Abstract
In pancreatic β cells, glucose stimulates the biosynthesis of insulin at transcriptional and post-transcriptional levels. The RNA-binding protein, polypyrimidine tract-binding protein 1 (PTBP1), also named hnRNP I, acts as a critical mediator of insulin biosynthesis through binding to the pyrimidine-rich region in the 3’-untranslated region (UTR) of insulin mRNA. However, the underlying mechanism that regulates its expression in β cells is unclear. Here, we report that glucose induces the expression of PTBP1 via the insulin receptor (IR) signaling pathway in β cells. PTBP1 is present in β cells of both mouse and monkey, where its levels are increased by glucose and insulin, but not by insulin-like growth factor 1. PTBP1 levels in immortalized β cells established from wild-type (βIRWT) mice are higher than levels in β cells established from IR-null (βIRKO) mice, and ectopic re-expression of IR-WT in βIRKO cells restored PTBP1 levels. However, PTBP1 levels were not altered in βIRKO cells transfected with IR-3YA, in which the Tyr1158/1162/1163 residues are substituted with Ala. Consistently, treatment with glucose or insulin elevated PTBP1 levels in βIRWT cells, but not in βIRKO cells. In addition, silencing Akt significantly lowered PTBP1 levels. Thus, our results identify insulin as a pivotal mediator of glucose-induced PTBP1 expression in pancreatic β cells.
Keywords: glucose, insulin, insulin receptor signaling, pancreatic β cell, PTBP1
INTRODUCTION
Insulin produced in and secreted from pancreatic β cells is the most crucial hormone for the control of glucose homeostasis in the blood circulatory system. Impaired biosynthesis and secretion of insulin leads to somatic damage via hyperglycemia. Therefore, the amount of insulin is tightly regulated in order to maintain a very narrow blood glucose range. The elevation of glucose levels induces calcium-mediated secretion of insulin, which, in turn, promotes the absorption of glucose from the blood into peripheral tissues, thereby reducing blood glucose levels. Intriguingly, insulin also regulates not only its own biosynthesis and secretion, but also β-cell mass, via intracellular signaling cascades, in which Akt is the prime mediator (Kulkarni et al., 1999; Otani et al., 2004; Paris et al., 2003; Withers et al., 1998). In addition to stimulating insulin secretion, glucose also stimulates the biosynthesis of insulin at the transcriptional and post-transcriptional levels (German and Wang, 1994; Giddings et al., 1982; Permutt and Kipnis, 1972). The acute production of insulin after short-term (< 2 h) glucose stimulation mostly occurs by the enhancement of pre-existing insulin mRNA stability and translation, rather than de novo synthesis of preproinsulin mRNA, whereas the subsequent production during long-term (> 2 h) glucose stimulation is mediated by both the former and latter processes (Brunstedt and Chan, 1982; Itoh and Okamoto, 1980; Welsh et al., 1985), thereby ensuring that sufficient insulin mRNA is present to serve as the substrate for rapid insulin biosynthesis under conditions of greater insulin demand. Indeed, insulin mRNA comprises a large proportion (> 30%) of the total mRNA pool in pancreatic β cells and has an exceptionally long half-life (> 24 h; Goodge and Hutton, 2000; Itoh and Okamoto, 1980). This increase in insulin mRNA stability and translation depends on its 5′ and 3′ untranslated regions (UTRs; Wicksteed et al., 2001).
Polypyrimidine tract binding protein 1 (PTBP1), which is also known as heterogeneous nuclear ribonucleoprotein I (hnRNP I), is a ubiquitous RNA-binding protein (RBP) that binds to the pyrimidine-rich region in the 3′ UTR of target mRNAs through four RNA recognition motifs (RRM) and contributes to their stability (Sawicka et al., 2008; Tillmar and Welsh, 2002; Tillmar et al., 2002). It is also known to function in diverse cellular processes, including splicing, polyadenylation, mRNA localization, and translation initiation (Sawicka et al., 2008). In pancreatic β cells, PTBP1 stabilizes insulin mRNA by binding to the pyrimidine-rich region in its 3’ UTR (Tillmar and Welsh, 2002; Tillmar et al., 2002), as also shown for iNOS and PGK2 mRNAs (Pautz et al., 2006; Xu and Hecht, 2007). This binding is increased by glucose stimulation (Tillmar and Welsh, 2002; Tillmar et al., 2002). Although PTBP1 mRNA levels have been reported to increase after glucose stimulation in mouse insulinoma MIN6 cells (Webb et al., 2000), the molecular mechanisms by which glucose regulates PTBP1 expression have not been clearly elucidated. Here, we provide evidence that glucose-stimulated PTBP1 expression is mediated by the insulin receptor (IR) signaling pathway via Akt.
