Abstract
Objective
The aim of this study was to evaluate minimal disease activity (MDA) assessments in patients with PsA during routine clinical care.
Methods
We used data from a multicentre observational study of patients with active PsA who initiated treatment with adalimumab during routine clinical practice and continued treatment for at least 6 months to evaluate achievement of MDA, individual MDA criteria (modified to conform to study assessments) and ACR responses during 24 months of therapy. Pearson correlation coefficients were used to evaluate the association between MDA and individual criteria at month 6; regression models were used to determine the influence of baseline MDA criteria on achievement of MDA at month 6.
Results
A total of 1684 patients were included in these analyses; most had long-standing disease. MDA was achieved by 597 patients (35.5%) at month 6. This proportion increased to 45.5% at month 24 in patients remaining on therapy. MDA status was stable over time; >75% of patients with MDA at month 6 recorded MDA at subsequent visits. Pain was the most difficult individual criterion to achieve, and enthesitis was the least difficult. Higher functional status and fewer tender joints at baseline predicted achievement of MDA at month 6. About half of patients (51.5%) with an ACR20 response at month 6 achieved MDA.
Conclusion
In this observational cohort of patients with long-standing disease, MDA provided a stable and valid assessment of clinical status over 24 months.
Trial registration
Clinicaltrials.gov, https://clinicaltrials.gov, NCT01111240
Keywords: adalimumab, psoriatic arthritis, minimal disease activity, outcomes research, treatment response, tumour necrosis factor inhibitor
Rheumatology key messages
Approximately 35% of PsA patients initiating treatment with adalimumab achieved minimal disease activity at month 6.
Pain was the most difficult PsA minimal disease activity criterion to achieve; enthesitis was the least difficult.
Minimal disease activity was a stable and valid PsA assessment during 24 months of routine clinical care.
Introduction
Low disease activity is a critical goal in the management of patients with rheumatological diseases, including PsA. Close monitoring of disease activity provides clinicians with information that guides the escalation or de-escalation of therapy. In 2010, Coates et al. [1] published minimal disease activity (MDA) criteria for PsA, which required the achievement of five of the seven following criteria: tender joint count (TJC) ⩽1, swollen joint count (SJC) ⩽1, Psoriasis Area and Severity Index ⩽1 or body surface area (BSA) ⩽3, patient pain visual analogue score (VAS) ⩽15, patient global disease activity (PGA) VAS ⩽20, HAQ-Disability Index (HAQ-DI) ⩽0.5 and tender entheseal points ⩽1. Subsequently, these criteria were validated using data from phase 2/3 trials of a TNF inhibitor (infliximab) in patients with PsA [2]. This validation study found that MDA reflected therapeutic response and was associated with a significant reduction in radiological progression. Similar support for MDA has come from the ADalimumab Effectiveness in Psoriatic Arthritis trial of another TNF inhibitor, adalimumab [3]. More recently, MDA has been successfully used in the open-label Tight Control of Inflammation in Early Psoriatic Arthritis study as the benchmark for the decision to escalate therapy in newly diagnosed patients [4]. Tight control of disease activity was found to result in significant improvements in joint outcomes at 48 weeks.
Although clinical trials are the gold standard for evaluating drug efficacy and safety, they are less successful in providing insights into real-world clinical usage. Observational studies can supplement clinical trials by providing data on the effectiveness of a drug during routine clinical use and over longer durations than typically employed in randomized trials [5]. Unlike randomized clinical trials, non-interventional studies do not have selection criteria for patients and do not regulate or restrict the dose of drug or its use in patients with comorbid conditions or concomitant medications. Although MDA has been proposed as a means of guiding treatment decisions during daily clinical practice [1, 2, 4], the use of these criteria in a large non-selected, multicentre population with long-standing disease has not been fully analysed. The availability of data from a large PsA cohort in an observational study of routine clinical care offered a unique opportunity to explore the stability and validity of MDA assessments during routine clinical care. The goal of this study was to investigate the potential value of MDA as an instrument for steering treatment decisions during daily clinical practice.
Methods
Study design and patient selection
This study utilized data from a multicentre non-interventional trial of patients with PsA who received adalimumab therapy at the decision of the clinician (Clinicaltrials.gov trial registration NCT01111240), as previously described by Behrens et al. [6, 7]. All patients were under the routine medical care of 279 physicians (rheumatologists: 63%; dermatologists: 14%; internists: 15%; and other specialists, mainly orthopaedic specialists: 8%) in Germany. Adult patients (⩾18 years of age) were required to have a diagnosis of active PsA, a clinical indication for treatment with a TNF inhibitor, and no contraindications. Patients were enrolled in this study between August 2005 and January 2013. The objective of the overall observational study was to examine the effectiveness and safety of adalimumab in adult patients with PsA during routine clinical care.
To be included in the analyses reported here, patients were required to have active disease (DAS28 ⩾3.2), axial involvement or ⩾1 swollen or tender joint, and adequate data for MDA calculations, including complete data for MDA criteria at month 6. Patients who discontinued or were lost to follow-up before month 6 were not included in these analyses. All patients were informed of the objectives of the observational study and provided written consent for their voluntary participation in the study and the anonymous use of personal data in statistical analyses. Because of the non-interventional nature of this study, ethics approval was not required by German law.
