Abstract
No previous study has evaluated the effects of RF on inflammatory and hematological indices of COPD patients. The main objective of the present pilot study was to assess the effects of RF on some inflammatory and hematological indices measured in male patients with stable COPD. Fifteen COPD patients (mean ± SD of age: 71 ± 6 years) who fasted during Ramadan 2017 volunteered for the study. Three sessions (Before-Ramadan, End-Ramadan and After-Ramadan) were selected. Spirometry tests and blood samples were consistently performed 2.5–4.5 hr before the interruption of the fasting. Assessment sessions comprised: spirometry, inflammatory [erythrocyte sedimentation rate (ESR); C-reactive protein (CRP)] and hematological [red and white blood cells (RBC, WBC); hemoglobin; hematocrit; mean corpuscular volume; mean corpuscular hemoglobin; platelets] indices. Findings were analyzed by applying Friedman ANOVA. The median (lower–upper quartiles) of ESR (Before-Ramadan: 3 (2–9), End-Ramadan: 7 (0–13), After-Ramadan: 9 (5–15) mm/h) and CRP (Before-Ramadan: 20 (11–38), End-Ramadan: 15 (9–34), After-Ramadan: 20 (12–46) mg/L) were not significantly affected by RF. Among all the hematological indices, RF influenced only hemoglobin (Before-Ramadan: 14.4 ± 2.2, End-Ramadan: 13.4 ± 1.3, After-Ramadan: 12.2 ± 0.9 g/dL), hematocrit (Before-Ramadan: 45 ± 7, End-Ramadan: 40 ± 4, After-Ramadan: 39 ± 4%), RBC (Before-Ramadan: 5.1 ± 1.0, End-Ramadan: 4.6 ± 0.7, After-Ramadan: 4.4 ± 0.5 106/mm3) and WBC (Before-Ramadan: 8,673 ± 1,911, End-Ramadan: 7,840 ± 1,526, After-Ramadan: 9,507 ± 2,190/mm3). Compared to the Before-Ramadan session, the End-Ramadan session values for hemoglobin, hematocrit, RBC and WBC were lower. Compared to the After-Ramadan session, the End-Ramadan session values for hemoglobin and WBC were higher and lower, respectively. In conclusion, RF caused significant reduction in hemoglobin, hematocrit, RBC and WBC. However, it did not induce any significant changes in the CRP and ESR indices.
Keywords: respiratory diseases, feasting, biochemical biomarkers, blood indices, anemia
Ramadan-fasting (RF) consists in alternating fasting and feasting periods. However, it is not only the self-restraint (from dawn to sunset) from all types of liquid and solid nutrient intake, but also from smoking and sexual contact. This restriction includes medications given via oral and parenteral route, but not the drugs used via inhalation (Official Website of The Presidency of Religious Affairs of the Republic of Turkey, n.d.). The fast is interrupted by taking two meals, pre-dawn (suhoor) and after-sunset (iftar). The RF average duration is generally 12–14 hr but can last up to 18 hr and even 22 hr in the summer in extreme latitude areas (Haouari et al., 2008). RF alters “considerably” daily routines. First, it induces changes in sleeping hours and/or in physical activities and/or in eating schedules (Barkia et al., 2011; Ramadan, 2002). Second, it alters the quality and amount of nutriment and liquefied ingestion (Barkia et al., 2011; Ramadan, 2002).
According to the Islamic principles, patients suffering from chronic conditions are discharged from RF (Official Website of The Presidency of Religious Affairs of the Republic of Turkey, n.d.). In practice, several patients with chronic respiratory diseases refuse these exemptions and insist on fasting (and/or want to fast and/or prefer fasting) during Ramadan (Adeli, Aghaali, & Nasab, 2015; Askari, Alavinezhad, & Boskabady, 2016; Aydin et al., 2014; Baay, 2017; Bener et al., 2006; Erkekol et al., 2006; Norouzy et al., 2013; Zouari et al., 2018). For example, almost 93% of Muslim with chronic obstructive pulmonary disease (COPD) are Ramadan fasters (Aydin et al., 2014). For COPD patients, the international guidelines (Celli et al., 2015; Vogelmeier et al., 2017) suggested to arrange the use of the symptom-relieving treatment as well as the disease-development anticipation (e.g., monitoring and education of patients, and avoidance of risk factors). The effects of spiritual beliefs weren’t addressed and no recommendations were advanced for individuals with COPD who wanted to fast during Ramadan.
The spirometry test is a valuable exploration for diagnosing and monitoring a variety of pulmonary diseases (Miller et al., 2005). During the month of Ramadan, it continues to be normally done on COPD patients who prefer fasting. As far as it is known, only one study examined the RF effects on the spirometric data of stable male COPD patients (Zouari et al., 2018). It appeared that RF did not bring about any important changes in COPD spirometric data (Zouari et al., 2018).
A recent systematic review indicated that anemia and inflammatory markers are becoming increasingly recognized as factors that contribute to the COPD pathogenesis (Hoepers, Menezes, & Frode, 2015). The markers are linked with an increased risk of hospitalization and mortality (Hoepers et al., 2015). Since RF limits the occurrence of meals per day to only two, it may cause numerous biochemical and hematological changes in individuals who fast (Al Hourani, Atoum, Akel, Hijjawi, & Awawdeh, 2009; Azizi, 2002; Dewanti, Watanabe, Sulistiawati, & Ohtsuka, 2006; El-Hazmi, Al-Faleh, & Al-Mofleh, 1987; Nasiri, Mahmoudzadeh, Kheiri, & Khoshdel, 2016; Sarraf-Zadegan et al., 2000; Sedaghat et al., 2017). Similarly, for the spirometry test, the inflammatory and hematological markers continue to be normally interpreted during the holy month. Therefore, the main question remains; how to interpret any possible biological data worsening during Ramadan in COPD patients? Is it caused by the effects of RF or by clinical deterioration? At the best of the authors knowledge, only one study, including asthmatic patients, evaluated the RF effects on inflammatory [erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)] and hematological [red blood cell (RBC); white blood cell (WBC); hemoglobin (Hb); hematocrit (Ht); mean corpuscular volume (MCV); mean corpuscular Hb (MCH) and platelets (Plt)] indices (Askari et al., 2016). It seemed that RF induced significant reduction in the serum CRP concentration of asthmatic patients (Askari et al., 2016).
Since correlation between health and various religious rituals is an important issue and in view of the above considerations, this pilot study completed during the summer 2017, mainly aimed to assess the effects of RF on some inflammatory (ESR and CRP) and hematological (RBC; WBC; Hb; Ht; MCV; MCH and Plt) indices measured in male subjects with a stable COPD. The null hypothesis was that RF induces significant changes in the aforementioned indices.
Population and Methods
Study Design
The present study was designed as a pilot cross-sectional and experimental study. It was executed at the Pulmonary Department of the Farhat HACHED University Hospital, Sousse (Tunisia). Approval for the study (number 405/2017) was granted from the Ethical Committee of the Farhat HACHED Hospital. All patients signed an informed written consent prior being included. Patients were individually informed about the study purposes, and were allowed to leave it any time they desired to. Patients were not charged any costs for the accomplished tests. The present study is part of a project aiming at evaluating the effects of RF on some biological data (e.g., inflammatory, hematological, oxidative stress) of COPD patients.
Information about the season and/or temperature and/or humidity during this study period is mentioned in the Supplementary Data section.
Sample Size
The sample size was appraised according to the following formula (Kang, Ragan, & Park, 2008): N = (Zα/2)2 s2/d2, where “s” is the standard deviation (SD = 1094.500/mm3) and “d” is the accuracy of estimate or how close it is to the true mean (=612.000/mm3). Given the pioneering nature of this study, the above two data were collected from a previous work including asthmatic patients (Askari et al., 2016), where the WBC values (mm3) were 7,157 ± 1,073 and 7,769 ± 1,116, respectively, during the Before-Ramadan (Before-R) and the After-Ramadan (After-R) sessions. “Zα/2” is the normal deviate for a two-tailed alternative hypothesis at a level of significance (Zα/2 equal to 1.96 at an error rate of 0.05%). The appraised sample size as N = (1.96)2 1,094.5002/(612.000)2 gives a sample of 13 COPD patients. The assumption of 40% for nonattendance during the second or the third session gives a revised sample of 22 COPD patients (22 = 13/(1.0−0.40)).
Study Population
Only male COPD patients aged 50 years and more were included. The sampling was done based on a convenience method. The patients’ recruitment was done in two ways. First, the folders/files of patients with COPD that were followed in the Pulmonary Diseases and Physiology and Functional Exploration departments (Farhat HACHED hospital) were verified. Second, some patients were directly addressed by three pulmonologists from the Basic Health Group of Sousse and from the regional hospital of Kalaa-Kebira of Sousse. At the beginning of the project, a letter of information, covering details about the project and visit dates, was given to the patients. Noninclusion criteria were: history of RF < 20 years; actual cigarette smoking; smoking history < 10 pack-years; tobacco cessation < four months; narghile-use, non-COPD patient (e.g., asthma); neuromuscular disease other than these related to COPD; diabetes-mellitus; congestive heart failure; malignancy; vertebral column or thoracic cage abnormalities; oral corticosteroid treatment and lack of cooperation during the spirometry test. Females were excluded because Muslim laws prohibit fasting during the menses and because lung function is somewhat lowered during menses cycle (Cotes, Chinn, & Reed, 1997). Absenteeism during the second or the third session and a post-bronchodilator (PBD) ratio between the first second forced expiratory volume (FEV1) and forced vital capacity (FVC) (FEV1/FVC ratio) ⩾ 0.70 were applied as exclusion criteria. To avoid confusing effects, patients with unstable respiratory state (e.g., respiratory tract infection or exacerbation) within four weeks prior to the beginning of the project were excluded. Patients were recommended to avoid using short-acting bronchodilators (BDs) 6 hr prior to the spirometry test.
