Abstract
Several factors such as chromosomal translocations, gene mutations, and polymorphisms are involved in the pathogenesis of leukemia/lymphoma. Recently, the role of vitamin D (VD) and vitamin D receptor (VDR) polymorphisms in hematologic malignancies has been considered. In this review, we examine the possible role of VD levels, as well as VDR polymorphisms as prognostic biomarkers in leukemia/lymphoma. Relevant English language literature were searched and retrieved from Google Scholar search engine (1985-2017). The following keywords were used: vitamin D, vitamin D receptor, leukemia, lymphoma, and polymorphism. Increased serum levels of VD in patients with leukemia are associated with a better prognosis. However, low VD levels are associated with a poor prognosis, and VDR polymorphisms in various leukemias can have prognostic value. VD biomarker can be regarded as a potential prognostic factor for a number of leukemias, including acute myeloblastic leukemia (AML), chronic lymphoblastic leukemia (CLL), and diffuse large B-cell lymphoma (DLBCL). There is a significant relationship between different polymorphisms of VDR (including Taq I and Fok I) with several leukemia types such as ALL and AML, which may have prognostic value.
Key words: Vitamin D, vitamin D receptor, leukemia, lymphoma, polymorphism
Introduction
Vitamin D (VD) is a fat-soluble vitamin and an endocrine hormone that plays a role in bone formation and integrity via calcium and phosphate absorption from the intestine and their transfer to bones. VD is also involved in proliferation and differentiation of different malignancies including prostate, breast, bone and leukemias. Effect of VD as hormone on metabolism and immunecell regulation of skin is also discussed. Studies have been reported immunomodulatory effect of VD and its analogues as skin protecting agents during exposure to UV.1 Also, modification of vitamin D receptor (VDR) can be a clinical approach in multiple cancers like leukemias.2 25-hydroxyvitamin D3 (25(OH) D3) and 1alpha 25-hydroxyvitamin D3 (1α 25(OH)2D3) are the most abundant and most active forms of VD, respectively. A low level of 25(OH)2D3 is associated with a poor function of the immune system. Several genes appear to respond to 1α 25(OH)2D3,3 and the fact that many genes are affected by VD further illustrates the importance of studying VD and its receptor polymorphisms. VD is involved in the process of cell proliferation, angiogenesis, and even metastasis through the regulation of gene expression.4 High serum levels of VD in different malignancies, including breast, colorectal, and prostate cancers, are associated with the reduced incidence risk of these cancers.5-10 It has also been shown that VD plays a positive role in preventing angiogenesis as well as metastasis and that it has the ability to modulate the innate and adaptive immune system, affecting the proliferation of T helper type 1 (Th1) lymphocytes by repressing immune factors such as interferon gamma (IFNγ). VD is also capable of controlling the differentiation of immune cells such as Dendritic Cells (DCs), which indicates its immunosuppressive role along with calcium (Ca) homeostasis in immune processes.3
VDR is a polymorphic gene. rs1544410 (Bsm I), rs7975232 (Apa I), rs2228570 (Fok I), rs731236 (Taq I), and rs11568820 (Cdx2) are among the polymorphisms specifically addressed in this review. Essentially, these polymorphisms are enzymes that are distinguished due to the variances in their restriction enzyme cleavage sites.11 VDR is a transcriptional factor (TF) binding 1α 25(OH)2D3 in extremely low concentrations, and the effects of VD hormone are thus mediated by VDR.3 The relationship between VDR and the pathogenesis of prostate, breast, melanoma, and colorectal cancers has been reported.5-7,12,13 In recent years, due to further attention to VDR, the prominent role of this TF in various signaling pathways, including mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/ATK/mTOR), has been demonstrated. The importance of this involvement is that the mentioned signaling pathways can lead to cellular differentiation during the signaling process in collaboration with VDR.10 Extensive evidence suggests the regulatory role of VDR in various signaling pathways. VDR can regulate different components of several pathways, thereby influencing apoptosis, autophagy, cellular adhesion, and other processes effective upon the pathophysiology of leukemia/lymphoma.3 It also affects the growth and proliferation of lymphocytes in the immune system by targeting immune mediators. 14,15
The above-mentioned findings demonstrate the evident role of VD in immune and hematopoietic functions, which, as previously mentioned, are mostly mediated by VDR. In this review, we will discuss the likely role of VD level as a prognostic biomarker in leukemia, we will refer to the significant relationship between VDR polymorphisms and various types of leukemia, and for the first time we will thoroughly review the relationship between VD and VDR polymorphisms in leukemia.
Vitamin D and gene regulation
In general, the involvement of VDR in nine signaling pathways has been reported. Among the cellular signaling pathways associated with VDR, three pathways have been studied more extensively than others: Lipid Signaling Pathway, PI3K Pathway, and MAPK pathway, in which VD plays an important role.4 Studies have identified a group of hematopoietic regulator networks, including a network with presence of VDR that is involved in the differentiation of granulocytes and monocytes. On the other hand, the involvement of 1α 25(OH)2D3 in monocyte differentiation has been indicated.3 In a study using Iregulon plugin in Cytoscape software, 1045 target genes have been recognized for VDR.16 Following the classification of these genes based on their biological roles, it was found that 68 genes were present in cancer-related pathways, and that 40 genes were among the markers dysregulated in various cancers. In addition, 54 genes were among the factors that played a role in MAPK signaling pathway (Table 1). This classification was based on the Kyoto encyclopedia of genes and genome (KEGG) database.17 MAPK pathway has been recognized as one of the most important pathways involved in the development of cancers, especially leukemias.18
Table 1.
