Abstract
Depression in men with prostate cancer is a significant and complex issue that can challenge clinicians’ diagnostic efforts. The objective of the current study was to evaluate prototypic and male-specific depression symptoms and suicidal ideation in men with a diagnosis of prostate cancer relative to those with and without comorbidity. The Patient Health Questionnaire-9 (PHQ-9) and Male Depression Risk Scale-22 (MDRS-22) were completed online along with demographic and background variables by 100 men with a diagnosis of prostate cancer (n = 54 prostatectomy, n = 33 receiving active treatment). Hierarchical logistic regression was used to examine recent (past 2 weeks) suicide ideation. Over one-third of the sample (38%) reported a comorbidity, and this group had significantly higher total depression scores on the PHQ-9 (Cohen’s d = 0.65), MDRS-22 emotion suppression (d = 0.35), and drug use subscales (d = 0.38) compared to respondents without comorbidity. A total of 14% reported recent suicidal ideation, of which 71.4% of cases were identified by the PHQ-9 “moderate” cut-off, and 85.7% of cases were identified by the MDRS-22 “elevated” cut-off. After control variables, MDRS-22 subscales accounted for 45.1% of variance in recent suicidal ideation. While limited by the exclusive use of self-report data, findings point to the potential benefits of evaluating male-specific symptoms as part of depression and suicide risk screening in men with prostate cancer and the need to be mindful of the heightened risk for depression among men with prostate cancer who have comorbidity.
Keywords: cancer, depression, depressive disorder, mental health, men’s health, oncology, prostate cancer
The majority of men diagnosed with prostate cancer have high 10-year survival rates due to improved screening, detection, and treatments (Wong et al., 2016). Unfortunately, these men have heightened risk for depression related to unmet psychosocial needs, prostate cancer–related symptoms, and treatment side effects that can include sexual dysfunction and urinary incontinence (Chambers et al., 2017). Many men with prostate cancer can suffer from additional health problems (i.e., sexual dysfunction and heart disease) that may contribute to depressive symptoms (D’Amico, Chen, Renshaw, Loffredo, & Kantoff, 2008; Saini et al., 2013). Depression among men with prostate cancer has emerged as a significant issue with prevalence reported at 16% to 30% (Christie & Sharpley, 2014; Sharp, O’Leary, Kinnear, Gavin, & Drummond, 2016; Sharpley, Bitsika, & Christie, 2010, 2013; Sharpley & Christie, 2007). Depression in prostate cancer is associated with men experiencing a loss of masculine identity (Sharpley, Bitsika, & Denham, 2014). Severe depression is a known risk for suicide, especially among older prostate cancer patients (Llorente et al., 2005). Although awareness of depression in prostate cancer has grown, the wider men’s health literature suggests depression among men is underdiagnosed (Oliffe & Phillips, 2008). Research attention has thus been increasingly devoted to the specificities of men’s depressive symptoms, the contexts in which they are experienced, and the methods and tools used to formally evaluate depression in men.
The generic diagnostic criteria for major depressive disorder, as per the Diagnostic and Statistical Manual (DSM-5), have been critiqued as gender neutral and insufficient to comprehensively evaluate depression in men (Sharpley, Bitsika, & Christie, 2014). At a broad level, such critiques have produced a focus on highlighting male-specific depressive symptoms and the design and testing of tools to more accurately diagnose and treat men’s depression. Systematic reviews and population studies support these efforts by arguing for the existence of a subtype of depression in men characterized by alcohol/substance use and externalizing symptoms including anger and irritability (Cavanagh, Wilson, Kavanagh, & Caputi, 2017; Martin, Neighbors, & Griffith, 2013). Expansion of the assessment of depression may be particularly important for men with prostate cancer. For example, Sharp et al. (2016) argued that psychological evaluation of men with prostate cancer should be informed by better understanding their cancer-related symptoms, rather than focusing on the major depressive disorder criteria. This was based on findings that men who experience prostate cancer–related urinary incontinence and those treated with androgen deprivation therapy, who in turn experienced fatigue and insomnia, were more likely to be depressed (Sharp et al., 2016). Unpleasant emotions and social withdrawal from others were also highlighted as significant factors associated with depression severity in a sample of 800 prostate cancer patients (Sharpley et al., 2010).
