016; Pahlajani, Raina, Jones, Ali, & Zippe, 2012).
Vacuum therapy has become an attractive proposition in the event of problems with the PDE-5i treatment, such as contraindications or significant side effects (Deng et al., 2017; Zippe & Pahlajani, 2008). Many recent clinical trials have indicated that early initiation of the vacuum erection device (VED) after RP is a simple and effective method for penile rehabilitation (Hoyland, Vasdev, & Adshead, 2013; Qian, Gao, Wei, & Yuan, 2016; Wang, 2017; Yuan, Lin, et al., 2010). VED can increase the amount of blood flowing into the penis by creating a negative pressure of approximately 150 to 200 mm Hg (Yuan, Hoang, et al., 2010). The VED-induced erection is not dependent upon functional nerves or a fully intact vascular supply to enhance blood flow (Engel, 2011). VED will also increase the confidence and enthusiasm of the patient and his partner sexual satisfaction, preserve penile length, and allow earlier return of spontaneous erection for post-RP men (Wang, 2017). The limited evidence for VED effectiveness and safety in randomized controlled trials (RCTs) has led to doubts over its usage.
This article reviewed available evidence on vacuum therapy to offer guidance for the early treatment of post-RP men. The result would be helpful to assess the effectiveness and safety of the early use of vacuum therapy for penile rehabilitation.
Methods
Inclusion and Exclusion Criteria
Studies were eligible for inclusion in the meta-analysis if they met all of the following criteria: (a) the study was conducted as a randomized, controlled clinical trial; (b) the study consisted of the men who underwent RP (nerve sparing or non-nerve sparing) as a treatment for prostate cancer; (c) all patients were initially evaluated with a comprehensive sexual history, physical examination, and pertinent laboratory testing; (d) the dosage of PDE-5i in the early VED group was equal to that in the control group, when PDE-5i in combination with VED; (e) a minimum follow-up of 3 months was required; and (f) authors were contacted if insufficient data were reported, and this article chose the latest and/or most informative one if any possible overlapping studies were found.
Search Strategy
This article performed database searches of PUBMED (1966 to Dec. 2017), EMBASE (1980 to Dec. 2017) through Ovid, CNKI database (1994 to Dec. 2017), Wanfang Data (1989 to Dec. 2017), VIP Information (1990 to Dec. 2017) and the Cochrane Library (issue 1, 2018) using the following keywords in combination with both medical subject headings terms and text words: VED or vacuum plus radical prostatectomy. There was no limitation on publication status or language. Reference lists of the included studies were checked manually to further identify related studies.
Bias Assessment
The methodological quality of included studies was appraised with the Cochrane Collaboration bias appraisal tool. The following factors were evaluated particularly: (a) Adequate sequence generation? (b) Allocation concealment? (c) Binding? (d) Incomplete outcome data addressed? (e) Free of selective reporting? (f) Free of other bias?
Selected Outcomes
Two predefined outcomes were assessed. The primary outcome was erectile function; it was measured with the five-item International Index of Erectile Function (IIEF-5). The secondary outcome was to assess Sexual Encounter Profile question 2 (SEP-2, were you able to insert your penis into your partner’s vagina? yes/no), Sexual Encounter Profile question 3 (SEP-3, did your erection last long enough for you to have successful intercourse? yes/no), penile length, and penile hardness.
Data Extraction
Two independent reviewers (FQ and SW) extracted data from the relevant trials using a standard data collection form, to avoid bias in the data abstraction process. All data were checked for internal consistency. Discrepancies were resolved through discussions between two reviewers (FQ and SW) and if needed, by seeking the opinion of a third reviewer (JY). Details abstracted from the reports included the name of the first author, date of publication, country of origin, study design, diagnostic criteria, preoperative erection quality, type of RP, type of the control group, treatment duration, number of participants, number of treatment responses, and adverse events in each arm. Where required, it was attempted to obtain additional information through collaboration with the original authors.