Evaluation of VDR targets by Iregulon plugin and cytoscape software.
| ID category | Category name | Benjamini | P-value | Fold enrichment | N. Genes |
|---|---|---|---|---|---|
| hsa05200 | Pathways in cancer | 2.53E-12 | 2.57E-15 | 2.762 | 68 |
| hsa04020 | Calcium signaling pathway | 2.22e-11 | 4.50e-14 | 3.4 | 46 |
| hsa04360 | Axon guidance | 1.61e-8 | 5.14e-11 | 3.484 | 34 |
| hsa04971 | Gastric acid secretion | 1.61e-8 | 6.55e-11 | 4.45 | 25 |
| hsa04010 | MAPK signaling pathway | 2.76e-8 | 1.40e-10 | 2.502 | 54 |
| hsa04725 | Cholinergic synapse | 5.79e-8 | 4.11e-10 | 3.56 | 30 |
| hsa05202 | Transcriptional dysregulation in cancer | 5.79e-8 | 4.02e-10 | 2.909 | 40 |
| hsa04960 | Aldosterone-regulated sodium reabsorption | 1.59e-7 | 1.29e-9 | 5.609 | 17 |
| hsa04510 | Focal adhesion | 2.66e-7 | 2.43e-9 | 2.667 | 42 |
| hsa05146 | Amoebiasis | 1.92e-6 | 1.95e-8 | 3.21 | 28 |
Vitamin D level in leukemia/lymphoma
Most patients with newly diagnosed acute myeloblastic leukemia (AML) show VD deficiency, and low levels of VD are significantly associated with poor disease outcomes, but higher VD levels are related with a better outcome.19 VD level seems to be related to translocation type; for example, most patients with internal tandem duplications of fms-like tyrosine kinase 3 (FLT3- ITD) are associated with low VD levels, and the improvement of VD serum levels can entail better outcomes in patients.20
VD dicreases proliferation and increases differentiation of hematopoietic stem cell (HSC) via modulation of main regulatory pathways for HSC proliferation and survival such as MAPK pathway and PI3K/AKT/mTOR.21 So hypothetically and based on this finding we can assume that VD and its analogues can inhibit excess proliferation of Leukemic stem cell (LSC) in leukemias. Also, it has been reported that co-treatment of leukemic cells with VD or its analogues modulate proliferation and differentiation of leukemic cells through changing the expression of hematopoietic growth factors, cytokines and their receptors.22 In addition, studies suggested that the effect of VD and its analogues as a differentiation therapy on leukemic cells relied on different cytogenetic abnormalities of leukemic blast cells.23 It is of note that combination of antioxidants and ceramide derivatives with VD or its analogues not only increased differentiation related cell cycle arrest of leukemic cells but also limited side effects of VD like hypercalcemia. Such differentiation agents in combination of VD can be an effective candidate for the treatment of leukemias.24,25 It is interesting that hematopoietic differentiation control of VD derivatives is attributed to inhibition and induction of erythroid and monocytic differentiation, respectively.26,27 It has been suggested that p27kip1, a cell cycle regulator of MAPK pathway, is a target gene of miR-181a and modulation of this miR can confer differentiation in cell lines such as HL60 and U937.28 So, hypothetically modulation of miR-181a could be of therapeutic importance in AML. Evaluation of low VD levels and their effects on Azacitidine, which is a chemotherapy drug used in patients with secondary oligoblastic AML and myelodysplastic syndrome (MDS), showed that elevating VD levels following treatment with this drug could lead to increased survival of patients. The in vitro synergistic antiproliferative effect of Azacitidine in combination with VD has likely led to this improvement.29 In children with acute lymphoblastic leukemia (ALL), it has also been shown that low levels of VD and the consequent defect in Ca homeostasis are directly related to clinical outcomes of ALL patients, including skeletomuscular pain.12
In Chronic Lymphoblastic Leukemia/Small Lymphoblastic Lymphoma (CLL/SLL), inadequate levels of VD have been associated with decreasing time to treatment and undesirable overall survival (OS) in patients. Assessment of the efficacy and safety of VD supplementation indicated that VD levels could be corrected without any risk for patients by administering different VD doses as required.30,31 The result of this study confirmed the prognostic role of VD levels in CLL/SLL since the VD levels have shown a significant correlation with OS. In Follicular Lymphoma (FL), there is a strong correlation between low VD levels and a poor outcome of FL.32 The study of cutaneous T-cell lymphoma (CTCL) patients with Mycosis Fungoides and Sezary’s Syndrome showed that the correction of VD deficiency and the type of supplement had no effect on overall clinical response, while vitamin deficiency affected the reduced synthesis of antimicrobial peptides mediated by VDR pathway, which was possibly associated with chronic infections in CTCL patients.33 Among Non-Hodgkin’s Lymphomas (NHL), Diffuse Large B-cell Lymphoma (DLBCL) patients having high interleukin 10 (IL-10) levels are associated with a poorer event-free survival (EFS) than those with lower IL- 10 levels.34 IL-10 is a target of VDR,35 and perhaps the use of VD and its