Abstract
Male breast cancer (MBC) is rare and known as a typical woman’s disease. This study is part of the N-MALE project (Male breast cancer: patient’s needs in prevention, diagnosis, treatment, rehabilitation and follow-up-care) and aims to investigate how MBC patients (MBCP) feel about suffering from a “woman’s disease,” what character the stigmatization has, and how it can be prospectively reduced. Therefore, a mixed methods design is applied including data of N = 27 qualitative interviews with MBCP and quantitative data of N = 100 MBCP. Findings identify a diverse picture, as stigmatization varies between contexts and patients: Most stigmatization concentrates on sexual stigmatization and ignorance of MBC and mostly occurs in cancer care systems and work-related contexts. The level of stigmatization varies with age and amount of treatment methods received, as reported within the created typology of different MBCP stigma types. To prospectively reduce stigmatization in MBCP, more publicity of MBC is needed, as well as gender-neutral communication and information material.
Keywords: stigma, (male) breast cancer, MBC, rare disease, medical sociology, health services research, mixed methods
Breast cancer in men is a rare disease that accounts for around 1% of breast cancer cases in the western world (Ly, Forman, Ferlay, Brinton, & Cook, 2013; Miao et al., 2011). However, the incidence has risen over the past decades (Hodgson, Button, Franceschi, Moffat, & Livingstone, 2004), and it has been suggested that this rise will continue in the future (Contractor, Kaur, Rodrigues, Kulkarni, & Singhal, 2008). Risk factors include a family history of breast cancer, genetic and hormonal aspects (da Silva, 2016; Giordano, 2018). As breast cancer is known as a typical woman’s disease, most research to date has focused on female breast cancer. Consequently, there is a need for more research on male breast cancer (MBC), especially concerning the psychosocial aspects of cancer care. Besides this female focus in breast cancer research, there is also a social construct that connects breasts in general and breast cancer with femaleness (da Silva, 2016). These social constructs can have significant implications for men who have breast cancer, wherein, besides having to cope with the disease, males also have to deal with gender aspects because of suffering from a perceived woman’s illness and feminization in therapy (da Silva, 2016). Emasculation is a big issue discussed in several studies of male breast cancer patients (MBCP) (da Silva, 2016; Donovan & Flynn, 2007; France et al., 2000; Iredale, Brain, Williams, France, & Gray, 2006; Smolin & Massie, 2002; Swergold, Murthy, & Chamberlain, 2014) and can lead to stigmatization.
Stigmatization as a sociological construct was first characterized by Goffman (Goffman, 1963). It has been variously defined as a process in which specific human characteristics, so-called stigmas, are stereotyped and negatively labeled (Link & Phelan, 2001; Esser et al., 2017). Stigmatization can lead to social exclusion, isolation, and changes in the life situation of the affected person such as employment opportunities or housing (Link & Phelan, 2006) and has also been reported to influence personal identity (Goffman, 1963; Link & Phelan, 2001). This labeling process, whether placed by others or oneself, often includes shame as part of self-stigmatization or disapprobation (Goffman, 1963). It is important to distinguish between self and external stigmatization, because stigmatized people sometimes do not identify themselves with the negative labeling of others (Ernst, 2016). Stigmatization has an orientation function in social interactions, as it helps to maintain norms and expectable behavior. To distance oneself from a stigmatized person retains our own identity as it normalizes the own identity or depreciates the other (Hohmeier, 1975). Also, as stated by Tang, Mayer, Chou, and Hsiao (2016), stigmatization is not consistent but depends on personal aspects, social relationships, and contexts, as to whether the stigmatized person interprets something as stigmatizing or not.
Health-related stigmatization means the labeling of people because of certain characteristics of illness (Fife & Wright, 2000; Goffman, 1963; Link & Phelan, 2001). Illness is a stigmatizing element for the reason that it is connected with (physical and/or mental) limitations (Fife & Wright, 2000). Stigmatization of individuals, based on health in general or illness, typically results in their exclusion from social roles or functions (Link & Phelan, 2001). As past studies identify, stigmatization is a very common aspect for cancer patients (Ernst, 2016; Fife & Wright, 2000; Lebel & Devins, 2008), as cancer is a disease that confounds the social norms of society and provokes fear and insecurity, which is reported and explained in detail elsewhere (Ernst, 2016).
Although several studies of stigmatization of (female) breast cancer patients (Meacham, Orem, Nakigudde, Zujewski, & Rao, 2016; Nyblade, Stockton, Travasso, & Krishnan, 2017; Tripathi, Datta, Agrawal, Chatterjee, & Ahmed, 2017; Trusson & Pilnick, 2017) exist, no studies, to date, have focused solely on stigmatization of MBCP. Instead, the issue of MBCP stigmatization is raised within the discussion of the patients (Brain, Williams, Iredale, France, & Gray, 2006; Donovan & Flynn, 2007; Iredale et al., 2006; Kipling, Ralph, & Callanan, 2014). Stigmatization plays an important role in MBCP. One issue of concern for MBCP surrounds the physical changes and changes in body image after treatment (Bunkley, Robinson, Bennett, & Gordon, 2000), especially because men often associate their body (and their chest) with masculinity (Donovan & Flynn, 2007; Pituskin, Williams, Au, & Martin-McDonald, 2007; Robinson, Metoyer, & Bhayani, 2008). Particularly, the scar on the breast is an important contributor to the altered body image, which is perceived controversially by the patients (France et al., 2000; Iredale et al., 2006; Pituskin et al., 2007; Robinson et al., 2008; Williams et al., 2003), and younger men tend to find it of greater concern than older patients do (Iredale et al., 2006). An altered body image is also connected with psychological distress (Brain et al., 2006). Additionally, the rareness of the disease, connected with a lack of awareness of MBC and the perception of breast cancer as a woman’s disease, can lead to isolation (Bunkley et al., 2000; Iredale et al., 2006) brought about by stigmatization and nondisclosure because of feared stigmatization (France et al., 2000; Iredale et al., 2006).
In the context of these aspects raised in the preceding text, four major areas were explored to examine how MBCP feel about suffering from a “woman’s disease”: (a) the contexts in which the stigmatization occurs; (b) what kind of stigmatization the patients experience; (c) how, from the patients’ perspective, stigmatization can be reduced; and (d) if different levels of stigmatization can be created and how they differ regarding demographical and cancer-related aspects.
Methods
Study Design
This study was approved by the Ethics Committee for Bonn (Germany). It was carried out by an interdisciplinary research team (psychology, sociology, and health economics) with members representing care providers, patient representatives, and a psychotherapist.
The study is part of the N-MALE project (Male breast cancer: patient’s needs in prevention, diagnosis, treatment, rehabilitation and follow-up care) conducted in Germany. N-MALE, which started in April 2016 and ends in March 2018, was undertaken to examine the medical and psychosocial needs of MBCP across the cancer care continuum (from prevention to follow-up). It is an interdisciplinary study involving the University Hospital of Bonn, the University Hospitals of Cologne and Munich and the German Cancer Society (DKG). The N-MALE study applies a mixed methods design capturing data via qualitative interviews with MBCP and a quantitative questionnaire. This triangulation of methods intends to create more depth and breadth in the analysis of data (Carell, 2005; Flick, 1992) and thereby gain more insight into the participants’ perspective (Carell, 2005; Denzin & Lincoln, 2003). In the following text, qualitative and quantitative methods are described successively based on the so-called between-method triangulation described by Carell (2005), where both methods—qualitative and quantitative—are treated equally. Mixed methods were chosen to exploit the strengths of both approaches: the exploratory and comprehending character of qualitative analysis since little is known about stigmatization of MBCP. The qualitative content analysis was performed according to Mayring (2016). Data collected from the quantitative analysis were used to support the qualitative results, as the results of more MBCP can be considered within this study because of a bigger number of participants within the quantitative sample. By using both methods, the results can validate each other. Furthermore, quantitative methods help to describe “facts” as demographic or disease-related characteristics of the participants. Results of both analyses are brought together at the end of the results section within a mixed methods matrix per patient to complement each other to make a typification of participants possible.
