Abstract
HIV-1-affected couples’ desire to have children and free sexual intercourses with the use of pre-exposure prophylaxis for the negative partner has emerged as an alternative option to assisted reproduction in aviremic patients under highly active antiretroviral therapy (HAART). It is already known that sperm quality may be impaired in HIV-infected men. The underlying physiopathological mechanism is still debated. The aim of this study was to evaluate the effects of HAART on sperm DNA fragmentation, comparing HIV-1-infected patients taking HAART versus naïve HIV-1-infected patients. This is a prospective case-control study. Sperm nuclear DNA fragmentation rate was evaluated by the sperm chromatin dispersion test in 77 HIV-infected men: 53 HIV-1 patients receiving HAART (Group 1) versus 24 naïve HIV-1 patients not receiving HAART (Group 2). Complete semen analysis was performed according to WHO 2010 recommendations. Patients with HBV infection or HCV infection coinfections and genital tract infections wre excluded. All the patients did not present any clinical signs of their disease. Seminal parameters were examined in the two groups, showing no significant differences. Increased sperm DNA fragmentation > 30% was demonstrated in 67.9% of patients in Group 1 and 37.5% of patients in Group 2, respectively (p = .02). A positive but nonsignificant trend toward increased fragmentation was reported with advancing patients’ age. In conclusion, sperm nuclear fragmentation rate is increased in HIV-1-infected patients taking HAART compared to HIV-1 patients not receiving HAART.
Keywords: HIV/AIDS, physiological and endocrine disorders, male infertility, physiological and endocrine disorders, male family planning, sexuality, sexually transmitted diseases/infections, physiological and endocrine disorders
The widespread use of highly active antiretroviral therapy (HAART) over the past years has dramatically increased the life expectancy and the quality of life of patients infected with HIV-1 (Yeni et al., 2002). Improvement in life expectancy has modified the family planning of serodiscordant couples and currently some couples in which the man is HIV-1-seropositive request assisted reproductive technology (ART), while other couples opt for free sexual intercourses and the use of pre-exposure prophylaxis (PreP) for the negative partner (Vernazza, Graf, Sonnenberg-Schwan, Geit, & Meurer, 2011). In both cases, since the semen quality is a key factor for reproductive success (Hunault et al., 2004; Van der Steeg et al., 2007), the possible effect of HAART on sperm DNA integrity is of main interest. Semen quality of HIV-1-infected men has been evaluated in many studies reporting conflicting results, mainly due to the difficulty to analyze such an heterogenic group of patients (Bujan et al., 2007; Crittenden, Handelsmann, & Stewart, 1992; Dondero et al., 1996; Dulioust et al., 2002; Garrido, Meseguer, Remohì, Simon, & Pellicer, 2005; Kehl et al., 2011; Muller, Coombs, & Krieger, 1998; Nicopoullos, Almeida, Ramsay, & Gilling-Smith, 2004). Sperm DNA fragmentation (SDF), defined as either single or double-strand breaks, is one of the major cause of male infertility. It has been linked to impaired fertilization, poor embryo quality, increased spontaneous abortion rates, and reduced pregnancy rates after assisted reproduction (Evenson, 2011; Henkel et al., 2003; Robinson et al., 2012; Seli, Gardner, Schoolcraft, Moffatt, & Sakkas, 2004). DNA fragmentation can be caused by a variety of factors such as infection, chemotherapy, radiotherapy, smoking, drug use, or advanced age (Fraga, Motchnik, Wyrobek, Rempel, & Ames, 1996; Sergerie, Mieusset, Croute, Daudin, & Bujan, 2007). One of the major contributors of DNA fragmentation is the increased level of reactive oxygen species and oxidative stress that can reduce sperm quality in HIV-1-infected men. Increasing evidence has identified an association between adverse effects of HAART and mitochondrial toxicity, including cellular respiratory dysfunction (Moyle, 2004; Pavili et al., 2010), decreased mitochondrial energy generation (Brinkman, ter Hofstede, Burger, Smeitink, & Koopmans, 1998), and changes in spermatozoa metabolism (Kehl et al., 2011). La Sala et al. (2007) reported an increase of mitochondrial DNA (mtDNA) content in spermatozoa obtained by the swim-up method in 19 HIV-1 patients all under HAART, while White, Mital, Taylor, and St John (2001) reported that sperm mtDNA deletions are a consequence of long-term HAART, analyzing six HIV patients under HAART versus four patients never treated. The underlying physiopathological mechanism linking poor semen quality and HAART is still debated. A longitudinal case control study was designed, in order to analyze the effects of HAART regimens on SDF, which could represent a very sensitive indicator of HAART toxicity. The aim of this study is the evaluation of the effects of HAART in HIV-1-infected patients in HAART therapy versus naïve HIV-1-infected patients, being able to exclude the influence previous therapies.