analogues repress this cytokine through VDR mediation. Investigation of the relationship between VD deficiency with DLBCL and T-cell lymphoma revealed that VD deficiency was associated with inferior OS and EFS in both diseases.36 In DLBCL patients treated with Rituximab, VD deficiency has been introduced as a risk factor, because VD deficiency inhibits the Rituximab-mediated toxicity; therefore, VD correction could increase the efficacy of Rituximab.37 There are also reports of the prognostic role of VD in other hematologic malignancies; for example, VD deficiency is an undesirable prognostic marker in multiple myeloma (MM).38,39 Thus, considering these findings, we can hypothesize that not only the prevalence of VD deficiency is high in hematologic malignancies, but it reduces the response of these patients to treatment. It is recommended to conduct clinical trials to evaluate the effect of VD supplementation on the therapeutic outcomes of these patients. Increasing Ca concentrations in CLL patients is associated with increased survival and proliferation of B-cells, as well as their resistance to apoptosis.40
Role of vitamin D receptor polymorphisms in leukemias
Acute leukemias
Apa I, Fok I, Taq I, and Bsm I are important polymorphisms of VDR gene, which have been closely correlated with AML. For example, Taq I expression is associated with Complete Remission (CR) and prognosis, so that 70% of CR patients have the TC genotype and 30% have TT genotype of Taq I polymorphism.41 In the study of children with ALL, Apa I, Taq I, Bsm I, Cxd2, and GATA polymorphisms have been evaluated. In ALL patients, Bone Mineral Density (BMD) is damaged due to corticosteroid and methotrexate (MTX) consumption. Since the Tt genotype of Taq I and Bb genotype of Bsm I are related with a higher BMD in ALL patients, it is likely that the patients harboring these polymorphisms show a better response to treatment and be more resistant to drug-induced damage42 (Table 2).
Table 2.
Different genotypes of Taq I polymorphism in acute leukemias.
| Effect of genotype | Chromosome | Leukemia | Ref. |
|---|---|---|---|
| Tt genotype is associated with higher BMD | 12q13.11 | ALL | 42 |
| TC and TT genotypes are associated with CR | 12q13.11 | AML | 41 |
ALL: acute lymphoblastic leukemia; AML: acute myeloblastic leukemia; BMD: Bone mineral density; CR: Complete remission.
Chronic leukemias
The analysis of Fok I polymorphism in Chronic Myeloblastic Leukemia (CML) patients showed that ff was the dominant genotype among patients.43 This allele has already been shown to be associated with an increased risk of T-cell lymphoma.44 According to these findings, it may be assumed that the f allele has an uncertain role in the pathogenesis of CML, and further research is needed to understand its role and effect on prognosis of the disease, while this allele might also be used as a prognostic factor because its presence is related with a higher risk of T-cell lymphoma.
The antagonistic effect of microRNA-214 (miR-214) on VDR signaling and inhibiting Hedgehog (Hh) signaling has been reported. 45 Studies have shown that Hh antagonists may play a role in the treatment of CML and B-ALL.46,47 VDR antagonists inhibiting Hh signaling are likely to treat patients with CML and even patients with other leukemia types. There is a relationship between the mutated Ff genotype of Fok I polymorphism and the increased risk of CLL, but this relationship has little effect on the clinical outcome of the disease48 (Table 3).
Table 3.
Different genotypes of Fok I polymorphism in chronic leukemias.
| Effect of genotype | Chromosome | Leukemia | Ref. |
|---|---|---|---|
| Ff genotype is associated with higher risk for CLL | 12q13.11 | CLL | 48 |
| Ff genotype has a significant correlation with CML | 12q13.11 | CML | 43 |
CLL: chronic lymphoblastic leukemia; CML: chronic myeloblastic leukemia.
Lymphomas
Fok I polymorphism in Plasmablastic Lymphoma (PBL) potentiates tumor growth inhibition.49 A relationship has been reported between Taq I, Fok I, and Bsm I variants with some types of NHL; for example, the B allele of Bsm I polymorphism and t allele of Taq I polymorphism are associated with DLBCL, and the f allele of Fok I polymorphism is related with T-cell lymphoma.44 The study of Hodgkin’s lymphoma (HL) and NHL cases showed that VDR was strongly expressed on HL tumor cells, and it was concluded that VDR was a diagnostic factor for HL. However, as there was not a high expression of VDR in NHL cases, the same function of VDR in NHL was not addressed by this study.50 In another study, no correlation was found between Fok I, Bsm I, Taq I, Apa I, and Cxd2 variants with disease progression in HL patients.51 Increased interleukin-6 (IL-6) is a major cause of anemia in HL,52 and because IL-6 gene is a target of VDR, it is possible to manipulate VDR and affect the expression of IL-6 gene to reduce anemia complications in HL patients. Figure 1 shows vitamin D metabolism and targets of vitamin D receptor.
Figure 1.