Experimental Design
The experimental design comprised three sessions: seven to 12 days Before-R (May, 15–23), four days at the End of Ramadan (End-R: June, 19–22) and 14 to 18 days After-R (July, 10–13). Throughout the Before-R session, all COPD patients answered three medical questionnaires (Ferris, 1978; Jones et al., 2009; Ninot et al., 2010). Then, the anthropometric data were measured and/or noted. After that, the spirometry test was executed and a blood sample was collected. During the second and the third session, only anthropometric, spirometric and blood samples data were collected.
Collected Data and Applied Definitions
The patients were questioned whether they had been RF for more than 20 years. If the response was “yes,” they were invited to answer additional questions assembled from the three questionnaires (Ferris, 1978; Jones et al., 2009; Ninot et al., 2010). The questions were asked in a Tunisian Arabic dialect by only one pulmonologist (HR in the authors’ list). They were linked to their socioeconomic and schooling levels, personal medical or surgical histories, chronic medication-use (especially BDs-use), dyspnea, cough, phlegm, physical activity, sleeping characteristics, smoking, and health-related-quality-of-life.
The nonvalidated short Arabic version of the American Thoracic Society division lung disease medical questionnaire assessed the patients’ medical and surgical histories (Ferris, 1978). Cigarette-smoking (pack-years, PY) was assessed. Dyspnea was evaluated according to the modified British Medical Research Council (mMRC) scale and two levels of dyspnea were arbitrary defined (mMRC < 2; mMRC ⩾ 2) (Fletcher, Elmes, Fairbairn, & Wood, 1959). Two schooling [low (illiterate, primary education) and high (secondary and university education)] and two socioeconomic [low (e.g., unskilled worker, jobless) and high (e.g., skilled worker, farmer, manager)] levels were determined. During the three sessions, the patients’ history of COPD exacerbation including hospitalizations was documented. COPD exacerbation was defined as an acute event characterized by a deterioration of the patient’s respiratory symptoms that are beyond normal day to day variations and which leads to a modification in the treatment (Vogelmeier et al., 2017). During the three sessions, COPD patients were asked about the schedules of their medication-use, latest meal and sleep durations. Meal-duration was defined as the period (in hour) between the last meal and the performed tests. Sleep-duration was defined as the period (in hour) of night sleep and naps before the performed tests.
The validated Arabic version of the COPD assessment-test (CAT) respiratory questionnaire aimed at quantifying the COPD impact on health status (Jones et al., 2009). According to the CAT score, COPD patients were classified into four groups [low (score < 10), medium (10 ⩽ score ⩽ 20), high (21 ⩽ score ⩽ 30) and very high (score > 30)] based on the impact level of disease on their health status.
The VQ11 is a valid French questionnaire that provides a reliable measure of COPD health-related-quality-of-life (Ninot et al., 2010). It includes 11 items distributed in three components (functional = 3; psychological = 4; relational = 4). Questions were translated into Arabic (by BSH in the authors’ list). The VQ11 score ranges from 11 to 55 and lower score (e.g., < 22) indicates better health-related-quality-of-life.
Decimal age was noted. Height (± 0.1 m) was measured with a mechanical scale (Seca Deutschland) with heels joined, and back straight. Weight (± 1 kg) was determined by a mechanical scale (HBS200-13, China). Body mass index (BMI = weight/height2, kg/m2) was calculated and the following obesity-status were categorized: underweight (BMI < 18.5 kg/m2), normal weight (18.5 kg/m2 to 24.9 kg/m2), overweight (25.0 kg/m2 to 29.9 kg/m2) and obesity (BMI ⩾ 30.0 kg/m2) (Tsai & Wadden, 2013).
Spirometry Measurements, COPD Diagnosis and Classification
All spirometric tests were done according to the international guidelines (Miller et al., 2005) at the same time of the day (between 12h00 and 15h00, approximately 455 to 275 min before sunset).
The spirometer was calibrated daily with a 3-L syringe. The spirometric data [FVC; FEV1; maximal mid-expiratory flow (MMEF), peak expiratory flow (PEF), FEV1/FVC] were expressed in percentages of local reference values (Ben Saad et al., 2013). The FVC maneuver was described elsewhere (Latiri et al., 2017; Zouari et al., 2018). The BD test was detailed elsewhere (Ben Saad, Prefaut, Tabka, Zbidi, & Hayot, 2008; Pellegrino et al., 2005; Zouari et al., 2018).
The COPD diagnosis was determined from a PBD FEV1/FVC ratio < 0.70 (Celli et al., 2015; Vogelmeier et al., 2017). The severity of the bronchial obstruction was classified as: mild (PBD FEV1 ⩾ 80%), moderate (50% ⩽ PBD FEV1 < 80%), severe (30% ⩽ PBD FEV1 < 50%) and very severe (PBD FEV1 < 30%) (Celli et al., 2015; Vogelmeier et al., 2017). The refined “ABCD” assessment tool (using patient’s health status and history of exacerbations but not spirometry) was applied, and COPD patients were classified into four groups (A, B, C, or D) (Vogelmeier et al., 2017).
Blood Samples
The blood sample was collected using a 20-ml syringe, upon completion of the spirometry test. It was divided into four tubes: hematological, ESR, chemical (CRP) and oxidative stress (total bilirubin, uric acid, Malondi-aldehyde, glutathione peroxidase) data.
Total RBC and WBC were counted via an automatized (Coulter LH 750 Analyzer, Beckman coulter). According to the staining and morphological criteria, differential cell analysis was carried out under a light microscope by counting 100 cells, and the percentage of each cell type was calculated. The following hematological data were measured/calculated: WBC (/mm3), RBC (106/mm3), Plt (/mm3), Hb level (g/dl), MCV (fl), MCH (pg) and Ht (%). Anemia was defined as Hb level < 13.0 g/dl (Cappellini & Motta, 2015). Patients were divided into two groups [0. No anemia; 1. Anemia]. Leukocytosis (Abramson & Melton, 2000) was defined as WBC count > 11 103/mm3. Patients were divided into two groups [0. No leukocytosis; 1. Leukocytosis].
The ESR analysis was performed according to the method of Westergren (Westergren, 1957). ESR values higher than 20 mm/hr were considered abnormal (Bottiger & Svedberg, 1967). CRP reagent was used to measure the CRP plasma concentration by a Turbidimetric method via the Beckman Coulter® DXC 600. Sensitivity for CRP determination was 5.0 mg/L (Boyden, Bolton, & Gemeroy, 1947). CRP values higher than 12 mg/L were considered abnormal (Colombet et al., 2010). Biological inflammation syndrome was defined by higher ESR and/or higher CRP and two groups of patients were defined [0. No; 1. Yes].
Statistical Analysis
The analysis of variable distribution was accomplished using the Kolmogorov–Smirnov test. When the distribution was normal and variances were identical, the results were expressed as mean ± SD (95% confidence interval [CI],). Otherwise, the results were expressed by their medians (lower-upper quartiles). Qualitative data were expressed by relative frequency.
Comparisons of the anthropometric and the spirometric data, sleep- and meal-durations and time of last BD-use were made between the three sessions. The results were obtained by applying repeated measures analysis of variance (Friedman ANOVA). When suitable, significant differences between means were tested using the Wilcoxon test.
Comparisons of the percentages of patients with anemia, leukocytosis or biological inflammation between the End-R session and the Before-R or the After-R sessions were accomplished via the Cochrane test. When applicable, significant differences between percentages were tested using the McNemar test. Analyses were carried out using the Statistica software (Statistica Kernel version 6; StatSoft, Paris, France). Alpha was set at p < 0.05.
Results
Among the 24 examined COPD, only 15 completed the three sessions. The cause for dropout was the absenteeism during the second (n = 6) or the third (n = 3) session.
Table 1 presents the baseline characteristics of the 15 COPD patients.
Table 1.
Baseline Characteristics of the 15 Stable Male COPD Patients.
| Low socioeconomic levela | 7 | |
| Low schooling levela | 10 | |
| Ramadan fasting experience (years)b | 57 ± 5 (54–60) | |
| Tobacco history (pack-years)b | 73 ± 44 (49–97) | |
| Smoking cessation duration (months)c | 84 (12–204) | |
| “Modified medical research council” ⩾ 2a | 13 | |
| Respiratory treatmentsa | Bronchodilators | 13 |
| Inhaled corticoid | 11 | |
| Mucoregulators | 1 | |
| Medical historiesa | Stable hypertension | 5 |
| Myocardial infarction | 1 | |
| Atrial fibrillation | 1 | |
| Anemia | 1 | |
| Dyslipidemia | 1 | |
| COPD assessment-test scoresc | 13 (9–15). | |
| COPD assessment-test classificationa | Low | 5 |
| Medium | 8 | |
| Very high | 2 | |
| VQ11 scoresc | 23 (18–32) | |
| Bad health-related-quality-of-lifea | 8 | |
| Last exacerbation (day)c | 120 (60–365) | |
| Age (years)b | 71 ± 6 (67–74) | |
| Height (m)c | 1.67 (1.65–1.70) | |
| Obesity statusa | Underweight | 4 |
| Normal weight | 3 | |
| Overweight | 6 | |
| Obese | 2 | |
| Severity of bronchial obstructiona | Mild to moderate | 7 |
| Severe to very severe | 8 | |
| Post bronchodilator spirometric datab | FEV1/FVC (absolute value) | 0.54 ± 0.13 (0.47–0.61) |
| FEV1 (%predicted) | 45% ± 18% (35–55 | |
| FVC (%predicted) | 70% ± 16% (61%–79%). | |
| Refined ABCD assessment toola | A | 0 |
| B | 2 | |
| C | 1 | |
| D | 12 |
Note. COPD = chronic obstructive pulmonary disease; FEV1 = first second forced expiratory volume; FVC = forced vital capacity. aNumber; bMean ± SD (95% CI); cMedian (lower–upper quartiles).