Assessments
Data on demographic and disease characteristics were collected at baseline. Criteria used to assess MDA were as previously published [1], with slight modifications as detailed below. Tender and swollen joint counts were performed on 78 and 76 joints, respectively. The study data did not include Psoriasis Area and Severity Index assessments, so skin evaluations were based on BSA as estimated by the examining clinician. Data for baseline BSA evaluations were recorded as pre-specified categories (<3, 3–10, 11–20 and >20%), whereas BSA data at subsequent visits were recorded as an estimated percentage without categorizations. For both the PGA and the patient-reported pain assessment, we used an 11-point categorical scale ranging from 0 (none) to 10 (severe). The PGA cut-off for MDA was ⩽2. For pain, the conservative value of ⩽1 was chosen as a cut-off (rather than ⩽15 on a 100-point VAS as originally published) [1]. Entheseal points were not assessed in our study, so we substituted the criterion of ⩽1 tender entheseal points with the absence of enthesitis as judged by the investigator. Instead of HAQ-DI, we used a comparable functional assessment, the self-administered Funktionsfragebogen Hannover (FFbH) patient questionnaire. The FFbH questionnaire indicates the remaining percentage of patient function and is scored on a scale of 0 (total loss of functional capacity) to 100 (maximal functional capacity) [8]; higher scores are better, which is opposite to the HAQ-DI. The FFbH questionnaire has been validated in patients with RA and is highly correlated to the HAQ-DI [9]; an English translation has been published [10]. The MDA criterion of HAQ-DI ⩽0.5, which corresponds to functional remission [11, 12], was substituted with the FFbH score for functional remission (⩾83%) [13].
We also assessed the proportions of patients achieving ACR response criteria for a 20, 50 or 70% improvement from baseline (ACR20, ACR50 and ACR70) [14]. CRP was used as the acute-phase reactant, and FFbH was used to assess physical function.
Stability of response was determined by evaluating the proportion of patients who maintained a 6-month MDA response at later time points. Validity was evaluated by determining the correlation between MDA and global assessments such as function and PGA, and by measuring the proportion of patients achieving both MDA and ACR responses.
Statistical analyses
Statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute, Cary, NC, USA). Descriptive statistics were computed as appropriate; missing data were not imputed. We used Pearson correlation coefficients to evaluate the association between the achievement of MDA at month 6 and the achievement of individual MDA criteria at month 6 (higher values indicate a closer linear relationship). To better understand the multivariate structure of the data, we used logistic regression analysis to determine the baseline MDA characteristics that influenced the achievement of MDA at month 6 [13, 15].
Results
Patients
A total of 1684 patients were included in the analyses presented here (supplementary Fig. S1, available at Rheumatology online). All of these patients completed at least 6 months of therapy and had data necessary for MDA calculation at month 6; patients who did not meet these criteria were excluded from the analyses reported here. Baseline characteristics of patients in this study were comparable to those in the overall population (data not shown). At baseline, most patients had long-standing disease (mean of 10 and 18 years for arthritis and skin symptoms, respectively) and extensive joint and skin involvement (Table 1). Adalimumab was the first biologic therapy for >80% of patients. During the 24 months of the study, 359 patients (21.3%) withdrew from the study and 472 (28.0%) were lost to follow-up. For patients with documented withdrawals, 203 (56.5%) withdrew due to lack of efficacy, 48 (13.4%) for adverse effects, three (0.8%) for both lack of efficacy and adverse effects, and 105 (29.2%) for other unspecified reasons. The numbers of patients assessed at months 12 and 24 were 1257 (74.6%) and 912 (54.2%), respectively (supplementary Table S1, available at Rheumatology online). As with all observational studies, missed visits were common, and not all assessments were performed at each visit. As a result, the number of patients with data for a given outcome varied within and among visits.
Table 1.
Baseline characteristics for all analysed patients and by MDA achievement at month 6
| Baseline characteristic | All patients (N = 1684) | MDA achievement at month 6 | |
|---|---|---|---|
| Yes (n = 597) | No (n = 1087) | ||
| Female sex, % | 50.6 | 39.3 | 56.8 |
| Age, years | 50.0 (11.5) | 46.7 (11.9) | 51.8 (10.9) |
| BMI, kg/m2 | 28.6 (5.6) | 27.4 (5.0) | 29.3 (5.7) |
| Duration of arthritis symptoms, years | 9.5 (8.6) | 9.0 (8.3) | 9.7 (8.8) |
| Duration of psoriasis symptoms, years | 18.1 (13.2) | 17.1 (11.9) | 18.6 (13.8) |
| DAS28 | 5.06 (1.08) | 4.80 (1.01) | 5.21 (1.09) |
| Tender joint count (78 joints) | 16.3 (15.4) | 12.2 (12.0) | 18.5 (16.6) |
| Swollen joint count (76 joints) | 9.3 (10.9) | 7.8 (8.8) | 10.1 (11.9) |
| Paina | 6.3 (2.1) | 5.5 (2.3) | 6.7 (1.9) |
| PGAa | 7.0 (1.6) | 6.7 (1.7) | 7.1 (1.6) |
| Function (% remaining)b | 67.6 (21.0) | 78.3 (17.5) | 61.7 (20.5) |
| Body surface area of psoriasis, percentage of patients | |||
| <3% | 34.4 | 38.5 | 32.2 |
| 3–10% | 33.2 | 31.0 | 34.4 |
| 11–20% | 16.4 | 16.0 | 16.6 |
| >20% | 16.0 | 14.5 | 16.8 |
| Dactylitis, percentage of patients | 48.0 | 47.7 | 48.2 |
| Enthesitis, percentage of patients | 27.3 | 23.6 | 29.3 |
| Number of previous biologic therapies, percentage of of patients | |||
| 0 | 82.1 | 87.9 | 78.8 |
| 1 | 15.4 | 10.6 | 18.0 |
| 2 or 3 | 2.6 | 1.5 | 3.1 |
Results are presented as mean (s.d.) unless otherwise indicated.