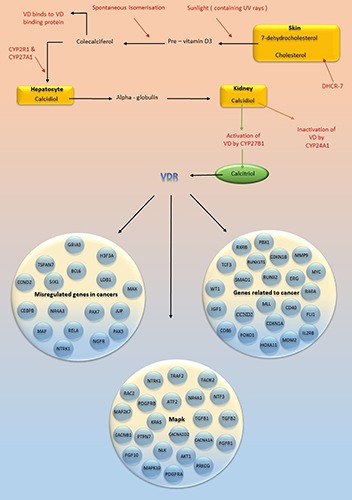
Vitamin D metabolism and targets of vitamin D receptor are shown in this figure. Vitamin D receptor targets different genes and thus affects biological functions. VD: vitamin D; CYP: cytochrome P; DHCR-7: 7-Dehydrocholesterol reductase; VDR: vitamin D receptor; TSPAN7: Tetraspanin-7; BCL6: B-cell lymphoma 6 protein; LDB1: LIM domain-binding protein 1; MAX: myc-associated factor X; CEBPB: CCAAT/enhancer-binding protein beta; NR4A3: nuclear receptor subfamily 4, group A, member 3; RXRB: Retinoid X receptor beta; TCF3: Transcription factor 3; CDKN1B: Cyclin-dependent kinase inhibitor 1B; MMP9: Matrix metallopeptidase 9; ERG: ETS-related gene; WT1: Wilm's tumor; IGF1: Insulin-like growth factor 1; MLL: myeloid/lymphoid or mixed-lineage leukemia; RARA; Retinoic acid receptor alpha;FLI1: Friend leukemia integration 1; MDM2: Mouse double minute 2 homolog; IL2RB: Interleukin-2 receptor subunit beta; TRAF2: TNF receptor-associated factor 2; RAC2: Ras-related C3 botulinum toxin substrate 2; PDGFR: platelet-derived growth factor receptor; ATF2: Activating transcription factor 2; TGFB: Transforming growth factor beta; PTPN7: Protein tyrosine phosphatase non-receptor type 7; AKT1: RAC-alpha serine/threonine-protein kinase; FGFR1: Fibroblast growth factor receptor 1 ; FGF10; Fibroblast growth factor 10; MAPK10: Mitogen-activated protein kinase 10; PRKCG: Protein kinase C gamma type.
Other blood disorder
In MM, the TT genotype of Taq I and the mutated C allele of Taq I have a significant relationship with increased risk of disease. 53 Another study reported that Fok I polymorphism inhibited tumor growth in MM patients.49 A study has indicated that DCs in MM patients have anabnormal function and that their defects are related to tumorigenicity in cancer. This study showed that enhanced IL-6 production by tumor, which was correlated with DC deficiency, led to the inhibition of precursor DC colonies and switched commitment of these CD34+ cells to monocytes.54 On the other hand, studies have shown that IL-6 is among VDR targets repressing this cytokine.15 Therefore, manipulation of VDR may reduce tumorigenesis of cancer by affecting IL-6.
In Aplastic Anemia (AA), GG genotype and G allele of Bsm I polymorphism are correlated with the increased risk of AA. Moreover, the supportive role of GA genotype and G allele of Bsm I has been raised in this disease. Carriers of TT genotype from Taq I polymorphism show a poor response to treatment and even have a higher risk of MDS/AML transformation,55 and Taq I polymorphism could be considered as a prognostic biomarker for AA. Decreased expression of VDR may be related with hyperimmunity of AA patients, and VD supplementation may be able to partially correct the abnormal immune function of patients through the effects of VDR signaling pathway (Table 4).56
Table 4.
VDR Polymorphisms related with leukemias.
| VDR gene polymorphism | Allele-genotypes | Chr. | Effection mechanism in prognosis | Leukemia | Ref. |
|---|---|---|---|---|---|
| Taq I | TT and TC | 12q13.11 | CR and GP | AML | 41 |
| Taq I | Tt | 12q13.11 | Higher BMD | ALL | 42 |
| Bsm I | Bb | 12q13.11 | Higher BMD | ||
| Fok I | F | 12q13.11 | Probable role in CML pathogenesis | CML | 43 |
| Fok I | Ff | 12q13.11 | Higher risk for CLL | CLL | 48 |
| - | - | - | Strong expression of VDR may be a marker for HL tumor cells | HL | 50 |
| Taq I | T | 12q13.11 | Correlated with DLBCL | DLBCL | 44 |
| Fok I | F | 12q13.11 | Correlated with T cell lymphoma | T cell lymphoma | 44 |
| Taq I | TT and C | 12q13.11 | Correlated with MM | MM | 53 |
| Bsm I | GG | 12q13.11 | Higher risk for AA | AA | 55 |
| Taq I | TT | 12q13.11 | Poor response to treatment |
ALL: acute lymphoblastic leukemia; AML: acute myeloblastic leukemia; CLL: chronic lymphoblastic leukemia; CML: chronic myeloblastic leukemia; CR: Complete Remission; GP: Good Prognosis; BMD: Bone Mineral Density; HL: Hodgkin Lymphoma; DLBCL: Diffuse Large B Cell Lymphoma; MM: Multiple Myeloma; AA: Aplastic Anemia.