The information provided about the ambient temperatures and the humidity throughout the three sessions was presented in Box 1 (see Supplementary Data section).
Table 2 presents some of the patients’ characteristics during the three sessions. Its main conclusions were: (a) There were no significant effect of RF on weight or BMI; (b) there was no significant difference between the three-sessions sleep-duration; and (c) there were significant differences between the three-sessions meal-duration and last BD-use: the End-R session data were significantly higher than those of Before-R and After-R.
Table 2.
Characteristics of the 15 Stable Male COPD Patients.
| Before-Ramadan | End-Ramadan | After-Ramadan | ANOVA | |
|---|---|---|---|---|
| Weight (kg)a | 71 ± 19 (61–81) |
71 ± 19 (60–82) |
72 ± 19 (61–82) |
0.679 |
| Body mass index (kg/m2)a | 25.4 ± 7.2 (21.4–29.4) |
25.4 ± 7.4 (21.3–29.6) |
25.7 ± 7.4 (21.6–29.8) |
0.678 |
| Last bronchodilator-use (h)a | 6 ± 2 (5–7) |
13 ± 2 (12–15) |
5 ± 2 (4–6) |
0.0001*c,d |
| Sleep-duration (h)b | 7(5–7) | 7(5–8) | 6 (6–7) | 0.864 |
| Meal-duration (h)b | 2(2–6) | 12(10–14) | 1(1–5) | <0.0001*c,d |
Note. ANOVA = analysis of variance; COPD = chronic obstructive pulmonary disease. aMean ± SD (95% CI); bMedian (lower-upper quartiles).
p < .05: Friedman ANOVA between the 3 sessions.
p < .05 (Wilcoxon test): cBefore-Ramadan vs. End-Ramadan; dEnd-Ramadan vs. After-Ramadan.
Table 3 presents the patients’ spirometric data expressed in percentages of predicted values. Merely the MMEF data were significantly modified by the RF: the After-R session data were higher than that Before-R.
Table 3.
Spirometric Data of the 15 Stable COPD Patients.
| Before-Ramadan | End-Ramadan | After-Ramadan | ANOVA | |
|---|---|---|---|---|
| FEV1 | 42 ± 15 | 40 ± 17 | 41 ± 16 | 0.420 |
| FVC | 66 ± 15 | 64 ± 16 | 64 ± 17 | 0.420 |
| FEV1/FVC | 67 ± 14 | 67 ± 16 | 68 ± 15 | 0.420 |
| PEF | 41 ± 13 | 37 ± 16 | 36 ± 13 | 0.090 |
| MMEF | 16 ± 8 | 17 ± 9 | 18 ± 9 | 0.006*a |
Note. ANOVA = Friedman analysis of variance between the three sessions; COPD = chronic obstructive pulmonary disease; FEV1 = first second forced expiratory volume; FVC = forced vital capacity; MMEF = maximal mid expiratory flow; PEF = peak expiratory flow. Data expressed in percentages of predicted values, were mean ± SD.
p < .05: Friedman ANOVA between the 3 sessions.
p < .05 (Wilcoxon test): aBefore-R vs. After-Ramadan.
Table 4 exposes the patients’ blood data. Hb, Ht, RBC and WBC were significantly modified by the RF. First, the End-R session values were lower than those of Before-R. Second, the Hb, Ht and RBC After-R session values were lower than those of the Before-R ones. Thirdly, the After-R session Hb value was lower than that of the End-R one. Fourthly, the End-R session WBC value was lower than that of the After-R one.
Table 4.
Blood Data of the 15 Stable COPD Patients.
| Before-Ramadan | End-Ramadan | After-Ramadan | ANOVA | |
|---|---|---|---|---|
| Hemoglobin (g/dl) | 14.4 ± 2.20 14.3 (12.5–15.1) |
13.4 ± 1.3 13.5 (12.0–13.9) |
12.2 ± 0.9 12.3 (11.9–12.9) |
0.0001*abc |
| Hematocrit (%) | 45 ± 7 44 (40–48) |
40 ± 4 40 (37–43) |
39 ± 4 38 (35–42) |
0.0001*ab |
| Red blood cells (106/mm3) | 5.1 ± 1.0 4.9 (4.6–5.4) |
4.6 ± 0.7 4.5 (4.1–5.0) |
4.4 ± 0.5 4.5 (4.0–4.8) |
0.0001*ab |
| Mean-corpuscular volume (fl) | 88.0 ± 5.1 88.0 (85.0–92.0) |
88.4 ± 5.7 89.7 (84.3–92.0) |
88.5 ± 5.8 89.1 (86.7–93.3) |
0.950 |
| Mean-corpuscular hemoglobin (pg) | 28.1 ± 2.4 29.0 (26.0–30.0) |
29.2 ± 2.4 29.1 (28.0–30.8) |
28.1 ± 2.8 27.9 (25.7–30.6) |
0.145 |
| White blood cells (/mm3) | 8, 673 ± 1,911 7,900 (7,700–9,700) |
7,840 ± 1,526 7,300 (6,900–8,500) |
9,507 ± 2,190 9,600 (7,800–11,300) |
0.0026*ac |
| Platelets (/mm3) | 2,26,000 ± 58,549 2,31,000 (1,76,000–2,78,000) |
2,46,200 ± 65,693 2,47,000 (2,02,000–2,94,000) |
2,44,400 ± 66,646 2,51,000 (1,76,000–2,86,000) |
0.534 |
Note. ANOVA = Friedman analysis of variance between the three sessions. COPD = chronic obstructive pulmonary disease. Data were expressed as mean ± SD and median (lower-upper quartiles).
p < .05: Friedman ANOVA between the 3 sessions.
p < .05 (Wilcoxon test): aBefore-Ramadan vs. End-Ramadan; bBefore-Ramadan vs. After-Ramadan; cEnd-Ramadan vs. After-Ramadan.
Figures 1 and 2 display, respectively, the patients’ ESR and CRP values during the three sessions. The median (lower-upper quartiles) of ESR [Before-R: 3 (2-9), End-R: 7 (0-13), After-R: 9 (5-15) mm/h] and CRP [Before-R: 20 (11-38), End-R: 15 (9-34), After-R: 20 (12-46), mg/L] were not significantly influenced by RF.
Figure 1.
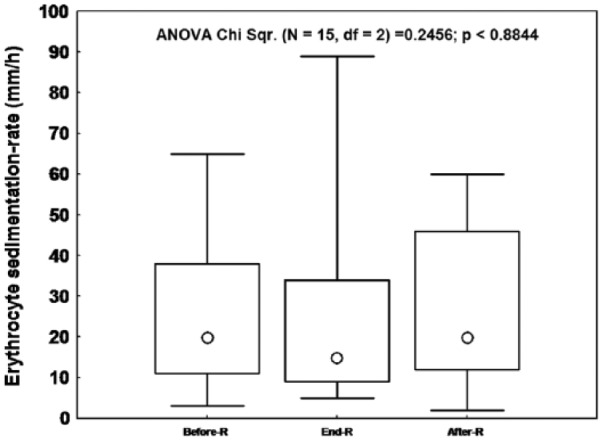
The effects of Ramadan (R) fasting on the erythrocyte sedimentation-rate of the 15 stable chronic obstructive pulmonary disease patients.
Data were median (Ο), lower–upper quartiles (□) and minimum-maximum (I). p: Friedman analysis of variance between the 3 sessions.
Figure 2.
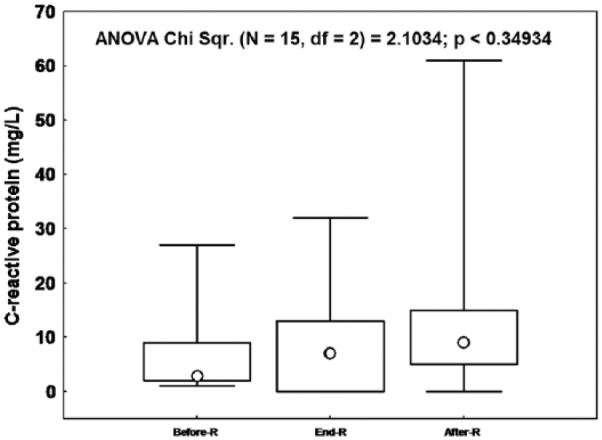
The effects of Ramadan (R) fasting on the C-reactive protein of the 15 stable chronic obstructive pulmonary disease patients.
Data were median (Ο), lower–upper quartiles (□) and minimum-maximum (I). p: Friedman analysis of variance between the 3 sessions.
The numbers of patients with anemia [Before-R (n = 4), End-R (n = 4), After-R (n = 12)] were significantly influenced by RF (Cochrane test = 14.22; p = .001). The difference was statistically significant between the After-R session and the Before-R or the End-R sessions (both p = .008).
The numbers of patients with leukocytosis [Before-R (n = 2), End-R (n = 1), After-R (n = 5), Cochrane test = 4.333; p = .115] or with biological inflammation syndrome [Before-R (n = 7), End-R (n = 5), After-R (n = 6), Cochrane test = 3.00; p = .223] weren’t significantly influenced by RF.