Nonetheless, depression scales that emphasize internalizing symptoms in the diagnosis of major depressive disorder continue to be used widely in prostate cancer patients. For example, the Patient Health Questionnaire-9 (PHQ-9) performed reliably in testing several aspects of depression in cancer patients (Hinz et al., 2016); however, according to Sharpley et al. (Sharpley, Bitsika, & Christie, 2014), the PHQ-9 does not fully capture the dimensions of depression as they manifest in men with prostate cancer. Specifically, the PHQ-9 and the Gotland Male Depression Scale (GMDS) were compared for their capacity to measure the prevalence of depression in 191 men with prostate cancer, and approximately 24% of the men screened as depressed on the GMDS were not identified by the PHQ-9 (Sharpley, Bitsika, & Christie, 2014). The GMDS has, however, drawn criticisms regarding psychometric validity, highlighting the need for measurement refinement amid the emergence of other male depression scales (Rice, Aucote, Möller-Leimkühler, & Amminger, 2017). Among the newer scales, the Male Depression Risk Scale-22 (MDRS-22) has shown much promise and has been validated in general populations of Canadian and Australian men to identify males at risk of depression (Rice et al., 2017; Rice, Oliffe, Kealy, & Ogrodniczuk, 2018). The scale assesses six broad externalizing domains of depression symptoms in men, including emotion suppression and anger and aggression, and may offer improved detection and early intervention among males at risk for depression and suicidal ideation.
The objective of the current study was to evaluate and compare PHQ-9 and MDRS-22 self-report data among men with prostate cancer relative to those with and without comorbidities (e.g., cardiovascular problems, arthritis, and chronic pain). The second aim was to evaluate the predictive ability of the MDRS-22 in identifying recent suicidal ideation in this population. Given evidence suggesting that men with prostate cancer who experience comorbidity report greater psychosocial stress and poorer outcomes (D’Amico et al., 2008; Saini et al., 2013), it was predicted that higher scores for prototypic and male-specific depression symptoms would be observed among men reporting comorbidity.
Methods
Participants
This study used a convenience sample of 100 Canadian men with prostate cancer, recruited online and via social media. Men self-identified as having prostate cancer and indicating year of diagnosis and providing information regarding current treatment and symptoms.
Measures
Demographic information included country and province of residence, age, employment status, education level, sexual identity, cultural affiliation, year diagnosed with prostate cancer and year treated, prostate cancer treatment[s], and current treatment. Data for comorbidity were collected using an item labeled “Other health challenges” to which respondents could indicate “yes” [Please specify] or “no.” The PHQ-9 (Kroenke, Spitzer, & Williams, 2001), developed from the diagnostic criteria for major depressive disorder, comprises nine items reflecting the diagnostic criteria in the DSM-V (American Psychological Association [APA], 2013). Respondents indicated how often (0 = “not at all,” 1 = “several days,” 2 = “more than half the days,” and 3 = “nearly every day”) they had been bothered by any of the listed problems over the last 2 weeks (see Table 1 for items). The PHQ-9 has excellent validity for individuals with mild, moderate, and severe depression (Kroenke et al., 2001), with aggregate scores ranging from 0 to 27 that map minimal depression (0–4), mild depression (5–9), moderate depression (10–14), moderately severe depression (15–19), and severe depression (20–27). The MDRS-22 comprises 22 items and six subscales focused on externalizing depression symptoms (Rice, Fallon, Aucote, & Möller-Leimkühler, 2013). Respondents, thinking back over the last month, responded to each item considering how often (0 = not at all to 7 = almost always) specific statements applied to them (see Table 2 for MDRS-22 subscales). The MDRS-22 exhibits test–retest stability and excellent psychometric properties (Rice et al., 2015) and has been validated in Australian and Canadian samples with the total score characterizing those in the low (0–31), elevated (32–50), high (51–86), and extreme ranges (87–154; Rice et al., 2017).
Table 1.
Group Comparison for PHQ-9 Items.
| Total sample, N = 100 | No comorbidity, n = 62 | Comorbidity, n = 38 | Group comparison | |||
|---|---|---|---|---|---|---|
| PHQ-9 itema | M (SD) | M (SD) | M (SD) | F | p | Cohen’s d |
| Little interest or pleasure in doing things | 0.72 (0.95) | 0.60 (0.91) | 0.92 (1.00) | 2.48 | .099 | 0.33 |
| Feeling down, depressed, or hopeless | 0.68 (0.82) | 0.52 (0.74) | 0.94 (0.87) | 7.00 | .010 | 0.52 |
| Trouble falling asleep or sleeping too much | 1.00 (1.03) | 0.79 (0.91) | 1.34 (1.15) | 7.11 | .009 | 0.53 |
| Feeling tired or having little energy | 1.14 (1.09) | 0.81 (0.97) | 1.68 (1.07) | 17.81 | <.001 | 0.85 |
| Poor appetite or overeating | 0.62 (0.87) | 0.50 (0.82) | 0.82 (0.92) | 3.15 | .079 | 0.36 |
| Feeling bad about yourself or that you are a failure or have let yourself or family down | 0.56 (0.70) | 0.47 (0.67) | 0.71 (0.73) | 2.88 | .093 | 0.34 |
| Trouble concentrating on things, such as reading the newspaper or watching television | 0.63 (0.87) | 0.48 (.80) | 0.86 (0.93) | 4.75 | .032 | 0.34 |
| Moving or speaking so slowly that other people could have noticed. Or the opposite being so fidgety or restless that you have been moving around a lot more than usual | 0.30 (0.58) | 0.19 (.44) | 0.47 (0.72) | 5.81 | .018 | 0.47 |
| Thoughts that you would be better off dead or of hurting yourself in some way | 0.20 (0.55) | 0.16 (.45) | 0.26 (0.55) | 0.81 | .372 | 0.20 |
Note. aPHQ-9 item scores range 0–3. PHQ-9 = Patient Health Questionnaire-9; SD = standard deviation.