Discussion
Several genes are affected by VD and its receptor (VDR), through which VD regulates gene expression in vital biological processes and metastasis; on the other hand, VDR plays a role in cell differentiation via signaling pathways such as MAPK and PI3K3,4,10 (Table 5). In this regard, anticancer activity of VDR is attributed to its inhibitory and promotion effect on proliferation and differentiation of malignant cells, respectively. Increased expression of p21 and p27 and consequently G0/G1 cell-cycle arrest is involved in differentiation process which is induced by VDR. So, VD, VDR and its analogues as differentiative inducer agents can be a therapeutical approach for leukemias in future.57 The above findings justify the role and effect of VD and VDR in leukemia/lymphoma. A higher level of VD in serum of AML patients is associated with a good response to treatment, longer survival, and better prognosis; therefore, low VD levels can be considered as a risk factor that can be readily and economically improved.19,20,29 Overall, low VD levels can be regarded as a biomarker of poor prognosis in patients with AML and ALL, which is associated with an unfavorable OS in patients with SLL/CLL and T-cell lymphoma.12,19,30,31,36 Low VD is also indicative of a poor prognosis in MM.38,39 Taq I seems to be a biomarker of good prognosis in ALL and AML, and mutated Fok I phenotypes due to the presence of f allele could be used as a prognostic marker in CML and CLL patients.41-43,48 This is while Fok I in MM and PBL may be associated with a good prognosis.49 Taq I polymorphism in MM is associated with an increased risk of associated disease and has prognostic value.53 In AA, Bsm I and Taq I are associated with a poor prognosis.55
Table 5.
VDR targets in leukemias.
| Target gene | Chro. | Type of effect | Leukemia | Ref. |
|---|---|---|---|---|
| CAMP | 3p21.31 | Unknown | AML | 65 |
| CDKN1B | 12p13.1 | Activation | AML | 62 |
| CYP1A1 | 15q24.1 | Unknown | AML | 64 |
| EGFR | 7p11.2 | Repression | AML | 64 |
| HOXA10 | 7p15.2 | Activation | AML | 67 |
| IL-6 | 7p15.3 | Repression | AML | 15 |
| SKP2 | 5p13.2 | Repression | AML | 68 |
| CDKN1B | 12p13.1 | Unknown | ALL | 63 |
| IL-10 | 1q32.1 | Repression | ALL | 35 |
| CDKN1B | 12p13.1 | Unknown | CML | 63 |
| CYP1A1 | 15q24.1 | Unknown | CML | 64 |
| HOXA10 | 7p15.2 | Activation | CML | 67 |
| IL-2 | 4q27 | Repression | CLL | 14 |
| IL-6 | 7p15.3 | Repression | CLL | 15 |
| BGLAP | 1q22 | Unknown | MM | 69 |
| CKN1B | 12p13.1 | Activation | MM | 62 |
| IL-6 | 7p15.3 | Repression | MM | 15 |
| SKP2 | 5p13.2 | Repression | MM | 68 |
| CDKN1B | 12p13.1 | Activation | HL | 63 |
| ERBB2 | 17q12 | Unknown | HL | 70 |
| IL-10 | 1q32.1 | Repression | HL | 35 |
CAMP: cathelicidin antimicrobial peptide; CDKN1B: cyclin dependent kinase inhibitor 1B; CYP1A1: cytochrome P450 family 1 subfamily A member 1; EGFR: Epidermal growth factor receptor; HOXA10: homeobox A10; IL-6: interleukin-6; SKP2: S-phase kinase associated protein 2; IL-10: interleukin-10; IL-2: interleukin-2; BGLAP: bone gamma-carboxyglutamate protein; ERBB2: erb-b2 receptor tyrosine kinase 2.
The association between different miRs and VD and VDR should be studied more because of possible therapeutic role of miRs. It has been suggested that the active form of VD exerts its anti-tumor effects by regulation of gene transcription and miR regulation. 58 MiR-214 is an antagonist of VDR. Studies indicate that miR-214 negatively regulates phosphatase and tensin homolog (PTEN) at protein level and activates the Akt signaling pathway, and that the reduced PTEN gene expression is related with a poor prognosis in AML. In patients with relapse, PTEN gene expression is lower than that of normal people. The role of miR-214 in the down regulation of PTEN gene has also been indicated in CLL.45,59-61 Thus, considering the fact that miR-214 is an antagonist of VDR, miR-214 manipulation could resolve the negative effect of this miR on VDR, improving the prognosis of AML and CLL patients by increasing the expression of PTEN gene. Furthermore, VDR can play an important role in future therapeutic strategies of cancer via targeting a number of targets, particularly IL-2, IL-6, IL-10 cytokines as well as CDKN1B gene.14,15,35,62-71 For instance, the aberrant IL-6 signaling contributes to cancer through signal transducer and activator of transcription 3 (STAT3). IL-2 and IL-6, which are among VDR targets, play a role in B-cell development. Therefore, the repression of these two cytokines by VDR following the consumption of VD and its analogues could have beneficial effects on CLL patients.14,15
Conclusion and future perspectives
In summary, it was shown in this review that VD and VDR polymorphisms could be used as prognostic biomarkers for various types of leukemia/lymphoma, including AML, CLL/SLL, DLBCL, T-cell lymphoma, MM and AA. There is a significant relationship between polymorphisms of Taq I, Fok I, and Bsm I with leukemia/lymphoma.
