Abstract
Objectives
Adherence to a treat to target (TTT) strategy is a recommended paradigm for RA; however, research shows there are many barriers to implementation. We conducted a trial to improve TTT implementation, and herein examine barriers to treatment adjustment within TTT among patient visits not in agreement with the TTT paradigm.
Methods
Chart review assessed TTT implementation based on documentation of four items: designation of a treatment target, recording a disease activity measure, shared-decision making when applicable and adjusting treatment when disease activity was not at target. A treatment decision not in agreement with the TTT paradigm was defined as lack of treatment adjustment when disease activity was not at the pre-determined treatment target. Providers were encouraged to report the barriers to treatment change; these were categorized and analysed by study staff. Multiple barriers were possible for one visit.
Results
Eighty-three visits not in agreement with the TTT strategy were observed in 74 patients, during which 90 reported barriers to treatment adjustment were noted. Common barriers to adjusting treatment included patient preference in 37.1% of visits and elevated disease activity measure despite no objective evidence of active RA in 38.6% of visits.
Conclusion
An elevated disease activity measure not reflective of RA disease activity and patient preference are the two leading barriers to treatment adjustment to TTT in RA. Understanding barriers to adherence should guide interventions aimed at using better markers of disease activity and improving alignment with patient preference, with the overarching goal of enhancing TTT adherence.
Keywords: treat to target, barriers, adherence, rheumatoid arthritis
Rheumatology key messages
Non-adherence to treat to target in RA is common for several reasons.
Leading barriers to treat to target in RA are patient preference and physicians questioning disease activity measures.
Introduction
Treat to target (TTT) is an accepted and recommended treatment strategy for patients with RA [1]. Adherence to the TTT paradigm requires: setting a disease target; regular monitoring of disease activity using a validated disease activity measure; shared decision-making, when applicable; and appropriate adjustment of therapy based on a predetermined target and disease activity score. TTT consistently demonstrates better outcomes when compared with routine care [2–5]. Patients within TTT cohorts tend to have higher percentages of low disease activity or remission, reach remission more rapidly and stay in remission for >1 year compared with RA patients receiving usual care [2]. In line with the evidence, TTT principles have been recommended by major professional societies in rheumatology [1, 6].
Although TTT has many proven benefits, studies find that TTT is not routinely practiced in the USA [7, 8]. Possible explanations for this include clinical inertia, work flow difficulties and patient-specific situations in which providers purposely elect not to use TTT. We conducted a quality improvement trial to test whether a Learning Collaborative (LC) would improve TTT implementation [9]. The trial demonstrated that the intervention improved implementation of TTT [9]. However, varying levels of adherence to the TTT paradigm were observed in the intervention group. Within the context of this trial, we explored the barriers to TTT adherence in the intervention arm.
Methods
Study design and population
The TRACTION trial (Treat-to-target in RA: Collaboration To Improve adOption and adhereNce; NCT02260778) was a randomized controlled clinical study with 11 US rheumatology sites, randomized into one of two study arms (see supplementary Table S1, available at Rheumatology online). The intervention sites received the LC during the first nine months of the trial, while control sites were wait-listed and received nothing during this period. The LC was guided by expert faculty and was aimed at teaching sites the TTT principles detailed in prior publications [9]. The LC utilized a face-to-face meeting at the beginning of each phase, monthly webinars and site-specific progress calls with TTT faculty. Each webinar was designed to focus on a specific TTT component or implementation barrier. For example, one monthly webinar focused on how to choose an appropriate disease activity measure while the next month focused on educating providers regarding shared decision-making. In addition, sites used a web-based collaborative tool where recorded learning sessions were made available and participants could post questions, share resources and display improvement metrics [9]. In the current analyses, we examined barriers to treatment adjustment as reported by providers in the intervention arm who received the LC during the first nine months.
This secondary analysis of the TRACTION trial studied the barriers to treatment adjustment that providers encountered within the TTT paradigm. We examined the providers and their visits in the intervention arm during the first nine months because they were the first to receive the necessary training regarding TTT principles. Providers within the wait-list control sites, who did not receive any intervention during this period, had no formal training in TTT practices, and therefore, they were not attempting to practice TTT and were not included in the current analyses.
At the end of the nine months, trained study staff performed a medical record review at both intervention and control sites. At least 30 patients with RA who had been seen during the study window were randomly chosen for each provider. The provider must have attended at least one learning session. The patients selected were chosen by a non-provider staff member to avoid selection bias. Rheumatology visit notes from September 2014 to December 2015 were reviewed. Staff evaluated each visit note for the following four items, which directly stem from the TTT paradigm: selection of a disease activity target; recording of RA disease activity measure using a validated measurement tool; documented evidence of shared decision-making, when necessary; and treatment adjustment based on the target and disease activity measure. When the fourth item was not met, providers were encouraged to describe the reason why, allowing for more than one reason. If documented, these reasons, or barriers to treatment adjustment, as well as the type of treatment deviation were recorded by study staff. Patient data such as age, disease characteristics, medications, comorbid conditions, family history, allergies and BMI were also collected.