MATERIALS AND METHODS
Animals and immunostaining
Male C57BL/6 mice were kept in an environmentally controlled room under a 12-h light-dark cycle and provided with chow and water ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Ajou University. Pancreata from eight-week-old male C57BL/6 mice and twenty-four-year-old adult rhesus monkeys (Macaca mulatta) were fixed in 4% paraformaldehyde solution overnight, embedded in paraffin, and sectioned onto glass slides. Paraffin sections were deparaffinized in OTTIX PLUS & OTTIX SHAPER (DiaPath, Martinengo BG, Italy) and after an antigen retrieval process, stained with primary antibodies against PTBP1 and insulin. Each bound antibody was detected using immunofluorescent staining with secondary antibodies or using the appropriate Elite ABC horseradish peroxidase (HRP)-diaminobenzidine (DAB) system (Vector Labs, USA). Nuclei were stained with DAPI. Slides were viewed using a Leica DMi8 microscope (Leica, USA). Details of primary antibodies are listed in Supplementary Table S1.
Plasmidd and site-directed mutagenesis
Myc-tagged PTBP1 plasmid was obtained from Addgene (USA). Human IR cDNA was amplified by RT-PCR from human pancreas RNA (Stratagene, USA) using an oligo-dT (18 bp) primer for reverse transcription. IR cDNA was incorporated into a 3 × Flag vector. An IR mutant (IR-3YA), in which Tyr1158/1162/1163 residues are substituted with Ala, was generated from wild-type IR (IR-WT) using a QuikChange II XL site-directed mutagenesis kit (Stratagene).
Cell culture, transfection, treatment, and insulin secretion
Mouse insulinoma βTC6 and human hepatocellular carcinoma Hep3B cells were cultured in high-glucose DMEM (Invitrogen, USA) supplemented with 10% FBS (HyClone, USA). βIRWT and βIRKO cells were established from wild-type (βIRWT) or β-cell-specific insulin receptor-deficient (βIRKO) mice (Assmann et al., 2009; Kim et al., 2011; 2012; Kulkarni et al., 1999; Lee et al., 2012). βIRWT and βIRKO cells were cultured in high-glucose DMEM (Invitrogen) supplemented with 10% FBS (HyClone). Transfection of siRNAs and plasmids was carried out using Lipofectamine RNAiMAX or 2000 (Invitrogen), according to the manufacturer’s instructions. Scrambled siRNA (Silencer Negative Control #1; Ambion, USA) or empty vector were transfected as negative controls. Details of the siRNAs are listed in Supplementary Table S2. For glucose, insulin, and IGF-1 treatment, cells were starved for 12 h in DMEM containing 5 mM glucose and 0.1% FBS and further incubated for 12 h with the indicated concentrations of glucose, insulin, or IGF-1. For insulin secretion assay, cells starved for 12 h in DMEM containing 5 mM glucose without FBS were washed three times in PBS and subsequently stimulated with the indicated concentration of glucose for 20 min. At the end of the experiment, the buffer was collected, centrifuged to remove cellular debris and saved for quantification of insulin. The insulin levels were measured using a Mouse Insulin ELISA Kit (ALPCO, USA) according to the manufacturer’s instructions. Transfections of the expression vectors and siRNA (Santa Cruz) for PTBP1 were carried out 24 and 48 h before treating with glucose using Lipofectamine 2000 and RNAiMAX (Invitrogen), respectively.
Western blot analysis
Whole-cell lysates prepared using RIPA buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% NP-40, 1 mM EDTA, and 0.1% SDS) were separated by SDS-PAGE, transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, USA), and incubated with primary antibodies to detect PTBP1, proinsulin, IRβ, p-IR (Tyr1162/1163), Akt, or β-actin. Membranes were then incubated with the corresponding HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, USA), which were detected using enhanced luminescence (GE Healthcare, UK). Sources and working dilutions of primary antibodies are listed in Supplementary Table S1.
Quantitative real-time PCR analysis (RT-qPCR)
Total RNAs were isolated from βTC6, βIRWT, and βIRKO cells using the TRIzol reagent (Thermo Fisher Scientific, USA), according to the manufacturer’s instructions. Quantitative real-time PCR (RT-qPCR) analysis was performed as previously described (Lee et al., 2012). A list of primer sequences is shown in Supplementary Table S3.
Cell synchronization and flow cytometry
Cells were synchronized by double thymidine block for 24 h and then released from growth arrest by adding fresh medium. For cell cycle analysis, cells were collected by trypsinization, washed with phosphate-buffered saline (PBS), and fixed with 70% ethanol. Fixed cells were washed with PBS and stained with propidium iodide solution. DNA content was measured by flow cytometry and cell cycle profiles were analyzed using Cell Quest and MOD Fit software (BD Bioscience, USA).