Materials and Methods
The study was designed as a prospective case control study including semen samples from two groups of men attending the Assisted Reproduction Unit in “Sacco Hospital” (University of Milan, Italy) asking reproductive assistance with their HIV negative partner. The couples were asking reproductive assistance as a strategy to reduce the risk of horizontal HIV transmission to their seronegative partner. None of them had previously attempted spontaneous conception. All patients were recruited between January 2014 and June 2016 and provided written informed consent prior to enrolment in the study. The Ethics Committee of the mentioned institution waived the need for ethics approval for the collection, analysis, and publication of the obtained and anonymized data for this non-interventional study.
Patients
Group 1: 53 HIV-1-infected patients taking antiretroviral therapy (HAART)
Group 2: 24 HIV-1-infected patients, who did not receive antiretroviral therapy (naïve controls). These patients were promptly referred from infectious disease specialists before the initiation of the therapy in order to receive adequate reproductive counseling and assistance to reduce the chance of horizontal transmission to the partner.
All the patients did not present any clinical signs of their disease. Patients with HBV infection or HCV infection coinfections were excluded. All available markers of HIV-1 disease like CDC stage, current CD4 + T lymphocytes count by flow cytometry, maximum and current HIV-1 RNA in blood by RT-PCR, duration of HAART, type of antiretroviral drugs were recorded. Patients received an extensive andrological work-up, including exclusion of varicocele by Doppler ultrasound assessment. Mycoplasma Genitalium, Chlamydia trachomatis, and Neisseria Gonorrhoeae infections were excluded by urethral swabs. For both groups, smoking and alcohol abuse was stopped at least 2 years before the inclusion in the study and body mass index was ⩽ 25 kg/m2. Only patients with a number of spermatozoa > 5.000.000/ml were included in the study.
Semen Analysis
Semen samples were obtained by masturbation after 3 to 7 days of sexual abstinence. All samples were analyzed by the same biologist and at the same laboratory. Samples were processed at the laboratory within 2 hr of ejaculation and parameters were assessed as outlined by the World Health Organization 2010 criteria (WHO, 2010). Semen sperm was bacteria-free in all patients, and the leukocyte count was under the WHO threshold (< 1 million/ml).
Nuclear Sperm DNA Fragmentation Analysis
Sperm nuclear DNA fragmentation rate was evaluated by the sperm chromatin dispersion (SCD) test, the Halosperm kit (INDAS laboratories, Madrid, Spain; Fernandez et al., 2003). In brief, an aliquot of a semen sample was diluted between 5 million/ml to 10 million/ml in phosphate-buffered saline (PBS) when needed. Eppendorf tubes, provided in the kit, were placed in a water bath at 90°–100°C; 60 ml of the diluted semen sample were added to the Eppendorf tube and mixed with the fused agarose. Of the semen-agarose mix, 20 microL were pipetted onto slides precoated with agarose and covered with a 22 by 22 mm coverslip. The slides were placed on a cold plate in the refrigerator (4° C) for 5 min to allow the agarose to produce a microgel with the sperm cells embedded within. After removing coverslips, the slides were immediately immersed horizontally in an acid solution, obtained by mixing 80 microL of HCL with 10 ml of distilled water and incubated for 7 min. The slides were horizontally immersed in 10 ml of the lysing solution for 25 min. After washing 5 min in a tray with abundant distilled water, the slides were dehydrated in increasing concentrations of ethanol (70%, 90%, 100%) for 2 min each and then air-dried. Slides may be stained for bright-field microscopy. For bright-filed microscopy in the improved SCD test (Halosperm kit), slides were horizontally covered with a mix of Wright’s staining solution (Merck, Darmstadt, Germany) and PBS (Merck; 1:1) for 15 min with continuous airflow. Slides were briefly washed in tap water and allowed to dry. Strong staining is preferred to easily visualize the periphery of the dispersed DNA loop halos. For this study, 500 spermatozoa per sample were scored under the X100 objective of the microscope. The samples from patients with SDF values above 30% were considered abnormal.
Statistical Analysis
All analyses were performed using IBM SPSS version 21.0 (Armonk, NY: IBM Corp) and R version 3.2.1 (The R Foundation for Statistical Computing). P values ⩽ .05 were considered significant. Appropriate descriptive statistics were calculated: median, minimum and maximum, quartiles for quantitative variables, and absolute frequencies and percentages for categorical variables. Population characteristics were compared using χ2 or exact tests for ordinal variables and Mann-Whitney U test for continuous variables. Mann–Whitney U test was used to compare medians of semen fragmentation rate, sperm motility, and sperm cells after capacitation between the two groups.
Comparison data are presented as odds ratio (OR; Mantel–Haenszel), p value and confidence interval (CI) and ORs refer to the effect of therapy on fragmentation, adjusted for the age of the patients.