Measured on a scale from 0 (none) to 10 (severe).
As assessed by FFbH on a scale of 0 (total loss of functional capacity) to 100 (maximal functional capacity). FFbH: Funktionsfragebogen Hannover; MDA: minimal disease activity; PGA: patient global disease activity assessment.
Patients who achieved MDA at month 6 varied in several key baseline characteristics compared with patients who did not achieve MDA at month 6, including lower values for DAS28, joint counts and pain, and improved function (Table 1). Patients who achieved MDA at month 6 were also more likely to be male and naïve to biologic therapy.
Achievement of MDA and stability of response over time
Of the 1684 patients who initiated treatment with adalimumab and continued treatment for 6 months, 597 (35.5%) achieved MDA at month 6. The mean (s.d.) time to achieving MDA in this group was 4.4 (1.6) months. The proportion of patients achieving MDA increased slightly at visits after month 6 to a maximum of 45.5% in patients remaining on therapy at month 24, perhaps due to responder bias (Table 2).
Table 2.
Achievement of MDA and individual MDA criteria at each visit
| Variable | Month 0 | Month 3 | Month 6 | Month 9 | Month 12 | Month 18 | Month 24 |
|---|---|---|---|---|---|---|---|
| MDA | 0.9 (14/1578) | 27.0 (402/1488) | 35.5 (597/1684) | 37.3 (456/1223) | 41.3 (454/1098) | 43.3 (398/920) | 45.5 (348/764) |
| TJC ≤1 | 4.2 (71/1684) | 39.6 (647/1635) | 46.6 (785/1684) | 50.5 (696/1377) | 52.5 (658/1253) | 54.4 (594/1091) | 57.8 (525/909) |
| SJC ≤1 | 19.1 (321/1684) | 64.0 (588/1635) | 70.1 (1180/1684) | 71.9 (990/1377) | 75.2 (942/1253) | 75.5 (824/1091) | 81.1 (737/909) |
| BSA ≤3%a | 34.4 (546/1587) | 57.0 (863/1513) | 64.8 (1901/1684) | 66.7 (838/1257) | 69.5 (781/1123) | 70.7 (666/924) | 70.8 (558/788) |
| Pain ≤1 | 2.3 (39/1684) | 17.1 (276/1614) | 21.1 (353/1671) | 22.6 (307/1361) | 23.9 (293/1228) | 24.0 (258/1073) | 24.5 (218/890) |
| PGA ≤2 | 0.6 (10/1674) | 31.5 (513/1629) | 40.1 (676/1684) | 42.3 (581/1374) | 45.1 (564/1251) | 47.2 (515/1092) | 49.3 (450/912) |
| Functional remissionb | 29.5 (493/1674) | 46.4 (750/1618) | 47.4 (798/1684) | 49.4 (670/1356) | 54.0 (662/1226) | 52.4 (562/1072) | 55.1 (489/888) |
| No enthesitis | 72.7 (1225/1684) | 87.1 (1425/1636) | 88.8 (1496/1684) | 90.3 (1254/1389) | 90.3 (1135/1257) | 90.6 (997/1100) | 92.0 (839/912) |
Data are presented as percentage of patients (number of patients who achieved criterion/number of patients with data for the specified outcome at this visit).
Baseline (month 0) MDA assessments were based on BSA <3% due to the use of pre-specified categories (<3, 3–10, 11–20 and >20%) for baseline BSA evaluations. Subsequent MDA assessments were based on BSA ≤3% as per the published MDA criterion due to BSA assessments at post-baseline visits as an estimated percentage without pre-specified categorizations.
FFbH ≥83% assessed on a scale of 0 (total loss of functional capacity) to 100 (maximal functional capacity). BSA: body surface area; FFbH: Funktionsfragebogen Hannover; MDA: minimal disease activity; PGA: patient global disease activity assessment; SJC: swollen joint count; TJC: tender joint count.