Statistical analysis
All values are expressed as the mean ± SEM. Differences between mean values were compared statistically by Student’s t-test. All statistical analyses were performed using GraphPad Prism (GraphPad Software). A P value < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Treatment with glucose or insulin increases PTBP1 levels in pancreatic β-cell lines
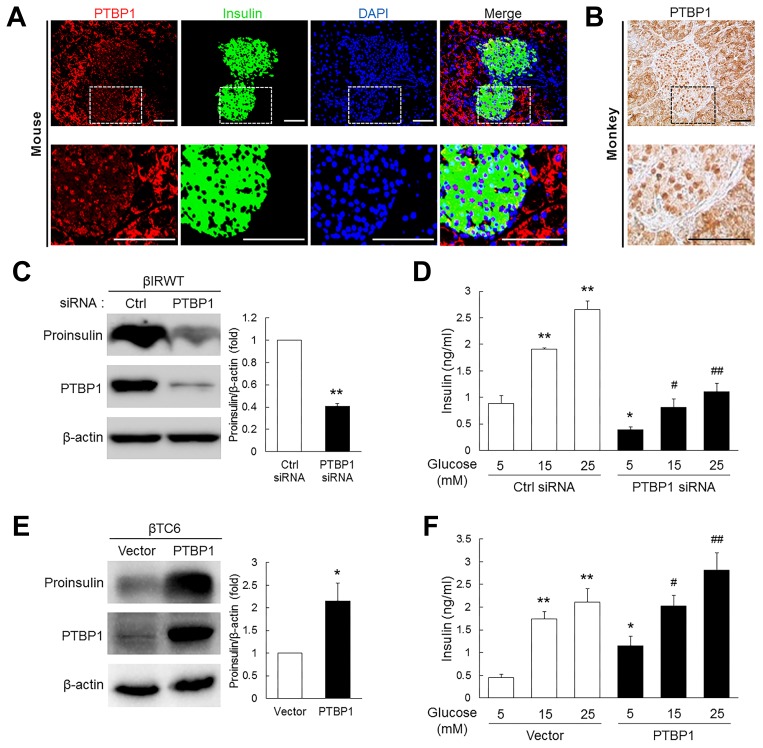
Although the presence of PTBP1 in insulin-producing mouse β-cell lines and isolated islets is well known (Knoch et al., 2004; 2006; Tillmar and Welsh, 2002; Tillmar et al., 2002; Webb et al., 2000), its expression pattern in vivo has not been firmly evaluated. We performed immunostaining analysis for PTBP1 in pancreatic sections from mice and monkeys after overnight fasting. We found that PTBP1 appeared to be present throughout the pancreas, including endocrine (islets) and exocrine tissues, but its subcellular localization differed between cell types. It was predominantly found in the nucleus of β cells, but in the cytoplasm of exocrine cells (Figs. 1A and 1B). This is in agreement with the notion that glucose induces the translocation of PTBP1 from the nucleus to the cytoplasm in insulinoma INS-1 cells and isolated rat islets and cytosolic PTBP1, in turn, prevents the degradation of insulin mRNA through binding to the pyrimidine-rich region in its 3′ UTR (Knoch et al., 2004). These results also suggest that the nucleocytoplasmic translocation of PTBP1 in β cells is more glucose-inducible than it is in the exocrine cells. Consistent with previous reports (Knoch et al., 2004; Tillmar and Welsh, 2002; Tillmar et al., 2002), silencing PTBP1 in immortalized β cells isolated from the pancreas of wild-type (βIRWT) mice (Assmann et al., 2009; Kim et al., 2011; 2012; Lee et al., 2012) lowered both mRNA and protein levels of proinsulin (Fig. 1C and Supplementary Fig. S1) and consistently reduced glucose-stimulated insulin secretion (GSIS) from mouse insulinoma βTC6 cells (Fig. 1D). Conversely, ectopic expression of PTBP1 increased proinsulin levels (Fig. 1E) and enhanced GSIS from βTC6 cells (Fig. 1F).
Fig. 1. Effects of PTBP1 on insulin expression in pancreatic β cells.