Discussion
This pilot study addressed the effects of a pious ritual on a group of 15 stable male COPD patients. It identified that RF did not bring about any significant variations in CRP and ESR data. Among all the hematological indices, RF influenced Hb, Ht, RBC and WBC: compared to the Before-R session, the End-R session values were lower. Compared to the After-R session, the End-R session Hb and WBC values were, respectively, higher and lower.
Ramadan has great communal value among Muslims all over the sphere. During this holy month Muslims are expected to refrain from drinking, eating, smoking and having sex, from suhoor to iftar (Sarraf-Zadegan et al., 2000). Although Islam exempts patients from fasting, a great number of them obviously fast, and this can cause their clinical circumstance to worsen due to a persistent gap between up-to-date expert information and decisive robust evidence regarding the pathophysiologic and metabolic modifications of fasting (Nematy et al., 2015). In humans, it seems that fasting helps reduce obesity, and helps treat hypertension and rheumatoid arthritis (Longo & Mattson, 2014). Moreover, fasting has the potential to delay aging, to aid prevent, and treat diseases, and to reduce the side effects caused by chronic dietary interventions (Longo & Mattson, 2014). For example, in a study of patients with a variety of malignancies, the combination of chemotherapy with fasting resulted in a decrease in a range of self-reported common side effects caused by chemotherapy compared to the same patients receiving chemotherapy while on a standard diet (Safdie et al., 2009). To the best of the authors knowledge, while the effects of RF on inflammatory and/or hematological indices were evaluated in several studies including healthy subjects (Al Hourani et al., 2009; Azizi, 2002; Dewanti et al., 2006; El-Hazmi et al., 1987; Nasiri et al., 2016; Sarraf-Zadegan et al., 2000; Sedaghat et al., 2017), only one study included asthmatics (Askari et al., 2016). The main characteristics and results of some of the above studies (Al Hourani et al., 2009; Askari et al., 2016; Sarraf-Zadegan et al., 2000; Sedaghat et al., 2017) are detailed in Tables 1S and 2S in the Supplementary Data. It seems that this study is the first one to treat such an issue in COPD patients.
Discussion of Methodology
Discussion concerning the season and the duration of RF, the timing of achieved tests and the number of sessions carried out were addressed in previous papers (Latiri et al., 2017; Zouari et al., 2018). Discussion about the spirometric measurements is available in the Supplementary Data section. Only the study design, the sample size, the subjects’ characteristics, the choice of inflammatory and hematological indices are discussed in the following sentences.
This study was an experimental one, as previously adopted in a local work aiming to evaluate the effects of RF on spirometric data of stable COPD patients (Zouari et al., 2018). One similar study, aiming to evaluate the effects of RF on hematological and inflammatory indices opted for a comparative design including asthmatics and healthy subjects (Askari et al., 2016). Cross-sectional studies are frequently used to check popular hypotheses about certain “risk factors” and their associations to disease (Suresh, Suresh, & Thomas, 2012).
The calculated sample size of this pilot study (n = 15) ‘‘seemed’’ to be reasonable. It is nearer to two similar ones aiming to evaluate the effects of RF either on spirometric data of COPD patients (n = 16) (Zouari et al., 2018) or on inflammatory and hematological indices of 15 asthmatics and 14 healthy subjects (Askari et al., 2016). However, it was lower than the sample sizes of some studies investigating the effects of RF on hematological indices of healthy adults [n = 22 males (Sarraf-Zadegan et al., 2000), n = 57 females (Al Hourani et al., 2009) and n = 51 males (Sedaghat et al., 2017)]. Due to its pilot design, the present study findings are preliminary, and should be useful in guiding researchers looking for the effects of RF on biological indices of COPD patients.
The mean age of our patients (71 ± 6 years) was similar to these of COPD patients included in North-African studies [e.g., 65 ± 8 years (Mosrane et al., 2017), 64 ± 7 years (Zouari et al., 2018), 61 ± 4 years (Rejbi et al., 2010)]. Nevertheless, the COPD patients of this study were older than asthmatic ones (49 ± 12 years) (Askari et al., 2016) or healthy subjects [18 to 29 years (Al Hourani et al., 2009), 30 to 45 years (Sarraf-Zadegan et al., 2000), 35 ± 9 years (Sedaghat et al., 2017)]. The frequencies of patients with low socioeconomic (7/15) or schooling levels (10/15) were similar to those reported in a previous local study (Zouari et al., 2018) (8/16 and 10/16, respectively). The mean tobacco consumption (73 ± 44 PY) was in-between these reported in previous local studies including COPD patients (e.g., 75 ± 32 PY (Ben Saad et al., 2014), 69 ± 38 PY (Ben Saad et al., 2008), 64 ± 27 PY (Ben Saad et al., 2014), 52 ± 31 PY (Mosrane et al., 2017)), but was higher than those reported by Zouari et al. (2018) (36 ± 27 PY). An important inclusion criterion should be highlighted (Ben Saad, 2016). It concerns the inclusion of only ex-smokers COPD patients, as done in one study (Zouari et al., 2018). This inclusion criterion could clarify some observed results. On the one hand, COPD systemic inflammation depends on the smoking status (Mosrane et al., 2017). On the other hand, smokers, compared with ex-smokers, are oversensitive during Ramadan (Kadri et al., 2000) and psychological stress affected the systemic inflammation (Lazzarino et al., 2016) and pulmonary function (Kang & Fox, 2000). Medical background of included COPD patients (Table 1) was in line with literature. For example, the frequencies of cardiovascular diseases, dyspnea, anemia and dyslipidemia, were respectively, 39% (Mosrane et al., 2017), 100% (Mosrane et al., 2017), 6 to 46% (Hoepers et al., 2015) and 6% (Zouari et al., 2018). As done by Zouari et al. (2018), a minimal of 20-years RF experience was applied as an inclusion criterion. This point neglected by some authors (Al Hourani et al., 2009; Askari et al., 2016; Sarraf-Zadegan et al., 2000; Sedaghat et al., 2017), could influence the subjects hematological and biological (Bragazzi, 2015; Trabelsi, Stannard, Shephard, Jamoussi, & Hakim, 2014) or respiratory (Fenneni et al., 2015) adaptations. While, oral medications, injections, ear and nose drops and suppositories are not allowed for patients who prefer fasting, the use of eye drops and inhalers are accepted as a process that does not invalidate the fast (Official Website of The Presidency of Religious Affairs of the Republic of Turkey, n.d.). Even with this information, numerous COPD patients change their medication routines during RF without consulting their physician (Fazel, 1998). For example, in a Turkish study, the majority of the COPD patients abandoned using their medications during Ramadan (Aydin et al., 2014). In this study, patients were asked about whether to keep using their usual medication or to regulate it to suhoor and iftar times. It seems that all inhaled medications used at the overhead times are not predictable to reduce the efficacy of the drugs and this method appears to be suitable for patients who refuse to use their drugs during fasting hours (Aadil, Houti, & Moussamih, 2004; Aydin et al., 2014).
CRP is an acute-phase protein that originates predominantly from hepatocytes in response to tissue damage or inflammation and, therefore, reflects the total systemic burden of inflammation of individuals’ infections (Pepys & Hirschfield, 2003). CRP is associated with an increased risk of incident cardiovascular diseases (Ridker, 2003) and is identified as an important protein related to the systemic inflammatory process during COPD (Dahl et al., 2007). Additional information concerning CRP is available in the Supplementary Data section. In asthmatic patients, Askari et al. (2016) opted for the use of high-sensitivity CRP (hs-CRP). However, it seems that CRP standard assay presents a reasonable alternative to hs-CRP (Helal et al., 2012).
ESR is a potential marker of COPD-related systemic inflammation (Corsonello et al., 2011). Yet, it is not related to COPD severity, but it might be more useful as a marker of COPD exacerbations (Corsonello et al., 2011). Askari et al. (2016) evaluated ESR at the first (ESR-1) and second (ESR-2) hours. It seems that ESR-2 has proved to have a higher sensitivity than the ESR-1 value (Putzki & Reichert, 1987). In practice, other biomarkers were used in patients with respiratory conditions (e.g., interleukins, tumor necrosis factor-α, fibrinogen) (Askari et al., 2016; Hoepers et al., 2015). Measured hematological indices, as explored by some authors (Al Hourani et al., 2009; Askari et al., 2016; Sarraf-Zadegan et al., 2000; Sedaghat et al., 2017), were WBC, RBC, Ht, Hb, MCV, MCH, and Plt. A systematic review indicated that anemia contributes to the pathogenesis of COPD (Hoepers et al., 2015). As highlighted by Askari et al. (2016), it was better to proceed to differential cell counts: neutrophil, lymphocyte, monocyte and eosinophil. Some other interesting hematological indices should be evaluated in future studies [e.g., blood ferritin, an acute phase inflammatory marker in COPD (Hoepers et al., 2015), certain hemostatic, fibrinolytic, platelet aggregation and coagulation factors (Sarraf-Zadegan et al., 2000), considered as coronary artery disease risk factors].
Effects of RF on Weight
Relative to Before-R data, there was no significant effect of RF on weight (Table 2). These findings correlated with a previous study evaluating the effects of RF on the lung function data of COPD patients (Zouari et al., 2018). In Askari et al. study (2016), data concerning weight were lacking (Table 2S). A systematic review, investigating the effects of RF on weight, concluded that RF could result in slight but significant weight loss (−1.24 kg) and most of the weight loss was recovered within few weeks After-R (Sadeghirad, Motaghipisheh, Kolahdooz, Zahedi, & Haghdoost, 2014).
