Abstract
Insulin resistance is closely associated with metabolic diseases such as type 2 diabetes, dyslipidemia, hypertension and atherosclerosis. Thiazolidinediones (TZDs) have been developed to ameliorate insulin resistance by activation of peroxisome proliferator-activated receptor (PPAR) γ. Although TZDs are synthetic ligands for PPARγ, metabolic outcomes of each TZD are different. Moreover, there are lack of head-to-head comparative studies among TZDs in the aspect of metabolic outcomes. In this study, we analyzed the effects of three TZDs, including lobeglitazone (Lobe), rosiglitazone (Rosi), and pioglitazone (Pio) on metabolic and thermogenic regulation. In adipocytes, Lobe more potently stimulated adipogenesis and insulin-dependent glucose uptake than Rosi and Pio. In the presence of pro-inflammatory stimuli, Lobe efficiently suppressed expressions of pro-inflammatory genes in macrophages and adipocytes. In obese and diabetic db/db mice, Lobe effectively promoted insulin-stimulated glucose uptake and suppressed pro-inflammatory responses in epididymal white adipose tissue (EAT), leading to improve glucose intolerance. Compared to other two TZDs, Lobe enhanced beige adipocyte formation and thermogenic gene expression in inguinal white adipose tissue (IAT) of lean mice, which would be attributable to cold-induced thermogenesis. Collectively, these comparison data suggest that Lobe could relieve insulin resistance and enhance thermogenesis at low-concentration conditions where Rosi and Pio are less effective.
Keywords: adipogenesis, inflammation, insulin resistance, PPARγ, thermogenesis
INTRODUCTION
Peroxisome proliferator-activated receptors (PPARs) are nuclear hormone receptors that are activated by numerous lipophilic ligands such as natural lipids and synthetic agonists (Gross et al., 2017). The PPAR family consists of three isoforms, PPARα, PPARβ/δ, and PPARγ. Activation of PPARα and PPARβ/δ stimulates metabolic catabolism by inducing fatty acid oxidative gene expression (Gross et al., 2017). However, PPARγ activation promotes lipid anabolism by enhancing adipogenic and lipogenic gene expression (Tontonoz and Spiegelman, 2008). In addition, PPARγ is known to be involved in glucose metabolism, insulin sensitivity, and anti-inflammatory responses (Yki-Järvinen, 2004).
Insulin resistance is a pathological state in which subjects cannot adequately respond to normal levels of insulin. Insulin resistance primarily occurs in key metabolic organs such as the muscles, liver, and adipose tissue (Taniguchi et al., 2006). Accumulating evidence suggest that it is closely associated with most metabolic diseases, including type 2 diabetes (T2D), hypertension, and coronary heart disease (Fonseca, 2009). To treat T2D, various medications have been developed to enhance insulin secretion and/or insulin action. For examples, sulphonylureas, benzoic acid derivatives, and thiazolidinediones (TZDs) have been developed as T2D medications (Jenssen and Hartmann, 2015). Sulphonylureas and benzoic acid derivatives stimulate β-cells to enhance insulin secretion by inhibiting ATP-sensitive K+ channels and activating Ca2+ channels (Sola et al., 2015). TZDs are synthetic agonists of PPARγ that improve insulin sensitivity in several types of target cells, including adipocytes and immune cells (Lehmann et al., 1995; Tontonoz and Spiegelman, 2008). As PPARγ is abundantly expressed in adipocytes, primary effects of TZDs are detectable in adipose tissue. In white adipocytes, TZDs promote adipocyte differentiation, insulin action, and beige adipocyte formation (Ahmadian et al., 2013). In brown adipose tissue (BAT), TZDs activate thermogenic activity (Nedergaard et al., 2005). In addition, TZDs trigger anti-inflammatory responses in immune cells. For instance, TZDs downregulate pro-inflammatory gene expression and induce alternatively activated macrophages (M2) polarization (Bouhlel et al., 2007).
Although the FDA has approved several TZDs, including troglitazone (Tro), rosiglitazone (Rosi), and pioglitazone (Pio), Tro has been withdrawn from the market because it was associated with increased the risk of liver failure (Kohlroser et al., 2000). About a decade ago, Rosi was reported to increase the risk of myocardial infarction (Nissen and Wolski, 2007). The cardiotoxic effects of high doses of Rosi appeared to be, at least partly, PPARγ-independent mitochondrial dysfunction (He et al., 2014). Pio also has adverse effects such as weight gain, edema, and osteoporosis (Berria et al., 2007). Nevertheless, it has been well-established that TZDs lower hemoglobin A1c and do not cause hypoglycemia like insulin or insulin secretagogues do (Yau et al., 2013). In the ADOPT randomized controlled trial, Rosi was found to induced more durable glycemic control than sulphonylureas or metformin which are first-line insulin sensitizers (Kahn et al., 2006). In addition, TZDs have several beneficial effects to treat atherosclerosis, polycystic ovary syndrome, and coronary artery disease (Yki-Järvinen, 2004). Because of these benefits of TZDs, there is a market-driven demand for the development of novel TZD with little adverse effects. Recently, a newly developed TZD, lobeglitazone (Lobe) was introduced (Lim et al., 2015). Thorough evaluation, characterization, and comparison of commercially available TZDs including the newly developed Lobe, have not been reported to date.
In this study, we assessed the effects of three major TZDs, Lobe, Rosi, and Pio on energy metabolism. At the same dose, Lobe more strongly enhanced adipocyte differentiation and inhibited pro-inflammatory responses than did Rosi and Pio. Lobe improved glucose intolerance through enhanced glucose uptake and decreased pro-inflammatory responses in white adipose tissue (WAT) of obese and diabetic db/db mice. Moreover, Lobe effectively promoted beige adipocyte formation upon cold exposure in lean mice. Collectively, these data suggest that Lobe would be a potent TZD to treat obesity-induced insulin resistance and metabolic complications.