Statistical Analysis
Meta-analysis was performed by using Review Manager (RevMan) version 5.3 (Cochrane Collaboration). Statistical heterogeneity among RCTs was evaluated with the χ2 and I2 tests. A fixed-effect model was used when heterogeneity was not detected (p > .1). Additionally, a random-effects model was applied if a significant heterogeneity between individual effect sizes was identified. Subgroup analysis was performed by stratifying the type of the control group. Otherwise, the data would be synthesized with descriptive statistics rather than quantitative assessment. The standardized mean difference (MD) and odds ratio (OR) with 95% confidence interval (CI) were used to investigate the effect sizes.
Results
Characteristics of the Individual Studies
An overview of the study selection process was summarized in Figure 1. Literature searches identified 116 potentially relevant abstracts after elimination of duplicates. Based on the inclusion and exclusion criteria, 99 articles were excluded after reading the titles and abstracts of the articles. Seven articles were not RCTs. Two articles lacked useful data. Two articles were not parallel design. In total, six studies with 273 patients (162 for the early use of VED, 111 for the control group) were finally included for pooling in the study (Engel, 2011; Hu, Hu, Zhao, & Shen, 2014; Köhler et al., 2007; Liu & Chen, 2016; Monga et al., 2006; Raina, Pahlajani, Agarwal, Jones, & Zippe, 2006). All six studies reported that there were no apparent differences between the groups in baseline characteristic. The baseline characteristics of the studies included in the meta-analysis are listed in Table 1.
Figure 1.
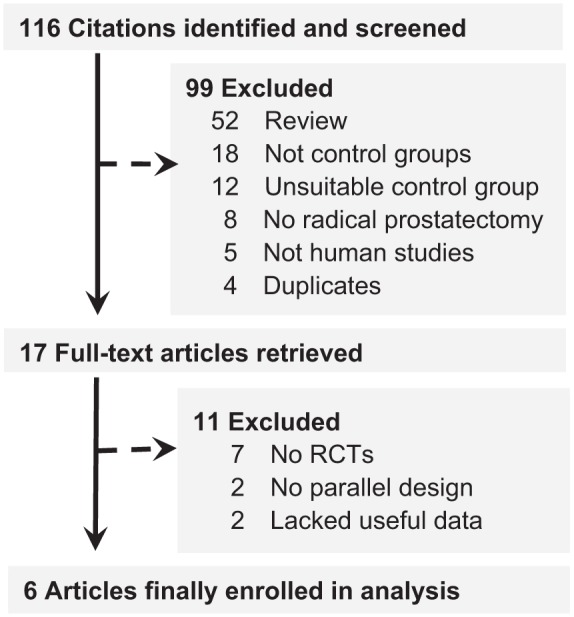
Study selection process for the meta-analysis with specifications of reasons.
Table 1.
Characteristics of the Included Studies in This Meta-Analysis.
| Study | Country | Study design | Participants (IIEF-5 score) |
Intervention |
Treatment courses (months) | Outcome | ||
|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | |||||
| Engel (2011) | USA | RCT | 13 BNS (mean 24.7 before RP) | 10 BNS (mean 24.7 before RP) | Vacuum + Tadalafil in 1 week after RP | Tadalafil in 1 week after RP | 12 | IIEF-5 score, SEP-2, SEP-3, penile hardness |
| Hu et al. (2014) | CHINA | RCT | 2 BNS, 3 UNS and 3 NNS (6.57 ± 0.58 after RP) | 2 BNS, 3 UNS and 1 NNS (6.34 ± 0.54 after RP) | Vacuum + Sildenafil in 4 months after RP | Sildenafil in 4 months after RP | 3 | IIEF-5 score, Penile length, penile hardness |
| Liu and Chen (2016) | CHINA | RCT | 17 BNS, 9 UNS and 6 NNS (6.49 ± 0.53 after RP) | 16 BNS, 9 UNS and 7 NNS (6.51 ± 0.58 after RP) | Vacuum + Sildenafil in 4 months after RP | Sildenafil in 4 months after RP | 3 | IIEF-5 score, Penile length, penile hardness |
| Raina et al. (2006) | USA | RCT | 31 BNS, 22 UNS and 21 NNS (>16 before RP) | 29 NS and 6 NNS (>16 before RP) | Vacuum in 2 weeks after RP | No treatment after RP | 9 | IIEF-5 score, SEP-2, SEP-3, penile length |
| Monga et al. (2006) | USA | RCT | The NS type was unknown (>11 before RP) | The NS type was unknown (>11 before RP) | Vacuum in 1 month after RP | Vacuum in 6 months after RP | 6 | IIEF-5 score, penile length |
| Köhler et al. (2007) | USA | RCT | 16 BNS and 1 UNS (21.1 ± 4.6 before RP) | 10 BNS and 1 UNS (22.3 ± 3.3 before RP) | Vacuum in 1 month after RP | Vacuum in 6 months after RP | 6 | Penile length, SEP-2 |
Note. RCT = randomized controlled trials; RP = radical prostatectomy; NS = nerve sparing; BNS = Bilateral nerve sparing; UNS = unilateral nerve sparing; NNS = non–nerve sparing; IIEF-5 = the five-item International Index of Erectile Function; SEP = sexual encounter profile question.
