Abstract
Although research has reported that prostate cancer (PCa) incidence and mortality rates are among the highest for African Americans, the data is inconclusive regarding PCa rates in native African men, Black men residing in other countries, and men in Asia, Europe, and the Americas. Data reveals that prostate-specific antigen (PSA) testing and disease incidence have risen significantly in developing and Asian countries, and PCa has become one of the leading male cancers in many of those nations.
The objective of this study was to review published peer-reviewed studies that address PCa in different regions of the world to get a better understanding of how PCa incidence, prevalence, detection, and mortality are influenced by race, ethnicity, and geography. A secondary goal was to compare PCa data from various world regions to contextualize how disproportionate the incidence and mortality rates are among men from the African diaspora versus men of European, Hispanic, and Asian descent, as well as to highlight the need for more robust screening and treatment guidelines in developing countries.
There are differences in incidence and mortality rates between men of African, Asian, Hispanic, and European ancestry, confirming the involvement of genetic factors. However, differences between men of the same race and ethnicity who live in different countries suggest that environmental factors may also be implicated. Availability and access to diagnostic and health-care services as well as recommendations regarding PCa testing vary from country to country and contribute to the variability in incidence and mortality rates.
Keywords: risk factors, behavioral issues, genetics, development and aging, PSA testing, general health and wellness, cultural disparity, psychosocial and cultural issues, men of color, special populations
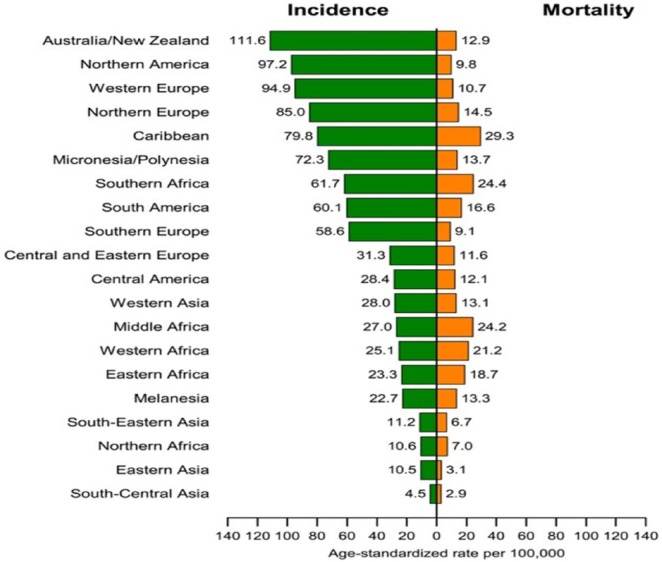
A systematic review of PCa incidence and mortality data based on geography, race, and ethnicity has yielded inconsistent and, in some cases, unreliable information. According to Torre et al. (2015) and as illustrated in Figure 1, Oceania, followed by Northern America, Western Europe, Northern Europe, and the Caribbean have among the highest PCa incidence rates in the world. Conversely, most African countries have incidence and mortality rates that are far below those reported in developed regions. It is estimated that in Europe and the United States, as many as 23%–42% of PCa cases may be due to overdiagnosis because of increased PSA testing (Quinn & Babb, 2002). Quinn and Babb theorized that much of the international variations in PCa incidence may be attributed to PSA testing.
Figure 1.
Prostate cancer incidence and mortality rates by geographical area. From “Global Cancer Statistics, 2012,” by L. A. Torre, F. Bray, R. Siegel, J. Ferlay, J. Lortet-Tievlent, and A. J. Jemal, 2015, CA: A Cancer Journal for Clinicians, 65, p. 87. Copyright 2015 by the American Cancer Society. Reprinted with permission.
PCa mortality rates have been declining in most Western countries as well as in some European countries such as Finland, Sweden, Portugal, Israel, Italy, the Netherlands, Norway, and France (Baade, Youlden, & Krnjacki, 2009). Although the reasons are not clear, Jemal, Center, DeSantis, and Ward (2010) postulated that it may be due to early detection and improved treatment. Hsing and Devesa (2001) theorized that variations in incidence and mortality rates reported for many countries may possibly be due to underdiagnosis, underreporting, differences in screening practices, differences in health-care access, gaps in knowledge and awareness, and attitudes toward PCa and associated screening. Conversely, Center, Jemal, Lortet-Tieulent, Ward, and Ferlay (2012) contended that PCa mortality rates and trends may be less affected by diagnostic practices but more so by differences in treatment worldwide and underlying risk. In developing countries, other factors such as a shorter life expectancy and possibly, a lower prevalence of risk factors may help explain some of the differences in documented statistics for men of African descent.
PCa is the fifth leading cause of death from cancer in men, with an estimated 307,000 deaths representing 6.6% of total male cancer mortality (Ferlay et al., 2015). An estimated 1.1 million men worldwide were diagnosed with PCa in 2012, accounting for 15% of the cancers diagnosed in men, with almost 70% of them (759,000) occurring in more developed regions. According to Jemal et al. (2010), the highest incidence rates have been reported in North America, Oceania, and Northern and Western Europe. Although the lowest rates are identified in Asia and North Africa, incidence and mortality rates are trending upward in countries such as Poland and some Asian countries such as Japan and Singapore where PSA testing is not commonly used (Baade et al., 2009).
In this document, I explore the differences in PCa detection methods, incidence, and mortality rates between races and ethnicities in various regions of the world. It is difficult to make such comparisons because of the difference in detection pathways and data collection, but it seems clear that men of African descent outside of the African continent are at a higher risk of developing PCa. The data is less definitive for Black men living in Africa.
This review is subdivided according to geographic region. Within each region, detection and known incidence and mortality rates are discussed. Generally, incidence and mortality data are presented under separate headings, but for several European countries, the Americas, and Oceania where less data is available, incidence and mortality trends are presented within the same section.
Evidence Acquisition
This review is a critical appraisal and summary of existing published literature, is limited to the English language, and includes observational and randomized studies. A systematic review of the electronic databases included the following: the National Center for Biotechnology Information; the National Library of Medicine; the Food and Drug Administration; CINAHL Plus; Medline; International Agency for Research on Cancer (IARC) as compiled in GLOBOCAN 2008 and 2012; Nursing and Allied Health; Google Scholar; the World Cancer Research Fund International Report—Continuous Update Project; the American Cancer Society—Annual Report on the Status of Cancer, Part II: Recent Changes in Prostate Cancer Trends and disease (Negoita et al., 2018); the Walden Library EBSCO database; and PubMed from 1990 to 2018.
Selected studies needed to provide comprehensive descriptions of the demographic and PCa disease characteristics using either quantitative or qualitative analyses. Incidence, detection, and mortality data based on geographic location, race, and ethnicity were gleaned from 147 full-text articles and 7 textbooks. Ninety-three articles and four textbooks were eligible after excluding those that were not relevant to the specific subject matter and published prior to 1990. Incidence and mortality data for specific countries and regions was limited to availability and accuracy of that information. The intent was to exclude any articles or studies published prior to 2005, but in several cases, more recent data was unavailable.
The literature review for this article included peer-reviewed articles and books that were secured using search terms such as prostate cancer; African-American; Afro-Caribbean; ethnic; genetic susceptibility; disparities; developing countries; developed countries; PSA; DRE; survival; morbidity; mortality; prostatic diseases; epidemiology; risk factors; etiology; diet; Africa; Australia; New Zealand; United Kingdom; Caucasian; European; Oceania; Northern Europe; Western Europe; Asian, screening; global; trends; patterns; immigration; ethnicities; environmental; racial quantitative; Native American; detection; diagnostic; preventative; beliefs; customs; heritage assessment; cultural competence; cultural diversity; cancer statistics; benign prostatic hyperplasia; and qualitative.
Those search terms used alone or in combination were valuable in identifying published peer-reviewed studies on PCa and men living in the United States, the Caribbean, Asia, Africa, Australia/New Zealand, the Americas, the United Kingdom, and other European countries. Those studies were useful by focusing on trends, incidence, mortality, morbidity, and global patterns of PCa in different ethnicities worldwide. Many of the most useful resources chosen were identified from the reference sections and citations provided in other peer-reviewed articles. In addition, available statistical and trend data on PCa incidence and death rates of all men in the United States, Europe, the Americas, the Caribbean, Oceania, Asia, Africa, and other ethnicities worldwide, national government data on PCa, and associated national and state statistics were secured from the following sources: http://www.gpo.gov/fdsys; http://catalog.gpo.gov; http://www.UnitedStates.gov; World Cancer Research Fund—Continuous Update Project; National Cancer Institute (NCI)—Quick cancer profiles (2013); SEER Cancer Statistics Review, 1975–2015; the National Cancer Intelligence Network—Cancer Incidence and Survival by Major Ethnic Group, England, 2002–2006; World Health Organization—World Health Statistics 2011; and Cancer Research U.K., 2008.
Implications of Available Evidence
PCa screening can detect early disease and it offers the potential to decrease morbidity and mortality. Despite potential and expected better outcomes from early detection, benefits from PCa screening remained unproven prior to 2018. Recent data from the U.S. Preventive Services Task Force (USPSTF) report documented that PSA screening offers a potential benefit of reducing the chance of death from PCa in some men aged 55–69 years. The Task Force now recommends that men should discuss the benefits and harms of screening with their doctor, so they can make the best choice for themselves based on their individual circumstances.