Data were collected between April 2016 and October 2017. This process included qualitative and quantitative data.
Inclusion Criteria and Participants
Inclusion criteria were the confirmed breast cancer diagnosis (C50.x or D05.x) and a written informed consent. Exclusion criteria for the interviews were defined as aspects that made it difficult to set up an interview and included, for example, deafness, speech or comprehension problems, psychosis, dementia, advanced cancer, and related issues like pain, difficulties in concentrating, or if the written declaration of consent was missing or withdrawn. The exclusion criterion for the quantitative questionnaire was a missing or withdrawn declaration of consent.
Access to the field was given through the Men with Breast Cancer Network (Netzwerk Männer mit Brustkrebs e.V.) and breast cancer centers that were certified in accordance with the criteria of North Rhine Westphalia State (Äkzert) and the requirements of the German Cancer Society (Deutsche Krebsgesellschaft, DKG) and the German Society for Breast Diseases (Deutsche Gesellschaft für Senologie). Furthermore, MBCP interested in taking part in the study contacted us via e-mail or telephone, as there were some invocations in the form of press releases and short articles.
From this number of interested MBCP, participants were selected for qualitative interviews. Sampling was done according to the precepts of the Glaser and Strauss model (2008) for theoretical sampling. This strategy is used to find cases as significant and contrasting as possible until theoretical saturation is reached (Glaser, Strauss, & Paul, 2008). For sampling, data of the standardized questionnaire was used.1 The interviewed subjects included participants varying in sociodemographic factors like age, family status (status of relationship, children), and education; facts of disease like date of diagnosis (recent and less recent), disease status (stage, relapse), and treatment (breast cancer center or hospital); and other aspects like contact with support group and experiences during treatment (positive or negative).
Reasons for nonresponse for the qualitative interviews were lack of interest in an interview in general, issues of the disease like a progressed stage or cancer-related problems (exclusion criteria), or death between sending the questionnaire and appointment for an interview. For the quantitative questionnaire, we have no information of reasons for nonresponse.
Data Collection
Qualitative interviews
The recruiting and the participant interviews were conducted by two female (and one male) research fellows (PhD candidates) of the N-MALE project who were trained in interviewing skills. One was experienced in interviewing and did an advanced intern training of the other interviewers. In addition, all interviewers completed an interviewer training with a psychotherapist, focusing on how to deal with serious situations that could arise within the interviews (e.g., strategies for talking about sensitive topics, like sexuality of the participants with those who have a different gender, how to deal with psychological stress that could arise from the interview).
The semistructured face-to-face interviews were done according to an interview guideline (Helfferich, 2011), which left enough space for open-ended answers and was structured along all steps of cancer therapy.2 The guideline was developed within the interdisciplinary N-MALE team, pilot-tested by three interviews with MBCP, and customized as discussed in the results of pretests in the project team. Each interview was between 1 and 2 hours and was audiotaped, anonymized, and transcribed toward specific rules according to Fuß and Karbach (Fuß & Karbach, 2014). After each interview, field notes on nonverbal aspects, abnormalities, first interpretations, and other information that could be helpful for the interpretation were recorded. The participants could choose the location of the interview, mostly their place of residence. To ensure an undisturbed atmosphere, care was taken to exclude others from the interview setting so that only the participant and the interviewer were present, except cases in which participants requested their partners. Before the interview, participants signed an informed consent and were advised about the procedure, the study objective, and the use of the data.
Quantitative data
The quantitative questionnaire was developed within the interdisciplinary N-MALE team and pretested with four MBCP. After the pretests, the questionnaire was modified and mailed to all interested participants (117 participants).3 Following Dillman’s total design survey method (1978), three reminders were sent at 1, 3 and 7 weeks, to achieve the highest response rate (Dillman, Smyth, & Christian, 2014).
Sociodemographic characteristics
Sociodemographic as-pects of the MBCP were measured. They included age, marital/relationship status, children, education, occupation, and residential area (urban, rural).
Disease-related characteristics and breast cancer treatment
The participants were asked about aspects of their disease, like date of diagnosis, first time breast cancer or relapse, and types of cancer treatment (current breast cancer treatment, surgery, chemotherapy, adjuvant radiation, hormonal therapy, rehabilitation), as well as contact with (MBCP) support group, experiences of cancer care (positive and negative), and comorbidities.
Measurement of stigmatization
Participants were questioned about stigmatization during the course of cancer treatment and in private surroundings. The questionnaire was structured in the different stages of cancer care, with filter questions about specific cancer care steps, in which the participants were simply required to indicate whether they have received in the past or will receive it in the future. Concerning stigmatization in the process of cancer care, the following five variables were used:
Have you felt excluded during hospitalization in terms of your breast cancer disease?
Have you felt excluded during chemotherapy in terms of your breast cancer disease?
Have you felt excluded during radiation therapy in terms of your breast cancer disease?
Have you felt excluded during medical rehabilitation in terms of your breast cancer disease?
Have you felt excluded during aftercare or follow-up survey in terms of your breast cancer disease?
Concerning the private surroundings, the survey asked about stigmatization in different circumstances like close and wider social relationships, with three variables in total:
Have you felt excluded from your family in terms of your breast cancer disease?
Have you felt excluded from your friends or acquaintances in terms of your breast cancer disease?
Have you felt excluded from your colleagues or superiors in terms of your breast cancer disease?
The answer categories for all questions were never, rarely, sometimes, often, and always with single selection. If men felt any stigmatization, the next question asked was in which form they experienced stigmatization to explain their experiences. This open-ended answer category was also included in the qualitative content analysis.
Data Analysis
Analysis of qualitative data
For qualitative analysis, transcript data of the interviews, after-interview notes, as well as the open-ended answer categories of the quantitative questionnaire (reasons for stigmatization) were analyzed using qualitative content analysis according to Mayring (Mayring, 2016). Coding was deductive and inductive4 using MAXQDA version 12.2.1 (VERBI GmbH, 2016) software for managing the data. The coding was described. One scientist who mainly conducted the interviews also did the coding process. During this process, there were regular consultations within the research team for validation. It was an alternating interviewing and analyzing process, where categories were developed and tested in the interviews that followed. This alternating strategy was also needed for the purposeful sampling (Helfferich, 2011). At the end of the coding process, the codes were discussed in a research workshop.
Statistical analysis
For statistical analysis, SPSS version 25 (IBM SPSS Statistics, 2017) was used. The sociodemographic, disease-related characteristics and breast cancer treatment were analyzed using descriptive statistics. For analysis of stigmatization, descriptive statistics were used as well. Mean values are reported separately for each item. Furthermore, t-tests were derived to estimate significant differences between items.
Mixed methods analysis
For mixed methods analysis, qualitative and quantitative data of 27 MBCP were included. Qualitative and quantitative data of those participants were merged, including codes of qualitative analysis (dimensions of stigmatization, no subjective stigma experienced) and data of quantitative analysis (context of stigmatization: results per person for Measurement of Stigmatization). Sociodemographic aspects (age, education) and disease-related aspects (breast cancer for the first time/ relapse, stage(s) in the cancer care system) were collected within the interviews.
Findings
Characteristics of Participants
Concerning quantitative data, the cleared response rate was 85,5% (N = 100).
Twenty-seven interviews with MBCP were conducted. Table 1 gives an overview of sociodemographic and disease-related characteristics of the quantitative sample and the (qualitative) subsample.