A) Representative images of immunostaining for PTBP1 (red), insulin (green), and DAPI (blue) in mouse pancreatic islets. Boxed areas are magnified and shown in the bottom panel. Scale bars, 50 μm. (B) Representative images of DAB staining for PTBP1 in monkey pancreatic islets. Boxed areas are magnified and shown in the bottom panel. Scale bars, 50 μm. (C) Levels of proinsulin and β-actin loading control in βIRWT cells transiently transfected with the indicated siRNAs. Relative densities for the indicated proteins are shown at the right. **P < 0.01 versus Ctrl siRNA group using Student’s t-test. (D) Effects of PTBP1 knockdown on glucose-stimulated insulin secretion in βTC6 cells. *P < 0.05, **P < 0.01 versus the 5 mM of Ctrl siRNA group; #P < 0.05, ##P < 0.01 versus the 5 mM of PTBP1 siRNA group using Student’s t-test. (E) Levels of proinsulin and β-actin in βTC6 cells transiently transfected with either empty vector (Vector) or PTBP1 cDNA construct (PTBP1). Relative densities for the indicated proteins are shown at the right. *P < 0.01 versus Vector group using Student’s t-test. (F) Effects of PTBP1 overexpression on glucose-stimulated insulin secretion in βTC6 cells. *P < 0.05, **P < 0.01 versus the 5 mM of Vector group; #P < 0.05, ##P < 0.01 versus the 5 mM of PTBP1 group using Student’s t-test. All data represent the mean ± SEM from at least three independent experiments.
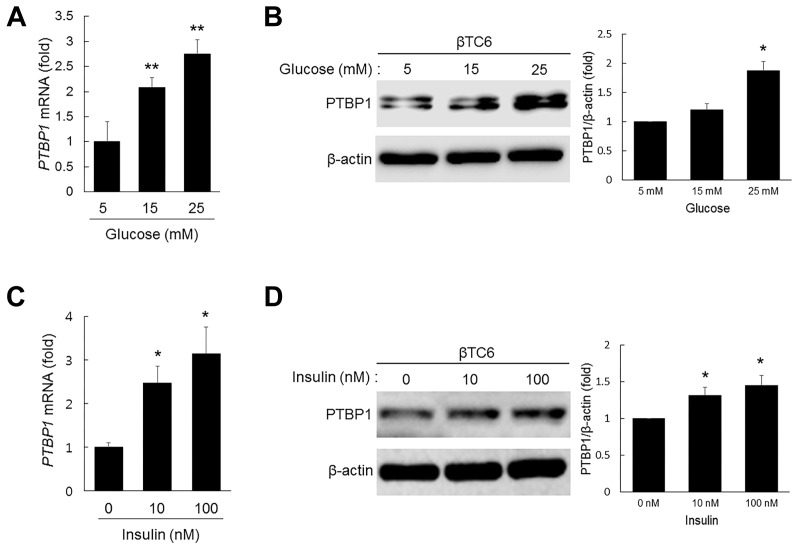
As glucose-induced up-regulation of PTBP1 mRNA levels in MIN6 cells has been reported (Webb et al., 2000), we evaluated mRNA and protein levels of PTBP1 after glucose stimulation and observed that glucose dose-dependently increased mRNA and protein levels of PTBP1 in βTC6 cells (Figs. 2A and 2B). Moreover, mRNA and protein levels of PTBP1 were also increased by insulin in a dose-dependent manner (Figs. 2C and 2D), but not by insulin-like growth factor 1 (IGF-1, Supplementary Fig. S2). Given that glucose stimulates insulin secretion, these results indicate that glucose-induced expression of PTBP1 is mediated, at least in part, by insulin released into the extracellular space.
Fig. 2. Effects of glucose and insulin on PTBP1 expression in pancreatic β cells.
(A, B) Levels of PTBP1 mRNA (A) and the protein (B) in βTC6 cells treated with glucose. βTC6 cells were cultured for 12 h in 5 mM glucose + 0.1% FBS and further cultured for 12 h with the indicated concentrations of glucose. Levels of PTBP1 mRNA and the protein were assessed by RT-qPCR analysis and western blot analysis, respectively. All mRNA amounts were normalized to the levels of 18S rRNA. (C, D) Levels of PTBP1 mRNA (C) and the protein (D) in βTC6 cells treated with insulin. βTC6 cells were cultured for 12 h in 5 mM glucose + 0.1% FBS and further cultured for 12 h with the indicated concentrations of insulin. Levels of PTBP1 mRNA and the protein were assessed by RT-qPCR analysis and western blot analysis, respectively. All mRNA amounts were normalized to the levels of 18S rRNA. Data represent the mean ± SEM from at least three independent experiments. *P < 0.05, **P < 0.01 versus the 5 mM group (A, B) or the vehicle group (C, D) using Student’s t-test.