Three studies reported the comparison between early VED plus PDE-5i with PDE-5i in monotherapy for post-RP men (Engel, 2011; Hu et al., 2014; Liu & Chen, 2016). Only one study was carried out to compare the efficacy of early VED with no treatment (Raina et al., 2006). Two studies were conducted to compare the efficacy of early VED (1 month after RP) with late VED (4 months after RP; Köhler et al., 2007; Monga et al., 2006). All six studies were RCTs.
Methodological Quality of Studies Included
According to the Cochrane risk of bias assessment tool, the methodologic quality item for all included studies were described in Figure 2. The methodological quality of the six studies was low, because all the studies were not double-blind and placebo-controlled trial. Three studies used a random number table for randomization (Engel, 2011; Hu et al., 2014; Liu & Chen, 2016), and the other studies did not provide detailed information about the random sequence generation. Three studies reported how many participants dropped out (Engel, 2011; Köhler et al., 2007; Raina et al., 2006), and the other studies did not mention this issue at all in the articles. In addition, all the studies failed to describe the allocation concealment, blinding of participants and personnel, and blinding of outcome assessors in details. None of the studies reported missing data.
Figure 2.
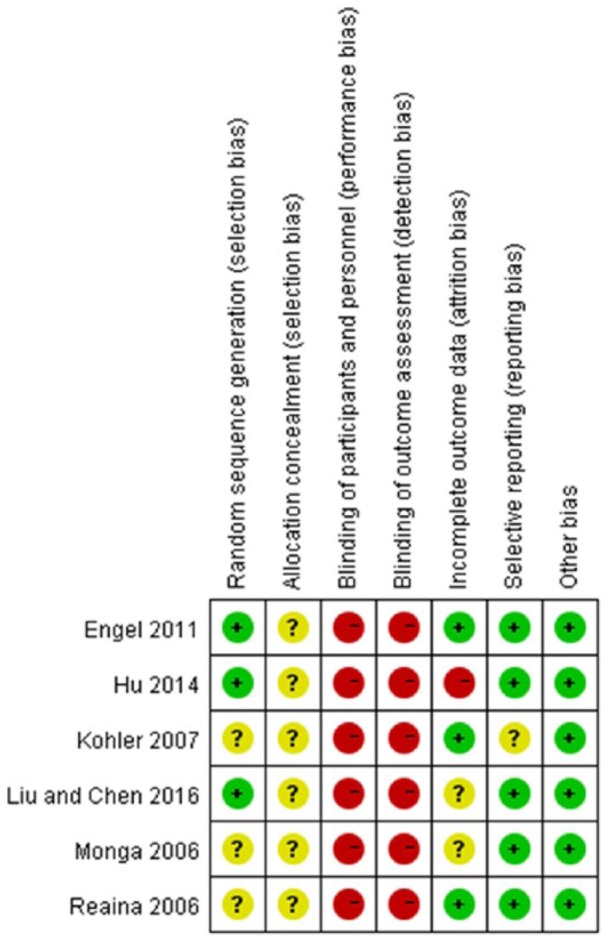
Methodological quality assessment of the risk of bias for each included study.  = low risk of bias;
= low risk of bias;  = unclear risk of bias;
= unclear risk of bias;  = high risk of bias.