Table 1.
Sample Characteristics of the Quantitative and Qualitative Sample of Male Breast Cancer Patients.
| N = 100 (N = 27) | N (N) | % (%) | Mean (mean) | Min (min) | Max (max) |
|---|---|---|---|---|---|
| Sociodemographic characteristics | |||||
| Age | |||||
| In years | 66.91 (64.8) | 39 (42) | 89 (89) | ||
| Missing | 2 (1) | 2 (3.7) | |||
| Living with a partner | |||||
| Yes | 82 (19) | 87.2 (79.2) | |||
| No | 12 (5) | 12.8 (20.8) | |||
| Missing | 6 (3) | 6.0 (11.1) | |||
| Children | |||||
| Yes | 79 (20) | 84.0 (76.9) | |||
| No | 15 (6) | 16.0 (23.1) | |||
| Missing | 6 (1) | 6.0 (3.7) | |||
| Education (multiple answers) | |||||
| No school certificate | 2 (0) | 2.0 (0.0) | |||
| Lower school certificate | 41 (11) | 41.8 (42.3) | |||
| Intermediate school certificate | 27 (8) | 27.6 (30.8) | |||
| Vocational diploma/university entrance certificate | 35 (11) | 35.7 (42.3) | |||
| Missing | 2 (1) | 2.0 (3.7) | |||
| Occupation | |||||
| Full-time | 26 (7) | 26.8 (26.9) | |||
| Part-time | 4 (1) | 4.1 (3.8) | |||
| Occupational rehabilitation | 2 (0) | 2.1 (.0) | |||
| Certified sick | 12 (6) | 12.4 (23.1) | |||
| (Early) retired | 54 (12) | 55.7 (46.2) | |||
| Unemployed | 1 (0) | 1.0 (.0) | |||
| Missing | 3 (1) | 3.0 (3.7) | |||
| Disease-related characteristics | |||||
| Time since first diagnosis | |||||
| In years | 3.61 (4.1) | <1 (<1) | 20 (17) | ||
| Missing | 5 (1) | 5 (3.7) | |||
| Types of treatment received | |||||
| Surgery | 97 (27) | 97.0 (100) | |||
| Chemotherapy | 56 (16) | 56.0 (59.3) | |||
| Radiation therapy | 65 (16) | 65.0 (59.3) | |||
| Antihormone therapy | 75 (22) | 75.0 (81.5) | |||
| I don’t know | 2 (1) | 2.0 (3.7) | |||
| Missing | 0 (0) | 0 (.0) | |||
| Newly diagnosed | |||||
| Yes | 92 (24) | 95.8 (96.0) | |||
| No | 4 (1) | 4.2 (4) | |||
| Missing | 4 (2) | 4.0 (7.4) | |||
Note. Quantitative sample N = 100; qualitative sample (subsample) N = 27. Numbers of qualitative sample are in brackets.
Context of Stigmatization
Findings based on the interviews with MBCP identify that most stigmatization occurs in the cancer care system. In addition, MBCP feel stigmatized by female breast cancer patients, especially if they have the feeling that the women are unaware of the disease in men:
“I remember that woman in the breast cancer center. She said: ‘What do YOU want here? (Laughing) You don’t belong here.’” (ID no. 63)
The statistical analyses (Table 2) indicate that in the cancer care system, most stigmatization takes place in rehabilitation settings (mean = 1.50), significantly more than during chemotherapy (p = .006), radiation (p = .019), follow-up survey (p = .031), and within family (p = .004)). In the cancer care system, the men experienced significantly higher stigmatization during hospitalization (mean = 1.20) than during chemotherapy (mean = 1.14; p = .049). The experienced stigmatization is higher within the cancer care system than within social surroundings. One exception from this finding was the feeling of exclusion in the working environment, which showed the highest value (mean = 1.69). The men felt significantly more excluded in the working environment than in hospital (p = .000), during chemotherapy (p = .000), radiation (p = .000), follow-up survey (p = .000), and within family (p = .000) and friends (p = .000). In qualitative data—in contrast—stigmatization is mostly being found within the cancer care system. In social surroundings, the closer the relationship, the less the stigmatization. That is, there is significantly less stigmatization with close family and friends than in broader social settings, for instance, with colleagues.
Table 2.
Stigmatization of Male Breast Cancer Patients (N = 100).
| N | Range | Minimum | Maximum | Mean value | Standard deviation | Significance in reference to (p)a | |
|---|---|---|---|---|---|---|---|
| Excluded during hospitalization | 97 | 1–5 | 1 | 4 | 1.20 | .606 | Chemo (.049), radiation (.017), family (.016), colleagues (–) (.000) |
| Excluded during chemotherapy | 57 | 1–5 | 1 | 3 | 1.14 | .398 | Rehab (–) (.006), colleagues (–) (.000) |
| Excluded during radiation therapy | 62 | 1–5 | 1 | 3 | 1.10 | .349 | Rehab (–) (.019), colleagues (–) (.000) |
| Excluded during medical rehabilitation | 48 | 1–5 | 1 | 5 | 1.50 | .968 | Follow-up (.031), family (.004) |
| Excluded during aftercare or follow-up survey | 80 | 1–5 | 1 | 4 | 1.14 | .497 | Colleagues (–) (.000) |
| Excluded from family | 100 | 1–5 | 1 | 3 | 1.05 | .297 | Friends (–) (.011), colleagues (–) (.000) |
| Excluded from friends or acquaintances | 100 | 1–5 | 1 | 3 | 1.16 | .487 | Colleagues (–) (.000) |
| Excluded from colleagues or superiors | 94 | 1–5 | 1 | 5 | 1.69 | .776 | |
| Valid terms (list wise) | 28 |
Note. aFor interpreting the terms: Positive values indicate higher feelings of exclusion within the term than within the comparative value.
Categories of Stigmatization
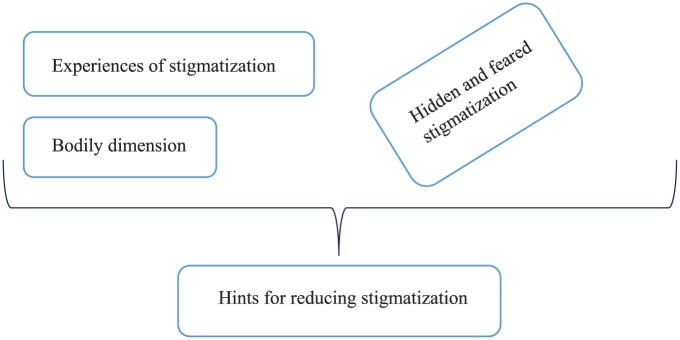
Within the interviews with MBCP, five main categories of stigmatization were identified and are shown in Figure 1.
Figure 1.
Categories of stigmatization of male breast cancer patients.
The category Experiences of stigmatization is a form of direct stigmatization. It describes situations in which MBCP were treated differently than other patients. The category Bodily dimension includes aspects associated with the changes to the body and body image after the surgery. In addition, there is a horizontal category of indirect stigmatization. Indirect stigmatization comprises situations that cause shame and indisposition and can lead to self-stigmatization. This category is called Hidden and feared stigmatization. Those direct and indirect dimensions led to the category Hints for reducing stigmatization given by the participants.
Experiences of stigmatization
The stigma aspects of most of the men are represented within this dimension. Sixteen men (59.26%; second highest stigma rate) experienced sexual stigmatization in the process of cancer care. This dimension of stigmatization occurs the most (Table 3). It includes the aspect that cancer care focuses on female breast cancer patients. Also, discrimination in treatment was experienced because of being male. For example, several men reported that some outpatient gynecologists who were specialists for breast cancer rejected them because treating a man might cause billing issues. Furthermore, some men were called by a female name in the waiting room:
“I think I was called as ‘Mrs. Miller’ once (laughing). Something like this is also unpleasant.” (ID no. 95)
Table 3.