Acknowledgments
We wish to thank all our colleagues at Allied Health Sciences School, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
References
- 1.Reichrath J, Lehmann B, Carlberg C, et al. Vitamins as hormones. Horm Metab Res 2007;39:71-84. [DOI] [PubMed] [Google Scholar]
- 2.Kulling PM, Olson KC, Olson TL, et al. Calcitriol-mediated reduction in IFN-γ output in T cell large granular lymphocytic leukemia requires vitamin D receptor upregulation. J Steroid Biochem Mol Biol 2018;177:140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berridge MJ. Vitamin D cell signalling in health and disease. Biochem Biophys Res Commun 2015;460:53-71. [DOI] [PubMed] [Google Scholar]
- 4.Carlberg C, Campbell MJ. Vitamin D receptor signaling mechanisms: integrated actions of a well-defined transcription factor. Steroids 2013;78:127-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor JA, Hirvonen A, Watson M, et al. Association of prostate cancer with vitamin D receptor gene polymorphism. Cancer Res 1996;56:4108-10. [PubMed] [Google Scholar]
- 6.Garland CF, Gorham ED, Mohr SB, et al. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol 2007;103:708-11. [DOI] [PubMed] [Google Scholar]
- 7.Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prevent Med 2007;32:210-6. [DOI] [PubMed] [Google Scholar]
- 8.Hutchinson PE, Osborne JE, Lear JT, et al. Vitamin D receptor polymorphisms are associated with altered prognosis in patients with malignant melanoma. Clin Cancer Res 2000;6:498-504. [PubMed] [Google Scholar]
- 9.Slattery ML, Herrick J, Wolff RK, et al. CDX2 VDR polymor phism and colorectal cancer. Cancer Epidemiol Prevent Biomark 2007;16:2752-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim M, Mirandola L, Pandey A, et al. Application of vitamin D and derivatives in hematological malignancies. Cancer Lett 2012;319:8-22. [DOI] [PubMed] [Google Scholar]
- 11.Hall AC, Juckett MB. The role of vitamin D in hematologic disease and stem cell transplantation. Nutrients 2013;5:2206-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halton JM, Atkinson SA, Fraher L, et al. Mineral homeostasis and bone mass at diagnosis in children with acute lymphoblastic leukemia. J Pediatr 1995;126:557-64. [DOI] [PubMed] [Google Scholar]
- 13.John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and Breast Cancer Risk: The NHANES I Epidemiologic Follow-up Study, 1971–1975 to 19921. Cancer Epidemiol Biomark Prev 1999;8:399-405. [PubMed] [Google Scholar]
- 14.Alroy I, Towers TL, Freedman LP. Transcriptional repression of the interleukin-2 gene by vitamin D3: direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol Cell Biol 1995;15:5789-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masood R, Nagpal S, Zheng T, et al. Kaposi sarcoma is a therapeutic target forvitamin D 3 receptor agonist. Blood 2000;96:3188-94. [PubMed] [Google Scholar]
- 16.Verfaillie A, Imrichová H, Van de Sande B, et al. iRegulon: from a gene list to a gene regulatory network using large motif and track collections. PLoS Computat Biol 2014;10:e1003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogata H, Goto S, Sato K, et al. KEGG: Kyoto encyclopedia of genes and genomes. Nucl Acids Res 1999;27:29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertacchini J, Ketabchi N, Mediani L, et al. Inhibition of Rasmediated signaling pathways in CML stem cells. Cell Oncol 2015;38:407-18. [DOI] [PubMed] [Google Scholar]
- 19.Seyedalipour F, Mansouri A, Vaezi M, et al. High Prevalence of Vitamin D Deficiency in Newly Diagnosed Acute Myeloid Leukemia Patients and Its Adverse Outcome. Int J Hematol- Oncol Stem Cell Res 2017;11:209-16. [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HJ, Muindi JR, Tan W, et al. Low 25 (OH) vitamin D3 levels are associated with adverse outcome in newly diagnosed, intensively treated adult acute myeloid leukemia. Cancer 2014;120:521-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Studzinski GP, Harrison JS, Wang X, et al. Vitamin D control of hematopoietic cell differentiation and leukemia. J Cell Biochem 2015;116:1500-12. [DOI] [PubMed] [Google Scholar]
- 22.James S, Williams M, Newland A, Colston K. Leukemia cell differentiation: cellular and molecular interactions of retinoids and vitamin D. General Pharmacol Vasc System 1999;32:143-54. [DOI] [PubMed] [Google Scholar]
- 23.Gocek E, Kiełbiński M, Baurska H, et al. Different susceptibilities to 1, 25-dihydroxyvitamin D3-induced differentiation of AML cells carrying various mutations. Leuk Res 2010;34:649-57. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Salman H, Danilenko M, Studzinski GP. Cooperation between antioxidants and 1, 25-dihydroxyvitamin D3 in induction of leukemia HL60 cell differentiation through the JNK/AP-1/Egr-1 pathway. J Cell Physiol 2005;204:964-74. [DOI] [PubMed] [Google Scholar]