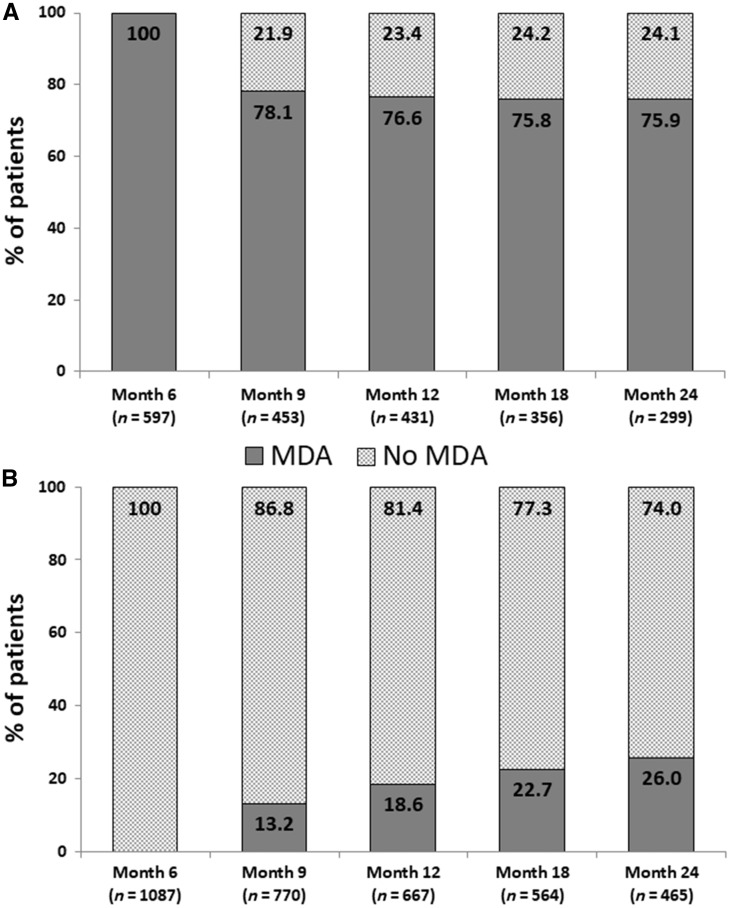
We evaluated the stability of MDA status in patients who did or did not achieve MDA at month 6. In patients with available data who remained on therapy (Fig. 1A), >75% of the group of patients with MDA at month 6 recorded an MDA response at subsequent visits (months 9, 12, 18 and 24). Of the 214 patients who achieved MDA at month 6 and had data available for all four subsequent visits, 118 (55.1%) had MDA at four visits and 43 (20.1%) had MDA at three visits. For the patient group who did not achieve an MDA response at month 6 and remained on therapy (Fig. 1B), an increasing proportion achieved MDA between month 9 (13.2%) and month 24 (26.0%).
Fig. 1.
Stability of MDA status
Stability of MDA status at subsequent visits in (A) patients who achieved an MDA response at month 6 (n = 597) and remained on therapy and (B) patients who did not achieve an MDA response at month 6 (n = 1087) and remained on therapy. Data are presented as percentage of patients with available data for the given visit. MDA: minimal disease activity.
Contribution of individual MDA criteria to achievement of MDA and correlations among MDA criteria
The individual MDA criterion that was most frequently met was the absence of enthesitis (∼90% of patients from months 6–24), followed by SJC ⩽1 (70–80%) and BSA ⩽3% (65–70%; Table 2). The individual MDA criterion that was least frequently met was pain (21–25% of patients from months 6–24). For TJC and SJC, the proportion of patients achieving that criterion increased by ⩾10% between months 6 and 24, suggesting that achievement of these outcomes might require a longer duration of therapy in some patients.
Among the patient population as a whole, fulfillment of the criterion of PGA ⩽2 had the highest correlation with the achievement of MDA at month 6 as evaluated by Pearson correlation coefficients (r = 0.72), followed by TJC ⩽1, pain and functional remission (Table 3). The absence of enthesitis had the lowest correlation with achievement of MDA (r = 0.21), followed by BSA ⩽3%. Among individual MDA criteria, the highest correlation was between pain ⩽1 and PGA ⩽2. Pain ⩽1 was also moderately correlated with functional remission, and PGA was correlated with TJC ⩽1 and functional remission. The two joint criteria, TJC ⩽1 and SJC ⩽1, showed a moderate correlation with each other. Overall, the absence of enthesitis had the weakest correlations with other MDA criteria. Correlations between BSA ⩽3% and other individual criteria were also weak.
Table 3.
Association (Pearson correlation coefficients) between month 6 MDA and individual MDA criteria (N = 1684)
| Variable | MDA | TJC ≤1 | SJC ≤1 | BSA ≤3% | Pain ≤1 | PGA ≤2 | FFbH ≥83%a | No enthesitis |
|---|---|---|---|---|---|---|---|---|
| MDA | 1.00 | 0.61 | 0.42 | 0.31 | 0.60 | 0.72 | 0.59 | 0.21 |
| TJC ≤1 | 1.00 | 0.45 | 0.14 | 0.36 | 0.44 | 0.32 | 0.15 | |
| SJC ≤1 | 1.00 | 0.12 | 0.20 | 0.33 | 0.22 | 0.16 | ||
| BSA ≤3% | 1.00 | 0.09 | 0.17 | 0.09 | 0.16 | |||
| Pain ≤1 | 1.00 | 0.51 | 0.46 | 0.06 | ||||
| PGA ≤2 | 1.00 | 0.43 | 0.10 | |||||
| FFbH ≥83%a | 1.00 | 0.07 | ||||||
| No enthesitis | 1.00 |
For Pearson correlation coefficients, higher values indicate a closer relationship. All P < 0.0001, except for the correlation between BSA and FFbH (P = 0.0002), pain and enthesitis (P = 0.0093) and FFbH and enthesitis (P = 0.0050).