Mixed Methods Matrix: Overview of Stigmatization of Male Breast Cancer Patients (N = 27).
| ID | Age | Educationa | Disease-related characteristicsb | Context of stigmatizationc | Dimensions of stigmatizationd | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiences of stigmatization | Bodily dimension | Hidden and feared stigma | ||||||||||||||||||||
| Clinic | Chemo | Radiation | Rehab | Aftercare | Family | Friends | Job | Sexual stigma | Only rooster in the yard | Ignorance | Scar | Loss of hair | Change in body image | Emaciated | Questioning glances | Being the odd one | Receiving different treatment | No subjective stigmae | ||||
| 5 | 87 | Diploma | First time (S,H) | 1 | np | np | np | 1 | 1 | 1 | 1 | x | x | |||||||||
| 9 | 73 | LS certificate | First time (S,C,A,R,H) | 1 | 1 | 1 | 4 | 1 | 1 | 1 | 2 | x | x | |||||||||
| 11 | 66 | LS certificate | 3x Relapse (S,C,A,H) | 1 | 1 | 1 | np | 1 | 1 | 1 | 2 | x | x | x | x | x | x | |||||
| 12 | 50 | Diploma | First time (S,C,A,H) | 1 | 2 | 999 | np | 1 | 1 | 1 | 2 | x | ||||||||||
| 14 | 79 | LS certificate | First time (S,H) | 1 | np | np | np | 1 | 1 | 1 | 1 | x | x | |||||||||
| 16 | 61 | Diploma | First time (S,C,A,R,H) | 3 | 1 | 1 | 3 | 1 | 1 | 1 | 2 | x | x | x | x | x | ||||||
| 18 | 59 | Diploma | First time (S,C,A,H) | 1 | 1 | 1 | np | 1 | 1 | 1 | 1 | x | x | |||||||||
| 19 | 57 | LS certificate | First time (S,C,A,R,H) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | x | x | x | ||||||||
| 22 | 88 | IS certificate | First time (S,C,A,R,H) | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | x | ||||||||||
| 28 | 61 | IS certificate | First time (S,C,A,R,H) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | x | x | (x) | x | x | x | |||||
| 31 | 70 | LS certificate | First time (S,C,A,R,H) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | x | x | (x) | x | x | ||||||
| 32 | 54 | IS Certificate | First time (S,C,A,R,H) | 4 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | x | x | x | x | |||||||
| 36 | 69 | LS certificate | First time (S) | 1 | np | np | np | 1 | 1 | 1 | 1 | x | ||||||||||
| 43 | 41 | LS Certificate | First time (S,C,A,R,H) | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 3 | x | x | x | ||||||||
| 48 | 73 | IS certificate | First time (S,H) | 999 | 999 | 999 | 999 | 999 | 999 | 999 | 999 | x | ||||||||||
| 52 | 59 | IS Certificate | Relapse (S,C,A,R,H) | 1 | 2 | 2 | 1 | 1 | 3 | 3 | 3 | x | x | x | ||||||||
| 55 | 51 | Diploma | First time (S,C,A,R,H) | 1 | 1 | 999 | 999 | fut. | 1 | 1 | 2 | x | x | |||||||||
| 63 | 74 | Diploma | First time (S) | 1 | np | np | np | 1 | 1 | 1 | 1 | x | ||||||||||
| 67 | 74 | LS certificate | First time (S,H) | 1 | np | np | np | 3 | 1 | 1 | 1 | x | x | x | x | x | x | x | ||||
| 74 | 75 | 999 | First time (S,A,H) | 1 | np | 1 | np | 1 | 1 | 1 | 1 | x | x | (x) | x | |||||||
| 77 | 63 | Diploma | First time (S,H,R) | 1 | np | np | 999 | 1 | 1 | 1 | 2 | (x) | x | |||||||||
| 78 | 60 | LS certificate | First time (S,C,A,R,H) | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 5 | x | x | x | x | x | x | |||||
| 82 | 56 | IS certificate | First time (S,C,A,R,H) | 4 | 3 | 2 | fut. | 999 | 3 | 3 | 1 | x | x | x | ||||||||
| 87 | 80 | IS certificate | First time (S,H) | 1 | np | np | np | 1 | 1 | 1 | 2 | x | x | |||||||||
| 91 | 65 | Diploma | First time (S,H,R) | 2 | np | np | 1 | 1 | 1 | 1 | 1 | x | x | x | (x) | |||||||
| 95 | 49 | LS certificate | First time (S,A,R,H) | 1 | np | 1 | 1 | 1 | 1 | 1 | 2 | x | ||||||||||
| 99 | 55 | Diploma | First time (S,C,H) | 1 | 1 | np | fut. | fut. | 1 | 1 | 2 | x | x | x | x | x | ||||||
| Total | 2 | 1 | 0 | 2 | 1 | 2 | 3 | 3 | 16 | 18 | 12 | 3(8) | 4(5) | 2 | 1 | 7 | 3 | 3 | 3(10) | |||
Note. aDiploma = vocational/university entrance diploma; LS certificate = lower school certificate; IS certificate = intermediate school certificate; 999 = missing.
First time = breast cancer for the first time; Relapse = relapse of breast cancer; S = surgery; C = chemotherapy; A = adjuvant radiation; R = rehabilitation; H = hormone therapy.
Quantitative stigma results of Table 2 per patient; np = not provided therapy; 999 = missing; fut. = treatment in the future; intensity of stigmatization: 1 = never, 2 = rarely, 3 = sometimes, 4 = often, 5 = always.
Qualitative stigma results per patient; x = stigmatization experienced within this dimension; As sociodemographic and disease related aspects were collected with different methods (questionnaire and interview), there are some deviations (if comparing data of qualitative sample within Tables 1 and 3).
Self-reporting that no stigmatization was experienced.
The highest stigma rate can be found within the dimension having the feeling of being the only rooster in the yard beside all the women in breast cancer therapy (occurs in 18 men; 66.67%). The participants experienced this stigma in two different ways. One, from a positive view:
“You then feel like an exotic. Many women. But me as the only man. [. . .] Anyway, you’re the only rooster in the yard.” (ID no. 9)
But also from a more negative one:
“I’ve been the only men among women. An exchange of experience was not possible at the rehabilitation center.” (ID no. 91)
Moreover, 12 men (44.44%; third highest stigma rate) experienced ignorance because nearly no one knows about breast cancer in men, both in their social and professional environments. Also, men experienced changes in social relationships, including social isolation, because some people do not know how to deal with a man having breast cancer.