- 25.Kim DS, Kim SH, Song JH, et al. Enhancing effects of ceramide derivatives on 1, 25-dihydroxyvitamin D3-induced differentiation of human HL-60 leukemia cells. Life Sci 2007;81:1638-44. [DOI] [PubMed] [Google Scholar]
- 26.Kolla SS, Moore DC, Studzinski GP. Vitamin D analogs inhibit erythroid differentiation and induce monocytic differentiation of leukemic cells with the same relative potency. Proc Soc Exp Biol Med 1991;197:214-7. [DOI] [PubMed] [Google Scholar]
- 27.Koeffler H. Vitamin D: myeloid differentiation and proliferation. Modern Trends in Human Leukemia VI New Results in Clinical and Biological Research Including Pediatric Oncology; Springer; 1985. p. 409-17. [Google Scholar]
- 28.Duggal J, S Harrison J, P Studzinski G, Wang X. Involvement of microRNA181a in differentiation and cell cycle arrest induced by a plant-derived antioxidant carnosic acid and vitamin D analog doxercalciferol in human leukemia cells. MicroRNA 2012;1:26-33. [DOI] [PubMed] [Google Scholar]
- 29.Radujkovic A, Schnitzler P, Ho AD, et al. Low serum vitamin D levels are associated with shorter survival after first-line azacitidine treatment in patients with myelodysplastic syndrome and secondary oligoblastic acute myeloid leukemia. Clin Nutr 2017;36:542-51. [DOI] [PubMed] [Google Scholar]
- 30.Shanafelt TD, Drake MT, Maurer MJ, et al. VitaminD insufficiency and prognosis in chronic lymphocytic leukemia. Blood 2011;117:1492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubeczko M, Nowara E, Spychałowicz W, et al. Efficacy and safety of vitamin D supplementation in patients withchronic lymphocytic leukemia. Postepy Hig Med Dosw (Online) 2016;70:534-41. [DOI] [PubMed] [Google Scholar]
- 32.Kelly JL, Salles G, Goldman B, et al. Low serum vitamin D levels are associated with inferior survival in follicular lymphoma: a prospective evaluation in SWOG and LYSA studies. J Clin Oncol 2015;33:1482-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talpur R, Cox KM, Hu M, et al. Vitamin D deficiency in mycosis fungoides and Sezary syndrome patients is similar to other cancer patients. Clin Lymph Myel Leuk 2014;14:518-24. [DOI] [PubMed] [Google Scholar]
- 34.Gupta M, Han JJ, Stenson M, et al. Elevated serum IL-10 levels in diffuse large B-cell lymphoma: a mechanism of aberrant JAK2 activation. Blood 2012;119:2844-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matilainen JM, Husso T, Toropainen S, et al. Primary effect of 1α, 25 (OH) 2 D 3 on IL-10 expression in monocytes is shortterm down-regulation. Biochim Biophys Acta Mol Cell Res 2010;1803:1276-86. [DOI] [PubMed] [Google Scholar]
- 36.Drake MT, Maurer MJ, Link BK, et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J Clin Oncol 2010;28:4191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bittenbring JT, Neumann F, Altmann B, et al. Vitamin Ddeficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J Clin Oncol 2014;32:3242-8. [DOI] [PubMed] [Google Scholar]
- 38.Drake MT, Ng AC. Vitamin D deficiency in multiple myeloma. Eur J Clin Med Oncol 2010;2:1. [Google Scholar]
- 39.Ng AC, Kumar SK, Rajkumar SV, Drake MT. Impact of vitamin D deficiency on the clinical presentation and prognosis of patients with newly diagnosed multiple myeloma. Am J Hematol 2009;84:397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Debant M, Hemon P, Brigaudeau C, et al. Calcium signaling and cell fate: how can Ca2+ signals contribute to wrong decisions for Chronic Lymphocytic Leukemic B lymphocyte outcome?. Int J Develop Biol 2015;59:379-89. [DOI] [PubMed] [Google Scholar]
- 41.Esfahani A, Ghoreishi Z. Is there any association between vitamin D receptor polymorphisms and acute myeloid leukemia? Ann Oncol 2016;27:suppl 6. [Google Scholar]
- 42.Tantawy M, Amer M, Raafat T, Hamdy N. Vitamin D receptor gene polymorphism in Egyptian pediatric acute lymphoblastic leukemia correlation with BMD. Meta Gene 2016;9:42-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Algadal SFS, Ali EW, Elamin HA, et al. Association of Vitamin D Receptor (VDR) Start Codon Fok-I Polymorphism with Chronic Myeloid Leukemia. IJAPBC 2015;4:228-32. [Google Scholar]
- 44.Purdue MP, Lan Q, Kricker A, et al. Vitamin D receptor gene polymorphisms and risk of non-Hodgkin’s lymphoma. Haematologica 2007;92:1145-6. [DOI] [PubMed] [Google Scholar]
- 45.Alimirah F, Peng X, Gupta A, et al. Crosstalk between the vitamin D receptor (VDR) and miR-214 in regulating SuFu, a hedgehog pathway inhibitor in breast cancer cells. Exp Cell Res 2016;349:15-22. [DOI] [PubMed] [Google Scholar]
- 46.Lin TL, Wang QH, Brown P, et al. Self-renewal of acute lymphocytic leukemia cells is limited by the Hedgehog pathway inhibitors cyclopamine and IPI-926. PLoS One 2010;5:e15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao C, Chen A, Jamieson CH, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature 2009;458:776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajab SA, Ibrahim IK, Abdelgader EA, et al. Vitamin D receptor gene (FokI) polymorphism in Sudanese patients with chronic lymphocytic leukaemia. Am J Res Commun 2015;3:71-8. [Google Scholar]