Effects of RF on spirometric data
Relative to Before-R data, RF disturbed only peripheral flows, such as MMEF. Studies investigating the RF effects on the spirometric data of patients with chronic respiratory conditions are scarce. As far as it is known, six studies were published [five included asthmatics (Adeli et al., 2015; Askari et al., 2016; Baay, 2017; Bener et al., 2006; Norouzy et al., 2013) and one included COPD patients (Zouari et al., 2018)]. Most studies performed on asthmatics concluded that they could tolerate the fast without significant modification of their spirometric data. However, one study (Norouzy et al., 2013) identified a significant increase in PEF during the End-R session compared to Before-R. An additional study (Baay, 2017) reported that RF seem to have detrimental effects as FEV1 and “forced expiratory flow rate at the 50% of FVC to be exhaled” demonstrated statistically significant differences Before- and After-R. This study results partly confirm these of Zouari et al. (2018) who concluded that RF did not bring about any significant changes in the spirometric data of stable COPD male patients, including the MMEF. The above issue was discussed in two previous studies including healthy or COPD adults (Latiri et al., 2017; Zouari et al., 2018).
Effects of RF on Inflammatory Data
RF did not bring about any significant changes in inflammatory biomarkers (ESR and CRP) (Figures 1 and 2). Moreover, the frequencies of subjects with biological inflammation were similar between the three sessions.
Given the pioneering nature of this study, only the data from a recent one (Askari et al., 2016) aiming at evaluating the impact of RF on inflammatory biomarkers of asthmatics will be reported. The abovementioned study reported that compared to Before-R, After-R CRP value was significantly reduced while no significant changes were noted for ESR-1 or ESR-2 values. When compared with the control-group, a significant change was noted for ESR-2: it was reduced during the After-R session. Although Askari et al. (2016) reported statistically significant changes in inflammatory marker-values, the readers were not sure that values were outside the normal laboratory reference range.
In a study (Johnson et al., 2007) where 10 asthmatic patients with a BMI > 30 kg/m2 were maintained for two months on a dietary regimen (they ate ad libitum every other day, while consuming less than 20% of their normal calorie intake on the intervening days), it was reported that serum markers of inflammation were reduced over a period of 2-4 weeks and that alternate day intermittent fasting resulted in significant reductions in serum tumor necrosis factor-α during the 2-month period. Thus, it seems that for many subjects able and willing to endure long-term fasting and to permanently modify their diet, fasting cycles would have the potential not only to increase but also to replace existing medical treatments.
Effects of RF on Hematological Indices
There was a significant effect of RF on Hb, Ht, RBC and WBC (Table 4). While, the frequencies of patients with anemia significantly increased between the Before- or the End-R sessions when compared to the After-R one, those of patients with leukocytosis were similar between the three sessions.
In healthy subjects, studies about the effects of RF on hematological indices, reported in the literature, have been conflicting and inconsistent (Al Hourani et al., 2009; Azizi, 2002; Dewanti et al., 2006; El-Hazmi et al., 1987; Nasiri et al., 2016; Sarraf-Zadegan et al., 2000; Sedaghat et al., 2017). For examples, while some studies (Al Hourani et al., 2009; Azizi, 2002; Sarraf-Zadegan et al., 2000), noted that RBC, Hb and Ht remained unchanged, others stated either a slight degree of hemoconcentration (El-Hazmi et al., 1987) or a significant decrease in Hb and Ht (Dewanti et al., 2006). Some studies identified a significant reduction in the Plt count (Al Hourani et al., 2009; Ramadan, Mousa, & Telahoun, 1994). Three studies are detailed in Tables 1S and 2S. In the study conducted by Sarraf-Zadegan et al. (2000), it was reported that compared to After-R data, the End-R mean levels were significantly higher for Hb, Ht and RBC; were significantly lower for Plt and were similar for WBC. In their study, Al-Hourani et al. (2009) concluded that compared to Before-R data; the Mid-R mean levels were significantly lower for Plt and were similar for Hb, Ht and RBC. In Sedaghat et al. study (2017), however, it was proved that compared to Before-R data, the After-R mean levels were significantly lower for Ht and MCV; were significantly higher for MCH and were similar for Hb, RBC, WBC and for Plt.
Given the pioneering nature of this study, only the data reported by Askari et al. (2016) in their study aiming at evaluating the impact of RF on hematological indices of asthmatics was reported in Table 2S. In the abovementioned study, and contrary to this one, compared to Before-R data, the After-R mean values were similar for RBC, Ht, Hb and WBC; and were significantly increased and reduced, respectively, for MCH and Plt. Concerning MCV data, similar results were confirmed by Askari et al. (2016) when comparing Before-R vs. After-R data. In this study, unlike Askari et al. (2016), differential WBC counts (neutrophil, lymphocyte, monocyte and eosinophil) were not evaluated. Askari et al. (2016) identified a significant difference only for the monocyte values: compared to the Before-R, the After-R monocyte values were significantly increased. When comparing the asthmatic and the control-group data, Askari et al. (2016) noted a significant change in WBC values determined during the After-R session: they were higher in the asthmatic group compared to the control (7,769 ± 1,116 vs. 6,572 ± 1,046/mm3, respectively).
How Can Changes in Hematological Indices During RF Be Explained?
Three hypotheses could be advanced to explain the decreases of Hb, Ht, RBC and WBC values during the After-R session when compared to the Before-R one (Table 4). The first one concerns the hydration status of the COPD patients (Hackney, Coyne, Pozos, Feith, & Seale, 1995) (Oppliger & Bartok, 2002). It seems “likely” that our patients were hyper-hydrated (hemodilution) during the End-R session. When compared with the Before- and/or the End-R sessions, the After-R one was characterized by a significantly higher ambient temperature and significantly lower humidity (Box 1, Supplementary Data section), and then a probably high water consumption. Nevertheless, the stability of the patients’ weight during the three sessions is against the above hypothesis. The second hypothesis concerns the way the meal is served. Previous studies reported that some serum data (e.g., cholesterol) was significantly higher when the daily food intake was served as one large meal (case of Ramadan month) instead of when it was divided into small meals (Fabry, Hejl, Fodor, Braun, & Zvolankova, 1964; Irwin & Feeley, 1967). The same phenomenon could appear for some hematological data (e.g., Hb, Ht and RBC). The third hypothesis concerns the nutritional status and/or the type of food consumed during Ramadan (Maughan et al., 2008; Sedaghat et al., 2017). During the holy month, there is a tendency towards increased consumption of fat and carbohydrate (mainly sucrose, especially to interrupt the fast and during the night). The significant decrease of Hb level during the End-R and the After-R sessions (Table 4) and the significant increase of the frequency of patients with anemia (After-R vs. Before-R or End-R) could be explained by a plausible low iron and/or acid folic and/or vitamin B12 diet during Ramadan (Anderson & Frazer, 2017; DeLoughery, 2017; Lall, Singh, Gulati, & Seth, 1999). Based on the previous literature, the most common nutritional deficiency responsible for anemia are iron and/or acid folic and/or vitamin B12 deficiency (Born, Elmadfa, & Schmahl, 1979). Since there was no significant effect of RF on the inflammatory status, the anemia cannot be qualified as “inflammatory” (Hoepers et al., 2015).
Study limitations
This pilot study presents five limitations. First, as reported in some relative studies (Table 1S), the convenience sampling was a main confounding factor (Sousa, Zauszniewski, & Musil, 2004). Convenience sampling is a type of nonprobability sampling method based on the judgment of the investigator (Sousa et al., 2004). It can lead to the under/over representation of specific groups inside the sample (eg; 12/15 patients belonged to group D, Table 1) and the ability to make generalizations from the present sample to the population being studied. While convenience sampling should be treated with attention, its low charge and comfort of use make it a favored choice for a significant number of investigators (Table 1S). It was much more efficient to apply for a sample random sampling study, as done by Sarraf-Zadegan et al. (2000) (Table 1S). Second, the noninclusion of a control-group of nonfasting patients could be considered a limitation because the interior validity of the results from this study and the variations in the data assessed cannot be attributed exclusively to RF (Latiri et al., 2017). In this regard, it has to be underlined that attaining nonfasting groups in Muslim countries is problematic, due to religious principles. Indeed, Aydin et al. (2014) reported that asthmatic or COPD patients do not feel their conditions to be an inhibitory factor when fasting during Ramadan. Nevertheless, among the 18 studies evaluating the effects of RF on the lung function and/or inflammatory and/or hematological data of healthy and/or asthmatics and/or COPD adults, only two achieved in India and Iran (Askari et al., 2016; Singha Roy & Bandyopadhyay, 2016) involved control-groups. The remaining 16 studies conducted in Muslim and non-Muslim states [Iran (Adeli et al., 2015; Moosavi, Kabir, Moghimi, Chehrei, & Rad, 2007; Norouzy et al., 2013; Sarraf-Zadegan et al., 2000; Sedaghat et al., 2017; Soori, Mohaghegh, Hajain, & Moraadi, 2016), Saudi-Arabia (Siddiqui, Sabir, & Subhan, 2005; Subhan, Siddiqui, Khan, & Sabir, 2006); Tunisia (Latiri et al., 2017; Zouari et al., 2018), Malaysia (Duncan, Husain, Raman, Cheah, & Ch’ng, 1990), Egypt (Abdel-aziz & Ibraheem, 2008), Jordan (Al Hourani et al., 2009), Irak (Baay, 2017), Qatar (Bener et al., 2006), India (Sayeed, Hazari, & Arifuddin, 2018)] used Before-R or During-R data as control. Thirdly, it was desirable to assess the COPD patients’ nutrition. In fact, dietary aberrations could mark the inflammatory and/or hematological systems and the lung structure and/or function (Anderson & Frazer, 2017; DeLoughery, 2017; Sedaghat et al., 2017). Fourthly, it was better to re-evaluate the CAT (Jones et al., 2009) and the VQ11 (Ninot et al., 2010) data during the second and third session. This re-evaluation could define the impact of RF on COPD respiratory symptoms and health-related-quality-of-life. Fifthly, it was desirable to estimate the physical activity status of the patients since it impacts the inflammatory and/or hematological systems and/or respiratory function (Ben Saad et al., 2008; Laveneziana & Palange, 2012; Nguyen et al., 2014). For example, moderate intensity and short duration exercise attenuate the inflammatory response (“anti-inflammatory” effect) (Laveneziana & Palange, 2012). However, in physically active individuals, the reported effects of RF on Ht and Hb have not been consistent (Trabelsi et al., 2014).