Although 10 men (37.04%) reported having no experiences of stigmatization, the codes (Table 3) reveal that this number is lower (N = 3). Hence, there is a difference between the self-reporting of stigmatization (when they were asked if they experienced stigmatization) and the results of coding concerning stigmatization.5
Bodily dimension
There were four aspects found in which the disease influences body issues related to stigmatization. Three of them are visible (e.g., the scar on the breast). Some men were ashamed to show themselves shirtless in public, for example, in a swimming pool. The loss of hair is also a problem for some men—on the head and especially on the face, that is, loss of the beard:
“This is a time when the disease is also disfiguring. Nobody sees the surgery. There is a shirt over it. You have your scars, [. . .] but you can hide them. But when the hair is gone, mustache away, eyebrows away.” (ID no. 32)
Not visible but also important for the men is the change in body image after the disease. Some men felt emaciated and less strong:
“This was a big problem for me at the beginning. Because I said, I’m distorted. The nipple is gone. [.] It was a learning process. [. . .] I have a certain body image of mine. I’m tall, I’m strong, I’m intact, I’m in working order. I’m reasonably good looking. And at that time, this body image got a first crack. [. . .] Some years ago, I had a hip replacement surgery. There is also a scar. That didn’t matter to me. Only here I had doubts.” (ID no. 77)
Hidden and feared stigmatization
Within this indirect dimension of stigmatization, some men mentioned receiving questioning glances while sitting in the waiting room of a gynecologist:
“[While sitting in the waiting room] the women are thinking: ‘He accompanies his wife. She’s in treatment.’ And when you’re being called: ‘Mr. Miller please.’ All heads are turning, and you feel kind of observed.” (ID no. 55)
Besides, participants had the feeling of being an oddity or outsider because of being the only male and not being integrated into the circle of female breast cancer patients:
“Well, in those occupational therapies there are predominantly women. [. . .] My impression was that they did not want to have men with them. [. . .] That’s why I kept out of it.” (ID no. 16)
In treatment, some men also had the sense of being treated differently from the women with breast cancer. Moreover, men reported fear of other people’s reactions that made them ashamed of their disease:
“I was ashamed at first [. . .]. Because men and breast cancer? [. . .] It’s the basis of several thoughts as: That could provoke mockery or strange questions. How can a man get breast cancer. [. . .] And I thought didn’t tell anyone at first.” (ID no. 99)
Hints for reducing stigmatization
From the men’s perspective, stigmatization can be reduced in two different ways. One is by increasing the awareness of MBC in the cancer care system and in public:
“Thus, enlightenment is the most important. In therapy, it should be taken for granted that also men can get breast cancer and that it isn’t extraordinary.” (ID no. 16)
“If the people were more enlightened, there would be less insecurity in social environment, I guess.” (ID no. 32)
Second, the men wish for equality of men and women in cancer care. Notably, the documents and information materials should be gender-neutral or contain aspects for both genders:
“As I said those forms. . . . It always annoys me. [. . .] Why it isn’t possible to create a form which says dear patient [female AND male salutation].” (ID no. 32)
Stigma Types
As the mixed methods matrix (Table 3) indicates, most of the 27 participants experienced stigmatization during their course of the disease. However, the level of stigmatization varies between the participants. The participants were divided into three different groups according to their experiences of stigmatization (how many experiences of stigmatization and number of areas the stigmatization was experienced).6 To allocate the MBCP into those three stigma groups, their answers within the questionnaire and the interview were added, concerning stigmatization.
The first group “not stigmatized” experienced no or minimum stigmatization. It includes participants who in the interview said they experienced no stigmatization and who had no hint of any stigma dimension within the coding of the interviews (or just one) and stated in the questionnaire that they did not experience any stigmatization among the different contexts of cancer care and social surroundings. This group consists of four participants (ID nos. 36, 48, 63 and 87), who have in common that they have breast cancer for the first time (no relapse) and have minimal therapy—surgery and, in some cases, hormone therapy. Another characteristic of this first group is their higher average age (74 years) compared to the other two groups. Additionally, two of the men are still suffering from other types of cancer or have already experienced another cancer.
In contrast to that group, another group of MBCP experienced much more stigmatization during their process of disease (“stigmatized”), as evidenced within the Dimensions of stigmatization and the Context of stigmatization (Table 3), having more aspects or rather higher rates in some contexts (cancer care and social surroundings). To be part of this group, the participants need to have experiences in ⩾5 categories of stigmatization (counted crosses [x] in Dimensions of stigmatization and all values ⩾3 in Context of stigmatization). Within this group, there are seven participants (ID nos. 11, 16, 32, 52, 67, 78, 82). All of them have received more types of therapy than the “not stigmatized” group and two of them had a relapse (all relapse cases are within this group). The average age of those men is much lower than in Group 1 (62 years).
The third group of MBCP represents those who experienced “average stigma levels,” as their level of stigmatization is located in the middle of the former two groups. It is the biggest group, consisting of all participants (N = 16) who experienced more than “no stigmatization” and less than a lot (i.e., stigmatization in ⩾2/3 and <5 categories). They have an average age of 68 years, which is between the ages of groups 1 and 2. The stage of cancer varies among the members of this group, from a lower to a higher stage in regard to the therapy. All participants within this group have breast cancer for the first time.
Discussion
This study aimed to determine how MBCP feel about suffering from a “woman’s disease.” To address this research question, it investigated (a) the surroundings in which the stigmatization is experienced; (b) the kind of stigmatization experienced; (c) how, from the patients’ perspective the stigmatization can be reduced; and (d) if there is a typology of different stigma types in terms of the level of stigmatization.
The results reveal that MBCP feel stigmatized in different settings within the cancer care system as well as in social surroundings. The men mention more stigmatization in the cancer care system and by female breast cancer patients within the care system than in their close social environment, based on the qualitative data. The quantitative data reveals a different picture, as most stigmatization was significantly experienced within the working environment, followed by stigmatization in the cancer care system—within rehabilitation. The reasons for this difference may be an issue of measurement. In general, stigma rates in the quantitative analysis are low. In comparison to the other rates, those of exclusion within rehabilitation and working environment are higher. Nevertheless, they are not high absolutely. Another explanation for this difference in the qualitative and quantitative results may be that stigma was measured indirectly, by the feeling of exclusion. Maybe for MBCP, feeling excluded is not the same as feeling stigmatized. For example, in the working environment the men might feel excluded because they are no longer participating in the lived-in working environment after their cancer diagnosis, but this does not have to mean that they feel stigmatized by colleagues and superiors. Results of other studies support this interpretation of the results, as they indicate that there is low stigmatization in the work context (Ernst, Mehnert, Taubenheim, et al., 2017; Fife & Wright, 2000).
Transferring the aspect of feeling excluded to the other high rate of stigma (i.e., rehabilitation), it can mean that MBCP are feeling excluded from the group of female breast cancer patients because they are not part of this group. In the absence of a specialized rehabilitation center for MBCP, they are going to the same institutions as women do and mostly constitute a minority among the female breast cancer patients. MBCP reported that often they are not allowed to visit the same programs as female breast cancer patients. For many MBCP it is the first time they are in such close contact with female breast cancer patients within rehabilitation programs. All these aspects mentioned may lead to the feeling of being excluded as a man in a rehabilitation surrounding that is dominated by women and designed for female breast cancer patients.
In the context of the private social surroundings, it was observed that MBCP experience significantly most stigma or exclusion within the work-related context. A reason this feeling is much higher than in other social surroundings, such as family or friends, may be connected to the role within the working environment. When getting ill, the role expectation as a working person can no longer be fulfilled, which may lead to stigmatization. At the same time, this role loss can be seen as a social effect of stigmatization, as Link and Phelan (2001) state within their theory of labeling approach. Stigmatization in the work-related context is not only the case for breast cancer but an issue of cancer in general (Ernst, Mehnert, Taubenheim, et al., 2017). A man might assume that he cannot continue to fulfill his role of feeding his family, which can lead to self-stigmatization. The working environment is not very intimately connected to the person, unlike friends or the family. Within those closer social environments, there is often more understanding toward the individual’s needs and worries and the efficiency aspect does not have the same priority as within the working context.
Referring to the different dimensions of stigmatization, it was identified that the category Experiences of stigmatization was most prevalent among the patients. It includes aspects demonstrating the unique position of the men regarding gender aspects. Many men feel they are in an exclusive position (Being the only rooster in the yard), which can be regarded as a positive kind of stigmatization because the men experienced more attention from the providers than other (female) patients did. Men are also experiencing Sexual stigmatization, as breast cancer is known as a typical women’s disease among the providers and the other patients. As a result, providers act according to these role expectations and regard MBCP as women initially. There is confusion about the role expected of a breast cancer patient if the disease is not automatically connected with femaleness anymore. This confusion is intensified by the fact presented within the category Ignorance that breast cancer in men is mostly unknown.