Table 2.
Group Comparison for MDRS-22 Subscales.
| Total sample, N = 100 | No comorbidity, n = 62 | Comorbidity, n=38 | Group comparison | |||
|---|---|---|---|---|---|---|
| MDRS-22 Subscalea | M (SD) | M (SD) | M (SD) | F | p | Cohen’s d |
| Emotion suppression | 8.73 (7.73) | 7.58 (6.96) | 10.61 (8.61) | 4.56 | .035 | 0.38 |
| Drug use | 1.03 (3.24) | 0.50 (1.87) | 1.89 (4.58) | 4.77 | .031 | 0.38 |
| Alcohol use | 2.51 (4.97) | 2.71 (5.35) | 2.18 (4.32) | 0.20 | .656 | 0.11 |
| Anger and aggression | 3.53 (5.18) | 2.91 (4.37) | 4.53 (6.22) | 2.71 | .103 | 0.30 |
| Somatic symptoms | 5.14 (5.35) | 4.56 (5.38) | 6.08 (5.25) | 1.22 | .140 | 0.28 |
| Risk-taking | 1.30 (2.61) | 1.37 (2.44) | 1.18 (2.88) | 0.04 | .843 | 0.07 |
Note. aMDRS-22 subscales range 0–28 for emotion suppression, alcohol use, anger and aggression, and somatic symptoms; MDRS-22 subscales range 0–21 for the drug use and risk-taking subscales. MDRS = Male Depression Risk Scale-22; SD = standard deviation.
Procedure
Following approval from the behavioral ethics review board at (University of British Columbia), the survey was embedded in an online prostate cancer psychosocial resource. The website and survey were launched in January 2017, highlighting the issue of depression in men with prostate cancer, and the opportunity for respondents to be entered into a $500 cash prize draw by completing the survey. The survey was available for 3 months to April 2017, and recruitment was aided by targeted Facebook ads and social media posts inviting men with prostate cancer to respond by clicking on the hyperlinked survey. The survey landing page provided details about the study including consent, confidentiality regards respondent’s demographic data, and its separate password protected storage from their survey responses, along with details about the aggregated data being shared as study findings in an academic publication and presentations. On completion of the survey, respondents were provided the URL of a men’s depression website (https://headsupguys.org/), which provided visitors with male-specific depression information and supports.
Data Analysis
Analyses were undertaken in SPSS 22.0 (IBM Corp.). Descriptive statistics were used to characterize the sample based on self-reported sexuality, cultural affiliation, and highest education. Independent sample t tests were used to compare groups (i.e., those with and without self-reported comorbidity) on age, and χ2 analyses were used to evaluate the associations between treatment groups (i.e., current active treatment and watchful waiting), recent diagnosis (i.e., last 12 months), and highest education level. Reliability for the PHQ-9 and MDRS-22 subscales was calculated using Cronbach’s α coefficients. Multivariate analysis of covariance was conducted on the PHQ-9 items and MDRS-22 subscales, controlling for current treatment and age. χ2 analysis evaluated established PHQ-9 categories (i.e., normal PHQ-9 = 0–4, minimal PHQ-9 = 5–9, mild PHQ-9 = 10–14, and moderate–severe PHQ-9 = 15–27) according to those with and without comorbidities. Hierarchical logistic regression was undertaken to predict recent (past 2 weeks) suicide ideation, as assessed by PHQ-9 item nine, controlling for age, recent diagnosis, current treatment, and sexuality (step 1) and comorbidity (step 2), with MDRS-22 subscales entered at step 3.
Results
Sample Characteristics
Mean age of the sample was 64.8 (standard deviation [SD] = 7.18) years, ranging 47 to 85. The vast majority of the sample identified as heterosexual (n=96), and four participants identified as gay, bisexual, or preferred not to say. Most (n = 87) participants identified as Caucasian. More than 60% of the sample reported higher education: some college/trade school (n = 19); graduation from college/trade school (n = 23); some university (n = 7); undergraduate degree (n = 17); and graduate degree (n = 21). Of the 38 participants indicating a comorbidity in addition to their prostate cancer diagnosis, only one reported a diagnosis of depression. A total of 11 participants reported multimorbidities. The remaining cases reported challenges with cardiovascular health (n = 10), arthritis (n = 7), chronic pain (n = 2), or other (n = 8; gallstones, diabetes). Recent suicidal ideation was relatively infrequent in the present sample (n = 14). One-third (n = 33) of the sample were receiving active treatment, and just over half (n=54) reported a prostatectomy.