Results
The baseline characteristics of HIV-1-infected patients of the two groups are reported in Table 1. At the time of semen collection in group 1, 16/53 men (30%) were receiving two or more Nucloside Reverse transcriptase inhibitor (NRTI), 18/53 (34%) men NRTI in combination with protease inhibitors therapy, and 19/53 men (36%) were under two NRTI in combination with efavirenz. The median therapy duration was 8 (1–14) years and therapy was only HAART. The median disease duration was 14 (3–32) years. Viral load was undetectable in 100% of patients and plasma CD4 + T lymphocyte count measured by flow cytometry was above 200/mm3 in both groups. In Group 2, the median disease duration was 5 (1–27) years. In the majority of cases, sexual transmission represented the main route of infection among men, accounting for 31/53 (58%) in Group 1 and 21/24 (88%) in Group 2. 20/53 (38%) patients in Group 1 and 2/24 (8%) in Group 2 were infected hematologically, while 2/53 (4%) and 1/24 (4%) were injection drug users, respectively. The median age in Group 1 was 42 (28–54) years, whereas in Group 2 was 39 (25–49) years. The influence of men’s age difference between the two groups was considered in the statistical analysis of sperm DNA fragmentation.
Table 1.
Population Characteristics.
| Group 1 - HAART (n = 53) |
Group 2 - NAÏVE (n = 24) |
p value | |
|---|---|---|---|
| Age (years); median (range) | 42 (28–54) | 39 (25–49) | NS |
| Duration of HIV-1 infection (years); median (range) | 14 (3–32) | 5 (1–27) | NS |
| Time under HAART (years); median (range) | 8 (1–14) | – | |
| CD4 count (cells/ml); median (range) | 470 (71–1670) | 445 (149–1086) | NS |
| Undetectable viral load, n (%) | 53 (100%) | 24 (100%) | |
| Educational level | |||
| High school, n (%) | 24 (45.3) | 12 (50) | NS |
| University, n (%) | 5 (9.4) | 3 (12.5) | NS |
| Unknown, n (%) | 24 (45.3) | 9 (37.5) | NS |
| Employed, n (%) | 48 (90.6) | 21 (87.5) | NS |
| Route of HIV-1 transmission | |||
| Injection drugs, n (%) | 2 (4%) | 1 (4%) | NS |
| Sexual transmission, n (%) | 31 (58%) | 21 (88%) | NS |
| Blood product transmission, n (%) | 20 (38%) | 2 (8%) | NS |
Note. The comparison between groups was performed using χ2 or exact tests for categorical variables and Mann–Whitney U test for continuous variables. Statistically significant difference as p < .05. HAART = highly-active antiretroviral therapy; NS = not significant.
Seminal parameters (sperm volume, total sperm count, progressive motility, and morphology) were examined in the two groups of patients and are reported in Table 2. No significant differences were found in any of the parameters assessed between samples of patients receiving HAART and samples of patients not taking antiretroviral therapy.
Table 2.
Different Seminal Parameters in Group 1 (HAART) Versus Group 2 (NAÏVE).
| Group 1 - HAART (n = 53) |
Group 2 - NAÏVE (n = 24) |
p value | OR (95% CI) | |
|---|---|---|---|---|
| Patients with ejaculate volume <1.5 ml, n (%) | 7 (13.2) | 1 (4.2) | .21 | 3.7 [0.42, 31.6] |
| Patients with concentration of spermatozoa < 39 × 106 /ejaculate, n (%) | 25 (47.2) | 16 (66.7) | .11 | 0.4 [0.16, 1.2] |
| Patients with progressive motility < 40%, n (%) | 20 (37.7) | 10 (41.7) | .71 | 0.8 [0.31, 2.2] |
| Patients with abnormal morphology < 4%, n (%) | 4 (7.5) | 5 (20.8) | .09 | 0.3 [0.07, 1.3] |
Note. The comparison between groups was performed using χ2 test.
Sperm Nuclear Fragmentation Evaluation
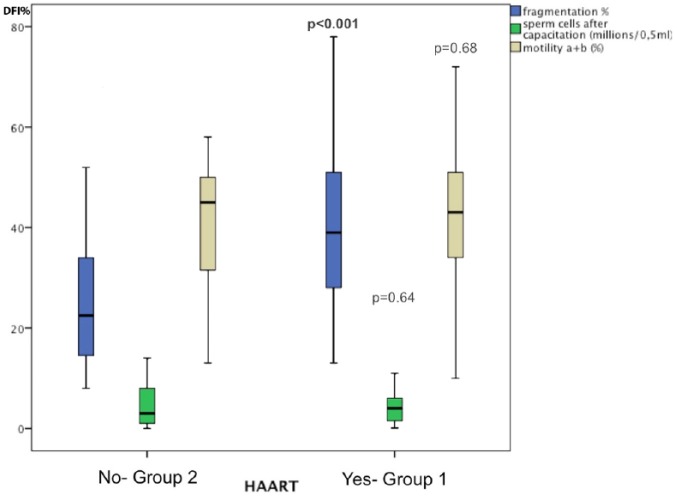
In Table 3, the analysis of the data indicates that the median of sperm nuclear fragmentation rate, expressed as DNA Fragmentation Index (DFI %), was significantly increased in HIV-1-infected patients taking antiretroviral therapy (Group 1) compared with the group of HIV-1-infected patients, who did not receive antiretroviral therapy (Group 2). In Group 1, 36/53 (67.9%) patients had SDF > 30%, with a median rate of fragmentation of 39%, whereas in Group 2, 9/24 (37.5%) patients had SDF > 30%, with a median rate of fragmentation of 22.5% (p = .0001; Table 3). The semen motility a + b and the sperm cells after capacitation expressed in Million/0, 5 were not significantly different. Figure 1 summarizes the main findings of seminal parameters and SDF in Group 1 versus Group 2.