Glucose-induced expression of PTBP1 in pancreatic β cells is mediated by the IR signaling pathway
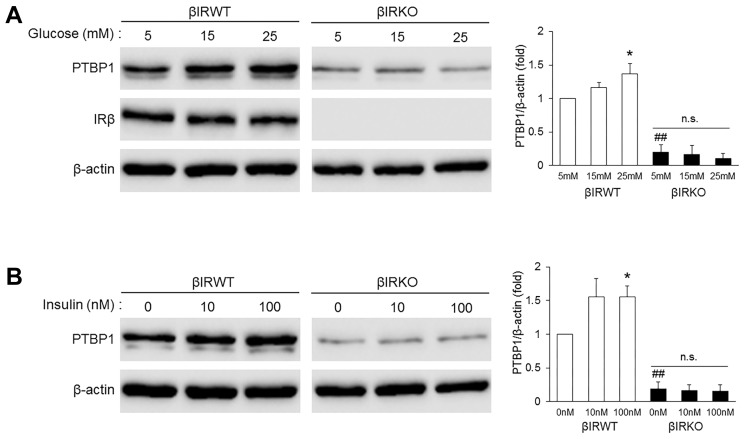
To investigate the potential role of insulin in mediating glucose-induced PTBP1 expression, we employed βIRWT and βIRKO cells established from WT and β-cell-specific insulin receptor (IR)-knockout mice, respectively (Assmann et al., 2009; Kim et al., 2011; 2012; Kulkarni, et al., 1999; Lee et al., 2012). Similar to the results in βTC6 cells (Fig. 2B), PTBP1 levels were dose dependently increased by glucose in βIRWT cells (Fig. 3A). By contrast, glucose-induced PTBP1 expression was not observed in βIRKO cells, where PTBP1 levels were significantly lower than that in βIRWT cells (Fig. 3A). Likewise, treatment with insulin elevated PTBP1 levels in βIRWT cells, but not in βIRKO cells (Fig. 3B). Neither insulin nor IGF-1 induced an increase in PTBP1 levels in human hepatocellular carcinoma Hep3B cells (Supplementary Figs. S3A and S3B). These results suggest that insulin has a role in mediating glucose-induced PTBP1 expression in β cells.
Fig. 3. PTBP1 expression in βIRWT and βIRKO cells.
(A, B) Levels of PTBP1, IR β subunit (IRβ), and β-actin were assessed by Western blot analysis following culture of βIRWT and βIRKO cells for 12 h in 5 mM glucose + 0.1% FBS and further culture with the indicated concentrations of glucose (A) or insulin (B) for 12 h. Relative densities for the indicated proteins are shown at the right. Data represent the mean ± SEM from three independent experiments. *P < 0.05 versus the 5 mM group in (A) or the vehicle group in (B); ##P < 0.01 versus the βIRWT group using Student’s t-test. n.s., not significant.
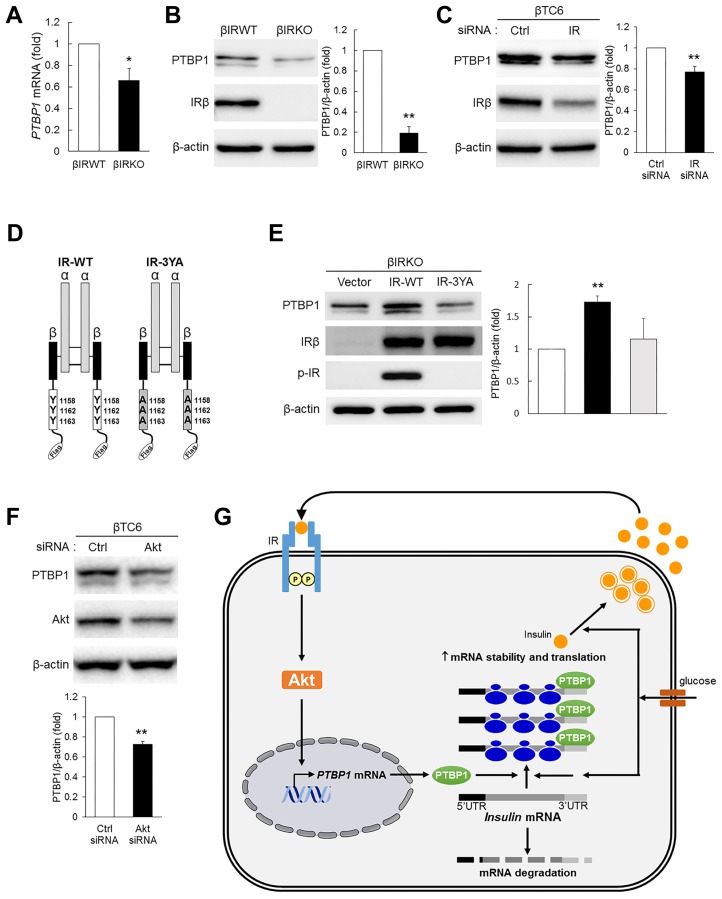
Next, we further confirmed the involvement of IR and its downstream molecules in insulin-induced PTBP1 expression. Consistent with the lower mRNA and protein levels of PTBP1 in βIRKO cells compared with βIRWT cells (Figs. 4A and 4B), silencing IR in βTC6 cells significantly lowered PTBP1 levels (Fig. 4C). Insulin binding to IR induces autophosphorylation at Tyr1158, Tyr1162, and Tyr1163 in the activation loop of the β subunits (IRβ), which increases the catalytic activity of the receptor and, in turn, transduces the signal to downstream signaling proteins (Hubbard, 1997; Tang et al., 2017; Taniguchi et al., 2006), including Akt, which is a key mediator of insulin responses such as gene expression, protein synthesis, proliferation, and glucose metabolism (Taniguchi et al., 2006). Mutation of these autophosphorylation sites reduces insulin-stimulated kinase activity and causes a parallel loss of biological function (Kim et al., 2011; 2012). Thus, we directly confirmed the effects of IR activity on PTBP1 expression by ectopic re-expression of Flag-tagged IR-WT and IR mutant (IR-3YA), in which the Tyr1158/1162/1163 residues are substituted with Ala (Fig. 