The study was approved by Partners Healthcare Human Subjects Panel. They deemed that no consent was necessary.
Barriers to treatment adjustment
Adherence to TTT was assessed using the four items described above. Reasons for non-adherence, or barriers to treatment adjustment, were noted only when TTT prerequisites were met. Thus, providers needed to demonstrate appropriate use of disease activity scores, treatment targets and shared decision making, if warranted. If these components were present but treatment was not adjusted when indicated (i.e. disease activity measure not at target), the barriers to treatment adjustment were documented. Control sites rarely documented treatment targets or disease activity scores and thus did not meet requirements for adherence or non-adherence with TTT. Thus, the reasons for non-adherence described in this paper are derived solely from intervention sites.
Study staff determined the types of treatment deviations from the TTT strategy, including not adjusting medication when indicated or tapering/discontinuing medication instead of continuing. For example, if a patient’s disease activity score indicated high disease activity and the target disease activity was remission, the physician would be expected to adjust therapy in accord with the TTT algorithm. Several potential barriers to treatment adjustment were described a priori based on previous work [5]. Provider documentation of the specific barriers was transcribed from visit notes verbatim. This documentation was qualitatively analysed and categorized by study staff. Barriers to treatment adjustment were categorized as follows: patient preference (e.g. financial barriers, insurance issues, fear of certain medications and/or reluctance to change); intolerance to a specific drug; elevated disease activity score not reflective of RA disease activity (i.e. pain due to comorbid condition or no objective joint swelling on clinical exam despite significant pain and/or tenderness); contraindications to therapy (e.g. infection, pregnancy, renal disease, chemotherapy); medication lag time (delayed treatment response); or unknown.
The analysis of these barriers was descriptive and examined frequencies across the sample of non-adherent visits. Patient characteristics were analysed for comparability with the total trial sample. All analyses used SAS (SAS Institute, Cary NC, version 9.4).
Results
Medical records were examined from the five geographically diverse intervention sites, all of which had teaching affiliations (see supplementary Table S1, available at Rheumatology online). Within these sites, a total of 23 providers’ notes were reviewed by trained research assistants. The providers ranged from senior physicians to fellows and included mid-level providers such as physician assistants and nurse practitioners. Study staff examined 2241 visits from 641 patients from both intervention and control sites. Of the 1270 visits from intervention sites, 136 visits adhered fully to the TTT paradigm, 1051 did not include a documented disease activity measure score and/or disease target as required in the TTT paradigm and 94 visits did not adhere to TTT (see supplementary Fig. S1, available at Rheumatology online). Eleven of the 94 visits were from control sites, so the remaining 83 visits form the basis for the current analyses. Of these 83 visits, two types of deviations from TTT were noted: not intensifying instead of intensifying (n = 81) and taper/discontinuing instead of continuing (n = 2).
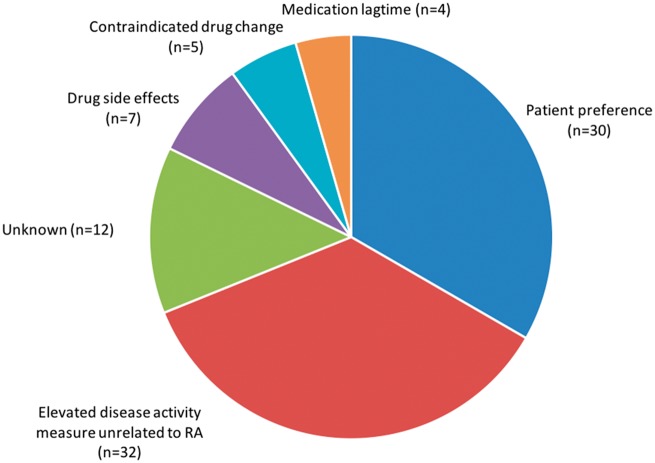
A total of 90 barriers to treatment adjustment were noted within the 83 visits described above. The most common documented barriers to TTT adherence were patient preference in 30 (37.1%) visits and elevated disease activity score not reflective of RA disease activity in 32 (38.6%) visits (see Fig. 1). The barrier to treatment adjustment was not recorded in 12 (4.8%) visits. All of these visits were with patients who were not at disease activity target based on either Clinical Disease Activity Index (CDAI) or Routine Assessment of Patient Index Data 3 (RAPID3) criteria. Of the 32 visits where TTT was not adhered to because of elevated disease activity unrelated to RA, only 1 visit used the CDAI and all others the RAPID3.
Fig. 1.