Table 3.
Semen Fragmentation, Motility, and Sperm Cells after Capacitation in Group 1 (HAART) Versus Group 2 (NAÏVE).
| Group 1 - HAART (n = 53) |
Group 2 - NAÏVE (n = 24) |
p value | |
|---|---|---|---|
| Fragmentation (DFI %); Median (range) | 39 (13–78) | 22.5 (8–66) | .0001 |
| Motility a + b (%); Median (range) | 43 (10–73) | 45 (13–58) | .67 |
| Sperm cells after capacitation, (Million /0,5); Median (range) | 4 (0.1–20) | 3 (0.025–14) | .64 |
Note. The comparison between groups was performed using Mann–Whitney U test.
DFI = DNA fragmentation index (%).
Figure 1.
Differences in semen parameters in Group receiving HAART therapy versus naive Group. Fragmentation index (DFI%) is significantly highest in HAART group compared to naive one.
In addition, in order to exclude the effect of the age of the patients on these results, DNA fragmentation was analyzed according to three different age groups, identifying a positive correlation between increasing age and DNA fragmentation rate in each group. Nevertheless, this correlation is not statistically significant. Table 4 indicates the number and percentages of patients, age-stratified, with SDF (DFI) > 30%.
Table 4.
Comparison of Sperm DNA Fragmentation in Group 1 (HAART) Versus Group 2 (NAÏVE) According to Age.
| Sperm DNA Fragmentation > 30% | Group 1- HAART (n = 53) |
Group 2 - NAÏVE (n = 24) |
|---|---|---|
| Age 25−38 years (n = 28) |
10/15 (66.7%) n = 15 |
5/13 (38.5%) n = 13 |
| Age 39−43 years (n = 24) |
11/17 (64.7%) n = 17 |
3/7 (42.9%) n = 7 |
| Age 44−54 years (n = 25) |
15/21 (71.4%) n = 21 |
1/4 (25%) n = 4 |
| Total HIV-1-infected patients |
36/53 (67.9%)
n = 53 |
9/24 (37.5%)
*
n = 24 |
Note. OR (Mantel–Haenszel): 3.51, p = .02, 95% CI [1.22, 10.12].
No correlation was found between increased DNA fragmentation and any classes of antiretroviral drugs in patients receiving therapy (Figure 2).
Figure 2.
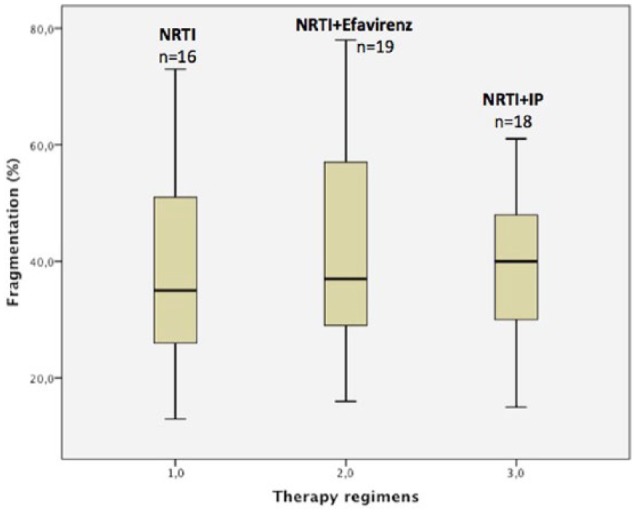
Differences in semen fragmentation index in three therapeutic regimens.
NRTI: Nucloside Reverse transcriptase inhibitor; NRTI + efavirenz; NRTI + IP (Protease inhibitor).