4D), in βIRKO cells. Ectopic IR-WT re-expression in βIRKO cells restored PTBP1 levels, but in IR-3YA-transfected βIRKO cells, PTBP1 levels were not altered (Fig. 4E). IR autophosphorylation at Tyr1158, Tyr1162, and Tyr1163 was only detected in IR-WT-transfected cells, not in IR-3YA-transfected cells, likely due to endogenous insulin secretion (Fig. 4E). In addition, silencing Akt in βTC6 cells significantly lowered PTBP1 levels (Fig. 4F). Together, these results indicate that PTBP1 is expressed in β cells under the control of the IR → Akt → PTBP1 pathway (Fig. 4G).
Fig. 4. Regulation of PTBP1 expression by insulin receptor activity.
(A, B) Levels of PTBP1 mRNA (A) and the protein (B) in βIRWT and βIRKO cells. All mRNA amounts were normalized to the levels of 18S rRNA. Relative densities for the indicated proteins are shown at the right. *P < 0.05, **P < 0.01 versus the βIRWT group using Student’s t-test. (C) Levels of PTBP1 and IRβ in βTC6 cells transiently transfected with the indicated siRNAs. Relative densities for the indicated proteins are shown at the right. **P < 0.01 versus the Ctrl siRNA group using Student’s t-test. (D) Diagram of constructs for Flag-tagged IR-WT and IR-3YA, in which the three autophosphorylation sites (Tyr1158/1162/1163) are substituted with Ala. (E) Western blot analysis for the indicated proteins in βIRKO cells transfected with empty vector, IR-WT, or IR-3YA. Relative densities for the indicated proteins are shown at the right. **P < 0.01 versus the vector group using Student’s t-test. (F) Levels of PTBP1 and Akt in βTC6 cells transfected with the indicated siRNAs. Relative densities for the indicated proteins are shown at the right. **P < 0.01 versus the Ctrl siRNA group using Student’s t-test. All data represent the mean ± SEM from three independent experiments. (G) Schematic illustration of the proposed mechanism by which IR signaling pathway regulates PTBP1 expression in pancreatic β cells.
PTBP1 expression correlates with insulin levels during cell cycle progression
Because insulin is a key regulator of β-cell proliferation, which is a major mechanism that contributes to maintaining adult β-cell mass (Assmann et al., 2009; Dor et al., 2004; Folli et al., 2011; Georgia and Bhushan, 2004; Kim et al., 2012; Kulkarni et al., 1999; Otani et al., 2004; Saltiel and Kahn, 2001; Withers et al., 1998), we investigated whether insulin and PTBP1 levels are correlated during cell cycle progression. To address this question, βIRWT and Hep3B cells were initially synchronized at G1/S phase by double thymidine block, after which they were released from growth arrest by adding fresh medium. During the cell cycle progression of βIRWT cells, PTBP1 expression decreased and was positively correlated with proinsulin levels (Supplementary Fig. S4). However, this change in PTBP1 levels was absent in Hep3B cells (Supplementary Fig. S3C).