MATERIALS AND METHODS
Animal experiments
All animal experiments were performed in accordance with the research guidelines of the Seoul National University Institutional Animal Care and Use Committee. Ten-week-old C57BLKS/J-Leprdb/Leprdb (db/db) male mice were purchased from DBL Co. (Korea) and were housed in 12-h light/12-h dark cycles. At 11 weeks of age, db/db mice were orally administered of 3 mg kg−1 body weight Lobe, Rosi, or Pio or equivalent volume of vehicle (5% DMSO in PBS) daily for 4 weeks. For glucose tolerance test, db/db mice were treated with drugs for 3 weeks, and then, after overnight fasting, they were administered glucose (1 g kg−1 body weight).
For cold tolerance test, 7-week-old C57BL/6J (Jackson Laboratory) male mice were intraperitoneally injected daily with 10 mg kg−1 Lobe, Rosi, or Pio or an equivalent volume of vehicle (5% DMSO in PBS) for 12 days. Then, the mice were placed at 4°C, and rectal temperature was measured at the indicated time points. After 24 h, the cold-challenged mice were sacrificed.
Cell culture
Raw264.7 macrophages were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin. Cells were maintained at 37°C in 5% CO2 atmosphere and were treated with 5 μM Lobe, Rosi, or Pio and vehicle (DMSO). To differentiate 3T3-L1 preadipocytes into adipocytes, confluent cells were incubated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 1 μM dexamethasone, 520 μM 3-isobutyl-1-methylzanthine, and 167 nM insulin (Alfadda et al., 2017). After 48 h, the culture medium was replaced with DMEM containing 10% FBS and 167 nM insulin. Then, the medium was replaced every other day with DMEM containing 10% FBS. 3T3-L1 cells were treated with 10 nM of Lobe, Rosi, Pio, or vehicle (DMSO) for the initial 48 h of differentiation or for 48 h after differentiation.
Flow cytometric analysis
Flow cytometry was performed as described previously (Sohn et al., 2018). Briefly, epididymal white adipose tissue (EAT) was chopped and incubated in collagenase buffer at 37°C for 20 min with shaking. After centrifugation, the fraction of pelleted stromal vascular cells (SVCs) was separated and red blood cells were eliminated with lysis buffer. SVCs were stained with monoclonal antibodies against CD11b (BD Biosciences), F4/80, and CD11c (eBioscience) for macrophage analysis. SVCs were analyzed using a FACS Canto II (BD Biosciences).
Glucose uptake assay and ex vivo glucose bioprobe uptake assay
Glucose and ex vivo glucose bioprobe uptake assays were performed as described previously (Kim et al., 2015a). For the glucose uptake assay, 3T3-L1 adipocytes were incubated in low-glucose DMEM containing 0.1% BSA at 37°C for 16 h. Cells were incubated with or without 100 nM insulin for 20 min and then, [14C]deoxyglucose in HEPES-buffered saline (140 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, 1 mM CaCl2, and 20 mM HEPES [pH 7.4]) was added for 10 min. For ex vivo glucose bioprobe uptake assays, chopped WATs were incubated in low-glucose DMEM containing 0.1% BSA at 37°C for 30 min and then, incubated with 10 μM GB-Cy3 for 30 min in the presence or absence of 1 μM insulin. The samples were observed under a confocal microscope (Carl Zeiss).
Quantitative reverse transcription (RT-q) PCR
RT-qPCR analysis was performed as described previously (Lee et al., 2014b). Briefly, Total RNA was isolated from 3T3-L1 adipocytes, RAW264.7 macrophages, and EAT. cDNA was synthesized using a reverse transcriptase kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. The primers used for quantitative RT-PCR were obtained from Bioneer (Korea).
Statistical analysis
All results are presented as means ± standard deviations (SD) or standard errors of the means (SEM). Means were compared using Student t test in Excel (Microsoft) or analysis of variance (ANOVA) in Prism (GraphPad). The statistical differences were considered significant at P < 0.05.
RESULTS
Effects of the three TZDs on adipogenesis and insulin-stimulated glucose uptake in adipocytes
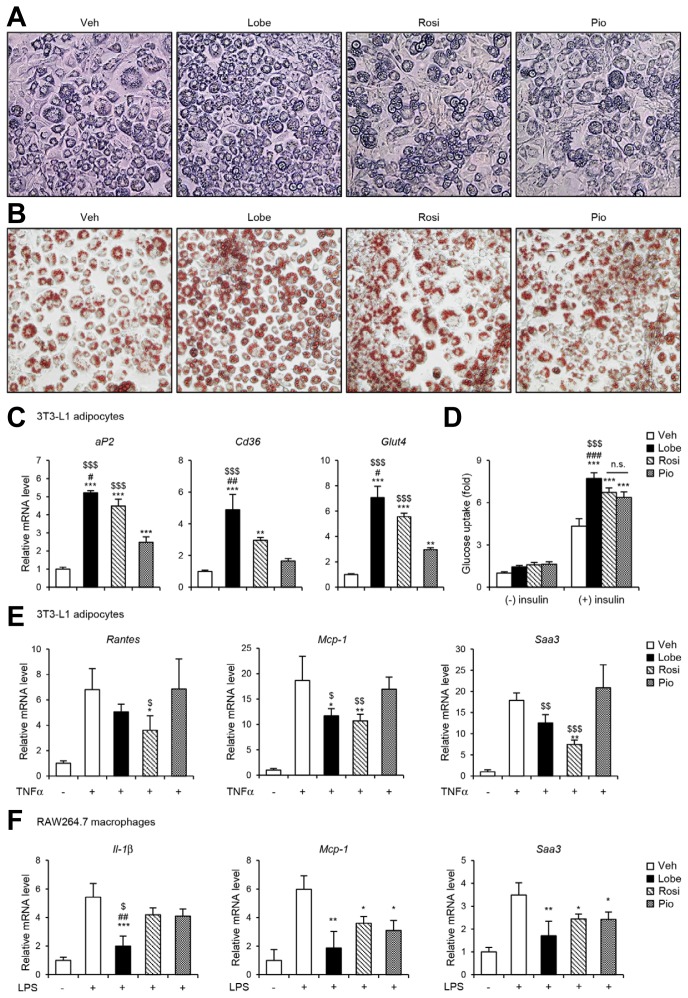
To compare the effects of the three TZDs, Lobe, Rosi, and Pio on adipogenesis, 3T3-L1 preadipocyte were differentiated into adipocytes in the presence or absence of each TZD. As expected, all three TZDs remarkably promoted adipocyte differentiation, accompanied with enhanced lipid droplet formation (Figs. 1A and 1B). However, Lobe stimulated adipocyte differentiation more effectively than did Rosi or Pio. Moreover, Lobe potently enhanced mRNA expression of the adipogenic genes aP2, Cd36, and Glut4 (Fig. 1C). In addition to adipogenic capacity, Lobe-treated adipocytes showed more strongly potentiated insulin-dependent glucose uptake ability than did Rosi- or Pio-treated adipocytes (Fig. 1D). These data suggest that Lobe stimulates adipocyte differentiation and insulin-dependent glucose uptake more potently than do Rosi or Pio, at least, at the same dose.