Discussion
The present study demonstrates elevated SDF in HIV-1-infected patients taking antiretroviral therapy compared to patients completely antiretroviral therapy naïve, excluding the bias of the effects of previous therapy. Furthermore, analyzing the three most common therapeutic regimes in the treatment of HIV infection disease, no differences in SDF rate were identified. To our knowledge, only Frainais et al. (2010) in their study examined the sperm DNA integrity comparing HIV-1-infected patients in therapy versus healthy uninfected patients, reporting an increased DNA fragmentation in HIV-1-infected men taking HAART. Frainais et al. (2010) hypothesized both a direct and indirect effect of the virus on DNA sperm integrity and the possibility of antiretroviral drug-mediated damage. The authors used healthy subjects as a control group and this could represent a bias in the interpretations of the results.
Several studies have reported semen alterations in HIV-infected men, such as decreased motility, sperm concentration, total sperm count, or volume ejaculate (Crittenden et al., 1992; Dondero et al., 1996; Dulioust et al., 2002; Kehl et al., 2011; Muller et al., 1998; Nicopoullos et al., 2004). Other reports did not identify, however, any difference (Bujan et al., 2007; Garrido et al., 2005). Since it is difficult to separate the role of HIV-1 infection from the adverse effects of therapy, the debate on this issue is still open.
There are several possible explanations for the observed sperm changes in patients receiving HAART. Therapeutic control of HIV-1 replication by antiretroviral medications is associated with increased oxidant stress in many patients and oxidant stress is not increased in patients with uncontrolled viral replication (Hulgan et al., 2003). Transport for drugs through the endothelial barrier can occur for passive diffusion, which is the primary driving force for drug transport to the lumen of the male genital tract, or for active cellular mechanism. Lowe et al. (2004), in contrast with previous suggestions (Bart et al., 2002; Holash, Harik, Perry, & Stewart, 1993), argue that the testicular extraluminal tissue is not a drug-deprived area and most antiretroviral drugs show good penetration in the male genital tract, potentially affecting spermatogenesis.
A second possible mechanism determining reduced sperm quality in HIV-1-infected men taking HAART, could be the changes in spermatozoa metabolism (Kehl et al., 2011) due to mitochondrial toxicity (Brinkman et al., 1998; Lewis & Dalakas, 1995). Maternally inherited, mitochondria are unique in that they contain their own circular double stranded genome, spanning 16.6 kb in length and encoding 13 subunits of the respiratory chain complexes, essential for the production of ATP in the cell (May-Panloup et al., 2003; Reynier et al., 2001). Disruption of mitochondrial electron transport flow in human spermatozoa results in generation of Reactive oxygen species (ROS) (Koppers, De Iuliis, Finnie, McLaughlin, & Aitken, 2008) and overproduction of ROS causes lipid peroxidation, responsible for sperm chromatin damage and DNA strand breaks (Aitken & Koppers, 2011; Blumer et al., 2012). A combination of antiretroviral therapy, duration of the therapy, duration of the infection and immunological status are all important parameters that should be taken into account when evaluating the semen quality (Bujan et al., 2007; Kehl et al., 2011; Nicopoullos, Almeida, Vourliotis, & Gilling-Smith, 2011; Pilatz et al., 2014; Robbins et al., 2001; Van Leeuwen et al., 2008). In addition, in order to exclude that the age of the patients could have influenced these results, DNA fragmentation was analyzed in relation to the men’s age. Although there is no clear consensus regarding the implication of male age on semen parameters, multiple studies have demonstrated that sperm DNA damage increases with advancing male age (Moskovtsev, Willis, White, & Mullen, 2007; Wyrobek et al., 2006). The results of the present study show a correlation between patients’ age and increased fragmentation, but the difference is not statistically significant. Previously published studies reported different findings regarding the impact of male age on semen quality. Kidd, Eskenazi, and Wyrobek (2001) reported a decline in semen volume, sperm motility, and morphology, while other studies identified a linear decline in semen volume (Dain, Auslander, & Dirnfeld, 2011). Sperm nuclear DNA fragmentation rate was evaluated by the SCD test. Several tests are available to analyze sperm nuclear DNA fragmentation, but no consensus has been reached yet with regard to which tests are the most predictive of infertility, due to the fact that all currently available techniques vary in their diagnostic accuracy and clinical value (Ribeiro et al., 2017). Ribas-Maynou et al. (2013) have reported a comprehensive analysis of SDF different assays and they demonstrate that the cut-off, sensitivity, and specificity results obtained by the SCD do not differ from previously published works (Fernandez et al., 2005; Nunez-Calonge et al., 2012; Velez de la Calle et al., 2008), showing a good capacity of this technique to assess male infertility. Feijó and Esteves (2014) conclude that the SCD test is more sensitive than the TUNEL assay for the assessment of DNA damage in men with unexplained infertility. In the study by Ribas-Maynou et al. (2013) the cut-off for SCD test is 22.75%, the sensitivity is 0.73, and specificity is 0.91. It can be concluded that the alkaline Comet assay, the SCD test, SCSA (Sperm Chromatin Structure Assay), and the TUNEL assay are useful to distinguish fertile and infertile patients, with a nonsignificant difference, except for the alkaline Comet assay being the best predictor of male infertility.