Figure 1 illustrates incidence and mortality rates by geographical area. The comparison of mortality to incidence ratio (MR/IR) is quite striking between developed and less developed countries. For example, the Caribbean has an MR/IR of 37%, while in Middle Africa, the ratio is as high as 90%. Conversely, the MR/IR ratio is 12% for Australia and New Zealand and 10% for Northern America. Even in regions where incidence rates are documented to be quite low such as South Central and Southeast Asia, the MR/IR ratio is as high as 64%.
These data seem to indicate that the detection of PCa and its relation to mortality does not show consistency and validate the continuing and highly relevant discussion around the relative value of PSA testing for detection. The MR/IR calculations have been very confusing and inconsistent from country to country and within regions. For example, in developed countries such as the United States and New Zealand, the mortality trend has been declining or stable. During the same period, PSA testing has declined based on the 2012 USPSTF recommendations. In contrast, many developing countries have seen a trend of increasing mortality rates, while their incidence rates have increased due to increased testing. If the correlation between MR and IR is a reliable measure of the most devastating result of PCa (i.e., death), then one should see a proportionate change. For example, if incidence increases, there should be a corresponding or predictable change (increase or decrease) in mortality. That is not necessarily the case and it supports the continuing discussion relating to the true relative value of PSA screening in men over 69 years, given the potentially devastating side effects of biopsies and various types of prostatectomies as a consequence of such screenings. For men over 69 years, the USPSTF maintained its 2012 conclusion with moderate certainty that the potential benefits of PSA-based screening for PCa do not outweigh the expected harms (Negoita et al., 2018).
PCa in the United States
Many men with PCa never have symptoms and unless they undergo screening or experience signs associated with the later stages, they may not know they have the disease. In the United States, the lifetime risk of being diagnosed with PCa is approximately 11%, and the lifetime risk of dying from PCa is 2.5% (Negoita et al., 2018).
African Americans and Non-Hispanic Whites
Incidence
Jemal et al. (2010) and several other researchers noted that African American men have among the highest incidence of PCa worldwide, are more likely to develop PCa at any age, and develop the disease earlier in life than men from all other racial and ethnic groups. Parkin et al. (as cited by Kheirandish & Chinegwundoh, 2011) noted that it is difficult to compare incidence rates among men of different races or compare rates between various countries because of differences in detection pathways and data collection.
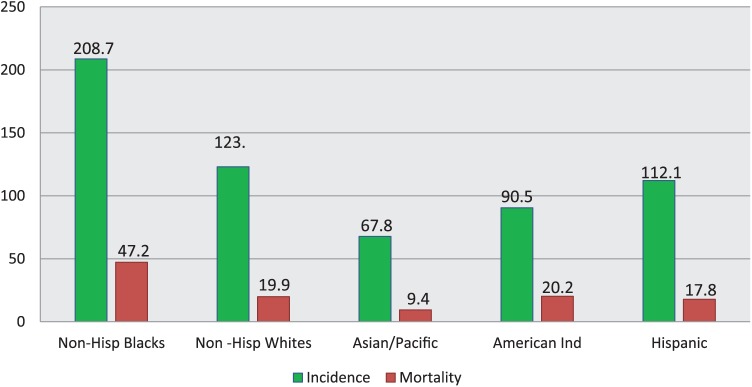
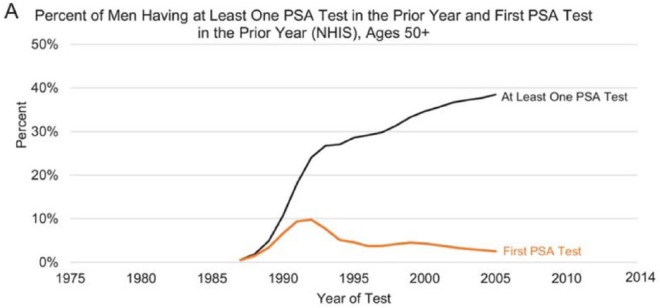
The overall incidence of PCa increased in the United States between 1988 and 1992, but starting in 2007, it decreased at a rate of 6.5% per year for all races combined (6.8% per year for Black men and 5.9% per year for White men; Negoita et al., 2018). The increase and decline in PCa incidence mimicked the dramatic increase and decrease in screening during the same years. That trend appears to be consistent with data from other studies. Figure 3 illustrates race-specific age-adjusted incidence rates of PCa in the U.S. population. Since the peak in 1992, PCa incidence has been decreasing in the United States with an accelerated rate of decrease since 2012. Several previous studies have suggested that the decline in incidence has been associated with recommendations by the 2012 USPSTF against routine PSA testing (Jemal et al., 2015). Figure 2 highlights the disparity between men over 50 years who received at least one PSA test and the same category of men who received their first PSA test between 1985 and 2005.
Figure 3.
Prostate cancer incidence and death rates in America by race and ethnicity, 2008–2012. Rates are per 100,000 population and age adjusted to the 2000 U.S. standard population. Non-White and non-Black race categories are not mutually exclusive of Hispanic origin. Adapted from “Cancer Statistics, 2016,” R. L. Siegel, K. D. Miller, and A. Jemal, 2016, CA: A Cancer Journal for Clinicians, 66, p. 7. Copyright 2015 by the American Cancer Society.
Figure 2.
Trends in the percent of U.S. men >50 years who received ⩾one PSA test in prior year and the first PSA test in the prior year. PSA = prostate-specific antigen. Adapted from “Annual Report to the Nation on the Status of Cancer, Part II: Recent Changes in Prostate Cancer Trends and Disease Characteristics,” by S. Negoita, E. J. Feuer, A. Mariotto, K. A. Cronin, V. I. Petkov, S. K. Hussey, … R. L. Sherman, 2018, Cancer, 124, p. 2801. Copyright 2018 by the American Cancer Society.
When PSA testing was introduced in the late 1980s, there was a rapid decrease in the incidence of distant-stage PCa. PSA testing use declined after 2008 for all races in the United States. Associated with the decline in PSA use, there was a reported increase in the incidence of distant-stage disease from 2008 to 2014. Negoita et al. (2018) postulated that when there is an increase in screening, several distant cases may be caught in the earlier stages, but with the lowered use of screening, such cases may be missed.
In its 2018 PCa screening recommendations, the USPSTF reported that there is adequate evidence from randomized clinical trials documenting that PSA-based screening in men aged 55–69 years might prevent approximately 1.3 deaths from PCa over approximately 13 years per 1,000 men screened. The evidence illustrated that screening programs might also prevent approximately 3 cases of metastatic PCa per 1,000 men screened. The USPSTF therefore revised its 2012 PSA screening rating and concluded that although the net benefit of PSA-based screening in men aged 55–69 years is small, screening offers a potential benefit of reducing the chance of death from PCa in some men. Consequently, for men aged 55–69 years, the decision to undergo periodic PSA screening should be an individual one in consultation with their clinician (U.S. Preventive Services Task Force, 2018). It seems likely that the latest revision can result in another change in PSA testing recommendations based on the findings regarding the increase in the incidence of distant-stage disease.
Mortality
PCa mortality increased slowly before 1987, but the trend moved more rapidly upward afterward for all races in the United States. For White men, the annual percent change (APC) was 3.1 and 3.2 for Black men. Figure 3 reveals race-specific age-adjusted mortality rates of PCa. Similar to the incidence data, mortality trends illustrate African American men having the highest rates, followed by non-Hispanic White (NHW) men (Siegel, Miller, and Jemal, 2016). The lifetime risk of dying from PCa is significantly higher for African American men at 4.2% versus 2.9% for Hispanic men, 2.3% for White men, and 2.1% for Asian and Pacific Islander men (Negoita et al., 2018). Those disproportionate incidence and mortality rates in African American men are likely rooted in poor knowledge, awareness, and medical mistrust, which may affect access and data collection downstream.
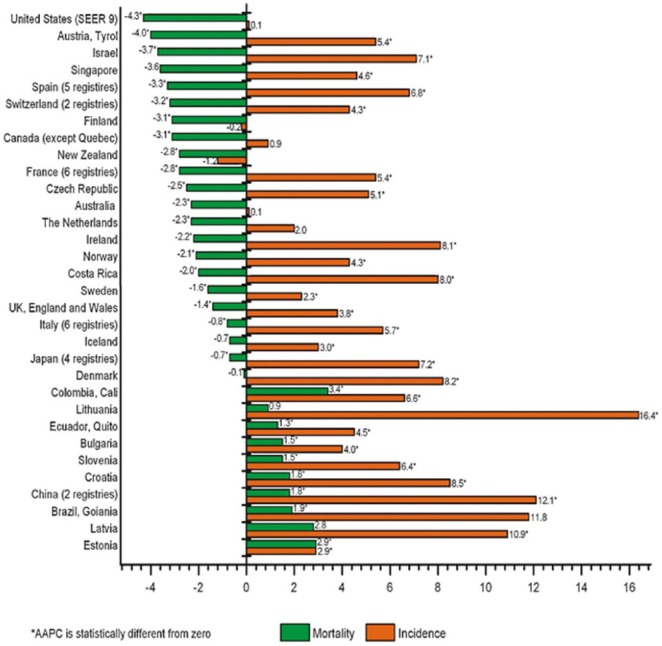
The United States experienced an overall decrease in PCa mortality of 4.3% per year (Figure 4) prior to 2012 as documented by Center et al. (2012). Negoita et al. (2018) provided more current data showing that overall PCa mortality stabilized from 2013 to 2015. The researchers noted that before 1987, PCa mortality increased slowly, but the trend increased at a steeper rate after 1987 in the United States. The highest mortality was observed in 1993 for all races at 39.3 per 100,000. Mortality per 100,000 for Black men peaked at 81.9 in 1993 and 2 years earlier for White men at 36.5 per 100,000.