Fig. 1. TZDs stimulate adipocyte function and reduce pro-inflammatory genes in adipocytes and macrophages.
3T3-L1 cells were treated with 10 nM of Lobe, Rosi, Pio, or vehicle (DMSO) during adipogenesis. (A) Microscopic images (magnification, 100×), (B) Oil-red-O staining (magnification, 100×) and (C) adipogenic gene expression were assessed by RT-qPCR. The data represent the mean ± SD for n = 3 in each group. **P < 0.01, ***P < 0.001 vs. vehicle group; #P < 0.05, ##P < 0.01 vs. Rosi group; $$$P < 0.001 vs. Pio group by one-way ANOVA followed by Tukey’s post-hoc test. Also, (D) insulin-stimulated glucose uptake was analyzed. The data represent the mean ± SD for n = 3 in each group. ***P < 0.001 vs. insulin-treated vehicle group; ###P < 0.001 vs. insulin-treated Rosi group; $$$P < 0.001 vs. insulin-treated Pio group by two-way ANOVA followed by Bonferroni’s post-hoc test. (E) Differentiated 3T3-L1 adipocytes were pretreated with 10 nM of Lobe, Rosi, or Pio for 4 h before TNFα (10 ng ml−1) treatment for 14 h. After treatment, pro-inflammatory gene expression was analyzed by RT-qPCR. (F) RAW264.7 cells were pretreated with 5 μM of Lobe, Rosi, or Pio for 4 h before LPS (10 ng ml−1) treatment for 6 h. After treatment, pro-inflammatory gene expression was analyzed by RT-qPCR. The data represent the mean ± SD (n = 4 per group). *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle, pro-inflammatory stimuli group; ##P < 0.01 vs. Rosi group; $P < 0.05, $$P < 0.01, $$$P < 0.001 vs. Pio group by one-way ANOVA followed by Tukey’s post-hoc test. All RT-qPCR data were normalized to the mRNA level of cyclophilin. Veh, vehicle; Lobe, lobeglitazone; Rosi, rosiglitazone; Pio, pioglitazone; n.s., not significant.
Effects of the three TZDs on pro-inflammatory gene regulation in adipocytes and macrophages
To investigate the potential suppressive effects of the three TZDs on pro-inflammatory gene expression, differentiated 3T3-L1 adipocytes and RAW264.7 macrophages were exposed to inflammatory stimuli such as TNFα or LPS, respectively, with or without TZDs, and then subjected to pro-inflammatory gene expression analysis. As shown in Fig. 1E, TNFα significantly induced mRNA levels of pro-inflammatory genes such as Rantes, Mcp-1, and Saa3 in adipocytes. In the presence of Lobe or Rosi, TNFα-induced pro-inflammatory gene expression was significantly downregulated. Similarly, Lobe-treated macrophages showed suppressed LPS-mediated pro-inflammatory gene expression (Fig. 1F). These results suggest that Lobe can suppress pro-inflammatory genes in both macrophages and adipocytes, as can Rosi or Pio.
Effects of the three TZDs on serum profiles and glucose metabolism in db/db mice
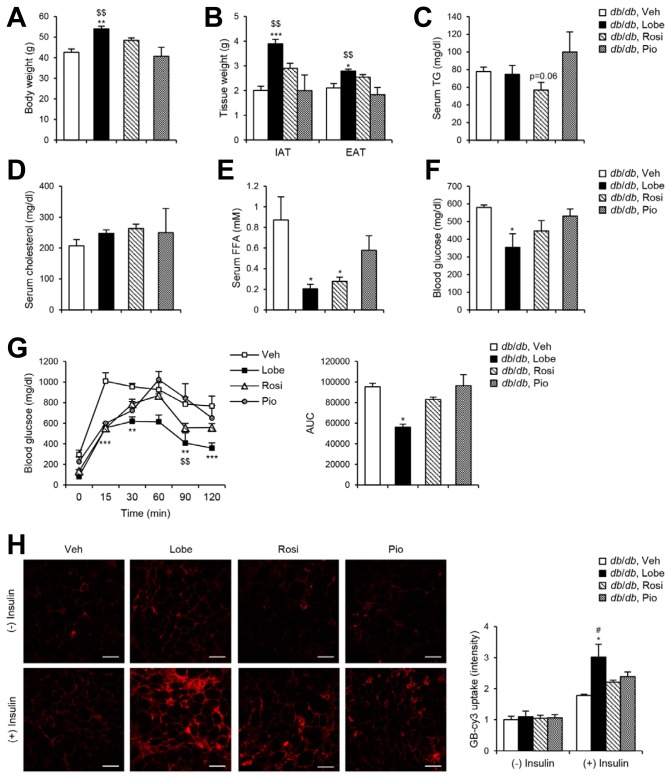
It has been well known that TZDs improve glucose homeostasis and insulin sensitivity in diabetic subjects (Yki-Järvinen, 2004). To investigate the effects of the three TZDs on various metabolic parameters, obese and diabetic db/db mice were given TZDs (3 mg kg−1) daily for four weeks. In db/db mice, Lobe induced significantly increased body weight and WAT mass compared to vehicle or the other TZDs (Figs. 2A and 2B), which might be attributable to a potent adipogenic activity of Lobe via PPARγ activity (Fig. 1). While the levels of serum triglycerides and cholesterol in db/db mice were not significantly altered by TZDs (Figs. 2C and 2D), Lobe and Rosi markedly reduced the level of serum free fatty acids (Fig. 2E). To assess the effects of TZDs on systemic glucose metabolism, ad libitum blood glucose levels in db/db mice were measured. Lobe remarkably alleviated hyperglycemia compared to the other TZDs (Fig. 2F). Next, we investigated whether Lobe might effectively ameliorate glucose intolerance in db/db mice. The effect of Lobe on glucose sensitivity was more potent than that of Rosi or Pio as indicated by a glucose tolerance test (Fig. 2G). To investigate the potency of TZDs to enhance insulin-dependent glucose uptake, ex vivo uptake of the glucose bioprobe GB-Cy3 was assessed in WAT of db/db mice. As shown in Fig. 2H, GB-Cy3 uptake was elevated more strongly in the WAT of Lobe-treated db/db mice than in that of mice treated with the other TZDs, which was consistent with in vitro glucose uptake data (Fig. 1D). These results suggest that Lobe more potently improves glucose and lipid dysregulation in obese and diabetic animals than Rosi or Pio.