Limitations
The limitations of this study must be acknowledged, principally linked to small size of patients included, and caution is warranted when interpreting results. In addition, the design of the study did not allow the assessment of SDF for the same patient before and after the initiation of antiretroviral therapy.
Conclusion
The results of this study demonstrate that sperm nuclear fragmentation rate is increased in HIV-1-infected patients taking antiretroviral therapy when compared to naïve HIV-1-infected patients. Furthermore, no correlations between antiretroviral therapy and semen parameters like total sperm count, progressive motility, or morphology has been observed. Further research is needed to define the mechanisms of the potential effect of HAART on sperm and sperm mitochondrial DNA.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Valeria Savasi  https://orcid.org/0000-0002-1476-7689
https://orcid.org/0000-0002-1476-7689
Arianna Laoreti  https://orcid.org/0000-0003-2648-195X
https://orcid.org/0000-0003-2648-195X
References
- Aitken R. J., Koppers A. J. (2011). Apoptosis and DNA damage in human spermatozoa. Asian Journal of Andrology, 13, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart J., Groen H. J., van der Graaf W. T., Hollema H., Hendrikse N. M., Vaalburg W., … de Vries E. G. (2002). An oncological view on the blood–testis barrier. The Lancet Oncology, 3, 357–363. [DOI] [PubMed] [Google Scholar]
- Blumer C. G., Restelli A. E., Giudice P. T., Soler T. B., Fraietta R., Nichi M., … Cedenho A. P. (2012). Effect of varicocele on sperm function and semen oxidative stress. British Journal of Urology International, 109, 259–265. [DOI] [PubMed] [Google Scholar]
- Brinkman K., ter Hofstede H. J. M., Burger D. M., Smeitink J. A. M., Koopmans P. P. (1998). Adverse effects of reverse transcriptase inhibitors: Mitochondrial toxicity as common pathway. AIDS, 12, 1735–1744. [DOI] [PubMed] [Google Scholar]
- Bujan L., Sergerie M., Moinard N., Martinet S., Porte L., Massip P., … Daudin M. (2007). Decreased semen volume and spermatozoa motility in HIV-1-infected patients under antiretroviral treatment. Journal of Andrology, 28, 444–452. [DOI] [PubMed] [Google Scholar]
- Crittenden J. A., Handelsmann D. J., Stewart G. J. (1992). Semen analysis in human immunodeficiency virus infection. Fertility and Sterility, 57(6), 1294–1299. [PubMed] [Google Scholar]
- Dain L., Auslander R., Dirnfeld M. (2011). The effect of paternal age on assisted reproduction outcome. Fertility and Sterility, 95, 1–8. [DOI] [PubMed] [Google Scholar]
- Dondero F., Rossi T., D’Offizzi G., Mazzilli F., Rosso R., Sarandrea N., … Aiuti F. (1996). Semen analysis in HIV seropositive men and in subjects at high risk for HIV infection. Human Reproduction, 17(8), 2112–2118. [DOI] [PubMed] [Google Scholar]
- Dulioust E., Du A. L., Costagliola D., Guibert J., Kunstmann J. M., Heard I., … De Almeida M. (2002). Semen alterations in HIV-1 infected men. Human Reproduction, 17, 2112–2118. [DOI] [PubMed] [Google Scholar]
- Evenson D. P. (2011). Sperm Chromatin Structure Assay (SCSA): 30 years of experience with the SCSA. In Zini A., Agarwal A. (Eds.), Sperm chromatin: Biological and clinical applications in male infertility and assisted reproduction (pp. 125–149). New York, NY: Springer. [Google Scholar]
- Feijó C. M., Esteves S. C. (2014). Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertility and Sterility, 101, 58–63. [DOI] [PubMed] [Google Scholar]
- Fernandez J. L., Muriel L., Goyanes V., Segrelles E., Gosalvez J., Enciso M., … De, Jonge C. (2005). Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertility and Sterility, 84, 833–842. [DOI] [PubMed] [Google Scholar]
- Fernandez J. L., Muriel L., Rivero M. T., Goyanes V., Vazquez R., Alvarez J. G. (2003). The sperm chromatin dispersion test: A simple method for the determination of sperm DNA fragmentation. Journal of Andrology, 24, 59–66. [PubMed] [Google Scholar]
- Fraga C. G., Motchnik P. A., Wyrobek A. J., Rempel D. M., Ames B. N. (1996). Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutation Research, 13, 199–203. [DOI] [PubMed] [Google Scholar]