Functional remission (FFbH ≥83%). FFbH is assessed on a scale of 0 (total loss of functional capacity) to 100 (maximal functional capacity). BSA: body surface area; FFbH: Funktionsfragebogen Hannover; MDA: minimal disease activity; PGA: patient global disease activity assessment; SJC: swollen joint count; TJC: tender joint count.
Predictors of MDA in patients initiating adalimumab therapy
We evaluated the impact of baseline MDA characteristics on the likelihood of achieving MDA at month 6 (Table 4). Patients with better function at baseline and those with lower TJC were significantly more likely to achieve MDA at month 6. Other baseline characteristics included in MDA assessments (BSA, pain, SJC, PGA and enthesitis) were not significantly associated with achievement of MDA at month 6.
Table 4.
Influence of baseline MDA characteristics on achieving MDA at month 6 in stepwise regression models
| Baseline variable | Odds ratioa (95% CI) | P-value |
|---|---|---|
| Significant predictors of MDA at month 6 | ||
| FFbHb | 1.042 (1.035, 1.050) | <0.0001 |
| TJCc | 0.974 (0.965, 0.984) | <0.0001 |
| Non-significant variables | ||
| BSA (by categories)d | 0.986 (0.972, 1.000) | 0.0561 |
| Pain | 0.947 (0.885, 1.012) | 0.1096 |
| SJCe | 1.007 (0.994, 1.020) | 0.2886 |
| PGA | 1.042 (0.962, 1.127) | 0.3128 |
| Enthesitis | 0.891 (0.685, 1.159) | 0.3896 |
Only patients with complete data sets (n = 1578) were included in the model. CIs and P-values were determined using the Wald test. Odds ratios are expressed as the ratio for a 1-U difference (risk equals the odds ratio to the power of x) except for enthesitis (ratio for presence vs absence).
FFbH is assessed on a scale of 0 (total loss of functional capacity) to 100 (maximal functional capacity).
78-joint count.
Based on pre-specified baseline categories of <3, 3–10, 11–20 and >20%.
76-joint count. BSA: body surface area; FFbH: Funktionsfragebogen Hannover; MDA: minimal disease activity; PGA: patient global disease activity assessment; SJC: swollen joint count; TJC: tender joint count.
Achievement of ACR vs MDA responses at month 6
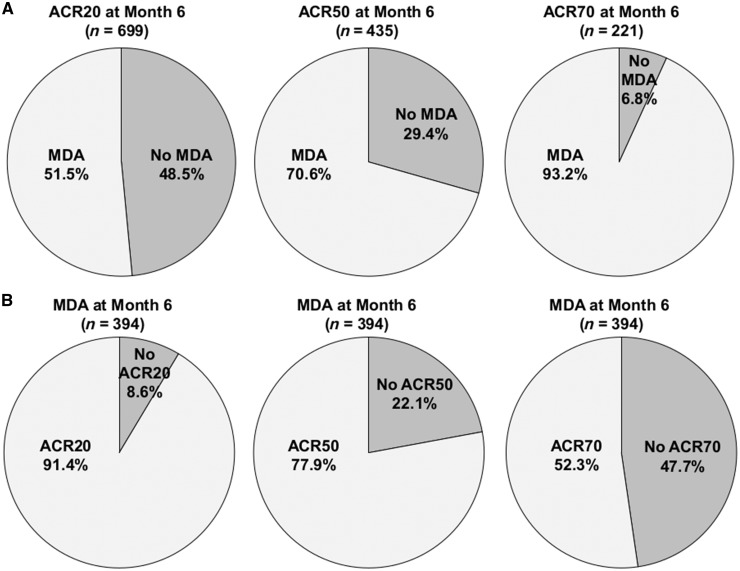
Of the 1166 patients at month 6 with sufficient data for calculation of an ACR response, 699 (59.9%) had an ACR20 response, 435 (37.3%) had an ACR50 response and 221 (19.0%) had an ACR70 response. We assessed the rates of MDA achievement at month 6 in each of these ACR groups (Fig. 2A) and found increasing rates of MDA achievement with more stringent ACR criteria. Of patients with an ACR20 response at month 6, approximately half (51.5%; 360/699) also achieved MDA. MDA achievement occurred in 70.6% of patients with an ACR50 response and 93.2% of patients with an ACR70 response.
Fig. 2.
Agreement between ACR and MDA responses at month 6
(A) Achievement of MDA at month 6 in patients who achieved the indicated ACR response. (B) Achievement of the indicated ACR response in patients who achieved MDA at month 6. Only patients with sufficient data for calculation of an ACR response were included in the analyses. MDA: minimal disease activity.