Fig. 2. Lobe ameliorates serum FFA level and glucose intolerance in db/db mice.
db/db mice were orally administered daily with 3 mg kg−1 of Lobe, Rosi, or Pio and vehicle for 4 weeks. (A) Body weight, (B) white fat mass, (C) serum triglyceride (TG), (D) serum cholesterol, (E) serum free fatty acid (FFA), and (F) blood glucose levels were measured. The data represent the mean ± SEM (n = 3–5). *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle group; $$P < 0.01 vs. Pio group by one-way ANOVA followed by Tukey’s post-hoc test. (G) Oral glucose tolerance test (OGTT) was performed. The data represent the mean ± SEM (n = 3–5). *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle group; $$P < 0.01 vs. Pio group by two-way ANOVA followed by Bonferroni’s post-hoc test. (H) EAT fragments isolated from drug-treated db/db mice were incubated with or without insulin, and then treated with glucose bioprobe (GB-Cy3). Scale bars, 100 μm. Relative GB-Cy3 intensity per cell was analyzed using ImageJ. The data represent the mean ± SD for n = 3 in each group. *P < 0.05 vs. insulin-treated vehicle group; #P < 0.05 vs. insulin-treated Rosi group by two-way ANOVA followed by Bon-ferroni’s post-hoc test.
Effects of the three TZDs on adipose tissue inflammation in db/db mice
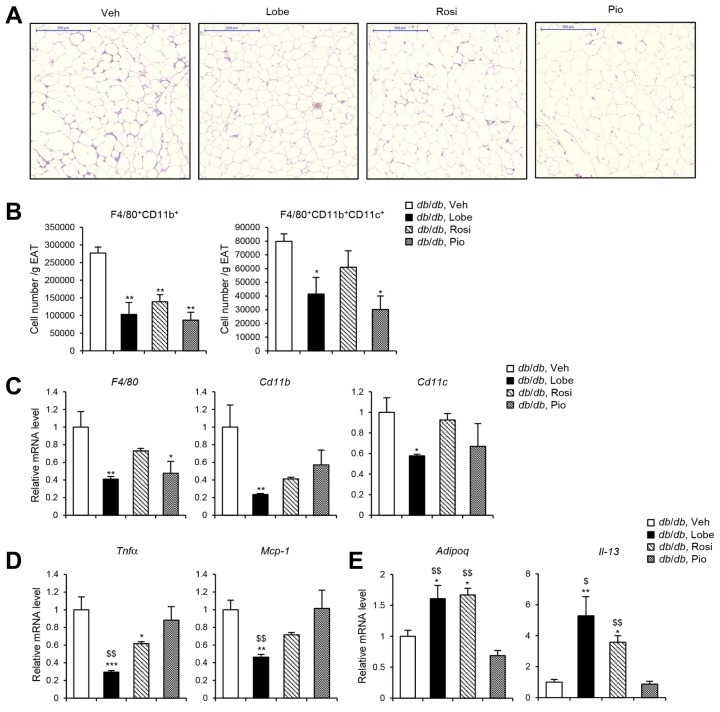
Adipose tissue inflammation is one of the major etiologies of obesity-induced insulin resistance (Choe et al., 2016; Huh et al., 2014; Olefsky and Glass, 2010). To evaluate potential inhibitory effects of TZDs on adipose tissue inflammation, we examined macrophage accumulation in EAT. In db/db mice, all three TZDs decreased the appearance of crown-like structures to a similar extent (Fig. 3A). All three TZDs reduced the total number of F4/80+CD11b+ cells (i.e., total macrophages) in the EAT to a similar extent reduced, whereas the total number of F4/80+CD11b+CD11c+ cells (i.e., M1-like macrophages) was more strongly decreased when mice had received Lobe or Pio (Fig. 3B). Consistent with these findings, Lobe more effectively downregulated the mRNA levels of macrophage genes including F4/80, Cd11b, and Cd11c, as well as those of the pro-inflammatory cytokine genes such as Tnfα and Mcp-1 than did Rosi or Pio (Figs. 3C and 3D). In contrast, the mRNA levels of the anti-inflammatory genes such as Adipoq and Il-13 in EAT of db/db mice appeared to be increased more strongly by Lobe or Rosi (Fig. 3E). These data suggest that Lobe effectively suppresses adipose tissue inflammation, eventually leading to improvement in insulin resistance in obese animals.
Fig. 3. TZDs attenuate adipose tissue inflammation in db/db mice.
Lobe, Rosi, or Pio was treated to db/db mice for 4 weeks. (A) Crown-like structure was detected in EAT by hematoxylin and eosin (H&E) staining. Scale bars, 500 μm. (B) Macrophage accumulation in EAT was assessed by flow cytometric analysis. Total numbers of F4/80+CD11b+ and F4/80+CD11b+CD11c+ cells in SVCs per gram of EATs were determined. Relative mRNA levels of (C) macrophage markers, (D) pro-inflammatory cytokine and chemokine genes, and (E) anti-inflammatory genes were determined in EAT by RT-qPCR. All data represent the mean ± SEM (n = 3–5). *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle group; $P < 0.05, $$P < 0.01 vs. Pio group by one-way ANOVA followed by Tukey’s post-hoc test. All RT-qPCR data were normalized to the mRNA level of cyclophilin.