There was no significant age difference between those without comorbidity (M = 64.55 years, SD = 7.07) and those reporting comorbidity (M = 65.24 years, SD = 7.42), p = .644. There was no association between those reporting comorbidity and current active treatment (n = 33, χ2(1) = 0.40, p = .522), watchful waiting (n = 22, χ2(1) = 1.72, p = .189), recent diagnosis of prostate cancer (i.e., last 12 months; n = 35, χ2(1) = 0.16, p = .690), highest education level (χ2(6) = 8.56, p = .200), or recent suicidal ideation (n = 6, χ2(1) = 0.16, p = .686).
Scale Reliability
The PHQ-9 demonstrated satisfactory internal consistency (Cronbach’s α = .89). For the MDRS-22, all subscales reported satisfactory internal consistency: emotion suppression (α =.88), drug use (α =.91), alcohol use (α =.91), anger and aggression (α =.92), somatic symptoms (α =.67), though internal consistency was low for the risk-taking subscale (α =.59). A subsequent sensitivity analysis of the risk-taking subscale, however, identified that the risk-taking subscale was internally consistent for those <65 years (n = 46, α =.70) relative to males ≥65 years (n = 54, α =.07).
Comparison of Symptom Profiles
For the PHQ-9 total score, those with comorbidity reported significantly higher depression scores (M = 8.02, SD = 5.56) compared to those without comorbidity (M = 4.52, SD = 5.19), F(1, 96) = 10.87, p = .001, Cohen’s d = 0.65. Neither current treatment (p = .738) or age (p = .097) was significant covariates. When the nine PHQ-9 symptoms were examined in a multivariate analysis, a significant omnibus effect was observed, Λ = 0.811, F(9, 90) = 2.33, p = .021, partial η2 = .189. Current treatment (p = .832) and age (p = .789) were not significant covariates. Significant univariate effects were observed for five major depression symptoms; higher scores were observed for those with comorbidity for low mood, sleep disturbance, fatigue, concentration difficulty, and psychomotor disturbance (see Table 1).
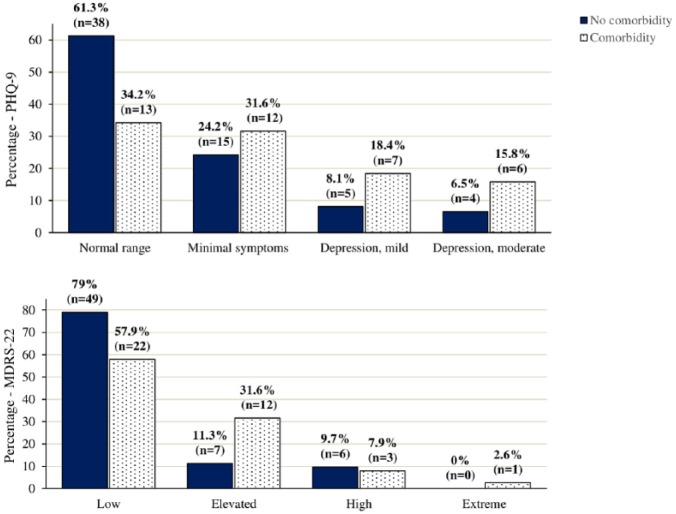
Categorical analysis was undertaken according to PHQ-9 depression categories. A significant association indicated that those with comorbidity were less likely to be in the normal range and more likely to be in the mild and moderate symptom ranges than those with no comorbidity χ2(3, N = 100) = 8.024, p = .046. The same association held for the MDRS-22 χ2(3, N = 100) = 8.30, p = .040 (see Figure 1).
Figure 1.
PHQ-9 and MDRS-22 categories (percentage with comorbidity).
For the MDRS-22 total score, there was a nonsignificant trend for those with comorbidity to report higher scores (M = 26.47, SD = 21.24) compared to those without comorbidity (M = 19.65, SD = 17.64), F(1, 96) = 3.92, p = .051, Cohen’s d = 0.35, with neither current treatment (p = .931) or age (p = .097) significant as covariates. When the MDRS-22 subscales were examined in a multivariate analysis, a significant multivariate effect was observed, Λ=.863, F(6, 91) = 2.41, p = .033, partial η2 = .132, with neither current treatment (p = .992) or age (p = .058) significant covariates. At the univariate level, there was a significant effect for the emotion suppression subscale, F(1, 96) = 4.56, p = .035, and the drug use subscale, F(1, 96) = 4.77, p = .031. In both instances, scores were higher among men with comorbidities.