- Frainais C., Vialard F., Rougier N., Aegerther P., Damond F., Ayel J. P., … Selva J. (2010). Impact of freezing/thawing technique on sperm DNA integrity in HIV-1 patients. Journal Assisted Reproduction and Genetics, 27, 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido N., Meseguer M., Remohì J., Simon C., Pellicer A. (2005). Semen characteristics in Human Immunodeficiency Virus (HIV) and hepatitis C (HCV) seropositive males: Predictors of the success of viral removal after sperm washing. Human Reproduction, 20, 1028–1034. [DOI] [PubMed] [Google Scholar]
- Henkel R., Kierspel E., Hajimohammad M., Stalf T., Hoogendijk C., Mehnert C., … Kruger T. F. (2003). DNA fragmentation of spermatozoa and assisted reproduction technology. Reproductive Biomedicine Online, 7, 477–484. [DOI] [PubMed] [Google Scholar]
- Holash J. A., Harik S. I., Perry G., Stewart P. A. (1993). Barrier properties of testis microvessels. Proceedings of the National Academy of Sciences of the United States of America, 90, 11069–11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulgan T., Morrow J., D’Aquila R. T., Raffanti S., Morgan M., Rebeiro P., Haas D. W. (2003). Oxidant stress is increased during treatment of human immunodeficiency virus infection. Clinical Infectious Diseases, 37, 1711–1717. [DOI] [PubMed] [Google Scholar]
- Hunault C. C., Habbema J. D., Eijkemans M. J., Collins J. A., Evers J. L., te Velde E. R. (2004). Two new prediction rules for spontaneous pregnancy leading to live birth among subfertile couples, based on the synthesis of three previous models. Human Reproduction, 19, 2019–2026. [DOI] [PubMed] [Google Scholar]
- Kehl S., Weigel M., Muller D., Gentili M., Hornemann A., Sütterlin M. (2011). HIV-1 infection and modern antiretroviral therapy impair sperm quality. Archives Gynecology Obstetrics, 284, 229–233. [DOI] [PubMed] [Google Scholar]
- Kidd S. A., Eskenazi B., Wyrobek A. J. (2001). Effects of male age on semen quality and fertility: A review of the literature. Fertility and Sterility, 75, 237–248. [DOI] [PubMed] [Google Scholar]
- Koppers A. J., De Iuliis G. N., Finnie J. M., McLaughlin E. A., Aitken R. J. (2008). Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. The Journal of Clinical Endocrinology & Metabolism, 93, 3199–3207. [DOI] [PubMed] [Google Scholar]
- La Sala G. B., Pilotti E., Nicoli A., Pinelli S., Villani M. T., Ronzi P., … Casoli C. (2007). Dynamics of HIV viral load in blood and semen of patients under HAART: Impact of therapy in assisted reproduction procedures. AIDS, 21, 377–379. [DOI] [PubMed] [Google Scholar]
- Lewis W., Dalakas M. C. (1995). Mitochondrial toxicity of antiviral drugs. Nature Medicine, 1, 417–422. [DOI] [PubMed] [Google Scholar]
- Lowe S. H., Sankatsing S. U., Repping S., van der Veen F., Reiss P., Lange J. M., Prins J. M. (2004). Is the male genital tract really a sanctuary site for HIV? Arguments that it is not. AIDS, 8(10), 1353–1362. [DOI] [PubMed] [Google Scholar]
- May-Panloup P., Chrétien M. F., Savagner F., Vasseur C., Jean M., Malthièry Y., Reynier P. (2003). Increased sperm mitochondrial DNA content in male infertility. Human Reproduction, 18(3), 550–556. [DOI] [PubMed] [Google Scholar]
- Moskovtsev S. I., Willis J., White J., Mullen J. B. M. (2007). Sperm survival relation-ship to age-related sperm DNA integrity in infertile men. Archives of Andrology, 53, 29–32. [DOI] [PubMed] [Google Scholar]
- Moyle G. (2004). Mitochondrial toxicity: Myths and facts. Journal of HIV Therapy, 9, 45–47. [PubMed] [Google Scholar]
- Muller C. H., Coombs R. W., Krieger J. N. (1998). Effects of clinical stage and immunological status on semen analysis results in human immunodeficiency virus type 1-seropositive men. Andrologia, 30(Suppl. 1), 15–22. [DOI] [PubMed] [Google Scholar]
- Nicopoullos J. D., Almeida P. A., Ramsay J. W., Gilling-Smith C. (2004). The effect of human immunodeficicency virus on sperm parameters and the outcome of intrauterine insemination following soperm washing. Human Reproduction, 19(10), 2289–2297. [DOI] [PubMed] [Google Scholar]
- Nicopoullos J. D., Almeida P., Vourliotis M., Gilling-Smith C. (2011). A decade of the sperm-washing programme: Correlation between markers of HIV and seminal parameters. HIV Medicine, 12, 195–201. [DOI] [PubMed] [Google Scholar]
- Nunez-Calonge R., Caballero P., Lopez-Fernandez C., Guijarro J. A., Fernandez J. L., Johnston S., Gosálvez J. (2012). An improved experimental model for understanding the impact of sperm DNA fragmentation on human pregnancy following ICSI. Reproductive Sciences, 19, 1163–1168. [DOI] [PubMed] [Google Scholar]