Provider reported barriers to treatment adjustment (n = 90)
We compared patient characteristics from visits when treatment was adjusted with visits without adjustment (see Table 1). Patients in both groups were similar across all measured characteristics. They had a similar number of comorbidities, similar age, a similar proportion were female, a similar proportion were seropositive, duration of RA was widely distributed but similar and the use of DMARDs was varied but similar across the two groups. It is possible that individual comorbidities may have affected providers’ ability to follow the TTT algorithm. However, we did not have detailed information regarding specific comorbidities. The RA disease activity distribution differed significantly between the two groups; a higher proportion of patients without any non-adherent visits were in remission, but a slightly higher proportion were in high disease activity.
Table 1.
Patient and visit characteristics
| Patient characteristics | Patients whose treatment was not adjusted but TTT recommended adjustment (n = 76) | Patients whose treatment was adjusted per TTT algorithm (n = 109) | P-value |
|---|---|---|---|
| Age, mean (s.d.), years | 61 (14) | 59 (13) | 0.42 |
| Gender, female | 60 (79) | 88 (81) | 0.70 |
| Seropositive status | 48 (80) | 81 (86) | 0.33 |
| Evidence of joint erosion | 35 (71) | 45 (52) | 0.052 |
| RA disease duration, years | 0.91 | ||
| <2 | 11 (24) | 15 (25) | |
| 2 − 5 | 13 (28) | 17 (28) | |
| 6–10 | 11 (24) | 15 (25) | |
| >10 | 11 (24) | 13 (22) | |
| Synthetic DMARD use | 61 (80) | 93 (85) | 0.68 |
| Biologic DMARD use | 36 (47) | 52 (48) | 0.84 |
| Number of comorbidities, mean (s.d.) | 6.5 (4.1) | 5.4 (3.2) | 0.55 |
| Number of comorbidities, median (IQR) | 6 (3–8) | 5 (3–8) | 0.068 |
| RA disease activity, by visita | <0.01 | ||
| Remission | 17 (20) | 72 (53) | |
| Low | 28 (34) | 26 (19) | |
| Moderate | 30 (36) | 28 (20) | |
| High | 8 (10) | 10 (7) |
Values are n (%) unless otherwise stated.
Disease activity considered at each visit for patients included. Among the 76 patients who had TTT non-adherence, there were 83 visits. Among the 109 patients who had TTT adherence, there were 136 visits. The median number of comorbidities is followed in parentheses by the interquartile range (IQR). P-values were estimated from Student’s t-test or Chi-square test.
Discussion
In the context of a larger trial to improve TTT implementation, we report barriers to treatment adjustment as indicated by TTT principles. The TTT paradigm is a recommended treatment strategy for patients with RA, but providers face many challenges with implementation and adherence [4, 8]. As others have found in both the typical clinical and trial settings, we identified multiple barriers to adherence to a TTT strategy [2, 3, 5, 8, 10].
A major barrier to treatment adjustment was patient preference. Patients play a central role within the TTT paradigm, as one of the core principles is shared decision-making. Past work has examined discrepancies between patient and provider approaches to treatment change, as well as discordance between patient satisfaction and RA-related outcomes [11–13]. Patient preference issues include concerns regarding side effects, cost and fear of losing control over their disease [11]. Beyond personal fears, patients may feel satisfied with their current state of disease, regardless of measured disease activity [11]. Patient resistance to treatment change can be difficult for providers to manage. Methods to overcome patient preference barriers should focus on integrating patients in the treatment process. We observed that only in about 9% of visits with shared decision-making did the provider mention the consequences of ongoing inflammation if treatment was not changed. Other work investigating the patient’s perspective has highlighted the importance of physicians as essential sources of information, particularity in early treatment, which could bolster the long-term patient–provider relationship, decision-making process and ultimately TTT adherence [14].
Due to our method of chart review, sufficient data were not available on the specific reasons why patients refused to change treatment (i.e. fears or cost), which may play a role in distinguishing between patients who adhere and do not adhere to a TTT paradigm [15]. Without these data, it is difficult to comment on specific strategies for how to address individual barriers.
One could also question whether patient preference should be considered a true barrier to treatment adjustment. If disease management is aligned with a well-informed patient’s preference, as encouraged through the use of shared decision-making, one might consider that TTT is being fully practiced regardless of whether disease activity is at target.
The second most common barrier resulting in provider deviation from the TTT strategy was elevated disease activity scores unrelated to RA, demonstrating that provider disagreement with disease activity measures also plays an important role in not following the prompt of a score value to change therapy. This may be dependent on the type of disease activity score used. In this study, providers were not required to choose a specific measure, but we found that most used a RAPID3 and not a CDAI. The RAPID3 is fully based on patient-reported outcomes. The lack of a formal joint count may contribute to discordance between the RAPID3 score and the need to escalate treatment. Although the RAPID3 and CDAI have been shown to be statistically correlated, they are fundamentally different scales and thus it is not surprising that discrepancies result.