Suicidal Ideation
Of the 14 respondents who endorsed suicidal ideation, the PHQ-9 cut-off score for “moderate depression” identified 71.4% (n = 10) of those men, while the MDRS-22 “elevated” cut-off identified 85.7% (n = 12). Bivariate correlations were calculated between PHQ-9 total scores and the MDRS-22 subscales. Significant correlations were observed for five of the MDRS-22 subscales; emotion suppression (r = .66, p < .001), drug use (r = .22, p = .025), anger and aggression (r = .59, p < .001), somatic symptoms (r = .46, p < .001), and risk-taking (r=.41, p < .001). The logistic regression model was not significant with the control variables entered at either step 1 (χ2(4) = 3.51, p =.447) or step 2 (χ2(5) = 3.77, p = .609) but was significant when the six MDRS-22 subscales were simultaneously entered at step 3 (χ2(11) = 33.75, p < .001). The model explained 51.8% of variance in recent suicide ideation, with the MDRS-22 subscales accounting for 45.1% of variance (Nagelkerke R2). Increasing MDRS-22 emotion suppression subscale scores were a significant predictor (Wald = 5.78, odds ratio [OR] = 1.18, p = .016), with a trend observed for the anger and aggression subscale (Wald = 3.80, OR = 1.19, p = .051).
Discussion
There was a consistent pattern of higher scores for major depression symptoms for those men with prostate cancer reporting comorbidities (i.e., cardiovascular disease and arthritis), with a robust moderate effect size for the group difference in PHQ-9 total scores. Those reporting comorbidities were more likely to be in the mild or moderate–severe range on the PHQ-9 than those without other health challenges. Specific effects were noted for low mood, sleep disturbance, fatigue, concentration difficulty, and psychomotor disturbance. This suggests that the likelihood of depression may increase in men experiencing prostate cancer who have comorbidities. These results indicate that it is not only the likelihood of depression severity that increases but also that the expression of depressive symptoms may be different for men with prostate cancer who also report comorbidity. Given that many men with prostate cancer are in their sixth, seventh, and eighth decades, this is an important consideration in routinely evaluating depression levels in men with prostate cancer. It is likely that many of these men were treated with polypharmacy agents, some of which in and of themselves might heighten their risk for depression (Higano, 2003). There was no significant difference between those with and without comorbidities with regards to the suicidal ideation item on the PHQ-9. While this may reflect an equivalent degree of suicidal ideation between the two groups, there may also be benefit in more fully evaluating suicidality in men with prostate cancer beyond the single PHQ-9 item, “Thoughts that you would be better off dead, or of hurting yourself in some way.”
Patterns for the MDRS-22 were less consistent. Those reporting additional health challenges had significantly higher scores for the emotion suppression and drug use subscales. The drug use effect might be explained by respondents with comorbidity being reliant on polypharmacy agents for a range of conditions. In this regard, the MDRS-22 likely yields diverse interpretations of the drug use items wherein some respondents may use illicit drugs recreationally, responding on that basis, while others such as the current sample might be responding based on their prescription drug use. Nonetheless, since men with comorbidity were markedly more distressed (based on PHQ-9 scores) than those without comorbidity, this difference remains cause for some concern—perhaps suggesting the overuse of drugs for emotional coping and/or for management of a higher pain burden. The effect for emotion suppression, which demonstrated the strongest correlation with the PHQ-9, suggests that those with comorbidity may attempt to self-censor and withhold the expression of negative emotion. Such responses may actually increase the likelihood of developing additional physical and mental health problems (Hoyt, Stanton, Irwin, & Thomas, 2013; Mauss & Gross, 2004). Furthermore, the current study findings indicate that the MDRS-22 risk-taking subscale may be less reliable in older men (i.e., those ≥65 years). This may be somewhat expected and likely reflects older men’s more conservative practices.
Beyond differences in symptom expression between men with and without comorbidities, unique associations were reported between overall depressive severity and two male-specific domains of the MDRS-22. Based on the regression analyses, anger and aggression also appeared to be salient among men with prostate cancer and comorbidity, while emotion suppression appeared to be an important factor associated with prototypic symptoms of depression in men with prostate cancer in general. As older men, respondents may have aligned to traditional masculine ideals around stoicism and relied on anger and aggression to express their emotions, which in turn can manifest as maladaptive coping. Experiencing prostate cancer and comorbidity may further threaten one’s sense of self as a man, evoking unmodulated anger (in protest and/or in compensation) for their compromised health. Results from the regression analyses indicated that the MDRS-22 subscales accounted for a large proportion of variance in the PHQ-9 scores, approximately 48% for those without comorbidity and 53% of variance for those with comorbidities. The current study findings suggest evaluation of emotion suppression in men with prostate cancer, along with anger and aggression in men with prostate cancer and comorbidity as important avenues of future research. That emotion suppression was identified to be a significant predictor of suicide ideation with a trend also observed for anger and aggression, suggests that additional research attention is paid to more fully apprehend and address these potential connections.