- Pavili L., Daudin M., Moinard N., Walschaerts M., Cuzin L., Massip P., … Bujan L. (2010). Decrease of mitochondrial DNA level in sperm from patients infected with human immunodeficiency virus-1 linked to nucleoside analogue reverse transcriptase inhibitors. Fertility and Sterility, 94, 2151–2156. [DOI] [PubMed] [Google Scholar]
- Pilatz A., Discher T., Lochnit G., Wolf J., Schuppe H. C., Schüttler C. G., … Diemer T. (2014). Semen quality in HIV patients under stable antiretroviral therapy is impaired compared to WHO 2010 reference values and on sperm proteome level. AIDS, 28, 875–880. [DOI] [PubMed] [Google Scholar]
- Reynier P., May-Panloup P., Chretien M. F., Morgan C. J., Jean M., Savagner F., … Malthiery Y. (2001). Mitochondrial DNA content affects the fertilizability of human oocytes. Molecular Human Reproduction, 7, 425–429. [DOI] [PubMed] [Google Scholar]
- Ribas-Maynou J., García-Peiró A., Fernández-Encinas A., Abad C., Amengual M. J., Prada E., … Benet J. (2013). Comprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral comet assay. Andrology, 1, 715–722. [DOI] [PubMed] [Google Scholar]
- Ribeiro S., Sharma R., Gupta S., Cakar Z., De Geyter C., Agarwal A. (2017). Inter- and intra-laboratory standardization of TUNEL assay for assessment of sperm DNA fragmentation. Andrology, 5, 477–485. [DOI] [PubMed] [Google Scholar]
- Robbins W. A., Witt K. L., Haseman J. K., Dunson D. B., Troiani L., Cohen M. S., … Bishop J. B. (2001). Antiretroviral therapy effects on genetic and morphologic end points in lymphocytes and sperm of men with human immunodeficiency virus infection. The Journal of Infectious Diseases, 184, 127–135. [DOI] [PubMed] [Google Scholar]
- Robinson L., Gallos I. D., Conner S. J., Rajkhowa M., Miller D., Lewis S., … Coomarasamy A. (2012). The effect of sperm DNA fragmentation on miscarriage rates: A systematic review and meta-analysis. Human Reproduction, 27, 2908–2917. [DOI] [PubMed] [Google Scholar]
- Seli E., Gardner D. K., Schoolcraft W. B., Moffatt O., Sakkas D. (2004). Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertility and Sterility, 82, 378–383. [DOI] [PubMed] [Google Scholar]
- Sergerie M., Mieusset R., Croute F., Daudin M., Bujan L. (2007). High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertility and Sterility, 88, 970.e1–970.e7. [DOI] [PubMed] [Google Scholar]
- Van der Steeg J. W., Steures P., Eijkemans M. J., Habbema J. D., Hompes P. G., Broekmans F. J., … Mol B. W. (2007). Pregnancy is predictable: A large-scale prospective external validation of the prediction of spontaneous pregnancy in subfertile couples. Human Reproduction, 22, 536–542. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen E., Wit F. W., Repping S., Eeftinck Schattenkerk J. K. M., Reiss P., van der Veen F., Prins J. M. (2008). Effects of antiretroviral therapy on semen quality. AIDS, 12, 637–642. [DOI] [PubMed] [Google Scholar]
- Velez de la Calle J. F., Muller A., Walschaerts M., Clavere J. L., Jimenez C., Wittemer C., Thonneau P. (2008). Sperm deoxyribonucleic acid fragmentation as assessed by the sperm chromatin dispersion test in assisted reproductive technology programs: Results of a large prospective multicenter study. Fertility and Sterility, 90, 1792–1799. [DOI] [PubMed] [Google Scholar]
- Vernazza P. L., Graf I., Sonnenberg-Schwan U., Geit M., Meurer A. (2011). Pre-exposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS, 25, 2005–2008. [DOI] [PubMed] [Google Scholar]
- White D. J., Mital D., Taylor S., St John J. C. (2001). Sperm mitochondrial DNA deletions as a consequence of long term highly active antiretroviral therapy. AIDS, 15, 1061–1062. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). (2010). WHO laboratory manual for the examination and processing of the human semen (5th ed.). Geneva: World Health Organization. [Google Scholar]
- Wyrobek A. J., Eskenazi B., Young S., Arnheim N., Tiemann-Boege I., Jabs E. W., … Evenson D. (2006). Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidiesin sperm. Proceedings of the National Academy of Sciences of the United States of America, 103, 9601–9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeni P. G., Hammer S. M., Carpenter C. C., Cooper D. A., Fischl M. A., Gatell J. M., … Volberding P. A. (2002). Antiretroviral treatment for adult HIV infection in 2002. The Journal of the American Medical Association, 288, 222–235. [DOI] [PubMed] [Google Scholar]