Figure 4.
AAPC in incidence and mortality rates for the past 10 years of available SEER data. AAPC = average annual percent change; SEER = Surveillance Epidemiology and End Results.
Reprinted from Center et al., 2012.
After the peak, the mortality decline in Black men was greater than that in White men, with the greatest rate of decline between 2001 and 2015 (Negoita et al., 2018). Subsequent to the mortality decline between 1994 and 1999, it slowed for White men and leveled off after 2013. The rate of decline among Black men continued to increase between 2001 and 2015 (Negoita et al., 2018). Negoita et al. documented that the leveled mortality trend was temporally associated with the fall in PSA testing and the rise of distant-stage PCa, but there may have been other contributing factors. For example, the timing and duration of the leveling may have been affected by improved treatments, earlier detection, and improved treatment of metastatic and castration-resistant PCa (Negoita et al., 2018).
Awareness and early detection in African Americans and NHWs
The primary goal of screening for PCa is to detect the disease early with the expectation that it can be managed with better outcomes before it reaches the later metastatic stages. Lim and Sherin (2008) noted that there is currently no convincing evidence that early screening, detection, and treatment improves mortality. Recent data from the USPSTF reported evidence that PSA screening offers a potential benefit of reducing the chance of death from PCa in some men aged 55–69 years (US Preventive Services Task Force, 2018). The data is less convincing for men over 70 years for all races, and USPSTF maintained that the benefits do not outweigh the risks (Negoita et al., 2018).
In a study by Oliver (2007), he concluded that African American men were significantly less likely than Caucasian men to correctly identify early symptoms of PCa and the basic components of a prostate checkup. African American men were also more likely to believe that “pain” was the first symptom of PCa and were less likely to undergo screening and other early diagnostic procedures such as PSA testing and digital rectal examinations (DRE) compared to Caucasian men (Oliver, 2007).
Given the Lim and Sherin (2008) findings and prior to the revised 2018 USPSTF report, several researchers have questioned whether the high mortality in African American men can actually be reduced by increasing awareness, screening, and treatment. There are several limitations to PCa screening, including potential adverse health effects associated with false positives, overdiagnosis, and possible side effects related to biopsies and treatment. Ries et al. (2006) posited that the PCa 5-year survival is nearly 100% when the disease is diagnosed at a local or regional stage but poor (32.6%) when diagnosed with distant metastases. Most men with PCa are diagnosed with local or regional disease (80%), but overall, both stage distribution and survival are poorer in African American men compared with White men (Ries et al., 2006).
Asian Americans, Native Hawaiians, and Pacific Islanders
PCa is the most commonly diagnosed cancer among Asian American (AA), Native Hawaiian, and Pacific Islander (AANHPI) men; A total of 4,550 new cases were expected in 2016, which would have accounted for approximately 18% of all cancers in this group during that period. As illustrated in Figure 3 and Table 1, incidence rates are approximately two times higher in NHWs and three times higher in Blacks than in AANHPIs. Similar to the trend in other U.S. racial groups, PCa incidence rates peaked among AANHPIs in the early 1990s because of the rapid uptake of PSA testing, followed by a steady decline (Potosky, Miller, Albertsen, & Kramer, 1995).
Table 1.
Cancer Incidence and Mortality Rates and Rate Ratios Comparing AANHPIs With NHWs, 2008–2012.
| Incidence | Mortality | ||||
|---|---|---|---|---|---|
| AANHPI | Rate ratio | NHW | AANHPI | Rate ratio | NHW |
| 67.8 | 0.6 | 123 | 9.4 | 0.5 | 19.9 |
Note. AANHPI = Asian American, Native Hawaiian, and Pacific Islander; NHW = non-Hispanic White.
Incidence
The population of AAs in 2014 was 20 million or 6.3% of the total U.S. population and is the fastest growing racial/ethnic group in the United States (Colby & Ortman, 2014). Although AAs, Native Hawaiians, and Pacific Islanders are distinct racial groups with very different cancer profiles, data are usually combined because of small numbers or for continuity with historical statistics. Consequently, this group is referred to as “Asian Americans, Native Hawaiians, and Pacific Islanders” (AANHPIs).
Mortality
PCa is the fifth leading cause of cancer death among AANHPI men. A total of 520 deaths were expected to occur in 2016 (Torre et al., 2015). Similar to incidence trends as seen in Figure 2, mortality rates are approximately two times higher in NHWs and three times higher in Blacks than in AANHPIs. The lifetime risk of dying from PCa for AANHPI men is 2.1% versus 4.2% for African American men; 2.9 for Hispanic men; and 2.3% for White men (Negoita et al., 2018). PCa death rates have been generally declining among AANHPIs since 1993, a trend that is similar to that seen in NHWs. These declines have been attributed to early detection and improvements in treatment, although Schröder et al. (2012) and others debate the relative contribution of each.
Awareness and early detection in AANHPIs
Currently, routine screening for PCa is not recommended for AANHPI men at average risk, but individuals and their physicians may modify how they approach PSA screening in the United States based on the 2018 USPSTF report. There is no nationwide data on the use of shared decision making for PSA testing among AANHPIs, although it is likely suboptimal given the low use of informed decision making (Han et al., 2013). Overall, 26% of AA men over 50 years underwent PSA testing in 2014 compared with 37% of NHWs (Fedewa, Sauer, Siegel, & Jemal, 2015).
PCa in Africa
Incidence
As evidenced by a wealth of data and discussed earlier in this document, the incidence of PCa in African American men is among the highest in the world. Based on estimates from the IARC (IARC, 2010), PCa is the leading cancer in terms of incidence and mortality in men of African descent. That is reflected in data for African American men, Caribbean men, and Black men in Europe. It is unclear if those rates are similarly high for Black men residing in Africa. Although reports suggest that PCa is a rare disease among Black men on the African continent, the disease is one of the most prevalent urological cancers in native African men. For example, PCa is more common than liver cancer, non-Hodgkin lymphoma, and lung cancer in Abidjan, Ivory Coast (Echimane et al., 2000). Approximately 27,400 cases were reported in Africa in 2000 despite low evidence of prevalent PCa screening (Parkin et al., 2003).
Major unexplained differences exist in PCa incidence between countries within the African continent. The uncertainty about the incidence and mortality data disparities in Africa is verified by early researchers such as Kambal (1977). Kambal postulated that prostatic diseases are almost nonexistent in Southern Sudan, while they are common in Northern and Central Sudan. In other examples, the age-standardized PCa incidence rate per 100,000 is 32 in Zimbabwe; 9.7 in Nigeria; 4.4 in Senegal; and 4.3 in Uganda. The incidence rates for South African Whites are similar to those of British men at approximately 32.6 per 100,000. These figures seem to suggest that in some locales, PCa incidence is higher for White South Africans than Blacks in other parts of Africa, but there is no additional data to support that supposition.
Chu at al. (2011) reported that PCa incidence rates among African Americans were as much as 40 times higher than those in Africa. The highest rates were documented in East Africa with incidence rates of 10.7–38.1 per 100,000. Southern African Blacks had a rate of 14.3–21.8 per 100,000, and West African countries had the lowest rate of 4.7–19.8 per 100,000. As an example of the stark differences in recorded incidence between American Blacks and native African men, The Gambia had an incidence rate of 4.7 during 1997–1998 versus an African American rate of between 80 and 195.3 during the same period (Chu et al., 2011).
Mortality
PCa mortality rates are reported to be the lowest in Asia and Northern Africa. This is increasingly becoming a public health concern because the majority of newly diagnosed cases present with advanced carcinomas, which are associated with poor prognoses (Osegbe 1997). While total PCa rates are consistently higher in the United States than in Africa, advanced-stage rates have been much higher in East Africa than those of African Americans in recent years (Chu et al., 2011). Osegbe reported that 64% of new PCa patients in a Nigerian Hospital died within 2 years of diagnosis. That compares to Surveillance Epidemiology and End Results (SEER) data, which documented that in 1975, the number of men in America who survived more than 5 years after diagnosis was 66%, and between 2008 and 2014, that number rose significantly to 98.2% (Noone et al., 2018). It is estimated that approximately 57,048 deaths associated with PCa will occur in Africa by the year 2030. That represents a 104% increase in the number of PCa deaths in Africa over the next 20 years (Ferlay et al., 2010).