Clinical Implications
The current study findings have implications for researchers and care providers. Both groups should be aware of comorbidities in men with prostate cancer as depression risk factors, given this appears to be associated with higher PHQ-9 scores in the current sample. Supporting this recommendation, depression also appears to be underdiagnosed in this population. For example, among those reporting comorbidity, only one participant self-reported a diagnosis of depression. Figure 1 shows that there were 13 cases (i.e., 34%) in the comorbidity subgroup achieving the PHQ-9 cut-off score for either mild or moderate–severe depression. The observation that 22 of the 100 respondents’ PHQ-9 scores also indicated mild to moderate–severe depression supports estimates of prevalence in the literature (Christie & Sharpley, 2014; Sharp et al., 2016; Sharpley et al., 2010, 2013; Sharpley & Christie, 2007). Furthermore, given their association with PHQ-9 scores, emotion suppression and anger and aggression in men should be taken into account by clinicians working with men who experience prostate cancer. This finding supports work by Sharpley and Bitsika (2014) and others (Oliffe & Phillips, 2008; Rice et al., 2013) indicating that a range of male-specific symptoms exist beyond those identified by generic depression screening tools, and that nuanced differences in the expression of such symptoms may emerge in the context of prostate cancer and comorbid conditions.
A proportion of men with prostate cancer will also experience suicidal ideation. Emotion suppression was found to be predictive of suicidal ideation in the present sample. When assessing for depression in this population, sensitive clinical enquiry regarding suicidal thoughts is suggested, along with assessing the likelihood of emotion suppression, given the potential association between these two constructs. Taking a wider view, adopting brief general screening tools such as the Distress Thermometer, a single-item measure validated in populations of men experiencing prostate cancer (Chambers, Zajdlewicz, Youlden, Holland, & Dunn, 2014), may also assist as a way to introduce clinical conversations about suicidal thinking.
In terms of prevention and treatment of depressive symptoms, Sharpley, Bitsika, Wootten, and Christie (2014) advocated for peer supports as an effective way to increase men’s resilience for coping with prostate cancer and alleviating depressive symptoms. A systematic review of randomized controlled trials drawn from programs to improve the psychological well-being of men with prostate cancer highlighted 11 effective interventions (Chambers et al., 2017). These included online psychoeducation and moderated peer forums; however, these interventions and their evaluations were not based on translational knowledge about masculinity and male-specific symptoms of depression (Chambers et al., 2017). Cormie et al. (2016) asserted the need to appeal to men’s masculine values through group-based physical exercise interventions. Such approaches may to promote social connectedness and reduce depression among men with prostate cancer (Cormie, Galvão, et al., 2015; Cormie, Turner, Kaczmarek, Drake, & Chambers, 2015). Building on these insights and recommendations by Sharpley et al. (2017), future work might also focus on prevention and treatments of depression among specific subgroups, with formal evaluation of prostate cancer psychosocial programs inclusive of end-users’ depressive symptoms and scores over time.
More broadly, findings have implications for the field of men’s depression research. In the present sample, the MDRS-22 cut-off identified additional cases of suicidality that fell below the PHQ-9 cut-off. As suggested by others (Rice, Kealy, Oliffe, & Ogrodniczuk, 2018), case identification may be improved by supplementing the use of standard depression symptom rating scales with male-specific measures. Furthermore, emotion suppression predicted suicidality in the present sample. This accords with recent population-based epidemiological work indicating that men’s stoicism is a robust predictor of suicidal ideation (Pirkis, Spittal, Keogh, Mousaferiadis, & Currier, 2017). Ongoing attention to men’s use of emotion suppression and tendency for stoicism is indicated.
Study Limitations
The current study has several limitations: the use of nonprobability sampling precludes generalizability of findings; the cross-sectional design prohibits causal inference; the exclusive use of self-report measures including the lack of follow-up screening and diagnosis by a clinician; and the small sample size. Furthermore, suicidal ideation was assessed by a single item from the PHQ-9; however, research has previously supported use of this item (Walker et al., 2010). Future studies should use large samples and longitudinal designs to fully examine the role of age, comorbidities, and treatment modalities in men’s depression across the prostate cancer trajectory.
Summary
To address depression in men with prostate cancer, more effective screening for depressive symptoms is needed, and future interventions should be designed and formally evaluated on understandings of relative depression risk and specific symptomatology in men with prostate cancer. By highlighting the increased risk for depression in men with prostate cancer who have comorbidity, and the predictive nature of emotion suppression and anger and aggression on PHQ-9 scores, the current study reveals the need for asking additional clinical and research questions of this subgroup of men who are vulnerable to depression.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Canadian Institutes of Health Research (Grant 11R06913).