Recommendations
Patients with stable COPD who want to fast during Ramadan are encouraged to consult their physician before-R to get personalized medical advice. In order to prevent anemia, physicians should advice their COPD patients to consume foods containing iron and/or folic acid and/or vitamin B12.
In future, research challenges should be made to abolish or reduce the effects of numerous confounders as it is only through alert control within the research design that consistent outcomes pertaining to the health effects of RF will be achieved. The authors recommend a large-scale coordinated studies, with standardization of research methods regarding the nutritional status and physical activity level of the patients, in order to explore the issue more comprehensively.
To conclude, this study including 15 stable COPD patients fasting the 2017 holy month of Ramadan, reported significant effects of RF on Hb, Ht, RBC, and WBC values. However, RF did not induce any significant changes in inflammatory (ESR and CRP) and other hematological (Plt, MCV, and MCH) indices. These findings emphasize the need for practitioners to take into account the patients’ religious rituals and beliefs when providing medical care. This study highlights the need to consider the hematological changes when physicians investigate COPD patients’ cases during and after the month of Ramadan.
Supplemental Material
Supplemental material, Supplementary_Data_CLEAN for The Effects of Ramadan-Fasting (RF) on Inflammatory and Hematological Indices of Stable Chronic Obstructive Pulmonary Disease (COPD) Male Patients: A Pilot Study by Hadhemi REJEB, Mouna BEN KHELIFA, Jihene BEN ABDALLAH, Sawssan MRAD, Mohamed BEN REJEB, Abdelaziz HAYOUNI, Mohamed BENZARTI, Khelifa LIMEM, Mondher KORTAS, Sonia ROUATBI and Helmi BEN SAAD in American Journal of Men’s Health
Acknowledgments
Authors wish to express their sincere gratitude to all participants for their cooperation. They also wish to thank Doctors Zeineb JAIDANE, Raoudha SFAXI, Radhia ZAYANI, and Nesrine ZAATIR for their help.
Footnotes
List of Abbreviations: ANOVA: Analysis of variance
BD: Bronchodilator
BMI: Body mass index
CAT: COPD assessment-test
CI: Confidence interval
COPD: Chronic obstructive pulmonary disease
CRP: C-reactive protein
ESR: Erythrocyte sedimentation rate
FEV1: First second forced expiratory volume
FVC: Forced vital capacity
GOLD: Global initiative for chronic obstructive lung disease
Hb: Hemoglobin
Ht: Hematocrit
MCH: Mean corpuscular hemoglobin
MCV: Mean corpuscular volume
MMEF: Maximal mid-expiratory flow
mMRC: Modified British medical research council
PBD: Post-bronchodilator
PEF: Peak expiratory flow
Plt: Platelets
PY: Pack-years
R: Ramadan
RBC: Red blood cell
RF: Ramadan-fasting
SD: Standard deviation
WBC: White blood cell
Authors’ Contributions: HR, MBK and HBS: Literature search, Data collection, Study design, Analysis of data, Manuscript preparation, and Review of manuscript.
JBA, SM and MBR: Study design, Analysis of data, Manuscript preparation, and Review of manuscript.
AH, MB, KL, MK and SR: Analysis of data, Manuscript preparation, and Review of manuscript.
All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HBS reports personal fees from AstraZeneca, Boehringer Ingelheim, INPHA-MEDIS and Chiesi. The remaining authors declare that they have no conflicts of interest concerning this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material is available for this article online.
ORCID iD: Helmi BEN SAAD  https://orcid.org/0000-0002-7477-2965
https://orcid.org/0000-0002-7477-2965
References
- Aadil N., Houti I. E., Moussamih S. (2004). Drug intake during Ramadan. British Medical Journal, 329(7469), 778–782. doi: 10.1136/bmj.329.7469.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-aziz I., Ibraheem A. (2008). Fasting during Ramadan: Does it alter pulmonary functions in healthy males? Al-Azhar Assiut Medical Journal, 6(3), 53−63. [Google Scholar]
- Abramson N., Melton B. (2000). Leukocytosis: Basics of clinical assessment. American Family Physician, 62(9), 2053–2060. [PubMed] [Google Scholar]
- Adeli S. H., Aghaali M., Nasab J. M. (2015). Studying the effects of fasting during Ramadan on pulmonary function test and asthma severity. Health Spirituality and Medical Ethics, 2, 2–5. [Google Scholar]
- Al Hourani H., Atoum M., Akel S., Hijjawi N., Awawdeh S. (2009). Effects of Ramadan fasting on some haematological and biochemical parameters. Jordan Journal of Biological Sciences, 2(3), 103–108. [Google Scholar]
- Anderson G. J., Frazer D. M. (2017). Current understanding of iron homeostasis. The American Journal of Clinical Nutrition, 106(Suppl. 6), 1559S–1566S. doi: 10.3945/ajcn.117.155804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari V. R., Alavinezhad A., Boskabady M. H. (2016). The impact of “Ramadan fasting period” on total and differential white blood cells, haematological indices, inflammatory biomarker, respiratory symptoms and pulmonary function tests of healthy and asthmatic patients. Allergologia et Immunopathologia, 44(4), 359–367. doi: 10.1016/j.aller.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Aydin O., Celik G. E., Onen Z. P., Yilmaz I., Ozdemir S. K., Yildiz O., … Demirel Y. S. (2014). How do patients with asthma and COPD behave during fasting? Allergologia et Immunopathologia, 42(2), 115–119. doi: 10.1016/j.aller.2012.07.010 [DOI] [PubMed] [Google Scholar]
- Azizi F. (2002). Research in Islamic fasting and health. Annals of Saudi Medicine, 22(3–4), 186–191. [DOI] [PubMed] [Google Scholar]
- Baay A. S. (2017). Study of the fasting of Ramadan on asthmatic patients and the outcome with treatment modification. Medical Journal of Babylon, 14(1), 216–224. doi:1812-156X-14-1 [Google Scholar]
- Barkia A., Mohamed K., Smaoui M., Zouari N., Hammami M., Nasri M. (2011). Change of diet, plasma lipids, lipoproteins, and fatty acids during Ramadan: A controversial association of the considered Ramadan model with atherosclerosis risk. Journal of Health, Population, and Nutrition, 29(5), 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bener A., Colakoglu B., Mobayed H., El Hakeem A., Al Mulla A. A., Sabbah A. (2006). Does hospitalization for asthma and allergic diseases occur more frequently in Ramadan fasting: A population based study (2000–2004). European Annals of Allergy and Clinical Immunology, 38(4), 109–112. [PubMed] [Google Scholar]
- Ben Saad H. (2016). Pulmonary function of young Muslim males during the month of Ramadan: Some points to highlight. American Journal of Men’s Health. Advance online publication. doi: 10.1177/1557988316662840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Saad H., Ben Amor L., Ben Mdalla S., Ghannouchi I., Ben Essghair M., Sfaxi R., … Rouatbi S. (2014. a). The importance of lung volumes in the investigation of heavy smokers. Revue des Maladies Respiratoires, 31(1), 29–40. doi: 10.1016/j.rmr.2013.05.009 [DOI] [PubMed] [Google Scholar]
- Ben Saad H., Ben Amor L., Ben Mdella S., Ghannouchi I., Ben Essghair M., Bougmiza I., … Rouatbi S. (2014. b). The diagnosis of COPD is recommendation dependent. La Tunisie Medicale, 92(7), 474–481. [PubMed] [Google Scholar]
- Ben Saad H., El Attar M. N., Hadj Mabrouk K., Ben Abdelaziz A., Abdelghani A., Bousarssar M., … Rouatbi S. (2013). The recent multi-Ethnic Global Lung Initiative 2012 (GLI2012) reference values don’t reflect contemporary adult’s North African spirometry. Respiratory Medicine, 107(12), 2000–2008. doi: 10.1016/j.rmed.2013.10.015 [DOI] [PubMed] [Google Scholar]
- Ben Saad H., Hamadou R., Ben Cheikh I., Chouchene A., Rejeb N., Zbidi A., Tabka Z. (2008. a). Respiratory rehabilitation of chronic obstructive pulmonary disease patients: Preliminary data of Tunisian experience. Journal de Réadaptation Médicale : Pratique et Formation en Médecine Physique et de Réadaptation, 28(4), 138–147. doi: 10.1016/j.jmr.2008.09.001 [DOI] [Google Scholar]
- Ben Saad H., Préfaut C., Tabka Z., Zbidi A., Hayot M. (2008. b). The forgotten message from GOLD: FVC is a primary clinical outcome measure of bronchodilator reversibility in COPD. Pulmonary Pharmacology & Therapeutic, 21(5), 767–773. doi: 10.1016/j.pupt.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Born M., Elmadfa I., Schmahl F. W. (1979). Effects of periodical fluid and food withdrawal: An inquiry conducted during the lenten month Ramadan on foreign workers. MMW, Munchener Medizinische Wochenschrift, 121(47), 1569–1572. [PubMed] [Google Scholar]