In relation to the Bodily dimension, the visible aspects of the disease, such as the scar on the breast or the loss of hair, were perceived differently in men. Pituskin et al. (2007) report similar results, stating that men differ in perceived stigmatization because of the scar. The loss of hair has not been mentioned in any studies on MBC so far, maybe because hair loss is seen as a typical problem for female (breast) cancer patients. Trusson and Pilnic (2017) note that hair loss can be very traumatic for female breast cancer patients, but physicians often underrate it. As some men within this study demonstrate, losing hair can also be a problem for MBCP especially losing the beard, as it is a typical element of masculinity. As a disease becomes outwardly visible (e.g., formation of the scar on the breast or the loss of hair after cancer therapy), it often interferes with the social interactions because from this point on, one is identified as being sick and cannot fulfill one’s role expectations in society as a functioning member of society (Parsons & Turner, 2005; Reuter, 2015). Cancer patients are seen as goners, who came back to life (Holmberg, 2005; Reuter, 2015). Physicality is gaining in importance in performance-oriented societies, not least because of the boom in health and fitness with its corresponding ideal of beauty (Reuter, 2015), and does not stop when it comes to fulfilling the roles of gender seen as typically female or male. Breast cancer can produce changes in body image, as some men feel emaciated due to the loss of physical strength (maleness) by cancer therapy.
In terms of indirect stigmatization, MBCP mentioned receiving questioning glances from other female patients and feeling observed within this women-dominated area of breast cancer care. This feeling may arise because breast cancer is connected with femaleness in society and being a man in this breast cancer care surrounding does not correspond to the social norms. As Hohmeier (1975) describes, stigmatization takes place to protect the social norm as well as the own identity—within this context—of female breast cancer patients.
Therefore, MBCP feel self-stigmatization, sensing they are intruding into a women’s area, and women or female breast cancer patients want to protect their identity as breast cancer patients and hence consider MBCP as intruders.
Some MBCP sensed they were receiving different treatment than female breast cancer patients. Prior studies arrived at similar outcomes, as MBCP felt ignored by physicians (Pituskin et al., 2007) or experienced isolation in treatment because they had to use separate entrances and waiting rooms in some clinics (Donovan & Flynn, 2007).
To reduce stigmatization in the future, the participants wish for awareness and equality of cancer care, so that breast cancer is not seen as an only woman’s disease anymore. In concurrence with the literature, increased awareness is needed to make the disease more public in general and in the cancer care system, as ignorance of MBC is a major issue within those areas (Iredale et al., 2006; Pituskin et al., 2007; White et al., 2011).
For equality in breast cancer care, the results revealed that the needs of MBCP should be considered in cancer care, in the form of gender-neutral documents and information materials. Similarly, other studies advocate the creation of breast cancer information material (France et al., 2000; Pituskin et al., 2007) or separate sections (Williams et al., 2003) within the information specifically for men.
The three stigma groups identified in the research help to explain stigmatization of MBCP as they reveal the effect of age and extent of cancer treatment: With increasing age, the experienced stigmatization seems to decrease, as demonstrated by the contrary groups, the “not stigmatized” and the “stigmatized.” Within the “not stigmatized” cohort, the comparatively older age of the participants seems to protect from stigmatization because of some aspects that are connected with older age. First, all of the men are retired, so they are not in the work context anymore and hence do not experience exclusion. Second, at a relatively older age one may have more experiences with cancer, as people in the social surroundings may have cancer and so it may seem more normal. Additionally, two of the men experienced cancer themselves, so they may have become hardened by that experience. A third explanation may be the perception of masculinity changes with age. Therefore, the men within this group might not have experienced any sexual stigmatization. Maybe the gender factor is not that important anymore for them compared to men of a younger age. Also, one of the men within this group is already suffering from another disease, which is dominating his life, so he did not attach too much importance to breast cancer. In contrast, the men within the “stigmatized” cohort have the youngest age on average and some of them are still working, so they also have a higher potential to feel excluded compared to the “not stigmatized” participants, who are retired.
Concerning the effect of extent of cancer treatment, the participants within the group of the “not stigmatized” had not had a long period of treatment and consequently had few points of contact (Bloom, Stewart, Chang, & Banks, 2004) with the cancer care system. In addition, none of them experienced chemotherapy or adjuvant radiation, so they did not lose any hair, and as such, the disease is not visible for others at first sight. It is only when the shirt is removed that the scar is visible. Among the three groups, the “stigmatized” members experienced more cancer treatment, so they have more potential to be stigmatized: first, within the different steps of cancer care and second, from the effects the treatment has on the body like losing hair because of chemotherapy. Therefore, the disease becomes visible for everyone in society and increases the potential for stigma attacks, as already discussed within the category Bodily dimension.
The literature recognizes that sociodemographic aspects like age can play an important role when it comes to stigmatization (Bloom et al., 2004; Moyer & Salovey, 1996). In some studies, associations with educational level and stigmatization were reported (Holman, 2015; Tripathi et al., 2017), but this effect cannot be observed in the current investigation.
Interestingly, some men reported in the interviews that they have not experienced any stigmatization over the course of their breast cancer journey, as Table 3 reveals (category: No subjective stigma). However, aspects of stigmatization could be identified within the interviews. Maybe this difference in subjective perception and narration is because those participants have not purposely acknowledged their experiences or feelings of stigmatization, and hence, these only appear within narrations. Besides, it can be regarded as a kind of protective mechanism to consider oneself as not stigmatized.
It can be recognized that other cancer patients are also stigmatized—not only MBCP—and there might exist worse stigmatizations than the men within this study experienced. As already stated, stigmatization is very individual and subjective. If someone conceives something as stigmatizing, it can sometimes also be connected with the individual’s perception. However, this study did not set out to compare the level of stigmatization of several groups. Instead, it aims to call attention to the problem of stigmatization of MBCP to raise the awareness of this issue among society and caretakers. The study also wanted to point out, from a patients’ perspective, how their experienced stigmatization can be reduced. It can be appreciated that stigmatization of MBCP often happens by mistake and in good faith, which highlights the importance of raising public awareness of the disease.
Strengths and Limitations
The strengths of this study are the diverse sample (due to the purposeful sampling), the high response rate of the MBCP, and the mixed methods design. Accordingly, the results could be described from a broad perspective and for a large sample of MBCP in Germany.
As limitations, it can be stated that this study is restricted to the German cancer care system because the health-care systems vary dramatically; therefore, the results cannot be transferred unrestrictedly to other countries with different cancer care systems. Furthermore, the data of disease-related characteristics and breast cancer treatment were gathered by the participants, introducing the possibility of mistakes if the participant’s knowledge was not accurate.
(Practical) Implications
As MBCP experience most stigmatization in the cancer care system, there is a need to devise strategies to manage this, including a need for creating awareness and providing equality of cancer care so that breast cancer is not seen only as a woman’s disease anymore. As MBCP within this study stated, specific needs of MBCP should be considered in cancer care.
Overall, there is a need for publicity and increased attention about MBCP to prevent stigmatization. As mentioned by (Ernst, Mehnert, Taubenheim, et al., 2017), cancer-specific approaches must be taken against stigmatization to avoid psychological and psychosocial problems, because every group of cancer patients has different needs (Ernst, Mehnert, Dietz, Hornemann, & Esser, 2017). For MBCP, one idea is to place a blue stripe on the pink-colored breast cancer ribbon as a symbol for MBC. Moreover, the restricted connection of breast cancer with femaleness must be dismissed, which demands a gender-neutral affiliation of breast cancer—in society as well as in breast cancer care.