References
- American Psychological Association. (2013). Diagnostic and statistical manual of mental disorders-V. Washington, DC: American Psychiatric Association. [Google Scholar]
- Cavanagh A., Wilson C. J., Kavanagh D. J., Caputi P. (2017). Differences in the expression of symptoms in men versus women with depression: A systematic review and meta-analysis. Harvard Review of Psychiatry, 25(1), 29–38. [DOI] [PubMed] [Google Scholar]
- Chambers S. K., Hyde M. K., Smith D. P., Hughes S., Yuill S., Egger S., … Dunn J. (2017). New challenges in psycho-oncology research III: A systematic review of psychological interventions for prostate cancer survivors and their partners: Clinical and research implications. Psycho-Oncology, 26(7), 873–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S. K., Zajdlewicz L., Youlden D. R., Holland J. C., Dunn J. (2014). The validity of the distress thermometer in prostate cancer populations. Psycho-Oncology, 23(2), 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie D. R., Sharpley C. F. (2014). Prostate cancer: Depression and prostate cancer – why do they show up together? Nature Reviews Urology, 11(1), 547–548. [DOI] [PubMed] [Google Scholar]
- Cormie P., Galvão D. A., Spry N., Joseph D., Chee R., Taaffe D. R., … Newton R. U. (2015). Can supervised exercise prevent treatment toxicity in prostate cancer patients initiating androgen deprivation therapy: A randomised controlled trial. BJU International, 115(2), 256–266. [DOI] [PubMed] [Google Scholar]
- Cormie P., Oliffe J. L., Wootten A. C., Galvao D. A., Newton R. U., Chambers S. K. (2016). Improving psychosocial health in men with prostate cancer through an intervention that reinforces masculine values – exercise. Psycho-Oncology, 25(2), 232–235. [DOI] [PubMed] [Google Scholar]
- Cormie P., Turner B., Kaczmarek E., Drake D., Chambers S. K. (2015). A qualitative exploration of the experiences of men with prostate cancer involved in supervised exercise programs. Oncology Nursing Forum, 42(1), 24–32. [DOI] [PubMed] [Google Scholar]
- D’Amico A. V., Chen M. H., Renshaw A. A., Loffredo M., Kantoff P. W. (2008). Androgen suppression and radiation vs radiation alone for prostate cancer: A randomized trial. JAMA, 299(3), 289–295. [DOI] [PubMed] [Google Scholar]
- Higano C. S. (2003). Side effects of androgen deprivation therapy: Monitoring and minimizing toxicity. Urology, 61(2), 32–38. [DOI] [PubMed] [Google Scholar]
- Hinz A., Mehnert A., Kocalevent R. D., Brähler E., Forkmann T., Singer S., … Schulte T. (2016). Assessment of depression severity with the PHQ-9 in cancer patients and in the general population. BMC Psychiatry, 16(1), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt M. A., Stanton A. L., Irwin M. R., Thomas K. S. (2013). Cancer-related masculine threat, emotional approach coping, and physical functioning following treatment for prostate cancer. Health Psychology, 32(1), 66–74. [DOI] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R., Williams J. (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16, 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente M. D., Burke M., Gregory G., Bosworth H. B., Grambow S. C., Homer R. D., … Olsen E. J. (2005). Prostate cancer: A significant risk factor for late-life suicide. American Journal of Geriatric Psychiatry, 13, 195–201. [DOI] [PubMed] [Google Scholar]
- Martin L. A., Neighbors H. W., Griffith D. M. (2013). The experience of symptoms of depression in men vs. women: Analysis of the national comorbidity survey replication. JAMA, 70, 1100–1106. [DOI] [PubMed] [Google Scholar]
- Mauss I. B., Gross J. J. (2004). Emotion suppression and cardiovascular disease. In Nyklícek I., Temoshok L., Vingerhoets A. (Eds.), Emotional expression and health: Advances in theory, assessment and clinical applications (p. 60). New York, NY: Brunner-Routledge. [Google Scholar]
- Oliffe J. L., Phillips M. J. (2008). Men, depression and masculinities: A review and recommendations. American Journal of Men’s Health, 5(3), 194–202. [Google Scholar]
- Pirkis J., Spittal M. J., Keogh L., Mousaferiadis T., Currier D. (2017). Masculinity and suicidal thinking. Social Psychiatry and Psychiatric Epidemiology, 52(3), 319–327. [DOI] [PubMed] [Google Scholar]
- Rice S. M., Aucote H., Moller-Leimkuhler A., Treeby M., Amminger G. P. (2015). Longitudinal sex differences of externalising and internalising depression symptom trajectories: Implications for assessment of depression in men. International Journal of Social Psychiatry, 61(3), 236–240. [DOI] [PubMed] [Google Scholar]