Effects of major TZDs on thermogenic activity and beige adipocyte formation
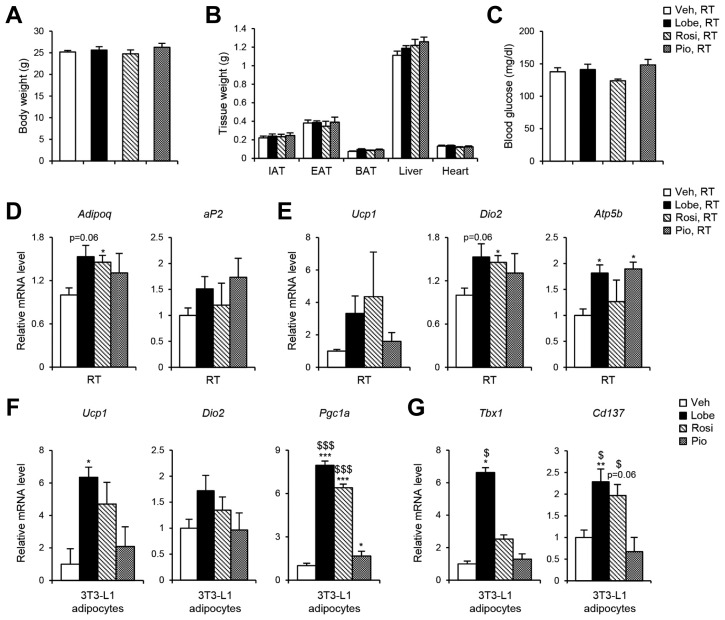
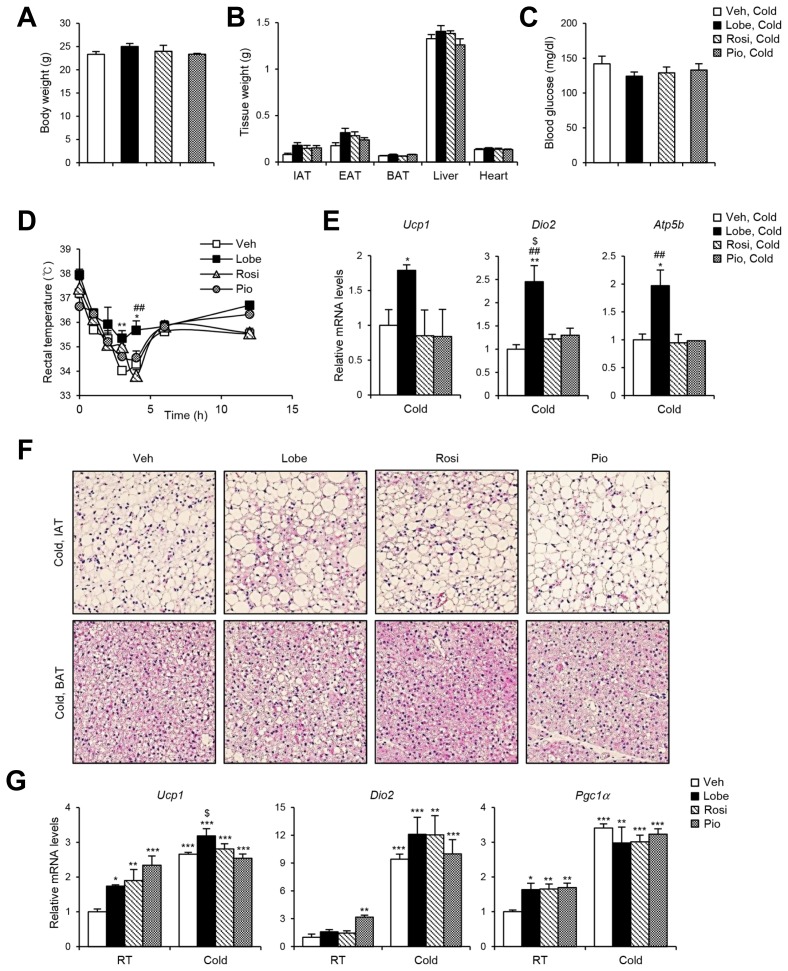
It has been demonstrated that PPARγ agonists augment thermogenic programming in WAT and BAT (Nedergaard et al., 2005; Ohno et al., 2012). To examine the capacity of the three TZDs to affect thermogenic gene expression, C57BL/6 lean mice were administered TZDs (10 mg kg−1) daily for 12 days. In lean mice, TZDs did not significantly alter body weight, tissue weight, and blood glucose level (Figs. 4A–4C). However, mRNA levels of the adipogenic genes such as Adi-poq and aP2 and the thermogenic genes such as Ucp1, Dio2, and Atp5b tended to be increased in inguinal white adipose tissue (IAT) of TZD-injected mice (Figs. 4D and 4E). To investigate the effects of TZDs on thermogenic gene regulation in vitro, expression of thermogenic genes including Ucp1, Dio2, Pgc1a and beige marker genes including Tbx1 and Cd137 were analyzed in TZDs-treated 3T3-L1 adipocytes. As shown in Figs. 4F and 4G, the levels of thermogenic and beige marker gene expression were potentiated by Lobe or Rosi. Compared to Rosi or Pio, Lobe appeared to be more potent to enhance thermogenic gene expression in adipocytes. To further investigate whether TZDs would stimulate thermogenic activity, TZDs-injected mice were exposed to cold temperature (4°C for 24 h. Upon cold exposure, body weight, weight of various metabolic tissue, and blood glucose level were not significantly different regardless of TZDs administration (Figs. 5A–5C). Consistent with gene expression data, Lobe-treated mice exhibited better cold-tolerance than the mice injected with the other TZDs (Fig. 5D). As indicated in Fig. 5E, thermogenic gene expression in IAT of Lobe-injected mice was more significantly upregulated upon cold than in other mice. Moreover, the formation of small and multilocular lipid droplets in IAT was more obvious in Lobe-treated mice (Fig. 5F). On the other hand, in BAT, histology and thermogenic gene expression profiles were not significantly different among all TZD-treated mice (Figs. 5F and 5G). Collectively, these results imply that Lobe might induce functional beige adipocyte formation in IAT, eventually leading to cold-induced thermogenesis.