We also performed the corresponding analysis of ACR response rates in patients who achieved MDA at month 6. Of the 597 patients who achieved MDA at month 6, 394 had sufficient data for assessment of ACR response. Of these patients, 360 (91.4%) achieved an ACR20 response at month 6, 77.9% achieved an ACR50 response and 52.3% achieved an ACR70 response (Fig. 2B). In the 772 patients who did not achieve MDA at month 6, ACR response rates were 43.9%, 16.6% and 1.9% for achievement of ACR20, ACR50 and ACR70, respectively.
Discussion
In controlled clinical trials, MDA has been shown to be a valuable tool for assessing the need for therapeutic changes in patients with PsA; however, the applicability of this evaluation to routine clinical care has not been well studied. Our findings indicate that MDA is a stable and valid assessment of disease activity during ‘real-world’ care; patients with PsA who achieved MDA at month 6 after initiation of adalimumab tended to maintain this status for at least 24 months. MDA, in conjunction with clinical judgement, thus appears to have the potential to provide a solid basis for decisions concerning therapeutic modifications in treat-to-target strategies during routine clinical care.
In this large observational study of 1684 patients with long-standing active PsA who initiated treatment with adalimumab and completed 6 months of therapy, 35.5% achieved MDA at month 6 as assessed by modified criteria. These results are in agreement with post hoc analyses from a randomized, placebo-controlled trial of adalimumab in patients with PsA, in which 39% of patients treated with adalimumab achieved MDA at month 6 vs 7% of patients who received placebo [3]. Compared with patients who did not achieve MDA at month 6, those who achieved MDA at month 6 in our study were more likely to be men and have less severe disease. Other studies have also found that male patients with PsA are more likely to achieve MDA than female patients [16–19]; the reasons for this discrepancy remain unclear.
As in other studies of patients with PsA treated with TNF inhibitors [20, 21], we noted an increase in the proportion of patients achieving MDA over time in patients remaining on therapy (from 35.5% at month 6 to 45.5% at month 24). Among patients who did not achieve MDA at month 6 and remained on therapy, 26% achieved MDA at month 24. These results may be due in part to responder bias, as patients benefitting from the drug are more likely to stay in the study. However, other studies also suggest that a longer treatment duration may be required to achieve MDA in some patients, including a retrospective study of patients treated with TNF inhibitors in clinical practice that reported a mean time to MDA of 1.30 years [19]. We observed particularly large increases (>10%) in the proportion of patients meeting MDA criteria for joints (SJC and TJC ⩽1) between 6 and 24 months. In contrast, the proportion of patients meeting criteria for skin and pain showed minimal increases over this time period. These findings suggest >6 months may be required to realize the full effect of adalimumab on joints in some patients.
More than 75% of patients with MDA at month 6 who stayed on therapy had MDA at subsequent visits, indicating that MDA status at month 6 is a stable indicator of therapeutic response over the following 18 months. There is evidence that sustained MDA is critical for optimal patient outcomes, as achievement of MDA at ⩾3 visits (minimum of 38 weeks) was significantly associated with reduced radiographic progression in a study of the TNF inhibitor golimumab [20].
In agreement with Lubrano and colleagues [21], who evaluated 124 patients with PsA receiving TNF inhibitor therapy, we found that the achievement of MDA was highly correlated with PGA ⩽2. We further found that TJC ⩽1, pain ⩽1 and functional remission (FFbH ⩾83%) were highly correlated with MDA. The high correlations between MDA and global assessments such as pain and functional remission support the validity of this instrument in routine daily care. Pain was the most difficult criterion to meet. Only 2.3% of patients had pain ⩽1 at baseline; this percentage increased to 21.1% at month 6. Pain was also the most commonly unmet MDA criterion in a study from the Biologic Treatment Registry Across Canada registry [22]. The influence of pain on therapeutic response in PsA is consistent with findings from our study of patients with RA, in which pain showed a high correlation with therapeutic response as assessed by DAS28 [23], and with a recent study of 83 patients with PsA who presented to a hospital clinic in Argentina, which also found that the pain score criterion was the least likely to be achieved (29% of patients overall and 56% of patients with MDA) [24]. In contrast, BSA ⩽3% and enthesitis showed only low correlations with MDA and did not correlate well with other MDA criteria. The most frequently met individual MDA criteria were absence of enthesitis, SJC ⩽1, and BSA ⩽3%. These findings may be relevant to future revisions of MDA criteria for PsA. For instance, in our study, the assessment of enthesitis appeared to have minimal contributions to MDA classification, perhaps due to difficulties in accurately assessing this disease characteristic.
In published studies, several baseline variables, including shorter symptom or disease duration, male sex, younger age, higher inflammatory marker levels and lower BMI [16–19, 22, 25, 26], have been associated with the achievement of MDA. Our method for evaluating predictors of MDA was different from these studies, as we confined our analyses to baseline characteristics included in subsequent MDA assessments. In agreement with other reports [16–18, 20], we found that a better functional status at baseline was a key predictor of MDA at month 6. It is possible that patients with better function have less irreversible bone damage and thus have a greater likelihood of benefitting from effective therapy. We also identified lower TJC at baseline as a predictor of MDA at month 6. The fact that only two of the seven MDA characteristics are significant predictors of MDA at month 6 suggests that baseline characteristics do not have an insurmountable influence on treatment outcome as assessed by MDA. Accordingly, even patients with extensive skin and SJC involvement may be able to achieve MDA during adalimumab treatment.