- Rice S. M., Aucote H. M., Möller-Leimkühler A. M., Amminger G. P. (2017). Confirmatory factor analysis of the gotland male depression scale in an Australian community sample. European Journal of Psychological Assessment, 33, 190–195. [Google Scholar]
- Rice S. M., Fallon B. J., Aucote H. M., Möller-Leimkühler A. M. (2013). Development and preliminary validation of the male depression risk scale: Furthering the assessment of depression in men. Journal of Affective Disorders, 151, 950–958. [DOI] [PubMed] [Google Scholar]
- Rice S. M., Kealy D., Oliffe J. L., Ogrodniczuk J. S. (2018). Externalizing depression symptoms among Canadian males with recent suicidal ideation: A focus on young men. Early Intervention in Psychiatry. doi: 10.1111/eip.12667 [DOI] [PubMed] [Google Scholar]
- Rice S. M., Ogrodniczuk J. S., Kealy D., Seidler Z. E., Dhillon H. M., Oliffe J. L. (2017). Validity of the Male Depression Risk Scale in a representative Canadian sample: sensitivity and specificity in identifying men with recent suicide attempt. Journal of Mental Health, 1–9. doi: 10.1080/09638237.2017.1417565 [DOI] [PubMed] [Google Scholar]
- Rice S. M., Oliffe J. L., Kealy D., Ogrodniczuk J. S. (2018). Male depression subtypes and suicidality: Latent profile analysis of internalising and externalising symptoms in a representative Canadian sample. Journal of Nervous and Mental Disease, 206, 169–172. doi: 10.1097/NMD.0000000000000739 [DOI] [PubMed] [Google Scholar]
- Saini A., Berruti A., Cracco C., Squazzotti E., Porpiglia F., Russo L., … Ostacoli L. (2013). Psychological distress in men with prostate cancer receiving adjuvant androgen-deprivation therapy. Urologic Oncology, 31(3), 352–358. [DOI] [PubMed] [Google Scholar]
- Sharp L., O’Leary E., Kinnear H., Gavin A., Drummond F. J. (2016). Cancer-related symptoms predict psychological wellbeing among prostate cancer survivors: Results from the PiCTure study. Psycho-Oncology, 25(3), 282–291. [DOI] [PubMed] [Google Scholar]
- Sharpley C. F., Bitsika V., Christie D. R. (2010). ‘Why I feel bad’: Refinement of the effects of prostate cancer upon lifestyle questionnaire and an initial exploration of its links with anxiety and depression among prostate cancer patients. Psycho-Oncology, 19(8), 839–846. [DOI] [PubMed] [Google Scholar]
- Sharpley C. F., Bitsika V., Christie D. R. (2013). The incidence and causes of different subtypes of depression in prostate cancer patients: Implications for cancer care. European Journal of Cancer Care, 22(6), 815–823. [DOI] [PubMed] [Google Scholar]
- Sharpley C. F., Bitsika V., Christie D. R. (2014). Diagnosing ‘male’ depression in men diagnosed with prostate cancer: The next step in effective translational psycho-oncology interventions? Psycho-Oncology, 23(9), 1042–1048. [DOI] [PubMed] [Google Scholar]
- Sharpley C. F., Bitsika V., Denham J. W. (2014). Factors associated with feelings of loss of masculinity in men with prostate cancer in the RADAR trial. Psycho-Oncology, 23(5), 524–530. [DOI] [PubMed] [Google Scholar]
- Sharpley C. F., Bitsika V., Warren A. K., Christie D. R. (2017). Using cluster analysis of anxiety-depression to identify subgroups of prostate cancer patients for targeted treatment planning. Psycho-Oncology, 26(11), 1846–1851. [DOI] [PubMed] [Google Scholar]
- Sharpley C. F., Bitsika V., Wootten A. C., Christie D. R. (2014). Predictors of depression in prostate cancer patients: A comparison of psychological resilience versus pre-existing anxiety and depression. Journal of Men’s Health, 11(3), 115–120. [Google Scholar]
- Sharpley C. F., Christie D. R. (2007). An analysis of the psychometric profile and frequency of anxiety and depression in Australian men with prostate cancer. Psycho-Oncology, 16(7), 660–667. [DOI] [PubMed] [Google Scholar]
- Walker J., Hansen C. H., Hodges L., Thekkumpurath P., O’Connor M., Sharma N., … Sharpe M. (2010). Screening for suicidality in cancer patients using Item 9 of the nine-item patient health questionnaire; does the item score predict who requires further assessment? General Hospital Psychiatry, 32(2), 218–220. [DOI] [PubMed] [Google Scholar]
- Wong M. C., Goggins W. B., Wang H. H., Fung F. D., Leung C., Wong S. Y., … Sung J. J. (2016). Global incidence and mortality for prostate cancer: Analysis of temporal patterns and trends in 36 countries. European Urology, 70(5), 862–874. [DOI] [PubMed] [Google Scholar]