The 28,000 deaths of all races in Africa due to PCa as reported by Rebbeck et al. (2013) were more than four times the 6,500 that occurred among Caribbean men of all races and exceeded deaths from any other cancers in Africa. That finding is not surprising given that PCa is more likely to be diagnosed at an advanced stage in developing countries because of the limited amount of screening done to detect the disease in its early stages. That presumption is supported by Chu et al. (2011) who noted that based on unpublished data, 75% of cases in Ghana and 47% of cases in Senegal were diagnosed at an advanced stage. Since the Caribbean Islands as a group are categorized as developing nations, it is difficult to explain such a significant difference in PCa mortality. One can theorize that proximity to the United States and the associated influence of “Westernization” as well as relatively greater access to medical services in the Caribbean region may be a few of the possible reasons.
Proposed reasons for differences in incidence and mortality in Africa
Several scholars such as Chu et al. (2011); Mohammed, Nwana, and Anjorin (2005); and Odedina et al. (2009) speculated that differences in lifestyle, access to medical care, quality of the registries, availability of incidence and mortality data, and minimal screening practices may contribute to the wide range of incidence and mortality rates for Black men living in Africa. In addition, poor availability of biopsy materials, reliance on hospital-based rather than population-based sampling, and lack of histopathologists may also be important contributing factors. Chu et al. posited that only 4% of Ghanaian men had access to medical care in 2004–2006 (unpublished data), compared to almost 80% of African American males who had some sort of health coverage in the same period. PCa is closely associated with increasing age, occurring primarily in men over 60 years in the United States. Consequently, one important factor for the lower incidence and mortality rates may be the fact that in some African countries, native men do not live long enough to develop or manifest signs of the disease.
Awareness and Early Detection in Africa
Based on the review and analysis of published studies and data, PCa incidence in Africa has been documented to be lower than that of African American men. PCa incidence rates in African men have increased between 1987 and 1992 and continue to increase over time (Chu et al., 2011). According to Rebbeck et al. (2013), no data exists on the prevalence of PSA testing in Africa, but it is generally held that early detection testing is not common. Given that PSA testing is relatively rare in several African countries such as Nigeria and The Gambia, PCa incidence rates are projected to increase as early detection, clinical diagnosis protocols, and economies improve. It is likely that improved availability and access to medical care and systems as well as better attainment, reporting, and documentation of cases may contribute to an increasing incidence rate trend (Chu et al., 2011; Rebbeck et al., 2013).
PCa in Europe
United Kingdom
Given the wealth of data that is available for the United Kingdom versus that for other parts of Europe, the United Kingdom is discussed separately.
Incidence
Between 1981 and 2011, PCa incidence rates in the United Kingdom increased almost threefold, but few studies have focused on the risk and incidence of PCa in Black men in the United Kingdom (Kheirandish & Chinegwundoh, 2011). In a study entitled “The Risk of PCa Amongst Black Men in the United Kingdom: The PROCESS Cohort Study,” Ben-Shlomo et al. (2008) compared incidence rates of first-generation Black migrants from Africa and the Caribbean with those of South Asian and European ethnic groups. Afro-Caribbean men in the PROCESS cohort study had an age-adjusted PCa incidence rate of 173 per 100,000 compared to a rate of 56.4 per 100,000 for U.K. White males and 139 for Black African men. In the PROCESS cohort study, it was determined that Afro-Caribbean men residing in the United Kingdom had the highest PCa incidence rate, were three times more likely to be diagnosed with the disease, and were diagnosed approximately 5 years earlier than U.K. Caucasian men despite having equal access to diagnostic services.
The National Cancer Intelligence Network (2006) reported that between 2002 and 2006, the cancer incidence for Black men in the United Kingdom ranged from 120.8 to 247.9 per 100,000 compared to 28.7 to 60.6 per 100,000 for White U.K. men. Chinegwundoh et al. (2006) reported age-adjusted rates in the United Kingdom of 647 per 100,000 for Afro-Caribbean men, 213 for Europeans, and 199 for South Asians. It is unclear why there is such a stark difference in age-adjusted incidence rates between the Chinegwundoh et al. study and other PCa studies of ethnic differences in the United Kingdom, but the threefold relative risk for the Black population in their study was much higher than that reported for African American men (ACS, 2013).
Mortality
There was a 10-fold difference in the uptake of PSA testing in the United Kingdom when compared to the United States in the 1990s, and consequently, detection of PCa was markedly different. A study by Collin et al. reported that although age-adjusted PCa mortality peaked for both countries in the early 1990s at almost identical rates, there was not the similar decline in mortality rates in the United Kingdom that was evident in the United States (Collin et al., 2008). Whereas the mortality decline in the United States was greatest and sustained in men over 75 years between 1994 and 1999 (Negoita et al., 2018), mortality rates continued to increase and did not plateau for the same group in the United Kingdom until 2000 (Collins et al., 2008). There is evidence that PCa tends to be treated more aggressively in the United States than in the United Kingdom, and therefore prolonged survival from increased medical intervention is associated with early diagnosis. Collin et al. contends that one can only speculate about the relative contributions of PCa detection and treatment methods (Collin et al., 2008).
Awareness and early detection in the United Kingdom
Screening for early PCa detection is not recommended in the United Kingdom by the U.K. National Health Service and the National Screening Committee because of lack of evidence of its effectiveness relative to its risks (Gavin et al., 2004). Men can be tested if they have read the evidence-based information on PSA testing. Based on the Gavin et al. findings, almost 33% of U.K. men over 50 years of age reported having at least one PSA test between 1994 and 1999. Unfortunately, there was not a breakdown by race or ethnicity in the Gavin et al. study, and available data on PSA testing in the United Kingdom according to ethnicity or race is difficult to locate.
Few PCa qualitative studies have been published specifically targeted at capturing the comparative experiences and views of various ethnicities in the United Kingdom. One study conducted by Rajbabu et al. (2007) compared the PCa knowledge and beliefs of African, Afro-Caribbean, and White men in the United Kingdom. Similar to studies conducted in the United States, Rajbabu et al. documented that even though Black men of African descent are at high risk of the disease, they had less accurate knowledge of PCa than White men. This finding is consistent with qualitative studies of African American men conducted in the United States by Allen, Kennedy, Wilson-Glover, and Gilligan (2007); Clarke-Tasker and Wade (2002); Thompson, Cavazos-Rehg, Tate, and Gaier (2008); and Kleier (2003).
Other European Countries
Incidence and mortality trends for European countries other than the United Kingdom are discussed together due to limited available data.
Northern Europe
Incidence and mortality. According to Center et al. (2012), PCa incidence rates increased in four of five Nordic countries (Denmark, Iceland, Norway, and Sweden) in the past decades, with the greatest average increase of 8.2% per year observed in Denmark from 1999 to 2008. In Finland, the incidence rate remained stable, while in Lithuania and Latvia there were steep increases in incidence of 16.4% and 10.9% per year, respectively. Similar to the United Kingdom, Ireland experienced increasing incidence trends over the past 10 years (Center et al., 2012).
In terms of mortality, Norway and Sweden experienced substantial decreases, but the trend was more stable in Denmark and Iceland (Center et al., 2012). In Finland, mortality has been decreasing at approximately 3.1% per year since 2000. Despite the increasing trend in incidence seen in Ireland, Lithuania, and Latvia, mortality has been decreasing in Ireland, while stable in both Lithuania and Latvia (Center et al., 2012).
Western Europe
Incidence and mortality. In Western Europe, the number of men diagnosed with PCa has been on the rise. This may be due to an increase in opportunistic screening, but other factors such as diet and low exposure to ultraviolet radiation (Vit D) may be involved (Mottet et al., 2017). Specifically, Austria, France, and Switzerland have seen increases in incidence rates of about 4%–5% per year since the mid-1990s. Rates were stable in the Netherlands from 1999 to 2008. In contrast, PCa mortality rates decreased in Austria, France, Switzerland, Germany, and the Netherlands, with the largest annual decline in Austria of 4.0 and the smallest in Germany and the Netherlands of 2.3 per annum (Center et al., 2012).
Southern Europe
Incidence and mortality. PCa incidence trends increased in Croatia, Italy, Slovenia, Malta, and Spain between 1998 and 2007, with Croatia exhibiting the largest increase of 8.5% per year. Mortality trends were more variable, with rates increasing in both Croatia and Slovenia and declining in Italy, Malta, and Spain (Center et al., 2012).
Central and Eastern Europe
Incidence and mortality. Incidence rates for PCa increased rapidly in Bulgaria, the Czech Republic, Hungary, Moldova, Romania, Russia, and Ukraine. The largest increase was seen in Poland, which experienced a rise of 7.7% per year. Mortality rates in most Central and Easter European countries also increased, with only the Czech Republic and Hungary exhibiting declining trends of around 2.5% per year from 2000 to 2009 (Center et al., 2012).
Awareness and early detection in other European countries
There is currently no evidence for introducing widespread population-based screening for early PCa detection in all men, and to date very few qualitative studies are available on awareness of PCa in European men. Screening has been associated with overdiagnosis, overtreatment, and side effects related to treatment. Consequently, there is strong advice against systematic population-based screening in Europe. Screening continues to be done on an individual basis in consultation with a physician, and diagnosis is by prostate biopsy. In low-risk PCa cases, active surveillance is an option and watchful waiting is an alternative to androgen deprivation therapy if locally advanced PCa does not require immediate local treatment (Heidenreich et al., 2014).