Here, we provide evidence that glucose induces PTBP1 expression and this is mediated by the IR signaling pathway via Akt (Fig. 4G). In addition to stimulating insulin secretion, glucose induces the rapid biosynthesis of insulin at the transcriptional and post-transcriptional levels (German and Wang, 1994; Giddings et al., 1982; Permutt and Kipnis, 1972) and the latter includes the enhancement of pre-existing insulin mRNA stability and translation (Brunstedt and Chan, 1982; Itoh and Okamoto, 1980; Welsh et al., 1985). In response to glucose, PTBP1 binds to the pyrimidine-rich region in the 3′ UTR of insulin mRNA, leading to its stabilization. It is likely that these posttranscriptional mechanisms mediated by PTBP1 are responsible for the acute increase in insulin biosynthesis in the 2 h following glucose stimulation (Itoh and Okamoto, 1980), while the subsequent production of insulin is mediated by an increase in PTBP1 transcription due to glucose stimulation. Taken together, our findings provide an important insight into the mechanism underlying glucose-induced PTBP1 expression in β cells. It will be important in future experiments to determine downstream molecules that is activated by the IR signaling pathway via Akt and directly participates in PTBP1 transcription.
Supplementary data
ACKNOWLEDGMENTS
We thank Dr. Josephine M. Egan (National Institute on Aging, USA) for monkey paraffin sections. This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future (2016R1E1A1A01941213). R.N.K. acknowledges support from National Institutes of Health grants (R01 DK67536 and R01 DK103215).
Footnotes
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Assmann A., Ueki K., Winnay J.N., Kadowaki T., Kulkarni R.N. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol Cell Biol. 2009;29:3219–3228. doi: 10.1128/MCB.01489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstedt J., Chan S.J. Direct effect of glucose on the preproinsulin mRNA level in isolated pancreatic islets. Biochem Biophys Res Commun. 1982;106:1383–1389. doi: 10.1016/0006-291x(82)91267-0. [DOI] [PubMed] [Google Scholar]
- Dor Y., Brown J., Martinez O.I., Melton D.A. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Folli F., Okada T., Perego C., Gunton J., Liew C.W., Akiyama M., D’Amico A., La Rosa S., Placidi C., Lupi R., et al. Altered insulin receptor signalling and β-cell cycle dynamics in type 2 diabetes mellitus. PLoS One. 2011;6:e28050. doi: 10.1371/journal.pone.0028050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgia S., Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest. 2004;114:963–968. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German M.S., Wang J. The insulin gene contains multiple transcriptional elements that respond to glucose. Mol Cell Biol. 1994;14:4067–4075. doi: 10.1128/mcb.14.6.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings S.J., Chirgwin J., Permutt M.A. Effects of glucose on proinsulin messenger RNA in rats in vivo. Diabetes. 1982;31:624–629. doi: 10.2337/diab.31.7.624. [DOI] [PubMed] [Google Scholar]
- Goodge K.A., Hutton J.C. Translational regulation of proinsulin biosynthesis and proinsulin conversion in the pancreatic b-cell. Semin Cell Dev Biol. 2000;11:235–242. doi: 10.1006/scdb.2000.0172. [DOI] [PubMed] [Google Scholar]
- Hubbard S.R. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997;16:5572–5581. doi: 10.1093/emboj/16.18.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Okamoto H. Translational control of proinsulin synthesis by glucose. Nature. 1980;283:100–102. doi: 10.1038/283100a0. [DOI] [PubMed] [Google Scholar]
- Kim W., Doyle M.E., Liu Z., Lao Q., Shin Y.K., Carlson O.D., Kim H.S., Thomas S., Napora J.K., Lee E.K., et al. Cannabinoids inhibit insulin receptor signaling in pancreatic β cells. Diabetes. 2011;60:1198–1209. doi: 10.2337/db10-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W., Lao Q., Shin Y.K., Carlson O.D., Lee E.K., Gorospe M., Kulkarni R.N., Egan J.M. Cannabinoids induce pancreatic β-cell death by directly inhibiting insulin receptor activation. Sci Signal. 2012;5:ra23. doi: 10.1126/scisignal.2002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch K.P., Bergert H., Borgonovo B., Saeger H.D., Altkrüger A., Verkade P., Solimena M. Polypyrimidine tract-binding protein promotes insulin secretory granule biogenesis. Nat Cell Biol. 2004;6:207–214. doi: 10.1038/ncb1099. [DOI] [PubMed] [Google Scholar]