Responses as assessed by MDA and ACR measure two distinct, but overlapping aspects of disease activity. MDA assesses the absolute level of disease activity, whereas ACR responses measure improvement from baseline in disease activity. These two types of evaluations are affected in opposite directions by high baseline disease activity: disease activity criteria such as MDA are more difficult to achieve with high baseline disease activity, whereas responder criteria are easier to achieve [27]. ACR criteria also differ from MDA in that they do not include skin or enthesitis. Our evaluation of the rate of MDA achievement in patients with ACR responses found that only approximately half of patients with an ACR20 response at month 6 also achieved MDA at this time point. This finding suggests that an ACR20 response does not signify effective control of disease activity; even an ACR50 response was not associated with MDA in almost 30% of patients. Conversely, over 90% of patients with an MDA response at month 6 also had an ACR20 response and 78% had an ACR50 response, supporting the validity of MDA in reflecting meaningful improvements. Although MDA and ACR may serve as complementary assessments during clinical trials, MDA appears to provide a better option for tracking disease activity during routine clinical care. In particular, it may provide a more objective perspective on patient health than clinical opinion. In a recent study conducted in the Netherlands, 35% of patients with PsA who were considered by their clinician to have an acceptable disease state did not fulfil criteria for MDA [28], a finding that ‘should challenge clinicians to incorporate these simple assessments into their routine patient care and strive for better outcomes for all patients’ [29].
Limitations of this study include reductions in the number of patients during the course of the study due to patients withdrawing or being lost to follow-up. In addition, in order to utilize data available for this population, MDA criteria were modified slightly. In particular, the criterion for pain used in this study (pain ⩽1) was more stringent than the published MDA criterion of pain ⩽1.5 cm [1]. However, a sensitivity analysis of the proportion of patients achieving the less stringent criterion of pain ⩽2 found that even with this definition, pain continued to be the most difficult MDA criterion to achieve (5.8% of patients at baseline and 36.3% of patients at month 6). We therefore feel confident that our use of a modified pain criterion did not substantially alter the MDA results.
In conclusion, MDA provides a stable assessment of disease activity in patients initiating treatment with adalimumab. Our findings thus support the use of MDA to help guide treatment decisions during routine clinical care. However, it is important to note that some patients may take longer than 6 months to achieve MDA. For patients who are tolerating therapy and showing signs of improvement but have not yet achieved MDA, it may be appropriate to consider continuing treatment for >6 months before suggesting a change in therapy, particularly if pain and skin parameters have improved but joint counts have not yet reached MDA status.
Supplementary Material
Acknowledgements
The authors wish to thank Sharon L. Cross, PhD, who provided medical writing services on behalf of the Centrum for Innovative Diagnostik und Therapie Rheumatologie/Immunologie (CIRI), Frankfurt am Main, Germany, under contract with AbbVie Deutschland GmbH & Co. KG for medical writing services.
Funding: This work was supported by AbbVie Deutschland GmbH & Co. KG, which provided funding for the observational studies, data analysis and manuscript support.
Disclosure statement: F.B., M.K., M.S., E.C.S., H.B. and H.-P.T. received speaker fees or compensation for consultation from AbbVie. H.G. is a paid consultant for AbbVie. G.G. is an employee of AbbVie.
References
- 1. Coates LC, Fransen J, Helliwell PS.. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 2. Coates LC, Helliwell PS.. Validation of minimal disease activity criteria for psoriatic arthritis using interventional trial data. Arthritis Care Res 2010;62:965–9. [DOI] [PubMed] [Google Scholar]
- 3. Mease PJ, Heckaman M, Kary S, Kupper H.. Application and modifications of minimal disease activity measures for patients with psoriatic arthritis treated with adalimumab: subanalyses of ADEPT. J Rheumatol 2013;40:647–52. [DOI] [PubMed] [Google Scholar]
- 4. Coates LC, Moverley AR, McParland L. et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicenter, open-label, randomised controlled trial. Lancet 2015;386:2489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silverman SL. From randomized controlled trials to observational studies. Am J Med 2009;122:114–20. [DOI] [PubMed] [Google Scholar]
- 6. Behrens F, Koehm M, Arndt U. et al. Does concomitant methotrexate impact treatment outcomes with adalimumab in psoriatic arthritis patients? An in-depth analysis of data from a large observational study. J Rheum 2016;43:632–9. [DOI] [PubMed] [Google Scholar]