PCa in the Caribbean
Incidence
PCa incidence rates as high as 304 per 100,000 have been documented for men in the Caribbean region (Bunker et al., 2006; Gibson & Gibson, 2010; Glover et al., 1998; Hennis et al., 2011; Mallick, Blanchet, & Multigner, 2005). Despite the effect on men’s health and mortality in the Caribbean region, information on PCa incidence rates, risk factors, and public health implications for Afro-Caribbean men is sparse and inconclusive (Hennis et al., 2011).
A PCa incidence rate of 304 per 100,000 was documented for Jamaican men by Glover et al. (1998). That represented the highest incidence rate in the world. More recent data from Gibson and Gibson (2010) and Ben-Shlomo et al. (2008) contradicted the accuracy of the Glover et al. rates. Gibson and Gibson reported that the Jamaican age-standardized rate (ASR) for PCa for the period 2003 to 2007 was 78.1 per 100,000, which is significantly below the Glover et al. rate.
Disparate and confusing incidence rates have been documented for Barbadian men (Hennis et al., 2011) and Afro-Caribbean men on the islands of Trinidad and Tobago (Bunker et al., 2006). Hennis et al. reported that the crude incidence rate in Barbados was 131.0 per 100,000 compared to 248.2 for African Americans; 158.0 for White Americans; 163.1 for Europeans; and 112.0 for the world. Hennis et al. noted that unlike in the United States where socioeconomic and health-care access issues need to be considered when investigating PCa and African American men, Barbados provides free and equal access to comprehensive health care. Based on the Hennis et al. (2011) data, the PCa incidence rate for Black Barbadian men between July 2002 and December 2008 was similar to that for White Americans at 160.4 per 100,000. The incidence rate for African Americans for the same period was approximately one and a half times higher than that for Black Barbadians (Hennis et al., 2011).
Researchers of Afro-Caribbean men on the islands of Martinique and Guadeloupe (colonies of France but with a mostly African heritage) have reported the PCa incidence rate to be among the highest in the world. Those incidence rates were 152.3 per 100,000 as noted by Mallick et al. (2005) and 173.7 per 100,000 as documented by Belpomme and Irigaray (2011). Belpomme and Irigaray reported that although the incidence rate in Martinique has been growing continuously at a rate similar to that of metropolitan France since 1985, it remains consistently higher at 173.7 per 100,000 than that of men in metropolitan France whose rate is 118.3 per 100,000. The researchers asserted that because the health-care system and access to testing techniques in Martinique are almost the same as in France, it is unlikely that the higher incidence rates may be due to a difference in screening. Instead, the differences in incidence may be due to a strong interaction between genetic and environmental factors (Belpomme & Irigaray, 2011). That association has been consistently reported in people from the same ethnicities such as Japan and China who now live in different geographic areas, under different environmental conditions, and illustrate different incidences in PCa than their native countries (Baade, Youlden, Cramb, Dunn, & Gardiner, 2013).
Mortality
Trinidad and Tobago has among the highest PCa mortality rates in the world. Their rates have been increasing by an average of 4.5% per year over the past decade (Center et al., 2012). While overall mortality rates for African American men declined between 2000 and 2006, the mortality rate for Black men (45% of population) in Trinidad and Tobago as well as for Black Barbadian men increased during the same period. The overall mortality rate from probable and definite PCa in Barbadian men between 1995 and 2008 ranged from 63.2 to 101.6 compared to 51.1 to 78.8 per 100,000 for the same time period for African American men (Hennis et al., 2011). Hennis et al. posited that the similar PCa mortality rates seen in Barbadian versus African American men should be a cause for concern, given the lower incidence rate in Barbadian men compared to African American men.
Awareness and Early Detection in the Caribbean
Bunker et al. (2002) conducted a study of Afro-Caribbean men living in Tobago. When compared to the data from a Richie et al. (1993) study of White men in the United States, the PCa prevalence detected in men in Tobago was three to four times higher than seen in the Richie et al. study. Bunker et al. (2002) theorized that the high Tobago prevalence rate may have been associated with the high biopsy rate of 90% seen in the study of Tobago men, compared with 69% in the Richie et al. (1993) U.S. study. It is important to note that the number of men recruited in the Tobago study was 80% of the total population of 5,121 men on the island, of which 2,492 (49%) aged 40–79 years completed PSA and/or DRE screening (Bunker et al., 2002).
Although specific data on the percentage of Afro-Caribbean men who undergo PSA and/or DRE screening is lacking, it is highly unlikely that such a high screening percentage recorded in the Tobago study is prevalent in other islands or among Afro-Caribbean men in other geographic locations. Few data are available for Caribbean populations, but similar to most developing countries, there is a lack of biopsy materials and histopathologists, few cancer registries, and reliance on hospital-based instead of population-based sampling. One of the main reasons that incidence rates may be higher in Afro-Caribbean populations than documented is due to fact that similar to most developing countries, screening for PCa is not prevalent among that group (Hennis et al., 2011).
PCa in Asia
Incidence
In contrast to the declining incidence of PCa in Western countries, PCa rates have increased in some Asian countries such as Japan and China even though PSA testing is not often used (Baade et al., 2009). Fourteen percent of all PCa diagnosed worldwide in 2008 was within the Asia-Pacific region. Approximately 60% of these PCa cases were diagnosed in either Japan (32%) or China (28%; Baade et al., 2013).
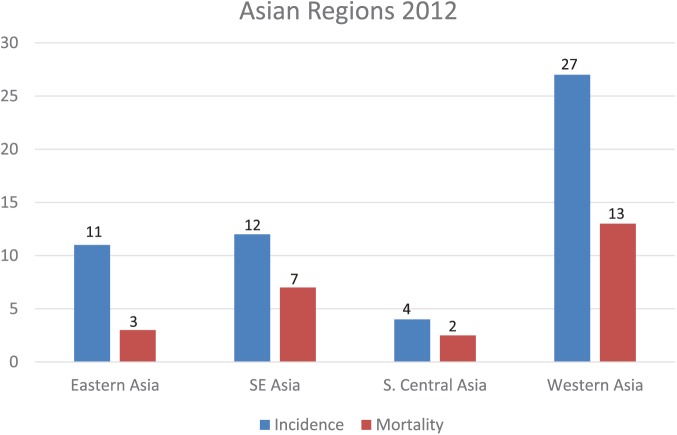
The incidence of PCa has risen significantly in many other Asian countries and PCa has become one of the leading male cancers in some of those nations. Figure 5 highlights the variability in incidence and mortality across the different Asian regions. In 2000, the age-adjusted incidence was over 10 per 100, 000 men in Japan, Taiwan, Singapore, Malaysia, the Philippines, and Israel (Pu et al., 2004). Sim and Cheng (2005) noted that incidence rates per 100,000 at centers in Japan rose from 6.3 to 12.7 (102%) between 1978 and 1997, while the incidence rates in Singaporean Chinese increased 118% from 6.6 to 14.4 within the same period. The lowest Asian-recorded incidence was in Shanghai, and the highest rates were in the Rizal Province in the Philippines during the same period (Sim & Cheng, 2005).
Figure 5.
Incidence and mortality rates (per 100,000) in different Asian regions in 2012. Data source: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Adapted from Chen et al., 2014, “Prostate Cancer in Asia: A Collaborative Report.”
Pu et al. (2004) and Baade et al. (2009) contended that although some of the increases in incidence rates may be the consequence of enhanced screening, in actuality, Westernization of lifestyle, reduced physical activity, and increased consumption of fat may be major contributors. Studies show that Asians living in the United States have higher incidence rates of PCa than the average rates of Asians living in their native countries (Baade et al., 2013). Based on such studies, Baade et al. concluded that the geographic variations in PCa incidence within the same ethnic groups living in different areas of the world would suggest that geography and environmental factors play a significant role as risk factors for PCa.
Mortality
According to Baade et al. (2013), PCa accounted for approximately 2% of all cancer-related deaths in the Asia-Pacific region in 2008. During that same period, more than 250,000 men have died from PCa worldwide; of these deaths, 42,000 (16%) occurred in the Asia-Pacific region. China accounted for 34% of PCa deaths within the region, followed by Japan (24%) and Indonesia (16%). New Caledonia had the highest country-specific mortality rate in the Asia-Pacific region of 45 deaths per 100,000 (Baade et al., 2013).
Increases in age-adjusted mortality rates per 100,000 person-years adjusted to the world standard ranged from 50% in Thailand to 260% in Korea between 1978 and 1997 (Sim & Cheng, 2005). The authors theorized that the increases may be partly due to genetic polymorphism in the androgen receptor and androgen metabolism pathway enzymes as well as dietary or environmental factors. Baade et al. (2013) contended that results from epidemiological and migrant study research have documented that environmental factors of a possible dietary and lifestyle change nature may be associated with PCa.
Awareness and Early Detection in Asia
Early diagnosis is the key to successful treatment of cancer, but a significant percentage of men with PCa in Asia (particularly in East Asia) are diagnosed at an advanced stage with poor prognoses. For example, in Malaysia, one institutional study reported over half of people diagnosed with PCa were already at Stage 4 (Baade et al., 2013). The percentage of men who received PSA testing throughout Asia is unknown, but in Japan, less than 20% of men over 50 years of age received PSA screening, while in South Korea, only 15% of men over 50 years in 2004 reported having been screened during the previous 2 years (Baade et al., 2013). The low incidence rate of PCa therefore does not reflect the actual statistics of this disease in Asia, and data from limited institutions in many Asian countries seem to bias the true incidence and mortality rates.