- 49.Gascoyne DM, Lyne L, Spearman H, Buffa FM, Soilleux EJ, Banham AH. Vitamin D Receptor Expression in Plasmablastic Lymphoma and Myeloma Cells Confers Susceptibility to Vitamin D. Endocrinol 2016;158:503-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Renné C, Benz AH, Hansmann ML. Vitamin D 3 receptor is highly expressed in Hodgkin’s lymphoma. BMC Cancer 2012;12:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tekgündüz SA, Yesil S, Ören AC, et al. Vitamin D Receptor (VDR) Polymorphisms in Pediatric Patients Presenting With Hodgkin’s Lymphoma. J Pediatr Hematol/Oncol 2017;39:e59- e61. [DOI] [PubMed] [Google Scholar]
- 52.Hohaus S, Massini G, Giachelia M, et al. Anemia in Hodgkin’s lymphoma: the role of interleukin-6 and hepcidin. J Clin Oncol 2010;28:2538-43. [DOI] [PubMed] [Google Scholar]
- 53.Rai V, Abdo J, Agrawal S, Agrawal DK. Vitamin D Receptor Polymorphism and Cancer: An Update. Anticancer Res 2017;37:3991-4003. [DOI] [PubMed] [Google Scholar]
- 54.Ratta M, Fagnoni F, Curti A, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood 2002;100:230-7. [DOI] [PubMed] [Google Scholar]
- 55.Yu W, Ge M, Shi J, et al. Role of vitamin D receptor gene polymorphisms in aplastic anemia: a case–control study from China. Int J Lab Hematol 2016;38:273-83. [DOI] [PubMed] [Google Scholar]
- 56.Yu W, Ge M, Lu S, et al. Decreased expression of vitamin D receptor may contribute to the hyperimmune status of patients with acquired aplastic anemia. Eur J Haematol 2016;96:507-16. [DOI] [PubMed] [Google Scholar]
- 57.Gocek E, Baurska H, Marchwicka A, Marcinkowska E. Regulation of leukemic cell differentiation through the vitamin D receptor at the levels of intracellular signal transduction, gene transcription, and protein trafficking and stability. Leuk Res Treat 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma Y, Trump LD, Johnson SC. Vitamin D and miRNAs in Cancer. Curr Gene Ther 2014;14:269-75. [DOI] [PubMed] [Google Scholar]
- 59.Yan X, Meng F, Zhao H, Yang J. Expression and clinical significance of MN1 and PTEN gene in patients with acute myeloid leukemia. Chinese-German J Clin Oncol 2011;10:232-4. [Google Scholar]
- 60.Yang H, Kong W, He L, et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res 2008;68:425-33. [DOI] [PubMed] [Google Scholar]
- 61.Zou Z-J, Fan L, Wang L, et al. miR-26a and miR-214 downregulate expression of the PTEN gene in chronic lymphocytic leukemia, but not PTEN mutation or promoter methylation. Oncotarget 2015;6:1276-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wlodarska I, Baens M, Peeters P, et al. Biallelic alterations of both ETV6 and CDKN1B genes in at (12; 21) childhood acute lymphoblastic leukemia case. Cancer Res 1996;56:2655-61. [PubMed] [Google Scholar]
- 63.Doig CL, Singh PK, Dhiman VK, et al. Recruitment of NCOR1 to VDR target genes is enhanced in prostate cancer cells and associates with altered DNA methylation patterns. Carcinogenesis 2012;34:248-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsunawa M, Akagi D, Uno S, et al. Vitamin D receptor activation enhances benzo [a] pyrene metabolism via CYP1A1 expression in macrophages. Drug Metab Dispos 2012;40:2059-66. [DOI] [PubMed] [Google Scholar]
- 65.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1, 25-dihydroxyvitamin D3. FASEB J 2005;19:1067-77. [DOI] [PubMed] [Google Scholar]
- 66.McGaffin KR, Chrysogelos SA. Identification and characterization of a response element in the EGFR promoter that mediates transcriptional repression by 1, 25-dihydroxyvitamin D3 in breast cancercells. J Mol Endocrinol 2005;35:117-33. [DOI] [PubMed] [Google Scholar]
- 67.Du H, Daftary GS, Lalwani SI, Taylor HS. Direct regulation of HOXA10 by 1, 25-(OH) 2D3 in human myelomonocytic cells and human endometrial stromal cells. Mol Endocrinol 2005;19:2222-33. [DOI] [PubMed] [Google Scholar]
- 68.Huang YC, Hung WC. 1, 25-dihydroxyvitamin D3 transcriptionally represses p45Skp2 expression via the Sp1 sites in human prostate cancer cells. J Cell Physiol 2006;209:363-9. [DOI] [PubMed] [Google Scholar]
- 69.Haussler MR, Haussler CA, Whitfield GK, et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J Steroid Biochem Mol Biol 2010;121:88-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Speer G, Dworak O, Cseh K, et al. Vitamin D receptor gene BsmI polymorphism correlates with erbB-2/HER-2 expression in human rectal cancer. Oncology 2000;58:242-7. [DOI] [PubMed] [Google Scholar]
- 71.Filippini T, Heck JE, Malagoli C, et al. A review and metaanalysis of outdoorair pollution and risk of childhood leukemia. J Environ Sci Health Part C 2015;33:36-66. [DOI] [PMC free article] [PubMed] [Google Scholar]