- 7. Behrens F, Koehm M, Thaçi D. et al. Anti-citrullinated protein antibodies are linked to erosive disease in an observational study of patients with psoriatic arthritis. Rheumatology 2016;55:1791–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raspe HH, Hagedorn U, Kohlmann T, Mattussek S.. Der Funktionsfragebogen Hannover (FFbH): Ein Instrument zur Funktionsdiagnostik bei polyartikulären Gelenkerkrankungen In: Siegrist J, ed. Wohnortnahe Betreuung Rheumakranker. Ergebnisse sozialwissenschaftlicher Evaluation eines Modellversuches. Stuttgart: Schattauer, 1990: 164–82. [Google Scholar]
- 9. Lautenschläger J, Mau W, Kohlmann T. et al. Comparative evaluation of a German version of the Health Assessment Questionnaire and the Hannover Functional Capacity Questionnaire. Z Rheumatol 1997;56:144–55. [DOI] [PubMed] [Google Scholar]
- 10. Westhoff G, Listing J, Zink A.. Loss of physical independence in rheumatoid arthritis; interview data from a representative sample of patients in rheumatologic care. Arthritis Care Res 2000;13:11–22. [PubMed] [Google Scholar]
- 11. Aletaha D, Machold KP, Nell VP, Smolen JS.. The perception of rheumatoid arthritis core set measures by rheumatologists. Results of a survey. Rheumatology 2006;45:1133–9. [DOI] [PubMed] [Google Scholar]
- 12. Nagasawa H, Kameda H, Sekiguchi N, Amano K, Takeuchi T.. Normalisation of physical function by infliximab in patients with RA: factors associated with normal physical function. Clin Exp Rheumatol 2010;28:365–72. [PubMed] [Google Scholar]
- 13. Listing J, Strangfeld A, Rau R. et al. Clinical and functional remission: even though biologics are superior to conventional DMARDs overall success rates remain low—results from RABBIT, the German biologics register. Arthritis Res Ther 2006;8:R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Felson DT, Anderson JJ, Boers M. et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. [DOI] [PubMed] [Google Scholar]
- 15. Woolson RF. . Multiple linear regression In: Woolson RF, ed. Statistical Methods for the Analysis of Biomedical Data. New York: John Wiley & Sons, Inc, 1987: 295–300. [Google Scholar]
- 16. Theander E, Husmark T, Alenius GM. et al. Early psoriatic arthritis: short symptom duration, male gender and preserved physical functioning at presentation predicts favourable outcome at 5-year follow-up. Results from the Swedish Early Psoriatic Arthritis Register. Ann Rheum Dis 2014;73:407–13. [DOI] [PubMed] [Google Scholar]
- 17. Perrotta FM, Marchesoni A, Lubrano E.. Minimal disease activity and remission in psoriatic arthritis patients treated with anti-TNF-α drugs. J Rheumatol 2016;43:350–5. [DOI] [PubMed] [Google Scholar]
- 18. Lubrano E, Parsons WJ, Perrotta FM.. Assessment of response to treatment, remission, and minimal disease activity in axial psoriatic arthritis treated with tumor necrosis factor inhibitors. J Rheumatol 2016;43:918–23. [DOI] [PubMed] [Google Scholar]
- 19. Haddad A, Thavaneswaran A, Ruiz-Arruza I. et al. Minimal disease activity and anti-tumor necrosis factor therapy in psoriatic arthritis. Arthritis Care Res 2015;67:842–7. [DOI] [PubMed] [Google Scholar]
- 20. Kavanaugh A, van der Heijde D, Beutler A. et al. Radiographic progression of patients with psoriatic arthritis who achieve minimal disease activity in response to golimumab therapy: results through 5 years of a randomized, placebo-controlled study. Arthritis Care Res 2016;68:267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lubrano E, Perrotta FM, Parsons WJ, Marchesoni A.. Patient’s global assessment as an outcome measure for psoriatic arthritis in clinical practice: a surrogate for measuring low disease activity? J Rheumatol 2015;42:2332–8. [DOI] [PubMed] [Google Scholar]
- 22. Rahman P, Zummer M, Bessette L. et al. Real-world validation of the minimal disease activity index in psoriatic arthritis: an analysis from a prospective, observational, biological treatment registry. BMJ Open 2017;7:e016619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scharbatke EC, Behrens F, Schmalzing M. et al. Association of improvement in pain with therapeutic response as determined by individual improvement criteria in patients with rheumatoid arthritis. Arthritis Care Res 2016;68:1607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marin J, Acosta Felquer ML, Ferreyra Garrott L. et al. Patients with psoriatic arthritis fulfilling the minimal disease activity do not have swollen and tender joints, but have active skin. J Rheumatol 2016;43:907–10. [DOI] [PubMed] [Google Scholar]
- 25. Iervolino S, Di Minno MN, Peluso R. et al. Predictors of early minimal disease activity in patients with psoriatic arthritis treated with tumor necrosis factor-α blockers. J Rheumatol 2012;39:568–73. [DOI] [PubMed] [Google Scholar]
- 26. Eder L, Thavaneswaran A, Chandran V, Cook RJ, Gladman DD.. Obesity is associated with a lower probability of achieving sustained minimal disease activity state among patients with psoriatic arthritis. Ann Rheum Dis 2015;74:813–7. [DOI] [PubMed] [Google Scholar]
- 27. Aletaha D, Landewe R, Karonitsch T. et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Ann Rheum Dis 2008;67:1360–4. [DOI] [PubMed] [Google Scholar]
- 28. van Mens LJJ, Turina MC, van de Sande MGH. et al. Residual disease activity in psoriatic arthritis: discordance between the rheumatologist’s opinion and minimal disease activity measurement. Rheumatology 2018;57:283–90. [DOI] [PubMed] [Google Scholar]
- 29. Coates LC. How routine use of a treat to target approach in PsA might impact on clinical decision making. Rheumatology 2018;57:209–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.