- Bottiger L. E., Svedberg C. A. (1967). Normal erythrocyte sedimentation rate and age. British Medical Journal, 2(5544), 85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden A., Bolton E., Gemeroy D. (1947). Precipitin testing with special reference to the photoelectric measurement of turbidity. Journal of Immunology, 57(3), 211–227. [PubMed] [Google Scholar]
- Bragazzi N. L. (2015. a). Ramadan fasting and biological biomarkers: The new opportunities of systems biology and omics sciences. In Chtourou H. (Ed.), Effects of Ramadan fasting on health and athletic performance (pp. 86–90). Retrieved from http://www.esciencecentral.org/ebooks/effects-of-ramadan-fasting/biological-biomarkers.php
- Cappellini M. D., Motta I. (2015). Anemia in clinical practice-definition and classification: Does hemoglobin change with aging? Seminars in Hematology, 52(4), 261–269. doi: 10.1053/j.seminhematol.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Celli B. R., Decramer M., Wedzicha J. A., Wilson K. C., Agusti A., Criner G. J. … ATS/ERS Task Force for COPD Research. (2015). An official American thoracic society/European respiratory society statement: Research questions in COPD. European Respiratory Journal, 45(4), 879–905. doi: 10.1183/09031936.00009015 [DOI] [PubMed] [Google Scholar]
- Colombet I., Pouchot J., Kronz V., Hanras X., Capron L., Durieux P., Wyplosz B. (2010). Agreement between erythrocyte sedimentation rate and C-reactive protein in hospital practice. The American Journal of Medicine, 123(9), 863.e7–863.e13. doi: 10.1016/j.amjmed.2010.04.021 [DOI] [PubMed] [Google Scholar]
- Corsonello A., Pedone C., Battaglia S., Paglino G., Bellia V., Incalzi R. A. (2011). C-Reactive Protein (CRP) and Erythrocyte Sedimentation Rate (ESR) as inflammation markers in elderly patients with stable Chronic Obstructive Pulmonary Disease (COPD). Archives of Gerontology and Geriatrics, 53(2), 190–195. doi: 10.1016/j.archger.2010.10.015 [DOI] [PubMed] [Google Scholar]
- Cotes J. E., Chinn D. J., Reed J. W. (1997). Lung function testing: Methods and reference values for Forced Expiratory Volume (FEV1) and transfer factor (TL). Occupational and Environmental Medicine, 54(7), 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl M., Vestbo J., Lange P., Bojesen S. E., Tybjaerg-Hansen A., Nordestgaard B. G. (2007). C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 175(3), 250–255. doi: 10.1164/rccm.200605-713OC [DOI] [PubMed] [Google Scholar]
- DeLoughery T. G. (2017). Iron deficiency anemia. The Medical Clinics of North America, 101(2), 319–332. doi: 10.1016/j.mcna.2016.09.004 [DOI] [PubMed] [Google Scholar]
- Dewanti L., Watanabe C., Sulistiawati & Ohtsuka R. (2006). Unexpected changes in blood pressure and haematological parameters among fasting and nonfasting workers during Ramadan in Indonesia. European Journal of Clinical Nutrition, 60(7), 877–881. doi: 10.1038/sj.ejcn.1602393 [DOI] [PubMed] [Google Scholar]
- Duncan M. T., Husain R., Raman A., Cheah S. H., Ch’ng S. L. (1990). Ventilatory function in Malaysian Muslims during normal activity and the Ramadan fast. Singapore Medical Journal, 31(6), 543–547. [PubMed] [Google Scholar]
- El-Hazmi M., Al-Faleh F., Al-Mofleh I. (1987). Effect of Ramadan fasting on the values of haematological and biochemical parameters. Saudi Medical Journal, 8(2), 171–176. [Google Scholar]
- Erkekol F. O., Celik G. E., Keskin O., Gullu E., Mungan D., Misirligil Z. (2006). Fasting: An important issue in asthma management compliance. Annals of Allergy, Asthma & Immunology, 97(3), 370–374. doi: 10.1016/S1081-1206(10)60803-4 [DOI] [PubMed] [Google Scholar]
- Fabry P., Hejl Z., Fodor J., Braun T., Zvolankova K. (1964). The frequency of meals its relation to overweight, hypercholesterolaemia, and decreased glucose-tolerance. Lancet, 284(7360), 614–615. [DOI] [PubMed] [Google Scholar]
- Fazel M. (1998). Medical implications of controlled fasting. Journal of the Royal Society of Medicine, 91(5), 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenneni M. A., Latiri I., Aloui A., Rouatbi S., Chamari K., Ben Saad H. (2015). Critical analysis of the published literature about the effects of Ramadan intermittent fasting on healthy children’s physical capacities. Libyan Journal of Medicine, 10(1), 28351. doi: 10.3402/ljm.v10.28351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris B. G. (1978). Epidemiology standardization project (American Thoracic Society). The American Review of Respiratory Disease, 118, 1–120. [PubMed] [Google Scholar]
- Fletcher C. M., Elmes P. C., Fairbairn A. S., Wood C. H. (1959). The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. British Medical Journal, 2(5147), 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney A. C., Coyne J. T., Pozos R., Feith S., Seale J. (1995). Validity of urine-blood hydrational measures to assess total body water changes during mountaineering in the sub-Arctic. Arctic Medical Research, 54(2), 69–77. [PubMed] [Google Scholar]
- Haouari M., Haouari-Oukerro F., Sfaxi A., Ben Rayana M. C., Kaabachi N., Mbazaa A. (2008). How Ramadan fasting affects caloric consumption, body weight, and circadian evolution of cortisol serum levels in young, healthy male volunteers. Hormone and Metabolic Research, 40(8), 575–577. doi: 10.1055/s-2008-1065321 [DOI] [PubMed] [Google Scholar]
- Helal I., Zerelli L., Krid M., ElYounsi F., Ben Maiz H., Zouari B., … Kheder A. (2012). Comparison of C-reactive protein and high-sensitivity C-reactive protein levels in patients on hemodialysis. Saudi Journal of Kidney Diseases and Transplantation, 23(3), 477–483. [PubMed] [Google Scholar]
- Hoepers A. T., Menezes M. M., Frode T. S. (2015). Systematic review of anaemia and inflammatory markers in chronic obstructive pulmonary disease. Clinical and Experimental Pharmacology & Physiology, 42(3), 231–239. doi: 10.1111/1440-1681.12357 [DOI] [PubMed] [Google Scholar]
- Irwin M. I., Feeley R. M. (1967). Frequency and size of meals and serum lipids, nitrogen and mineral retention, fat digestibility, and urinary thiamine and riboflavin in young women. The American Journal of Clinical Nutrition, 20(8), 816–824. doi: 10.1093/ajcn/20.8.816 [DOI] [PubMed] [Google Scholar]
- Johnson J. B., Summer W., Cutler R. G., Martin B., Hyun D. H., Dixit V. D., … Mattson M. P. (2007). Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radical Biology & Medicine, 42(5), 665–674. doi: 10.1016/j.freeradbiomed.2006.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. W., Harding G., Berry P., Wiklund I., Chen W. H., Kline Leidy N. (2009). Development and first validation of the COPD assessment test. European Respiratory Journal, 34(3), 648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- Kadri N., Tilane A., El Batal M., Taltit Y., Tahiri S. M., Moussaoui D. (2000). Irritability during the month of Ramadan. Psychosomatic Medicine, 62(2), 280–285. [DOI] [PubMed] [Google Scholar]
- Kang D. H., Fox C. (2000). Neuroendocrine and leukocyte responses and pulmonary function to acute stressors. Annals of Behavioral Medicine : A Publication of the Society of Behavioral Medicine, 22(4), 276–285. doi: 10.1007/BF02895663 [DOI] [PubMed] [Google Scholar]
- Kang M., Ragan B. G., Park J. H. (2008). Issues in outcomes research: An overview of randomization techniques for clinical trials. Journal of Athletic Training, 43(2), 215–221. doi: 10.4085/1062-6050-43.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall S. B., Singh B., Gulati K., Seth S. D. (1999). Role of nutrition in toxic injury. Indian Journal of Experimental Biology, 37(2), 109–116. [PubMed] [Google Scholar]
- Latiri I., Sandid S., Fennani M. A., Hadrich M., Masmoudi T., Maatoug C., … Ben Saad H. (2017). The effects of Ramadan fasting on the spirometric data of healthy adult males. American Journal of Men’s Health, 11(4), 1214–1223. doi: 10.1177/1557988316675091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laveneziana P., Palange P. (2012). Physical activity, nutritional status and systemic inflammation in COPD. European Respiratory Journal, 40(3), 522–529. doi: 10.1183/09031936.00041212 [DOI] [PubMed] [Google Scholar]
- Lazzarino A. I., Hamer M., Gaze D., Collinson P., Rumley A., Lowe G., Steptoe A. (2016). The interaction between systemic inflammation and psychosocial stress in the association with cardiac troponin elevation: A new approach to risk assessment and disease prevention. Preventive Medicine, 93, 46–52. doi: 10.1016/j.ypmed.2016.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo V. D., Mattson M. P. (2014). Fasting: Molecular mechanisms and clinical applications. Cell Metabolism, 19(2), 181–192. doi: 10.1016/j.cmet.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan R. J., Leiper J. B., Bartagi Z., Zrifi R., Zerguini Y., Dvorak J. (2008). Effect of Ramadan fasting on some biochemical and haematological parameters in Tunisian youth soccer players undertaking their usual training and competition schedule. British Journal of Sports Medicine, 26(Suppl. 3), S39-S46. doi: 10.1080/02640410802491368 [DOI] [PubMed] [Google Scholar]