- Knoch K.P., Meisterfeld R., Kersting S., Bergert H., Altkrüger A., Wegbrod C., Jäger M., Saeger H.D., Solimena M. cAMP-dependent phosphorylation of PTB1 promotes the expression of insulin secretory granule proteins in b cells. Cell Metab. 2006;3:123–134. doi: 10.1016/j.cmet.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Kulkarni R.N., Brüning J.C., Winnay J.N., Postic C., Magnuson M.A., Kahn C.R. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- Lee E.K., Kim W., Tominaga K., Martindale J.L., Yang X., Subaran S.S., Carlson O.D., Mercken E.M., Kulkarni R.N., Akamatsu W., et al. RNA-binding protein HuD controls insulin translation. Mol Cell. 2012;45:826–835. doi: 10.1016/j.molcel.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani K., Kulkarni R.N., Baldwin A.C., Krutzfeldt J., Ueki K., Stoffel M., Kahn C.R., Polonsky K.S. Reduced beta-cell mass and altered glucose sensing impair insulin-secretory function in betaIRKO mice. Am J Physiol Endocrinol Metab. 2004;286:E41–E49. doi: 10.1152/ajpendo.00533.2001. [DOI] [PubMed] [Google Scholar]
- Pautz A., Linker K., Hubrich T., Korhonen R., Altenhofer S., Kleinert H. The polypyrimidine tract-binding protein (PTB). is involved in the post-transcriptional regulation of human inducible nitric oxide synthase expression. J Biol Chem. 2006;281:32294–32302. doi: 10.1074/jbc.M603915200. [DOI] [PubMed] [Google Scholar]
- Paris M., Bernard-Kargar C., Berthault M.F., Bouwens L., Ktorza A. Specific and combined effects of insulin and glucose on functional pancreatic beta-cell mass in vivo in adult rats. Endocrinology. 2003;144:2717–2727. doi: 10.1210/en.2002-221112. [DOI] [PubMed] [Google Scholar]
- Permutt M.A., Kipnis D.M. Insulin biosynthesis. I. On the mechanism of glucose stimulation. J Biol Chem. 1972;247:1194–1199. [PubMed] [Google Scholar]
- Saltiel A.R., Kahn C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Sawicka K., Bushell M., Spriggs K.A., Willis A.E. Polypyrimidine-tract-binding protein: a multifunctional RNA-binding protein. Biochem Soc Trans. 2008;36:641–647. doi: 10.1042/BST0360641. [DOI] [PubMed] [Google Scholar]
- Tang C.Y., Man X.F., Guo Y., Tang H.N., Tang J., Zhou C.L., Tan S.W., Wang M., Zhou H.D. IRS-2 partially compensates for the insulin signal defects in IRS-1−/− mice mediated by miR-33. Mol Cells. 2017;40:123–132. doi: 10.14348/molcells.2017.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi C.M., Emanuelli B., Kahn C.R. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Tillmar L., Welsh N. Hypoxia may increase rat insulin mRNA levels by promoting binding of the polypyrimidine tract-binding protein (PTB). to the pyrimidine-rich insulin mRNA 3′-untranslated region. Mol Med. 2002;8:263–272. [PMC free article] [PubMed] [Google Scholar]
- Tillmar L., Carlsson C., Welsh N. Control of insulin mRNA stability in rat pancreatic islets. Regulatory role of a 3′-untranslated region pyrimidine-rich sequence. J Biol Chem. 2002;277:1099–1106. doi: 10.1074/jbc.M108340200. [DOI] [PubMed] [Google Scholar]
- Webb G.C., Akbar M.S., Zhao C., Steiner D.F. Expression profiling of pancreatic b cells: Glucose regulation of secretory and metabolic pathway genes. Proc Natl Acad Sci USA. 2000;97:5773–5778. doi: 10.1073/pnas.100126597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M., Nielsen D.A., MacKrell A.J., Steiner D.F. Control of insulin gene expression in pancreatic beta-cells and in an insulin-producing cell line, RIN-5F cells. II. Regulation of insulin mRNA stability. J Biol Chem. 1985;260:13590–13594. [PubMed] [Google Scholar]
- Wicksteed B., Herbert T.P., Alarcon C., Lingohr M.K., Moss L.G., Rhodes C.J. Cooperativity between the preproinsulin mRNA untranslated regions is necessary for glucose-stimulated translation. J Biol Chem. 2001;276:22553–22558. doi: 10.1074/jbc.M011214200. [DOI] [PubMed] [Google Scholar]
- Withers D.J., Gutierrez J.S., Towery H., Burks D.J., Ren J.M., Previs S., Zhang Y., Bernal D., Pons S., Shulman G.I., et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- Xu M., Hecht N.B. Polypyrimidine tract binding protein 2 stabilizes phosphoglycerate kinase 2 mRNA in murine male germ cells by binding to its 3′ UTR. Biol Reprod. 2007;76:1025–1033. doi: 10.1095/biolreprod.107.060079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.