Fig. 4. Lobe promotes expressions of thermogenic genes in IAT.
C57BL/6J mice were injected with 10 mg kg−1 of Lobe, Rosi, or Pio for 12 days. (A) Body weight, (B) tissue weights, (C) blood glucose level were measured. Relative mRNA levels of (D) PPARγ target genes and (E) thermogenic genes were measured in IAT by RT-qPCR. The RT-qPCR data were normalized to the mRNA level of Tbp. (F) Thermogenic and (G) beige marker gene expressions were measured in 3T3-L1 adipocytes by RT-qPCR. The RT-qPCR data were normalized to the mRNA level of Gapdh. All data represent the mean ± SEM (n = 3–4 per group). *P < 0.05, **P < 0.01, ***P < 0.01 vs. vehicle group; $P < 0.05, $$$P < 0.001 vs. Pio group by one-way ANOVA followed by Tukey’s post-hoc test.
Fig. 5. Lobe increases cold-induced thermogenesis and beige adipocyte formation.
C57BL/6J mice were injected with Lobe, Rosi, or Pio for 12 days, and then placed at 4° for 24 h. (A) Body weight, (B) tissue weights, (C) blood glucose level were assessed. The data represent the mean ± SEM (n = 4 per group). (D) Rectal temperature was measured after cold exposure. The data represent the mean ± SEM (n = 4 per group). *P < 0.05, **P < 0.01 vs. vehicle group; ##P < 0.01 vs. Rosi group by two-way ANOVA followed by Bonferroni’s post-hoc test. After 24 h cold exposure, (E) mRNA levels of thermogenic genes in IAT and (F) H&E staining of IAT and BAT (200× magnification) were analyzed. The data represent the mean ± SEM (n = 4 per group). *P < 0.05, **P < 0.01 vs. vehicle group; ##P < 0.01 vs. Rosi group; $P < 0.05 vs. Pio group by one-way ANOVA followed by Tukey’s post-hoc test. The RT-qPCR data were normalized to the mRNA level of Tbp. (G) Levels of thermogenic gene expression were analyzed. The data represent the mean ± SEM (n = 4 per group). *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle, RT group; $P < 0.05 vs. Pio, Cold group by two-way ANOVA followed by Bonferroni’s post-hoc test. The RT-qPCR data were normalized to the mRNA level of cyclophilin.
DISCUSSION
In WAT, PPARγ plays crucial roles in the regulation of glucose homeostasis and lipid metabolism (Tontonoz and Spiegelman, 2008). Pharmacological modulation of PPARγ activity is a well-established strategy to treat metabolic diseases (Semple et al., 2006). TZDs share a 2, 4-TZD chemical ring structure and are used as insulin-sensitizers (Soccio et al., 2014). In addition to TZDs, several other chemicals have been reported to act as PPARγ agonists. For example, sulfonamides are structurally distinct from TZDs with different PPARγ binding properties, but they exhibit insulin-sensitizing effects similar to those of TZDs (Li et al., 2008). Moreover, chemical modulators of PPARγ phosphorylation have been reported to have insulin sensitizing and glucose lowering effects (Choi et al., 2010). Among TZDs, Rosi and Pio had been approved by the FDA for the treatment of T2D in adults. However, none of these drug is currently approved to treat prediabetes or metabolic disorders because of their detrimental side effects, such as heart failure, liver toxicity, and potential bladder cancer risk (Rizos et al., 2009), implying that TZDs can have different effects in different organs, depending on dosage. Thus, it is important to comparatively analyze the efficacies the TZDs at the same concentration in metabolic organs.
Several lines of evidence suggest that the newly available TZD, Lobe, effectively ameliorates insulin resistance in obese and diabetic animals. Firstly, Lobe more potently increased insulin-dependent glucose uptake in adipocytes and adipose tissue than did Rosi or Pio. Secondly, Lobe significantly inhibited pro-inflammatory gene expression in macrophages and adipocytes. Lastly, Lobe efficiently improved whole-body energy metabolism, including glucose tolerance and lipid metabolism, in db/db mice.
Lobe seemed to exhibit favorable effects on metabolic control compared with conventional TZDs. One of the plausible explanations is the different affinity of TZDs for PPARγ. Very recently, it has been reported that Lobe shows higher affinity for PPARγ than Rosi or Pio (Lee et al., 2017). Lobe was designed by modification of the chemical structure of Rosi through pyrimidine substitution (Lee et al., 2009). This modification induces another hydrophobic interaction besides the canonical ligand binding site of PPARγ, resulting in enhanced affinity toward PPARγ (Jang et al., 2018; Lee et al., 2017). We found that Lobe more strongly enhanced adipocyte differentiation as compared to Rosi or Pio by more strongly inducing PPARγ target genes, which might result from increased PPARγ activity owing to the higher affinity for PPARγ. For the same reason, it is plausible to assume that Lobe would exhibit clinical effects at relatively lower doses than conventional TZDs (Jin et al., 2015; Lee et al., 2005). In turn, the usage of lower doses in clinic can be expected to alleviate the incidence of adverse effects such as cardiovascular disease and bladder cancer (Kim et al., 2017b; 2015b; Lee et al., 2014a; Moon et al., 2014).