= high risk of bias.
Improvement of erectile function
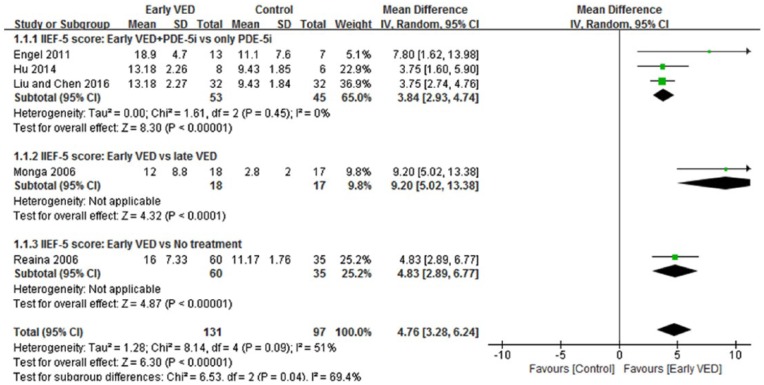
A total of five trials (228 patients) tested the IIEF-5 score of the early VED use against the control group in patients after RP (Engel, 2011; Hu et al., 2014; Liu & Chen, 2016; Monga et al., 2006; Raina et al., 2006). All trials reported effects in favor of early VED compared to control group at the end of treatment. As presented in Figure 3, the meta-analysis identified a significant increase of the IIEF-5 score compared to control group (MD = 4.76, 95% CI [3.28, 6.24], p < .00001). The χ2 test for homogeneity was performed (χ2 = 8.14, df = 4; p = 0.09) and demonstrated no statistical significant differences in the five trials. Similar results were reported in the subgroup analysis (Figure 3), the early VED+PDE-5i group had a significantly better erectile function than only PDE-5i group (MD = 3.84, 95% CI [2.93, 4.74], p < .00001), and the early VED group also had a significantly better erectile function than the late VED group (MD = 9.20, 95% CI [5.02, 13.38], p < .0001) and no treatment group (MD = 4.83, 95% CI [3.89, 0.77], p < .00001).
Figure 3.
Forest plot for meta-analysis of the IIEF-5 score in patients after RP. CI = confidence interval; IV = intravenous; SD = standard deviation.
Successful vaginal penetration
Three RCTs tested the successful vaginal penetration rate of the early VED group against the control group in patients after RP (SEP-2: “Were you able to insert your penis into your partner’s vagina?” yes/no; Engel, 2011; Hu et al., 2014; Liu & Chen, 2016). As presented in Figure S1, a meta-analysis of the three trials (143 patients) identified that the early VED demonstrated no improvement in the SEP-2 compared with the control group (OR = 1.18, 95% CI [0.55, 2.54], p = .64).
Successful intercourse
Two RCTs (115 participants) tested the successful intercourse rate of the early VED group against the control group in patients after RP (SEP-3: “Did your erection last long enough for you to have successful intercourse?” yes/no; Engel, 2011; Hu et al., 2014). No heterogeneity was reported between the trials (Figure S2). The pooled OR was 18.99 and the 95% CI was 2.27–158.92 (p = .007). This result suggests that the early VED reported significantly improvement in the SEP-3 compared with the control group. There was statistical homogeneity between the two trials (χ2 = 0.15, df = 1, p = .70; I2 = 0%).