- Miller M. R., Hankinson J., Brusasco V., Burgos F., Casaburi R., Coates A., … Force A. E. T. (2005). Standardisation of spirometry. European Respiratory Journal, 26(2), 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- Moosavi S. A., Kabir A., Moghimi A., Chehrei A., Rad M. B. (2007). Evaluation of the effect of Islamic fasting on lung volumes and capacities in the healthy persons. Saudi Medical Journal, 28(11), 1666–1670. [PubMed] [Google Scholar]
- Mosrane Y., Bougrida M., Alloui A. S., Martani M., Rouabah L., Bourahli M. K., … Ben Saad H. (2017). Systemic inflammatory profile of smokers with and without COPD. Revue de pneumologie clinique, 73(4), 188–198. doi: 10.1016/j.pneumo.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Nasiri J., Mahmoudzadeh M., Kheiri S., Khoshdel A. (2016). The effect of Ramadan fasting on haematological parameters. Journal of Fasting and Health, 4(4), 145–151. doi: 10.22038/jfh.2017.20133.1073 [DOI] [Google Scholar]
- Nematy M., Mazidi M., Rezaie P., Kazemi M., Norouzy A., Mohajeri S. A. R., Razavi A. (2015). Ramadan fasting: Do we need more evidence? Journal of Fasting and Health, 3(1), 4–10. doi: 10.22038/jfh.2015.4170 [DOI] [Google Scholar]
- Nguyen H. Q., Chu L., Amy Liu I. L., Lee J. S., Suh D., Korotzer B., … Gould M. K. (2014). Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Annals of the American Thoracic Society, 11(5), 695–705. doi: 10.1513/AnnalsATS.201401-017OC [DOI] [PubMed] [Google Scholar]
- Ninot G., Soyez F., Fiocco S., Nassih K., Morin A. J., Prefaut C. (2010). The VQ11, a short health-related quality of life questionnaire for routine practice in COPD patients. Revue des Maladies Respiratoires, 27(5), 472–481. doi: 10.1016/j.rmr.2010.03.020 [DOI] [PubMed] [Google Scholar]
- Norouzy A., Karimirad R., Sabety Baygi Z., Amini M., Attaran D., Mohajeri S. M. R., … Nematy M. (2013). Effects of Ramadan fasting on spirometric values and clinical symptoms in asthmatic patients. Journal of Fasting and Health, 1(1), 23–27. doi: 10.22038/jfh.2013.303 [DOI] [Google Scholar]
- Official Website of The Presidency of Religious Affairs of the Republic of Turkey. (n.d.). Retrieved from http://www.diyanet.gov.tr
- Oppliger R. A., Bartok C. (2002). Hydration testing of athletes. Sports Medicine, 32(15), 959–971. [DOI] [PubMed] [Google Scholar]
- Pellegrino R., Viegi G., Brusasco V., Crapo R. O., Burgos F., Casaburi R., … Wanger J. (2005). Interpretative strategies for lung function tests. European Respiratory Journal, 26(5), 948–968. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Hirschfield G. M. (2003). C-reactive protein: A critical update. The Journal of Clinical Investigation, 111(12), 1805–1812. doi: 10.1172/JCI18921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzki H., Reichert B. (1987). Blood cell sedimentation rate is the 2-hour value still necessary? Zeitschrift fur die Gesamte Innere Medizin und ihre Grenzgebiete, 42(18), 530–532. [PubMed] [Google Scholar]
- Ramadan J. (2002). Does fasting during Ramadan alter body composition, blood constituents and physical performance? Medical Principles and Practice, 11(Suppl. 2), 41–46. doi: 10.1159/000066413 [DOI] [PubMed] [Google Scholar]
- Ramadan J., Mousa M., Telahoun G. (1994). Effect of Ramadan fasting on physical performance, blood and body composition. Medical Principles and Practice, 4(4), 204–212. [Google Scholar]
- Rejbi I. B., Trabelsi Y., Chouchene A., Ben Turkia W., Ben Saad H., Zbidi A., … Tabka Z. (2010). Changes in six-minute walking distance during pulmonary rehabilitation in patients with COPD and in healthy subjects. International Journal of Chronic Obstructive Pulmonary Disease, 5, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker P. M. (2003). Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation, 107(3), 363–369. [DOI] [PubMed] [Google Scholar]
- Sadeghirad B., Motaghipisheh S., Kolahdooz F., Zahedi M. J., Haghdoost A. A. (2014). Islamic fasting and weight loss: A systematic review and meta-analysis. Public Health Nutrition, 17(2), 396–406. doi: 10.1017/S1368980012005046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdie F. M., Dorff T., Quinn D., Fontana L., Wei M., Lee C., … Longo V. D. (2009). Fasting and cancer treatment in humans: A case series report. Aging, 1(12), 988–1007. doi: 10.18632/aging.100114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarraf-Zadegan N., Atashi M., Naderi G. A., Baghai A. M., Asgary S., Fatehifar M. R., … Zarei M. (2000). The effect of fasting in Ramadan on the values and interrelations between biochemical, coagulation and haematological factors. Annals of Saudi Medicine, 20(5–6), 377–381. [DOI] [PubMed] [Google Scholar]
- Sayeed A., Hazari M. A. H. H., Arifuddin M. S. (2018). Immediate and delayed effect of Ramadan fasting on spirometry parameters. Annals of Medical Physiology, 2(1), 7–10. doi: 10.23921/amp.2018v2i1.279619 [DOI] [Google Scholar]
- Sedaghat M., Askarizadeh F., Heravian J., Rakhshandadi T., Nematy T., Mahmoodi Z., … Amirakalali-Sijavandi M. (2017). The effects of Islamic fasting on blood haematological-biochemical parameters. Journal of Fasting and Health, 5(2), 56–62. doi: 10.22038/JFH.2017.22778.1085 [DOI] [Google Scholar]
- Siddiqui Q. A., Sabir S., Subhan M. M. (2005). The effect of Ramadan fasting on spirometry in healthy subjects. Respirology, 10(4), 525–528. doi: 10.1111/j.1440-1843.2005.00744.x [DOI] [PubMed] [Google Scholar]
- Singha Roy A., Bandyopadhyay A. (2016). Pulmonary function of young Muslim males during the month of Ramadan. American Journal of Men’s Health, 12(4), 828–836. doi: 10.1177/1557988316643292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soori M., Mohaghegh S., Hajain M., Moraadi B. (2016). Effects of Ramadan fasting on inspiratory muscle function. Asian Journal of Sports Medicine, 7(3), e35201. doi: 10.5812/asjsm.35201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa V. D., Zauszniewski J. A., Musil C. M. (2004). How to determine whether a convenience sample represents the population. Applied Nursing Research, 17(2), 130–133. [PubMed] [Google Scholar]
- Subhan M. M., Siddiqui Q. A., Khan M. N., Sabir S. (2006). Does Ramadan fasting affect expiratory flow rates in healthy subjects? Saudi Medical Journal, 27(11), 1656–1660. [PubMed] [Google Scholar]
- Suresh K., Suresh G., Thomas S. V. (2012). Design and data analysis 1 study design. Annals of Indian Academy of Neurology, 15(2), 76–80. doi: 10.4103/0972-2327.94987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabelsi K., Stannard S., Shephard R., Jamoussi K., Hakim A. (2014). Body composition, haematological and biochemical modifications during Ramadan fasting. In Chtourou H. (Ed.), Effects of Ramadan fasting on health and athletic performance (pp. 108–116). Retrieved from http://www.esciencecentral.org/ebooks/effects-of-ramadan-fasting/body-composition.php
- Tsai A. G., Wadden T. A. (2013). In the clinic: Obesity. Annals of Internal Medicine, 159(5), ITC3. doi: 10.7326/0003-4819-159-5-201309030-01003 [DOI] [PubMed] [Google Scholar]
- Vogelmeier C. F., Criner G. J., Martinez F. J., Anzueto A., Barnes P. J., Bourbeau J., … Agusti A. (2017). Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine, 195(5), 557–582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- Westergren A. (1957). Diagnostic tests: The erythrocyte sedimentation rate range and limitations of the technique. Triangle, 3(1), 20–25. [PubMed] [Google Scholar]
- Zouari H., Latiri I., Mahjoub M., Boussarsar M., Benzarti M., Abdelghani A., Ben Saad H. (2018). The effects of Ramadan Intermittent Fasting (RIF) on spirometric data of stable COPD patients: A pilot study. American Journal of Men’s Health, 12(2), 359–369. doi: 10.1177/1557988317734131 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Data_CLEAN for The Effects of Ramadan-Fasting (RF) on Inflammatory and Hematological Indices of Stable Chronic Obstructive Pulmonary Disease (COPD) Male Patients: A Pilot Study by Hadhemi REJEB, Mouna BEN KHELIFA, Jihene BEN ABDALLAH, Sawssan MRAD, Mohamed BEN REJEB, Abdelaziz HAYOUNI, Mohamed BENZARTI, Khelifa LIMEM, Mondher KORTAS, Sonia ROUATBI and Helmi BEN SAAD in American Journal of Men’s Health