Body image problems of MBCP like the scar on the breast or the loss of hair should also be taken more seriously by the health-care professionals, as these can be traumatic for all cancer patients. For instance, periwigs for male patients should be offered by physicians. Implications for further research are the multivariate testing of the stated hypotheses, notably, the correlation of stigmatization, age, and intensity of cancer care.
Acknowledgments
We thank all male breast cancer patients who participated, for sharing their experiences. Thanks to breast cancer centers, the Netzwerk Männer mit Brustkrebs e.V., and all those involved in supporting this study and recruiting their patients. In addition, we are thankful for the administrative, cognitive, and motivational support of our colleagues Christian Heuser, Hannah Nakata, Sherin Dörner, and Lydia Chorus.
Within this standardized questionnaire, the men were asked for their willingness to conduct an interview. If they agreed, the men were called, during which their desire was confirmed and an appointment made for a personal interview.
The interview guideline included questions about how the participants experienced the cancer care during their course of disease, which persons were involved in what way, how the participants experienced their disease, and how they dealt with their breast cancer disease. The narrative-generating introducing question was “If you think back to the time before you were diagnosed with breast cancer. What was it like when you noticed signs of physical change/possible illness in your body for the first time?”
Because we did not know if the participants would meet the exclusion criteria before having contacted them personally, we sent the questionnaire to all interested MBCP we had established contact with. Also, the declaration of consent was sent with the questionnaire.
The questionnaire was sent before the interviews were conducted to facilitate a purposeful selection of participants for the qualitative interviews as described earlier.
Coding was inductive from the data and deductive based on the literature, especially from Link and Phelan (2001) and Reuter (2015).
For coding of stigmatization within the interview material, a systematic coding system was developed. Every aspect within the transcripts that described stigmatization, according to the definition stated within this article, was coded as stigmatization (among the related subcategories). Further information about the coding process is available from the authors.
For interpreting Table 3: The qualitative and quantitative results of stigmatization and as well demographic aspects of participants are merged within this table per participant. The heading Context of stigmatization comprises the results of the quantitative analysis of experiences of stigmatization during the course of cancer treatment and in private surroundings described in Table 2. The values 1–5 demonstrate the intensity of stigmatization (1) never, (2) rarely, (3) sometimes, (4) often, and (5) always. The aspect Dimensions of stigmatization shows the results of the qualitative analysis from the interview per participant, which are the five main categories of stigmatization that could be found within the interviews. The category No subjective stigma means that the participant stated within the interview that he experienced no stigmatization concerning his breast cancer disease.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CK is an employee of the German Cancer Society.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The N-MALE project was funded by German Cancer Aid (Grant Number: 111742).
References
- Bloom J. R., Stewart S. L., Chang S., Banks P. J. (2004). Then and now: Quality of life of young breast cancer survivors. Psycho-Oncology, 13(3), 147–160. doi: 10.1002/pon.794 [DOI] [PubMed] [Google Scholar]
- Brain K., Williams B., Iredale R., France L., Gray J. (2006). Psychological distress in men with breast cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 24(1), 95–101. doi: 10.1200/JCO.2006.10.064 [DOI] [PubMed] [Google Scholar]
- Bunkley D. T., Robinson J. D., Bennett J. N. E., Gordon S. (2000). Breast cancer in men: Emasculation by association? Journal of Clinical Psychology in Medical Settings, 7(2), 91–97. doi: 10.1023/A:1009553613564 [DOI] [Google Scholar]
- Carell A. (2005). Triangulation durch empirisch begründete Typenbildung am Beispiel der Evaluation eines virtuellen Hochschulseminars. In Kruse E., Küchler U., Kuhl M. (Eds.), Unbegrenztes Lernen – Lernen über Grenzen? Generierung und Verteilung von Wissen in der Hochschulentwichlung. Reihe Hochschule und Innovation (pp. 119–130). Berlin: Lit-Verlag. [Google Scholar]
- Contractor K. B., Kaur K., Rodrigues G. S., Kulkarni D. M., Singhal H. (2008). Male breast cancer: Is the scenario changing. World Journal of Surgical Oncology, 6, 58. doi: 10.1186/1477-7819-6-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva T. L. (2016). Male breast cancer: Medical and psychological management in comparison to female breast cancer. A review. Cancer Treatment Communications, 7, 23–34. doi: 10.1016/j.ctrc.2016.03.004 [DOI] [Google Scholar]
- Denzin N. K., Lincoln Y. S. (2003). Handbook of qualitative research (2nd ed.). Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Dillman D. A. (1978). Mail and telephone surveys: The total design method. A Wiley-Interscience publication. New York, NY: Wiley; Retrieved from http://www.loc.gov/catdir/enhancements/fy0607/78000581-b.html [Google Scholar]
- Dillman D. A., Smyth J. D., Christian L. M. (2014). Internet, phone, mail, and mixed-mode surveys: The tailored design method (4th ed.). s.l.: Wiley; Retrieved from http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=827492 [Google Scholar]
- Donovan T., Flynn M. (2007). What makes a man a man? The lived experience of male breast cancer. Cancer Nursing, 30(6), 464–470. doi: 10.1097/01.NCC.0000300173.18584.37 [DOI] [PubMed] [Google Scholar]
- Ernst J. (2016). Stigmatisierung und Krebs. In Mehnert A., Koch U. (Eds.), Handbuch Psychoonkologie (pp. 689–700). Göttingen: Hogrefe. [Google Scholar]
- Ernst J., Mehnert A., Dietz A., Hornemann B., Esser P. (2017). Perceived stigmatization and its impact on quality of life - results from a large register-based study including breast, colon, prostate and lung cancer patients. BMC Cancer, 17(1), 741. doi: 10.1186/s12885-017-3742-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J., Mehnert A., Taubenheim S., Rentsch A., Hornemann B., Esser P. (2017). Stigmatisierung von erwerbstätigen Patienten mit Brust-, Darm-, Prostata- und Lungenkrebs [Stigmatization in Employed Patients with Breast, Intestinal, Prostate and Lung Cancer]. Psychotherapie, Psychosomatik, medizinische Psychologie, 67(7), 304–311. doi: 10.1055/s-0043-110138 [DOI] [PubMed] [Google Scholar]
- Esser P., Mehnert A., Johansen C., Hornemann B., Dietz A., Ernst J. (2017). Body image mediates the effect of cancer-related stigmatization on depression: A new target for intervention. Psycho-Oncology (Advance online publication). doi: 10.1002/pon.4494 [DOI] [PubMed]
- Fife B. L., Wright E. R. (2000). The dimensionality of stigma: A comparison of its impact on the self of persons with HIV/AIDS and cancer. Journal of Health and Social Behavior, 41(1), 50. doi: 10.2307/2676360 [DOI] [PubMed] [Google Scholar]