Improvement of penile length
Five studies evaluated the effectiveness of different treatments on the improvement of penile length (Hu et al., 2014; Köhler et al., 2007; Liu & Chen, 2016; Monga et al., 2006; Raina et al., 2006). As presented in Figure S3, three trials (Köhler et al., 2007; Monga et al., 2006; Raina et al., 2006) reported that early VED could partially reverse penile shrinkage in post-RP patients. Pooled data from the three trials identified that the number of patients with reversed the penile shrinkage in early VED group was 5.44 times more than that in control group (OR = 5.44, 95% [CI 2.67, 11.08], p < .00001). There was statistical homogeneity among the three trials (χ2 = 0.06, df = 2, p = .97; I2 = 0%). As presented in Figure S4, two trials (Hu et al., 2014; Liu & Chen, 2016) reported that early VED could increase the penile length in patients after RP. Pooled data from the two trials identified that the penile length (unit: cm) was longer significantly in early VED+PDE-5i group than that in only PDE-5i group (MD = 1.26, 95% CI [0.78, 1.74], p < .00001). There was statistical homogeneity among the trials evaluated (χ2 = 0.00, df = 1, p = .99; I2 = 0%).
Improvement of penile hardness
Summary estimates of three trials (Engel, 2011; Hu et al., 2014; Liu & Chen, 2016) identified a significant increment of penile hardness in early VED+PDE-5i group compared with only PDE-5i group; the increment was statistically significant (MD = 0.85, 95% CI [0.68, 1.03], p < .00001; Figure S5). There was statistical homogeneity for this outcome (χ2 = 0.33, df = 2, p = .85, I2 = 0%).
Adverse Events
Of the six articles, four trials included data on adverse events. No serious adverse events were reported in any of these trials. Reaina et al. (2006) reported that discomfort (9.9%) and cyanosis of the penis (3.6%) were the main side effect after VED. Engel reported that the main side effect after tadalafil (PDE-5i) was headache, flushing, and muscle ache during treatment, while the main side effect after the VED was minor local discomfort (Engel, 2011). Hu et al. (2014) reported that four patients had experienced headache and erubescence (two in the VED+PDE-5i group; and two in only PDE-5i group, which were considered to be the adverse effects of sildenafil during treatment), and one patient had numbness of the penis (Hu et al., 2014). A similar side effect has been described in the study of Liu and Chen (2016). In addition to the above side effects, cyanosis of the penis also occurred in two patients after VED treatment. The other two reports did not refer to the occurrence of adverse events at all.
Discussion
In total, this study assessed the efficacy and safety of vacuum therapy for early penile rehabilitation in post-RP men. Review Manager 5.3 software was used to analyze the clinical data from six RCTs, with a total of 273 men. This also is the first meta-analysis on the use of vacuum therapy for early penile rehabilitation of post-RP men. Although the sample size is relatively small, the meta-analysis revealed that the early use of vacuum therapy could significantly improve erectile function (which was measured by the IIEF-5 score) and penile shrinkage in post-RP patients. Moreover, no serious side effects were observed in any RCT.
Accumulating studies have reported that many men continue to suffer from ED and penile shortening after RP, due to neuropraxia (Lin, Yang, Zhang, Dai, & Wang, 2013; Moskovic, Miles, Lipshultz, & Khera, 2011; Mulhall, 2005). It is associated with significant reduction in health-related quality of life in these men. The most popular initial choice for those post-RP men is oral PDE-5i therapies. The European guidelines have listed the use of a VED as a first-line option for ED patients, when standard oral PDE-5i treatment fails (Hatzimouratidis et al., 2014). VED is the one of three methods used in the clinical setting that improve erectile function and is the only PR method which may preserve penile length (Naccarato et al., 2016; Zippe & Pahlajani, 2008). Despite the limited evidence for their effectiveness, the early use of VED is frequently recommended in ED patients after RP. Although some RCTs comparing early VED with the control in post-RP men report that early VED are better, no formal meta-analysis has been performed to date.
The use of validated questionnaires such as the IIEF-5 scores can be useful as an “icebreaker” to initiate the conversation about ED (Albersen, Weyne, & Bivalacqua, 2013). The IIEF-5 is an extensively validated questionnaire covering five domains of male sexual function, including erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction. The current data indicated that early VED had excellent therapeutic effect in ED patients after RP, when compared with the control. Early VED can significantly improve the IIEF-5 scores (Figure 3), with significantly more positive answers for the SEP-3 (Figure S2).