PPARγ agonists induce thermogenic gene expression in subcutaneous adipose tissue including IAT (Ohno et al., 2012). Although most TZD studies on thermogenic gene regulation have been performed under room temperature (RT) condition (Carriere et al., 2014; Qiang et al., 2012; Rothwell et al., 1987), little information is available whether PPARγ agonist-induced beige adipocyte formation would be thermogenic upon cold exposure in lean mice. Our data suggest that Lobe would be involved in the upregulation of body temperature and thermogenic gene expression upon cold exposure. While three TZDs were able to stimulate thermogenic gene expression in BAT and IAT under RT condition, Lobe more potently induced beige adipocyte formation with augmented thermogenic gene expression and altered lipid droplet morphology upon cold exposure, compared to Rosi or Pio. In IAT, Lobe effectively stimulated cold-induced thermogenesis, accompanied by functional beige adipocyte formation. Given that beige adipocytes appear to attenuate obesity-induced metabolic disorders by enhancing whole-body energy expenditure (Ikeda et al., 2018), it is likely that Lobe might ameliorate systemic glucose intolerance, at least in part, through activating beige adipocyte formation. Accordingly, it has been very recently reported that 20 weeks of Lobe (1 mg kg−1) treatment increased thermogenic potentials in db/db mice (Kim et al., 2017a). Therefore, it remains to be elucidated whether energy expenditure might be increased in Lobe-treated lean mice.
In conclusion, our data suggest that Lobe is a potent TZD that acts to enhance glucose uptake and reduce adipose tissue inflammation in obese and diabetic animals. In view of the development of novel PPARγ targeting drugs, its high efficiency at low concentration represents one of the key advantages of Lobe. This study focused on effects of the TZDs in adipose tissue, comparative effects in other metabolic organs remain to be investigated in future.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the South Korea government (Ministry of Science, ICT, and Future Planning; no. 2011-0018312).
REFERENCES
- Ahmadian M., Suh J.M., Hah N., Liddle C., Atkins A.R., Downes M., Evans R.M. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;99:557. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfadda A.A., Sallam R.M., Gul R., Hwang I., Ka S. Endophilin A2: a potential link to adiposity and beyond. Mol Cells. 2017;40:855–863. doi: 10.14348/molcells.2017.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berria R., Glass L., Mahankali A., Miyazaki Y., Monroy A., De Filippis E., Cusi K., Cersosimo E., Defronzo R.A., Gastaldelli A. Reduction in hematocrit and hemoglobin following pioglitazone treatment is not hemodilutional in Type II diabetes mellitus. Clin Pharmacol Therap. 2007;82:275–281. doi: 10.1038/sj.clpt.6100146. [DOI] [PubMed] [Google Scholar]
- Bouhlel M.A., Derudas B., Rigamonti E., Dièvart R., Brozek J., Haulon S., Zawadzki C., Jude B., Torpier G., Marx N., et al. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metabol. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Carriere A., Jeanson Y., Berger-Muller S., Andre M., Chenouard V., Arnaud E., Barreau C., Walther R., Galinier A., Wdziekonski B., et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes. 2014;63:3253–3265. doi: 10.2337/db13-1885. [DOI] [PubMed] [Google Scholar]
- Choe S.S., Huh J.Y., Hwang I.J., Kim J.I., Kim J.B. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol. 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.H., Banks A.S., Estall J.L., Kajimura S., Bostrom P., Laznik D., Ruas J.L., Chalmers M.J., Kamenecka T.M., Bluher M., et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca V.A. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151–156. doi: 10.2337/dc09-S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross B., Pawlak M., Lefebvre P., Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. 2017;13:36–49. doi: 10.1038/nrendo.2016.135. [DOI] [PubMed] [Google Scholar]
- He H., Tao H., Xiong H., Duan S.Z., McGowan F.X., Jr, Mortensen R.M., Balschi J.A. Rosiglitazone causes cardiotoxicity via peroxisome proliferator-activated receptor gamma-independent mitochondrial oxidative stress in mouse hearts. Toxicol Sci. 2014;138:468–481. doi: 10.1093/toxsci/kfu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh J.Y., Park Y.J., Ham M., Kim J.B. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells. 2014;37:365–371. doi: 10.14348/molcells.2014.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K., Maretich P., Kajimura S. The common and distinct features of brown and beige adipocytes. Trends Endocrinol Metabol. 2018;29:191–200. doi: 10.1016/j.tem.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J.Y., Bae H., Lee Y.J., Choi Y.I., Kim H.J., Park S.B., Suh S.W., Kim S.W., Han B.W. Structural basis for the enhanced anti-diabetic efficacy of lobeglitazone on PPARγ. Sci Rep. 2018;8:31. doi: 10.1038/s41598-017-18274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenssen T., Hartmann A. Emerging treatments for post-transplantation diabetes mellitus. Nat Rev Nephrol. 2015;11:465–477. doi: 10.1038/nrneph.2015.59. [DOI] [PubMed] [Google Scholar]
- Jin S.M., Park C.Y., Cho Y.M., Ku B.J., Ahn C.W., Cha B.S., Min K.W., Sung Y.A., Baik S.H., Lee K.W., et al. Lobeglitazone and pioglitazone as add-ons to metformin for patients with type 2 diabetes: a 24-week, multicentre, randomized, double-blind, parallel-group, active-controlled, phase III clinical trial with a 28-week extension. Diabetes Obes Metabol. 2015;17:599–602. doi: 10.1111/dom.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn S.E., Haffner S.M., Heise M.A., Herman W.H., Holman R.R., Jones N.P., Kravitz B.G., Lachin J.M., O’Neill M.C., Zinman B., et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- Kim J.I., Huh J.Y., Sohn J.H., Choe S.S., Lee Y.S., Lim C.Y., Jo A., Park S.B., Han W., Kim J.B. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol Cell Biol. 2015a;35:1686–1699. doi: 10.1128/MCB.01321-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Kim S.G., Kim D.M., Woo J.T., Jang H.C., Chung C.H., Ko K.S., Park J.H., Park Y.S., Kim S.J., et al. Safety and efficacy of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 52 weeks: An open-label extension study. Diabetes Res Clin Pract. 2015b;110:e27–30. doi: 10.1016/j.diabres.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Kim G., Lee Y.H., Yun M.R., Lee J.Y., Shin E.G., Lee B.W., Kang E.S., Cha B.S. Effects of lobeglitazone, a novel thiazolidinedione, on adipose tissue remodeling and brown and beige adipose tissue development in db/db mice. Int J Obes. 2017a;42:545–551. doi: 10.1038/ijo.2017.222. [DOI] [PubMed] [Google Scholar]