- Flick U. W. (1992). Triangulation revisited: Strategy of validation or alternative? Journal for the Theory of Social Behaviour, 22(2), 175–197. doi: 10.1111/j.1468-5914.1992.tb00215.x [DOI] [Google Scholar]
- France L., Michie S., Barrett-Lee P., Brain K., Harper P., Gray J. (2000). Male cancer: A qualitative study of male breast cancer. Breast (Edinburgh, Scotland), 9(6), 343–348. doi: 10.1054/brst.2000.0173 [DOI] [PubMed] [Google Scholar]
- Fuß S., Karbach U. (2014). Grundlagen der Transkription: Eine praktische Einführung. utb-studi-e-book: Vol. 4185. Opladen, Stuttgart: Budrich; UTB; Retrieved from http://www.utb-studi-e-book.de/9783838541853 [Google Scholar]
- Giordano S. H. (2018). Breast cancer in men. The New England Journal of Medicine, 378(24), 2311–2320. doi: 10.1056/NEJMra1707939 [DOI] [PubMed] [Google Scholar]
- Glaser B. G., Strauss A. L., Paul A. T. (2008). Grounded theory: Strategien qualitativer Forschung: Gesundheitswissenschaften Methoden (1. Nachdr. der 2., korrigierten Aufl.). Bern: Huber. [Google Scholar]
- Goffman E. (1963). Stigma: Notes on the management of spoiled identity. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- Helfferich C. (2011). Die Qualität qualitativer Daten: Manual für die Durchführung qualitativer Interviews (4. Auflage). Wiesbaden: VS Verlag für Sozialwissenschaften / Springer Fachmedien Wiesbaden GmbH Wiesbaden. doi: 10.1007/978-3-531-92076-4 [DOI] [Google Scholar]
- Hodgson N. C. F., Button J. H., Franceschi D., Moffat F. L., Livingstone A. S. (2004). Male breast cancer: Is the incidence increasing? Annals of Surgical Oncology, 11(8), 751–755. doi: 10.1245/ASO.2004.01.001 [DOI] [PubMed] [Google Scholar]
- Hohmeier J. (1975). Stigmatisierung als sozialer Definitions-prozess. In Brusten M., Hohmeier J. (Eds.), Stigmat-isierung 1: Zur Produktion gesellschaftlicher Randgruppen (pp. 5–24). Darmstadt: Hermann Luchterhand Verlag. [Google Scholar]
- Holman D. (2015). Exploring the relationship between social class, mental illness stigma and mental health literacy using British national survey data. Health (London, England: 1997), 19(4), 413–429. doi: 10.1177/1363459314554316 [DOI] [PubMed] [Google Scholar]
- Holmberg C. (2005). Diagnose Brustkrebs: Eine ethnografische Studie über Krankheit und Krankheitserleben. Zugl: Berlin, Humboldt-Univ; (Diss., 2002. Kultur der Medizin: Vol. 13 Frankfurt: Campus-Verl.) [Google Scholar]
- Iredale R., Brain K., Williams B., France E., Gray J. (2006). The experiences of men with breast cancer in the United Kingdom. European Journal of Cancer (Oxford, England: 1990), 42(3), 334–341. doi: 10.1016/j.ejca.2005.09.027 [DOI] [PubMed] [Google Scholar]
- Kipling M., Ralph J. E. M., Callanan K. (2014). Psychological impact of male breast disorders: Literature review and survey results. Breast Care (Basel, Switzerland), 9(1), 29–33. doi: 10.1159/000358751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel S., Devins G. M. (2008). Stigma in cancer patients whose behavior may have contributed to their disease. Future Oncology (London, England), 4(5), 717–733. doi: 10.2217/14796694.4.5.717 [DOI] [PubMed] [Google Scholar]
- Link B. G., Phelan J. C. (2001). Conceptualizing stigma. Annual Review of Sociology, 27(1), 363–385. doi: 10.1146/annurev.soc.27.1.363 [DOI] [Google Scholar]
- Link B. G., Phelan J. C. (2006). Stigma and its public health implications. The Lancet, 367(9509), 528–529. doi: 10.1016/S0140-6736(06)68184-1 [DOI] [PubMed] [Google Scholar]
- Ly D., Forman D., Ferlay J., Brinton L. A., Cook M. B. (2013). An international comparison of male and female breast cancer incidence rates. International Journal of Cancer, 132(8), 1918–1926. doi: 10.1002/ijc.27841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayring P. (2016). Einführung in die qualitative Sozialforschung: Beltz Studium (6., neu ausgestattete Aufl.). Weinheim: Beltz, J. [Google Scholar]
- Meacham E., Orem J., Nakigudde G., Zujewski J. A., Rao D. (2016). Exploring stigma as a barrier to cancer service engagement with breast cancer survivors in Kampala, Uganda. Psycho-Oncology, 25(10), 1206–1211. doi: 10.1002/pon.4215 [DOI] [PubMed] [Google Scholar]
- Miao H., Verkooijen H. M., Chia K.-S., Bouchardy C., Pukkala E., Larønningen S., … Hartman M. (2011). Incidence and outcome of male breast cancer: An international population-based study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 29(33), 4381–4386. doi: 10.1200/JCO.2011.36.8902 [DOI] [PubMed] [Google Scholar]
- Moyer A., Salovey P. (1996). Psychosocial sequelae of breast cancer and its treatment. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine, 18(2), 110–125. doi: 10.1007/BF02909583 [DOI] [PubMed] [Google Scholar]
- Nyblade L., Stockton M., Travasso S., Krishnan S. (2017). A qualitative exploration of cervical and breast cancer stigma in Karnataka, India. BMC Women’s Health, 17(1), 58. doi: 10.1186/s12905-017-0407-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T., Turner B. S. (2005). The social system: Routledge sociology classics (2nd ed., transferred to digital printing). Abingdon: Routledge. [Google Scholar]
- Pituskin E., Williams B., Au H.-J., Martin-McDonald K. (2007). Experiences of men with breast cancer: A qualitative study. The Journal of Men’s Health & Gender, 4(1), 44–51. doi: 10.1016/j.jmhg.2006.12.002 [DOI] [Google Scholar]
- Reuter T. (2015). Darüber spricht man(n) nicht? über Stigma-Erleben und Stigma-Management an Darmkrebs erkrankter Männer (Dissertation). Helmut-Schmidt-Universität/Universität der Bundeswehr Hamburg, Hamburg. [Google Scholar]
- Robinson J. D., Metoyer K. P., Bhayani N. (2008). Breast cancer in men: A need for psychological intervention. Journal of Clinical Psychology in Medical Settings, 15(2), 134–139. doi: 10.1007/s10880-008-9106-y [DOI] [PubMed] [Google Scholar]
- Smolin Y., Massie M. J. (2002). Male breast cancer: A review of the literature and a case report. Psychosomatics, 43(4), 326–330. doi: 10.1176/appi.psy.43.4.326 [DOI] [PubMed] [Google Scholar]
- Swergold N., Murthy V., Chamberlain R. S. (2014). Males at high risk for breast cancer: Who are they and how should we screen them? Surgical Science, 5(7), 320–331. doi: 10.4236/ss.2014.57054 [DOI] [Google Scholar]
- Tang P. L., Mayer D. K., Chou F. H., Hsiao K. Y. (2016). The experience of cancer stigma in Taiwan: A qualitative study of female cancer patients. Archives of Psychiatric Nursing, 30(2), 204–209. [DOI] [PubMed] [Google Scholar]
- Tripathi L., Datta S. S., Agrawal S. K., Chatterjee S., Ahmed R. (2017). Stigma perceived by women following surgery for breast cancer. Indian Journal of Medical and Paediatric Oncology: Official Journal of Indian Society of Medical & Paediatric Oncology, 38(2), 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusson D., Pilnick A. (2017). Between stigma and pink positivity: Women’s perceptions of social interactions during and after breast cancer treatment. Sociology of Health & Illness, 39(3), 458–473. doi: 10.1111/1467-9566.12486 [DOI] [PubMed] [Google Scholar]
- White J., Kearins O., Dodwell D., Horgan K., Hanby A. M., Speirs V. (2011). Male breast carcinoma: Increased awareness needed. Breast Cancer Research: BCR, 13(5), 219. doi: 10.1186/bcr2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. G., Iredale R., Brain K., France E., Barrett-Lee P., Gray J. (2003). Experiences of men with breast cancer: An exploratory focus group study. British Journal of Cancer, 89(10), 1834–1836. doi: 10.1038/sj.bjc.6601305 [DOI] [PMC free article] [PubMed] [Google Scholar]