Many studies have suggested that a large percentage of men reported penile shrinkage in patients undergoing RP (Moskovic et al., 2011; Mulhall, 2005). The exact cause of this change is unclear, and most likely is multi-factorial in etiology as previously discussed in several publications. The theories to explain this phenomenon include cavernosal nerve injury and its associated structural alterations in the penis, cavernosal hypoxia and its induction of structural changes in the penis, and sympathetic hyper-innervation (Mulhall, 2005). The ability to maintain penile health after RP, such as in preserving penile length, may potentially impact on the ultimate recovery of erectile function as well. Some scholars believe that early use of VED is the only method in penile rehabilitation which may be utilized to preserve penile length, but the evidence is inadequate and the proposition is doubtful at present (Lin et al., 2013; Yuan, Lin, et al., 2010). The question still remains on whether the early use of VED as a penile rehabilitation regimen would improve penile shrinkage in patients after RP. According to the study findings, the early use of VED was observed to be significantly greater improvement for penile length (Figures S3 and S4), when compared with control group in post-RP men.
VED is a more aggressive therapy when used in combination with other forms of treatment. Oral PDE-5i therapy is not always optimal in terms of efficacy and adverse effects, and the use of a VED will often increase patient compliance and satisfaction (Sun, Peng, Yu, Liu, & Chen, 2014; Zippe & Pahlajani, 2008). The British Society for Sexual Medicine guidelines on ED management recommend PDE-5i as well as a VED as first-line management for ED after RP (Hackett et al., 2008). This study did not provide adequate evidence to conclude whether the early use of VED was superior, inferior, or the same as PDE-5i in terms of efficacy and safety for penile rehabilitation in post-RP men. However, the IIEF-5 score, penile shrinkage, and penile hardness were significantly improved when PDE-5i was combined with VED, compared to the only PDE-5i group. The current study suggests that early addition of the VED treatment to standard PDE-5i therapy appears to offer advantages to monotherapy with PDE-5i when treating ED and penile shrinkage after RP.
Recently, there was also a great variability in the time at which the penile rehabilitation program was applied in post-RP men. Vacuum therapy is not suitable for post-RP men before the catheter removing (about 1–2 weeks). Normally, the best performance time for penile rehabilitation is 1 week to 1 month after RP, which was adopted in the previous numerical studies (Engel, 2011; Raina et al., 2006; Monga et al., 2006; Köhler et al., 2007). Fraiman et al. (1999) first examined the penile morphometrics for the patients after RP and indicated that denervation muscular atrophy is most apparent between the first 4–8 months after RP. The time point for penile rehabilitation in the current study was 4 months after surgery; six RCTs occurred within this period.
Vacuum therapy is a drug-free program and with limited side effects. However, there are numerous known drawbacks to the VED, some of which are instability at the base of the penis, leading to unnatural pivoting, a bluish or cyanotic appearance, and a cooler erection due to constriction of the blood flow (Raina, Pahlajani, Agarwal, Jones, & Zippe, 2010). Safety data from the included RCTs in this meta-analysis suggest that VED is generally well-tolerated.
The present study has several potential limitations that should be addressed. First, there are no high quality RCTs in the present study; all included studies are of low to moderate quality. To our knowledge, these trials of vacuum therapy could not be double-blind design because there was no way of disguising administration of the early use of VED and the control. Second, considering the small sample size in the present meta-analysis, the results need to be interpreted with caution. Better methodological quality, longer follow-up period, and larger sample size are expected to further verify the therapeutic effect of vacuum therapy.
Conclusion
This study suggests that initiating an early use of vacuum therapy appears to be an effective strategy for improving ED and penile shrinkage in post-RP men. Moreover, when vacuum therapy are combined with PDE-5i, the benefits of penile rehabilitation seem to be significantly enhanced. The severity of the adverse events was mild and easy to control in post-RP men. As this study is conducted on a small sample, the results need to be interpreted with caution. Better methodological, large controlled trials are expected to further verify the therapeutic effect of vacuum therapy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Natural Science Foundation of China (No. 81671453 & 81270691) and Sichuan Science and Technology Program (No. 2018SZ0019 & 2018TJPT0018).