To improve awareness and early detection, Zhang et al. (2011) argued that it would be important and helpful to incorporate PSA screening for PCa as well as constructing a nationwide cancer registration system. Consequently, there have been calls for increasing PSA testing in several Asia-Pacific countries, but appropriate screening strategies would probably need to differ from those in Western countries because PCa is diagnosed at a later stage in the disease. In addition, PCa screening protocols that are recommended for use in Asia may need to be individualized based on the epidemiological features and socioeconomic status of each country (Ito, 2014).
PCa in India
Incidence
Previously it was thought that incidence of PCa in India was far lower than in Western countries. Data specific to PCa is not readily available, and the disease is not often diagnosed in its early stages. The age-adjusted incidence of PCa in Mumbai was approximately 18 times less than that observed in African Americans in the 1990s (Hsing, Tsao, & Devesa, 2000). More recent data has now reported that with the increased migration of rural populations to urban areas, changing lifestyles, increased awareness, and easier access to medical facilities, more cases of PCa are being identified. The incidence of PCa in India has been increasing across most registry regions over the past two decades, and it is now theorized that it is not far behind the rate observed in Western countries (Jain, Saxena, & Kumar, 2014).
Mortality
A study at the Bombay Hospital Institute of Medical Sciences between 1986 and 1992 reported that 84% of diagnosed PCa cases were at an advanced stage (Srinivas et al., 1995). This contrasts with the United States, where only about 15% of patients were diagnosed at an advanced stage at initial presentation during the same period (Esper et al., 1997). More recent data has reported that PCa is the second leading cancer among males in large Indian cities like Delhi, Kolkata, Pune, and Thiruvananthapuram; the third leading male cancer in cities like Bangalore and Mumbai; and among the top 10 leading cancers in the rest of India based on the population-based cancer registries (PBCRs) of India. The data shows that almost all regions of India are equally affected by PCa, but although incidence rates of this cancer are constantly and rapidly increasing in all the PBCRs, available PCa mortality rates as retrieved from the World Health Organization (WHO) mortality data bank were not available for India (Hsing et al., 2000).
Awareness and Early Detection in India
In countries such as India and most of Asia, screening is seldom used to identify PCa. Consequently, in India, the disease is not identified until in its later stages. More recently, Jain et al. documented that PBCRs are reporting new information that indicate a major increase in PCa incidences in the coming years due to greater access to medical services and more affordable and improved diagnostic and detection technologies (Jain et al., 2014).
PCa in Oceania
Incidence
PCa was the most common male cancer in many parts of Oceania, including Australia, Fiji, New Caledonia, and New Zealand, while it ranked second in Brunei, Micronesia, Polynesia, and the Solomon Islands (Ferlay et al., 2010). Testing in New Zealand using PSA increased in the early 1990s at a rate similar to the United States, but was stable between 1995 and 2004. Australia experienced an annual increase of about 6% between 1998 and 2008 after being stable prior to 2000 (Baade et al., 2013).
Mortality
There was considerable variation in the age-standardized mortality rate for PCa, with mortality in the Oceania subregion six times greater than in Eastern Asia and three times greater than in Southeastern Asia (Baade et al., 2013). Despite those comparative numbers, mortality rates decreased in Australia by 2.3% between 1995 and 2004 and by 2.8% per year on average in New Zealand between 1998 and 2007 (Center et al., 2012).
Awareness and Early Detection in Oceania
Since the early 1990s, PSA testing has served as the primary screening tool for diagnosis of PCa in Australia and New Zealand. In 1996, the Australian Health Technology Advisory Committee recommended against screening asymptomatic men for PCa. In 2008/2009, 21%–25% of all men in Australia aged 50–75 years had a PSA test (Zargar et al., 2017). Since the introduction of the USPSTF recommendations in 2012, there has been a steady nationwide decline in PSA testing, prostate biopsies, and prostatectomies based on Australian Medicare data. The impact of that change, as well as whether the 2018 USPSTF recommendations will impact testing protocols in Oceania, remains to be seen.
In New Zealand, most general practitioners (GPs) recommend screening for PCa, although there was no evidence that screening improves life expectancy and quality of life (Durham, Low, & McLeod, 2003). Forty-nine percent of New Zealand men aged 40–74 years reporting having at least one PSA test, with 22% having one within the previous 5 years (Baade et al., 2013). Seventy-four percent of New Zealand GPs would recommend a PSA test for a 55-year-old man presenting for an annual checkup or requesting advice about screening. If the same man had a family history of prostate or breast cancer, 93% of GPs would perform a PSA test. Most New Zealand GPs seemed to overestimate the effectiveness of screening for PCa and were uncertain about the consequences and importance of associated risk factors (Durhan, Low, & McLeod, 2003). It is interesting to note that the current approach to PSA screening by GPs in New Zealand is very similar to the new 2018 USPSTF recommendations.
PCa in The Americas
Incidence
Columbia, Costa Rico, and Ecuador have experienced increasing PCa trends from 1993 to 2002. Incidence rates in Brazil have been relatively high but have remained rather stable in more recent years following an increase in the 1990s (Center et al., 2012). The incidence of PCa in Cuba was the highest in the Americas and increased from 20.7 in 1977 to 28.6 per 100,000 in 1999 (Fernández et al., 2005). Alvarez, Yi, Garrote, and Rodríguez (2004) provided a different PCa incidence statistic of 34.9 per 100,000 for Cuba for the same year. Those different statistics once again highlight the disparities in available PCa data. Fernández et al. (2005) theorized that since neither screening nor PSA testing is conducted on the island, the increase in incidence was unlikely influenced by diagnostic practices. Although there is no definitive data, Fernández et al. theorized that the increases in incidence support the hypothesis that sexual behavior and a history of sexually transmitted diseases have been associated with the risk of PCa (Fernández et al., 2005).
Mortality
Mortality trends in the Americas are variable. Rates are increasing in Brazil, Colombia, and Ecuador, decreasing in Argentina, Costa Rica, and Chile, and stable in Mexico (Center et al., 2012). Between 2005 and 2009, Cuba, Uruguay, and Venezuela had the highest PCa mortality rates in the Americas of more than 18 per 100,000 overall. Chile, Argentina, Costa Rica, Puerto Rico, Colombia, and Brazil had overall rates between 13 and 17 per 100,000 during the same period (Chatenoud et al., 2014). In Argentina, Chile, Costa Rica, and Mexico, there has been a slight decline in PCa mortality since 1990. Sierra, Soerjomataram, and Forman (2016) contended that any declines in PCa mortality in the Americas may have been related to improvements in treatment and early detection, but that is yet to be validated.
The lowest mortality rates of 12–13 per 100,000 have been observed in Brazil and Mexico in 2000, but mortality rates have been steadily increasing in Cuba, Columbia, Ecuador, and Brazil between 1980 and 2010 (Bosetti et al., 2005; Sierra et al., 2016). Since 2004, the overall PCa mortality in Latin American countries has reported a moderate upward trend. Since no appreciable increases were observed in ages below 65 years, Bosetti et al. (2005) argued that it is likely that the upward trends in countries such as Chile, Costa Rica, Ecuador, and Venezuela are influenced by changes in diagnosis and certification in the elderly.
Awareness and Early Detection in the Americas
PCa incidence essentially reflects the variable adoption of PSA testing. As in most developing countries, PCa screening is not common because of economic reasons as well as the lack of available health-care resources. In order to reduce mortality, PCa needs to be detected in its early stages by adopting diagnostic and modern therapeutic practices within the Americas (Bosetti et al., 2005).
Discussion
This review confirms the lack of conformity regarding PCa detection practices, quality of registries, and availability/accuracy of incidence and mortality data based on race, ethnicity, and geographic location. The published data demonstrate that there is a difference in incidence and mortality rates between men of African descent, Asian men, Indian men, men of the Americas, and men of European ancestry, validating the involvement of genetic factors. There are differences between men of the same race and ethnicity who live in different countries, suggesting that other factors such as diet and socioeconomic status are implicated. That is particularly evident based on conflicting PCa data for men of African descent who live in America, the Caribbean, and the United Kingdom. The same is true for Asian men who live in different Asian countries versus those who have migrated to other countries such as the United States and Hawaii. Other complicating issues relate to the differences in diagnostic and screening services, treatment availability, and differences in technologies and recommendations regarding PCa testing. Estimated incidence rates remain highest in developed countries, while mortality rates are highest in developing countries.
Regardless of the current pattern that indicates reducing incidence and mortality in developed countries, there is a general global trend suggesting that PCa incidence is increasing worldwide when variables such as immigration, acculturalization, and increase in life expectancy are considered. In addition, the geographic and temporal variation of PCa rates seen in most developing countries seem to reflect differences in diagnostic practices, disease awareness, availability and access to health care, and recording certification of the cause of death. Increasing use of screening practices in those countries will likely show an increase in documented PCa incidence. The impact of increased screening on reducing mortality continues to be strongly debated, but new evidence from randomized trials suggest that although the net benefit of PSA-based screening in men aged 55–69 years is small, screening offers a potential benefit of reducing the chance of death from PCa in some men.