Conclusion
Adherence to TTT is complex and barriers to treatment adjustment can be difficult to overcome. Patient preference and elevated disease activity score unrelated to active RA were observed in 37 and 39% of visits, respectively. Future work should flesh out specific patient-centred concerns regarding treatment change, so that appropriate education regarding the TTT can be provided. Providers may find adherence to TTT more difficult with certain patients in specific clinical settings. However, our findings from the original trial suggest that TTT adherence can be improved [9]. Providers must also carefully contemplate which disease activity measure is most appropriate for their clinical setting, as the individual components of each measure can affect whether patients meet target or not.
Supplementary Material
Acknowledgements
A.Z., C.C. and D.H.S. analysed the data and drafted the manuscript. A.B., L.F., L.H. and J.S.S. revised the manuscript and suggested analyses. All authors read and approved the final manuscript. The datasets generated and/or analysed during the current study are not yet publicly available because other secondary analyses are still being completed. However, they will be made available from the corresponding author on reasonable request.
Funding: This work was supported by the National Institutes of Health [NIH-P60-AR047782]. Research reported in this publication also supported by [NIH-K24-AR060231].
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Smolen JS, Breedveld FC, Burmester GR. et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016;75:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schipper LG, Vermeer M, Kuper HH. et al. A tight control treatment strategy aiming for remission in early rheumatoid arthritis is more effective than usual care treatment in daily clinical practice: a study of two cohorts in the Dutch Rheumatoid Arthritis Monitoring registry. Ann Rheum Dis 2012;71:845–50. [DOI] [PubMed] [Google Scholar]
- 3. Grigor C, Capell H, Stirling A. et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263–9. [DOI] [PubMed] [Google Scholar]
- 4. Schipper LG, van Hulst LT, Grol R. et al. Meta-analysis of tight control strategies in rheumatoid arthritis: protocolized treatment has additional value with respect to the clinical outcome. Rheumatology (Oxford) 2010;49:2154–64. [DOI] [PubMed] [Google Scholar]
- 5. Wailoo A, Hock ES, Stevenson M. et al. The clinical effectiveness and cost-effectiveness of treat-to-target strategies in rheumatoid arthritis: a systematic review and cost-effectiveness analysis. Health Technol Assess 2017;21:1–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh JA, Saag KG, Furst D.. Reply: to PMID 22473917. Arthritis Care Res 2013;65:832–3. [DOI] [PubMed] [Google Scholar]
- 7. Harrold LR, Harrington JT, Curtis JR. et al. Prescribing practices in a US cohort of rheumatoid arthritis patients before and after publication of the American College of Rheumatology treatment recommendations. Arthritis Rheum 2012;64:630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tymms K, Zochling J, Scott J. et al. Barriers to optimal disease control for rheumatoid arthritis patients with moderate and high disease activity. Arthritis Care Res (Hoboken) 2014;66:190–6. [DOI] [PubMed] [Google Scholar]
- 9. Solomon DH, Losina E, Lu B. et al. Implementation of treat-to-target in rheumatoid arthritis through a learning collaborative: results of a randomized controlled trial. Arthritis Rheumatol 2017;69:1374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vermeer M, Kuper HH, Bernelot Moens HJ. et al. Adherence to a treat-to-target strategy in early rheumatoid arthritis: results of the DREAM remission induction cohort. Arthritis Res Ther 2012;14:R254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolfe F, Michaud K.. Resistance of rheumatoid arthritis patients to changing therapy: discordance between disease activity and patients’ treatment choices. Arthritis Rheum 2007;56:2135–42. [DOI] [PubMed] [Google Scholar]
- 12. Desthieux C, Hermet A, Granger B, Fautrel B, Gossec L.. Patient-physician discordance in global assessment in rheumatoid arthritis: a systematic literature review with meta-analysis. Arthritis Care Res (Hoboken) 2016;68:1767–73. [DOI] [PubMed] [Google Scholar]
- 13. Studenic P, Radner H, Smolen JS, Aletaha D.. Discrepancies between patients and physicians in their perceptions of rheumatoid arthritis disease activity. Arthritis Rheum 2012;64:2814–23. [DOI] [PubMed] [Google Scholar]
- 14. Meyfroidt S, Van der Elst K, De Cock D. et al. Patient experiences with intensive combination-treatment strategies with glucocorticoids for early rheumatoid arthritis. Patient Educ Couns 2015;98:384–90. [DOI] [PubMed] [Google Scholar]
- 15. Ter Wee MM, Coupe VM, den Uyl D. et al. Cost-utility of COBRA-light versus COBRA therapy in patients with early rheumatoid arthritis: the COBRA-light trial. RMD Open 2017;3:e000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.