References
- Albersen M., Weyne E., Bivalacqua T. J. (2013). Stem cell therapy for erectile dysfunction: Progress and future directions. Sexual Medicine Reviews, 1, 50–64. [DOI] [PubMed] [Google Scholar]
- Deng H., Liu D., Mao X., Lan X., Liu H., Li G. (2017). Phosphodiesterase-5 inhibitors and vacuum erection device for penile rehabilitation after laparoscopic nerve-preserving radical proctectomy for rectal cancer: A prospective controlled trial. American Journal of Men’s Health, 11, 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. D. (2011). Effect on sexual function of a vacuum erection device post-prostatectomy. Canadian Journal of Urology, 18, 5721–5725. [PubMed] [Google Scholar]
- Fraiman M. C., Lepor H., McCullough A. R. (1999). Changes in penile morphometrics in men with erectile dysfunction after nerve-sparing radical retropubic prostatectomy. Molecular Urology, 3, 109–115. [PubMed] [Google Scholar]
- Hackett G., Kell P., Ralph D., Dean J., Price D., Speakman M., … Wylie K. (2008). British society for sexual medicine guidelines on the management of erectile dysfunction. Journal of Sexual Medicine, 5, 1841–1865. [DOI] [PubMed] [Google Scholar]
- Hatzimouratidis K., Amar E., Eardley I., Giuliano F., Hatzichristou D., Montorsi F., … European Association of Urology. (2010). Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. European Urology, 57, 804–814. [DOI] [PubMed] [Google Scholar]
- Hoyland K., Vasdev N., Adshead J. (2013). The use of vacuum erection devices in erectile dysfunction after radical prostatectomy. Reviews in Urology, 15, 67–71. [PMC free article] [PubMed] [Google Scholar]
- Hu D., Hu Y., Zhao J., Shen Z. (2014). Efficacy and safety of vacuum erection device in the treatment of erectile dysfunction after radical prostatectomy. Chinese Journal of Human Sexuality, 23, 14–16. (in Chinese) [Google Scholar]
- Köhler T. S., Pedro R., Hendlin K., Utz W., Ugarte R., Reddy P., … Monga M. (2007). A pilot study on the early use of the vacuum erection device after radical retropubic prostatectomy. BJU International, 100, 858–862. [DOI] [PubMed] [Google Scholar]
- Lee J. K., Sjoberg D. D., Miller M. I., Vickers A. J., Mulhall J. P., Ehdaie B. (2018). Improved recovery of erectile function in younger men after radical prostatectomy: Does it justify immediate surgery in low-risk patients? European Urology, 73, 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. C., Yang W. L., Zhang J. L., Dai Y. T., Wang R. (2013). Penile rehabilitation with a vacuum erectile device in an animal model is related to an antihypoxic mechanism: Blood gas evidence. Asian Journal of Andrology, 15, 387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Lopez D. S., Chen M., Wang R. (2017). Penile rehabilitation therapy following radical prostatectomy: A meta-analysis. Journal of Sexual Medicine, 14, 1496–1503. [DOI] [PubMed] [Google Scholar]
- Liu T., Chen X. (2016). Clinical diagnosis and treatment of erectile dysfunction after radical prostatectomy. Chinese Journal of Human Sexuality, 25, 10–3. (in Chinese) [Google Scholar]
- Monga M., Kohler T., Hendlin K., Ryndin I., Canales B., Weiland D., … Ugarte R. (2006). Early use of the vacuum constriction device following radical retropubic prostatectomy: A randomized clinical trial. Urology, 68, 262. [Google Scholar]
- Montorsi F., Guazzoni G., Strambi L. F., Da Pozzo L. F., Nava L., Barbieri L., … Miani A. (1997). Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: Results of a prospective, randomized trial. Journal of Urology, 158, 1408–1410. [PubMed] [Google Scholar]