Overdiagnosis rates in the range of 23%–42% have been estimated in the United States and Europe, strongly contributing to the argument that more men than necessary are being treated for PCa as a consequence of PSA testing. That argument is a valid one, but how does one address the issue confronting developing countries, where the incidence is seen as quite low but the mortality is high because PCa is not discovered until symptoms are observed? The solution is somewhere in the middle. I contend that men at risk should have a baseline PSA, but treatment should not be based on a single PSA result. That may not be feasible for many countries but should be utilized wherever possible. The importance of raising the overall awareness of PCa in developing countries and its potentially devastating effects if not diagnosed and treated early cannot be overemphasized.
Finally, this research did not explore the impact of diet on global trends and PCa, but it is important to mention that the World Cancer Research Fund Continuous Update Project (CUP) recently released its revised 2018 findings on diet, nutrition, physical activity, and PCa. The CUP reported that there is now strong evidence that obesity and height (being tall) are strong factors associated with increasing the risk of advanced PCa. The CUP also reported limited but suggestive evidence that higher consumption of dairy products, diets high in calcium, low plasma alpha-tocopherol concentration, and low plasma selenium concentration might increase the risk of PCa (World Cancer Research Fund, CUP − 2018).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Allen J. D., Kennedy M., Wilson-Glover A., Gilligan T. D. (2007). African-American men’s perceptions about prostate cancer: Implications for designing educational interventions. Social Science and Medicine, 64(11), 2189–2200. doi: 10.1016/j.socscimed.2007.01.007 [DOI] [PubMed] [Google Scholar]
- Alvarez Y. G., Yi M. G., Garrote L. F., Rodríguez R. C. (2004). Incidence, mortality and survival from prostate cancer in Cuba, 1977–1999. European Journal of Cancer Prevention, 13(5), 377–381. [DOI] [PubMed] [Google Scholar]
- American Cancer Society (ACS). (2013). Cancer facts and figures. Retrieved from http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2013/index
- Baade P. D., Youlden D. R., Cramb S. M., Dunn J., Gardiner R. A. (2013). Epidemiology of prostate cancer in the Asia-Pacific region. Prostate International, 1(2), 47–58. doi: 10.12954/PI.12014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baade P. D., Youlden D. R., Krnjacki L. J. (2009). International epidemiology of prostate cancer: Geographical distribution and secular trends. Molecular Nutrition and Food Research, 53(2), 171–184. doi: 10.1002/mnfr.200700511 [DOI] [PubMed] [Google Scholar]
- Belpomme D., Irigaray P. (2011). Environment as a potential key determinant of the continued increase of prostate cancer incidence in Martinique. Prostate Cancer, 2011, 1–8. doi: 10.1155/2011/819010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shlomo Y., Evans S., Ibrahim F., Patel B., Anson K., Chinegwundoh F., … Persad R. (2008). The risk of prostate cancer amongst Black men in the United Kingdom: The PROCESS cohort study. European Urology, 53(1), 99–105. doi: 10.1016/j.eururo.2007.02.047 [DOI] [PubMed] [Google Scholar]
- Bosetti C., Malvezzi M., Chatenoud L., Negri E., Levi F., La Vecchia C. (2005). Trends in cancer mortality in the Americas, 1970–2000. Annals of Oncology, 16(3), 489–511. [DOI] [PubMed] [Google Scholar]
- Bunker C. H., Patrick A. L., Konety B. R., Dhir R., Brufsky A. M., Vivas C. A., … Kuller L. H. (2002). High prevalence of screening-detected prostate cancer among Afro-Caribbeans: The Tobago prostate cancer survey. Cancer Epidemiology Biomarkers & Prevention, 11(8), 726–729. [PubMed] [Google Scholar]
- Bunker C. H., Zmuda J. M., Patrick A. L., Wheeler V. W., Weissfeld J. L., Kuller L. H., Cauley J. A. (2006). High bone density is associated with prostate cancer in older Afro-Caribbean men: Tobago prostate survey. Cancer Causes & Control, 17(8), 1083–1089. doi: 10.1007/s10552-006-0047-1 [DOI] [PubMed] [Google Scholar]
- Cancer Research U.K. (2008). Cancer statistics. Retrieved from http://info.cancerresearchuk.org/cancerstats/incidence/commoncancers
- Center M. M., Jemal A., Lortet-Tieulent J., Ward E., Ferlay J. (2012). International variation in prostate cancer incidence and mortality rates. European Urology, 61(6), 1079–1092. doi: 10.1016/j.eururo.2012.02.054 [DOI] [PubMed] [Google Scholar]
- Chatenoud L., Bertuccio P., Bosetti C., Malvezzi M., Levi F., Negri E., La Vecchia C. (2014). Trends in mortality from major cancers in the Americas: 1980–2010. Annals of Oncology, 25(9), 1843–1853. [DOI] [PubMed] [Google Scholar]
- Chen R., Ren S., Yiu M. K., Fai N. C., Cheng W. S., Ian L. H., Chiu J. Y. (2014). Prostate cancer in Asia: a collaborative report. Asian Journal of Urology, 1(1), 15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinegwundoh F., Enver M., Lee A., Nargund V., Oliver T., Ben-Shlomo Y. (2006). Risk and presenting features of prostate cancer amongst Afro-Caribbean, South Asian, and European men in North-East London. British Journal of Urology International, 98, 1216–1220. 10.1111/j.1464-410X.2006.06503.x [DOI] [PubMed] [Google Scholar]
- Chu L. W., Ritchey J., Devesa S. S., Quraishi S. M., Zhang H., Hsing A. W. (2011). Prostate cancer incidence rates in Africa. Prostate Cancer, 2011, 1–6. doi: 10.1155/2011/947870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke-Tasker V. A., Wade R. (2002). What we thought we knew: African-American males’ perceptions of prostate cancer and screening methods. ABNF Journal, 13(3), 56–60. [PubMed] [Google Scholar]
- Colby S. L., Ortman J. M. (2014). Projections of the size and composition of the US Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau. [Google Scholar]
- Collin S. M., Martin R. M., Metcalfe C., Gunnell D., Albertsen P. C., Neal D., … Donovan J. (2008). Prostate-cancer mortality in the USA and UK in 1975–2004: An ecological study. The lancet Oncology, 9(5), 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham J., Low M., McLeod D. (2003). Screening for prostate cancer: A survey of New Zealand general practitioners. The New Zealand Medical Journal (Online), 116(1176), 476–484. [PubMed] [Google Scholar]
- Echimane A. K., Ahnoux A. A., Adoudi I., Hien S., M’Bra K., D’Horpock A., … Parkin D. M. (2000). Cancer incidence in Abidjan, Ivory Coast. Cancer, 89(3), 653–663. [DOI] [PubMed] [Google Scholar]
- Esper P., Mo F., Chodak G., Sinner M., Cella D., Pienta K. J. (1997). Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology, 50(6), 920–928. [DOI] [PubMed] [Google Scholar]
- Fedewa S. A., Sauer A. G., Siegel R. L., Jemal A. (2015). Prevalence of major risk factors and use of screening tests for cancer in the United States. Cancer Epidemiology and Prevention Biomarkers, 24(4), 637–652. [DOI] [PubMed] [Google Scholar]
- Ferlay J., Shin H. R., Bray F., Forman D., Mathers C., Parkin D. M. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer, 127(12), 2893–2917. doi: 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., … Bray F. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer, 136(5), E359–E386. [DOI] [PubMed] [Google Scholar]
- Fernández L., Galán Y., Jiménez R., Gutiérrez Á., Guerra M., Pereda C., … González C. (2005). Sexual behavior, history of sexually transmitted diseases, and the risk of prostate cancer: A case–control study in Cuba. International Journal of Epidemiology, 34(1), 193–197. [DOI] [PubMed] [Google Scholar]
- Gavin A., McCarron P., Middleton R. J., Savage G., Catney D., O’Reilly D., … Murray L. J. (2004). Evidence of prostate cancer screening in a UK region. British Journal of Urology International, 93(6), 730–734. doi: 10.1111/j.1464-410X.2003.04716.x [DOI] [PubMed] [Google Scholar]
- Gibson T. N., Gibson D. (2010). Age-specific incidence of prostate cancer in Kingston and St. Andrew, Jamaica, 2003–2007. West Indian Medical Journal, 59(5), 456–464. [PubMed] [Google Scholar]
- Glover F. E., Coffey D. S., Douglas L. L., Cadogan M., Russell H., Tulloch T., … Walsh P. C. (1998). The epidemiology of prostate cancer in Jamaica. Journal of Urology, 159(6), 1986–1987. [DOI] [PubMed] [Google Scholar]
- Han P. K., Kobrin S., Breen N., Joseph D. A., Li J., Frosch D. L., Klabunde C. N. (2013). National evidence on the use of shared decision making in prostate-specific antigen screening. The Annals of Family Medicine, 11(4), 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich A., Bastian P. J., Bellmunt J., Bolla M., Joniau S., van der Kwast T., … Mottet N. (2014). EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent—update 2013. European urology, 65(1), 124–137. [DOI] [PubMed] [Google Scholar]