- Kim K.M., Jin H.J., Lee S.Y., Maeng H.J., Lee G.Y., Oh T.J., Choi S.H., Jang H.C., Lim S. Effects of lobeglitazone, a new thiazolidinedione, on osteoblastogenesis and bone mineral density in mice. Endocrinol Metabol. 2017b;32:389–395. doi: 10.3803/EnM.2017.32.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlroser J., Mathai J., Reichheld J., Banner B.F., Bonkovsky H.L. Hepatotoxicity due to troglitazone: report of two cases and review of adverse events reported to the United States Food and Drug Administration. Am J Gastroenterol. 2000;95:272–276. doi: 10.1111/j.1572-0241.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- Lee H.W., Kim B.Y., Ahn J.B., Kang S.K., Lee J.H., Shin J.S., Ahn S.K., Lee S.J., Yoon S.S. Molecular design, synthesis, and hypoglycemic and hypolipidemic activities of novel pyrimidine derivatives having thiazolidinedione. Eur J Med Chem. 2005;40:862–874. doi: 10.1016/j.ejmech.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Woo Y.A., Hwang I.C., Kim C.Y., Kim D.D., Shim C.K., Chung S.J. Quantification of CKD-501, lobeglitazone, in rat plasma using a liquid-chromatography/tandem mass spectrometry method and its applications to pharmacokinetic studies. J Pharm Biomed Anal. 2009;50:872–877. doi: 10.1016/j.jpba.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Lee H.S., Chang M., Lee J.E., Kim W., Hwang I.C., Kim D.H., Park H.K., Choi H.J., Jo W., Cha S.W., et al. Carcinogenicity study of CKD-501, a novel dual peroxisome proliferator-activated receptors alpha and gamma agonist, following oral administration to Sprague Dawley rats for 94–101 weeks. Regul Toxicol Pharmacol. 2014a;69:207–216. doi: 10.1016/j.yrtph.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Lee G.Y., Jang H., Choe S.S., Koo S.H., Kim J.B. RNF20 regulates hepatic lipid metabolism through PKA-dependent SREBP1c degradation. Hepatology 6. 2014b:844–857. doi: 10.1002/hep.27011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.A., Tan L., Yang H., Im Y.G., Im Y.J. Structures of PPARgamma complexed with lobeglitazone and pioglitazone reveal key determinants for the recognition of antidiabetic drugs. Sci Rep. 2017;7:16837. doi: 10.1038/s41598-017-17082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J.M., Moore L.B., Smith-Oliver T.A., Wilkison W.O., Willson T.M., Kliewer S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang Z., Furukawa N., Escaron P., Weiszmann J., Lee G., Lindstrom M., Liu J., Liu X., Xu H., et al. T2384, a novel antidiabetic agent with unique peroxisome proliferator-activated receptor gamma binding properties. J Biol Chem. 2008;283:9168–9176. doi: 10.1074/jbc.M800104200. [DOI] [PubMed] [Google Scholar]
- Lim S., Lee K.S., Lee J.E., Park H.S., Kim K.M., Moon J.H., Choi S.H., Park K.S., Kim Y.B., Jang H.C. Effect of a new PPAR-gamma agonist, lobeglitazone, on neointimal formation after balloon injury in rats and the development of atherosclerosis. Atherosclerosis. 2015;243:107–119. doi: 10.1016/j.atherosclerosis.2015.08.037. [DOI] [PubMed] [Google Scholar]
- Moon K.S., Lee J.E., Lee H.S., Hwang I.C., Kim D.H., Park H.K., Choi H.J., Jo W., Son W.C., Yun H.I. CKD-501, a novel selective PPARgamma agonist, shows no carcinogenic potential in ICR mice following oral administration for 104 weeks. J Appl Toxicol. 2014;34:1271–1284. doi: 10.1002/jat.2918. [DOI] [PubMed] [Google Scholar]
- Nedergaard J., Petrovic N., Lindgren E.M., Jacobsson A., Cannon B. PPARγ in the control of brown adipocyte differentiation. Biochim Biophys Acta. 2005;1740:293–304. doi: 10.1016/j.bbadis.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Eng J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Ohno H., Shinoda K., Spiegelman B.M., Kajimura S. PPAR agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metabol. 2012;15:395–404. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Ann Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Qiang L., Wang L., Kon N., Zhao W., Lee S., Zhang Y., Rosenbaum M., Zhao Y., Gu W., Farmer S.R., et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizos C.V., Elisaf M.S., Mikhailidis D.P., Liberopoulos E.N. How safe is the use of thiazolidinediones in clinical practice? Exp Opin Drug Safety. 2009;8:15–32. doi: 10.1517/14740330802597821. [DOI] [PubMed] [Google Scholar]
- Rothwell N.J., Stock M.J., Tedstone A.E. Effects of ciglitazone on energy balance, thermogenesis and brown fat activity in the rat. Mol Cell Endocrinol. 1987;51:253–257. doi: 10.1016/0303-7207(87)90035-9. [DOI] [PubMed] [Google Scholar]
- Semple R.K., Chatterjee V.K., O’Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soccio R.E., Chen E.R., Lazar M.A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metabol. 2014;20:573–591. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn J.H., Lee Y.K., Han J.S., Jeon Y.G., Kim J.I., Choe S.S., Kim S.J., Yoo H.J., Kim J.B. Perilipin 1 (Plin1) deficiency promotes inflammatory responses in lean adipose tissue through lipid dysregulation. J Biol Chem. 2018 doi: 10.1074/jbc.RA118.003541. pii: jbc.RA118.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola D., Rossi L., Schianca G.P.C., Maffioli P., Bigliocca M., Mella R., Corlianò F., Fra G.P., Bartoli E., Derosa G. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11:840–848. doi: 10.5114/aoms.2015.53304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi C.M., Emanuelli B., Kahn C.R. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Tontonoz P., Spiegelman B.M. Fat and beyond: the diverse biology of PPARgamma. Ann Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Yau H., Rivera K., Lomonaco R., Cusi K. The future of thiazolidinedione therapy in the management of type 2 diabetes mellitus. Curr Diabetes Rep. 2013;13:329–341. doi: 10.1007/s11892-013-0378-8. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]