- Moskovic D. J., Miles B. J., Lipshultz L. I., Khera M. (2011). Emerging concepts in erectile preservation following radical prostatectomy: A guide for clinicians. International Journal of Impotence Research, 23, 181–192. [DOI] [PubMed] [Google Scholar]
- Mulhall J. P. (2005). Penile length changes after radical prostatectomy. BJU International, 96, 472–474. [DOI] [PubMed] [Google Scholar]
- Naccarato A. M., Reis L. O., Ferreira U., Denardi F. (2016). Psychotherapy and phosphodiesterase-5 inhibitor in early rehabilitation after radical prostatectomy: A prospective randomised controlled trial. Andrologia, 48, 1183–1187. [DOI] [PubMed] [Google Scholar]
- Pahlajani G., Raina R., Jones S., Ali M., Zippe C. (2012). Vacuum erection devices revisited: Its emerging role in the treatment of erectile dysfunction and early penile rehabilitation following prostate cancer therapy. Journal of Sexual Medicine, 9, 1182–1189. [DOI] [PubMed] [Google Scholar]
- Primeau C., Paterson C., Nabi G. (2017). A qualitative study exploring models of supportive care in men and their partners/caregivers affected by metastatic prostate cancer. Oncology Nursing Forum, 44, E241–E249. [DOI] [PubMed] [Google Scholar]
- Qian S. Q., Gao L., Wei Q., Yuan J. (2016). Vacuum therapy in penile rehabilitation after radical prostatectomy: Review of hemodynamic and antihypoxic evidence. Asian Journal of Andrology, 18, 446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F., Gao L., Qian S., Fu F., Yang Y., Yuan J. (2018). Advantages and limitations of sleep-related erection and rigidity monitoring: A review. International Journal of Impotence Research, doi: 10.1038/s41443-018-0032-8 [DOI] [PubMed] [Google Scholar]
- Raina R., Agarwal A., Ausmundson S., Lakin M., Nandipati K. C., Montague D. K., … Zippe C. D. (2006). Early use of vacuum constriction device following radical prostatectomy facilitates early sexual activity and potentially earlier return of erectile function. International Journal of Impotence Research, 18, 77–81. [DOI] [PubMed] [Google Scholar]
- Raina R., Pahlajani G., Agarwal A., Jones S., Zippe C. (2010). Long-term potency after early use of a vacuum erection device following radical prostatectomy. BJU International, 106, 1719–1722. [DOI] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2017). Cancer statistics, 2017. CA: A Cancer Journal for Clinicians, 67, 7–30. [DOI] [PubMed] [Google Scholar]
- Sun L., Peng F. L., Yu Z. L., Liu C. L., Chen J. (2014). Combined sildenafil with vacuum erection device therapy in the management of diabetic men with erectile dysfunction after failure of first-line sildenafil monotherapy. International Journal of Urology, 21, 1263–1267. [DOI] [PubMed] [Google Scholar]
- Wang R. (2017). Vacuum erectile device for rehabilitation after radical prostatectomy. Journal of Sexual Medicine, 14, 184–186. [DOI] [PubMed] [Google Scholar]
- Wilt T. J., Jones K. M., Barry M. J., Andriole G. L., Culkin D., Wheeler T., … Brawer M. K. (2017). Follow-up of prostatectomy versus observation for early prostate cancer. New England Journal of Medicine, 377, 132–142. [DOI] [PubMed] [Google Scholar]
- Yuan J., Hoang A. N., Romero C. A., Lin H., Dai Y., Wang R. (2010). Vacuum therapy in erectile dysfunction–science and clinical evidence. International Journal of Impotence Research, 22, 211–219. [DOI] [PubMed] [Google Scholar]
- Yuan J., Lin H., Li P., Zhang R., Luo A., Berardinelli F., … Wang R. (2010). Molecular mechanisms of vacuum therapy in penile rehabilitation: A novel animal study. European Urology, 58, 773–780. [DOI] [PubMed] [Google Scholar]
- Zippe C. D., Pahlajani G. (2008). Vacuum erection devices to treat erectile dysfunction and early penile rehabilitation following radical prostatectomy. Current Urology Reports, 9, 506–513. [DOI] [PubMed] [Google Scholar]