- Hennis A. J. M., Hambleton I. R., Wu S. Y., Skeete D. H. A., Nemesure B., Leske M. C. (2011). Prostate cancer incidence and mortality in Barbados, West Indies. Prostate Cancer, 2011, 1–10. doi: 10.1155/2011/565230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing A. W., Devesa S. S. (2001). Trends and patterns of prostate cancer: What do they suggest? Epidemiology Review, 23(1), 60–67. [DOI] [PubMed] [Google Scholar]
- Hsing A. W., Tsao L., Devesa S. S. (2000). International trends and patterns of prostate cancer incidence and mortality. International Journal of Cancer, 85(1), 60–67. doi: [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC). (2010). GLOBOCAN 2008. Retrieved from http://globocan.iarc.fr
- Ito K. (2014). Prostate cancer in Asian men. Nature Reviews Urology, 11(4), 197. [DOI] [PubMed] [Google Scholar]
- Jain S., Saxena S., Kumar A. (2014). Epidemiology of prostate cancer in India. Meta Gene, 2, 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A., Center M. M., DeSantis C., Ward E. M. (2010). Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiology Biomarkers and Prevention, 19(8), 1893–1907. doi: 10.1158/1055-9965.EPI-10-0437 [DOI] [PubMed] [Google Scholar]
- Jemal A., Fedewa S. A., Ma J., Siegel R., Lin C. C., Brawley O., Ward E. M. (2015). Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA, 314(19), 2054–2061. [DOI] [PubMed] [Google Scholar]
- Kambal A. (1977). Prostatic obstruction in Sudan. British Journal of Urology, 49(2), 139–141. [DOI] [PubMed] [Google Scholar]
- Kheirandish P., Chinegwundoh F. (2011). Ethnic differences in prostate cancer. British Journal of Cancer, 105(4), 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleier J. A. (2003). Prostate cancer in Black men of Afro-Caribbean descent. Journal of Cultural Diversity, 10(2), 56–61. [PubMed] [Google Scholar]
- Lim L.S., Sherin K. (2008). Screening for prostate cancer in U.S. men. American Journal of Preventive Medicine, 34(2), 164–170. [DOI] [PubMed] [Google Scholar]
- Mallick S., Blanchet P., Multigner L. (2005). Prostate cancer incidence in Guadeloupe, a French Caribbean archipelago. European Urology, 47(6), 769–772. doi: 10.1016/j.eururo.2005.02.020 [DOI] [PubMed] [Google Scholar]
- Mohammed A. Z., Nwana E. J. C., Anjorin A. S. (2005). Histological pattern of prostatic diseases in Nigerians. African Journal of Urology, 11(1), 33–38. [Google Scholar]
- Mottet N., Bellmunt J., Bolla M., Briers E., Cumberbatch M. G., De Santis M., … Lam T. B. (2017). EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. European Urology, 71(4), 618–629. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. (2013). Quick cancer profiles. Retrieved from http://statecancerprofiles.cancer.gov/cgi-bin/quickprofiles/profile.pl?27&066
- National Cancer Intelligence Network. (2006). Cancer incidence and survival by major ethnic group, England. Retrieved from http://www.cancerresearchuk.org/cancer-info/cancerstats
- Negoita S., Feuer E. J., Mariotto A., Cronin K. A., Petkov V. I., Hussey S. K., … Sherman R. L. (2018). Annual report to the nation on the status of cancer, part II: Recent changes in prostate cancer trends and disease characteristics. Cancer, 124(13), 2801–2814. doi: 10.1002/cncr.31549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noone A. M., Howlader N., Krapcho M., Miller D., Brest A., Yu M., . . . Cronin K. A. (Eds). SEER cancer statistics review, 1975-2015. Bethesda, MD: National Cancer Institute; Retrived from https://seer.cancer.gov/csr/1975_2015/,basedonNovember2017SEERdatasubmission,postedtotheSEERwebsite,April2018. [Google Scholar]
- Odedina F. T., Yu D., Akinremi T. O., Reams R. R., Freedman M. L., Kumar N. (2009). Prostate cancer cognitive-behavioral factors in a West African population. Journal of Immigrant Minority Health, 11(4), 258–267. doi: 10.1007/s10903-008-9212-9 [DOI] [PubMed] [Google Scholar]
- Oliver J. S. (2007). Attitudes and beliefs about prostate cancer and screening among rural African-American men. Journal of Cultural Diversity, 14(2), 74–80. [PubMed] [Google Scholar]
- Osegbe D. N. (1997). Prostate cancer in Nigerians: Facts and non-facts. The Journal of Urology, 157(4), 1340–1343. [PubMed] [Google Scholar]
- Parkin D. M., Ferlay J., Hamdi-Chirif M., Sitas F., Thomas J. O., Wabinga H., … Whelan S. L. (Eds.). (2003). Cancer in Africa: Epidemiology and prevention. 153 Lyons. France: IARC Scientific Publications. [Google Scholar]
- Potosky A. L., Miller B. A., Albertsen P. C., Kramer B. S. (1995). The role of increasing detection in the rising incidence of prostate cancer. JAMA, 273(7), 548–552. [PubMed] [Google Scholar]
- Pu Y. S., Chiang H. S., Lin C. C., Huang C. Y., Huang K. H., Chen J. (2004). Changing trends of prostate cancer in Asia. The Aging Male, 7(2), 120–132. [DOI] [PubMed] [Google Scholar]
- Quinn M., Babb P. (2002). Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: International comparisons. British Journal of Urology International, 90(2), 162–173. doi: 10.1046/j.1464-410X.2002.2822.x [DOI] [PubMed] [Google Scholar]
- Rajbabu K., Chandrasekera S., Zhu G., Dezylva S., Grunfeld E. A., Muir G. H. (2007). Racial origin is associated with poor awareness of prostate cancer in U.K. men, but can be increased by simple information. Prostate Cancer and Prostatic Diseases, 10(3), 256–260. doi: 10.1038/sj.pcan.4500961 [DOI] [PubMed] [Google Scholar]
- Rebbeck T. R., Devesa S. S., Chang B. L., Bunker C. H., Cheng I., Cooney K., … Zeigler-Johnson C. M. (2013). Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of African descent. Prostate Cancer, 2013, 560857. doi: 10.1155/2013/560857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie J. P., Catalona W. J., Ahmann F. R., Hudson M. L. A., Scardino P. T., Flanigan R. C., … Southwick P. C. (1993). Effect of patient age on early detection of prostate cancer with serum prostate-specific antigen and digital rectal examination. Urology, 42(4), 365–374. doi: 10.1016/0090-4295(93)90359-I [DOI] [PubMed] [Google Scholar]
- Ries L. A., Harkins D., Krapcho M., Mariotto A., Miller B. A., Feuer E. J., … Hayat M. (2006). SEER cancer statistics review, 1975–2003. Bethesda, MD: National Cancer Institute. [Google Scholar]
- Schröder F. H., Hugosson J., Roobol M. J., Tammela T. L., Ciatto S., Nelen V., … Denis L. J. (2012). Prostate-cancer mortality at 11 years of follow-up. New England Journal of Medicine, 366(11), 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2016). Cancer statistics, 2016. CA: A Cancer Journal for Clinicians, 66(1), 7–30. [DOI] [PubMed] [Google Scholar]
- Sierra M. S., Soerjomataram I., Forman D. (2016). Prostate cancer burden in Central and South America. Cancer Epidemiology, 44, S131–S140. [DOI] [PubMed] [Google Scholar]
- Sim H. G., Cheng C. W. (2005). Changing demography of prostate cancer in Asia. European Journal of Cancer, 41(6), 834–845. [DOI] [PubMed] [Google Scholar]
- Srinivas V., Mehta H., Amin A., Choudary R., Gadgil N., Ravishanker D., Phadke A. G. (1995). Carcinoma of the prostate—state at initial presentation. International Urology and Nephrology, 27(4), 419–422. [DOI] [PubMed] [Google Scholar]
- Thompson V. L. S., Cavazos-Rehg P., Tate K. Y., Gaier A. (2008). Cancer information seeking among African-Americans. Journal of Cancer Education, 23(2), 92–101. doi: 10.1080/08858190701849429 [DOI] [PubMed] [Google Scholar]
- Torre L .A., Bray F., Siegel R., Ferlay J., Lortet-Tievlent J., Jemal A. J. (2015). Global cancer statistics, 2012. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- US Preventive Services Task Force. (2018). Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA, 319(18), 1901–1913. doi: 10.1001/jama.2018.3710 [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund. (2018). Prostate cancer: How diet, nutrition, and physical activity affect prostate cancer risk. Retrieved from; https://www.wcrf.org/dietandcancer/prostate-cancer
- World Health Organization. (2011). WHO statistics: Haiti. Retrieved from http://who.int/country/hti/en
- Zargar H., Bergh R., Moon D., Lawrentschuk N., Costello A., Murphy D. (2017). The impact of the United States Preventive Services Task Force (USPSTF) recommendations against Prostate-Specific Antigen (PSA) testing on PSA testing in Australia. BJU international, 119(1), 110–115. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yang B. X., Zhang H. T., Wang J. G., Wang H. L., Zhao X. J. (2011). Prostate cancer: An emerging threat to the health of aging men in Asia. Asian Journal of Andrology, 13(4), 574. [DOI] [PMC free article] [PubMed] [Google Scholar]